Abstract
Autophagy is a highly conserved process by which cells can recycle organelles and proteins by degrading them in the lysosomes. Although autophagy is considered a dynamic system responsible for cellular renovation and homeostasis under physiological conditions, it is increasingly clear that autophagy is directly relevant to clinical disease. During disease progression, autophagy not only serves as a cellular protective mechanism but also can represent a harmful event under certain conditions. In addition, although autophagy can act as a nonselective bulk degradation process, recent research shows that autophagy can selectively degrade specific proteins, organelles, and invading bacteria, in processes termed “selective autophagy.” Selective autophagy has drawn the attention of researchers because of its potential importance in clinical diseases. In this article, we outline the most recent studies implicating autophagy and selective autophagy in human lung diseases, including chronic obstructive pulmonary disease, pulmonary hypertension, idiopathic pulmonary fibrosis, and sepsis. We also discuss the relationship between autophagy and other molecular mechanisms related to disease progression, including programmed necrosis (necroptosis) and the inflammasome, an inflammatory signaling platform that regulates the secretion of IL-1β and IL-18. Finally, we examine the dual nature of autophagy and selective autophagy in the lung, which have both protective and injurious effects for human lung disease.
Keywords: autophagy, ciliophagy, inflammasome, mitophagy, necroptosis
Autophagy is a lysosomal degradation system that engulfs cytoplasm and organelles by complex reorganization of subcellular membranes to form a new organelle: the autophagosome (Figure 1). Autophagosomes fuse with lysosomes and thereby deliver their contents for degradation (1). Although autophagy can function as a recycling system for metabolic precursors to promote cell survival, this process has also been implicated in clinical diseases (2). Autophagy is known to exert both protective and injurious effects in a variety of different models of disease, suggesting that its role in human diseases is complex (Table 1).
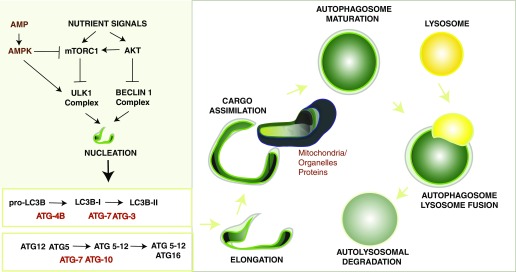
Figure 1.
Autophagy pathway. Autophagy is a regulated process that responds to regulation by nutrient signals. Autophagy responds to negative regulation by growth factor stimuli that activate the phosphatidylinositol-3-kinase (PI3K/AKT) pathway, which up-regulates the mTOR pathway and down-regulates the Beclin1 complex. Autophagy responds to up-regulation by depletion of cellular energy charge through the activation of the adenosine monophosphate (AMP)-activated protein kinase (AMPK). In response to elevated AMP levels, AMPK can inhibit mTORC1 and directly phosphorylate ULK1, leading to activation of autophagy. The antibiotic rapamycin induces autophagy by inactivating mTORC1. The initiation of autophagosome formation is also regulated by the autophagy protein Beclin 1 (Atg6). Beclin 1 associates with a macromolecular complex that includes hVps34, a class III phosphatidylinositol-3 kinase. Autophagosome elongation requires two ubiquitin-like conjugation systems, the ATG5-12 conjugation system, and the ATG8 (LC3) conjugation system, which are regulated by various ATG proteins. Autophagy protein LC3-II remains associated with the maturing autophagosome. The basic sequence of steps of autophagy include: (1) initiation and autophagosomal nucleation (formation of the phagophore); (2) elongation of the nascent autophagosomal membrane, to capture a cargo such as mitochondria; (3) maturation of the double-membraned autophagosomal structure with cargo assimilation; and (4) autophagosome-lysosome fusion, which is concluded by degradation of the autolysosomal contents.
Table 1.
Role of autophagy in human lung diseases
| Disease | Strain/Manipulation | Phenotype/Observation | References |
|---|---|---|---|
| COPD | C57Bl/6; chronic CS | Increased airspace enlargement associated with increased autophagy markers | 7, 12 |
| LC3B−/− mice, chronic CS | Decreased epithelial cell apoptosis and airspace enlargement | 7 | |
| Becn1+/− mice, acute CS | Decreased cilia loss and airways dysfunction | 4 | |
| PINK1−/− mice, chronic and acute CS | Decreased epithelial cell necroptosis and airspace enlargement; decreased airways dysfunction | 3 | |
| Human COPD lung | Increased autophagosome formation and autophagy markers | 12 | |
| Human smokers (alveolar macrophages) | Impaired autophagy | 14 | |
| Human COPD bronchial epithelial cells | Increased basal autophagy, decreased autophagy in response to CS stimulation, accelerated cell senescence | 18 | |
| PAH | C57Bl/6; hypoxia | Increased autophagy associated with indices of vascular dysfunction | 23 |
| LC3B−/− mice, hypoxia | Worsened indices of pulmonary hypertension | 23 | |
| Human PAH | Increased autophagy markers | 23 | |
| ILD | C57Bl/6; bleomycin, rapamycin | Reduced lung fibrosis relative to bleomycin alone | 25 |
| PINK1−/− mice | Increased lung fibrosis in aging mice | 31 | |
| PINK1−/− mice, bleomycin | Increased susceptibility to bleomycin-induced pulmonary fibrosis relative to wild-type mice | 32 | |
| Human ILD | Reduced autophagy markers | 25 | |
| Sepsis | C57Bl/6; CLP | Increased autophagy markers | 47 |
| LC3B−/−, LPS | Increased mortality, increased inflammasome cytokine production | 9 | |
| Beclin 1+/−, CLP sepsis | Increased mortality | 47 |
Definition of abbreviations: CLP = cecal ligation and puncture; COPD = chronic obstructive pulmonary disease; CS = cigarette smoke; ILD = interstitial lung disease; LPS = lipopolysaccharide; PAH = pulmonary arterial hypertension.
