To the Editors
There are an estimated 2.9 million 10–24-year-olds living with HIV in sub-Saharan Africa,1 and these numbers are expected to increase with improved survival among perinatally infected children and as a result of new behaviorally acquired infections among older adolescents and youth.2 Enrolling and retaining adolescents and youth in HIV care and treatment services remain a significant challenge.3–5 Targeted services tailored to the specific needs of adolescents and youth, including dedicated adolescent care clinics, peer support groups, and sexual and reproductive services, have been cited as a way to improve care outcomes.6 Evaluations of the impact of these services, particularly in resource-limited settings where most HIV-infected young people, have been very limited.
Routinely collected deidentified patient-level data from electronic databases at health facilities participating in the Optimal Models for HIV Care in Africa Study in the Nyanza region of Kenya were used to examine whether the implementation of youth and adolescent friendly services (YAFS) improved retention among HIV-infected 10–24-year-olds. All facilities provided HIV services as per Kenyan national guidelines and received technical support from ICAP-Columbia University. Six facilities implemented YAFS starting in March 2013 which included (1) training and mentorship for health care providers on care for adolescents/youth, (2) a dedicated day for adolescent/youth HIV clinic at least once monthly which provided integrated sexual and reproductive health services, including gynecologic examinations, condoms, and hormonal contraception and, (3) support groups and education programs run by youth and adult peer educators.
The impact of YAFS on retention of patients was examined by comparing incidence of loss to follow-up (LTF) in the first 12 months for all newly enrolled patients 10–24 years of age in 2 periods; the “pre-YAFS” period before introduction of YAFS services (March–December 2011) and the “post-YAFS” period after YAFS implementation (March–December 2013). In addition, LTF outcomes were examined in the pre-and post-YAFS periods at 28 health facilities that did not implement YAFS (“Non-YAFS”) to examine changes in LTF in the same periods which were unrelated to YAFS.
The analysis examined LTF before antiretroviral therapy (ART) initiation among all newly enrolled patients before ART initiation (pre-ART). Pre-ART LTF was defined as not attending any visits within 12 months (and not recorded as dead or transferred to a different facility). LTF after ART initiation in the first 6 months was examined among patients who started treatment at least 6 months before the end of data collection. Competing risk estimators were used to calculate cumulative incidence of LTF, with death and ART initiation treated as competing risks for pre-ART LTF and only death for LTF after ART initiation. Subdistributional modified Cox proportional hazards models were used to compare cumulative incidence of LTF in the pre and post-YAFS periods and between YAFS and control facilities, adjusting for facility clustering. Analyses were conducted in SAS 9.3 and STATA 12. Ethics review and approval were received from institutional review boards at Columbia University Medical Center, US Centers for Disease Control and Prevention (CDC), and the Kenya Medical Research Institute (KEMRI).
At the 6 YAFS facilities, 426 patients 10–24 years enrolled in care in the pre-YAFS period and 304 were enrolled in the post-YAFS period. At the 28 non-YAFS facilities, 1017 patients enrolled in the pre-YAFS period and 574 enrolled post-YAFS. Facilities that implemented YAFS were purposively selected based on higher enrollment of adolescents/youth and were more likely to be secondary level (rather than primary) and to be in urban/semiurban areas compared with the facilities that did not implement YAFS.
Across both periods and facility types, the median age at enrollment in care was 21 years (interquartile range: 19–23) and most enrolled adolescents/youth (85.4%) were female, about half of whom (51.8%) were pregnant at enrollment in care. There were no differences in ages, sex, or pregnancy status among patients enrolled at YAFS and non-YAFS facilities or by period. Among those with CD4+ cell count (CD4+) (43%) and World Health Organization (WHO) stage (80%) data at enrollment, the overall median CD4+ was 429 cells per cubic millimeter (interquartile range: 241–638), 79.7% of patients had a CD4+ ≥200 cells per cubic millimeter at enrollment and 70.3% were WHO stage I or II. There were no differences in enrollment CD4+ or WHO stage across YAFS/non-YAFS facilities or by period. In total, 46.9% of patients started ART with no differences in proportions starting by YAFS status or period.
