Abstract
Asthma in the elderly (>65 yr old) is common and associated with higher morbidity and mortality than asthma in younger patients. The poor outcomes in this group are due, in part, to underdiagnosis and undertreatment. There are a variety of factors related to aging itself that affect the presentation of asthma in the elderly and influence diagnosis and management. Structural changes in the aging lung superimposed on structural changes due to asthma itself can worsen the disease and physiologic function. Changes in the aging immune system influence the cellular composition and function in asthmatic airways. These processes and differences from younger individuals with asthma are not well understood. Phenotypes of asthma in the elderly have not been clearly delineated, but it is likely that age of onset and overlap with chronic obstructive pulmonary disease impact disease characteristics. Physiologic tests and biomarkers used to diagnose and follow asthma in the elderly are generally similar to testing in younger individuals; however, whether they should be modified in aging has not been established. Confounding influences, such as comorbidities (increasing the risk of polypharmacy), impaired cognition and motor skills, psychosocial effects of aging, and age-related adverse effects of medications, impact both diagnosis and treatment of asthma in the elderly. Future efforts to understand asthma in the elderly must include geriatric-specific methodology to diagnose, characterize, monitor, and treat their disease.
Keywords: aging, reactive airways disease, immunosenescence, lung function, phenotype
Contents
Overview
Introduction
Methods
Results
Update on the Epidemiology of asthma in the elderly (AIE)
Update on the Effects of Aging on Lung Structure and Function as it Pertains to Asthma
Update on the Effects of Aging on Immune Function and Airway Inflammation
Current Understanding of Phenotypes of AIE
Physiologic Tests and Biomarkers in the Diagnosis and Management of Elderly Patients with Asthma
Update on Strategies to Manage the Elderly Patient with Asthma
Comorbidities That Influence Disease Severity, Diagnosis, and Management of AIE
Limitations and Future Directions
Conclusions
Overview
There are now more Americans over the age of 65 years than at any other time in United States history, and their numbers increased 15.1% in the past decade (1). In 2014, 14% of the U.S. population was older than 65 years, and in other countries this number was considerably higher (2). The U.S. aging population is expected to increase rapidly over the next decade. Active asthma is common in patients older than 65 years of age and can be severe and disabling, with marked ventilatory impairment (3–5) and negative impact on quality of life (QOL) (6). Asthma in older patients may persist from childhood or may begin in adulthood, even at an advanced age. Across the American lifespan, those who were 65 years and older had the largest increase in the prevalence of current asthma, from 6.0% in 2001 to 8.1% in 2010 (7). Importantly, this age group had the highest rate of asthma deaths and asthma-based physician office visits and the second highest rate of asthma hospitalizations (7, 8).
Asthma in the elderly (AIE) is underdiagnosed, misdiagnosed, and frequently undertreated (4, 9). There is limited information regarding many aspects (e.g., diagnosis, pathophysiology, and treatment) of asthma in this age group. Although there are many clinical and physiologic features of AIE that are similar to asthma in younger individuals, a number of confounding influences, such as comorbidities, impaired cognition and motor skills, and psychosocial effects of aging, complicate its management. A workshop by the National Institute on Aging (NIA) in September 2008 explored the pathophysiology, recognition, and care of AIE and identified a number of knowledge gaps (10). The American Thoracic Society (ATS) sponsored a workshop in May 2015 to discuss discoveries about AIE subsequent to the NIA proceedings to identify future directions to advance knowledge in the field.
Major conclusions from the workshop included the following:
1. Epidemiology of AIE
- •
-
•
Has high rates of morbidity and mortality; the most vulnerable older individuals with asthma are low-income African-American and Hispanic women.
2. Structural changes of the aging lung may worsen physiologic function in asthma
-
•
With aging, there is loss of elastic recoil, increased airway remodeling in the smaller airways, and increased thickness of the central airway wall, which may act synergistically with asthma to worsen airflow obstruction.
3. Immune function and airway inflammation in older patients with asthma
-
•
Aging itself is associated with altered immune response and increased systemic inflammation (“inflamm-aging”).
-
•
Differences in airway inflammation between older and younger patients with asthma are not well characterized.
-
•
Atopy is common in older patients with asthma; however, the role of antigen sensitization and exposure on disease severity is not defined.
4. Phenotypes of AIE
-
•
Understanding of phenotypes of AIE is limited and must address age of asthma onset and overlap with chronic obstructive pulmonary disease (COPD), termed asthma–COPD overlap syndrome (ACOS).
5. Diagnosis of AIE
-
•
There are no specific physiologic tests or biomarkers for the diagnosis and monitoring of AIE.
-
•
Future AIE studies should include global geriatric assessment tools.
6. Treatment of AIE
-
•
AIE treatment is complex due to potential reductions in cognitive and physical function, comorbidities, polypharmacy, and psychosocial challenges associated with aging.
-
•
There is limited knowledge on optimal pharmacological management strategies and response to asthma medication in older adults, largely due to their exclusion from clinical trials.
-
•
Adverse effects of asthma medications are more common in the elderly.
Introduction
Asthma was long considered a childhood disease. Little attention was given to asthma in elderly subjects until the Tucson epidemiologic study of obstructive lung disease (12–14). This longitudinal study reported that asthma is common, often severe, and associated with a high death rate in people older than 65 years of age. Subsequent work has demonstrated that asthma in the elderly (AIE) is generally poorly understood and, therefore, underdiagnosed or misdiagnosed and undertreated (15, 16). An NIA workshop in September 2008 identified multiple gaps in understanding of the disease process of AIE and its management (10). One major question raised was whether the pathophysiology of AIE differs from asthma in younger patients. An ATS Workshop on The Evaluation and Management of Asthma in the Elderly in May 2015 addressed knowledge gained since the 2008 NIA conference. In this meeting, a group of clinicians and basic scientists with expertise on asthma and aging reviewed the current state-of-the-art knowledge and identified future directions for research. This article presents the summary of the workshop findings on AIE (i.e., defined as asthma in those older than age 65 yr).
Methods
Clinicians and researchers from various backgrounds (pulmonary medicine, allergy and immunology, geriatrics, and epidemiology) were selected for this workshop on the basis of their recognized interests and contributions in the field of AIE (Table 1). Key subtopics pertaining to AIE were selected by the Chair and assigned to workshop group members in alignment with their expertise. The Program Officer for Lung Biology from the NIA provided guidance for interfacing with the National Institutes of Health and for developing strategies for multidisciplinary research initiatives. Literature searches were performed with independence regarding search strategies, inclusion/exclusion criteria, and subtopic materials for discussion. Participants presented the current science in their field of expertise relevant to the aging lung and AIE. Discussion followed each presentation, and roundtable interactions helped achieve consensus on current understanding of AIE, limitations in knowledge, and future directions. The findings of the workshop were written by the participants and synthesized in the current report. Workshop participants who had industry relationships (e.g., industry-funded research) were recused from writing, editing, and commenting on related portions of the manuscript.
Table 1.
Methodology
| Methods Checklist | Yes | No |
|---|---|---|
| Panel assembly | ||
| Included experts from relevant clinical and nonclinical fields | x | |
| Included individuals who represented patients and society at large | x | |
| Included methodologist with appropriate expertise (documented expertise in development of conducting systematic reviews to identify the evidence base and development of evidence-based recommendations) | x | |
| Literature review | ||
| Performed in collaboration with a librarian | x | |
| Searched multiple electronic databases | x | |
| Reviewed reference list of retrieved article | x | |
| Evidence synthesis | ||
| Applied preselected inclusion and exclusion criteria | x | |
| Evaluated included articles for sources bias | x | |
| Explicitly summarized benefits and harms | x | |
| Used PRISMA1 to report systematic review | x | |
| Used GRADE to describe quality of evidence | x | |
| Generation of recommendations | x | |
| Used GRADE to rate the strength of recommendations | x | |
Definition of abbreviations: GRADE = Grades of Recommendation Assessment, Development, and Evaluation; PRISMA1 = Preferred Reporting Items for Systematic Reviews and Metaanalyses 1.
Results
Update on the Epidemiology of AIE
Asthma imposes a substantial health burden worldwide, affecting 300 million people and ranking 22nd in disability-adjusted life-years (17). In addition, 250,000 people worldwide die of asthma each year, due in part to limited access to treatment (17). Regions with high rates of asthma include those with increased urbanization and an aging population (17). Persons 65 years and older are especially vulnerable to adverse health outcomes including asthma-related events and reduced quality of life (QOL), given age-related reductions in cognitive and physical function, and increased rates of comorbid conditions (18–23). In addition, older Americans often live in urban environments (24), with higher traffic pollution predicting poorer asthma-related QOL (6).
The United States National Surveillance of Asthma report (7) shows that across the American lifespan, those aged 65 years or older had the: (1) largest increase in the prevalence of current asthma, (2) highest rate of asthma-related deaths, (3) second-highest rate of asthma-based physician office visits and hospitalizations, but unexpectedly (4) lowest rate of a reported asthma attack in the previous year or an emergency department (ED) visit for asthma. The most vulnerable older Americans were women, African Americans, Hispanics, and low-income groups. Although these observations may apply to other countries, similar worldwide epidemiologic investigations are needed for confirmation (17).
Age-related factors may impact the epidemiology of AIE, including the discordant finding of lower rates of asthma attacks and asthma-based ED visits relative to higher rates of asthma deaths, hospitalizations, and physician office visits. Establishing an asthma diagnosis and determining asthma-related health care utilization may be confounded by several factors: (1) concurrent age-related increases in comorbidities (e.g., heart disease, chronic obstructive pulmonary disease [COPD], etc.), (2) multifactorial geriatric health conditions (i.e., cognitive and physical impairments), (3) adverse effects from polypharmacy, and (4) psychosocial factors (i.e., lower level of education, sedentary status, social isolation, and being homebound) (18–21, 25–27). Furthermore, evaluation of asthma-based symptoms may be limited by age-related reductions in awareness of symptoms (e.g., perception of wheezing) (28–30) and by the “paradox of well-being” (31). The latter phenomenon refers to high levels of life satisfaction in older persons, which can coexist with lower health expectations (31), thus increasing the likelihood that symptoms are missed or minimized in severity (i.e., delaying awareness of an asthma attack). This underrecognition of symptoms may lead to decreased utilization of ED services, possibly leading to higher rates of hospitalization and death.
Update on the Effects of Aging on Lung Structure and Function as it Pertains to Asthma
To understand the interaction of asthma and aging on lung structure and function, it is critical to first review the effects of aging in the normal lung. It is implicit that there is biologic heterogeneity of the aging lung, characterized by great interindividual variability in chronologic physiologic change (10). With normal aging, the collagen fiber network that coils around the alveolar ducts changes, producing alveolar duct dilation and homogenous enlargement of alveolar air spaces (32–36). The alveolar air space enlargement that occurs in the “senile” lung differs from emphysema, because there is no associated inflammation or alveolar wall destruction (34). Alveolar enlargement decreases alveolar surface tension and, in turn, decreases elastic recoil pressure. Degenerative changes of the spine contribute to kyphosis and, in combination with increased convexity of the sternum, increase the anteroposterior diameter of the chest (33, 36). Concurrently, chest wall compliance decreases due to the spinal changes and to stiffening of the rib cage and reduced thickness of the parietal muscles (33–35, 37, 38). Respiratory muscle strength deteriorates due to decreased curvature of the diaphragm, sarcopenia (i.e., loss of muscle mass and function), and inadequate nutrition (32–34, 36–39).
