Abstract
Rationale: The use of neuromuscular blocking agents (NMBAs) has been shown to be valuable in improving successful tracheal intubation in the operating room and emergency department. However, data on NMBA use in critically ill intensive care unit (ICU) patients are lacking. Furthermore, there are no data on NMBA use with video laryngoscopy.
Objectives: To evaluate the effect of NMBA use on first-attempt success (FAS) with tracheal intubation in the ICU.
Methods: Single-center observational study of 709 consecutive patients intubated in the medical ICU of a university medical center from January 1, 2012 to June 30, 2014. Data were collected prospectively through a continuous quality improvement program on all patients intubated in the ICU over the study period. Data relating to patient demographics, intubation, and complications were analyzed. We used propensity score (propensity to use an NMBA) matching to generate 5,000 data sets of cases (failed first intubation attempts) matched to controls (successful first attempts) and conditional logistic regression to analyze the results.
Measurements and Main Results: There were no significant differences in patient demographics, except median total difficult airway characteristics were higher in the non–NMBA group (2 vs. 1, P < 0.001). There were significant differences in the sedative used between groups and the operator level of training. More patients who were given NMBAs received etomidate (83 vs. 35%) and more patients in the non–NMBA group received ketamine (39 vs. 9%) (P < 0.001). The FAS for NMBA use was 80.9% (401/496) compared with 69.6% (117/168) for non–NMBA use (P = 0.003). The summary odds ratio for FAS when an NMBA was used from the propensity matched analyses was 2.37 (95% confidence interval, 1.36–4.88). In the subgroup of patients intubated with a video laryngoscope, propensity-adjusted odds of FAS with the use of an NMBA was 2.50 (1.43–4.37; P < 0.001). There were no differences in procedurally related complications between groups.
Conclusions: After controlling for potential confounders, this propensity-adjusted analysis demonstrates improved odds of FAS at intubation in the ICU with the use of an NMBA. This improvement in FAS is seen even with the use of a video laryngoscope.
Keywords: neuromuscular blockade, rapid sequence intubation, intubation, intensive care, airway management
Tracheal intubation in critically ill patients is a commonly required procedure that is fraught with risk. Patients intubated in the intensive care unit (ICU) are at risk of procedurally related complications, and do not have the physiologic reserve to tolerate prolonged intubation attempts (1–6). These intubations are frequently unplanned and often require emergent management with little time for preparation. As a result, complications related to airway management in the ICU are more frequent and severe than complications from airway management in the operating room and emergency department (3, 5, 7–9). Previous literature has shown that intubation-related complications increase with each successive attempt (10–13), yet there remains significant controversy over who should perform intubations in the ICU, what device(s) should be used, and what the optimal method of pharmacologic assistance is (7, 14–33).
Neuromuscular blocking agent (NMBA) use has been shown to improve first-attempt success (FAS) and decrease procedurally related complications in the operating room and emergency department, yet still remains controversial in the ICU (4, 34–36). Furthermore, there is no literature to our knowledge describing the effect of neuromuscular blockade when using video laryngoscopy (VL), which is becoming more widely available in the ICU. The goal of this study was to compare FAS in patients intubated with the use of an NMBA in the medical ICU with patients intubated without an NMBA. Subgroup analyses were identified a priori, which included comparing FAS and intubating conditions achieved with succinylcholine versus rocuronium, and the effect of neuromuscular blockade on the patients intubated with VL.
Methods
Study Design
This is a single-center prospective observational analysis of 664 consecutive ICU intubations prospectively recorded in a continuous quality improvement database from January 1, 2012 to June 30, 2014 at a major academic referral center with a 20+ bed medical ICU. This project was granted exemption from full review and approved by the University of Arizona’s Institutional Review Board.
Setting and Population
This ICU service is affiliated with accredited 3-year pulmonary/critical care medicine (CCM) and two-year CCM fellowship programs with a total of 16 fellows. Each teaching team is staffed with an attending (pulmonary/CCM or CCM), a fellow (Postgraduate Year [PGY] 4–6), and residents (Internal Medicine PGY 1–3 and Emergency Medicine PGY 2). Occasionally, fellows from anesthesiology or surgical critical care fellowships rotate through the medical ICU service. All intubations are performed under supervision by faculty skilled in airway management. All fellows in this program participate in an ongoing didactic airway curriculum consisting of lectures and a rigorous monthly simulation laboratory experience aimed at the recognition and management of the difficult airway.
Selection of Participants
All patients intubated using direct laryngoscopy (DL) or VL as the initial device were included in this study. Patients were excluded if they were intubated with a flexible fiberoptic bronchoscope or other alternative device. For the duration of the study period, DL was available in all sizes of Macintosh and Miller Blades. VL was available throughout the entire study period. Our ICU has the following video laryngoscopes: GlideScope (Verathon, Bothell, WA) with both reusable and disposable blade configurations, with blade sizes 3 and 4, and the C-MAC (Karl Storz, Tuttlingen, Germany) with Macintosh-type blade, sizes 3 and 4. Recently, we added the McGrath MAC (Covidien, Mansfield, MA) with Macintosh-type blade, sizes 2–4, and have trialed the King Vision (King Systems, Nobelsville, IN). When the C-MAC or McGrath MAC were used as a direct laryngoscope, the attempt was considered a VL attempt regardless of whether the operator looked at the monitor during the attempt.
Methods of Measurement
After each intubation, the operator completed a data collection form, which included the following information: patient demographics, operator specialty, operator PGY, indication for intubation, paralytic agent, sedative agent, device(s) used, presence of certain difficult airway characteristics (DACs), preoxygenation methods, the Cormack-Lehane (CL) view and percentage of glottic opening (POGO) score of the airway, number of attempts at intubation, and the outcome of each attempt, including complications.
Methods of intubation included rapid sequence intubation in which a paralytic agent was used, oral intubation in which a sedative agent only was used, and oral intubation in which no medications were used. For the purposes of this analysis, intubations using sedation only or no medications were combined into one cohort and compared with intubations in which a paralytic was used.
Standard preoperative difficult airway predictors have been shown to be challenging to apply in the emergency setting, due to lack of patient cooperation and the urgency to complete the intubation rapidly (37, 38). These difficult airway predictors and scoring systems, such as the MACOCHA (Mallampati score III or IV, apnea syndrome [obstructive], cervical spine limitation, mouth opening <3 cm, coma, hypoxia, anesthesiologist nontrained) score were developed primarily for DL and have not been validated with VL, which has become our primary device (26). Thus, we used a list of DACs that were feasible for the operator to determine before intubation in an emergent setting by simple examination of the patient. These include both anatomic and physiologic DACs. The anatomic DACs include: the presence of blood, vomit, or secretions in the airway, cervical immobility (intrinsic or due to a cervical collar), obesity, large tongue, short neck, small mandible, facial or neck trauma, airway edema, and limited mouth opening. Physiologic DACs include hypotension and hypoxemia, which may make the process of intubation more challenging.