Once autophagy was simply considered as a nonspecific homeostatic cellular process; however, increasing evidence suggests that autophagy can represent a more selective process than originally anticipated. Recently, we and others have revealed the importance of selective autophagy pathways in chronic obstructive pulmonary disease (COPD); including mitophagy and ciliophagy (Figure 2) (3–5). Mitophagy refers to the specific autophagic elimination of mitochondria in which damaged or depolarized mitochondria are targeted for degradation (6). Parkin and phosphatase and tensin homolog (PTEN)-induced putative kinase protein 1 (PINK1) have been identified as two major mitophagy-related regulatory proteins. Ciliophagy is an autophagy-dependent pathway that regulates cilia length (4). Impaired airway clearance caused by ciliophagy-regulated cilia shortening may prevent the elimination of pathogens from the airways, resulting in recurrent respiratory infections that exacerbate COPD. Other forms of selective autophagy that may be relevant to human diseases include the removal of pathogens (xenophagy) and the processing of protein aggregates (aggrephagy). Selective autophagy is an emerging field that, as discussed in this article, is expected to provide new insight into the pathogenesis of human lung disease.
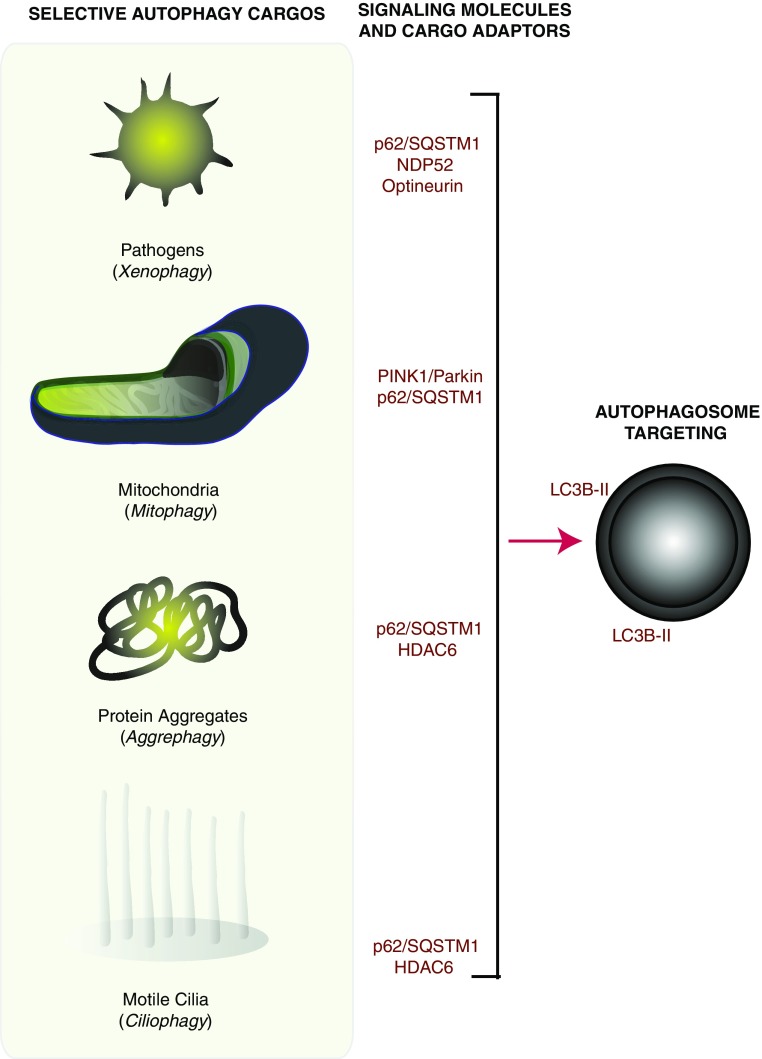
Figure 2.