Age-related alterations in lung structure impact physiologic function (32–39). The reduction in static elastic recoil pressure decreases expiratory flow (i.e., FEV1). In healthy nonsmokers, there is an ∼30-ml decline in FEV1 yearly starting after age 30 years (32, 35, 38), with an accelerated decline in both FEV1 and FVC between age 65 and 93 years (40). The FEV1/FVC ratio also declines with aging (34), producing a more “obstructive” flow volume loop (39). Residual volume (RV) increases by about 50% between ages 20 and 70 years as a result of early airway closure secondary to reduced lung recoil combined with reduction in chest wall compliance and respiratory muscle strength (34, 35, 38, 39). By age 65 years, closing volume approaches FRC, so that airways close even during tidal breathing (34, 39). This results in ventilation–perfusion mismatch and widening of the alveolar–arterial gradient. FRC also increases with aging, whereas total lung capacity remains relatively unchanged, because the increased RV is counterbalanced by the decreased vital capacity. Diaphragmatic strength in a 76-year-old is about 25% lower than in a 20- to 30-year-old (35), and inspiratory and expiratory respiratory muscle strength steadily declines between ages 65 to older than 85 years in both sexes (39). The decreased airway caliber in the elderly that results from these mechanical factors may enhance the consequences of smooth muscle shortening after inhalation of a bronchoconstrictor stimulus, contributing to increased airway hyperresponsiveness (AHR) (41) with aging.
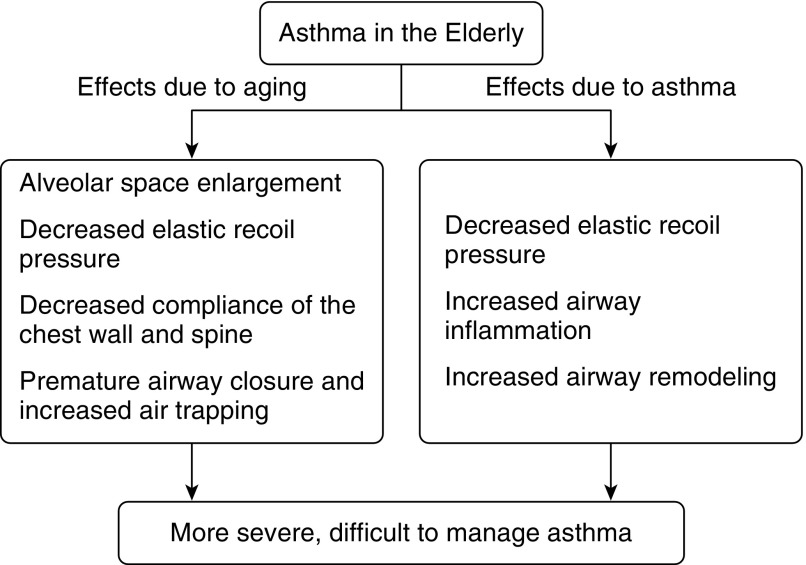
In elderly patients with asthma, pathologic changes of asthma may synergize with those of normal aging to affect lung structure and function (Figure 1). Loss of elastic recoil is reported in chronic asthma in varying age groups (42, 43). This may contribute to airflow reduction independent of airway remodeling, a characteristic feature of asthma in patients of all ages. Studies of small and large airway wall area and thickness in older versus younger individuals with asthma have shown conflicting results by computed tomography (CT) scans and autopsy assessments (44, 45). It is likely that aging alone does not lead to airway remodeling; however, longer duration of asthma may lead to increased airway narrowing due to progressive remodeling and increase in airway smooth muscle volume (45). Thus, factors attributable to asthma alone, combined with those due to normal aging, may account for the accelerated decline in FEV1 in asthma noted longitudinally in some large population studies (46, 47). The impact of age of asthma onset and of disease duration on airway function in the elderly requires further study.
Figure 1.
In the elderly patient with asthma, pathologic changes of asthma may synergize with those of normal aging to affect both lung structure and function. This may potentially lead to more severe and difficult-to-control disease.
Update on the Effects of Aging on Immune Function and Airway Inflammation
With advanced age, there are alterations in both innate and adaptive immune responses termed “immunosenescence” (Table 2). In addition, aging is associated with low-grade, chronic, systemic inflammation, referred to as “inflamm-aging,” characterized by increased IL-1β, IL-6, and tumor necrosis factor–α (48). Effects of immunosenescence and inflamm-aging on airway inflammation and its regulation in older individuals with asthma are not well established. However, there are some studies that have provided some insight.
Table 2.
Features of immunosenescence: potential impact on asthma in the elderly
| Type of Immunity | Cell Type | Observations |
|---|---|---|
| Innate | Eosinophil | ↑ Peripheral eosinophilia and AHR in men (Normative Aging Study) |
| ↓ Degranulation of peripheral eosinophils in older patients with asthma | ||
| Neutrophil | ↑ BALF neutrophils and neutrophil elastase activity with aging in patients without asthma | |
| ↑ Sputum neutrophils in older versus younger patients with asthma | ||
| ↑ Levels of sputum neutrophil mediators in older patients with asthma (e.g., IL-8, MMP-9, neutrophil elastase) | ||
| Adaptive cellular immunity | Tregs | ↓ Peripheral Tregs in older patients with asthma versus aged control subjects |
| T cells | ↑ Proinflammatory cytokines with aging (inflamm-aging) | |
| Adaptive humoral immunity | Humoral immunity | ↓ Antibody response to vaccines |
Definition of abbreviations: AHR = airway hyperresponsiveness; BALF = bronchoalveolar lavage fluid; MMP = matrix metallopeptidase; Tregs = regulatory T cells.
Eosinophils from aged subjects may have some decreased effector functions, but data on their role in AHR are conflicting. The development of AHR later in life was associated with elevated peripheral blood eosinophil counts in men (mean age, 60 yr) enrolled in the Normative Aging Study (49). However, peripheral eosinophils from subjects with asthma (55–80 yr) exhibited decreased degranulation in response to IL-5 stimulation and a trend for decreased superoxide production when compared with cells from patients 20 to 40 years of age (50), whereas there was no difference in eosinophil leukotriene C4 production (51). In a mouse model of asthma, although antigen-sensitized and airway-challenged aged mice developed greater bronchoalveolar fluid eosinophilia than younger mice, AHR was lower in the former, suggesting that increased airway eosinophilia was not correlated with AHR (52). Taken together, these studies suggest that although age-associated changes in eosinophils exist, the clinical implications of these changes and their relationship to asthma phenotypes in older patients remain unclear.
The number of airway neutrophils increases in older individuals without airway disease (53–55), and peripheral neutrophils have increased primary granule release and neutrophil elastase activity, which could lead to increased tissue damage (56). Older compared with younger patients with asthma have increased sputum neutrophils, but the impact of inflamm-aging on this increase has not been addressed (44, 50, 57, 58). Airway neutrophilia in older patients with asthma corresponds to increased levels of sputum neutrophil mediators, including matrix metallopeptidase-9, neutrophil elastase, and IL-8 (58), as well as increased systemic inflammation (e.g., C-reactive protein and IL-6) (59). This resembles changes seen in a severe asthma phenotype noted in some younger adults (60). Aged mouse models of asthma have demonstrated increased expression of airway IL-8 and cytokines associated with Th17 cells (61, 62). This could contribute to increased airway neutrophilia. Determining the type of underlying airway inflammation in older adults with asthma is important, as neutrophilic asthma is often less responsive to corticosteroid treatment (63, 64).
The role of regulatory T cells (Tregs) in younger patients with asthma, though not clearly established, most likely suppresses airway inflammation and AHR (65–67). Peripheral Treg cell numbers are lower in younger patients with asthma than in age-matched healthy subjects (68). Another study reported that older patients with asthma had decreased peripheral Treg cells compared with age-matched normal control subjects (69), but the importance of Treg cells in AIE has not been widely investigated. We are unaware of any studies that directly compare Tregs in older versus younger patients with asthma. However, aged mice fed low-dose antigen before sensitization (to induce oral tolerance and increase Treg expression) and challenge developed suppressed features of asthma, including decreased bronchoalveolar fluid total cell count and eosinophils, cytokine production, and AHR, suggesting that Tregs maintained function with aging (70).
Telomere shortening is believed to be associated with normal aging (71). During replicative senescence, telomere shortening may signal cell cycle arrest or apoptosis (72, 73) or release proinflammatory proteins (74). Shortening of telomere length in peripheral blood mononuclear cells correlated with increased asthma severity in a small group of patients with asthma (aged 25–60 yr) (75). We are not aware of any study that specifically examined telomere shortening in AIE.
Current Understanding of Phenotypes of AIE
Phenotypes, the outward manifestation of an individual’s underlying genetics, have been widely accepted as a way to characterize patients with asthma (76–81) and include clinical, physiologic, inflammatory, and molecular features. Stratification of asthma subtypes by phenotype and endotype (i.e., specific biologic mechanisms) represents the cutting edge for advancing asthma treatment. Despite the significant health burden of AIE, we are aware of only one study exclusively characterizing this population. Park and colleagues (82) evaluated 872 patients older than 65 years of age using cluster analysis to segregate them into groups with common features and to assess the risk of asthma exacerbation in each cluster. A key finding from the study was that the cluster with the longest duration of asthma was more at risk for acute exacerbation than clusters with more recent asthma onset and more normal lung function. Long duration of asthma and smoking (two distinct clusters) were associated with accelerated lung function decline. Although other investigators, including those from the Severe Asthma Research Program (76), have used a similar analytic approach and have identified patients with “late-onset asthma” (LOA), very few of the individuals included exceeded age 65 years. In one study (83), patients with “longstanding asthma” (LSA) reportedly had more atopy than those with “late-onset disease,” but the latter category was not limited to elderly subjects. Others have found that staphylococcal enterotoxin might be a risk factor for LOA that may, in some cases, be nonatopic (32, 84). One “simplistic” approach to phenotyping elderly asthma would be to separately evaluate those with LSA, LOA, and ACOS (85). This may be challenging because of lack of uniform definitions of LSA and LOA as well as uncertainty about the exact definition, prevalence, and overall significance of ACOS (86). Distinguishing patients with ACOS is important, because they experience more frequent exacerbations and poorer QOL (86). AIE displays many of the hallmarks of COPD (87), and patients with LOA are often misdiagnosed with COPD or other diseases, such as congestive heart failure (85). There are no biomarkers that definitively distinguish elderly from younger patients with asthma, although sputum cellularity is generally more neutrophilic in AIE. Thus, current methodologies to phenotype AIE are insufficient. In addition, several cohort studies have demonstrated that over long follow up, it is not uncommon for patients to change from one cluster designation to another (88–91). Investigations dedicated to phenotypic and endotypic characterization of AIE are therefore needed to facilitate diagnosis and treatment.