An intubation attempt was defined as insertion of the laryngoscope blade into the oropharynx regardless of whether an attempt was made to pass the endotracheal tube (ETT). Successful intubation was defined as correct placement of the ETT in the trachea, as confirmed by capnometry, pulse oximetry, chest auscultation, observation of chest excursion, absence of epigastric sounds, and misting of the ETT. If there was uncertainty about ETT placement by the operator and the tube was removed and replaced, it was considered an esophageal intubation. First attempt success was defined as successful tracheal intubation on the initial laryngoscope insertion.
Complications evaluated include: hypotension, desaturation, esophageal intubation, aspiration, and airway trauma. Hypotension was defined as any drop in systolic blood pressure (SBP) requiring intervention, such as fluid resuscitation or initiation, or titration of vasopressors that occurs during or within 5 minutes of the intubation. Desaturation was defined as a decline in oxygen saturation greater than 10% from the baseline during the intubation procedure. Aspiration included any witnessed aspiration of gastric contents during the intubation attempt. Airway trauma included any lacerations, swelling, edema, or dental injury related to the airway manipulation.
The data were then entered into the electronic database (Excel for Macintosh 2011; Microsoft, Redmond, WA).
Outcome Measures
The primary outcome measured was successful first-attempt intubation. Secondary outcome measures were glottic view obtained (CL and POGO), total attempts required, and procedurally related complications. Planned subgroup analyses were conducted comparing succinylcholine to rocuronium and examining the effect of paralysis on rate of FAS and grade of laryngoscopic view in patients intubated with VL.
Primary Data Analysis
Summary statistics were generated for patient, intubation, and operator characteristics using Fisher’s exact test for categorical variables, Kruskal-Wallis test, and Student’s t test where appropriate. A propensity score for receiving an NMBA was generated from prespecified variables expected to affect the decision to use a paralytic using the “pscore” command with logistic regression in Stata v.12.0 (StataCorp LP, College Station, TX): patient age and sex; sedative choice; number of DACs; presence of blood or vomit; cervical immobility; facial or neck trauma; airway edema; small mandible; obesity; large tongue; short neck; hypotension; hypoxemia; operator level of training; and intubation device chosen.
Certain variables might affect both the clinician’s decision to use an NMBA and simultaneously affect the likelihood of FAS. For example, a clinician might be less likely to administer an NMBA to a patient with a DAC such as obesity while obesity may simultaneously decrease the likelihood of FAS. Propensity score matching/adjustment seeks to eliminate this type of confounding by first generating a propensity score, which estimates the probability of treatment assignment, and then using the propensity score to adjust the analyses examining the treatment effect on the outcome.
The distribution of the propensity score was used to divide all intubation attempts into blocks, and the distribution of all covariates was compared between the NMBA and the non-NMBA groups within each block to satisfy the balancing property (39). Covariates that did not meet the balancing property were recoded and rechecked or excluded from propensity score generation until the balancing property was met. A P value of 0.001 was used for statistical significance when rejecting the balancing property for each covariate to account for multiple hypothesis testing. This threshold was still conservative, given the nominal corrected P value was 5.9 × 10−4 (17 covariates compared across 5 blocks [85 comparisons overall]).
We used propensity score matching to match each failed first intubation attempt (cases) to controls (FASs) with similar propensity scores. We used caliper matching with replacement using a random selection of controls within the caliper range, a method found to be a relatively robust choice when compared with other propensity score matching algorithms (40). We matched each case to one to three controls (i.e., a potentially variable number of controls per case) following the recommendations of Linden and Samuels (41) for 1 to N matching. The initial caliper width used was 0.01 propensity score units as a starting value. Controls were randomly chosen by assigning each control a random number using a random number seed (the time of day), then sorting by the random number, and then choosing the first one to three cases that were within the caliper range. For cases that did not have at least one match (i.e., there were no controls within the caliper range), we incrementally increased the caliper width by 0.005 units until at least one control was matched to the remaining cases.
We used conditional logistic regression, with each group being a control matched to one to three cases, along with clustered robust standard errors, to estimate the odds ratio (OR) of FAS given the use of an NMBA. Given that any one iteration of the matching algorithm could result in an OR different from another iteration because of the random selection of controls, we used bootstrapping to sample 5,000 case–control data sets from the original data set using the propensity score matching algorithm described previously here. Conditional logistic regression results from the 5,000 matching iterations were summarized as a final point estimate as the median of the regression coefficients (log ORs) along with the 95% confidence interval (CI; simply the lower fifth and upper 95th percentile of the distribution of the log ORs) for FAS when using an NMBA compared with not using an NMBA. The final summary OR and 95% CI was simply the three values (median, lower fifth, and upper 95th percentiles) raised to the power of e, the base of the natural logarithm.
We also used standard multivariable logistic regression using the propensity score as a covariate as a sensitivity analysis. Both methods (conditional logistic regression using propensity score–matched cases and controls and multivariable logistic regression using the propensity score as a risk adjuster) reduce the chance of bias due to the influence of nonrandomized treatment selection, and assure that, conditional on the propensity score, the distribution of measured baseline prognostic variables and confounders will be similar between the two treatment groups.
We calculated various model residual and diagnostic statistics for the conditional logistic regression models (e.g., influence and leverage residuals) and standard multivariable logistic regression models (e.g., Pearson, deviance, and Ascombe residuals), and checked all covariate patterns (cases with identical diagnostic values) that were outliers by first checking for potentially miscoded cases and then deleting individual covariate patterns one at a time and rerunning each model. Covariate patterns that changed model regression coefficients for our main independent variable (use of NMBA) by more than 20% were considered for exclusion. We used the Hosmer-Lemeshow goodness-of-fit test and calculated the area under the receiver operating characteristics curve as a measure of model discrimination for standard logistic regression models.
We used fractional polynomials to test if patient age was linearly associated with the log odds of first intubation attempt success, a requirement for continuous variables for logistic regression, and used the best fit fractional polynomial transformation of age if it significantly improved model fit.
