Abstract
Discovery of alpha-1 antitrypsin (A1AT) as the principal circulating inhibitor of neutrophil elastase was critical to the appreciation of protease/antiprotease imbalance involvement in the pathogenesis of emphysema. Additional targets of A1AT have been uncovered, along with their contribution to alveolar wall destruction induced by cigarette smoke exposure. We highlight in this report mechanisms of A1AT antiapoptotic effects on structural lung endothelial cells. This function was largely dependent on uptake of the protein from the circulation via clathrin- and, in part, caveolae-mediated endocytosis and on specific interactions with cysteine proteases such as capsase-3, -6, and -7. Exposures to cigarette smoke diminished A1AT intracellular uptake and its anticaspase action, suggesting that even in A1AT-suficient individuals, cigarette smoke may weaken the serpin’s endothelial prosurvival effect. In addition, cigarette smoke exposure or genetic mutations known to induce posttranslational modifications such as oxidation or polymerization may alter A1AT bidirectional intracellular traffic in endothelial cells and thus determine its functional bioavailability in certain lung compartments. Uncovering and harnessing the A1AT canonical and noncanonical mechanisms will advance our understanding of the pathogenesis of emphysema and may provide means to improve the effectiveness of therapies in both A1AT-sufficient and A1AT-deficient individuals.
Keywords: alpha-1 antitrypsin, apoptosis, caspase, cigarette smoke, emphysema
Alpha-1 antitrypsin (A1AT) deficiency or dysfunction has been associated with the development of chronic obstructive pulmonary disease characterized (in part) by permanent destruction of the alveolar unit distal of the terminal bronchioles (emphysema) and increased risk for infectious exacerbations (1, 2). A1AT function as a serine protease inhibitor with great specificity against neutrophil elastase (3, 4) led to the development of the protease/antiprotease paradigm as central to the pathogenesis of emphysema (5). Reduced serum A1AT levels typically occur from the inheritance of two protease inhibitor–deficient alleles (Pi) at the A1AT locus (ZZ genotype), leading to polymerization of the protein in hepatocytes and a classical phenotype of A1AT deficiency (1). In addition, exposure to oxidants such as cigarette smoke (CS) leads to posttranslational modifications of the A1AT molecule (e.g., polymerization, nitrosylation, or oxidation of Met-358 within the reactive center loop of the serpin) both in A1AT-deficient and -sufficient individuals and explains the concept of qualitative (as opposed to quantitative) A1AT deficiency (6). These posttranslational modifications are associated with loss of A1AT canonical functions, such as elastase inhibition, and may contribute to the pathogenesis of cigarette smoke–induced emphysema (7, 8).
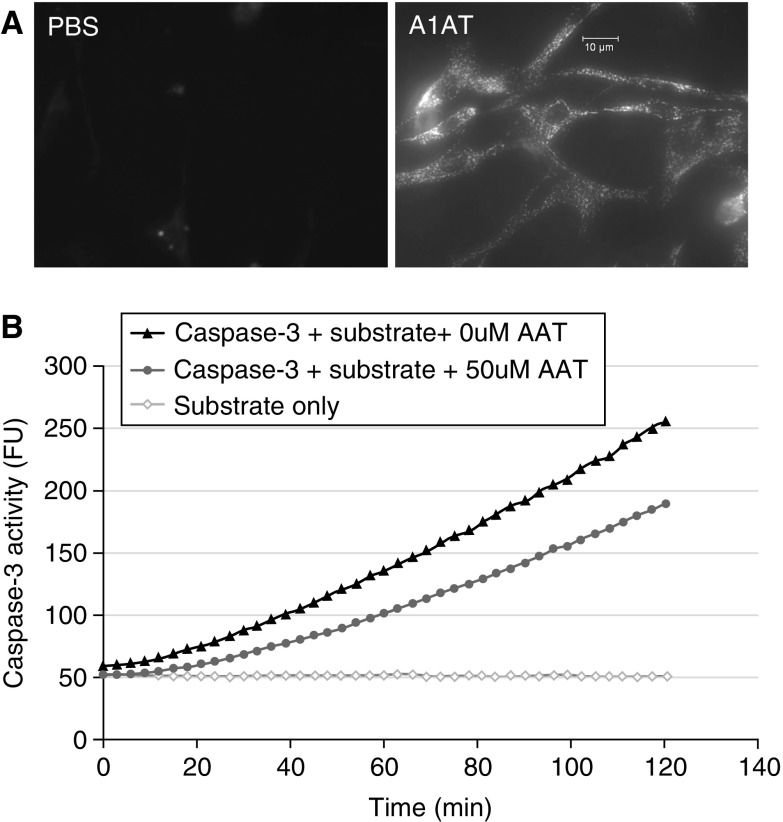
