Abstract
AIM
The prevalence of benign paroxysmal positional vertigo (BPPV) is higher in people with type 2 diabetes (DM). The impact of DM on mobility, balance, and management of BPPV is unknown. This prospective study compared symptom severity, mobility and balance before and after the canalith repositioning maneuver (CRM) in people with posterior canal BPPV canalithiasis, with and without DM.
METHODS
Fifty participants, BPPV (n=34) and BPPV+DM (n=16) were examined for symptom severity (Dizziness Handicap Inventory, DHI), mobility (Functional Gait Assessment, FGA), and postural sway (using an accelerometer in five conditions) before and after the CRM. The number of maneuvers required for symptom resolution was recorded.
RESULTS
At baseline, no differences in DHI or FGA scores were seen between groups, however, people with BPPV+DM had higher sway velocity in the medio-lateral direction in tandem stance (p<0.01). After treatment, both groups improved in DHI and FGA scores (p<0.01), with no differences between groups. Decrease in sway velocity in the mediolateral direction (p=0.003) were seen in tandem stance in persons with BPPV+DM. There were no differences between the groups in the number of CRMs provided.
CONCLUSIONS
This pilot study showed no differences in symptom severity, mobility deficits or efficacy of CRM treatments in people with posterior canal BPPV canalithiasis with and without DM. Future studies examining the impact of the severity and duration of diabetes, as well as the influence of diabetic peripheral neuropathy on functional performance are essential.
Keywords: Canalith repositioning maneuvers, type 2 diabetes, positional vertigo
1. Introduction
The vestibular system plays an important role in maintaining balance in static and dynamic conditions. It provides information about the position and motion of the body with respect to earth’s vertical (Minor, 1998). Within the peripheral vestibular system, the semicircular canals provide sensory input regarding head velocity; while the otolith organs (utricle and saccule) register linear acceleration and head tilt.
Vestibular dysfunction has been recognized as a complication of type 2 diabetes (DM), and has been reported to be 70% higher in people with DM, compared to age matched controls (Agrawal, Carey, Della Santina, Schubert, & Minor, 2009). In people with diabetes, vestibular dysfunction, and complaints of dizziness, the risk of falls is two times higher after accounting for peripheral neuropathy and retinopathy (Agrawal, Carey, Della Santina, Schubert, & Minor, 2010). Both central and peripheral vestibular dysfunction have been observed in type 1 and type 2 diabetes (D’Silva, Lin, Staecker, Whitney, & Kluding, 2015; Gawron, Pospiech, Orendorz-Fraczkowska, & Noczynska, 2002; Klagenberg, Zeigelboim, Jurkiewicz, & Martins-Bassetto, 2007; Myers, 1998; Myers & Ross, 1987; Nicholson, King, Smith, & Darlington, 2002; Ward et al., 2015). Metabolic stress due to hyperglycemia has been shown to cause loss of type 1 hair cells in the saccule (Myers & Ross, 1987), and lysis of the myelin of the vestibulocochlear nerve (Myers, 1998) in experimentally induced diabetic animal models; while clinical studies have shown peripheral vestibular organ dysfunction (Konukseven et al., 2014; Ward et al., 2015).
One common vestibular condition, benign paroxysmal positional vertigo (BPPV) has been seen in higher frequency in people with diabetes. Cohen et al. reported 14% of the individuals in their sample with BPPV had a history of diabetes (Cohen, Kimball, & Stewart, 2004), while D’Silva et al. noted that BPPV was seen in 46% of individuals with type 2 diabetes compared to 37% without diabetes (D’Silva et al., 2016). In a histopathology study examining human temporal bones, Yoda et al. found a significantly higher prevalence of BPPV in people with type 1 diabetes compared to age matched controls (Yoda et al., 2011). In addition, the presence of comorbidities like diabetes and hypertension have been shown to increase the risk of recurrence of BPPV significantly (De Stefano et al., 2013). The combination of hypertension, diabetes, and osteoarthritis increased the recurrence rate of BPPV 4.55 times (De Stefano et al., 2013). In BPPV, calcium carbonate crystals called otoconia, fall off the gelatinous membrane of the utricle and saccule and enter the semicircular canals. One reason for the detachment of the otoconia is underlying degeneration of the utricle and saccule (Bhattacharyya et al., 2008; von Brevern, Schmidt, Schonfeld, Lempert, & Clarke, 2006). The otoconia fragments enter the semicircular canal causing movement of the endolymph within the semicircular canal. Endolymph movement is perceived as vertigo (spinning), even after head movements have ceased (Bhattacharyya et al., 2008; Hall, Ruby, & McClure, 1979; Schuknecht, 1969). Otoconia fragments can enter the anterior, posterior or lateral semicircular canals. Posterior canal BPPV is the most common variant, as it is seen in 91% of patients compared to 9% in the lateral canal (Steenerson, Cronin, & Marbach, 2005). Another variant of BPPV is based on whether the otoconia are free floating in the semicircular canal (canalithiasis) or attached to the cupula (cupulolithiasis). Successful treatment of BPPV depends on identifying the canal involved, making the diagnosis of canalithiasis or cupulolithiasis, and providing the specific treatment maneuver. The gold standard test for diagnosing posterior canal BPPV is the Dix-Hallpike test (refer to Bhattacharya et al. for a description of the test) (Bhattacharyya et al., 2008). The treatment of choice for treating posterior canal BPPV canalithiasis is the canalith repositioning maneuver (CRM) (Epley, 1992; Korres, Balatsouras, Papouliakos, & Ferekidis, 2007). The success rate of treating posterior canal BPPV canalithiasis with the CRM is significantly higher compared to the treatment of anterior, or lateral canal BPPV canalithiasis or cupulolithiasis. The CRM is a series of head movements to guide the otoconia back into the utricle and has a success rate of up to 85% with a single treatment maneuver (Bhattacharyya et al., 2008; Prokopakis et al., 2013).