Descriptive statistics were calculated for measured variables as means and SDs, medians and interquartile ranges (IQR), or proportions with 95% CIs, using the “exact” method, as appropriate. All statistical analyses, including logistic regression analyses, calculation of propensity scores, and case–control matching, were performed with Stata.
Results
Over the 30-month study period, a total of 711 patients were intubated. There were 42 patients excluded for flexible fiberoptic intubations, 1 patient for nasal intubation, and 4 patients because the intubation was performed by a medical student. The remaining 664 patients made up the study population. Of these patients, 496 were intubated with an NMBA, and 168 without an NMBA. Table 1 summarizes the patient and operator demographics, along with intubation characteristics. There were no differences in age, sex, individual DACs, and reason for intubation between groups. In patients for whom no NMBA was used, there was a higher median number of DACs (2 vs. 1, P <0.001) and higher use of ketamine (39.3 vs. 8.9%, P <0.001) (Table 1). Patients intubated without an NMBA were intubated by physicians with a relatively higher level of postgraduate training (P = 0.005; Table 1).
Table 1.
Patient and operator demographics
| Characteristic |
Paralytic % |
95% CI |
No Paralytic % |
95% CI |
P Value |
|---|---|---|---|---|---|
| (n = 496) | (n = 168) | ||||
| Age, yr, median, IQR | 59 | IQR, 51–68 | 59 | IQR, 47–70 | 0.62 |
| Patient sex | |||||
| Male | 59.7 (296) | 47–67 | 50.6 (84) | 52–69 | 0.05 |
| DACs | |||||
| Total DACs, median, IQR | 1 | IQR, 1–3 | 2 | IQR, 1–4 | <0.001 |
| None | 21.7 (108) | 18.2–25.6 | 15.6 (26) | 10.4–21.97 | 0.10 |
| Cervical immobilization | 2.2 (11) | 1.1–3.9 | 4.2 (7) | 1.7–8.4 | 0.18 |
| Blood in airway | 14.9 (74) | 11.9–18.3 | 16.2 (27) | 10.9–22.6 | 0.71 |
| Vomit in airway | 5.03 (25) | 3.3–7.3 | 7.2 (12) | 3.8–12.2 | 0.33 |
| Facial/neck trauma | 0.6 (3) | 0.12–1.8 | 0.6 (1) | 0.02–3.3 | 1.00 |
| Obesity | 27.8 (138) | 23.9–31.9 | 33.5 (56) | 26.4–41.2 | 0.17 |
| Short neck | 22.5 (112) | 18.9–26.5 | 28.1 (47) | 21.5–35.6 | 0.14 |
| Large tongue | 12.1 (60) | 9.3–15.3 | 16.8 (28) | 11.4–23.3 | 0.15 |
| Airway edema | 7.0 (35) | 4.95–9.7 | 10.8 (18) | 6.5–16.5 | 0.14 |
| Small mandible | 13.9 (69) | 10.97–17.24 | 19.2 (32) | 13.5–25.96 | 0.11 |
| Hypoxemia | 26.2 (130) | 22.3–30.3 | 29.9 (50) | 23.1–37.5 | 0.37 |
| Hypotension | 21.3 (106) | 17.8–25.2 | 28.1 (47) | 21.5–35.6 | 0.07 |
| Sedatives | |||||
| None | 0.2 (1) | 0.0–1.1 | 11.3 (19) | 6.95–17.1 | |
| Etomidate | 83.3 (414) | 79.9–86.6 | 34.5 (58) | 27.4–42.2 | |
| Ketamine | 8.9 (44) | 6.5–11.7 | 39.3 (66) | 31.9–47.1 | <0.001 |
| Midazolam | 2.4 (12) | 1.3–4.2 | 2.4 (4) | 0.65–5.98 | |
| Propofol | 5.0 (25) | 3.3–7.4 | 11.3 (19) | 6.9–17.1 | |
| Combination | 0 (0) | 0–0.07 | 1.2 (2) | 0.14–4.2 | |
| Paralytics | — | ||||
| None | 0 (0) | 0–0.74 | 100 (168) | — | |
| Succinylcholine | 65.1 (323) | 60.7–69.3 | — | — | |
| Rocuronium | 32.7 (162) | 28.5–36.98 | — | — | |
| Cisatracurium | 2.2 (11) | 1.1–3.9 | — | ||
| Reason for intubation | 0.55 | ||||
| Airway protection | 21.3 (106) | 17.8–25.2 | 21.4 (36) | 15.5–28.4 | |
| Respiratory failure | 47.2 (234) | 42.7–51.7 | 42.9 (72) | 35.3–50.7 | |
| Patient control | 1.2 (6) | 0.4–2.6 | 1.8 (3) | 0.4–5.1 | |
| Cardiac arrest | 1.0 (5) | 0.3–2.3 | 7.1 (12) | 3.7–12.1 | |
| Hypoxemia | 22.1 (110) | 18.6–26.1 | 20.8 (35) | 15.0–27.8 | |
| Hemodynamic instability | 3.2 (16) | 1.9–5.2 | 3.6 (6) | 1.3–7.6 | |
| Severe acidosis | 3.8 (19) | 2.3–5.9 | 2.4 (4) | 0.6–5.9 | |
| Operator PGY level | 0.005 | ||||
| 1 | 7.7 (38) | 5.5–10.4 | 6.0 (10) | 2.9–10.7 | |
| 2 | 19.8 (98) | 16.3–23.5 | 11.9 (20) | 7.4–17.8 | |
| 3 | 12.9 (64) | 10.1–16.2 | 10.7 (18) | 6.5–16.4 | |
| 4 | 24 (119) | 20.3–28.0 | 27.0 (45) | 20.3–34.2 | |
| 5 | 24.2 (120) | 20.5–28.2 | 27.4 (46) | 20.8–34.8 | |
| 6 | 9.7 (48) | 7.2–12.6 | 12.5 (21) | 7.9–18.5 | |
| Attending | 1.8 (9) | 0.8–3.4 | 4.8 (8) | 2.1–9.2 | |
| Device used | 0.10 | ||||
| Direct laryngoscopy | 20.3 (101) | 17–24.2 | 11.3 (19) | 6.9–17.1 | |
| GlideScope | 17.5 (87) | 14.3–21.2 | 20.2 (34) | 14.4–27.1 | |
| C-MAC | 61 (302) | 56.4–65.2 | 67.2 (113) | 59.6–74.3 | |
| Other video laryngoscope | 1.2 (6) | 0.4–2.6 | 1.2 (2) | 0.1–4.2 |
Definition of abbreviations: CI = confidence interval; DACs = difficult airway characteristics; IQR = interquartile range; PGY = postgraduate year of resident.
