Abstract
Rationale: Abnormalities on chest computed tomography (CT) in children with cystic fibrosis (CF) have been shown to correlate with short-term measures of lung disease. Chest CT scores offer promise as a potential surrogate end point in CF; however, there is limited information available on the ability of chest CT scores to predict future morbidity.
Objectives: Determine whether chest CT scores are associated with the rate of pulmonary exacerbations over the next 10 years.
Methods: Ten years of follow-up data were obtained from the CF Foundation Patient Registry for 60 children enrolled in the Pulmozyme Early Intervention Trial and who had chest CT scans at baseline.
Measurements and Main Results: Multivariable Poisson regression was used to compare Brody CT scores and the number of pulmonary exacerbations in the following 10 years. At the time of the chest CT, the mean (SD) age was 10.6 (1.7) years. A 1-point increase in the Brody CT score was associated with an increase in the mean (95% confidence interval) rate of pulmonary exacerbations of 1.39 (1.15, 1.67) (P < 0.001). Brody CT scores were more strongly associated with the number of pulmonary exacerbations than FEV1 % predicted at the time of the chest CT (P = 0.037 by chi-square test).
Conclusions: There is a significant association between Brody CT scores and the rate of pulmonary exacerbations up to 10 years later. This association is stronger than for FEV1 obtained at the time of the CT, suggesting that chest CT scores offer improved ability to predict future outcomes.
Keywords: Brody scores, FEV1, bronchiectasis, epidemiology
In cystic fibrosis (CF), as in many other diseases (1), the establishment of surrogate end points has been challenging (2). To be characterized as a surrogate end point by the U.S. Food and Drug Administration (FDA), a laboratory measurement or physical sign must be biologically plausible, reflect clinical severity, improve rapidly with effective therapy, and correlate with true clinical end points—direct measures of how a patient feels, functions, or survives (3–5). In CF, FEV1 is the only surrogate end point accepted by the FDA (6, 7). Mortality, pulmonary exacerbations, and quality of life are recognized as clinical end points. This paucity of available end points is magnified for young children who cannot perform traditional spirometry, have fewer pulmonary exacerbations (8), and for whom CF-specific observer/parent-reported outcome measures are not yet fully validated.
Chest computed tomography (CT) scores are potential surrogate end points for CF lung disease. Chest CT is the most sensitive method of detecting structural lung disease in children with CF and is the “gold standard” for detecting bronchiectasis (9–12). Chest CT scores are associated with the frequency of pulmonary exacerbations (10, 13); worse quality of life (14); and in patients with end-stage lung disease, higher mortality (15). From a practical standpoint, CT is routinely performed clinically in all ages, and nearly all centers have CT scanners—neither of which may be true for other recently developed potential surrogate outcome measures in CF (e.g., lung clearance index, infant pulmonary function tests [PFTs], magnetic resonance imaging). However, no studies correlate chest CT with the long-term prognosis in CF: most studies that have evaluated chest CT have not explored associations with clinical end points beyond 2 years of follow-up (10, 13, 15). Although CT scanning has limitations, including cost, concerns about radiation risk, and the need for sedation in young children, chest CT is a promising method that may allow clinicians to individualize the care for patients with CF at an early age to prevent future progression of lung disease (16, 17). Current therapeutic recommendations in CF are applied universally, without regard to current disease severity or future morbidity, in part because of the lack of surrogate end points to guide clinical decisions.
To evaluate the possibility that CT may qualify as a meaningful surrogate end point we sought to determine whether chest CT scores are associated with the rate of pulmonary exacerbations over a 10-year follow-up period. To address this objective, we used chest CT data from scans obtained in a standardized protocol and scored by two thoracic radiologists with extensive experience with CF and the Brody score (18). We combined these data with follow-up data from the CF Foundation Patient Registry (CFFPR) (19). We hypothesized that chest CT scores would be associated with the rate of pulmonary exacerbations over a 10-year follow-up period.