Physiologic Tests and Biomarkers in the Diagnosis and Management of Elderly Patients with Asthma
Many of the same tests used in younger patients with asthma are useful to characterize AIE (Table 3). Spirometry and airway reactivity measurements are first-line methods for diagnosing and monitoring AIE, as in younger patients, although the nature of the airway disease may differ in the elderly. Many studies show lower FEV1 and more severe airflow limitation in older patients with asthma (44, 92, 93). Bronchoprovocation testing can detect airway reactivity in older as in younger patients (44, 94), and older patients tend to have more peripheral airway closure during bronchoconstriction (measured by the reduction in FVC) than younger patients with asthma (92). When evaluating spirometry in older patients, age-adjusted values are essential, particularly when interpreting the FEV1/FVC ratio, to avoid overdiagnosing respiratory impairment (95). Although predicted values for spirometry are available for the elderly (96), data for nonwhite individuals and for those older than 75 years are sparse. An important limitation of spirometry, and thus also of standard bronchoprovocation tests in the elderly, is that these involve effort-dependent maneuvers. Although studies (97, 98) have shown that 80% or more of older persons can achieve ATS-acceptable spirometry, this may be hard for some frail elderly patients (99–101). The FEV1/FEV6 may be a more easily obtained surrogate for FEV1/FVC in such individuals (102, 103). Bronchoprovocation challenges may be contraindicated in some elderly patients due to low baseline lung function and cardiac comorbidities.
Table 3.
Diagnostic and treatment comparison of elderly and younger patients with asthma
| Test/Characteristic | Elderly | Young |
|---|---|---|
| Spirometry | May be less useful in frail patients; reference standards not widely available | Generally useful tool to assess asthma severity |
| Bronchodilator responsiveness | May be less pronounced | Variable but generally greater |
| eNO | May be useful | May be useful |
| Methacholine challenge | Less often used because of more frequent contraindications (e.g., cardiovascular disease) | Useful; overall fewer contraindications |
| Atopy | Less common | Common |
| Comorbidities | COPD, heart disease more common | Allergic rhinitis more common |
| Phenotypes | Limited knowledge, but late-onset asthma, long-standing asthma, and ACOS described | Multiple phenotypes described |
| Sputum cellularity | Generally more neutrophilic | Generally more eosinophilic |
| Therapy | No age-specific guidelines | Guideline-specific regimens in place that address the needs of most patients |
| Optimal regimen unknown | ||
| More susceptible to adverse effects due to comorbidities, drug-to-drug interactions, and polypharmacy | ||
| Inability to use certain inhalers due to lack of dexterity and reduced inspiratory flow |
Definition of abbreviations: ACOS = asthma–COPD overlap syndrome; COPD = chronic obstructive pulmonary disease; eNO = exhaled nitric oxide.
Forced oscillation may be useful to follow older patients with asthma, as it is provides an effort-independent evaluation of respiratory system mechanics. Forced oscillation measures changes in lung impedance, and data obtained at different frequencies of applied pressure provide insight into abnormalities in different lung regions. Inoue and colleagues found that elderly patients with asthma had significantly greater resistance of the respiratory system at 5 Hz, significantly greater frequency dependence of resistance (resistance at 5–20 Hz), and more negative reactance at 5 Hz than younger patients with asthma (44). These observations suggest an abnormality particularly in the lung periphery, perhaps reflective of increased heterogeneity or enhanced airway closure during quiet breathing in elderly patients with asthma.
Imaging may have utility in evaluating AIE. Chest CT is a noninvasive modality used to assess lung structure, and measurements at different lung volumes can be used to make inferences about lung function. Chest CT in elderly patients with asthma shows increased wall thickness and increased air trapping compared with younger patients with asthma (44). Xenon ventilation CT in the elderly shows that dyspnea severity correlates with xenon gas–measured air trapping, and decreases in gas trapping correlate with improvements in FEV1. However, these techniques are limited by the complexity of the measurements and the need for specialized imaging and analysis algorithms not readily available in clinical practice. The expense and risks of radiation exposure also obviate CT use.
Exhaled nitric oxide (eNO) is a biomarker in younger patients with asthma. Bozek and colleagues found slightly higher eNO levels in very elderly patients with asthma (≥80 yr) than in a younger cohort of patients with asthma (18–30 yr) (104). Other studies report similar levels in older and younger patients with asthma (44). Porsbjerg and colleagues found that eNO levels correlated with airway reactivity in the elderly (105); this has been demonstrated in younger individuals as well (106, 107). Overall, these studies suggest that eNO likely has similar diagnostic and monitoring capabilities in both older and younger patients with asthma.
Update on Strategies to Manage the Elderly Patient with Asthma
Current evidence-based guidelines for asthma (86, 108) are derived from studies on younger individuals, as older patients are frequently excluded from clinical trials (109). Only a few reports have addressed the management of the elderly population with asthma (10, 110). It is widely accepted that asthma is often undertreated in older patients (111–113), likely due to multiple factors (Table 3), including incomplete understanding of pathophysiology and most appropriate age-related therapy, decreased asthma self-management with aging, misdiagnosis of AIE, poor access to health care, comorbidities, medication costs, fear of corticosteroid use, and poor medication delivery technique (114–117). The appropriate management of any chronic disease in the elderly, including asthma, should include multidimensional assessment (MDA) of physical, psychological, cognitive, and social factors that may impact successful treatment.
Nonpharmacological management strategies
Optimal asthma management for all patients incorporates avoidance of known asthma triggers. Elderly patients may have allergy-triggered asthma, although the role of allergen sensitization in the population is less well defined than in younger patients. Detecting and managing comorbidities that may exacerbate asthma are essential to optimize asthma control. Although influenza vaccination is recommended for all patients with asthma (118), it is underutilized in the elderly patient with asthma (119). Furthermore, the immune response to the vaccine may wane with aging, especially in patients on high-dose inhaled corticosteroids (120–122). The long-term benefit of pulmonary rehabilitation in elderly asthma, although described in few published reports, is unknown (123–126).
Asthma education is important in managing asthma but may be more difficult in older populations due to physical and cognitive function decline. Unfortunately, asthma education in elderly patients is poorly implemented (127). An MDA to asthma care in older patients has been shown to identify additional significant clinical issues compared with a diagnosis-centered approach (128). The MDA should include: (1) standardized evaluation of comorbidities and screening for frailty and psychosocial impediments to care using geriatric-specific tools, (2) assessment of barriers to adherence to inhaled therapy, (3) individualization of treatment incorporating age- and disease-specific factors, and (4) multiple points of care and assessment including social workers, pharmacists, nurses, certified asthma educators, and physicians.
Pharmacological management strategies
Current pharmacological management of AIE is based on guidelines developed for younger patients with asthma with Th2-high or eosinophilic airway inflammation. Studies have shown that Th2 immune deviation may not be generalizable to all patients with asthma, particularly the elderly (58, 63, 129). Limited inclusion of older patients in clinical trials and alterations in medication pharmacodynamics and pharmacokinetics associated with aging impedes conclusions on optimal asthma management in this population. Traditional lung function measurements to evaluate asthma medications in clinical trials may be less useful in the elderly due to increased prevalence of irreversible obstruction. Outcomes that are symptom-driven may be more effective (130, 131). Furthermore, careful monitoring of inhaler technique (due to decreased muscle strength and loss of coordination), medication adherence, and potential adverse reactions to asthma medications are essential (130, 132–134).
Rescue medications
Although β2-receptor responsiveness and affinity may decline with age, this has not been consistently established (135, 136). However, elderly patients may be more sensitive to adverse effects of β2-agonists, particularly those with unstable cardiovascular disorders (137, 138). Animal models suggest that parasympathetic activity decreases with aging due to reduced receptor numbers or postreceptor coupling, but relatively little is known about the effect of aging on anticholinergic responses in humans. Short-acting anticholinergic medications may be useful bronchodilators in the elderly and do not have the cardiac side effects of β2-agonists. Although more likely with oral agents, cognitive impairment, falls, symptomatic urinary outlet obstruction, and closed-angle glaucoma are potential risks of inhaled anticholinergics and should be used with caution (139).
Controller therapies
Inhaled corticosteroids (ICS) constitute the cornerstone of chronic asthma management, yet they are underutilized in elderly patients (112, 140, 141). ICS use reduces hospital admissions and mortality in this population (142). However, corticosteroids may be less effective in older patients with asthma with predominantly neutrophilic inflammation; this needs further study. Older patients receiving higher-dose ICS should be monitored closely for potential decreased bone mineral density, increased fracture risk, and cataracts (143, 144). The safety of long-acting β-agonist use in older patients, particular those with underlying cardiovascular disease, has been most studied in COPD, and results are inconsistent. Some studies suggest that when used, especially as monotherapy, there is increased risk of cardiovascular events (145, 146), whereas other studies have not shown this (147, 148). Studies specifically focused on the safety of this class of drugs in elderly patients with asthma are lacking; however, the black box warning for use of long-acting β-agonist as monotherapy applies to all patients with asthma. Long-acting muscarinic antagonists have been shown to be efficacious as add-on therapy in patients with asthma up to age 75 years old (149) and may be especially helpful in patients with concomitant COPD (ACOS).
A few studies have reported that leukotriene modifying agents may benefit AIE (131, 150). Although significant improvement in asthma indices was observed in older age groups, it was less pronounced than in younger patients with asthma (131) and in older patients treated with ICS (150). Some older patients with asthma have demonstrated clinical improvement after treatment with anti-IgE therapy (151, 152). Specific immunotherapy has also been effective in older patients and should be considered, but risks and benefits must be weighed carefully (153, 154).
Comorbidities That Influence Disease Severity, Diagnosis, and Management of AIE
Comorbidities in asthma worsen disease severity and QOL in all individuals (155), but more likely to a greater degree in older patients (156, 157). Concomitant medical conditions are more common in older patients (158). Some comorbidities of older patients with asthma that typically differ from those of younger patients include atrial fibrillation (159), congestive heart failure (157), and COPD (15). Distinguishing AIE from COPD (15) in older adults is difficult, and some older patients may have components of both diseases, or ACOS (160). The prevalence of bronchiectasis increases with aging (161), and coexistence with asthma is associated with more severe asthma (162), more hospitalizations, and increased risk of chronic respiratory failure (163). As in younger patients with asthma, chronic rhinosinusitis in people older than 65 years is strongly associated with the diagnosis of asthma (84, 164, 165), in particular when associated with IgE sensitization to staphylococcal enterotoxin (84). Obesity, often prevalent in the elderly, is associated with poor asthma control and asthma exacerbations (166). Gastroesophageal reflux disease increases with age (167), likely due to age-associated reductions in lower esophageal sphincter pressure, and this may contribute to asthma exacerbations (168). Cognitive impairment and change of mood (depression and/or anxiety) are also common in geriatric patients, decrease QOL (169), and negatively influence adherence to asthma treatment (169, 170). Most importantly, they have been shown to be independent correlates of mortality in AIE (137). Sleep disorders are more prevalent in elderly patients with asthma than in age-matched control subjects (171) and in young patients with asthma (172) and have been associated with low QOL (171, 172).