Besides the protease/antiprotease imbalance causing lung matrix damage due to unopposed activation of neutrophil elastases and other proteinases such as proteinase-3, cathepsins, and metalloproteinases, other mechanisms of alveolar wall destruction are involved in emphysema, including lung inflammation, autoimmunity (9), and excessive cell death of structural cells comprising the alveolus (10–12). Canonical (irreversible binding of serine proteases) and noncanonical functions of A1AT may be involved in regulating these mechanisms, as well. For example, antiinflammatory functions of A1AT have been shown to occur via binding and inhibition of CS-induced thrombin and plasmin in the airway (2, 13), inhibition of secreted metalloproteinase MMP-12 from activated alveolar macrophages (13), or inhibition of sheddase tumor necrosis factor (TNF)-α–converting enzyme at the polymorphonuclear neutrophils plasma membrane (14), decreased LPS-induced macrophage activation (15), and direct binding of cytokines TNF-α and IL-8 (14). The role of A1AT in modulating the excessive death of structural lung cells after CS exposure was first uncovered in a simplified model of emphysema that allowed the study of apoptosis in the absence of exuberant inflammation, induced via receptors or signaling inhibition for vascular endothelial growth factor (VEGF), an essential growth factor for lung endothelial cell (EC) survival (16, 17). Using the model of VEGF blockade in mice or rats, we showed that transduction of human A1AT via replication-deficient adeno-associated virus attenuated airspace enlargement and emphysema, and suppressed caspase-3 activation and oxidative stress (18). These findings were the first evidence of a prosurvival action of native A1AT on structural lung endothelial cells that lack the ability to synthesize their own pool of intracellular A1AT and are entirely dependent on circulating A1AT levels. Because VEGF is predominantly EC trophic, we focused on the prosurvival effect of A1AT on lung ECs using ex vivo models. Since ECs are not a source of A1AT synthesis, we investigated whether the antiapoptotic effects of A1AT in these models required the internalization of the protein by ECs. These studies identified intracytoplasmic uptake of A1AT (Figure 1A) and at least partial colocalization with caspase-3, the main executioner caspase involved in apoptosis. Using cell-free studies we showed that native A1AT, but not conformers lacking an active reactive center loop, or posttranslationally modified A1AT preincubated with CS extract, dose- and time-dependently inhibited the interaction between active caspase-3 and a specific substrate (Figure 1B) (19, 20). A1AT inhibited other executioner caspases activity such as caspase-6 and -7, but not initiator caspases such as caspase-8 and -9 (20). Whereas other groups have shown that A1AT inhibits caspase-1 (21), cysteine proteinase associated with inflammasome activity, we and others have not detected an A1AT inhibitory effect on caspase-1 activity, suggesting specific interactions between the native A1AT molecule and certain apoptotic caspases (20, 22). However, the A1AT prosurvival effect is not cell type specific; it has been shown to inhibit TNF-α–induced hepatocyte apoptosis (23), beta cell apoptosis after pancreatic islet graft transplantation (24), and pulmonary artery endothelial cell apoptosis after ischemia–reperfusion-induced lung injury (25), and to correct accelerated neutrophil apoptosis in A1AT-deficient individuals (26).
Figure 1.