Methods
As described previously, the Pulmozyme Early Intervention Trial (PEIT) enrolled children 6–10 years of age with FVC greater than or equal to 85% predicted, the ability to perform reproducible pulmonary function tests, no dornase alfa use for 6 months before enrollment, and no pulmonary exacerbations within 60 days before enrollment between 1997 and 2000 (20). Chest CT scans were obtained during periods of clinical stability at the beginning and end of the 2-year study and scored using the Brody scoring system for 60 of the subjects in the PEIT (18). For this study, we used data from the CT obtained at the end of the PEIT study. PFTs were obtained on the same day as the chest CT. Inspiratory images were obtained at 10-mm intervals and four expiratory images were obtained at the following: 0.5 cm above the aortic arch, the carina, at the inferior margin of the hilum, and 1 cm above the diaphragm.
CT scans were scored independently by two thoracic radiologists using the Brody scoring system (18). The radiologists were blinded to the patient identities, severity of lung disease, and treatment group. The Brody scoring system has been reported as a total score with a maximal possible value of 207 (18) and as a score representing the average severity of each of the six lobes, including the lingula as a separate lobe, with a maximum of 40.5 (21). In this study, we report the average scores of the two radiologists based on a maximal score of 40.5.
Data from the time of the chest CT in 1999 through 2009 were obtained from the CFFPR and linked to the original chest CT data. The CFFPR is a well-described database that contains data on more than 40,000 people with CF in the United States (19) Pulmonary exacerbations were defined as hospitalizations treated with intravenous antibiotics for an increase in respiratory signs and symptoms, and/or if the “pulmonary exacerbation” box was checked on the CFFPR encounter form. The current study was approved by the institutional review boards at the University of Cincinnati (Cincinnati, OH) and University of Wisconsin (Madison, WI).
We used multivariable Poisson regression models to determine the association between the Brody chest CT scores in 1999 and the rate of pulmonary exacerbations between 1999 and 2009. Regression models were adjusted for important potential confounders, including sex (22), genotype (categorized as homozygous F508del and other), FEV1 expressed as a percentage of the predicted value (FEV1 % predicted), and positive cultures for Staphylococcus aureus and mucoid Pseudomonas aeruginosa (23) at the time of the chest CT. To determine whether chest CT scores were more strongly associated with the rate of pulmonary exacerbations than FEV1 % predicted, we compared the magnitudes of the slopes of the chest CT score and FEV1 in the multivariable Poisson regression model with a chi-square test. We performed similar analyses to determine whether the bronchiectasis subscore was associated with the rate of pulmonary exacerbations between 1999 and 2009. Finally, we used multivariable linear regression models to determine the association between the Brody chest CT scores in 1999 and FEV1 % predicted in 2009. Statistical significance was defined as a two-sided P value less than 0.05.
Results
The 60 children who underwent chest CT as part of the PEIT study were similar to a typical CF population in the United States (Table 1), with the exception that, in line with PEIT inclusion criteria of FVC greater than or equal to 85% predicted, the mean FEV1 % predicted was higher than the mean FEV1 % predicted for 6- to 10-year-old children with CF in the United States in 1999. In this subject group, the mean FEV1 was 99% predicted, similar to the national average for 10-year-old children born in 2002–2006 (19). Only one patient was positive for methicillin-resistant Staphylococcus aureus at the time of the chest CT. The mean (SD) Brody chest CT score was 3.8 (1.9), out of a possible 40.5. The mean (SD) bronchiectasis subscore was 0.6 (0.8), out of a possible 12. Both of these scores indicate generally mild, but abnormal, values.
Table 1.