Selective autophagy pathways. Selective autophagy pathways have been described that are specific for the targeting and autophagosomal delivery of specific cargo. Selective autophagy cargoes and pathways may include pathogens (e.g., bacteria, viruses) (xenophagy), mitochondria (mitophagy), protein aggregates (aggrephagy), and motile cilia (ciliophagy). Phosphatase and tensin homolog (PTEN)-induced putative kinase protein 1 (Pink1) and Parkin are known activators of mitophagy. Selective autophagy cargo adaptors (e.g., p62/SQSTM1) have been identified that facilitate these pathways. Nuclear domain 10 protein 52 (NDP52) and optineurin have been associated with xenophagic processing, whereas histone deacetylase 6 (HDAC6) has been implicated in the autophagic processing of protein aggregates and of cilia.
Recent studies have revealed that autophagy or selective autophagy can regulate other cellular pathways. In lung diseases, autophagy-regulated apoptosis may contribute to the pathogenesis of COPD (7). Although apoptosis was previously recognized as the sole form of programmed cell death, necrosis was considered as an uncontrolled cell death induced by extreme physical or chemical stress. However, emerging studies have demonstrated the existence of a genetically programmed and regulated form of necrosis, termed necroptosis (8). Interestingly, necroptosis is regulated by mitophagy, which may contribute to the pathogenesis of COPD (3). Moreover, autophagy has been implicated in the regulation of the inflammasome pathway (9). Inflammasomes represent an inflammatory signaling platform that regulates the maturation and secretion of proinflammatory cytokines (e.g., IL-1β and IL-18), which are implicated in sepsis (9, 10). The role of autophagy and selective autophagy processes, whether protective or deleterious, depend on the disease, and sometimes differ between cell types. In this review, we examine the considerable emerging evidence for the contribution of autophagy and selective autophagy in the pathogenesis of complex lung diseases. A better understanding of autophagy and selective autophagy as “double-edged swords” in disease pathogenesis will help design personalized therapies for the treatment of lung diseases.
Autophagy: Regulation and Function in Experimental and Human Lung Diseases
Chronic Obstructive Pulmonary Disease
The World Health Organization reported that more than 3 million people died of COPD in 2012, which is equal to 6% of all deaths globally that year; however, the pathogenesis of this disease remains incompletely understood. We previously analyzed comprehensive gene expression profiles in Global Initiative for Chronic Obstructive Lung Disease stage 2 versus stage 0 smokers, which revealed that autophagy-related protein, ATG8/microtubule-associated protein-1 light chain-3 (LC3), was a candidate gene that may serve as a potential molecular target in COPD (11). Our further investigation demonstrated a pivotal role for autophagy proteins in cigarette smoke (CS)-induced emphysema (7, 12). We demonstrated that autophagic vacuoles (autophagosomes/autolysosomes) were dramatically increased in COPD lung tissues using electron microscopy, a gold-standard method for the determination of autophagy, whereas little vacuole formation was evident in control tissues (12). The expression of the active form of LC3B, LC3B-II, as well as that of additional autophagy-related proteins Atg4, Atg5-Atg12, and Atg7 increased in human lung specimens from patients with COPD. Genetic depletion of the essential autophagy mediators, LC3B and Beclin 1, reduced cigarette smoke extract (CSE)-induced epithelial cell death. Moreover, LC3B-deficient mice exhibited significant basal airspace enlargement relative to wild-type littermate mice (7). To determine whether increased autophagosome formation correlated with autophagic activity in the lungs of CS-treated mice, we applied in vivo autophagic flux assays developed in our laboratory (13). Analysis of LC3B steady-state levels in a lysosome-enriched fraction of lung homogenates revealed the time-dependent increase in leupeptin-sensitive LC3B degradation in vivo, which persisted 24 hours after CS exposure, supporting the conclusion that CS caused a cumulative increase in autophagic flux in lung tissue (4). These data suggest that the CS-induced autophagy pathway may promote epithelial cell death, associated with emphysematous airspace enlargement, in chronic CS-exposed mice and in patients with COPD.
On the other hand, a previous study reported defective autophagy in CS-exposed macrophages (14). Such a deficit in autophagy was found in the alveolar macrophages of smokers, suggesting that impaired delivery of bacteria to lysosomes may lead to recurrent infections in patients with COPD. Interestingly, it has also been suggested that autophagy regulates cellular senescence. Although there is evidence that autophagy induction may accelerate the development of senescence, other studies suggest the opposite, that autophagy inhibition is permissive for senescence (15). As CS-induced cell senescence has been implicated in the pathogenesis of COPD (16, 17), a previous study has evaluated autophagy-regulated senescence in bronchial epithelial cells (18). Although 3-MA, an inhibitor of autophagy, enhanced CSE-induced senescence in primary human bronchial epithelial cells (HBEC), Torin-1, an inducer of autophagy, suppressed CSE-induced HBEC senescence (18). Furthermore, although baseline autophagy activity in HBEC from patients with COPD was significantly increased compared with those of HBEC from nonsmokers and smokers without COPD, autophagy induction in response to CSE exposure was significantly attenuated in HBEC from patients with COPD compared with that observed in HBEC from nonsmokers and smokers without COPD (18). These findings implicate that autophagy is insufficient in the lungs of patients with COPD, which accelerates epithelial cell senescence. Additionally, mTOR signaling has been linked to CS induced COPD/emphysema (19). mTOR is an evolutionarily conserved serine-threonine kinase that acts as a sensor of environmental and cellular nutrition and energy status, which also plays a crucial role in regulating autophagy (20). Rtp801, a stress-related protein triggered by adverse environmental conditions, was overexpressed in human emphysematous lungs and in lungs of mice exposed to CS (19). Rtp801 stabilized the assembly of the mTOR inhibitory complex TSC1-TSC2, resulting in the exacerbation of oxidative stress–induced cell death (19). In this study, the mTOR inhibitor rapamycin was protective in wild-type mice exposed to smoke, reducing alveolar inflammation. In contrast, rapamycin increased the number of apoptotic and inflammatory cells in room air–exposed wild-type mice and abrogated the protective effects of Rtp801 knockout in mice exposed to CS. These studies highlight that the timing and lung cell targets of mTOR inhibition may therefore be essential to define its beneficial and pathological roles in disease.