Alpha-1 antitrypsin (A1AT) intracellular uptake and anti–cysteine proteases activity. (A) Fluorescence micrographs of mouse endothelial cells after incubation with phosphate-buffered saline (PBS; left) or with DyLight (547 NHS ester; Thermo Scientific, Waltham, MA)-labeled h1AT (purified human A1AT from Sigma-Aldrich [St. Louis, MO]; right). Note the intracytoplasmic uptake of labeled human A1AT (hA1AT). (B) Activity of recombinant caspase-3/7 incubated with its specific fluorescently tagged DEVD substrate, measured in cell-free systems by the release of fluorescence signal over time. The kinetic curves show caspase-3/7 activity in the absence of hA1AT (solid triangles) or the presence of hA1AT (gray circles; 50 μM/ml; Aralast, from Baxter, Deerfield, IL). Note the inhibitory effect of hA1AT on caspase-3 activity. Fluorescence signal of the substrate in the absence of enzyme is shown (line of open diamonds).
Having shown that, similar to its effect on A1AT antielastase activity, CS-induced posttranslational A1AT changes affect its antiapoptotic activity in vitro, we investigated whether circulating A1AT from active smokers has diminished anticaspase action. We have demonstrated that A1AT immune-purified from current smokers, independent of the chronic obstructive pulmonary disease status, exhibited decreased anti–caspase-3 and anti–caspase-6 activity (20). This finding, together with the report by Aldonyte and colleagues that A1AT protects against CS-induced lung endothelial cell apoptosis, suggests that even in A1AT-suficient individuals cigarette smoking disables the endothelial prosurvival effect of A1AT, which may contribute to chronic lung damage in susceptible individuals (20, 27).
Our studies of the prosurvival effect of A1AT on lung endothelial cells led us to uncover mechanisms of intracellular uptake of A1AT in the endothelium. We have shown that A1AT is internalized by lung endothelial cells in a time-, dose-, and conformer-dependent manner and is detected intracellularly in the endothelial cells of nondiseased human lungs. A1AT internalization is actively regulated via endocytosis, through both clathrin- and, in part, caveolae-mediated endocytosis as demonstrated by loss-of-function experiments with pharmacological inhibitors of endocytotic pathways or small interfering RNA knockdown of clathrin heavy chains. Inhibition of clathrin-mediated endocytosis significantly decreased both A1AT uptake and its ability to inhibit caspase-3 activity, suggesting A1AT internalization is required for its protective effects in endothelial cells (28). Time-lapse confocal microscopy indicated partial A1AT localization in Golgi bodies and increased intracellular A1AT retention after inhibition of the classical secretory pathway with tunicamycin (29). These experiments were followed up with evidence of bidirectional A1AT transport in ECs, with apical and basolateral secretion after initial uptake. Confluent rat lung ECs cocultured on Corning Transwells across epithelial cells revealed A1AT transcytosis and transfer to adjacent epithelial cells via basolateral uptake, but not via apical epithelial surface uptake (29). Although the precise mechanism and pathways that lead to A1AT uptake and intracellular and transcellular transport require further investigations, the A1AT association with circulatory lipoproteins such as high-density lipoprotein suggest that scavenging receptors may be involved (our unpublished data). Evidence for transcytosis across the lung microcirculation was also obtained in vivo by two-photon excitation microscopy of the mouse lung (29). CS exposure decreased A1AT uptake by lung endothelial cells, as determined by fluorescence microscopy (29). A1AT endothelial uptake and bidirectional intracellular traffic may determine the A1AT functional bioavailability in specific lung compartments under homeostatic conditions and in lung injury.