FAS was significantly higher in patients intubated using an NMBA (80.9%, 401/496, 95% CI, 77–84%) compared with those intubated without an NMBA (69.6%, 117/168, 95% CI, 62–76%) (P = 0.003; Table 2). There was no significant difference in CL grade of view or POGO score between groups (Table 2).
Table 2.
Intubation success and grade of laryngoscopic view by use of neuromuscular blocking agent
| Outcome | Paralytic |
No Paralytic |
P Value | ||
|---|---|---|---|---|---|
| % (n/N) | 95% CI | % (n/N) | 95% CI | ||
| First attempt success, | 80.9 (401/496) | 77–84 | 69.6 (117/168) | 62–76 | 0.003 |
| More than two attempts | 3.6 (18) | 2.2–5.7 | 5.4 (9) | 2.5–9.9 | 0.36 |
| CL I or II | 82.1 (407/496) | 78–85 | 78.0 (131/168) | 71–84 | 0.26 |
| POGO score, mean | 72% | 69–75 | 69% | 63–74 | 0.29 |
Definition of abbreviations: CI = confidence interval; CL = Cormack-Lehane grade of view; POGO = percentage of glottic opening.
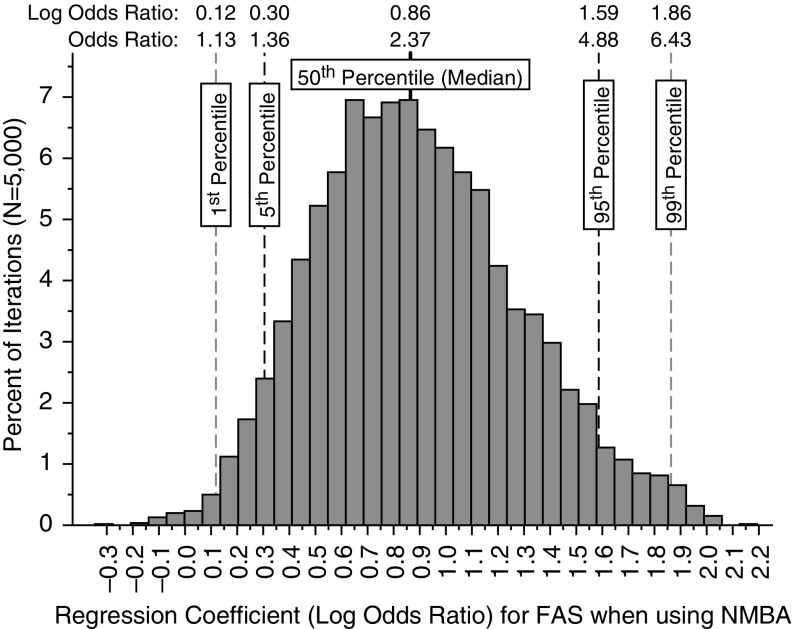
For the propensity score generation, the DAC category “Other” did not meet the balancing property across the five generated blocks (strata) of the propensity score, and it was eliminated from analyses. All remaining variables met the balancing property. The summary OR for FAS when using an NMBA, from the conditional logistic regression analyses of 5,000 propensity score–matched cases (first intubation attempt failure) and controls (FAS) was 2.37 (95% CI, 1.36–4.88; see Figure 1). For the sensitivity analysis, the unadjusted OR for FAS using an NMBA was 1.84 (95% CI, 1.24–2.74), and the propensity score–adjusted OR (controlling for potential confounders as well) was 2.22 (95% CI, 1.32–3.75; see Table 3). Model diagnostics for both the conditional logistic regression and the ordinary logistic regression identified several different outliers representing potentially miscoded cases; however, no evidence of miscoding was found, and removal of these outliers did not change regression coefficients by more than 5%. Thus, all cases were included in the final models.
Figure 1.
Histogram of the regression coefficients (natural log of the odds ratio [OR]) from the conditional logistic regression analyses of 5,000 propensity score–matched case–control data sets sampled from the complete data set. Shown are the 1st, 5th, 50th, 95th, and 99th percentiles of the regression coefficients from the. 5,000 iterations along with the associated ORs (OR = log OR raised to the power of e, the base of the natural logarithm). FAS = first-attempt success; NMBA = neuromuscular blocking agent.
Table 3.
Propensity-adjusted multivariate regression model for first-attempt success
| Variable | Successful Tube Placement on First Intubation Attempt |
|||
|---|---|---|---|---|
| Unadjusted (Crude) |
Adjusted* |
|||
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| Paralytic use | 1.84 | 1.24–2.74 | 2.22 | 1.32–3.75 |
| Male sex | 1.06 | 0.73–1.54 | 0.81 | 0.53–1.24 |
| Age per 1 yr increase | 1.01 | 0.95–1.08 | 1.02 | 0.95–1.09 |
| Total number of DACs | ||||
| 0 | [Reference] | [Reference] | ||
| 1 | 0.56 | 0.31–1.02 | 0.50 | 0.27–0.95 |
| 2 | 0.53 | 0.28–0.99 | 0.53 | 0.27–1.05 |
| 3 | 0.60 | 0.30–1.20 | 0.61 | 0.29–1.29 |
| 4+ | 0.38 | 0.20–0.72 | 0.33 | 0.16–0.66 |
| Sedative | ||||
| None | [Reference] | [Reference] | ||
| Etomidate | 0.71 | 0.20–2.47 | 0.05 | 0.002–0.079 |
| Ketamine | 0.43 | 0.12–1.57 | 0.11 | 0.01–0.66 |
| Midazolam | 0.76 | 0.13–4.43 | 0.11 | 0.01–1.79 |
| Propofol | 0.42 | 0.15–1.68 | 0.08 | 0.01–0.66 |
| Device | ||||
| Direct laryngoscopy | [Reference] | [Reference] | ||
| Glidescope video laryngoscopy | 2.58 | 1.39–4.81 | 5.36 | 2.35–12.27 |
| C-MAC video laryngoscopy | 1.87 | 1.19–2.93 | 3.99 | 1.91–8.33 |
| Other video laryngoscopes† | 2.41 | 0.27–21.31 | 3.20 | 0.31–33.16 |
| Operator PGY | ||||
| 1 | [Reference] | [Reference] | ||
| 2 | 1.53 | 0.74–3.19 | 2.00 | 0.90–4.43 |
| 3 | 0.96 | 0.45–2.05 | 0.91 | 0.40–2.07 |
| 4 | 2.32 | 1.13–4.79 | 3.20 | 1.45–7.04 |
| 5 | 2.46 | 1.19–5.09 | 3.33 | 1.49–7.42 |
| 6 | 1.8 | 0.79–4.12 | 2.61 | 1.05–6.51 |
| Attending | 8.0 | 0.97–65.82 | 22.57 | 2.43–209.7 |
| Propensity score | — | 9.55 | 0.48–188.59 | |
Definition of abbreviations: CI = confidence interval; DACs = difficult airway characteristics; PGY = postgraduate year of resident.