Cohort characteristics
| Patient Characteristic |
At Time of Chest CT (1999): Total n = 60 |
At Most Recent Time Point (2009): Total n = 55 |
|---|---|---|
| [n (%) or mean (SD)] | [n (%) or mean (SD)] | |
| Female sex | 24 (40) | 21 (38) |
| Age, yr | 10.6 (1.7) | 20.5 (1.7) |
| Homozygous F508del | 30 (50) | 26 (47) |
| Heterozygous F508del | 23 (38) | 22 (40) |
| Other | 7 (12) | 7 (13) |
| Pancreatic insufficient | 58 (97) | 53 (96) |
| FEV1 % predicted | 99.2 (14.2) | 77.1 (23.9) |
| Culture positive for Pseudomonas aeruginosa | 30 (50) | 39 (74) |
| Culture positive for mucoid P. aeruginosa | 20 (33) | 29 (55) |
| Culture positive for Staphylococcus aureus | 40 (67) | 24 (45) |
| Dornase alfa | 29 (48) | 46 (84) |
| Inhaled tobramycin | 19 (32) | 32 (58) |
| Chronic macrolides | 0 | 36 (65) |
| Hypertonic saline | 0 | 15 (27) |
Definition of abbreviation: CT = computed tomography.
Data were available in the CFFPR for all patients between the time of the chest CT and 2005. Data were missing for one subject in 2006, two in 2007, three in 2008, and five in 2009. One patient died before 2009. Overall, the mean (SD) observation time after the chest CT was 9.8 (0.7) years. The mean FEV1 decreased by 2.2% predicted per year over this time period (Table 1). During the observation period, subjects had between 0 and 63 pulmonary exacerbations treated with intravenous antibiotics and recorded in the CFFPR. The mean (SD) was 7.7 (11.7) pulmonary exacerbations per patient, and the median was 3 pulmonary exacerbations.
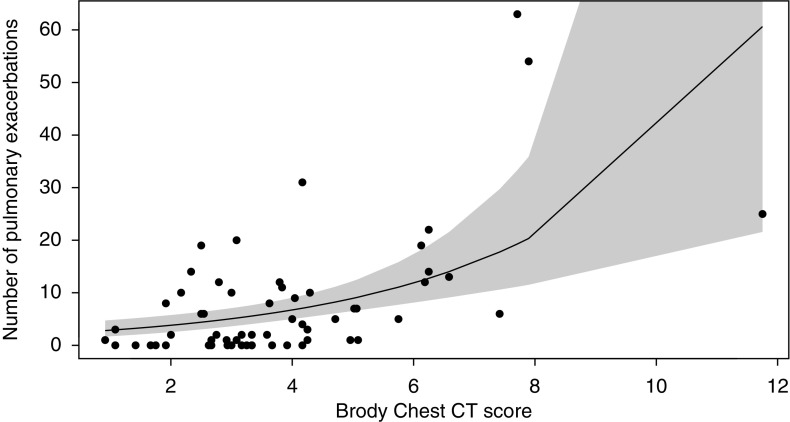
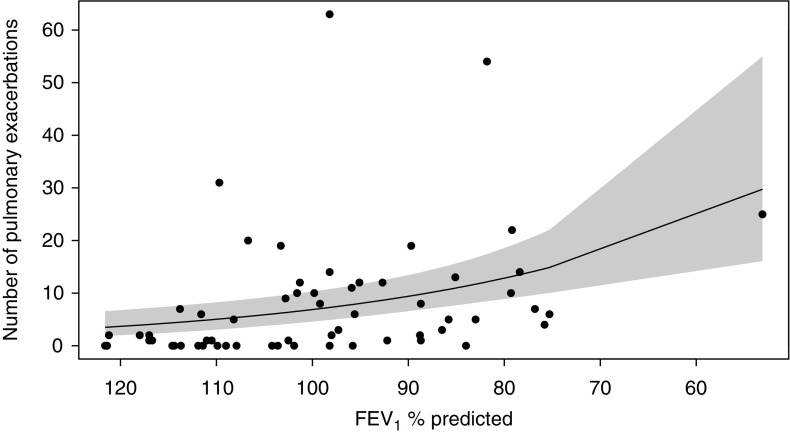
In multivariable Poisson regression models, the Brody chest CT scores (Figure 1), bronchiectasis subscores, and FEV1 % predicted (Figure 2) at the time of the chest CT were each associated with the number of pulmonary exacerbations during the 10 years of observation (Table 2). For every 1-point increase in Brody chest CT score, the mean (95% confidence interval) rate of pulmonary exacerbations during the observation period increased by a factor of 1.39 (1.15, 1.67) (P < 0.001). Results were similar when outlier patients were excluded (data not shown). A 1-point difference in the Brody chest CT score was more strongly associated with the rate of pulmonary exacerbations between 1999 and 2009 than a 5% predicted difference in FEV1 % predicted at the time of the chest CT (P = 0.037 by chi-square test). There were no differences in the strengths of the association between the Brody bronchiectasis subscore and FEV1 % predicted in 1999 with the rate of pulmonary exacerbations between 1999 and 2009 (P = 0.3 by chi-square test).