Benign paroxysmal positional vertigo causes a position dependent vertigo and is associated with loss of balance and frequent falls (Blatt, Georgakakis, Herdman, Clendaniel, & Tusa, 2000; Dix & Hallpike, 1952; Gananca et al., 2010). Due to vertigo, individuals with BPPV restrict their daily activities with increased missed days at work resulting in decreased productivity (Benecke, Agus, Kuessner, Goodall, & Strupp, 2013; von Brevern et al., 2007).
Presently, we do not have a clear understanding of how DM may affect the clinical presentation of people with BPPV when they are symptomatic, or the efficacy of the CRM to resolve BPPV symptoms. The main objective of this pilot study was to identify if the presence of diabetes affected symptom severity, mobility and balance in people with BPPV when symptomatic, and changes in these symptoms after the resolution of vertigo with the CRM in people with posterior canal BPPV canalithiasis. Our second objective was to determine if the presence of diabetes influenced the efficacy of the CRM. The efficacy of treatment is significantly higher in people with posterior canal BPPV canalithiasis, hence, for this study we excluded individuals who had anterior or lateral canal BPPV, people with bilateral BPPV, and those with the cupulolithiasis variant of BPPV.
The findings of this study will be useful to health care professionals to recognize mobility and balance deficits due to diabetes in the presence of BPPV and the efficacy of the CRM in this population.
2. Methods
2.1. Study Design
This prospective study, evaluated two groups of participants, those with BPPV and those with BPPV+DM, at baseline and after treatment. In this single-blinded study, the primary investigator (LD) was blinded to the diabetes status of participants during both data collection time points. The Human Subjects Committee at the University of Kansas Medical Center approved the research protocol. All participants signed institutionally approved written informed consent prior to participation in the study.
2.2. Participants
Participants were recruited through physician referral from the Kansas University neuro-otology clinic, as well as internal medicine, and family medicine clinics in the area. Individuals between 40 and 80 years of age with a diagnosis of unilateral posterior canal BPPV canalithiasis were recruited for this study.
The diagnosis of posterior canal BPPV was based on the presence of torsional up beating nystagmus in the Dix-Hallpike position, where the patient was supine-lying with the head rotated 45 degrees and extended 30 degrees, using videonystagmography (Micromedical, Visual Eyes 2002). The nystagmus had a brief latency, lasted less than 60 seconds, and was associated with complaints of vertigo (Bhattacharyya et al., 2008). Participants were excluded if they presented with any of the following: (1) a history of neurological disease including stroke, multiple sclerosis, Parkinson’s disease, intracranial tumor, (2) a history of Meniere’s disease, (3) received chemotherapy or ototoxic and/or neurotoxic medications, (4) a history of traumatic head injury, (5) a BMI greater than 45kg/m2, or (6) musculoskeletal or integumentary conditions that would impair balance. In addition, participants were excluded if videonystagmography revealed anterior or lateral canal BPPV canalithiasis, cupulolithiasis in any canal, or bilateral BPPV.
2.3. Study Procedure
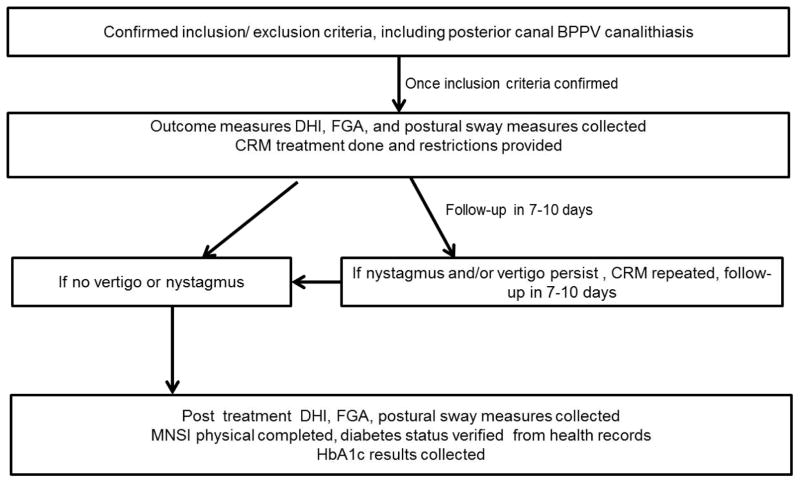
Figure 1 illustrates the sequence of study procedures. Once inclusion/ exclusion criteria and the diagnosis of unilateral posterior canal BPPV canalithiasis were confirmed, baseline outcome measures were collected.
Figure 1.
Study sequence flow sheet.
After collection of baseline outcome measures, all participants received the CRM. Subjects were given restrictions to follow for 48 hours, which included no bending, no looking overhead, and no sleeping on the affected side (Cakir, Ercan, Cakir, & Turgut, 2006).
Participants returned for follow up between 7 to 10 days of the initial evaluation, and the Dix-Hallpike was repeated using videonystagmography to determine if the BPPV had resolved. If nystagmus and vertigo persisted, participants continued to receive treatment maneuvers with follow up between 7 to 10 days, until resolution of symptoms. Once participants had no complaints of vertigo and a negative Dix-Hallpike test, they were considered symptom-free, and post-treatment outcome measures were collected. Next, all participants were screened for the presence of sensory impairment using the Michigan Neuropathy Screening Instrument (MNSI) (Jacobson & Newman, 1990). Participants were classified as having peripheral neuropathy if their physical exam score was ≥ 2.0 (sensitivity of 65%, specificity of 83%) (Moghtaderi, Bakhshipour, & Rashidi, 2006).