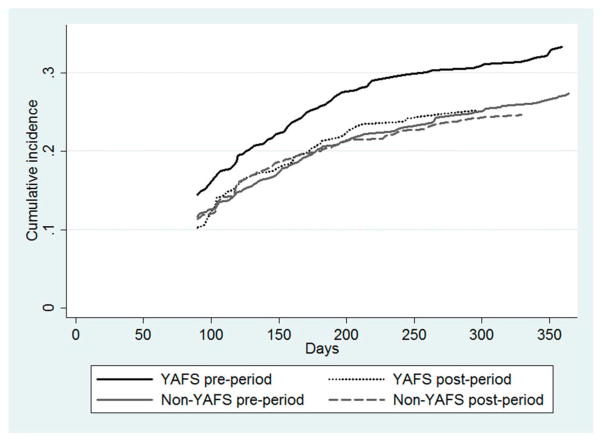
In the period before YAFS at the 6 facilities that later implemented YAFS, pre-ART LTF among enrolled 10–24-year-olds was 14.5% [95% confidence interval (CI): 11.2% to 18.1%] at 3 months, 25.5% (95% CI: 21.1 to 29.6) at 6 months, and 33.2% (95% CI: 28.6 to 37.9) by 12 months (Fig. 1). In the period after implementation of YAFS, pre-ART LTF at 3, 6, and 12 months was 10.3% (95% CI: 7.1 to 14.1), 20.6% (95% CI: 16.2 to 25.5), and 25.2% (95% CI: 20.4 to 30.3), respectively (Fig. 1). Although there was an observed decrease in LTF between the pre- and post-YAFS periods, the difference was not significant (P = 0.15). At the 28 non-YAFS facilities, pre-ART LTF was 11.7% (95% CI: 9.7 to 13.8), 20.3% (95% CI: 17.8 to 22.7), and 27.3% (95% CI: 24.5 to 30.2) at 3, 6, and 12 months in the pre-YAFS period, and in the post-YAFS period was 11.3% (95% CI: 8.8 to 14.2), 19.9% (95% CI: 16.6 to 23.3), and 24.6% (95% CI: 21.0 to 28.3), respectively (Fig. 1). The difference in pre-ART LTF between the before and after periods at the non-YAFS health facilities was also not significant (P = 0.28). There was no difference in pre-ART LTF between YAFS and non-YAFS facilities in the pre-YAFS period (P = 0.08) nor were there significant differences in LTF in the post-YAFS periods between facilities with and without YAFS (P = 0.87). There were few deaths reported (mortality is likely under ascertained) with no difference in mortality over time or between YAFS and non-YAFS facilities.
FIGURE 1.
Cumulative pre-ART LTF over 12 months after enrollment among adolescents/youth at Kenyan health facilities before and after implementation of YAFS at facilities that did and did not implement YAFS (N = 2321). Figure note: Patients were not considered LTF pre-ART until 90 days after the last attended visit (if they did not return for next visit) therefore the first LTF event occurs at day 90.
The analysis of LTF in the 6 months after ART initiation included 274 adolescents/youth from the YAFS facilities (172 from the before and 102 from the after period) and 576 from the control facilities (410 from the before and 166 from the after period). In the pre-YAFS period at YAFS facilities, LTF at 3 and 6 months was 8.8% (95% CI: 5.1 to 13.6) and 11.9% (95% CI: 7.5 to 17.3), respectively, and in the post-YAFS period was 13.9% (95% CI: 8.0 to 21.4) and 17.0% (95% CI: 10.4 to 25.0) (Fig. 1). At the non-YAFS facilities, pre-YAFS LTF was 6.2% (95% CI: 4.1 to 8.8) and 10.8% (95% CI: 8.0 to 14.0) at 3 and 6 months, and in the post-YAFS period was 10.4% (95% CI: 6.3 to 15.7) and 16.2% (95% CI: 11.0 to 22.3), respectively. There was no significant difference in LTF in the before and after periods at the YAFS facilities (P = 0.19) however for health facilities that did not have YAFS, LTF observed in the after period was significantly higher than in the before period (P = 0.04). There was no significant difference in LTF among patients who started ART between YAFS and non-YAFS facilities in either the pre- (P = 0.73) or post-YAFS periods (P = 0.77).
In this analysis of ICAP-supported health facilities in Kenya, the introduction of services targeted to adolescents and youth did not improve retention either before ART or in the first 6 months after treatment initiation. Although there seemed to be some overall improvement in retention among pre-ART adolescents/youth at all health facilities, both YAFS and control sites, between the before and after periods, the differences were not significant. Among adolescents/youth who started ART, we observed somewhat higher LTF by 6 months after the start of treatment in the later period at health facilities that did not implement YAFS.
