Abstract
Objective
We evaluated ST segment monitoring to detect clinical decompensation in infants with single ventricle anatomy. We proposed a signal processing algorithm for ST segment instability and hypothesized that instability is associated with cardiopulmonary arrests.
Design
Retrospective observational study.
Setting
Tertiary children’s hospital 21-bed cardiovascular intensive care unit (CVICU) and 36-bed step-down unit.
Patients
Twenty single ventricle infants who received stage 1 palliation surgery between January 2013 and January 2014. Twenty rapid response events resulting in cardiopulmonary arrests (arrest group) were recorded in 13 subjects and 9 subjects had no interstage cardiopulmonary arrest (control group).
Interventions
None.
Measurements and Main Results
Arrest data were collected over the 4-hour time window prior to cardiopulmonary arrest. Control data were collected from subjects with no interstage arrest using the 4-hour time window prior to CVICU discharge. A paired subgroup analysis was performed comparing subject 4-hour windows prior to arrest (pre-arrest group) with 4-hour windows prior to discharge (post-arrest group). Raw values of ST segments were compared between groups. A 3-dimensional ST segment vector was created using 3 quasi-orthogonal leads (II, aVL, and V5). Magnitude and instability of this continuous vector were compared between groups.
There was no significant difference in mean unprocessed ST segment values in the arrest and control groups. Utilizing signal processing, there was an increase in ST vector magnitude (p = 0.02) and instability (p = 0.008) in the arrest group. In the paired subgroup analysis, there was an increase in ST vector magnitude (p = 0.05) and instability (p = 0.05) in the pre-arrest state compared to the post-arrest state prior to discharge.
Conclusions
In single ventricle patients, increased ST instability and magnitude was associated with rapid response events which required intervention for cardiopulmonary arrest, whereas conventional ST segment monitoring did not differentiate an arrest from control state.
Keywords: Electrocardiography, Heart Defects, Congenital, Left Heart Syndrome, Hypoplastic, Cardiopulmonary Arrest
INTRODUCTION
Infants with single ventricle physiology pose a significant challenge in perioperative management. The palliative surgical approach for single ventricle lesions first seeks, in the neonatal period, to balance pulmonary and systemic flows from the common ventricular chamber in stage I surgery. Stage 2 surgical palliation, typically performed between 3 and 5 months of life, reduces the volume load of parallel circulation with a superior cavo-pulmonary anastomosis. Stage 3 palliation is the Fontan completion, after which a serial circulation is achieved with minimal or no residual ventricular mixing of systemic and pulmonary venous return. The single ventricle palliation pathway can be applied to lesions with right or left ventricular hypoplasia. In either case, the most unstable period is the interstage between stage I and stage II, when volume loading caused by parallel systemic and pulmonary circulations places strain on the single (or common) ventricle.
In the case of hypoplastic left heart syndrome (HLHS), stenosis or atresia of the aortic valve, stenosis or atresia of the mitral valve, and hypoplasia of the ascending aorta or aortic arch render a vulnerable systemic circulation that is incompatible with life after closure of the ductus arteriosus (1). The stage I palliation described by Norwood in 1983 has transformed the management and outcomes of single ventricle patients with left-sided lesions (2). Over the years the HLHS interstage mortality rate has fallen from >90% to 11–15% in tertiary centers (3–5). This mortality rate is still disproportionately high compared with other congenital cardiac diagnoses, and HLHS patients are at the highest risk of death (33%) during the interstage period (6, 7).
Infants with right ventricular hypoplasia have a volume-overloaded, parallel circulation that is also vulnerable to hemodynamic instability in the interstage. Infants with single ventricle anatomy, whether right or left sided morphologically, are closely monitored during the first interstage period in an effort to recognize subtle signs of hemodynamic instability intrinsic to the parallel circulation created by stage 1 palliation. Effective ICU monitoring in this population is made difficult by “abnormal” monitor values in patients despite optimized hemodynamics. Consequently, a monitoring platform specific for balanced circulation lesions, and a care team with experience in the management of these lesions is required.
Continuous electrocardiography (ECG) monitoring is commonly used in the perioperative period to identify signs of cardiac ischemia or arrhythmias and ideally allows for the early identification and intervention to prevent potentially fatal interstage cardiac arrests (8). Though continuous ECG monitoring is useful in identifying life-threatening arrhythmias, the ECG obtained from infants with single ventricle anatomy is highly variable in appearance. There is often ST segment depression or elevation at baseline in univentricular hearts due to cardiac conduction abnormalities either native to the patient or following surgery (9). An array of ECG abnormalities have been documented in this population, including right or left axis deviation, first-degree atrioventricular block, prolonged PR intervals, prolonged QRS complexes, and nonspecific ST segment and T wave changes (10, 11). Ambiguous ECG baselines limit the ability to detect states of cardiac ischemia and strain with conventional bedside monitoring. The single ventricle population is uniquely vulnerable to cardiac ischemia due to various combinations of the following: low coronary perfusion pressure from diastolic runoff to the pulmonary artery, increased wall stress from the volume overload created by parallel systemic and pulmonary circulation, and retrograde aortic flow to coronary arteries arising from a diminutive, native aortic root (in the case of hypoplastic left heart syndrome). An improved monitoring technique to indicate inadequate coronary perfusion in this population could allow for early intervention.
We utilized signal processing methods to analyze continuous monitoring of the ECG waveform and ST segment during the high-risk interstage for single ventricle patients. We hypothesized that dynamic changes in the ST segment would be associated with a cardiopulmonary pre-arrest state. To assess this, a signal processing algorithm was created which distilled 3 single-dimension ECG leads into a single, 3-dimensional ST segment vector. Instability of the 3-dimensional ST vector was quantified and association with arrest was evaluated.