Comorbidities may interact with asthma therapy by modifying the pharmacokinetics and pharmacodynamics of asthma medications. Renal or hepatic diseases, for example, can impair the absorption, distribution, metabolism, and excretion of drugs, thus increasing risk of side effects (173). Even patients with normal serum creatinine can have reduced glomerular filtration rates, so that the risk of side effects related to renal dysfunction should not be underestimated (174). Furthermore, some medications used to treat comorbidities may worsen asthma (e.g., β-blockers, aspirin, nonsteroidal antiinflammatory drugs, cholinergic agents, etc.). Comorbidities are also invariably associated with polypharmacotherapy, an important risk factor for adverse drug reactions in the elderly. Aging, per se, is also responsible for pharmacokinetic changes (175), which should be accounted for when developing a pharmacotherapy plan.
Limitations and Future Directions
Advances in knowledge about AIE have been hindered by multiple factors (Table 4). Diagnosing and phenotyping AIE is confounded by the effects of normal age-related changes in the lung, by underreporting of symptoms, and by comorbidities. In addition, absence of a precise definition for AIE, lack of appropriate models to study the disease, and failure to include older adults in clinical trials contribute to gaps in understanding. Although there has been some progress since the NIA proceedings, major research questions remain unanswered. Recommendations for future directions were established by consensus of the current workshop group.
Table 4.
Progress of understanding of asthma in the elderly: limitations and future directions
| Topic | Limitation | Advances Since NIA Workshop | ATS Workshop Recommendations for Future Directions |
|---|---|---|---|
| Epidemiology | Absence of age-specific asthma guidelines for older patients resulting in under- and misdiagnosis | Not accomplished | Develop geriatric-specific guidelines and geriatric- specific survey instruments for the diagnosis of asthma. Develop a more precise definition of AIE. |
| Distinguishing health care utilization records to identify care of asthma vs. other comorbid conditions | Develop a survey instrument to better capture health care utilization for AIE. | ||
| Effect of aging and asthma on lung structure and function | Incomplete understanding of structural and functional changes in aging plus asthma vs. aging or asthma alone | Studies using HRCT scans of the lung and forced oscillation testing | Define normal structural and functional age- related changes in the lung (from the cellular to whole-organ level). |
| Establish interval imaging of cohorts of aging individuals to better understand structural changes in the lung. | |||
| Effect of aging and AIE on immune function | Confounding effects of changes in immune function and inflammation with aging (i.e., inflamm-aging). | Additional data on neutrophilic inflammation in AIE | Investigate systemic and lung-specific inflammatory changes in normal aging and in aging plus asthma. |
| Lack of understanding of potential differences in airway inflammation between older and younger patients with asthma | Define the roles of allergen sensitization and exposure in onset, progression, and exacerbation of AIE. | ||
| Unclear role of allergen sensitization and exposure in older patients with asthma | |||
| Appropriate models for studying AIE | Difficulty obtaining lung tissue and BALF from older patients | Some new animal model data on neutrophilic inflammation in AIE | Develop novel allergic and nonallergic models of asthma, reflecting altered immune cell and cytokine milieu of the aging lung. |
| Translation of aged animal studies to human studies. | Establish biobanks of lung tissue, sputum, blood, and other samples from older subjects with and without asthma. | ||
| Asthma phenotypes | Lack of uniform definitions and incomplete characterization of longstanding versus late-onset AIE | Generally not accomplished. Staphylococcal enterotoxin has been identified as a potential risk factor for late-onset asthma. | Design studies in older patients with asthma of different ages of onset, incorporating clinical/historical (e.g., tobacco use), physiology, imaging, indices of airway inflammation, molecular biomarkers, and response to treatment in order to characterize phenotypes and endotypes in this population. |
| Incomplete understanding of the ACOS | Although ACOS is not completely understood, the term was first proposed after the NIA workshop. | Reach consensus definition on late-onset asthma. | |
| Physiologic tests and biomarkers in AIE | Confounding effects of aging on physiologic testing on AIE (i.e., comorbidities, decreased respiratory muscle strength, cognitive impairment) | Useful references standards for individuals up to age 95 yr were published in 2012, after the NIA workshop. However, data for nonwhite individuals and for individuals >75 yr of age are still lacking. | Establish age-appropriate reference standards for better interpretation of lung function in the elderly, particularly for nonwhite individuals and those of extreme age. |
| Develop more age-appropriate (i.e., effort independent) physiologic tests to assess AIE. | |||
| Investigate the role of currently available biomarkers in older patients and identify novel biomarkers to increase insight into AIE. | |||
| Management strategies for AIE | Complexity of management of AIE due to comorbidities, polypharmacy, medication adverse effects, and psychosocial factors | Not accomplished despite increased awareness of the complexity of AIE | Develop specific guidelines for managing AIE, incorporating a multidisciplinary approach. |
| Lack of inclusion of older patients in clinical trials for new asthma therapies | Enroll elderly patients in clinical trials to better understand age-specific treatment response and safety factors. | ||
| Potential age-related altered response to existing asthma therapies (e.g., ICS) | Explore novel routes of medication delivery (e.g., inhaled delivery devices not dependent on technique or inspiratory flow, transdermal, etc.) | ||
| Incorporation of geriatric care into asthma care | Common geriatric health issues (e.g., impaired cognition, reduced strength including reduced lung function, and frailty) may limit use of certain therapies. | Not accomplished, although emphasized more specifically in the current report than in the NIA proceedings | Develop studies incorporating comorbidities in asthma treatment to assess the effect of multifactorial geriatric health conditions on the clinical trajectory of asthma in older persons. |
| Explore role of pulmonary rehabilitation in AIE, as patients commonly suffer from physical dysfunction. |
Definition of abbreviations: ACOS = asthma–COPD overlap syndrome; AIE = asthma in the elderly; ATS = American Thoracic Society; BALF = bronchoalveolar lavage fluid; HRCT = high-resolution computed tomography; ICS = inhaled corticosteroids; NIA = National Institute on Aging.
Conclusions
In May 2015, the ATS sponsored a workshop to evaluate and report on discoveries about AIE since the NIA workshop and to identify future directions to advance knowledge in this field. AIE, a complex entity with poorly understood phenotypic heterogeneity (including inflammatory profiles), is impacted by comorbidities of advancing age, such as heart disease, concomitant lung disease (e.g., COPD), cognitive impairment, and depression, and also by natural changes of the aging respiratory and immune systems. Although asthma in persons aged 65 years and older has had the largest increase in prevalence and the highest mortality of any age group, geriatric-specific guidelines are not available for diagnosis and treatment of AIE. Physiologic tests for elderly patients with asthma are the same as those used in younger individuals, and age-appropriate inflammatory biomarkers have not been identified. As the population is aging, AIE will present a greater future management issue. Therefore, it is imperative that research efforts focus on characterization of AIE to enhance diagnostic and treatment strategies for this vulnerable population.
Acknowledgments
This official workshop report was prepared by an ad hoc subcommittee of the Assembly on Allergy, Immunology and Inflammation.
Members of the subcommittee are as follows:
Gwen S. Skloot, M.D. (Chair)
Paula J. Busse, M.D.
Sidney S. Braman, M.D.
Elizabeth J. Kovacs, Ph.D.
Anne E. Dixon, B.M., B.Ch.
Carlos A. Vaz Fragoso, M.D.
Nicola Scichilone, M.D., Ph.D.
Y. S. Prakash, M.D., Ph.D.
Christina M. Pabelick, M.D.
Sameer K. Mathur, M.D., Ph.D.
Nicola A. Hanania, M.D., M.S.
Wendy C. Moore, M.D.
Peter G. Gibson, M.B. B.S.
Susan Zieman, M.D., Ph.D.
Betina B. Ragless
Acknowledgment
The authors thank Ms. Joan Adler for participating in the workshop conference and contributing from the patient’s perspective. They also thank Ms. Janette Birmingham, who assembled the references for the manuscript and assisted with formatting of tables.
Footnotes
Supported by the American Thoracic Society and National Institutes of Health grant R01 AG018859 (E.J.K.).
The views expressed in this article do not communicate an official position of the Department of Veteran Affairs, National Institute on Aging, or the American Lung Association.
Author Contributions: Each author contributed to the concepts presented through their participation in the workshop, revised the draft, approved the final version of the report, and agreed to be accountable for the information presented.
Author Disclosures: S.S.B. was a speaker and an advisory committee member for AstraZeneca, consultant to Boehringer Ingelheim, GlaxoSmithKline, and Sunovion, and a speaker for Teva Pharmaceuticals. A.E.D. was on a data and safety monitoring board of Genentech and received research support from Pfizer. S.K.M. was on a data and safety monitoring board of Teva. P.G.G. was a speaker for AstraZeneca, GlaxoSmithKline, and Novartis. G.S.S., P.J.B., E.J.K., C.A.V.F., N.S., Y.S.P., C.M.P., N.A.H., W.C.M., S.Z., B.B.R. reported no relationships with relevant commercial interests.