Figure 1.
Scatterplot and Poisson regression for the Brody chest computed tomography (CT) score in 1999 and the cumulative number of pulmonary exacerbations in 1999–2009. Shaded area represents 95% confidence intervals.
Figure 2.
Scatterplot and Poisson regression for FEV1 % predicted in 1999 and the cumulative number of pulmonary exacerbations in 1999–2009. Shaded area represents 95% confidence intervals.
Table 2.
Multivariable regression estimates of rate ratios for number of pulmonary exacerbations in 1999–2009 and differences in FEV1 % predicted in 2009
| Lung Disease Measure in 1999 | RR for Pulmonary Exacerbations* [mean (95% CI)] | P Value | Difference in FEV1 % Predicted† [mean (95% CI)] | P Value |
|---|---|---|---|---|
| Brody chest CT score, 1-point increase | 1.39 (1.15, 1.67) | <0.001 | −4.76 (–7.80, –1.72) | 0.003 |
| Bronchiectasis subscore, 1-point increase | 1.38 (0.99, 1.92) | 0.06 | −7.88 (–15.29, –0.48) | 0.037 |
| FEV1 % predicted, 5-point decrease | 1.19 (1.10, 1.30) | <0.001 | −4.47 (–6.48, –2.46) | <0.001 |
Definition of abbreviations: CI = confidence interval; CT = computed tomography; RR = rate ratio.
Multivariable Poisson model adjusted for sex, genotype, and FEV1 and mucoid P. aeruginosa status at the time of the chest CT. Robust SE used to account for overdispersion.
Multivariable linear regression model adjusted for sex, genotype, and FEV1 and mucoid P. aeruginosa status at the time of the chest CT.
In linear regression models, the Brody chest CT score, bronchiectasis subscore, and FEV1 % predicted at the time of the chest CT were each significantly associated with FEV1 % predicted in 2009 (Table 2). There were no differences in the strengths of the association between the Brody chest CT score and FEV1 % predicted in 1999 with FEV1 % predicted in 2009 (P = 0.4 by F test).
Discussion
In an analysis of rigorously determined chest CT scores obtained as part of the prospective PEIT study and 10 years of data obtained during routine care, we have shown that chest CT scores are associated with the rate of pulmonary exacerbations over a 10-year follow-up period. Chest CT scores were more strongly associated with the rate of pulmonary exacerbations than FEV1 obtained at the same time as the chest CT. This study provides the strongest evidence yet that chest CT scores correlate with true clinical end points over an extended follow-up period, supporting the use of chest CT scanning as an outcome surrogate for CF lung disease (24). This study is unique in that chest CT scores were evaluated as predictors of later lung disease measured by a true outcome measure, the number of pulmonary exacerbations over the 10 years after the CT scan. This 10-year follow-up period exceeds all studies of the ability of CT scans or PFTs to predict future morbidity and mortality. This study further contributes to our knowledge of CT scanning as an outcome surrogate by comparing the predictive ability of CT scanning with FEV1.
In contrast with our experience with chest CT, our experience with the ability of traditional PFTs to predict future morbidity and mortality is much greater, particularly for adolescents and adults. FEV1 at age 20 years can accurately discriminate between patients with mild and severe lung disease and is predictive of duration of survival (7). FEV1 of less than 30% predicted was as predictive of 2-year survival as a well-fitted multivariable logistic regression model that incorporated age, height, FEV1, microbiology, and pulmonary exacerbations (25). Our results are particularly important, as the chest CT scans were obtained in children who had generally normal PFTs. It is well known that chest CT scans demonstrate abnormalities in children with CF who have normal PFTs (9, 18). Here, we have shown that the presence and severity of these abnormalities are important predictors of future clinical end points.