Autophagy may play a complex role in the lung, where it can have both protective and injurious effects on the progression of COPD. At present, there is no unifying explanation for the discrepancies between various studies. The timing and lung cell targets for autophagy may be essential to define its beneficial and pathologic roles in a context-specific manner. A better understanding of the balance between the cytoprotective and pro-death functions of autophagy in response to CS will be required for the therapeutic targeting of this process in COPD.
Pulmonary Hypertension
Pulmonary hypertension (PH) refers to elevated pulmonary arterial pressure that can represent a progressive, fatal disease if untreated (21). Hypoxia causes secondary PH, and hypoxic PH is a progressive and often fatal complication of chronic lung disease (22). We have demonstrated the elevated occurrence of autophagy in lung tissue from patients with PH (23). Mice genetically deficient in LC3B were subjected to chronic hypoxia for determination of the involvement of autophagy proteins in PH and their impact on measured indices of PH. Hypoxic lung tissues displayed increased autophagic vacuoles compared with lung tissue from normoxic mice. After chronic hypoxia, LC3B−/− mice showed increased indices of PH, right ventricular systolic pressure and Fulton’s index, relative to wild-type mice. These results suggest that the autophagy protein LC3B may perform a protective function during the pathogenesis of hypoxic pulmonary hypertension.
Idiopathic Pulmonary Fibrosis
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive lung disease that predominantly affects older patients and has a death rate worse than that of many cancers. However, therapeutic options to alter the course of this disease remain lacking (24). Lung tissues from patients with IPF showed decreased autophagic activity as assessed by LC3B-II expression and few or no autophagosomes by electron microscopy (25). Insufficient autophagy may accelerate cellular senescence in epithelial cells and potentiate myofibroblast differentiation (26). To evaluate the role of autophagy in bleomycin-induced experimental fibrosis, mice were given oral administration of rapamycin, an inducer of autophagy (20). Mice receiving rapamycin in addition to bleomycin displayed significantly lower levels of lung hydroxyproline as a measure of collagen content than mice treated with bleomycin alone. These results suggest that regulating autophagy might alter the course of experimental IPF.
Selective Autophagy: Mitophagy and Ciliophagy in Experimental and Human Lung Disease
Chronic Obstructive Pulmonary Disease
Selective autophagy pathways deliver a wide range of cargo to the lysosome for degradation, including protein aggregates, whole organelles (e.g., mitochondria), and intracellular pathogens (27). Ubiquitin (Ub)-positive substrates, such as protein aggregates (inclusion bodies), mitochondria, and invading bacteria not dealt with by the proteasome system are selectively degraded by autophagy, suggesting ubiquitination is general tag for selective autophagy (28, 29). Autophagy receptors, such as p62 and neighbor of BRCA1 gene 1 (NBR1), which simultaneously bind monoubiquitinated or polyubiquitinated substrates, act as adaptors between ubiquitination and autophagy (30). Recently, we have demonstrated that mitophagy regulates programmed necroptosis, also called necroptosis, which contributes to the pathogenesis of COPD (Figure 3) (3). CSE, an in vitro model of CS exposure, caused significant mitochondrial depolarization and induced mitophagy in lung epithelial cells. We demonstrated that the mitochondrial division/mitophagy inhibitor Mdivi-1 protected against CS-induced cell death by reducing the phosphorylation of mixed lineage kinase domain-like protein (MLKL), a substrate for the receptor-interacting serine/threonine-protein kinase 3 (RIP3) in the necroptosis pathway. Mice genetically deficient in the mitophagy regulator PINK1 were protected against mitochondrial dysfunction, airspace enlargement, and mucociliary clearance disruption during CS exposure. Our results suggest that CS-activated mitophagy may alter mitochondrial membrane integrity and lead to the induction of mitophagy and necroptosis in pulmonary epithelial cells. Moreover, recent studies have suggested that CS-induced mitophagy may regulate cellular senescence in COPD pathogenesis (5). Genetically blocking mitophagy resulted in enhanced CS-induced mitochondrial reactive oxygen species (ROS) production and cellular senescence in HBEC. A precise mechanism by which mitophagy regulates the relationship between necroptosis and senescence remains obscure; however, one possible hypothesis is that mitophagy may lead to either senescence or necroptosis depending on the degree of cellular injury (5).