References
- 1.Vincent GK, Velkoff VAU.S. Census BureauThe next four decades: the older population in the United States: 2010 to 2050. Washington, D.C.: U.S. Deptartment of Commerce, Economics, and Statistics Administration, U.S. Census Bureau; 2010 [Google Scholar]
- 2.United Nations Department of Economic and Social Affairs Population DivisionWorld Population Prospects: The 2012 Revision, key findings and advance tables. Working paper no. ESA/P/WP.227. New York, NY: United Nations; 2013
- 3.Braman SS, Hanania NA. Asthma in older adults. Clin Chest Med. 2007;28:685–702, v. doi: 10.1016/j.ccm.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Mathur SK. Allergy and asthma in the elderly. Semin Respir Crit Care Med. 2010;31:587–595. doi: 10.1055/s-0030-1265899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milgrom H, Huang H. Allergic disorders at a venerable age: a mini-review. Gerontology. 2014;60:99–107. doi: 10.1159/000355307. [DOI] [PubMed] [Google Scholar]
- 6.Kannan JA, Bernstein DI, Bernstein CK, Ryan PH, Bernstein JA, Villareal MS, Smith AM, Lenz PH, Epstein TG. Significant predictors of poor quality of life in older asthmatics. Ann Allergy Asthma Immunol. 2015;115:198–204. doi: 10.1016/j.anai.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moorman JE, Akinbami LJ, Bailey CM, Zahran HS, King ME, Johnson CA, Liu X. National surveillance of asthma: United States, 2001-2010. Vital Health Stat 3. 2012;35:1–58. [PubMed] [Google Scholar]
- 8.Tsai CL, Lee WY, Hanania NA, Camargo CA., Jr Age-related differences in clinical outcomes for acute asthma in the United States, 2006-2008. J Allergy Clin Immunol. 2012;129:1252–1258.e1. doi: 10.1016/j.jaci.2012.01.061. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Garcia M, Caballero A, Jaramillo C, Maldonado D, Torres-Duque CA. Prevalence, risk factors and underdiagnosis of asthma and wheezing in adults 40 years and older: a population-based study. J Asthma. 2015;52:823–830. doi: 10.3109/02770903.2015.1010733. [DOI] [PubMed] [Google Scholar]
- 10.Hanania NA, King MJ, Braman SS, Saltoun C, Wise RA, Enright P, Falsey AR, Mathur SK, Ramsdell JW, Rogers L, et al. Asthma in Elderly workshop participants. Asthma in the elderly: current understanding and future research needs--a report of a National Institute on Aging (NIA) workshop. J Allergy Clin Immunol. 2011;128:S4–S24. doi: 10.1016/j.jaci.2011.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akgün KM, Crothers K, Pisani M. Epidemiology and management of common pulmonary diseases in older persons. J Gerontol A Biol Sci Med Sci. 2012;67:276–291. doi: 10.1093/gerona/glr251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodge RR, Burrows B. The prevalence and incidence of asthma and asthma-like symptoms in a general population sample. Am Rev Respir Dis. 1980;122:567–575. doi: 10.1164/arrd.1980.122.4.567. [DOI] [PubMed] [Google Scholar]
- 13.Burrows B, Barbee RA, Cline MG, Knudson RJ, Lebowitz MD. Characteristics of asthma among elderly adults in a sample of the general population. Chest. 1991;100:935–942. doi: 10.1378/chest.100.4.935. [DOI] [PubMed] [Google Scholar]
- 14.Burrows B, Lebowitz MD, Barbee RA, Cline MG. Findings before diagnoses of asthma among the elderly in a longitudinal study of a general population sample. J Allergy Clin Immunol. 1991;88:870–877. doi: 10.1016/0091-6749(91)90243-h. [DOI] [PubMed] [Google Scholar]
- 15.Bellia V, Battaglia S, Catalano F, Scichilone N, Incalzi RA, Imperiale C, Rengo F. Aging and disability affect misdiagnosis of COPD in elderly asthmatics: the SARA study. Chest. 2003;123:1066–1072. doi: 10.1378/chest.123.4.1066. [DOI] [PubMed] [Google Scholar]
- 16.Gibson PG, McDonald VM, Marks GB. Asthma in older adults. Lancet. 2010;376:803–813. doi: 10.1016/S0140-6736(10)61087-2. [DOI] [PubMed] [Google Scholar]
- 17.Bousquet J, Khaltaev N. Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. Geneva, Switzerland: World Health Organization; 2007. [Google Scholar]
- 18.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 19.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fried TR, Vaz Fragoso CA, Rabow MW. Caring for the older person with chronic obstructive pulmonary disease. JAMA. 2012;308:1254–1263. doi: 10.1001/jama.2012.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat 10. 2014;260:1–161. [PubMed] [Google Scholar]
- 23.Jia H, Lubetkin EI. Impact of nine chronic conditions for US adults aged 65 years and older: an application of a hybrid estimator of quality-adjusted life years throughout remainder of lifetime. Qual Life Res. 2016;25:1921–1929. doi: 10.1007/s11136-016-1226-5. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg S (for the Administration on Aging, U.S. Department of Health and Human Services). A profile of older Americans: 2010 [accessed 2015 Sep]. Available from: http://www.aoa.acl.gov/
- 25.Ornstein KA, Leff B, Covinsky KE, Ritchie CS, Federman AD, Roberts L, Kelley AS, Siu AL, Szanton SL. Epidemiology of the homebound population in the United States. JAMA Intern Med. 2015;175:1180–1186. doi: 10.1001/jamainternmed.2015.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cigolle CT, Langa KM, Kabeto MU, Tian Z, Blaum CS. Geriatric conditions and disability: the Health and Retirement Study. Ann Intern Med. 2007;147:156–164. doi: 10.7326/0003-4819-147-3-200708070-00004. [DOI] [PubMed] [Google Scholar]
- 27.Brault MW. Americans with disabilities (2010) Current Population Reports. U.S. Department of Commerce Economics and Statistics Administration, U.S. Census Bureau. 2012;(July 2012 Issue):70–131. [Google Scholar]
- 28.Joo JH, Lim GI, Seo MJ, Park SJ, Lee JH, Uh ST, Kim YH, Park CS. Perception of wheezing in the elderly asthmatics. Korean J Intern Med. 2001;16:260–264. doi: 10.3904/kjim.2001.16.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen SC, Khattab A. The airflow resistance sensing threshold during tidal breathing rises in old age in patients with asthma. Age Ageing. 2012;41:557–560. doi: 10.1093/ageing/afs041. [DOI] [PubMed] [Google Scholar]
- 30.Connolly MJ, Crowley JJ, Charan NB, Nielson CP, Vestal RE. Reduced subjective awareness of bronchoconstriction provoked by methacholine in elderly asthmatic and normal subjects as measured on a simple awareness scale. Thorax. 1992;47:410–413. doi: 10.1136/thx.47.6.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy BR. Mind matters: cognitive and physical effects of aging self-stereotypes. J Gerontol B Psychol Sci Soc Sci. 2003;58:203–211. doi: 10.1093/geronb/58.4.p203. [DOI] [PubMed] [Google Scholar]
- 32.Yáñez A, Cho SH, Soriano JB, Rosenwasser LJ, Rodrigo GJ, Rabe KF, Peters S, Niimi A, Ledford DK, Katial R, et al. Asthma in the elderly: what we know and what we have yet to know. World Allergy Organ J. 2014;7:8. doi: 10.1186/1939-4551-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hochhegger B, Meirelles GS, Irion K, Zanetti G, Garcia E, Moreira J, Marchiori E. The chest and aging: radiological findings. J Bras Pneumol. 2012;38:656–665. doi: 10.1590/s1806-37132012000500016. [DOI] [PubMed] [Google Scholar]
- 34.Vaz Fragoso CA, Gill TM. Respiratory impairment and the aging lung: a novel paradigm for assessing pulmonary function. J Gerontol A Biol Sci Med Sci. 2012;67:264–275. doi: 10.1093/gerona/glr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller MR. Structural and physiological age-associated changes in aging lungs. Semin Respir Crit Care Med. 2010;31:521–527. doi: 10.1055/s-0030-1265893. [DOI] [PubMed] [Google Scholar]
- 36.Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Respir J. 1999;13:197–205. doi: 10.1034/j.1399-3003.1999.13a36.x. [DOI] [PubMed] [Google Scholar]
- 37.Lowery EM, Brubaker AL, Kuhlmann E, Kovacs EJ. The aging lung. Clin Interv Aging. 2013;8:1489–1496. doi: 10.2147/CIA.S51152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma G, Goodwin J. Effect of aging on respiratory system physiology and immunology. Clin Interv Aging. 2006;1:253–260. doi: 10.2147/ciia.2006.1.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janssens JP. Aging of the respiratory system: impact on pulmonary function tests and adaptation to exertion. Clin Chest Med. 2005;26:469–484, vi–vii. doi: 10.1016/j.ccm.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Sorino C, Battaglia S, Scichilone N, Pedone C, Antonelli-Incalzi R, Sherrill D, Bellia V. Diagnosis of airway obstruction in the elderly: contribution of the SARA study. Int J Chron Obstruct Pulmon Dis. 2012;7:389–395. doi: 10.2147/COPD.S31630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scichilone N, Messina M, Battaglia S, Catalano F, Bellia V. Airway hyperresponsiveness in the elderly: prevalence and clinical implications. Eur Respir J. 2005;25:364–375. doi: 10.1183/09031936.05.00080204. [DOI] [PubMed] [Google Scholar]
- 42.Gelb AF, Yamamoto A, Verbeken EK, Nadel JA. Unraveling the pathophysiology of the asthma-COPD overlap syndrome: unsuspected mild centrilobular emphysema is responsible for loss of lung elastic recoil in never smokers with asthma with persistent expiratory airflow limitation. Chest. 2015;148:313–320. doi: 10.1378/chest.14-2483. [DOI] [PubMed] [Google Scholar]
- 43.Gelb AF, Licuanan J, Shinar CM, Zamel N. Unsuspected loss of lung elastic recoil in chronic persistent asthma. Chest. 2002;121:715–721. doi: 10.1378/chest.121.3.715. [DOI] [PubMed] [Google Scholar]
- 44.Inoue H, Niimi A, Takeda T, Matsumoto H, Ito I, Matsuoka H, Jinnai M, Otsuka K, Oguma T, Nakaji H, et al. Pathophysiological characteristics of asthma in the elderly: a comprehensive study. Ann Allergy Asthma Immunol. 2014;113:527–533. doi: 10.1016/j.anai.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Senhorini A, Ferreira DS, Shiang C, Silva LF, Dolhnikoff M, Gelb AF, Mauad T. Airway dimensions in fatal asthma and fatal COPD: overlap in older patients. COPD. 2013;10:348–356. doi: 10.3109/15412555.2012.752806. [DOI] [PubMed] [Google Scholar]
- 46.Bai TR, Cooper J, Koelmeyer T, Paré PD, Weir TD. The effect of age and duration of disease on airway structure in fatal asthma. Am J Respir Crit Care Med. 2000;162:663–669. doi: 10.1164/ajrccm.162.2.9907151. [DOI] [PubMed] [Google Scholar]
- 47.James AL, Palmer LJ, Kicic E, Maxwell PS, Lagan SE, Ryan GF, Musk AW. Decline in lung function in the Busselton Health Study: the effects of asthma and cigarette smoking. Am J Respir Crit Care Med. 2005;171:109–114. doi: 10.1164/rccm.200402-230OC. [DOI] [PubMed] [Google Scholar]