Thus, the addition of a carefully interpreted chest CT scan to a CF care plan can provide benefit by identifying patients at risk for future pulmonary exacerbations and progression of lung disease that would likely not be identified for at least several years. Pulmonary exacerbations are clinical end points in CF associated with higher mortality (6, 25), progression of lung disease (26–28), lower quality of life (29), and increased health care expenditures (30). These benefits can be weighed against the ongoing concerns over radiation exposure and the potential future risk of malignancy (31, 32). Chest CT scores may allow more personalized care for children with CF early in life, before irreversible disease occurs. Current recommendations are for a “one-size-fits-all” approach: it is recommended that all children 6 years old and over use dornase alfa, hypertonic saline, azithromycin, and ibuprofen, and that all children 6 years old and over infected with P. aeruginosa also use inhaled tobramycin and inhaled aztreonam (33). The use of all of these medications together may lead to a substantial treatment burden and increase the risk of nonadherence (34, 35). Many of the subjects in the current study had no pulmonary exacerbations requiring intravenous antibiotics over a 10-year period, in an era when many of these therapies were not yet available. Thus, patients with near-normal chest CT scores, who are unlikely to have more than a few pulmonary exacerbations, may do well without one or more of these therapies. Newer modalities such as the Lung Clearance Index and magnetic resonance imaging may provide similar diagnostic and prognostic information as chest CT scores (36, 37), without exposure to excessive ionizing radiation. However, neither of these modalities is available for clinical care or been established as surrogate end points for clinical trials. There are several barriers to overcome before chest CT scores can be applied routinely in clinical practice to adjust therapeutic regimens (38). Despite the wide use of routine CT scanning, there are no data that show that alterations in care based on CT findings result in improved outcomes, either in improved health or reduced treatment burden. The Brody score was designed as a research tool and provides highly granular data at the cost of complexity and a tedious scoring process. Improvements will be needed before this system can be used for routine clinical care.
There are limitations to our study. The chest CT in the current study was obtained, on average, at about 10 years of age, so we are unable to comment on the optimal timing of a CT, either in terms of age, severity of PFTs, or predictive ability. To attain a long follow-up period, we studied outcomes after chest CT scans that were obtained in 1999, before many of the current CF therapies were available. As with any study with a long follow-up period, this could limit the generalizability of our findings and the ability to predict future events, especially as additional therapies become available, although it should be noted that the frequency of pulmonary exacerbations has not changed appreciably in at least 20 years (19). Moreover, the children in the current study were healthier than the average child with CF born in the same era: in 2000, we would have expected a mean FEV1 in this age group of approximately 90% predicted (19). This improvement in FEV1 for the average 10-year-old with CF was not achieved until 2012 (19). Although our study cohort had a similar severity of lung disease as measured by spirometry to more recent birth cohorts of children with CF, it is possible that our subjects are not representative of more recent birth cohorts, because the study cohort achieved normal spirometry without the benefit of current CF therapies. The severity of bronchiectasis in these subjects was generally mild, if present at all, which may have resulted in a type II error in our comparisons between bronchiectasis and FEV1 % predicted. Clinicians would not have been aware of the Brody CT score, as the Brody score was not shared with the treating providers. However, it is possible that clinicians may have used CT images qualitatively to guide therapy, although therapeutic options were more limited at the time. We cannot assess whether these therapeutic decisions affected outcomes 10 years later. Outcomes after chest CT scans are scored quantitatively have not been evaluated in comparison with qualitative assessments of chest CT scans. There were some missing data, although FEV1 measurements were available for all patients for at least 6 years after the chest CT, and measurements were available through 2009 for 55 patients. It is possible that some exacerbations occurred but were not entered into the CFFPR, although this number is likely to be quite low, and pulmonary exacerbations treated without intravenous antibiotics are not recorded in the Registry. Because of the limited number of subjects, we were limited in the number of variables that could be included in regression models. Adjusting for body mass index at the time of the CT did not affect the regression models substantially (data not shown); at the time of the CT, few patients were underweight, reflecting the generally good health of subjects in the PEIT study.