Lastly, the assessor was unblinded and for all participants a detailed medical history, list of medications, the presence or absence of diabetes, hypertension and BMI was collected through history taking, which was also confirmed through the electronic health records. To characterize diabetes severity, glycosylated hemoglobin (HbA1c) was tested via a disposable finger stick testing kit (Metrika A1cNow+ Bayer, Tarrytown NY) for all participants.
2.4. Outcome Measures
The following outcome measures were collected at baseline and after resolution of vertigo
Dizziness Handicap Inventory (DHI)
This is a standardized measure of self-report activity limitation and participation restriction due to either dizziness or unsteadiness (Jacobson & Newman, 1990). It is a 25-item questionnaire with three subscales: functional, emotional and physical. It has a high test-retest reliability (r=0.9) (Jacobson & Newman, 1990), and it is responsive to change in the vestibular population (Friscia, Morgan, Sparto, Furman, & Whitney, 2014; Lopez-Escamez, Gamiz, Fernandez-Perez, & Gomez-Finana, 2005; Lopez-Escamez, Gamiz, Fernandez-Perez, Gomez-Finana, & Sanchez-Canet, 2003) with an 18-point change on the DHI considered clinically meaningful (Jacobson & Newman, 1990). A DHI total score between 0–30 is considered a mild perception of handicap; 31–60 is moderate, and 61–100 points’ severe perception of handicap (Whitney, Wrisley, Brown, & Furman, 2004). Persons with higher scores on the DHI have been shown to have greater functional impairments and higher number of falls (Whitney et al., 2004).
Functional Gait Assessment (FGA)
The FGA is a 10-item functional mobility test (Wrisley & Kumar, 2010; Wrisley, Marchetti, Kuharsky, & Whitney, 2004), that is scored on a four point ordinal scale (0–3) with a higher score indicating greater stability during functional mobility. The various items on the test include: walking at normal speed, change in speed while walking, walking with head turns and head tilts, pivot turns, stepping over an obstacle, tandem walking, walking with eyes closed, walking backwards, and stair climbing. The FGA has moderate to strong correlations with other standard tests of balance (Wrisley & Kumar, 2010). A cut-off score of 22/30 on the FGA provides optimum validity for classifying fall risk in older adults (Wrisley & Kumar, 2010).
Postural sway
Pelvic accelerations were measured using an inertial motion sensor (IMU; Xsens North America, USA) sized 5.25 × 3.75 × 2 cm. The sensor was centered posteriorly on the third vertebra of the lumbar spine (L3) and held firmly in place using an elastic belt. Participants were assessed in quiet standing for 30 seconds in five conditions: Condition 1: standing on a firm surface with feet together, eyes open; Condition 2: standing on a firm surface with feet together, eyes closed; Condition 3: standing on a foam pad (Alimed balance pad elite™) with feet together, eyes open; Condition 4: standing on a foam pad with feet together, eyes closed; and Condition 5: tandem standing with eyes open on a firm surface. One trial was performed for each condition and all conditions were performed in the same order for all participants.
Data was acquired using the MT Manager software (Xsens North America Inc., Culver City, CA, USA) at a sampling frequency of 120 Hz. The three-axial (X, Y, and Z) acceleration data was recorded during the 30 seconds of data collection. The accelerations in Y and Z directions, corresponding to medial-lateral (ML) and anterior-posterior (AP) directions respectively, were used to calculate the variables that represented postural sway. The data was filtered with Butterworth 2nd order low-pass filter at 20 Hz and corrected for offset. The following variables were analyzed: 1) range of acceleration (cm/s2) in the anterior-posterior (range-AP) and medial-lateral (range-ML) directions, 2) peak velocity (from integrated acceleration) (cm/s) in the anterior-posterior (PV-AP), and medial-lateral (PV-ML) direction 3) root mean square of acceleration (RMS) in the anterior-posterior (RMS-AP) and medial-lateral (RMS-ML) direction. All variables were calculated using customized Matlab code (Matlab R2015b, Mathworks Inc., Natick, MA, USA).
Number of treatment maneuvers
All maneuvers that a participant required to achieve complete resolution of symptoms were added to get a total count of the number of CRMs performed. Participants who felt nauseous or had increased symptoms of dizziness after the CRM received only one treatment maneuver. In those participants who could tolerate the procedure without increased symptoms, the Dix-Hallpike was repeated in the same session. If the person had vertigo or nystagmus during the repeat Dix-Hallpike, another CRM was performed.
3. Statistical Analysis
Descriptive statistics (mean, standard deviation, %) are used to present participant demographics. Differences in demographics between groups were examined using ANOVA for continuous and chi-square tests for categorical variables. ANCOVA was used to compare DHI and FGA scores at baseline between groups with age, BMI, and presence of neuropathy as covariates; while change scores were examined after resolution of vertigo. A general linear mixed model was used to examine baseline postural sway measures comparing the conditions as within subject factor and group as between subject factor, while change in postural sway measures were examined after treatment taking covariates into account. Main effects are reported as F-values, and p-values. Effect size is reported as partial eta squared, where 0.01 represents a small effect size, 0.06 a medium effect size and 0.14 a large effect size. For a significant model, post hoc comparisons were conducted. For postural sway measures, for the trials in which participants were unable to complete the test, we used an intention to treat model, and values were assigned based on the group mean + 2SD. Number of treatment maneuvers required between groups was compared using Mann-Whitney U tests. Data were analyzed using SPSS 20.0 (SPSS, Inc., Chicago, IL) with significance level set at 0.05.