METHODS
Study population and design
Approval of this study was obtained from the Baylor College of Medicine Institutional Review Board with a waiver of consent prior to the start of the study (H-28829 and H-33066, Houston, TX). A retrospective, observational study was conducted at Texas Children’s Hospital (TCH), a 456-bed, tertiary care pediatric hospital with a 21-bed cardiovascular intensive care unit (CVICU) and 36-bed cardiovascular step-down unit. From January 2013 to January 2014, all patients admitted to the hospital with single ventricle physiology (a cardiac lesion with single morphologic left or right ventricle) who received stage I palliation were included in the study until stage II palliation was performed or death occurred. Subjects were identified from a cardiology diagnosis database and chart review.
Exclusion criteria included subjects without the 5-lead ECG monitoring required to carry out acquisition and subsequent analysis of the ECG waveform and ST segment changes. This occurred when the patient had a rapid response event in a location where ECG signals were not being captured by the data collection system or ECG signals could not render a value for ST segment change (elevation or depression).
The interstage was chosen as the period of study because it is the period of greatest risk of hemodynamic instability and cardiopulmonary arrest. For the purposes of this study, arrests were defined as rapid response team (RRT) activation (i.e. code team activation) which led to cardiopulmonary resuscitation and/or endotracheal intubation. Rapid response events were identified through chart review. The timing of each rapid response event was confirmed by physician review of collected vital signs. The study period utilized for the arrest group was the four hours prior to identified arrest. Subjects who did not have a rapid response event during the interstage were classified in the control group. The study period for the control group was four hours prior to discharge from the CVICU, a time when a physician has determined the subject stable enough to not require intensive monitoring and care.
Due to the challenges in defining study and control groups, a subgroup analysis was performed comparing metrics from each subject’s 4-hour pre-arrest state to post-arrest recovery state 4 hours prior to CVICU discharge. In this paired analysis, each subject’s ST signals prior to discharge when they were in a state of “health” after recovery from arrest was utilized for comparison against the pre-arrest 4-hour window.
Data Collection
Interstage 5-lead ECG signals were collected with the SickbayTM platform (Medical informatics Corp; Houston, TX, USA). Signals are recorded at the monitor refresh rate, which is 240 Hz for ECG, pulse oximetry, and blood pressure. ST segments are refreshed at 0.25 Hz and were captured at this rate. All data were stored on site, behind a Health Insurance Portability and Accountability Act (HIPAA) compliant firewall maintained by Baylor College of Medicine Information Technology. ST segment values were determined based on the GE monitor output algorithm, performed as the J point plus 60 – 80 ms depending on the age of the patient (12, 13). To reduce bias, all available data from patients that met inclusion criteria were included for analysis.
Signal processing and construction of 3-dimensional ST segment vector
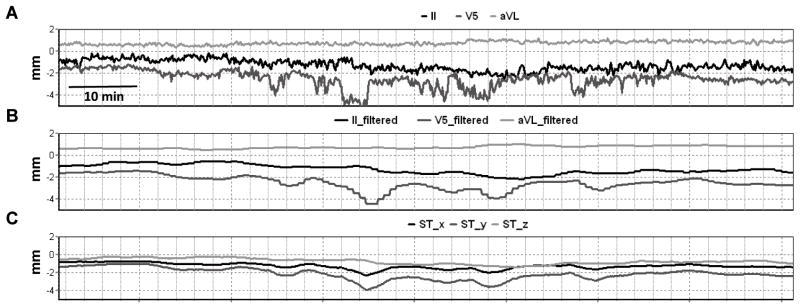
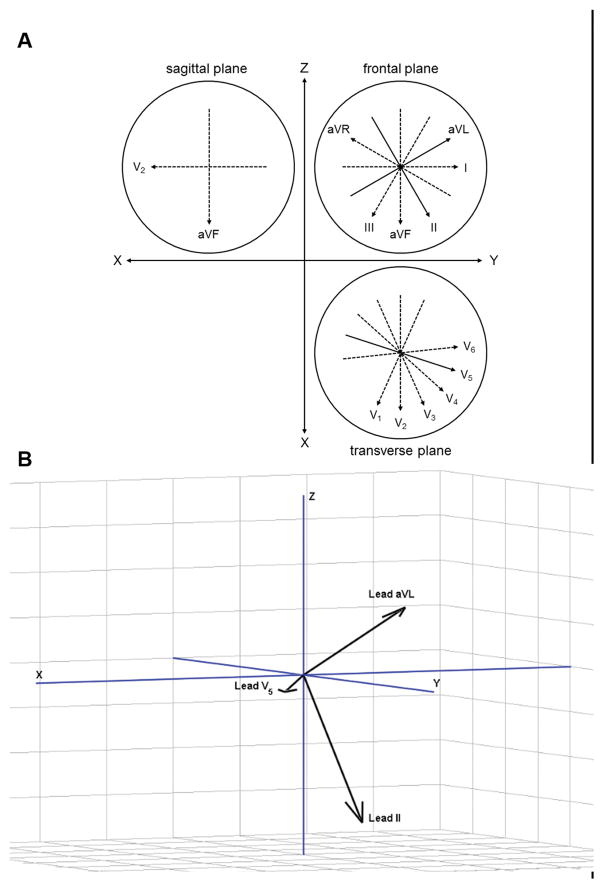
To capture movement of the ST segment, a 3-dimensional ST vector was constructed from the collected raw ST segment output (figure 1A). Leads II, V5, and aVL were chosen for analysis due to their quasi-orthogonal orientation. A 4-minute moving average filter was applied to the raw signals. This filter was utilized to remove rapid, non-physiologic artifacts, such as artifact caused by subject movement. The result captures the low-frequency change in the ST segment that was of interest (figure 1B). Using the relative geometries of the ECG leads (figure 2A), an ST segment vector was constructed from the filtered signals with x-, y-, and z- coordinates of the instantaneous ST vector (figure 2B) defined by:
| (Eqn. 1) |
| (Eqn. 2) |
| (Eqn. 3) |
where II, V5, and aVL are the values of the filtered ST segments of the associated ECG leads. The position of the ST vector in x-, y-, and z- dimensions over the window of time is demonstrated in the example (figure 1C). The magnitude of the ST segment vector was calculated from equation 4.