We have shown that there is a significant association between chest CT scores in young school-age children and the rate of pulmonary exacerbations over the next 10 years. The association with pulmonary exacerbations is stronger than that of FEV1 obtained at the same time as the CT. This indicates the predictive potential of chest CT scores in children with mild PFT abnormalities and confirms and extends previous findings that support the use of chest CT as a surrogate end point. Chest CT scores may assist clinicians in individualizing the care of patients by recognizing the risk of progression of lung disease early in life, before PFT abnormalities become apparent.
Acknowledgments
Acknowledgment
The authors thank Bruce Marshall, Emily Knapp, the Cystic Fibrosis Foundation, and the Cystic Fibrosis Foundation Patient Registry Committee for providing the CF Foundation Registry data. The authors thank Phil Farrell for critical evaluations of the manuscript.
Footnotes
Supported by the Cystic Fibrosis Foundation (SANDERS11A0).
Author Contributions: D.B.S., Z.L., and A.S.B. all contributed to the conception and design of the work, interpretation of data for the work, acquisition and/or analysis of data, and drafting the work. D.B.S., Z.L., and A.S.B. each provided final approval and agree to be accountable for all aspects of the work.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? Ann Intern Med. 1996;125:605–613. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]
- 2.Rosenfeld M. An overview of endpoints for cystic fibrosis clinical trials: one size does not fit all. Proc Am Thorac Soc. 2007;4:299–301. doi: 10.1513/pats.200611-178HT. [DOI] [PubMed] [Google Scholar]
- 3.Nimmo WS, Tucker GT. Chichester, UK: John Wiley & Sons; 1995. Clinical measurement in drug evaluation. [Google Scholar]
- 4.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 5.Ramsey BW. Use of lung imaging studies as outcome measures for development of new therapies in cystic fibrosis. Proc Am Thorac Soc. 2007;4:359–363. doi: 10.1513/pats.200611-183HT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153:345–352. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schluchter MD, Konstan MW, Drumm ML, Yankaskas JR, Knowles MR. Classifying severity of cystic fibrosis lung disease using longitudinal pulmonary function data. Am J Respir Crit Care Med. 2006;174:780–786. doi: 10.1164/rccm.200512-1919OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandevanter DR, Yegin A, Morgan WJ, Millar SJ, Pasta DJ, Konstan MW. Design and powering of cystic fibrosis clinical trials using pulmonary exacerbation as an efficacy endpoint. J Cyst Fibros. 2011;10:453–459. doi: 10.1016/j.jcf.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jong PA, Nakano Y, Lequin MH, Mayo JR, Woods R, Paré PD, Tiddens HA. Progressive damage on high resolution computed tomography despite stable lung function in cystic fibrosis. Eur Respir J. 2004;23:93–97. doi: 10.1183/09031936.03.00006603. [DOI] [PubMed] [Google Scholar]
- 10.Brody AS, Sucharew H, Campbell JD, Millard SP, Molina PL, Klein JS, Quan J. Computed tomography correlates with pulmonary exacerbations in children with cystic fibrosis. Am J Respir Crit Care Med. 2005;172:1128–1132. doi: 10.1164/rccm.200407-989OC. [DOI] [PubMed] [Google Scholar]
- 11.de Jong PA, Lindblad A, Rubin L, Hop WC, de Jongste JC, Brink M, Tiddens HA. Progression of lung disease on computed tomography and pulmonary function tests in children and adults with cystic fibrosis. Thorax. 2006;61:80–85. doi: 10.1136/thx.2005.045146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansell DM. Bronchiectasis. Radiol Clin North Am. 1998;36:107–128. doi: 10.1016/s0033-8389(05)70009-9. [DOI] [PubMed] [Google Scholar]