Adjusted for all other variables shown; Hosmer-Lemeshow goodness-of-fit P value = 0.53; area under the receiver operating characteristics curve = 0.718.
Other video laryngoscopes includes the McGrath MAC and the King Vision.
There was no statistical difference in either individual complications or total complications in patients intubated with an NMBA versus those who were not (Table 4). In the subgroup analysis comparing rocuronium and succinylcholine, there were no statistically significant differences in outcomes (Table 5).
Table 4.
Procedurally related complications
| Complications |
Paralytic |
95% CI |
No Paralytic |
95% CI |
P Value |
|---|---|---|---|---|---|
| % (n/N) | % (n/N) | ||||
| Hypotension | 7.5 (37/496) | 5–10% | 8.3 (14/168) | 5–14% | 0.74 |
| Desaturation | 19.2 (95/496) | 16–23% | 19.6 (33/168) | 14–26% | 0.91 |
| Esophageal intubation | 2.2 (11/496) | 1–4% | 3.0 (5/168) | 1–7% | 0.57 |
| Aspiration | 1.8 (9/496) | 1–3% | 1.2 (2/168) | 0–4% | 0.74 |
| Airway trauma | 0.2 (1/496) | 0–1% | 0.6 (1/168) | 0–3% | 0.44 |
| Other | 1.6 (8/496) | 1–3% | 3.6 (6/168) | 1–8% | 0.13 |
| Total complications, mean | 0.33 | 0.27–0.38 | 0.37 | 0.27–0.47 | 0.43 |
Definition of abbreviation: CI = confidence interval.
Table 5.
Rocuronium compared to succinylcholine
| Outcome | Succinylcholine | 95% CI | Rocuronium | 95% CI | P Value |
|---|---|---|---|---|---|
| First attempt success, % (n/N) | 82.7 (267/323) | 78–87% | 77.2 (125/162) | 70–83% | 0.18 |
| CL I or II, % (n/N) | 84.5 (273/323) | 80–88% | 78.4 (127/162) | 71–84% | 0.10 |
| POGO Score, mean % | 71 | 68–75% | 73 | 67–78% | 0.67 |
Definition of abbreviations: CI = confidence interval; CL = Cormack-Lehane; POGO = percentage of glottic opening.
Of the patients intubated with VL, NMBA use resulted in higher FAS (84.6%, 334/395, 95% CI, 81–88% versus 69.1%, 103/149, 95% CI, 61–76%, P < 0.001) and higher percentage of patients with a CL I or II grade of view (86.6%, 343/395, 95% CI, 83–90% versus 77.9%, 116/149, 95% CI, 70–84%, P = 0.02) (Table 6). NMBA use was associated with an adjusted odds of FAS of 2.50 (95% CI, 1.43–4.37, P < 0.001; Table 7).
Table 6.
Video laryngoscopy subgroup
| Outcome | Paralytic | 95% CI | No Paralytic | 95% CI | P Value |
|---|---|---|---|---|---|
| First attempt success, % (n/N) | 84.6 (334/395) | 81–88% | 69.1 (103/149) | 61–76% | <0.001 |
| CL I or II, % (n/N) | 86.6 (343/395) | 83–90% | 77.9 (116/149) | 70–84% | 0.02 |
| POGO score, mean % | 77 | 74–79% | 71 | 65–76% | 0.05 |
Definition of abbreviations: CI = confidence interval; CL = Cormack-Lehane; POGO = percentage of glottic opening.
Table 7.
Propensity-adjusted multivariate regression model for video laryngoscopy subgroup
| Variable | Successful Tube Placement on First Intubation Attempt |
|||
|---|---|---|---|---|
| Unadjusted (Crude) |
Adjusted* |
|||
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| Paralytic use | 2.44 | 1.57–3.80 | 2.50 | 1.43–4.37 |
| Total number of DACs | ||||
| 0 | [Reference] | — | [Reference] | — |
| 1 | 0.61 | 0.30–1.22 | 0.53 | 0.25–1.11 |
| 2 | 0.63 | 0.30–1.34 | 0.66 | 0.29–1.50 |
| 3 | 0.49 | 0.23–1.07 | 0.49 | 0.21–1.17 |
| 4+ | 0.33 | 0.16–0.68 | 0.30 | 0.12–0.74 |
| Sedative | ||||
| None | [Reference] | — | [Reference] | — |
| Etomidate | 0.48 | 0.06–3.88 | 0.32 | 0.04–2.98 |
| Ketamine | 0.24 | 0.12–1.99 | 0.32 | 0.03–3.29 |
| Midazolam | 0.23 | 0.13–2.73 | 0.23 | 0.01–4.55 |
| Propofol | 0.32 | 0.15–3.01 | 0.33 | 0.02–6.30 |
| Operator PGY | ||||
| 1 | [Reference] | — | [Reference] | |
| 2 | 1.53 | 0.65–3.62 | 1.44 | 0.59–3.57 |
| 3 | 0.75 | 0.33–1.71 | 0.72 | 0.30–1.78 |
| 4 | 2.34 | 1.02–5.32 | 2.81 | 1.17–6.85 |
| 5 | 2.17 | 0.97–4.87 | 2.63 | 1.10–6.32 |
| 6 | 2.34 | 0.88–6.22 | 3.16 | 1.11–8.98 |
| Attending | 1 | — | 1 | — |
| Propensity score | — | — | 2.30 | 0.14–38.34 |
Definition of abbreviations: CI = confidence interval; DACs = difficult airway characteristics; PGY = postgraduate year of resident.
Adjusted for all other variables shown; Hosmer-Lemeshow goodness-of-fit P value = 0.12.
Discussion
These data show that the use of an NMBA during intubation in the ICU is associated with an improvement in FAS of non–anesthesiologist intensivists from 70 to 81%. When controlling for identified factors that may influence the decision to give a paralytic as well as factors that may influence FAS, the use of an NMBA was associated with over two times the odds (adjusted OR, 2.37) of FAS compared with not using an NMBA. These data are consistent with other reports suggesting that NMBA use should be considered for intubations in the ICU (4, 41).