4. Results
A total of 107 patients were screened, of which 53 were included in the study. Thirty-six participants had BPPV while 17 had BPPV+DM. Two participants with BPPV dropped out from initial evaluation to final collection of outcome measures due to transportation difficulties. One participant with BPPV+DM dropped out because of recent rotator cuff surgery that caused pain during the CRM technique. When the participant rolled to side lying, on to the affected shoulder, as part of the treatment maneuver, significant pain in the shoulder did not allow for the technique to be performed correctly, hence, she requested that she discontinue the study. All three participants were excluded from the study, including baseline analysis. Postural sway data were collected at baseline and at the final visit on 42 out of 50 total participants due to equipment issues. One participant in the BPPVDM could not stand on foam, eyes closed or tandem stance, while another was unable to complete tandem stance, due to loss of balance.
Participant demographics are presented in Table 1. There were no differences between the groups in age or gender. Significant differences were seen in HbA1c (p<0.001), BMI (p=0.002), and neuropathy (p=0.04) between the groups.
Table 1.
Participant Characteristics
| BPPV (n=34) | BPPV+DM (n=16) | p | |
|---|---|---|---|
| Age (years) | 58.85 ± 10.65 | 62 ± 7.8 | p=0.29 |
| Gender (Female/ male) | 29/5 | 10/6 | p=0.08 |
| HbA1c (unit-%) (mmol/mol) | 5.66 ± 0.41 39 |
6.93 ± 1.4 52 |
p<0.001* |
| BMI (kg/m2 ) | 29.56 ± 7.6 | 36.27 ± 4.4 | p=0.002* |
| Peripheral neuropathy | 6 (17%) | 8 (50%) | p=0.04* |
HbA1c- glycosylated hemoglobin level, BMI- body mass index, BPPV-benign paroxysmal positional vertigo, BPPV+DM- BPPV and concurrent type 2 diabetes. Continuous variables were examined using independent sample t-test, chi-square tests examined categorical variables.
indicates significant differences between groups.
Dizziness Handicap Inventory
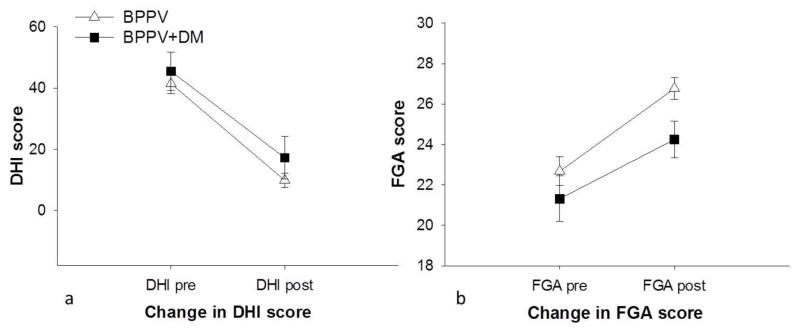
At baseline, after taking age, BMI, and neuropathy into account, there was no difference in DHI scores between the BPPV group (41.5 ± 18.5) and BPPV+DM (45.5 ± 24.9) group (p=0.89). The DHI score changed significantly with treatment (p<0.001). Change in DHI scores after treatment were not different between groups (p=0.18). The average decrease in DHI score in the BPPV group was 31.6 ± 16.8, with 27 of the 34 (79%) participants showing an improvement >18 points. In the BPPV+DM group, the average decrease in DHI score was 28.5 ± 26.8 with 9 of 16 (45%) participants showing an improvement >18 points. Figure 2a shows DHI scores before and after treatment in both groups.
Figure 2. Change in 2a) DHI and 2b) FGA scores in both groups before and after treatment.
DHI-Dizziness Handicap Inventory, FGA-Functional Gait Assessment, BPPV-benign paroxysmal positional vertigo, BPPV+DM-BPPV and type 2 diabetes.
Functional Gait Assessment
At baseline, after taking age, BMI, and neuropathy into account, there were no differences in FGA scores between the BPPV (22.7 ± 4.1) and BPPV+DM (21.3 ± 4.5) groups (p=0.79). The FGA scores improved significantly with treatment (p<0.001). The FGA change scores between the BPPV (4.09 ± 2.4) and the BPPV+DM groups (2.9 ± 2.9), was not significant (p=0.12). Figure 2b shows FGA scores in both groups before and after treatment.
Postural sway variables
At baseline, range of acceleration-AP showed no main effects for condition, group, or interaction between condition and group, age, BMI or DPN.
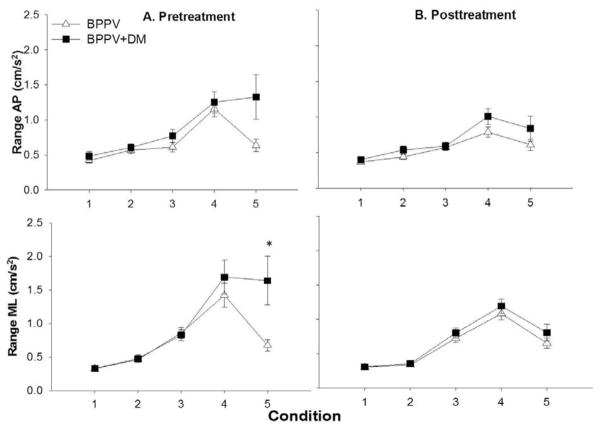
Range-ML showed interaction between condition and group (F1,42: 2.3, p=0.01, partial eta squared: 0.08). Post-hoc analysis showed that range-ML was significantly higher in tandem stance in the BPPV+DM group (1.6 ± 1.3 cm/s) compared to the BPPV group (0.67 ± 0.5cm/s) (p=0.001) (Figure 3A).
Figure 3. Means and standard errors of range in the anteroposterior (AP) and mediolateral (ML) direction in the two groups across the five testing conditions, before and after treatment.