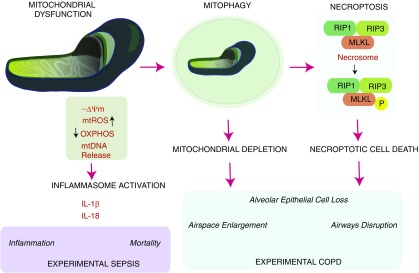
Figure 3.
Significance of mitochondrial dysfunction in human disease pathogenesis. Mitochondria have been implicated in the pathogenesis of human disease. Mitochondrial dysfunction associated with depolarization of –ΔΨ, increased mitochondrial ROS (mtROS) production, decline in oxidative phosphorylation (OXPHOS) and energy charge, and increased release of mitochondrial DNA (mtDNA) has been linked to pathogenic processes. In human sepsis models, mitochondrial dysfunction associated with mtDNA release leads to activation of the inflammasome and secretion of proinflammatory cytokines (IL-1β, IL-18). These events may be associated with sepsis mortality and increased inflammation. In chronic obstructive pulmonary disease (COPD) models, mitochondrial dysfunction was associated with activation of the mitophagy program and subsequent activation of the necroptosis mode of cell death. Necroptosis is regulated by formation of the receptor-interacting serine/threonine-protein kinase (RIP)1/RIP3-containing necrosome, which activates (phosphorylates) mixed lineage kinase domain-like protein (MLKL). These phenomena were linked to increased airspace enlargement, airways dysfunction, and epithelial cell loss in COPD.
We also reported that ciliophagy, the consumption of cilia components by autophagy, regulates cilia length during CS exposure (4). We showed that autophagy-impaired (Becn1+/– or Map1lc3B–/–) mice, as well as tracheal epithelial cells isolated from these mice, display a reduction of CS-induced cilia shortening. We identified the cytosolic deacetylase histone deacetylase 6 (HDAC6) as a critical regulator of autophagy-mediated cilia shortening during CS exposure. Importantly, analysis of human COPD specimens demonstrated epigenetic deregulation of HDAC6 by hypomethylation and increased protein expression in the airways. These data suggest that ciliophagy, an HDAC6-dependent selective autophagy pathway, may represent a novel pathway that is critical to cilia homeostasis in response to CS exposure.
It remains to be elucidated how cells use “nonselective autophagy” and “selective autophagy” pathways in response to CS exposure. The term “nonselective autophagy” refers to bulk degradation of cytosol and is implicated as a survival mechanism in starvation. Emerging studies, however, implicate that most substrate degradation by autophagy is assisted by selective processes. We speculate that CS induces nonselective autophagy coincident with injury to organelles (e.g., mitochondria, cilia). Thus, nonselective autophagy and selective autophagy may likely proceed simultaneously in a single cell. In addition, either process may be impacted by cell type and stimulus intensity. Future studies are needed for understanding the relationship between nonselective autophagy and selective autophagy in responses to CS exposure.
Pulmonary Fibrosis
Along with Bueno and colleagues, we have demonstrated that the lungs of patients with IPF exhibit marked accumulation of damaged mitochondria (31, 32). In lung epithelial cells, genetic deficiency of the mitophagy regulator PINK1 potentiated transforming growth factor-β–induced mitochondrial ROS production and cell death (32). PINK1 overexpression also ameliorated endoplasmic reticulum stress–induced mitochondrial accumulation and depolarization (31). Moreover, Pink1−/− mice were more susceptible than control mice to bleomycin-induced lung fibrosis (32). These data suggest that induction of mitophagy may improve mitochondrial function in the lungs of patients with IPF. Therapeutic targeting of mitophagy pathway may be useful in the treatment of fibrotic lung diseases.