When controlling for potential confounders in the VL subgroup with a propensity-adjusted multivariable regression model, the use of a neuromuscular blocker was associated with an improvement in FAS with an adjusted OR of 2.50. To our knowledge, this is the first evaluation of the effect of an NMBA when using VL.
Succinylcholine was the paralytic of choice during this observational period, being used twice as often as rocuronium (65 versus 33%). However, our findings show no statistical difference between these two NMBAs. Although comparisons between succinylcholine and rocuronium are mixed (42–49), the fact that we did not show a difference in intubating conditions and the concern for a “can’t intubate, can’t oxygenate” situation in patients receiving an NMBA, succinylcholine is an attractive option in the absence of contraindications to induce short-term paralysis to facilitate intubation.
Wilcox and colleagues (34) demonstrated that the use of an NMBA reduced the incidence of procedurally related complication rates. We found no difference in the incidence of complications between patients intubated with or without an NMBA. In general, the procedurally related complications (esophageal intubation, aspiration, and airway trauma) are similar to other reports of ICU intubations (7, 17, 50). VL is the primary device of choice in our institution, given the data on improved FAS (28, 29, 31, 32) and the ability of the supervising faculty to recognize and correct errors in real time. For example, esophageal intubations are usually immediately recognized by the supervising faculty rather than after failed ventilation, negative placement confirmation strategies, and potential aspiration as with DL.
Although we report higher desaturation and hypotension rates in our observational period than were noted in other studies (17, 34, 50), these discrepancies are most likely due to differences in definitions. Other studies defined hypotension as SBP less than 70 mm Hg (34, 50) or less than 65 mm Hg (17), whereas our definition is any drop in SBP requiring fluid bolus or vasopressor adjustment. Regarding hypoxemia (desaturation), our definition is any drop in oxygen saturation greater than 10% from the baseline, compared with any saturation less than 80% in the other studies. We chose these definitions, as they are potentially more sensitive to complications from prolonged attempts rather than weighted by the severity of the patient’s illness. Only one-half of patients in our cohort with desaturations greater than 10% had a lowest saturation under 80%. Using the conventional definition would bring our desaturation rate to 12% for patients with NMBA use and 9% for those without, similar to other studies.
There are several limitations to consider when interpreting these data. Most importantly, this is an observational study, studying nonrandomized interventions, and is prone to bias. In addition, some of the variables studied are self-reported, and findings must be interpreted with caution, as self-reported data are subject to bias. To reduce the chance of bias, a random sample of data collection forms are cross-referenced to the medical record to ensure accuracy of objective data, such as number of attempts, oxygen saturation, complications, and pharmacologic agents used. Furthermore, even though a propensity score was calculated for the likelihood of receiving a paralytic, it is impossible to control for every factor considered in the decision, such as physician comfort with the intubation, which cannot be accounted for in the model, and severity of illness, which is not available in our dataset. Similarly, we attempt to account for operator level of training; however, PGY of the operator may not be a completely accurate measure of operator experience. In addition, the current widespread use of VL makes it difficult to compare these data with previous studies in which DL is the primary device of choice. Although we used propensity score adjustment and matching to minimize the chance of bias from analyzing a nonrandomized intervention, it is possible that an unmeasured (and thus unaccounted for) confounder or risk factor may be responsible for our findings. Finally, our findings merely indicate that there is a significant, independent association between the presence of an NMBA and FAS. This nonrandomized observational study cannot prove causation. Given these limitations, a randomized controlled trial is warranted to further evaluate the effect of neuromuscular blockers on ICU intubations.
Conclusions
In this observational study of intubations in the ICU, the use of an NMBA to facilitate intubation was associated with higher odds of FAS using a propensity score–matched analysis to reduce the chance of bias. In the VL subgroup, NMBA use was also associated with higher odds of FAS and improved laryngoscopic view. These data suggest that an NMBA is an attractive option when faced with intubation in a critically ill patient in the ICU.
Acknowledgments
Acknowledgment
The authors thank Jose Guillen, M.S., for programming and statistical assistance, and the University of Arizona College of Medicine Medical Student Research Program for providing the funding for the summer research internship for H.C.
Footnotes
Supported in part by National Institutes of Health grant T35HL07479.
This work was presented in abstract form at the Society of Critical Care Medicine 2015 annual congress in Phoenix, Arizona.
Author Contributions: J.M.M., J.W.B., J.M., C.D.H., and J.C.S. conceived the study; J.M.M. designed the data collection instrument and managed the database; J.M.M., U.S., C.D.H., H.C., and J.W.B. performed statistical analysis in the study; J.M.M., J.C.S., U.S., C.D.H., H.C., J.M., and J.W.B. contributed to the drafting of the manuscript; J.M.M. takes responsibility for the paper as a whole.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Mort TC. Complications of emergency tracheal intubation: hemodynamic alterations—part I. J Intensive Care Med. 2007;22:157–165. doi: 10.1177/0885066607299525. [DOI] [PubMed] [Google Scholar]