Condition 1: Firm surface, eyes open, Condition 2: Firm surface, eyes closed, Condition 3: Foam, eyes open, Condition 4: Foam, eyes closed, Condition 5: Tandem stance. BPPV-benign paroxysmal positional vertigo, BPPV+DMBPPV and concurrent type 2 diabetes
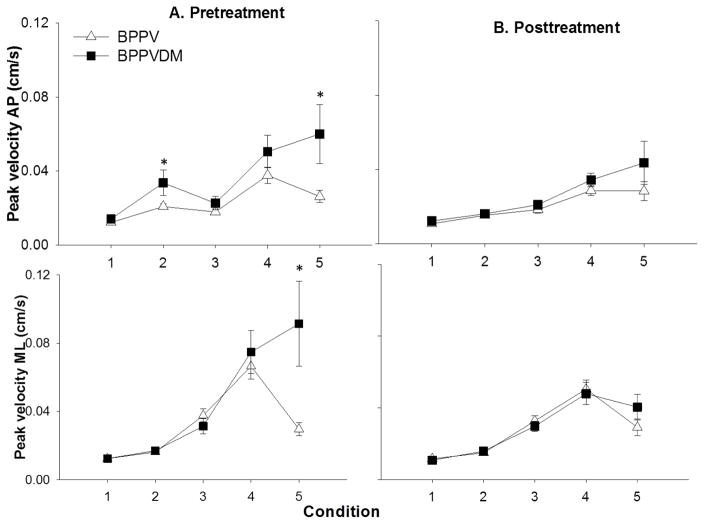
A significant main effect for group was seen for PV-AP (F1, 42: 5.8, p=0.02, partial eta squared=0.14). The BPPV+DM group had higher sway velocity with standing on firm ground with eyes closed (p=0.02) and in tandem stance (p=0.007) compared to the BPPV group.
PV-ML showed a significant interaction between condition and group (F 1,42: 4.0, p=0.004, partial eta squared: 0.1), and condition and DPN (F 1,42: 4.6, p= 0.002, partial eta squared= 0.11). Post hoc analysis showed that the BPPV+DM group had higher PV-ML in tandem stance (p=0.002) compared to people with BPPV (Figure 4A); people with DPN had significantly higher PV-ML in tandem stance (p=0.001) compared to those without DPN.
Figure 4. Means and standard errors of peak velocity in the anteroposterior (AP) and mediolateral (ML) direction in the two groups across the five testing conditions, before and after treatment.
Condition 1: Firm surface, eyes open, Condition 2: Firm surface, eyes closed, Condition 3: Foam, eyes open, Condition 4: Foam, eyes closed, Condition 5: Tandem stance. BPPV-benign paroxysmal positional vertigo, BPPV+DM- BPPV and type 2 diabetes
No effect of condition, group, or interactions were noted for RMS at baseline. Analysis of data following the treatment (change scores) revealed no main effects or interactions for the AP postural sway change scores (range, velocity, or RMS).
Change scores for range-ML and RMS-ML showed no effect of condition, group or interactions (Figure 3B). Change scores of PV-ML showed a significant effect of interaction between condition and neuropathy (F 1,42: 3.4, p=0.012, partial eta squared=0.08), significant change in PV-ML was seen in tandem standing (p=0.004) in people with neuropathy (Figure 4B).
Treatment Maneuvers
In the BPPV group, the average number of CRMs performed was 1.8 ± 1.4, with a range between 1 and 7. In the BPPV+DM group, the average number of CRM performed were 2.0 ± 1.2, with a range between 1 and 5. The difference was not statistically significant (p= 0.37). No adverse events were noted in any participants.
5. Discussion
The results of this pilot study showed no differences in symptom severity, mobility deficits or the efficacy of CRM treatments in people with posterior canal BPPV canalithiasis with or without type 2 diabetes.
People in both the BPPV and BPPV+DM groups showed significant improvement (>18 points) in the Dizziness Handicap Inventory scores after the treatment maneuver, which is a clinically meaningful change (Jacobson & Newman, 1990). However, at baseline and after resolution of vertigo, no differences were noted between the people with BPPV only and people who had the additional influence of diabetes. Our study found that residual symptoms persisted in both groups in all three domains, but particularly in the functional domain. In the BPPV group, 16 participants had DHI scores >0 and <30, while three had scores >30 and <60 at levels that would indicate mild-moderate restriction of physical activity due to dizziness. In the BPPV+DM group, 5 participants had mild restriction, 3 had moderate and 1 had severe perception of handicap persist after the CRM. Results of our study are similar to others that have shown improvement in DHI scores after resolution of vertigo in people with BPPV (Kasse et al., 2010), with persistence of residual symptoms (Lee, Kwon, & Ban, 2009; Silva, Ribeiro, Freitas, Ferreira, & Guerra, 2016).
Functional mobility based on the Functional Gait Assessment improved significantly after treatment in both the BPPV and BPPV+DM groups, showing lower risk of falls (Wrisley & Kumar, 2010). Although we could not find studies that have examined the FGA after treatment of BPPV, a recent study showed improvement in the Dynamic Gait Index after treatment of BPPV with the CRM (Silva et al., 2016). Like the DHI, there were no differences in performance of functional mobility tasks between groups either before or after resolution of BPPV. It was interesting to note that participants with BPPV performed better than people with BPPV+DM on the FGA (higher FGA scores), at baseline as well as after treatment. All participants with BPPV showed an improvement in functional mobility after the CRM, however, not all participants with BPPV+DM improved in their mobility after the treatment maneuver. Both groups did not achieve optimal scores on the FGA (30/30), indicating that functional mobility did not improve to its full potential although the symptoms of BPPV had resolved. Based on these results it appears that although the symptoms of BPPV had resolved, participants in both groups continued to have residual symptoms and mobility deficits. Future longitudinal studies exploring the patterns of recovery of patient symptoms and functional performance will help determine if people with diabetes require more time for complete recovery, as well as if they may need specific balance and gait training interventions for optimal recovery, compared to people without diabetes.