Autophagy: A Critical Regulator of Cell Death
Basal autophagy is considered as a cellular protective mechanism to degrade cytoplasmic materials and provide energy for cellular renovation and homeostasis (1). Autophagy has also been considered as a suicidal mechanism, and the term “autophagic cell death” was used to indicate instances of cell death associated with excessive cytoplasmic vacuolization (33). However, “autophagic cell death” is not currently recognized as a distinct form of programmed cell death, but rather autophagy can be regarded as a phenomenon commonly associated with cell death pathways (34). In contrast, a previous study suggested that treatment with zVAD, a pan-caspase inhibitor with broad specificity, induced autophagy and the death of L929 cells. This process required RIP1, suggesting that autophagy is involved in necroptosis (35). Subsequently, autophagy has been shown to regulate necroptosis in several models. In childhood acute lymphoblastic leukemia cells, the induction of autophagy-dependent necroptosis was required to overcome glucocorticoid resistance (36). In endothelial cells, the inhibition of autophagy rescued palmitic acid–induced necroptosis (37). Moreover, apoptosis can occur at the same time as autophagy, suggesting a common regulatory mechanism (38, 39). Several studies indicated that proapoptotic signaling molecules induce autophagy, including tumor necrosis factor (40), tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) (41), Fas-associated protein with death domain (FADD) (42), dynamin-related protein-1 (DRP-1) (43), and death-associated protein kinase (DAPK) (44). In the CS exposure model, we observed reduced epithelial apoptosis coincident with Beclin1 or LC3B knockdown (7, 12). Therefore, an emerging hypothesis is that autophagy proteins can regulate programmed cell death, and vice versa, including necroptosis and apoptosis in a context-specific fashion. We have described that autophagy and selective autophagy can regulate apoptosis and necroptosis in COPD. We demonstrated that dynamic interactions of the autophagic protein LC3B with Cav-1 and Fas regulate CS-induced lung epithelial cell apoptosis, leading to emphysematous airspace enlargement (7). More recently, we also demonstrated that CS-induced mitophagy may alter mitochondrial membrane integrity and lead to the induction of necroptosis (3). However, the precise mechanisms by which mitophagy can aggravate mitochondrial injury or alter mitochondrial dynamics or biogenesis in the CS exposure model remain unclear. Moreover, as dose-dependent effects of autophagy/mitophagy have been proposed, we cannot completely exclude the possibility that autophagy/mitophagy may also contribute to mitochondrial quality control in a prosurvival role during mild CS exposure (45, 46). Further studies are necessary to improve the understanding of the relationship between autophagy/mitophagy and programmed cell death.
Autophagy: A Critical Regulator of Inflammasomes
Sepsis
Autophagy has been implicated in the regulation of inflammation, in particular the regulation of the inflammasome, an inflammatory signaling platform for the secretion of IL-1β and IL-18. Consistent with the findings of Zhou and colleagues, we have revealed that autophagy maintains mitochondrial integrity and prevents damaged ROS-producing mitochondria from activating inflammasome complexes (9, 10). The NLRP3 inflammasome and mitochondrial ROS production regulate cytosolic translocation of mitochondrial DNA (mtDNA) in macrophages, which in turn regulate the secretion of IL-1β and IL-18 (Figure 3). We found that depletion of autophagy proteins promoted the accumulation of dysfunctional mitochondria and increased the cytosolic translocation of mtDNA in response to LPS and ATP stimulation in macrophages (9). Furthermore, we evaluated the function of autophagy in severe sepsis using endotoxemia, commonly used as a model of septic shock, and cecal ligation and puncture (CLP), a more clinically relevant model of polymicrobial sepsis. Mice genetically deficient in LC3B were more susceptible to both LPS- and CLP-induced lethality than control mice. Serum concentrations of IL-1β and IL-18 were significantly higher in endotoxemic LC3B−/− mice than in their wild-type littermates (9). These data suggest that autophagy may regulate inflammasome activity by preventing cytosolic translocation of mtDNA commonly associated with severe sepsis, implicating that autophagy has beneficial antiinflammatory effects. Similarly, Becn1+/– mice were more susceptible to CLP-induced sepsis. Recently, we have reported that carbon monoxide (CO) confers protection in sepsis by enhancing Beclin1-dependent autophagy and phagocytosis (47). Although CO enhanced bacterial phagocytosis in mice, this effect was reduced in Becn1+/– mice. These results suggest that CO gas may induce xenophagy and phagocytosis, representing a novel therapy for patients with sepsis.
Translational/Clinical Implications
Previously we showed that circulating IL-1β and IL-18 levels increased in critically ill patients with both sepsis and adult respiratory distress syndrome and that increases in circulating IL-18 correlated with disease severity and mortality (48). Consistent with our experimental verification that mtDNA is implicated in inflammasome activity, we also demonstrated that increased mtDNA levels in plasma are associated with intensive care unit mortality and that inclusion of mtDNA level improves risk prediction in medical intensive care unit patients (9). These data suggest that the evaluation of inflammasome activity, including measurements of IL-18 and circulating cell-free mtDNA levels, could provide new diagnostic and/or therapeutic approaches for patients with critical illness.
Conclusions
Accumulating evidence demonstrates that autophagy and selective autophagy exert previously unknown functions during the pathogenesis of human lung diseases. It is now clear that autophagy plays a complex role in the lung, where it can have both protective and injurious effects on the progression of lung disease. Careful consideration and further research are needed for the design of strategies to manipulate autophagy as a valid therapeutic intervention.