- 2.Mort TC. Complications of emergency tracheal intubation: immediate airway-related consequences: part II. J Intensive Care Med. 2007;22:208–215. doi: 10.1177/0885066607301359. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz DE, Matthay MA, Cohen NH. Death and other complications of emergency airway management in critically ill adults: a prospective investigation of 297 tracheal intubations. Anesthesiology. 1995;82:367–376. doi: 10.1097/00000542-199502000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds SF, Heffner J. Airway management of the critically ill patient: rapid-sequence intubation. Chest. 2005;127:1397–1412. doi: 10.1378/chest.127.4.1397. [DOI] [PubMed] [Google Scholar]
- 5.Benedetto WJ, Hess DR, Gettings E, Bigatello LM, Toon H, Hurford WE, Schmidt U. Urgent tracheal intubation in general hospital units: an observational study. J Clin Anesth. 2007;19:20–24. doi: 10.1016/j.jclinane.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Gudzenko V, Bittner EA, Schmidt UH. Emergency airway management. Respir Care. 2010;55:1026–1035. [PubMed] [Google Scholar]
- 7.Jaber S, Amraoui J, Lefrant JY, Arich C, Cohendy R, Landreau L, Calvet Y, Capdevila X, Mahamat A, Eledjam JJ. Clinical practice and risk factors for immediate complications of endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Crit Care Med. 2006;34:2355–2361. doi: 10.1097/01.CCM.0000233879.58720.87. [DOI] [PubMed] [Google Scholar]
- 8.Le Tacon S, Wolter P, Rusterholtz T, Harlay M, Gayol S, Sauder P, Jaeger A. Complications of difficult tracheal intubations in a critical care unit [Article in French] Ann Fr Anesth Reanim. 2000;19:719–724. doi: 10.1016/s0750-7658(00)00316-6. [DOI] [PubMed] [Google Scholar]
- 9.Cook TM, Woodall N, Harper J, Benger J Fourth National Audit Project. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2: intensive care and emergency departments. Br J Anaesth. 2011;106:632–642. doi: 10.1093/bja/aer059. [DOI] [PubMed] [Google Scholar]
- 10.Mort TC. Emergency tracheal intubation: complications associated with repeated laryngoscopic attempts. Anesth Analg. 2004;99:607–613. (table of contents). doi: 10.1213/01.ANE.0000122825.04923.15. [DOI] [PubMed] [Google Scholar]
- 11.Jabre P, Avenel A, Combes X, Kulstad E, Mazariegos I, Bertrand L, Lapostolle F, Adnet F. Morbidity related to emergency endotracheal intubation—a substudy of the KETAmine SEDation trial. Resuscitation. 2011;82:517–522. doi: 10.1016/j.resuscitation.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa K, Shigemitsu K, Hagiwara Y, Chiba T, Watase H, Brown CA, III, Brown DF. Japanese Emergency Medicine Research Alliance Investigators. Association between repeated intubation attempts and adverse events in emergency departments: an analysis of a multicenter prospective observational study. Ann Emerg Med. 2012;60:749–754.e2. doi: 10.1016/j.annemergmed.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Sakles JC, Chiu S, Mosier J, Walker C, Stolz U. The importance of first pass success when performing orotracheal intubation in the emergency department. Acad Emerg Med. 2013;20:71–78. doi: 10.1111/acem.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leibowitz AB. Tracheal intubation in the intensive care unit: extremely hazardous even in the best of hands. Crit Care Med. 2006;34:2497–2498. doi: 10.1097/01.CCM.0000235993.47514.8F. [DOI] [PubMed] [Google Scholar]
- 15.Walz JM, Zayaruzny M, Heard SO. Airway management in critical illness. Chest. 2007;131:608–620. doi: 10.1378/chest.06-2120. [DOI] [PubMed] [Google Scholar]
- 16.Griesdale DE, Bosma TL, Kurth T, Isac G, Chittock DR. Complications of endotracheal intubation in the critically ill. Intensive Care Med. 2008;34:1835–1842. doi: 10.1007/s00134-008-1205-6. [DOI] [PubMed] [Google Scholar]
- 17.Jaber S, Jung B, Corne P, Sebbane M, Muller L, Chanques G, Verzilli D, Jonquet O, Eledjam JJ, Lefrant JY. An intervention to decrease complications related to endotracheal intubation in the intensive care unit: a prospective, multiple-center study. Intensive Care Med. 2010;36:248–255. doi: 10.1007/s00134-009-1717-8. [DOI] [PubMed] [Google Scholar]
- 18.Divatia JV, Khan PU, Myatra SN. Tracheal intubation in the ICU: life saving or life threatening? Indian J Anaesth. 2011;55:470–475. doi: 10.4103/0019-5049.89872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griesdale DE, Henderson WR, Green RS. Airway management in critically ill patients. Lung. 2011;189:181–192. doi: 10.1007/s00408-011-9278-3. [DOI] [PubMed] [Google Scholar]
- 20.Byhahn C, Cavus E. Airway management disasters in the ICU—lessons learned? Crit Care. 2012;16:162. doi: 10.1186/cc11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doerschug KC. Counterpoint: should an anesthesiologist be the specialist of choice in managing the difficult airway in the ICU? Not necessarily. Chest. 2012;142:1375–1377. doi: 10.1378/chest.12-2196. [DOI] [PubMed] [Google Scholar]
- 22.Griesdale DE, Chau A, Isac G, Ayas N, Foster D, Irwin C, Choi P Canadian Critical Care Trials Group. Video-laryngoscopy versus direct laryngoscopy in critically ill patients: a pilot randomized trial. Can J Anaesth. 2012;59:1032–1039. doi: 10.1007/s12630-012-9775-8. [DOI] [PubMed] [Google Scholar]
- 23.Noppens RR, Geimer S, Eisel N, David M, Piepho T. Endotracheal intubation using the C-MAC® video laryngoscope or the Macintosh laryngoscope: a prospective, comparative study in the ICU. Crit Care. 2012;16:R103. doi: 10.1186/cc11384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walz JM. Point: should an anesthesiologist be the specialist of choice in managing the difficult airway in the ICU? Yes. Chest. 2012;142:1372–1374. doi: 10.1378/chest.12-2194. [DOI] [PubMed] [Google Scholar]
- 25.De Jong A, Clavieras N, Conseil M, Coisel Y, Moury PH, Pouzeratte Y, Cisse M, Belafia F, Jung B, Chanques G, et al. Implementation of a combo videolaryngoscope for intubation in critically ill patients: a before–after comparative study. Intensive Care Med. 2013;39:2144–2152. doi: 10.1007/s00134-013-3099-1. [DOI] [PubMed] [Google Scholar]
- 26.De Jong A, Molinari N, Terzi N, Mongardon N, Arnal JM, Guitton C, Allaouchiche B, Paugam-Burtz C, Constantin JM, Lefrant JY, et al. AzuRéa Network for the Frida-Réa Study Group. Early identification of patients at risk for difficult intubation in the intensive care unit: development and validation of the MACOCHA score in a multicenter cohort study. Am J Respir Crit Care Med. 2013;187:832–839. doi: 10.1164/rccm.201210-1851OC. [DOI] [PubMed] [Google Scholar]