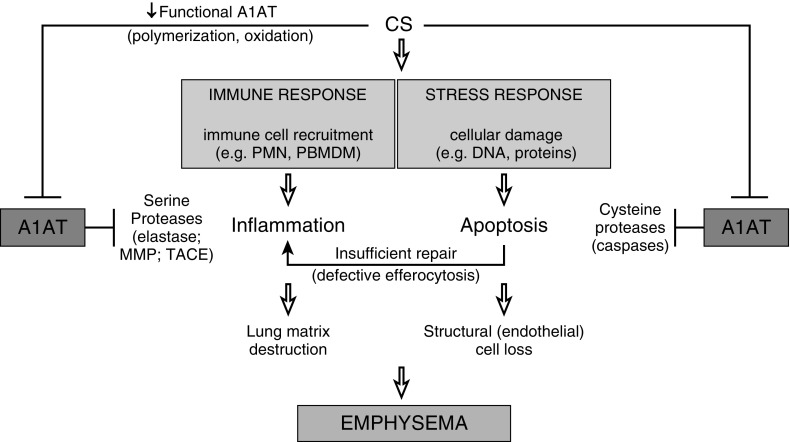
There is increasing appreciation of the role of apoptotic cell clearance by macrophages via efferocytosis in lung repair and homeostasis. The timely removal of apoptotic cells prevents proinflammatory cytokine release from apoptotic cells undergoing secondary necrosis and is associated with an antiinflammatory secretory phenotype of the macrophages that engulf the apoptotic cells. Ineffective efferocytosis after CS exposure combined with a high apoptotic burden, is expected during infectious exacerbations of the disease, and may contribute to sustained inflammation and inadequate tissue repair. Work from several groups suggests that monocyte and macrophage functions during inflammation are modulated by A1AT (13, 15, 30, 31). This work led us to extend our investigations on the clearance of apoptotic cells by specialized phagocytic cells, such as alveolar macrophages. These antiinflammatory and immune-modulatory A1AT effects, added to its well-described antimatrix proteolytic and antiapoptotic effects, are not surprising, given the major protective role of this serpin against the development of such a complex chronic disease as emphysema (Figure 2). Understanding and harnessing the mechanisms by which A1AT protects the lung and microvasculature will advance our understanding of the pathogenesis of emphysema and its comorbidities and provide the means to improve therapy effectiveness in both A1AT-sufficient and -deficient individuals.
Figure 2.
Proposed protective role of alpha-1 antitrypsin (A1AT) in cigarette smoke (CS)–induced lung injury. CS exposure induces oxidative stress and cellular damage. Lung responses to injury include proinflammatory cytokine secretion, immune cell recruitment, and cell death. Insufficient repair mechanisms (such as efferocytosis) combined with ongoing injury leads to emphysematous lung tissue destruction characterized by disintegration of lung matrix and structural cell loss. A1AT protects lung matrix and cells via antiinflammatory and endothelial prosurvival functions mediated by its antiprotease function against neutrophil elastase, macrophage metalloproteinases, TACE, and endothelial cell caspases, among others. MMP = matrix metalloproteinase; PBMDM = peripheral blood monocyte–derived macrophages; PMN = polymorphonuclear neutrophils; TACE (ADAM-17) = tumor necrosis factor-α–converting enzyme.
Footnotes
Supported by the 2012 Junior Investigator Alpha-1 Foundation and a CHEST Foundation Clinical Research grant (K.A.S.), an Alpha-1 Foundation 2014 Gordon L. Snider Scholar Award (K.A.S.), a VA Merit Review Research Award (I.P.), the Alpha-1 Foundation (I.P.), and Baxter Healthcare, US (I.P.).
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Stoller JK, Aboussouan LS. α1-Antitrypsin deficiency. Lancet. 2005;365:2225–2236. doi: 10.1016/S0140-6736(05)66781-5. [DOI] [PubMed] [Google Scholar]
- 2.Tuder RM, Janciauskiene SM, Petrache I. Lung disease associated with α1-antitrypsin deficiency. Proc Am Thorac Soc. 2010;7:381–386. doi: 10.1513/pats.201002-020AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lomas DA. The selective advantage of α1-antitrypsin deficiency. Am J Respir Crit Care Med. 2006;173:1072–1077. doi: 10.1164/rccm.200511-1797PP. [DOI] [PubMed] [Google Scholar]
- 4.Turino GM, Seniorrm, Garg BD, Keller S, Levi MM, Mandl I. Serum elastase inhibitor deficiency and α1-antitrypsin deficiency in patients with obstructive emphysema. Science. 1969;165:709–711. doi: 10.1126/science.165.3894.709. [DOI] [PubMed] [Google Scholar]
- 5.Stockley RA. Chronic bronchitis: the antiproteinase/proteinase balance and the effect of infection and corticosteroids. Clin Chest Med. 1988;9:643–656. [PubMed] [Google Scholar]