Another observation of this study was that the presence of neuropathy did not affect FGA scores. One reason for not detecting an effect of neuropathy on mobility scores may be due to the small sample of individuals with diabetes and neuropathy as well as the presence of neuropathy in people without DM. Additionally, the Michigan Neuropathy Screening Instrument though easily performed in the clinic setting is not the gold standard for diagnosing peripheral neuropathy, and may not have identified participants with neuropathy accurately. Future studies with a larger sample examining the influence of DPN on functional mobility are necessary to identify if mobility deficits and if the risk of falls may be higher in people with DPN.
To control balance the central nervous system uses information from the somatosensory, visual, and vestibular receptors, thus, information from all these sensory systems complement each other. However, if one of these sensory inputs is unavailable or deprived, it is critical for the central nervous system be able to extract sensory information from the other available sensory systems (sensory reweighting) to maintain postural stability and avoid a fall (Herdman, 2013). Based on this literature, we expected people with BPPV to have higher sway when standing on foam with eyes closed, since the somatosensory and visual systems are deprived, which makes it difficult to compensate for the vestibular deficit. We found that before treatment, both people with BPPV and BPPV+DM had the highest postural sway values when standing on foam with eyes closed. These findings are similar to those of other researchers who have examined balance in people with BPPV (Blatt et al., 2000; Chang, Hsu, Yang, & Wang, 2006; Di Girolamo et al., 1998; Giacomini, Alessandrini, & Magrini, 2002). However, because of the ability to successfully reweight the somatosensory system when standing on firm ground, even though people with BPPV were symptomatic, they had lower sway velocity in tandem standing with eyes open on firm ground. However, people with BPPV+DM had a higher velocity of sway, i.e. less stability, when standing in tandem stance, even though it was on firm ground. Their inability to maintain postural stability may be due to their inability to use somatosensation effectively, which may have been an effect of sensory impairment due to neuropathy.
After successful resolution of vertigo due to the maneuvers, all participants showed less postural sway, especially while standing on foam with eyes closed. Results of our study are similar to Celebisoy et al (Celebisoy, Bayam, Gulec, Kose, & Akyurekli, 2009) who showed a significant reduction in sway after the canalith repositioning maneuver. People with BPPV+DM had a significant decrease in sway velocity in the mediolateral direction after treatment in tandem standing. This indicates that once the effect of BPPV was resolved, they may have been able to use the vestibular system to compensate for their sensory deficits in the feet, which reflected in an improvement in postural control. We could not determine if the post treatment postural sway values in our BPPV and BPPV+DM groups approached normative values of healthy controls since we did not assess balance in a matched control group using the accelerometry technique in this study. However, studies have shown that despite successful resolution of BPPV, postural stability continues to be impaired in some participants (Blatt et al., 2000; Zhang, Fan, Han, Yu, & Wang, 2010), with balance training required to achieve normal dynamic balance. Both studies did not specifically examine the presence of diabetes as a comorbidity or the influence of neuropathy. Future studies examining the influence of DPN on postural sway after treatment of BPPV will determine if postural sway returns to baseline or if balance training may be necessary to improve postural control.
Limitations
One limitation of this study is that our sample size though adequate to identify differences between groups in sensitive measures like postural sway was not able to show differences in functional mobility. For FGA change scores in particular, we have a power of 28% to detect between group changes with our sample size, indicating a possibility for a type II error. Our participants had well controlled diabetes, hence, we were unable to examine the effect of poor glycemic control on their performance. Future studies examining balance and mobility in people with uncontrolled diabetes or with prolonged duration of diabetes will help to elucidate the relationship between these important diabetes related variables and mobility and balance. Adults with diabetes are not only at a higher risk for falling, but they sustain more severe injuries after a fall, hence, improving postural stability is essential in this patient population.
6. Conclusion
People with posterior canal BPPV canalithiasis with and without type 2 diabetes have functional deficits in daily activity, mobility, and balance when symptomatic. Fortunately, these symptoms and functional deficits respond well and with the same number of treatment maneuvers in both groups.
Acknowledgments
This work was conducted at the University of Kansas Medical Center, and was supported by an institutional grant T32HD057850 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. REDCap at the University of Kansas Medical Center is supported by CTSA grant (CTSA Award # UL1TR000001) from NCRR and NCATS awarded to the University of Kansas Medical Center. The authors would like to extend their appreciation to the Kansas Partners in Progress and the International Scientific Partnership Program ISPP at King Saud University for funding this work through ISPP#00. The authors would like to thank A. Yahya and D. Leist for their assistance with data collection.