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:1845–1846. doi: 10.1056/NEJMc1303158. [DOI] [PubMed] [Google Scholar]
- 3.Mizumura K, Cloonan SM, Nakahira K, Bhashyam AR, Cervo M, Kitada T, Glass K, Owen CA, Mahmood A, Washko GR, et al. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J Clin Invest. 2014;124:3987–4003. doi: 10.1172/JCI74985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam HC, Cloonan SM, Bhashyam AR, Haspel JA, Singh A, Sathirapongsasuti JF, Cervo M, Yao H, Chung AL, Mizumura K, et al. Histone deacetylase 6-mediated selective autophagy regulates COPD-associated cilia dysfunction. J Clin Invest. 2013;123:5212–5230. doi: 10.1172/JCI69636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito S, Araya J, Kurita Y, Kobayashi K, Takasaka N, Yoshida M, Hara H, Minagawa S, Wakui H, Fujii S, et al. PARK2-mediated mitophagy is involved in regulation of HBEC senescence in COPD pathogenesis. Autophagy. 2015;11:547–559. doi: 10.1080/15548627.2015.1017190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen ZH, Lam HC, Jin Y, Kim HP, Cao J, Lee SJ, Ifedigbo E, Parameswaran H, Ryter SW, Choi AM. Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. Proc Natl Acad Sci USA. 2010;107:18880–18885. doi: 10.1073/pnas.1005574107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 9.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 11.Ning W, Li CJ, Kaminski N, Feghali-Bostwick CA, Alber SM, Di YP, Otterbein SL, Song R, Hayashi S, Zhou Z, et al. Comprehensive gene expression profiles reveal pathways related to the pathogenesis of chronic obstructive pulmonary disease. Proc Natl Acad Sci USA. 2004;101:14895–14900. doi: 10.1073/pnas.0401168101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen ZH, Kim HP, Sciurba FC, Lee SJ, Feghali-Bostwick C, Stolz DB, Dhir R, Landreneau RJ, Schuchert MJ, Yousem SA, et al. Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. Plos One. 2008;3:e3316. doi: 10.1371/journal.pone.0003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haspel J, Shaik RS, Ifedigbo E, Nakahira K, Dolinay T, Englert JA, Choi AMK. Characterization of macroautophagic flux in vivo using a leupeptin-based assay. Autophagy. 2011;7:629–642. doi: 10.4161/auto.7.6.15100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monick MM, Powers LS, Walters K, Lovan N, Zhang M, Gerke A, Hansdottir S, Hunninghake GW. Identification of an autophagy defect in smokers’ alveolar macrophages. J Immunol. 2010;185:5425–5435. doi: 10.4049/jimmunol.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gewirtz DA. Autophagy and senescence in cancer therapy. J Cell Physiol. 2014;229:6–9. doi: 10.1002/jcp.24420. [DOI] [PubMed] [Google Scholar]
- 16.Mercado N, Ito K, Barnes PJ. Accelerated ageing of the lung in COPD: new concepts. Thorax. 2015;70:482–489. doi: 10.1136/thoraxjnl-2014-206084. [DOI] [PubMed] [Google Scholar]
- 17.Tuder RM, Kern JA, Miller YE. Senescence in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2012;9:62–63. doi: 10.1513/pats.201201-012MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujii S, Hara H, Araya J, Takasaka N, Kojima J, Ito S, Minagawa S, Yumino Y, Ishikawa T, Numata T, et al. Insufficient autophagy promotes bronchial epithelial cell senescence in chronic obstructive pulmonary disease. OncoImmunology. 2012;1:630–641. doi: 10.4161/onci.20297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida T, Mett I, Bhunia AK, Bowman J, Perez M, Zhang L, Gandjeva A, Zhen L, Chukwueke U, Mao T, et al. Rtp801, a suppressor of mTOR signaling, is an essential mediator of cigarette smoke-induced pulmonary injury and emphysema. Nat Med. 2010;16:767–773. doi: 10.1038/nm.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YC, Guan KL. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125:25–32. doi: 10.1172/JCI73939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–1665. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 22.Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 23.Lee SJ, Smith A, Guo L, Alastalo TP, Li M, Sawada H, Liu X, Chen ZH, Ifedigbo E, Jin Y, et al. Autophagic protein LC3B confers resistance against hypoxia-induced pulmonary hypertension. Am J Respir Crit Care Med. 2011;183:649–658. doi: 10.1164/rccm.201005-0746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunninghake GM. A new hope for idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2142–2143. doi: 10.1056/NEJMe1403448. [DOI] [PubMed] [Google Scholar]
- 25.Patel AS, Lin L, Geyer A, Haspel JA, An CH, Cao J, Rosas IO, Morse D. Autophagy in idiopathic pulmonary fibrosis. Plos One. 2012;7:e41394. doi: 10.1371/journal.pone.0041394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Araya J, Kojima J, Takasaka N, Ito S, Fujii S, Hara H, Yanagisawa H, Kobayashi K, Tsurushige C, Kawaishi M, et al. Insufficient autophagy in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304:L56–L69. doi: 10.1152/ajplung.00213.2012. [DOI] [PubMed] [Google Scholar]