- 48.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69:S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 49.Annema JT, Sparrow D, O’Connor GT, Rijcken B, Koëter GH, Postma DS, Weiss ST. Chronic respiratory symptoms and airway responsiveness to methacholine are associated with eosinophilia in older men: the Normative Aging Study. Eur Respir J. 1995;8:62–69. doi: 10.1183/09031936.95.08010062. [DOI] [PubMed] [Google Scholar]
- 50.Mathur SK, Schwantes EA, Jarjour NN, Busse WW. Age-related changes in eosinophil function in human subjects. Chest. 2008;133:412–419. doi: 10.1378/chest.07-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nyenhuis SM, Schwantes EA, Mathur SK. Characterization of leukotrienes in a pilot study of older asthma subjects. Immun Ageing. 2010;7:8. doi: 10.1186/1742-4933-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Busse PJ, Zhang TF, Srivastava K, Schofield B, Li XM. Effect of ageing on pulmonary inflammation, airway hyperresponsiveness and T and B cell responses in antigen-sensitized and -challenged mice. Clin Exp Allergy. 2007;37:1392–1403. doi: 10.1111/j.1365-2222.2007.02775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meyer KC, Rosenthal NS, Soergel P, Peterson K. Neutrophils and low-grade inflammation in the seemingly normal aging human lung. Mech Ageing Dev. 1998;104:169–181. doi: 10.1016/s0047-6374(98)00065-7. [DOI] [PubMed] [Google Scholar]
- 54.Meyer KC, Soergel P. Variation of bronchoalveolar lymphocyte phenotypes with age in the physiologically normal human lung. Thorax. 1999;54:697–700. doi: 10.1136/thx.54.8.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pignatti P, Ragnoli B, Radaeli A, Moscato G, Malerba M. Age-related increase of airway neutrophils in older healthy nonsmoking subjects. Rejuvenation Res. 2011;14:365–370. doi: 10.1089/rej.2010.1150. [DOI] [PubMed] [Google Scholar]
- 56.Sapey E, Greenwood H, Walton G, Mann E, Love A, Aaronson N, Insall RH, Stockley RA, Lord JM. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood. 2014;123:239–248. doi: 10.1182/blood-2013-08-519520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brooks CR, Gibson PG, Douwes J, Van Dalen CJ, Simpson JL. Relationship between airway neutrophilia and ageing in asthmatics and non-asthmatics. Respirology. 2013;18:857–865. doi: 10.1111/resp.12079. [DOI] [PubMed] [Google Scholar]
- 58.Nyenhuis SM, Schwantes EA, Evans MD, Mathur SK. Airway neutrophil inflammatory phenotype in older subjects with asthma. J Allergy Clin Immunol. 2010;125:1163–1165. doi: 10.1016/j.jaci.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wood LG, Baines KJ, Fu J, Scott HA, Gibson PG. The neutrophilic inflammatory phenotype is associated with systemic inflammation in asthma. Chest. 2012;142:86–93. doi: 10.1378/chest.11-1838. [DOI] [PubMed] [Google Scholar]
- 60.Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, Wenzel SE, Peters SP, Meyers DA, Bleecker ER. National Heart, Lung, and Blood Institute’s Sever Asthma Research Program. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2014;133:1557–1563. doi: 10.1016/j.jaci.2013.10.011. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Birmingham JM, Gillespie VL, Srivastava K, Li XM, Busse PJ. Influenza A infection enhances antigen-induced airway inflammation and hyperresponsiveness in young but not aged mice. Clin Exp Allergy. 2014;44:1188–1199. doi: 10.1111/cea.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brandenberger C, Li N, Jackson-Humbles DN, Rockwell CE, Wagner JG, Harkema JR. Enhanced allergic airway disease in old mice is associated with a Th17 response. Clin Exp Allergy. 2014;44:1282–1292. doi: 10.1111/cea.12388. [DOI] [PubMed] [Google Scholar]
- 63.Nair P, Aziz-Ur-Rehman A, Radford K. Therapeutic implications of ‘neutrophilic asthma’. Curr Opin Pulm Med. 2015;21:33–38. doi: 10.1097/MCP.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 64.Kane B, Fowler SJ, Niven R. Refractory asthma: beyond step 5, the role of new and emerging adjuvant therapies. Chron Respir Dis. 2015;12:69–77. doi: 10.1177/1479972314562210. [DOI] [PubMed] [Google Scholar]
- 65.Hartl D, Koller B, Mehlhorn AT, Reinhardt D, Nicolai T, Schendel DJ, Griese M, Krauss-Etschmann S. Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J Allergy Clin Immunol. 2007;119:1258–1266. doi: 10.1016/j.jaci.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 66.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. 2005;202:1539–1547. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kearley J, Robinson DS, Lloyd CM. CD4+CD25+ regulatory T cells reverse established allergic airway inflammation and prevent airway remodeling. J Allergy Clin Immunol. 2008;122:617–624. doi: 10.1016/j.jaci.2008.05.048. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, Carr VA, Robinson DS. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet. 2004;363:608–615. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 69.Vale-Pereira S, Todo-Bom A, Geraldes L, Schmidt-Weber C, Akdis CA, Mota-Pinto A. FoxP3, GATA-3 and T-bet expression in elderly asthma. Clin Exp Allergy. 2011;41:490–496. doi: 10.1111/j.1365-2222.2010.03640.x. [DOI] [PubMed] [Google Scholar]
- 70.Birmingham JM, Patil S, Li XM, Busse PJ. The effect of oral tolerance on the allergic airway response in younger and aged mice. J Asthma. 2013;50:122–132. doi: 10.3109/02770903.2012.753455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murray MA, Chotirmall SH. The impact of immunosenescence on pulmonary disease. Mediators Inflamm 2015. 2015. p. 692546. [DOI] [PMC free article] [PubMed]
- 72.d’Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP, Jackson SP. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–198. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 73.Gilson E, Géli V. How telomeres are replicated. Nat Rev Mol Cell Biol. 2007;8:825–838. doi: 10.1038/nrm2259. [DOI] [PubMed] [Google Scholar]
- 74.Passos JF, Simillion C, Hallinan J, Wipat A, von Zglinicki T. Cellular senescence: unravelling complexity. Age (Dordr) 2009;31:353–363. doi: 10.1007/s11357-009-9108-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kyoh S, Venkatesan N, Poon AH, Nishioka M, Lin TY, Baglole CJ, Eidelman DH, Hamid Q. Are leukocytes in asthmatic patients aging faster? A study of telomere length and disease severity. J Allergy Clin Immunol. 2013;132:480–482. doi: 10.1016/j.jaci.2013.02.010. e2. [DOI] [PubMed] [Google Scholar]
- 76.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D’Agostino R, Jr, Castro M, Curran-Everett D, Fitzpatrick AM, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fajt ML, Wenzel SE. Asthma phenotypes and the use of biologic medications in asthma and allergic disease: the next steps toward personalized care. J Allergy Clin Immunol. 2015;135:299–310, quiz 311. doi: 10.1016/j.jaci.2014.12.1871. [DOI] [PubMed] [Google Scholar]
- 78.Menzella F, Lusuardi M, Galeone C, Zucchi L. Tailored therapy for severe asthma. Multidiscip Respir Med. 2015;10:1. doi: 10.1186/2049-6958-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Agache IO. From phenotypes to endotypes to asthma treatment. Curr Opin Allergy Clin Immunol. 2013;13:249–256. doi: 10.1097/ACI.0b013e32836093dd. [DOI] [PubMed] [Google Scholar]
- 80.Kontakioti E, Domvri K, Papakosta D, Daniilidis M. HLA and asthma phenotypes/endotypes: a review. Hum Immunol. 2014;75:930–939. doi: 10.1016/j.humimm.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 81.Wenzel S. Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy. 2012;42:650–658. doi: 10.1111/j.1365-2222.2011.03929.x. [DOI] [PubMed] [Google Scholar]
- 82.Park HW, Song WJ, Kim SH, Park HK, Kim SH, Kwon YE, Kwon HS, Kim TB, Chang YS, Cho YS, et al. Classification and implementation of asthma phenotypes in elderly patients. Ann Allergy Asthma Immunol. 2015;114:18–22. doi: 10.1016/j.anai.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 83.Baptist AP, Ross JA, Clark NM. Older adults with asthma: does age of asthma onset make a difference? J Asthma. 2013;50:836–841. doi: 10.3109/02770903.2013.816967. [DOI] [PubMed] [Google Scholar]
- 84.Song WJ, Sintobin I, Sohn KH, Kang MG, Park HK, Jo EJ, Lee SE, Yang MS, Kim SH, Park HK, et al. Staphylococcal enterotoxin IgE sensitization in late-onset severe eosinophilic asthma in the elderly. Clin Exp Allergy. 2016;46:411–421. doi: 10.1111/cea.12652. [DOI] [PubMed] [Google Scholar]
- 85.Al-Alawi M, Hassan T, Chotirmall SH. Advances in the diagnosis and management of asthma in older adults. Am J Med. 2014;127:370–378. doi: 10.1016/j.amjmed.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 86.Reddel HK, Bateman ED, Becker A, Boulet LP, Cruz AA, Drazen JM, Haahtela T, Hurd SS, Inoue H, de Jongste JC, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J. 2015;46:622–639. doi: 10.1183/13993003.00853-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Diaz-Guzman E, Mannino DM. Airway obstructive diseases in older adults: from detection to treatment. J Allergy Clin Immunol. 2010;126:702–709. doi: 10.1016/j.jaci.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 88.European Community Respiratory Health Survey II Steering Committee. The European Community Respiratory Health Survey II. Eur Respir J. 2002;20:1071–1079. doi: 10.1183/09031936.02.00046802. [DOI] [PubMed] [Google Scholar]
- 89.Siroux V, Boudier A, Bousquet J, Bresson JL, Cracowski JL, Ferran J, Gormand F, Just L, Le Moual N, Morange S, et al. Phenotypic determinants of uncontrolled asthma. J Allergy Clin Immunol. 2009;124:681–687. doi: 10.1016/j.jaci.2009.06.010. e3. [DOI] [PubMed] [Google Scholar]
- 90.Ackermann-Liebrich U, Kuna-Dibbert B, Probst-Hensch NM, Schindler C, Felber Dietrich D, Stutz EZ, Bayer-Oglesby L, Baum F, Brändli O, Brutsche M, et al. SAPALDIA Team. Follow-up of the Swiss Cohort Study on Air Pollution and Lung Diseases in Adults (SAPALDIA 2) 1991-2003: methods and characterization of participants. Soz Praventivmed. 2005;50:245–263. doi: 10.1007/s00038-005-4075-5. [DOI] [PubMed] [Google Scholar]
- 91.Boudier A, Curjuric I, Basagaña X, Hazgui H, Anto JM, Bousquet J, Bridevaux PO, Dupuis-Lozeron E, Garcia-Aymerich J, Heinrich J, et al. Ten-year follow-up of cluster-based asthma phenotypes in adults: a pooled analysis of three cohorts. Am J Respir Crit Care Med. 2013;188:550–560. doi: 10.1164/rccm.201301-0156OC. [DOI] [PubMed] [Google Scholar]