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001–2004. Arch Intern Med. 2009;169(10):938–944. doi: 10.1001/archinternmed.2009.66. [DOI] [PubMed] [Google Scholar]
- Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Diabetes, vestibular dysfunction, and falls: analyses from the National Health and Nutrition Examination Survey. Otol Neurotol. 2010;31(9):1445–1450. doi: 10.1097/MAO.0b013e3181f2f035. [DOI] [PubMed] [Google Scholar]
- Benecke H, Agus S, Kuessner D, Goodall G, Strupp M. The Burden and Impact of Vertigo: Findings from the REVERT Patient Registry. Front Neurol. 2013;4:136. doi: 10.3389/fneur.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya N, Baugh RF, Orvidas L, Barrs D, Bronston LJ, Cass S, … Haidari J. Clinical practice guideline: benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 2008;139(5 Suppl 4):S47–81. doi: 10.1016/j.otohns.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Blatt PJ, Georgakakis GA, Herdman SJ, Clendaniel RA, Tusa RJ. The effect of the canalith repositioning maneuver on resolving postural instability in patients with benign paroxysmal positional vertigo. Am J Otol. 2000;21(3):356–363. doi: 10.1016/s0196-0709(00)80045-9. [DOI] [PubMed] [Google Scholar]
- Cakir BO, Ercan I, Cakir ZA, Turgut S. Efficacy of postural restriction in treating benign paroxysmal positional vertigo. Arch Otolaryngol Head Neck Surg. 2006;132(5):501–505. doi: 10.1001/archotol.132.5.501. [DOI] [PubMed] [Google Scholar]
- Celebisoy N, Bayam E, Gulec F, Kose T, Akyurekli O. Balance in posterior and horizontal canal type benign paroxysmal positional vertigo before and after canalith repositioning maneuvers. Gait Posture. 2009;29(3):520–523. doi: 10.1016/j.gaitpost.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Chang WC, Hsu LC, Yang YR, Wang RY. Balance ability in patients with benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 2006;135(4):534–540. doi: 10.1016/j.otohns.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Cohen HS, Kimball KT, Stewart MG. Benign paroxysmal positional vertigo and comorbid conditions. ORL J Otorhinolaryngol Relat Spec. 2004;66(1):11–15. doi: 10.1159/000077227. [DOI] [PubMed] [Google Scholar]
- D’Silva LJ, Lin J, Staecker H, Whitney SL, Kluding PM. Impact of Diabetic Complications on Balance and Falls: Contribution of the Vestibular System. Phys Ther. 2015 doi: 10.2522/ptj.20140604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Silva LJ, Staecker H, Lin J, Sykes KJ, Phadnis MA, McMahon TM, … Kluding PM. Retrospective data suggests that the higher prevalence of benign paroxysmal positional vertigo in individuals with type 2 diabetes is mediated by hypertension. J Vestib Res. 2016;25(5–6):233–239. doi: 10.3233/ves-150563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefano A, Dispenza F, Suarez H, Perez-Fernandez N, Manrique-Huarte R, Ban JH, … Croce A. A multicenter observational study on the role of comorbidities in the recurrent episodes of benign paroxysmal positional vertigo. Auris Nasus Larynx. 2013 doi: 10.1016/j.anl.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Di Girolamo S, Paludetti G, Briglia G, Cosenza A, Santarelli R, Di Nardo W. Postural control in benign paroxysmal positional vertigo before and after recovery. Acta Otolaryngol. 1998;118(3):289–293. doi: 10.1080/00016489850183340. [DOI] [PubMed] [Google Scholar]
- Dix MR, Hallpike CS. The pathology symptomatology and diagnosis of certain common disorders of the vestibular system. Proc R Soc Med. 1952;45(6):341–354. doi: 10.1177/003591575204500604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epley JM. The canalith repositioning procedure: for treatment of benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 1992;107(3):399–404. doi: 10.1177/019459989210700310. [DOI] [PubMed] [Google Scholar]
- Friscia LA, Morgan MT, Sparto PJ, Furman JM, Whitney SL. Responsiveness of self-report measures in individuals with vertigo, dizziness, and unsteadiness. Otol Neurotol. 2014;35(5):884–888. doi: 10.1097/mao.0000000000000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gananca FF, Gazzola JM, Gananca CF, Caovilla HH, Gananca MM, Cruz OL. Elderly falls associated with benign paroxysmal positional vertigo. Braz J Otorhinolaryngol. 2010;76(1):113–120. doi: 10.1590/S1808-86942010000100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawron W, Pospiech L, Orendorz-Fraczkowska K, Noczynska A. Are there any disturbances in vestibular organ of children and young adults with Type I diabetes? Diabetologia. 2002;45(5):728–734. doi: 10.1007/s00125-002-0813-x. [DOI] [PubMed] [Google Scholar]
- Giacomini PG, Alessandrini M, Magrini A. Long-term postural abnormalities in benign paroxysmal positional vertigo. ORL J Otorhinolaryngol Relat Spec. 2002;64(4):237–241. doi: 10.1159/000064130. 64130. [DOI] [PubMed] [Google Scholar]
- Hall SF, Ruby RR, McClure JA. The mechanics of benign paroxysmal vertigo. J Otolaryngol. 1979;8(2):151–158. [PubMed] [Google Scholar]
- Herdman SJ. Vestibular rehabilitation. Curr Opin Neurol. 2013;26(1):96–101. doi: 10.1097/WCO.0b013e32835c5ec4. [DOI] [PubMed] [Google Scholar]
- Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1990;116(4):424–427. doi: 10.1001/archotol.1990.01870040046011. [DOI] [PubMed] [Google Scholar]