Figure 1.
A: Example of four hours of ST segment electrocardiogram values from quasi-orthogonal leads II, V5, and aVL in subject prior to clinical deterioration. B: Four minute moving average filter applied to ST segment values. C: Applied algorithm to determine ST vector x-, y-, z- coordinates in Cartesian system (see Figure 2).
Figure 2.
A: Relative geometries of Einthoven and Wilson precordial ECG lead system in sagittal, frontal, and transverse plane. Quasi-orthogonal leads utilized in model depicted as solid arrow. B: Orientation of quasi-orthogonal leads in 3-dimiensional, right handed Cartesian system.
| (Eqn. 4) |
All data analysis was performed with Matlab™ (MathWorks, Natick, MA).
Measuring instability
Instability was quantified based on the range of the ST vector values in 3-dimensions using a moving 20-minute time window updated every minute.
| (Eqn. 5) |
Displacements over 20-minute moving windows of time were chosen based on observations made by physicians in the CVICU noting that there were low-frequency changes in the ST segment which could be grossly observed in the GE monitor trends over 20–30 minutes (figure 1).
Statistical analysis
Data are presented with median and interquartile ranges unless otherwise specified. Based on the study design, number of comparisons, and Shapiro-Wilk testing, non-parametric statistics were applied. Characteristics of the subjects in each group were compared at baseline using Fisher’s exact test for categorical variables and Wilcoxon rank-sum test for continuous variables.
Mean unprocessed ST segment values were compared between the study and control groups. Next, the mean absolute value of each of the leads were compared between the study and control groups. Finally, comparisons were made using the ST vector magnitude (eqn. 4) and ST vector instability (eqn. 5) utilizing the same 4-hour window prior to arrest in the study group and 4-hour window prior to CVICU discharge in the control group. Comparisons for group differences (arrest vs. control) were made with a Wilcoxon rank-sum test. When differences were statistically significant (p value < 0.05), a Receiver Operating Characteristic (ROC) analysis was utilized to evaluate the performance of the ST segment analysis method (14). Statistical analyses were performed with Stata 14 (College Station, TX; USA) and graphically displayed using GraphPad Prism 5 (La Jolla, CA; USA).
For the paired subgroup analysis, the same ST metrics were compared utilizing the subject’s pre-arrest state (4-hour window prior to arrest) against the post-arrest recovery state (4-hour window prior to CVICU discharge) utilizing a Wilcoxon signed-rank test. Subjects who did not survive to discharge had to be excluded in this analysis due to the inability to define a post-arrest recovery state of “health.” In the case where a subject had multiple arrests, the arrest data were averaged prior to comparison.
RESULTS
Study Population
Between January 2013 and January 2014, there were 24 single ventricle patients who received palliative surgery at TCH. After inclusion and exclusion criteria were applied, 20 infants were included for analysis; 4 patients were excluded due to lack of ST segment output from the GE monitor. In the study population, 13 (65%) of the subjects had an anatomical lesion characterized as an underdeveloped left ventricle, and 7 (35%) had a lesion characterized as an underdeveloped right ventricle (table 1a). The stage 1 palliation procedures included: Norwood procedure with Blalock-Taussig shunt (8; 40%), Norwood procedure with Sano shunt (4; 20%), pulmonary artery banding (4; 20%), and Blalock-Taussig shunt (3; 15%) (table 1b). There was no difference in the study population between the control and pre-arrest group characteristics when comparing average age, sex, cardiopulmonary bypass time, aortic cross clamp time, circulatory arrest time, shunt type, or interstage length; in the pre-arrest group, the median gestational age was 38.1 weeks versus 39 weeks in the control group (p = 0.009) (table 1b).
Table 1.
A) Anatomical characteristics, surgical procedures performed on the study population; associated number of arrests, median interstage length, and survival rates. B) Characteristics of study population.