- 27.Stolz A, Ernst A, Dikic I. Cargo recognition and trafficking in selective autophagy. Nat Cell Biol. 2014;16:495–501. doi: 10.1038/ncb2979. [DOI] [PubMed] [Google Scholar]
- 28.Kirkin V, McEwan DG, Novak I, Dikic I. A role for ubiquitin in selective autophagy. Mol Cell. 2009;34:259–269. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 29.Ichimura Y, Komatsu M. Selective degradation of p62 by autophagy. Semin Immunopathol. 2010;32:431–436. doi: 10.1007/s00281-010-0220-1. [DOI] [PubMed] [Google Scholar]
- 30.Kim PK, Hailey DW, Mullen RT, Lippincott-Schwartz J. Ubiquitin signals autophagic degradation of cytosolic proteins and peroxisomes. Proc Natl Acad Sci USA. 2008;105:20567–20574. doi: 10.1073/pnas.0810611105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bueno M, Lai YC, Romero Y, Brands J, St Croix CM, Kamga C, Corey C, Herazo-Maya JD, Sembrat J, Lee JS, et al. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest. 2015;125:521–538. doi: 10.1172/JCI74942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel AS, Song JW, Chu SG, Mizumura K, Osorio JC, Shi Y, El-Chemaly S, Lee CG, Rosas IO, Elias JA, et al. Epithelial cell mitochondrial dysfunction and PINK1 are induced by transforming growth factor-beta1 in pulmonary fibrosis. Plos One. 2015;10:e0121246. doi: 10.1371/journal.pone.0121246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 36.Bonapace L, Bornhauser BC, Schmitz M, Cario G, Ziegler U, Niggli FK, Schäfer BW, Schrappe M, Stanulla M, Bourquin JP. Induction of autophagy-dependent necroptosis is required for childhood acute lymphoblastic leukemia cells to overcome glucocorticoid resistance. J Clin Invest. 2010;120:1310–1323. doi: 10.1172/JCI39987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan MJ, Rizwan Alam M, Waldeck-Weiermair M, Karsten F, Groschner L, Riederer M, Hallström S, Rockenfeller P, Konya V, Heinemann A, et al. Inhibition of autophagy rescues palmitic acid-induced necroptosis of endothelial cells. J Biol Chem. 2012;287:21110–21120. doi: 10.1074/jbc.M111.319129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levine B, Sinha S, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008;4:600–606. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lalaoui N, Lindqvist LM, Sandow JJ, Ekert PG. The molecular relationships between apoptosis, autophagy and necroptosis. Semin Cell Dev Biol. 2015;39:63–69. doi: 10.1016/j.semcdb.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Jia L, Dourmashkin RR, Allen PD, Gray AB, Newland AC, Kelsey SM. Inhibition of autophagy abrogates tumour necrosis factor alpha induced apoptosis in human T-lymphoblastic leukaemic cells. Br J Haematol. 1997;98:673–685. doi: 10.1046/j.1365-2141.1997.2623081.x. [DOI] [PubMed] [Google Scholar]
- 41.Herrero-Martín G, Høyer-Hansen M, García-García C, Fumarola C, Farkas T, López-Rivas A, Jäättelä M. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 2009;28:677–685. doi: 10.1038/emboj.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pyo JO, Jang MH, Kwon YK, Lee HJ, Jun JI, Woo HN, Cho DH, Choi B, Lee H, Kim JH, et al. Essential roles of Atg5 and FADD in autophagic cell death: dissection of autophagic cell death into vacuole formation and cell death. J Biol Chem. 2005;280:20722–20729. doi: 10.1074/jbc.M413934200. [DOI] [PubMed] [Google Scholar]
- 43.Inbal B, Bialik S, Sabanay I, Shani G, Kimchi A. DAP kinase and DRP-1 mediate membrane blebbing and the formation of autophagic vesicles during programmed cell death. J Cell Biol. 2002;157:455–468. doi: 10.1083/jcb.200109094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou F, Yang Y, Xing D. Bcl-2 and Bcl-xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J. 2011;278:403–413. doi: 10.1111/j.1742-4658.2010.07965.x. [DOI] [PubMed] [Google Scholar]
- 45.Frank M, Duvezin-Caubet S, Koob S, Occhipinti A, Jagasia R, Petcherski A, Ruonala MO, Priault M, Salin B, Reichert AS. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochim Biophys Acta. 2012;1823:2297–2310. doi: 10.1016/j.bbamcr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 46.Schiavi A, Ventura N. The interplay between mitochondria and autophagy and its role in the aging process. Exp Gerontol. 2014;56:147–153. doi: 10.1016/j.exger.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 47.Lee S, Lee SJ, Coronata AA, Fredenburgh LE, Chung SW, Perrella MA, Nakahira K, Ryter SW, Choi AM. Carbon monoxide confers protection in sepsis by enhancing beclin 1-dependent autophagy and phagocytosis. Antioxid Redox Signal. 2014;20:432–442. doi: 10.1089/ars.2013.5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, Massaro AF, Rogers A, Gazourian L, Nakahira K, et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med. 2012;185:1225–1234. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]