- 6.Janciauskiene S. Conformational properties of serine proteinase inhibitors (serpins) confer multiple pathophysiological roles. Biochim Biophys Acta. 2001;1535:221–235. doi: 10.1016/s0925-4439(01)00025-4. [DOI] [PubMed] [Google Scholar]
- 7.Ogushi F, Fells GA, Hubbard RC, Straus SD, Crystal RG. Z-type α1-antitrypsin is less competent than M1-type α1-antitrypsin as an inhibitor of neutrophil elastase. J Clin Invest. 1987;80:1366–1374. doi: 10.1172/JCI113214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hubbard RC, Ogushi F, Fells GA, Cantin AM, Jallat S, Courtney M, Crystal RG. Oxidants spontaneously released by alveolar macrophages of cigarette smokers can inactivate the active site of α1-antitrypsin, rendering it ineffective as an inhibitor of neutrophil elastase. J Clin Invest. 1987;80:1289–1295. doi: 10.1172/JCI113204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taraseviciene-Stewart L, Scerbavicius R, Choe KH, Moore M, Sullivan A, Nicolls MR, Fontenot AP, Tuder RM, Voelkel NF. An animal model of autoimmune emphysema. Am J Respir Crit Care Med. 2005;171:734–742. doi: 10.1164/rccm.200409-1275OC. [DOI] [PubMed] [Google Scholar]
- 10.Ikari Y, Mulvihill E, Schwartz SM. α1-Proteinase inhibitor, α1-antichymotrypsin, and α2-macroglobulin are the antiapoptotic factors of vascular smooth muscle cells. J Biol Chem. 2001;276:11798–11803. doi: 10.1074/jbc.M008503200. [DOI] [PubMed] [Google Scholar]
- 11.Van Molle W, Denecker G, Rodriguez I, Brouckaert P, Vandenabeele P, Libert C. Activation of caspases in lethal experimental hepatitis and prevention by acute phase proteins. J Immunol. 1999;163:5235–5241. [PubMed] [Google Scholar]
- 12.Elliott PR, Pei XY, Dafforn TR, Lomas DA. Topography of a 2.0 Å structure of α1-antitrypsin reveals targets for rational drug design to prevent conformational disease. Protein Sci. 2000;9:1274–1281. doi: 10.1110/ps.9.7.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Churg A, Wang X, Wang RD, Meixner SC, Pryzdial EL, Wright JL. α1-Antitrypsin suppresses TNF-α and MMP-12 production by cigarette smoke–stimulated macrophages. Am J Respir Cell Mol Biol. 2007;37:144–151. doi: 10.1165/rcmb.2006-0345OC. [DOI] [PubMed] [Google Scholar]
- 14.Bergin DA, Reeves EP, Meleady P, Henry M, McElvaney OJ, Carroll TP, Condron C, Chotirmall SH, Clynes M, O’Neill SJ, et al. α-1 Antitrypsin regulates human neutrophil chemotaxis induced by soluble immune complexes and IL-8. J Clin Invest. 2010;120:4236–4250. doi: 10.1172/JCI41196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janciauskiene S, Larsson S, Larsson P, Virtala R, Jansson L, Stevens T. Inhibition of lipopolysaccharide-mediated human monocyte activation, in vitro, by α1-antitrypsin. Biochem Biophys Res Commun. 2004;321:592–600. doi: 10.1016/j.bbrc.2004.06.123. [DOI] [PubMed] [Google Scholar]
- 16.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311–1319. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuder RM, Wood K, Taraseviciene L, Flores SC, Voekel NF. Cigarette smoke extract decreases the expression of vascular endothelial growth factor by cultured cells and triggers apoptosis of pulmonary endothelial cells. Chest. 2000;117(5) Suppl 1:241S–242S. doi: 10.1378/chest.117.5_suppl_1.241s. [DOI] [PubMed] [Google Scholar]