- Kasse CA, Santana GG, Scharlach RC, Gazzola JM, Branco FC, Dona F. Results from the balance rehabilitation unit in benign paroxysmal positional vertigo. Braz J Otorhinolaryngol. 2010;76(5):623–629. doi: 10.1590/S1808-86942010000500015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagenberg KF, Zeigelboim BS, Jurkiewicz AL, Martins-Bassetto J. Vestibulocochlear manifestations in patients with type I diabetes mellitus. Braz J Otorhinolaryngol. 2007;73(3):353–358. doi: 10.1016/S1808-8694(15)30079-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konukseven O, Polat SB, Karahan S, Konukseven E, Ersoy R, Cakir B, … Aksoy S. Electrophysiologic vestibular evaluation in type 2 diabetic and prediabetic patients: Air conduction ocular and cervical vestibular evoked myogenic potentials. Int J Audiol. 2014:1–8. doi: 10.3109/14992027.2014.971887. [DOI] [PubMed] [Google Scholar]
- Korres SG, Balatsouras DG, Papouliakos S, Ferekidis E. Benign paroxysmal positional vertigo and its management. Med Sci Monit. 2007;13(6):CR275–282. [PubMed] [Google Scholar]
- Lee NH, Kwon HJ, Ban JH. Analysis of residual symptoms after treatment in benign paroxysmal positional vertigo using questionnaire. Otolaryngol Head Neck Surg. 2009;141(2):232–236. doi: 10.1016/j.otohns.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Lopez-Escamez JA, Gamiz MJ, Fernandez-Perez A, Gomez-Finana M. Long-term outcome and health-related quality of life in benign paroxysmal positional vertigo. Eur Arch Otorhinolaryngol. 2005;262(6):507–511. doi: 10.1007/s00405-004-0841-x. [DOI] [PubMed] [Google Scholar]
- Lopez-Escamez JA, Gamiz MJ, Fernandez-Perez A, Gomez-Finana M, Sanchez-Canet I. Impact of treatment on health-related quality of life in patients with posterior canal benign paroxysmal positional vertigo. Otol Neurotol. 2003;24(4):637–641. doi: 10.1097/00129492-200307000-00018. [DOI] [PubMed] [Google Scholar]
- Minor LB. Physiological principles of vestibular function on earth and in space. Otolaryngol Head Neck Surg. 1998;118(3 Pt 2):S5–15. doi: 10.1016/S0194-59989870002-6. [DOI] [PubMed] [Google Scholar]
- Moghtaderi A, Bakhshipour A, Rashidi H. Validation of Michigan neuropathy screening instrument for diabetic peripheral neuropathy. Clin Neurol Neurosurg. 2006;108(5):477–481. doi: 10.1016/j.clineuro.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Myers SF. Myelin-sheath abnormalities in the vestibular nerves of chronically diabetic rats. Otolaryngol Head Neck Surg. 1998;119(5):432–438. doi: 10.1016/s0194-5998(98)70098-1. [DOI] [PubMed] [Google Scholar]
- Myers SF, Ross MD. Morphological evidence of vestibular pathology in long-term experimental diabetes mellitus. II. Connective tissue and neuroepithelial pathology. Acta Otolaryngol. 1987;104(1–2):40–49. doi: 10.3109/00016488709109045. [DOI] [PubMed] [Google Scholar]
- Nicholson M, King J, Smith PF, Darlington CL. Vestibulo-ocular, optokinetic and postural function in diabetes mellitus. Neuroreport. 2002;13(1):153–157. doi: 10.1097/00001756-200201210-00035. [DOI] [PubMed] [Google Scholar]
- Prokopakis E, Vlastos IM, Tsagournisakis M, Christodoulou P, Kawauchi H, Velegrakis G. Canalith repositioning procedures among 965 patients with benign paroxysmal positional vertigo. Audiol Neurootol. 2013;18(2):83–88. doi: 10.1159/000343579. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF. Cupulolithiasis. Arch Otolaryngol. 1969;90(6):765–778. doi: 10.1001/archotol.1969.00770030767020. [DOI] [PubMed] [Google Scholar]
- Silva CN, Ribeiro KM, Freitas RV, Ferreira LM, Guerra RO. Vertiginous Symptoms and Objective Measures of Postural Balance in Elderly People with Benign Paroxysmal Positional Vertigo Submitted to the Epley Maneuver. Int Arch Otorhinolaryngol. 2016;20(1):61–68. doi: 10.1055/s-0035-1565915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenerson RL, Cronin GW, Marbach PM. Effectiveness of treatment techniques in 923 cases of benign paroxysmal positional vertigo. Laryngoscope. 2005;115(2):226–231. doi: 10.1097/01.mlg.0000154723.55044.b5. [DOI] [PubMed] [Google Scholar]
- von Brevern M, Radtke A, Lezius F, Feldmann M, Ziese T, Lempert T, Neuhauser H. Epidemiology of benign paroxysmal positional vertigo: a population based study. J Neurol Neurosurg Psychiatry. 2007;78(7):710–715. doi: 10.1136/jnnp.2006.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Brevern M, Schmidt T, Schonfeld U, Lempert T, Clarke AH. Utricular dysfunction in patients with benign paroxysmal positional vertigo. Otol Neurotol. 2006;27(1):92–96. doi: 10.1097/01.mao.0000187238.56583.9b. [DOI] [PubMed] [Google Scholar]
- Ward BK, Wenzel A, Kalyani RR, Agrawal Y, Feng AL, Polydefkis M, … Carey JP. Characterization of Vestibulopathy in Individuals with Type 2 Diabetes Mellitus. Otolaryngol Head Neck Surg. 2015;153(1):112–118. doi: 10.1177/0194599815576717. [DOI] [PubMed] [Google Scholar]
- Whitney SL, Wrisley DM, Brown KE, Furman JM. Is perception of handicap related to functional performance in persons with vestibular dysfunction? Otol Neurotol. 2004;25(2):139–143. doi: 10.1097/00129492-200403000-00010. [DOI] [PubMed] [Google Scholar]
- Wrisley DM, Kumar NA. Functional gait assessment: concurrent, discriminative, and predictive validity in community-dwelling older adults. Phys Ther. 2010;90(5):761–773. doi: 10.2522/ptj.20090069. [DOI] [PubMed] [Google Scholar]
- Wrisley DM, Marchetti GF, Kuharsky DK, Whitney SL. Reliability, internal consistency, and validity of data obtained with the functional gait assessment. Phys Ther. 2004;84(10):906–918. [PubMed] [Google Scholar]
- Yoda S, Cureoglu S, Yildirim-Baylan M, Morita N, Fukushima H, Harada T, Paparella MM. Association between type 1 diabetes mellitus and deposits in the semicircular canals. Otolaryngol Head Neck Surg. 2011;145(3):458–462. doi: 10.1177/0194599811407610. [DOI] [PubMed] [Google Scholar]
- Zhang DG, Fan ZM, Han YC, Yu G, Wang HB. Clinical value of dynamic posturography in the evaluation and rehabilitation of vestibular function of patients with benign paroxysmal positional vertigo. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010;45(9):732–736. [PubMed] [Google Scholar]