- 92.Cuttitta G, Cibella F, Bellia V, Grassi V, Cossi S, Bucchieri S, Bonsignore G. Changes in FVC during methacholine-induced bronchoconstriction in elderly patients with asthma: bronchial hyperresponsiveness and aging. Chest. 2001;119:1685–1690. doi: 10.1378/chest.119.6.1685. [DOI] [PubMed] [Google Scholar]
- 93.Ducharme ME, Prince P, Hassan N, Nair P, Boulet LP. Expiratory flows and airway inflammation in elderly asthmatic patients. Respir Med. 2011;105:1284–1289. doi: 10.1016/j.rmed.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 94.Renwick DS, Connolly MJ. The relationship between age and bronchial responsiveness: evidence from a population survey. Chest. 1999;115:660–665. doi: 10.1378/chest.115.3.660. [DOI] [PubMed] [Google Scholar]
- 95.Vaz Fragoso CA, McAvay G, Van Ness PH, Casaburi R, Jensen RL, MacIntyre N, Gill TM, Yaggi HK, Concato J. Phenotype of normal spirometry in an aging population. Am J Respir Crit Care Med. 2015;192:817–825. doi: 10.1164/rccm.201503-0463OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, et al. ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bellia V, Battaglia S, Matera MG, Cazzola M. The use of bronchodilators in the treatment of airway obstruction in elderly patients. Pulm Pharmacol Ther. 2006;19:311–319. doi: 10.1016/j.pupt.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 98.Luoto JA, Elmståhl S, Wollmer P, Pihlsgård M. Incidence of airflow limitation in subjects 65-100 years of age. Eur Respir J. 2016;47:461–472. doi: 10.1183/13993003.00635-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Allen SC, Ragab S. Ability to learn inhaler technique in relation to cognitive scores and tests of praxis in old age. Postgrad Med J. 2002;78:37–39. doi: 10.1136/pmj.78.915.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Allen SC, Yeung P. Inability to draw intersecting pentagons as a predictor of unsatisfactory spirometry technique in elderly hospital inpatients. Age Ageing. 2006;35:304–306. doi: 10.1093/ageing/afj090. [DOI] [PubMed] [Google Scholar]
- 101.Bellia V, Pistelli R, Catalano F, Antonelli-Incalzi R, Grassi V, Melillo G, Olivieri D, Rengo F. Quality control of spirometry in the elderly: the SA.R.A. study. SAlute Respiration nell’Anziano = Respiratory Health in the Elderly. Am J Respir Crit Care Med. 2000;161:1094–1100. doi: 10.1164/ajrccm.161.4.9810093. [DOI] [PubMed] [Google Scholar]
- 102.Melbye H, Medbø A, Crockett A. The FEV1/FEV6 ratio is a good substitute for the FEV1/FVC ratio in the elderly. Prim Care Respir J. 2006;15:294–298. doi: 10.1016/j.pcrj.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bhatt SP, Kim YI, Wells JM, Bailey WC, Ramsdell JW, Foreman MG, Jensen RL, Stinson DS, Wilson CG, Lynch DA, et al. FEV(1)/FEV(6) to diagnose airflow obstruction: comparisons with computed tomography and morbidity indices. Ann Am Thorac Soc. 2014;11:335–341. doi: 10.1513/AnnalsATS.201308-251OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bozek A, Filipowski M, Fischer A, Jarzab J. Characteristics of atopic bronchial asthma in seniors over 80 years of age. Biomed Res Int 2013. 2013. p. 689782. [DOI] [PMC free article] [PubMed]
- 105.Porsbjerg CM, Gibson PG, Pretto JJ, Salome CM, Brown NJ, Berend N, King GG. Relationship between airway pathophysiology and airway inflammation in older asthmatics. Respirology. 2013;18:1128–1134. doi: 10.1111/resp.12142. [DOI] [PubMed] [Google Scholar]
- 106.Langley SJ, Goldthorpe S, Craven M, Morris J, Woodcock A, Custovic A. Exposure and sensitization to indoor allergens: association with lung function, bronchial reactivity, and exhaled nitric oxide measures in asthma. J Allergy Clin Immunol. 2003;112:362–368. doi: 10.1067/mai.2003.1654. [DOI] [PubMed] [Google Scholar]
- 107.Dweik RA, Sorkness RL, Wenzel S, Hammel J, Curran-Everett D, Comhair SA, Bleecker E, Busse W, Calhoun WJ, Castro M, et al. National Heart, Lung, and Blood Institute Severe Asthma Research Program. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am J Respir Crit Care Med. 2010;181:1033–1041. doi: 10.1164/rccm.200905-0695OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 109.Battaglia S, Basile M, Spatafora M, Scichilone N. Are asthmatics enrolled in randomized trials representative of real-life outpatients? Respiration. 2015;89:383–389. doi: 10.1159/000375314. [DOI] [PubMed] [Google Scholar]
- 110.NAEPP (National Heart, Lung, and Blood Institute). Working Group on Asthma in the Elderly. Consideration for diagnosing and managing asthma in the elderly. Bethesda, MD: National Institutes of Health, National Heart, Lung, and Blood Institute; 1996
- 111.Stupka E, deShazo R. Asthma in seniors: part 1. Evidence for underdiagnosis, undertreatment, and increasing morbidity and mortality. Am J Med. 2009;122:6–11. doi: 10.1016/j.amjmed.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 112.Enright PL, McClelland RL, Newman AB, Gottlieb DJ, Lebowitz MD Cardiovascular Health Study Research Group. Underdiagnosis and undertreatment of asthma in the elderly. Chest. 1999;116:603–613. doi: 10.1378/chest.116.3.603. [DOI] [PubMed] [Google Scholar]
- 113.Parameswaran K, Hildreth AJ, Chadha D, Keaney NP, Taylor IK, Bansal SK. Asthma in the elderly: underperceived, underdiagnosed and undertreated; a community survey. Respir Med. 1998;92:573–577. doi: 10.1016/s0954-6111(98)90311-0. [DOI] [PubMed] [Google Scholar]
- 114.O’Conor R, Wolf MS, Smith SG, Martynenko M, Vicencio DP, Sano M, Wisnivesky JP, Federman AD. Health literacy, cognitive function, proper use, and adherence to inhaled asthma controller medications among older adults with asthma. Chest. 2015;147:1307–1315. doi: 10.1378/chest.14-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sofianou A, Martynenko M, Wolf MS, Wisnivesky JP, Krauskopf K, Wilson EA, Goel MS, Leventhal H, Halm EA, Federman AD. Asthma beliefs are associated with medication adherence in older asthmatics. J Gen Intern Med. 2013;28:67–73. doi: 10.1007/s11606-012-2160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bouwmeester C, Kraft J, Bungay KM. Optimizing inhaler use by pharmacist-provided education to community-dwelling elderly. Respir Med. 2015;109:1363–1368. doi: 10.1016/j.rmed.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 117.Battaglia S, Benfante A, Scichilone N. Asthma in the older adult: presentation, considerations and clinical management. Expert Rev Clin Immunol. 2015;11:1297–1308. doi: 10.1586/1744666X.2015.1087850. [DOI] [PubMed] [Google Scholar]
- 118.The safety of inactivated influenza vaccine in adults and children with asthma. N Engl J Med. 2001;345:1529–1536. doi: 10.1056/NEJMoa011961. [DOI] [PubMed] [Google Scholar]
- 119.Lu PJ, Euler GL, Callahan DB. Influenza vaccination among adults with asthma: findings from the 2007 BRFSS survey. Am J Prev Med. 2009;37:109–115. doi: 10.1016/j.amepre.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 120.Panda A, Qian F, Mohanty S, van Duin D, Newman FK, Zhang L, Chen S, Towle V, Belshe RB, Fikrig E, et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010;184:2518–2527. doi: 10.4049/jimmunol.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet. 2005;366:1165–1174. doi: 10.1016/S0140-6736(05)67339-4. [DOI] [PubMed] [Google Scholar]
- 122.Hanania NA, Sockrider M, Castro M, Holbrook JT, Tonascia J, Wise R, Atmar RL American Lung Association Asthma Clinical Research Centers. Immune response to influenza vaccination in children and adults with asthma: effect of corticosteroid therapy. J Allergy Clin Immunol. 2004;113:717–724. doi: 10.1016/j.jaci.2003.12.584. [DOI] [PubMed] [Google Scholar]
- 123.Lingner H, Ernst S, Groβhennig A, Djahangiri N, Scheub D, Wittmann M, Schultz K. Asthma control and health-related quality of life one year after inpatient pulmonary rehabilitation: the ProKAR Study. J Asthma. 2015;52:614–621. doi: 10.3109/02770903.2014.996650. [DOI] [PubMed] [Google Scholar]
- 124.Trevor JL, Bhatt SP, Wells JM, Kirkpatrick d, Schumann C, Hitchcock J, Dransfield MT. Benefits of completing pulmonary rehabilitation in patients with asthma. J Asthma. 2015;52:969–973. doi: 10.3109/02770903.2015.1025410. [DOI] [PubMed] [Google Scholar]
- 125.Rochester CL, Fairburn C, Crouch RH. Pulmonary rehabilitation for respiratory disorders other than chronic obstructive pulmonary disease. Clin Chest Med. 2014;35:369–389. doi: 10.1016/j.ccm.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 126.Renolleau-Courtois D, Lamouroux-Delay A, Delpierre S, Badier M, Lagier-Tessonnier F, Palot A, Gouitaa M, Tummino C, Charpin D, Molinari N, et al. Home-based respiratory rehabilitation in adult patients with moderate or severe persistent asthma. J Asthma. 2014;51:552–558. doi: 10.3109/02770903.2014.885039. [DOI] [PubMed] [Google Scholar]
- 127.Baptist AP, Deol BB, Reddy RC, Nelson B, Clark NM. Age-specific factors influencing asthma management by older adults. Qual Health Res. 2010;20:117–124. doi: 10.1177/1049732309355288. [DOI] [PubMed] [Google Scholar]
- 128.McDonald VM, Simpson JL, Higgins I, Gibson PG. Multidimensional assessment of older people with asthma and COPD: clinical management and health status. Age Ageing. 2011;40:42–49. doi: 10.1093/ageing/afq134. [DOI] [PubMed] [Google Scholar]
- 129.Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma: evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest. 2001;119:1329–1336. doi: 10.1378/chest.119.5.1329. [DOI] [PubMed] [Google Scholar]
- 130.Buist AS, Vollmer WM, Wilson SR, Frazier EA, Hayward AD. A randomized clinical trial of peak flow versus symptom monitoring in older adults with asthma. Am J Respir Crit Care Med. 2006;174:1077–1087. doi: 10.1164/rccm.200510-1606OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Korenblat PE, Kemp JP, Scherger JE, Minkwitz MC, Mezzanotte W. Effect of age on response to zafirlukast in patients with asthma in the Accolate Clinical Experience and Pharmacoepidemiology Trial (ACCEPT) Ann Allergy Asthma Immunol. 2000;84:217–225. doi: 10.1016/S1081-1206(10)62759-7. [DOI] [PubMed] [Google Scholar]
- 132.Baptist AP, Ross JA, Yang Y, Song PX, Clark NM. A randomized controlled trial of a self-regulation intervention for older adults with asthma. J Am Geriatr Soc. 2013;61:747–753. doi: 10.1111/jgs.12218. [DOI] [PubMed] [Google Scholar]
- 133.Baptist AP, Talreja N, Clark NM. Asthma education for older adults: results from the National Asthma Survey. J Asthma. 2011;48:133–138. doi: 10.3109/02770903.2010.535880. [DOI] [PubMed] [Google Scholar]
- 134.Jarvis S, Ind PW, Shiner RJ. Inhaled therapy in elderly COPD patients: time for re-evaluation? Age Ageing. 2007;36:213–218. doi: 10.1093/ageing/afl174. [DOI] [PubMed] [Google Scholar]