| A | ||||
|---|---|---|---|---|
| Cardiac Anatomy | Patients | Arrests | Interstage (days) | Survival |
| Morphologic LV Hypoplasia | 13 | 17 | 97 [91 – 166] | 11 (89%) |
| Hypoplastic Left Heart Syndrome | 9 | 13 | 95 [89 – 132] | 8 (89%) |
| Heterotaxy with Pulmonary Atresia | 1 | 0 | 256 | 1 (100%) |
| Unbalanced AV Canal | 1 | 0 | 148 | 1 (100%) |
| Double Outlet Right Ventricle | 2 | 4 | 97 | 1 (50%) |
| Morphologic RV Hypoplasia | 7 | 3 | 179 [153 – 182] | 7 (100%) |
| Tricuspid Atresia | 4 | 2 | 154 [122 – 169] | 4 (100%) |
| Pulmonary Atresia | 2 | 1 | 160 [134 – 186] | 2 (100%) |
| Double Inlet Left Ventricle | 1 | 0 | 179 | 1 (100%) |
|
| ||||
| Surgical Procedure | ||||
|
| ||||
| Norwood with Blalock-Taussig Shunt | 8 | 12 | 95 [92 – 97] | 6 (89%) |
| Norwood with Sano Shunt | 4 | 3 | 97 [83 – 175] | 4 (100%) |
| Hybrid Norwood | 1 | 3 | 167 | 1 (100%) |
| Pulmonary Artery Band | 4 | 1 | 179 [156 – 182] | 4 (100%) |
| Blalock-Taussig Shunt | 3 | 1 | 148 [134 – 186] | 3 (100%) |
|
| ||||
| Overall | 20 | 20 | 141 [94 – 175] | 18 (90%) |
| B | |||
|---|---|---|---|
| Characteristic | Control (n = 9) | Arrest (n = 11) | p |
| Age (days) | 8 [5 – 47] | 5 [4 – 9] | 0.40 |
| Male sex | 6 | 4 | 0.22 |
| Weight (kg) | 3.3 [3.1 – 3.7] | 3.1 [2.7 – 3.6] | 0.22 |
| Gestational age at birth (weeks) | 39 [39 – 39.1] | 38.2 [36.8 – 38.9] | 0.007 |
| CPB time (min) | 168 [140 – 210] | 178 [153 – 209] | 0.84 |
| AoX time (min) | 81 [77 – 110] | 89.5 [85 – 109] | 0.41 |
| ACP time (min) | 73 [67 – 101] | 72.5 [69 – 83] | 0.68 |
| CA time (min) | 9 [8 – 10] | 9 [7 – 15] | 0.77 |
| Shunt type | |||
| Blalock-Taussig | 2 | 6 | |
| Sano | 2 | 2 | 0.55 |
| Interstage (days) | 156 [97 – 182] | 97 [92 – 153] | 0.20 |
Values are median [IQR25 – IQR75] or number reported when applicable. LV = left ventricle. AV = atrioventricular. RV = right ventricle. CPB = cardiopulmonary bypass. AoX = aortic cross clamp. ACP = antegrade cerebral perfusion. CA = circulatory arrest.
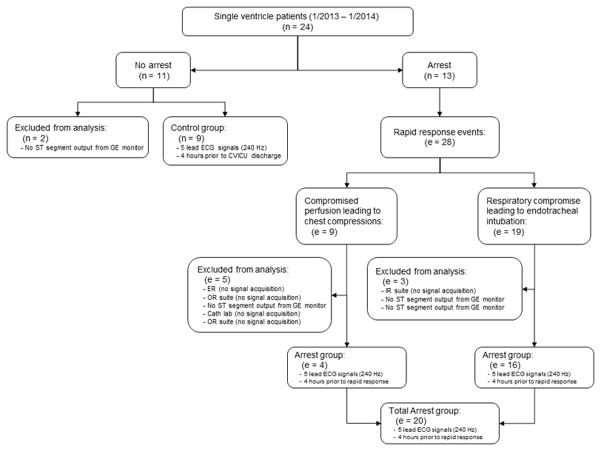
In 9 subjects, there was no deterioration event and they were classified as controls. In the remaining 11 subjects, there were a total of 28 rapid response events: 9 of which were due to compromised perfusion leading to chest compressions and 19 of which were due to respiratory or perfusion compromise leading to endotracheal intubation. However, a total of 8 RRT events were excluded from analysis due to absence of signal acquisition (figure 3). This was due to the event occurring in a location where the SickbayTM platform was not installed and thus, data could not retrospectively be analyzed. In 4 RRT events (14%), the GE monitor did not produce ST segment output. This could have been attributed to faulty leads, leads which were not completely on subject, or motion causing artifacts in the ECG signal. A total of 20 clinical deterioration events were utilized as the study group, 4 (20%) of which led to cardiopulmonary resuscitation with chest compressions, and 16 (80%) of which led to endotracheal intubation.
Figure 3.
Study design. TCH = Texas Children’s Hospital. ER = emergency room. IR = interventional radiology.
For the subgroup analysis, there were 9 subjects that had an arrest event and survived to CVICU discharge to have comparison data available. In this cohort, there were 17 cardiopulmonary arrests; 5 patients had multiple pre-arrest events. The multiple 4-hour windows were averaged to create a single pre-arrest value for each patient in comparison to the 4-hour post-arrest recovery state.
ST segment analysis
The average ST segments over the 4-hour window in the arrest and control groups were compared in the lateral leads: I, aVL, V5 and inferior leads: II, III, aVF, and there were no differences in unprocessed ST segments (table 2a, see figure - supplemental digital content 1). When the absolute value was calculated for each lead, there was an increase in ST position in the arrest vs. control group in leads I, II, III, and aVF (p<0.05, table 2b). The ROC area under curve for the absolute value of leads I, II, III, and aVF were 0.73, 0.81, 0.74, and 0.76, respectively (table 2b). For the subgroup analysis, there were no differences in unprocessed ST segments (table 3a, see figure – supplemental digital content 2) and no differences absolute value of leads in the pre-arrest vs. post-arrest states (table 3b).
Table 2.