- 27.Ghamande SA, Arroliga AC, Ciceri DP. Let’s make endotracheal intubation in the intensive care unit safe: difficult or not, the MACOCHA score is a good start. Am J Respir Crit Care Med. 2013;187:789–790. doi: 10.1164/rccm.201301-0099ED. [DOI] [PubMed] [Google Scholar]
- 28.Kory P, Guevarra K, Mathew JP, Hegde A, Mayo PH. The impact of video laryngoscopy use during urgent endotracheal intubation in the critically ill. Anesth Analg. 2013;117:144–149. doi: 10.1213/ANE.0b013e3182917f2a. [DOI] [PubMed] [Google Scholar]
- 29.Lakticova V, Koenig SJ, Narasimhan M, Mayo PH. Video laryngoscopy is associated with increased first pass success and decreased rate of esophageal intubations during urgent endotracheal intubation in a medical intensive care unit when compared to direct laryngoscopy. J Intensive Care Med. 2015;30:44–48. doi: 10.1177/0885066613492641. [DOI] [PubMed] [Google Scholar]
- 30.Larsson A, Dhonneur G. Videolaryngoscopy: towards a new standard method for tracheal intubation in the ICU? Intensive Care Med. 2013;39:2220–2222. doi: 10.1007/s00134-013-3118-2. [DOI] [PubMed] [Google Scholar]
- 31.Mosier JM, Whitmore SP, Bloom JW, Snyder LS, Graham LA, Carr GE, Sakles JC. Video laryngoscopy improves intubation success and reduces esophageal intubations compared to direct laryngoscopy in the medical intensive care unit. Crit Care. 2013;17:R237. doi: 10.1186/cc13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jharap B, de Boer NK, Stokkers P, Hommes DW, Oldenburg B, Dijkstra G, van der Woude CJ, de Jong DJ, Mulder CJ, van Elburg RM, et al. Dutch Initiative on Crohn and Colitis. Intrauterine exposure and pharmacology of conventional thiopurine therapy in pregnant patients with inflammatory bowel disease. Gut. 2014;63:451–457. doi: 10.1136/gutjnl-2012-303615. [DOI] [PubMed] [Google Scholar]
- 33.Mosier JM, Law JA. Airway management in the critically ill. Intensive Care Med. 2014;40:727–729. doi: 10.1007/s00134-014-3261-4. [DOI] [PubMed] [Google Scholar]
- 34.Wilcox SR, Bittner EA, Elmer J, Seigel TA, Nguyen NT, Dhillon A, Eikermann M, Schmidt U. Neuromuscular blocking agent administration for emergent tracheal intubation is associated with decreased prevalence of procedure-related complications. Crit Care Med. 2012;40:1808–1813. doi: 10.1097/CCM.0b013e31824e0e67. [DOI] [PubMed] [Google Scholar]
- 35.Di Filippo A, Gonnelli C. Rapid sequence intubation: a review of recent evidences. Rev Recent Clin Trials. 2009;4:175–178. doi: 10.2174/157488709789957556. [DOI] [PubMed] [Google Scholar]
- 36.Vincent F, Gonzalez F, Cohen Y. Endotracheal intubation in the intensive care unit: non-anesthesiologists know rapid sequence intubation, but is it accurate in all cases? Crit Care Med. 2007;35:983–984, author reply 984. doi: 10.1097/01.ccm.0000257470.40571.92. [DOI] [PubMed] [Google Scholar]
- 37.Bair AE, Caravelli R, Tyler K, Laurin EG. Feasibility of the preoperative Mallampati airway assessment in emergency department patients. J Emerg Med. 2010;38:677–680. doi: 10.1016/j.jemermed.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 38.Levitan RM, Everett WW, Ochroch EA. Limitations of difficult airway prediction in patients intubated in the emergency department. Ann Emerg Med. 2004;44:307–313. doi: 10.1016/j.annemergmed.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Linden A, Samuels SJ. Using balance statistics to determine the optimal number of controls in matching studies. J Eval Clin Pract. 2013;19:968–975. doi: 10.1111/jep.12072. [DOI] [PubMed] [Google Scholar]
- 40.Austin PC. Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am J Epidemiol. 2010;172:1092–1097. doi: 10.1093/aje/kwq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tourtier JP, Falzone E, Hoffmann C, Auroy Y. Rapid sequence intubation: a safe and effective technique. J Intensive Care Med. 2012;27:66–67; authors reply 68. doi: 10.1177/0885066611410375. [DOI] [PubMed] [Google Scholar]
- 42.Laurin EG, Sakles JC, Panacek EA, Rantapaa AA, Redd J. A comparison of succinylcholine and rocuronium for rapid-sequence intubation of emergency department patients. Acad Emerg Med. 2000;7:1362–1369. doi: 10.1111/j.1553-2712.2000.tb00493.x. [DOI] [PubMed] [Google Scholar]
- 43.Perry J, Lee J, Wells G. Rocuronium versus succinylcholine for rapid sequence induction intubation. Cochrane Database Syst Rev. 2003;(1):CD002788. doi: 10.1002/14651858.CD002788. [DOI] [PubMed] [Google Scholar]
- 44.Larsen PB, Hansen EG, Jacobsen LS, Wiis J, Holst P, Rottensten H, Siddiqui R, Wittrup H, Sørensen AM, Persson S, et al. Intubation conditions after rocuronium or succinylcholine for rapid sequence induction with alfentanil and propofol in the emergency patient. Eur J Anaesthesiol. 2005;22:748–753. doi: 10.1017/s0265021505001249. [DOI] [PubMed] [Google Scholar]
- 45.Sluga M, Ummenhofer W, Studer W, Siegemund M, Marsch SC. Rocuronium versus succinylcholine for rapid sequence induction of anesthesia and endotracheal intubation: a prospective, randomized trial in emergent cases. Anesth Analg. 2005;101:1356–1361. doi: 10.1213/01.ANE.0000180196.58567.FE. [DOI] [PubMed] [Google Scholar]
- 46.Perry JJ, Lee JS, Sillberg VA, Wells GA. Rocuronium versus succinylcholine for rapid sequence induction intubation. Cochrane Database Syst Rev. 2008;(2):CD002788. doi: 10.1002/14651858.CD002788.pub2. [DOI] [PubMed] [Google Scholar]
- 47.Curley GF. Rapid sequence induction with rocuronium—a challenge to the gold standard. Crit Care. 2011;15:190. doi: 10.1186/cc10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marsch SC, Steiner L, Bucher E, Pargger H, Schumann M, Aebi T, Hunziker PR, Siegemund M. Succinylcholine versus rocuronium for rapid sequence intubation in intensive care: a prospective, randomized controlled trial. Crit Care. 2011;15:R199. doi: 10.1186/cc10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seupaul RA, Jones JH. Evidence-based emergency medicine: does succinylcholine maximize intubating conditions better than rocuronium for rapid sequence intubation? Ann Emerg Med. 2011;57:301–302. doi: 10.1016/j.annemergmed.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Mayo PH, Hegde A, Eisen LA, Kory P, Doelken P. A program to improve the quality of emergency endotracheal intubation. J Intensive Care Med. 2011;26:50–56. doi: 10.1177/0885066610384070. [DOI] [PubMed] [Google Scholar]