- 135.Connolly MJ. Ageing, late-onset asthma and the beta-adrenoceptor. Pharmacol Ther. 1993;60:389–404. doi: 10.1016/0163-7258(93)90029-d. [DOI] [PubMed] [Google Scholar]
- 136.Connolly MJ, Crowley JJ, Charan NB, Nielson CP, Vestal RE. Impaired bronchodilator response to albuterol in healthy elderly men and women. Chest. 1995;108:401–406. doi: 10.1378/chest.108.2.401. [DOI] [PubMed] [Google Scholar]
- 137.Bellia V, Pedone C, Catalano F, Zito A, Davì E, Palange S, Forastiere F, Incalzi RA. Asthma in the elderly: mortality rate and associated risk factors for mortality. Chest. 2007;132:1175–1182. doi: 10.1378/chest.06-2824. [DOI] [PubMed] [Google Scholar]
- 138.Gupta P, O’Mahony MS. Potential adverse effects of bronchodilators in the treatment of airways obstruction in older people: recommendations for prescribing. Drugs Aging. 2008;25:415–443. doi: 10.2165/00002512-200825050-00005. [DOI] [PubMed] [Google Scholar]
- 139.Collamati A, Martone AM, Poscia A, Brandi V, Celi M, Marzetti E, Cherubini A, Landi F. Anticholinergic drugs and negative outcomes in the older population: from biological plausibility to clinical evidence. Aging Clin Exp Res. 2016;28:25–35. doi: 10.1007/s40520-015-0359-7. [DOI] [PubMed] [Google Scholar]
- 140.Hartert TV, Togias A, Mellen BG, Mitchel EF, Snowden MS, Griffin MR. Underutilization of controller and rescue medications among older adults with asthma requiring hospital care. J Am Geriatr Soc. 2000;48:651–657. doi: 10.1111/j.1532-5415.2000.tb04723.x. [DOI] [PubMed] [Google Scholar]
- 141.Sin DD, Tu JV. Underuse of inhaled steroid therapy in elderly patients with asthma. Chest. 2001;119:720–725. doi: 10.1378/chest.119.3.720. [DOI] [PubMed] [Google Scholar]
- 142.Sin DD, Tu JV. Inhaled corticosteroid therapy reduces the risk of rehospitalization and all-cause mortality in elderly asthmatics. Eur Respir J. 2001;17:380–385. doi: 10.1183/09031936.01.17303800. [DOI] [PubMed] [Google Scholar]
- 143.Etminan M, Sadatsafavi M, Ganjizadeh Zavareh S, Takkouche B, FitzGerald JM. Inhaled corticosteroids and the risk of fractures in older adults: a systematic review and meta-analysis. Drug Saf. 2008;31:409–414. doi: 10.2165/00002018-200831050-00005. [DOI] [PubMed] [Google Scholar]
- 144.Battaglia S, Cardillo I, Lavorini F, Spatafora M, Scichilone N. Safety considerations of inhaled corticosteroids in the elderly. Drugs Aging. 2014;31:787–796. doi: 10.1007/s40266-014-0213-1. [DOI] [PubMed] [Google Scholar]
- 145.Gershon A, Croxford R, Calzavara A, To T, Stanbrook MB, Upshur R, Stukel TA. Cardiovascular safety of inhaled long-acting bronchodilators in individuals with chronic obstructive pulmonary disease. JAMA Intern Med. 2013;173:1175–1185. doi: 10.1001/jamainternmed.2013.1016. [DOI] [PubMed] [Google Scholar]
- 146.Gershon AS, Campitelli MA, Croxford R, Stanbrook MB, To T, Upshur R, Stephenson AL, Stukel TA. Combination long-acting β-agonists and inhaled corticosteroids compared with long-acting β-agonists alone in older adults with chronic obstructive pulmonary disease. JAMA. 2014;312:1114–1121. doi: 10.1001/jama.2014.11432. [DOI] [PubMed] [Google Scholar]
- 147.Bermingham M, O’Callaghan E, Dawkins I, Miwa S, Samsudin S, McDonald K, Ledwidge M. Are beta2-agonists responsible for increased mortality in heart failure? Eur J Heart Fail. 2011;13:885–891. doi: 10.1093/eurjhf/hfr063. [DOI] [PubMed] [Google Scholar]
- 148.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 149.Kerstjens HA, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, Sigmund R, Seibold W, Moroni-Zentgraf P, Bateman ED. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012;367:1198–1207. doi: 10.1056/NEJMoa1208606. [DOI] [PubMed] [Google Scholar]
- 150.Creticos P, Knobil K, Edwards LD, Rickard KA, Dorinsky P. Loss of response to treatment with leukotriene receptor antagonists but not inhaled corticosteroids in patients over 50 years of age. Ann Allergy Asthma Immunol. 2002;88:401–409. doi: 10.1016/S1081-1206(10)62372-1. [DOI] [PubMed] [Google Scholar]
- 151.Maykut RJ, Kianifard F, Geba GP. Response of older patients with IgE-mediated asthma to omalizumab: a pooled analysis. J Asthma. 2008;45:173–181. doi: 10.1080/02770900701247277. [DOI] [PubMed] [Google Scholar]
- 152.Korn S, Schumann C, Kropf C, Stoiber K, Thielen A, Taube C, Buhl R. Effectiveness of omalizumab in patients 50 years and older with severe persistent allergic asthma. Ann Allergy Asthma Immunol. 2010;105:313–319. doi: 10.1016/j.anai.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 153.Asero R. Efficacy of injection immunotherapy with ragweed and birch pollen in elderly patients. Int Arch Allergy Immunol. 2004;135:332–335. doi: 10.1159/000082328. [DOI] [PubMed] [Google Scholar]
- 154.Cox L, Nelson H, Lockey R, Calabria C, Chacko T, Finegold I, Nelson M, Weber R, Bernstein DI, Blessing-Moore J, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127:S1–S55. doi: 10.1016/j.jaci.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 155.Chen W, Lynd LD, FitzGerald JM, Marra CA, Rousseau R, Sadatsafavi M. The added effect of comorbidity on health-related quality of life in patients with asthma. Qual Life Res. 2015;24:2507–2517. doi: 10.1007/s11136-015-0995-6. [DOI] [PubMed] [Google Scholar]
- 156.van Manen JG, Bindels PJ, IJzermans CJ, van der Zee JS, Bottema BJ, Schadé E. Prevalence of comorbidity in patients with a chronic airway obstruction and controls over the age of 40. J Clin Epidemiol. 2001;54:287–293. doi: 10.1016/s0895-4356(01)00346-8. [DOI] [PubMed] [Google Scholar]
- 157.Diette GB, Krishnan JA, Dominici F, Haponik E, Skinner EA, Steinwachs D, Wu AW. Asthma in older patients: factors associated with hospitalization. Arch Intern Med. 2002;162:1123–1132. doi: 10.1001/archinte.162.10.1123. [DOI] [PubMed] [Google Scholar]
- 158.Zhang T, Carleton BC, Prosser RJ, Smith AM. The added burden of comorbidity in patients with asthma. J Asthma. 2009;46:1021–1026. doi: 10.3109/02770900903350473. [DOI] [PubMed] [Google Scholar]
- 159.Chan WL, Yang KP, Chao TF, Huang CC, Huang PH, Chen YC, Chen TJ, Lin SJ, Chen JW, Leu HB. The association of asthma and atrial fibrillation: a nationwide population-based nested case-control study. Int J Cardiol. 2014;176:464–469. doi: 10.1016/j.ijcard.2014.07.087. [DOI] [PubMed] [Google Scholar]
- 160.Hardin M, Cho M, McDonald ML, Beaty T, Ramsdell J, Bhatt S, van Beek EJ, Make BJ, Crapo JD, Silverman EK, et al. The clinical and genetic features of COPD-asthma overlap syndrome. Eur Respir J. 2014;44:341–350. doi: 10.1183/09031936.00216013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Whitters D, Stockley RA. Bronchiectasis in older patients with chronic obstructive pulmonary disease: prevalence, diagnosis and therapeutic management. Drugs Aging. 2013;30:215–225. doi: 10.1007/s40266-013-0053-4. [DOI] [PubMed] [Google Scholar]
- 162.Reed CE. The natural history of asthma. J Allergy Clin Immunol. 2006;118:543–548, quiz 549–550. doi: 10.1016/j.jaci.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 163.Oguzulgen IK, Kervan F, Ozis T, Turktas H. The impact of bronchiectasis in clinical presentation of asthma. South Med J. 2007;100:468–471. doi: 10.1097/SMJ.0b013e31802fa16f. [DOI] [PubMed] [Google Scholar]
- 164.Pite H, Pereira AM, Morais-Almeida M, Nunes C, Bousquet J, Fonseca JA. Prevalence of asthma and its association with rhinitis in the elderly. Respir Med. 2014;108:1117–1126. doi: 10.1016/j.rmed.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 165.Jarvis D, Newson R, Lotvall J, Hastan D, Tomassen P, Keil T, Gjomarkaj M, Forsberg B, Gunnbjornsdottir M, Minov J, et al. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy. 2012;67:91–98. doi: 10.1111/j.1398-9995.2011.02709.x. [DOI] [PubMed] [Google Scholar]
- 166.Schatz M, Zeiger RS, Yang SJ, Chen W, Sajjan S, Allen-Ramey F, Camargo CA., Jr Prospective study on the relationship of obesity to asthma impairment and risk. J Allergy Clin Immunol Pract. 2015;3:560–565.e1. doi: 10.1016/j.jaip.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 167.Mold JW, Reed LE, Davis AB, Allen ML, Decktor DL, Robinson M. Prevalence of gastroesophageal reflux in elderly patients in a primary care setting. Am J Gastroenterol. 1991;86:965–970. [PubMed] [Google Scholar]
- 168.Shimizu Y, Dobashi K, Kusano M, Mori M. Different gastoroesophageal reflux symptoms of middle-aged to elderly asthma and chronic obstructive pulmonary disease (COPD) patients. J Clin Biochem Nutr. 2012;50:169–175. doi: 10.3164/jcbn.11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Krauskopf KA, Sofianou A, Goel MS, Wolf MS, Wilson EA, Martynenko ME, Halm EA, Leventhal H, Feldman JM, Federman AD, et al. Depressive symptoms, low adherence, and poor asthma outcomes in the elderly. J Asthma. 2013;50:260–266. doi: 10.3109/02770903.2012.757779. [DOI] [PubMed] [Google Scholar]
- 170.Bozek A, Jarzab J. Adherence to asthma therapy in elderly patients. J Asthma. 2010;47:162–165. doi: 10.3109/02770900903497204. [DOI] [PubMed] [Google Scholar]
- 171.Bellia V, Catalano F, Scichilone N, Incalzi RA, Spatafora M, Vergani C, Rengo F. Sleep disorders in the elderly with and without chronic airflow obstruction: the SARA study. Sleep. 2003;26:318–323. doi: 10.1093/sleep/26.3.318. [DOI] [PubMed] [Google Scholar]
- 172.Teodorescu M, Polomis DA, Gangnon RE, Fedie JE, Consens FB, Chervin RD. Teodorescu MC. Asthma control and its relationship with obstructive sleep apnea (OSA) in older adults. Sleep Disord 2013. 2013. p. 251567. [DOI] [PMC free article] [PubMed]
- 173.Onder G, Petrovic M, Tangiisuran B, Meinardi MC, Markito-Notenboom WP, Somers A, Rajkumar C, Bernabei R, van der Cammen TJ. Development and validation of a score to assess risk of adverse drug reactions among in-hospital patients 65 years or older: the GerontoNet ADR risk score. Arch Intern Med. 2010;170:1142–1148. doi: 10.1001/archinternmed.2010.153. [DOI] [PubMed] [Google Scholar]
- 174.Corsonello A, Pedone C, Corica F, Mussi C, Carbonin P, Antonelli Incalzi R Gruppo Italiano di Farmacovigilanza nell’Anziano (GIFA) Investigators. Concealed renal insufficiency and adverse drug reactions in elderly hospitalized patients. Arch Intern Med. 2005;165:790–795. doi: 10.1001/archinte.165.7.790. [DOI] [PubMed] [Google Scholar]
- 175.Wooten JM. Pharmacotherapy considerations in elderly adults. South Med J. 2012;105:437–445. doi: 10.1097/SMJ.0b013e31825fed90. [DOI] [PubMed] [Google Scholar]