- 13.Loeve M, Gerbrands K, Hop WC, Rosenfeld M, Hartmann IC, Tiddens HA. Bronchiectasis and pulmonary exacerbations in children and young adults with cystic fibrosis. Chest. 2011;140:178–185. doi: 10.1378/chest.10-1152. [DOI] [PubMed] [Google Scholar]
- 14.Tepper LA, Utens EM, Caudri D, Bos AC, Gonzalez-Graniel K, Duivenvoorden HJ, van der Wiel EC, Quittner AL, Tiddens HA. Impact of bronchiectasis and trapped air on quality of life and exacerbations in cystic fibrosis. Eur Respir J. 2013;42:371–379. doi: 10.1183/09031936.00137612. [DOI] [PubMed] [Google Scholar]
- 15.Loeve M, Hop WC, de Bruijne M, van Hal PT, Robinson P, Aitken ML, Dodd JD, Tiddens HA Computed Tomography Cystic Fibrosis Survival Study Group. Chest computed tomography scores are predictive of survival in patients with cystic fibrosis awaiting lung transplantation. Am J Respir Crit Care Med. 2012;185:1096–1103. doi: 10.1164/rccm.201111-2065OC. [DOI] [PubMed] [Google Scholar]
- 16.Davis SD, Ferkol T. Identifying the origins of cystic fibrosis lung disease. N Engl J Med. 2013;368:2026–2028. doi: 10.1056/NEJMe1303487. [DOI] [PubMed] [Google Scholar]
- 17.Sanders DB, Li Z, Brody AS, Farrell PM. Chest computed tomography scores of severity are associated with future lung disease progression in children with cystic fibrosis. Am J Respir Crit Care Med. 2011;184:816–821. doi: 10.1164/rccm.201105-0816OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brody AS, Klein JS, Molina PL, Quan J, Bean JA, Wilmott RW. High-resolution computed tomography in young patients with cystic fibrosis: distribution of abnormalities and correlation with pulmonary function tests. J Pediatr. 2004;145:32–38. doi: 10.1016/j.jpeds.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 19.Cystic Fibrosis Foundation. Bethesda, MD: Cystic Fibrosis Foundation; 2013. Cystic Fibrosis Foundation Patient Registry: 2012 annual data report. [Google Scholar]
- 20.Quan JM, Tiddens HA, Sy JP, McKenzie SG, Montgomery MD, Robinson PJ, Wohl ME, Konstan MW Pulmozyme Early Intervention Trial Study Group. A two-year randomized, placebo-controlled trial of dornase alfa in young patients with cystic fibrosis with mild lung function abnormalities. J Pediatr. 2001;139:813–820. doi: 10.1067/mpd.2001.118570. [DOI] [PubMed] [Google Scholar]
- 21.Brody AS, Kosorok MR, Li Z, Broderick LS, Foster JL, Laxova A, Bandla H, Farrell PM. Reproducibility of a scoring system for computed tomography scanning in cystic fibrosis. J Thorac Imaging. 2006;21:14–21. doi: 10.1097/01.rti.0000203937.82276.ce. [DOI] [PubMed] [Google Scholar]
- 22.Corey M, Edwards L, Levison H, Knowles M. Longitudinal analysis of pulmonary function decline in patients with cystic fibrosis. J Pediatr. 1997;131:809–814. doi: 10.1016/s0022-3476(97)70025-8. [DOI] [PubMed] [Google Scholar]
- 23.Farrell PM, Collins J, Broderick LS, Rock MJ, Li Z, Kosorok MR, Laxova A, Gershan WM, Brody AS. Association between mucoid Pseudomonas infection and bronchiectasis in children with cystic fibrosis. Radiology. 2009;252:534–543. doi: 10.1148/radiol.2522081882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loeve M, Krestin GP, Rosenfeld M, de Bruijne M, Stick SM, Tiddens HA. Chest computed tomography: a validated surrogate endpoint of cystic fibrosis lung disease? Eur Respir J. 2013;42:844–857. doi: 10.1183/09031936.00051512. [DOI] [PubMed] [Google Scholar]