The lack of improvement in retention after introduction of YAFS may be a result of several factors. Data from the YAFS facilities were evaluated for the period immediately after implementation of the program and more time may be needed to scale-up services, fully engage adolescents/youth, and evaluate outcomes to see results. In addition, there may have been heterogeneity across facilities regarding quality of services and participation. It is possible that some health facilities implemented more robust YAFS programs and may have had better results, however because of a small number of sites and small sample sizes at the facilities, we were unable to examine interfacility differences. In our analysis, facilities that implemented YAFS were larger and more likely to be urban, factors that have been associated with poorer retention outcomes in adults.7,8 In addition, differences in retention have been previously observed by age, with younger adolescents (10–14) being less likely to be LTF compared with older youth.3,5,9 There may be differences in the impact of YAFS on retention for adolescents compared with older youth at the health facilities we evaluated, however we were unable to examine this given our small sample size. It is also possible that some of the adolescents identified as lost to follow-up transferred to care at other health facilities. Further data on quality of services, longer follow-up time, and larger patient numbers are needed to fully measure the impact of services targeted to adolescents and youth.
Our results suggest that offering a basic set of YAFS, including dedicated clinics and support groups targeted to adolescents and youth, may not be adequate to surmount the retention barriers faced by young people living with HIV. Other factors may also contribute to the retention of this group. A recent study also conducted in Nyanza found that many youth reported disengaging from care as a result of stigma and fear of disclosure to parents, teachers, and clinicians.10 More data assessing the specific clinical and psychosocial needs of adolescents and youth living with HIV are needed to understand the specific barriers they face in remaining in HIV care and treatment services. In addition, broader interventions targeting families and engaging communities may also be necessary to improve program and individual health outcomes for this very vulnerable group.
Acknowledgments
Supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention under the terms of Cooperative Agreement Number 5U62PS223540 and 5U2GPS001537.
Footnotes
The authors have no conflicts of interest to disclose.
The contents are solely the responsibility of the authors and do not necessarily represent the official views of PEPFAR or the Centers for Disease Control and Prevention.
References
- 1.UNAIDS. The Gap Report. Geneva, Switzerland: WHO; 2014. [Google Scholar]
- 2.Idele P, Gillespie A, Porth T, et al. Epidemiology of HIV and AIDS among adolescents: current status, inequities, and data gaps. J Acquir Immune Defic Syndr. 2014;66(suppl 2):S144–S153. doi: 10.1097/QAI.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 3.Bygrave H, Mtangirwa J, Ncube K, et al. Antiretroviral therapy outcomes among adolescents and youth in rural Zimbabwe. PLoS One. 2012;7:e52856. doi: 10.1371/journal.pone.0052856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nachega JB, Hislop M, Nguyen H, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr. 2009;51:65–71. doi: 10.1097/QAI.0b013e318199072e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koech E, Teasdale CA, Wang C, et al. Characteristics and outcomes of HIV-infected youth and young adolescents enrolled in HIV care in Kenya. AIDS. 2014;28:2729–2738. doi: 10.1097/QAD.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lall P, Lim SH, Khairuddin N, et al. Review: an urgent need for research on factors impacting adherence to and retention in care among HIV-positive youth and adolescents from key populations. J Int AIDS Soc. 2015;18:19393. doi: 10.7448/IAS.18.2.19393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nuwagaba-Biribonwoha H, Jakubowski A, Mugisha V, et al. Low risk of attrition among adults on antiretroviral therapy in the Rwandan national program: a retrospective cohort analysis of 6, 12, and 18 month outcomes. BMC Public Health. 2014;14:889. doi: 10.1186/1471-2458-14-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fatti G, Grimwood A, Bock P. Better anti-retroviral therapy outcomes at primary healthcare facilities: an evaluation of three tiers of ART services in four South African provinces. PLoS One. 2010;5:e12888. doi: 10.1371/journal.pone.0012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans D, Menezes C, Mahomed K, et al. Treatment outcomes of HIV-infected adolescents attending public-sector HIV clinics across Gauteng and Mpumalanga, South Africa. AIDS Res Hum Retroviruses. 2013;29:892–900. doi: 10.1089/aid.2012.0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolf HT, Halpern-Felsher BL, Bukusi EA, et al. “It is all about the fear of being discriminated [against]…the person suffering from HIV will not be accepted”: a qualitative study exploring the reasons for loss to follow-up among HIV-positive youth in Kisumu, Kenya. BMC Public Health. 2014;14:1154. doi: 10.1186/1471-2458-14-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]