Primary analysis comparing arrest and control groups with A) unprocessed signals. B) absolute value of signals. C) signal processing ST vector algorithm applied.
| A | |||
|---|---|---|---|
| Unprocessed lead | Arrest | Control | p |
| Lateral (mm) | |||
| I | 0.2 [−0.9 – 0.7] | 0.0 [−0.5 – 0.3] | 0.58 |
| aVL | −0.4 [−1.4 – 2.4] | −0.1 [−0.6 – −0.1] | 0.70 |
| V5 | 0.1 [−0.7 – 0.7] | 0.3 [−1.0 – 0.4] | 0.78 |
| Inferior (mm) | |||
| II | 0.9 [−0.2 – 2.6] | 0.4 [0.0 – 1.1] | 0.21 |
| III | 0.9 [−0.2 – 1.8] | 0.4 [−0.1 – 1.1] | 0.38 |
| aVF | 1.3 [−0.2 – 1.8] | 0.4 [0.2 – 0.8] | 0.39 |
| B | |||||
|---|---|---|---|---|---|
| Absolute value of lead | Arrest | Control | p | ROC area | 90% specificity threshold |
| Lateral (mm) | |||||
| |I| | 0.7 [0.4 – 1.5] | 0.3 [0.0 – 0.7] | 0.05 | 0.73 | 1.0 |
| |aVL| | 0.7 [0.4 – 1.5] | 0.5 [0.1 – 0.6] | 0.06 | ||
| |V5| | 0.6 [0.4 – 1.7] | 0.4 [0.3 – 1.1] | 0.46 | ||
| Inferior (mm) | |||||
| |II| | 1.3 [0.8 – 2.9] | 0.5 [0.1 – 1.1] | 0.006 | 0.81 | 1.4 |
| |III| | 1.2 [0.3 – 2.0] | 0.4 [0.2 – 1.1] | 0.04 | 0.74 | 1.1 |
| |aVF| | 1.6 [0.6 – 1.8] | 0.4 [0.3 – 0.8] | 0.03 | 0.76 | 1.0 |
| C | |||||
|---|---|---|---|---|---|
| 3-dimensional lead | Arrest | Control | p | ROC area | 90% specificity threshold |
| ST vector length (mm) | 2.1 [1.4 – 4.6] | 1.3 [1.1 – 1.6] | 0.02 | 0.78 | 1.7 |
| ST vector instability (mm) | 3.8 mm / 20 min [2.4 – 25.4] | 2.4 mm / 20 min [1.8 – 2.7] | 0.008 | 0.81 | 2.8 |
Values are presented as median [IQR25 – IQR75]. Area under the receiving operating characteristic curve and 90% specificity thresholds were calculated when there was a statistically significant difference between groups, p < 0.05.
Table 3.
Subgroup analysis comparing subject pre-arrest and post-arrest state with A) unprocessed signals. B) absolute value of signals. C) signal processing ST vector algorithm applied.
| A | |||
|---|---|---|---|
| Unprocessed lead | Pre-arrest | Post-arrest | p |
| Lateral (mm) | |||
| I | 0.0 [−0.6 – 0.6] | 0.4 [−0.3 – 0.5] | 0.21 |
| aVL | −0.3 [−0.7 – 0.1] | −0.1 [−0.7 – 0.6] | 0.07 |
| V5 | −0.4 [−0.9 – −0.02] | −0.2 [−1.1 – 0.5] | 0.51 |
| Inferior (mm) | |||
| II | 0.3 [−0.2 – 3.5] | 0.8 [−0.4 – 1.9] | 0.37 |
| III | −0.1 [−0.2 – 0.8] | −0.3 [−0.9 – 1.1] | 0.17 |
| aVF | 0.4 [−0.3 – 1.8] | −0.0 [−1.2 – 1.2] | 0.37 |
| B | |||
|---|---|---|---|
| Absolute value of lead | Pre-arrest | Post-arrest | p |
| Lateral (mm) | |||
| |I| | 0.6 [0.5 – 1.5] | 0.5 [0.4 – 0.9] | 0.52 |
| |aVL| | 0.5 [0.3 – 1.8] | 0.7 [0.4 – 0.7] | 0.44 |
| |V5| | 0.6 [0.4 – 1.3] | 0.8 [0.6 – 1.1] | 0.77 |
| Inferior (mm) | |||
| |II| | 1.3 [0.3 – 3.5] | 1.9 [0.8 – 2.1] | 0.31 |
| |III| | 0.4 [0.2 – 0.8] | 1.1 [0.9 – 1.6] | 0.26 |
| |aVF| | 0.8 [0.5 – 2.8] | 1.2 [1.1 – 1.6] | 0.68 |
| C | |||
|---|---|---|---|
| 3-dimensional lead | Pre-arrest | Post-arrest | p |
| ST vector length (mm) | 2.8 [1.4 – 8.5] | 2.0 [1.4 – 2.8] | 0.05 |
| ST vector instability (mm) | 4.3 mm / 20 min [2.5 – 15.4] | 3.1 mm / 20 min [2.6 – 5.0] | 0.05 |
Values are presented as median [IQR25 – IQR75].