- 18.Petrache I, Fijalkowska I, Zhen L, Medler TR, Brown E, Cruz P, Choe KH, Taraseviciene-Stewart L, Scerbavicius R, Shapiro L, et al. A novel antiapoptotic role for α1-antitrypsin in the prevention of pulmonary emphysema. Am J Respir Crit Care Med. 2006;173:1222–1228. doi: 10.1164/rccm.200512-1842OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrache I, Fijalkowska I, Medler TR, Skirball J, Cruz P, Zhen L, Petrache HI, Flotte TR, Tuder RM. α-1 antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am J Pathol. 2006;169:1155–1166. doi: 10.2353/ajpath.2006.060058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lockett AD, Van Demark M, Gu Y, Schweitzer KS, Sigua N, Kamocki K, Fijalkowska I, Garrison J, Fisher AJ, Serban K, et al. Effect of cigarette smoke exposure and structural modifications on the α-1 antitrypsin interaction with caspases. Mol Med. 2012;18:445–454. doi: 10.2119/molmed.2011.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, He Y, Abraham B, Rouhani FN, Brantly ML, Scott DE, Reed JL. Cytosolic, autocrine alpha-1 proteinase inhibitor (A1PI) inhibits caspase-1 and blocks IL-1β dependent cytokine release in monocytes. PLoS One. 2012;7:e51078. doi: 10.1371/journal.pone.0051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahman MA, Mitra S, Sarkar A, Wewers MD. Alpha 1-antitrypsin does not inhibit human monocyte caspase-1. PLoS One. 2015;10:e0117330. doi: 10.1371/journal.pone.0117330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Molle W, Libert C, Fiers W, Brouckaert P. Alpha 1-acid glycoprotein and alpha 1-antitrypsin inhibit TNF-induced but not anti-Fas–induced apoptosis of hepatocytes in mice. J Immunol. 1997;159:3555–3564. [PubMed] [Google Scholar]
- 24.Abecassis A, Schuster R, Shahaf G, Ozeri E, Green R, Ochayon DE, Rider P, Lewis EC. α1-Antitrypsin increases interleukin-1 receptor antagonist production during pancreatic islet graft transplantation. Cell Mol Immunol. 2014;11:377–386. doi: 10.1038/cmi.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao W, Zhao J, Kim H, Xu S, Chen M, Bai X, Toba H, Cho HR, Zhang H, Keshavjeel S, et al. α1-Antitrypsin inhibits ischemia reperfusion–induced lung injury by reducing inflammatory response and cell death. J Heart Lung Transplant. 2014;33:309–315. doi: 10.1016/j.healun.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 26.Hurley K, Lacey N, O’Dwyer CA, Bergin DA, McElvaney OJ, O’Brien ME, McElvaney OF, Reeves EP, McElvaney NG. Alpha-1 antitrypsin augmentation therapy corrects accelerated neutrophil apoptosis in deficient individuals. J Immunol. 2014;193:3978–3991. doi: 10.4049/jimmunol.1400132. [DOI] [PubMed] [Google Scholar]
- 27.Aldonyte R, Hutchinson TE, Jin B, Brantly M, Block E, Patel J, Zhang J. Endothelial alpha-1-antitrypsin attenuates cigarette smoke induced apoptosis in vitro. COPD. 2008;5:153–162. doi: 10.1080/15412550802092936. [DOI] [PubMed] [Google Scholar]
- 28.Sohrab S, Petrusca DN, Lockett AD, Schweitzer KS, Rush NI, Gu Y, Kamocki K, Garrison J, Petrache I. Mechanism of α-1 antitrypsin endocytosis by lung endothelium. FASEB J. 2009;23:3149–3158. doi: 10.1096/fj.09-129304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lockett AD, Brown MB, Santos-Falcon N, Rush NI, Oueini H, Oberle AJ, Bolanis E, Fragoso MA, Petrusca DN, Serban KA, et al. Active trafficking of alpha 1 antitrypsin across the lung endothelium. PLoS One. 2014;9:e93979. doi: 10.1371/journal.pone.0093979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aldonyte R, Jansson L, Janciauskiene S. Concentration-dependent effects of native and polymerised α1-antitrypsin on primary human monocytes, in vitro. BMC Cell Biol. 2004;5:11. doi: 10.1186/1471-2121-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nita IM, Serapinas D, Janciauskiene SM. α1-Antitrypsin regulates CD14 expression and soluble CD14 levels in human monocytes in vitro. Int J Biochem Cell Biol. 2007;39:1165–1176. doi: 10.1016/j.biocel.2007.02.017. [DOI] [PubMed] [Google Scholar]