- 25.Mayer-Hamblett N, Rosenfeld M, Emerson J, Goss CH, Aitken ML. Developing cystic fibrosis lung transplant referral criteria using predictors of 2-year mortality. Am J Respir Crit Care Med. 2002;166:1550–1555. doi: 10.1164/rccm.200202-087OC. [DOI] [PubMed] [Google Scholar]
- 26.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 27.Sanders DB, Bittner RC, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med. 2010;182:627–632. doi: 10.1164/rccm.200909-1421OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanders DB, Bittner RC, Rosenfeld M, Redding GJ, Goss CH. Pulmonary exacerbations are associated with subsequent FEV1 decline in both adults and children with cystic fibrosis. Pediatr Pulmonol. 2011;46:393–400. doi: 10.1002/ppul.21374. [DOI] [PubMed] [Google Scholar]
- 29.Britto MT, Kotagal UR, Hornung RW, Atherton HD, Tsevat J, Wilmott RW. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest. 2002;121:64–72. doi: 10.1378/chest.121.1.64. [DOI] [PubMed] [Google Scholar]
- 30.Briesacher BA, Quittner AL, Fouayzi H, Zhang J, Swensen A. Nationwide trends in the medical care costs of privately insured patients with cystic fibrosis (CF), 2001–2007. Pediatr Pulmonol. 2011;46:770–776. doi: 10.1002/ppul.21441. [DOI] [PubMed] [Google Scholar]
- 31.O’Connell OJ, McWilliams S, McGarrigle A, O’Connor OJ, Shanahan F, Mullane D, Eustace J, Maher MM, Plant BJ. Radiologic imaging in cystic fibrosis: cumulative effective dose and changing trends over 2 decades. Chest. 2012;141:1575–1583. doi: 10.1378/chest.11-1972. [DOI] [PubMed] [Google Scholar]
- 32.Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, Howe NL, Ronckers CM, Rajaraman P, Sir Craft AW, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380:499–505. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mogayzel PJ, Jr, Naureckas ET, Robinson KA, Mueller G, Hadjiliadis D, Hoag JB, Lubsch L, Hazle L, Sabadosa K, Marshall B Pulmonary Clinical Practice Guidelines Committee. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med. 2013;187:680–689. doi: 10.1164/rccm.201207-1160oe. [DOI] [PubMed] [Google Scholar]
- 34.Sawicki GS, Ren CL, Konstan MW, Millar SJ, Pasta DJ, Quittner AL Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Treatment complexity in cystic fibrosis: trends over time and associations with site-specific outcomes. J Cyst Fibros. 2013;12:461–467. doi: 10.1016/j.jcf.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Modi AC, Quittner AL. Barriers to treatment adherence for children with cystic fibrosis and asthma: what gets in the way? J Pediatr Psychol. 2006;31:846–858. doi: 10.1093/jpepsy/jsj096. [DOI] [PubMed] [Google Scholar]
- 36.Owens CM, Aurora P, Stanojevic S, Bush A, Wade A, Oliver C, Calder A, Price J, Carr SB, Shankar A, et al. London Cystic Fibrosis Collaboration. Lung Clearance Index and HRCT are complementary markers of lung abnormalities in young children with CF. Thorax. 2011;66:481–488. doi: 10.1136/thx.2010.150375. [DOI] [PubMed] [Google Scholar]
- 37.Wielpütz MO, Puderbach M, Kopp-Schneider A, Stahl M, Fritzsching E, Sommerburg O, Ley S, Sumkauskaite M, Biederer J, Kauczor HU, et al. Magnetic resonance imaging detects changes in structure and perfusion, and response to therapy in early cystic fibrosis lung disease. Am J Respir Crit Care Med. 2014;189:956–965. doi: 10.1164/rccm.201309-1659OC. [DOI] [PubMed] [Google Scholar]
- 38.Calder AD, Bush A, Brody AS, Owens CM. Scoring of chest CT in children with cystic fibrosis: state of the art. Pediatr Radiol. 2014;44:1496–1506. doi: 10.1007/s00247-013-2867-y. [DOI] [PubMed] [Google Scholar]