ST segment vector analysis
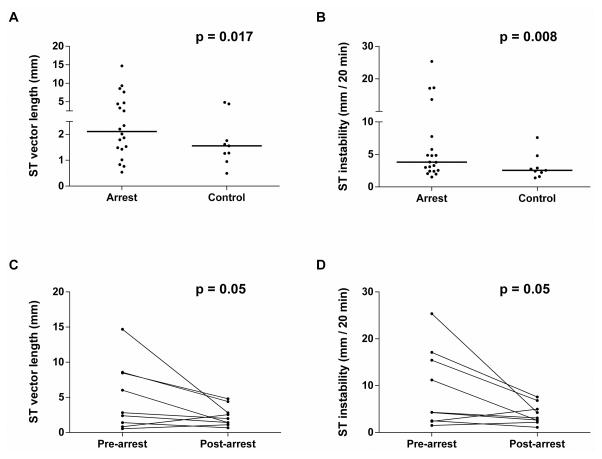
A sample time lapse video of ST vector behavior in an arrest subject is available for comparison with a control subject (see Video – Supplemental Digital Content 3A and 3B, respectively). After signal processing (eqns. 1–4) was applied to the ST segment, the median ST vector length was 62% greater in the arrest group compared to the control group (2.1 mm [1.4 – 4.6 mm] vs. 1.3 mm [1.1 – 1.6 mm], p = 0.02) (figure 4a, table 2c). For ST instability (eqn. 5), there was a 54% increase in arrest subjects compared to control subjects (3.8 mm / 20 min [2.4 – 25.4] vs. 2.4 mm / 20 min [1.8 – 2.7], p = 0.008) (figure 4b, table 2c). The ROC area under the curve for ST vector length and ST vector instability were 0.78 and 0.81, respectively. For the subgroup analysis, median ST vector length was 40% greater in the pre-arrest state compared to the post-arrest recovery group (2.8 mm [1.4 – 8.5 mm] vs. 2.0 mm [1.4 – 2.8 mm], p = 0.05) (figure 4c, table 3c). For ST instability, there was a 39% increase in ST instability in subjects in the pre-arrest state compared to the post-arrest recovery group (4.3 mm / 20 min [2.5 – 15.4] vs. 3.1 mm/20 min [2.6 – 5.0], p = 0.05) (figure 4d, table 3c).
Figure 4.
Primary analysis of 4-hour window in arrest vs. control group average A: ST segment vector length and B: ST segment instability. Subgroup analysis of paired 4-hour window of subjects’ pre-arrest state vs. post-arrest recovery state prior to cardiovascular intensive care unit discharge. Signal processing demonstrated statistically significant increase in ST vector length and ST segment instability in pre-arrest vs. control group (p < 0.05) in all analyses.
DISCUSSION
Through signal processing of 3 quasi-orthogonal ECG lead ST segment trends, an algorithm reconstructing a 3-dimensional ST segment vector was defined in this study. In this preliminary study, the magnitude and instability of this ST vector is associated with cardiopulmonary arrest in this cohort of subjects with single ventricle parallel circulations where unprocessed ST segments were not. Processing the ECG in this way may provide clinically relevant information from the otherwise confounded ST segment in the single ventricle population. Following stage 1 palliation, residual or progressive anatomic lesions such as a restrictive atrial septum, stenosis of the systemic to pulmonary shunt or aortic arch place the infant at high risk for cardiac ischemia even after surgical correction (15). In addition, infants have minimal cardiac reserve when faced with respiratory tract infections, gastroenteritis, or fever which could lead to hypovolemia or hypoxemia (16). Though ST segment changes have been traditionally used to identify myocardial ischemia or infarction in biventricular physiology, monitoring ST segment changes is limited in single ventricle physiology where the ST segment is frequently confounded by a non-zero ST segment baseline and conduction abnormalities in the His-Purkinje system (10, 17, 18).
Our findings support the notion of limited clinical utility in current, bedside ECG ST segment changes in single ventricle patients as there was no difference in unprocessed ST segment values in the arrest versus control groups (and pre-arrest versus post-arrest states) for any monitored leads (I, aVL, V5, II, III, aVF). When the absolute value of the ST segments were computed, there was an increase in ST segment values in lateral lead I, and inferior leads II, III, and aVF, with lead II showing the greatest sensitivity and specificity for arrest physiology. While this represented an improvement in the potential identification of infants prior to arrest, ischemic vulnerability can occur in any direction. This could be due to the anatomic variability in the cohort which included both left and right sided lesions. The derived, ST vector combines information about ST segment deviations from multiple leads into one value. ST vector length had fair discrimination and ST vector instability had good discrimination with an area under the receiver operator characteristic curve of 0.78 and 0.81 respectively. The association of increased ST vector length and instability with clinical deterioration suggest that these new signal processing algorithms may have clinical utility in forecasting (within 4 hours) an RRT event requiring CPR or intubation in the setting of single ventricle physiology, which may otherwise be difficult to detect with traditional ST segment monitoring.
Spatial vectorcardiography (VCG) has been utilized to reproduce electrical activity in X, Y, and Z directions using a Frank lead system (19). Though this method has been found to be superior in the detection of myocardial infarction compared to standard ECG analysis, the clinical use of VCG has been limited in the United States (20). Previous studies have converted ECG lead systems to VCG recordings, but the methods are imprecise due to the difference in magnitude between Einthoven and Wilson precordial leads (21 – 24). In our 3-dimensional method of examining the ST segment, this issue is circumvented by creating a single vector of the relative ST segment displacement in quasi-orthogonal leads. We anticipate that the ST segment vector could be a valid surrogate for coronary ischemia, particularly in conditions where there is no ST segment baseline.
A challenge in this study was in defining study versus control groups. Our primary goal in this initial analysis was to define candidate metrics in predicting cardiopulmonary arrest for a larger multivariate analysis. The study design best differentiates between a state of deterioration (4 hours prior to arrest) and health (4 hours prior to discharge from ICU in a subject that never experienced arrest) for screening of candidate metrics. However, to further evaluate ST instability, a subgroup analysis was performed, comparing patients in a pre-arrest state against their post-arrest recovery state prior to ICU discharge. The results reflect a significant increase in ST vector length and magnitude, though the level of statistical significance achieved was not as great compared to our primary analysis. This was likely due to the exclusion of subjects with “disease” who did not survive to discharge to have a definable state of “health.” In addition, the results may reflect that the arrest subjects may never fully recover and achieve the same baseline ST stability as those who never experienced cardiopulmonary arrest.
In this preliminary study, there are several limitations to consider. There were instances where no data was captured on subjects (8 out of 36 events, 22%). These instances are more likely to occur in locations remote from the intensive care unit, so future studies confirming these results in a low-acuity or home-monitoring setting would be informative. ECG lead location was not standardized by the study, and incorrect placement may have confounded the data. To identify the study group, any activation of an RRT event was utilized that resulted in either chest compressions or endotracheal intubation. Endotracheal intubations were included in this study population because airway interventions are often performed as a form of support in compromised cardiac disease. With a greater number of patients, the degree of ST segment instability may be compared between respiratory versus cardiac arrests. In addition, this cohort included any patient with single ventricle anatomy and even in this population, there are variations in physiology based on anatomy and surgical palliation performed. This likely led to the large interquartile range of ST variability seen in our study. While there are possibly different mechanisms of ischemia and strain based on the physiology and shunt type, our results indicate that the ST instability metric performed well in distinguishing an arrest from control conditions. The absolute values of ST displacements in individual leads were variable performers to detect impending arrest; in this cohort lead II rendered the best receiver operator characteristic, which was the same as the ST instability algorithm (AUC 0.81). Larger cohorts will be required to definitively delineate the relative performance of individual leads against the 3-dimensional lead presented.
We did not perform a time series to examine ST behavior in subjects who experienced cardiac arrest remotely from the event. Therefore, we cannot say definitively that the ST instability observed was a sustained feature of the subjects that ultimately experienced decompensation, or that it occurred as a precursor to deterioration. This study design limits the ability to perform predictive value assessments of the variables studied. Nonetheless, the metrics of the ST segment vector identified in this pilot study may be useful candidates for future multivariate predictive analytics. In future studies of entire hospital admissions, the algorithms may be applied to ultimately determine positive and negative predictive value.
In conclusion, we have developed a signal processing algorithm for ST segment instability that is associated with cardiac and respiratory arrests in the interstage for single ventricle patients. In a population where the ST segment is commonly confounded by arrhythmias and the lack of a baseline, this novel monitoring technique may be a noninvasive method for the early detection of cardiac ischemia. With continued development, the ST instability algorithm may be incorporated into advanced predictive monitoring techniques and benefit the perioperative care of the unstable single ventricle patient.
Supplementary Material
Sample time-lapse video at 45 fps demonstrating 3-dimensional ST segment vector movement in four-hour window A: prior to cardiopulmonary arrest and B: in control subject who did not experience cardiopulmonary arrest. Created in MatlabTM. mpeg4.
Primary analysis of 4-hour window in arrest vs. control groups evaluating average unprocessed ST segment position in A: lateral leads I, aVL, V5 and B: inferior leads II, III, aVF. There was no statistically significant difference observed between groups (p > 0.05).
Subgroup analysis of subjects’ 4-hour pre-arrest state compared 4-hour post-arrest recovery state prior to cardiovascular intensive care unit discharge evaluating average unprocessed ST segment position in A: lateral leads I, aVL, V5 and B: inferior leads II, III, aVF in subgroup analysis. There was no statistically significant difference observed between groups (p > 0.05).
Acknowledgments
Sources of Funding: Funding for this investigation was provided by departmental funds and an NIH Pediatric Heart Network Mentored Research Award #U09-HL068270 (Craig Rusin). Data was analyzed by the primary author while supported by a Foundation for Anesthesia Education and Research (FAER) Fellowship Award.
Footnotes
Conflicts of Interest
Craig Rusin is the co-founder of Medical Informatics Corporation, which developed the physiologic data collection system. There are no conflicts for the other investigators.
References
- 1.Feinstein JA, Benson DW, Dubin AM, et al. Hypoplastic left heart syndrome: current considerations and expectations. J Am Coll Cardiol. 2012;59(1 Suppl):S1–42. doi: 10.1016/j.jacc.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norwood WI, Lang P, Hansen DD. Physiologic repair of aortic atresia-hypoplastic left heart syndrome. N Engl J Med. 1983;308(1):23–26. doi: 10.1056/NEJM198301063080106. [DOI] [PubMed] [Google Scholar]
- 3.Furck AK, Uebing A, Hansen JH, et al. Outcome of the Norwood operation in patients with hypoplastic left heart syndrome: a 12-year single-center survey. J Thorac Cardiovasc Surg. 2010;139(2):359–365. doi: 10.1016/j.jtcvs.2009.07.063. [DOI] [PubMed] [Google Scholar]
- 4.Ghanayem NS, Allen KR, Tabbutt S, et al. Interstage mortality after the Norwood procedure: Results of the multicenter Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144(4):896–906. doi: 10.1016/j.jtcvs.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tweddell JS, Hoffman GM, Mussatto KA, et al. Improved survival of patients undergoing palliation of hypoplastic left heart syndrome: lessons learned from 115 consecutive patients. Circulation. 2002;106(12 Suppl 1):I82–89. [PubMed] [Google Scholar]
- 6.Jaquiss R, Imamura M. Single ventricle physiology: surgical options, indications and outcomes. Curr Opin Cardiol. 2009;24(2):113–118. doi: 10.1097/HCO.0b013e328323d85a. [DOI] [PubMed] [Google Scholar]
- 7.Ohye RG, Schonbeck JV, Eghtesady P, et al. Cause, timing, and location of death in the Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144(4):907–914. doi: 10.1016/j.jtcvs.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drew BJ, Califf RM, Funk M, et al. Practice standards for electrocardiographic monitoring in hospital settings: an American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation. 2004;110(17):2721–2746. doi: 10.1161/01.CIR.0000145144.56673.59. [DOI] [PubMed] [Google Scholar]
- 9.Khairy P, Poirier N, Mercier LA. Univentricular heart. Circulation. 2007;115(6):800–812. doi: 10.1161/CIRCULATIONAHA.105.592378. [DOI] [PubMed] [Google Scholar]
- 10.Monaco MA, Liberman L, Starc TJ, et al. Defining the electrocardiogram in the neonate with hypoplastic left heart syndrome. Pediatr Cardiol. 2015;36(5):1014–1018. doi: 10.1007/s00246-015-1112-x. [DOI] [PubMed] [Google Scholar]
- 11.Noonan JA, Nadas AS. The hypoplastic left heart syndrome; an analysis of 101 cases. Pediatr Clin North Am. 1958;5(4):1029–1056. doi: 10.1016/s0031-3955(16)30727-1. [DOI] [PubMed] [Google Scholar]
- 12.Ehrenwerth J, Eisenkraft JB, Berry JM. Anesthesia equipment: principles and applications. 2. Philadelphia, PA: Saunders; 2013. Electrocardiographic Monitoring. [Google Scholar]
- 13.12-lead ST Monitoring (White paper) GE Healthcare; Milwaukee, WI: [Accessed December 22, 2015]. Available at: http://clinicalview.gehealthcare.com/download.php?obj_id=187. [Google Scholar]
- 14.Zou KH, O’Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115(5):654–657. doi: 10.1161/CIRCULATIONAHA.105.594929. [DOI] [PubMed] [Google Scholar]
- 15.Bartram U, Grunenfelder J, Van Praagh R. Causes of death after the modified Norwood procedure: a study of 122 postmortem cases. Ann Thorac Surg. 1997;64(6):1795–1802. doi: 10.1016/s0003-4975(97)01041-2. [DOI] [PubMed] [Google Scholar]
- 16.Mahle WT, Spray TL, Wernovsky G, et al. Survival after reconstructive surgery for hypoplastic left heart syndrome: A 15-year experience from a single institution. Circulation. 2000;102(19 Suppl 3):III136–141. doi: 10.1161/01.cir.102.suppl_3.iii-136. [DOI] [PubMed] [Google Scholar]
- 17.Sprung J, Abdelmalak B, Gottlieb A, et al. Analysis of risk factors for myocardial infarction and cardiac mortality after major vascular surgery. Anesthesiology. 2000;93(1):129–140. doi: 10.1097/00000542-200007000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Reckova M, Rosengarten C, deAlmeida A, et al. Hemodynamics is a key epigenetic factor in development of the cardiac conduction system. Circ Res. 2003;93(1):77–85. doi: 10.1161/01.RES.0000079488.91342.B7. [DOI] [PubMed] [Google Scholar]
- 19.Frank E. An accurate, clinically practical system for spatial vectorcardiography. Circulation. 1956;13(5):737–749. doi: 10.1161/01.cir.13.5.737. [DOI] [PubMed] [Google Scholar]
- 20.McConahay DR, McCallister BD, Hallermann FJ, et al. Comparative quantitative analysis of the electrocardiogram and the vectorcardiogram. Correlations with the coronary arteriogram. Circulation. 1970;42(2):245–259. doi: 10.1161/01.cir.42.2.245. [DOI] [PubMed] [Google Scholar]
- 21.Einthoven W. Weiteres über das Elektrokardiogram. Pflüger Arch ges Physiol. 1908 [Google Scholar]
- 22.Wilson FN, Johnston FD, et al. On Einthoven’s triangle, the theory of unipolar electrocardiographic leads, and the interpretation of the precordial electrocardiogram. Am Heart J. 1946;32:277–310. doi: 10.1016/0002-8703(46)90791-0. [DOI] [PubMed] [Google Scholar]
- 23.Draper HW, Peffer CJ, Stallmann FW, et al. The corrected orthogonal electrocardiogram and vectorcardiogram in 510 normal men (Frank lead system) Circulation. 1964;30:853–864. doi: 10.1161/01.cir.30.6.853. [DOI] [PubMed] [Google Scholar]
- 24.Strauss DG, Olson CW, Wu KC, et al. Vectorcardiogram synthesized from the 12-lead electrocardiogram to image ischemia. J Electrocardiol. 2009;42(2):190–197. doi: 10.1016/j.jelectrocard.2008.12.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sample time-lapse video at 45 fps demonstrating 3-dimensional ST segment vector movement in four-hour window A: prior to cardiopulmonary arrest and B: in control subject who did not experience cardiopulmonary arrest. Created in MatlabTM. mpeg4.
Primary analysis of 4-hour window in arrest vs. control groups evaluating average unprocessed ST segment position in A: lateral leads I, aVL, V5 and B: inferior leads II, III, aVF. There was no statistically significant difference observed between groups (p > 0.05).
Subgroup analysis of subjects’ 4-hour pre-arrest state compared 4-hour post-arrest recovery state prior to cardiovascular intensive care unit discharge evaluating average unprocessed ST segment position in A: lateral leads I, aVL, V5 and B: inferior leads II, III, aVF in subgroup analysis. There was no statistically significant difference observed between groups (p > 0.05).