Abstract
Oxygen delivery and carbon dioxide removal being critical to cell survival, mammals have developed collateral vascular and ventilation systems to ensure tissue viability. Collateral ventilation, defined as ventilation of alveoli via pathways that bypass normal airways, is present in humans and many other species. The presence of collateral ventilation can be beneficial in certain disease states, whereas its relative absence can predispose to other diseases. These well defined anatomical pathways contribute little to ventilation in normal humans, but modulate ventilation perfusion imbalance in a variety of diseases, including obstructive diseases, such as asthma and emphysema. These pathways can be affected by pharmaceuticals and inhaled gas compositions. The middle lobe and lingula, constrained by their isolated, segmental anatomy, have reduced collateral ventilation, which predisposes them to disease. Recently, attempts to improve the quality of life of patients with emphysema, by performing nonsurgical lung volume reduction via use of endobronchial valves, have led to mixed results, because the role of collateral ventilation in the success or failure of the procedure was not initially appreciated. This review describes the anatomical pathways of collateral ventilation, their physiology and relationship to disease states, their modulatory effects on gas exchange, treatment considerations, and their effect on diagnostic procedures.
Keywords: pulmonary gas exchange, asthma, pulmonary emphysema, middle lobe syndrome, broncoalveolar lavage
Oxygen uptake and carbon dioxide (CO2) removal are critical to survival, leading many mammals to have developed redundant collateral respiratory and vascular pathways to meet cellular needs. Collateral ventilation, defined as the ventilation of alveoli via pathways that bypass normal airways, is present in many species, including humans (1). Its presence or absence can be beneficial or harmful, depending on the clinical circumstances (2, 3). This review describes the anatomy, physiology, relationship to disease states, modulatory effects on gas exchange, and the role of collateral ventilation in recent treatment advances.
Anatomy of Collateral Ventilation
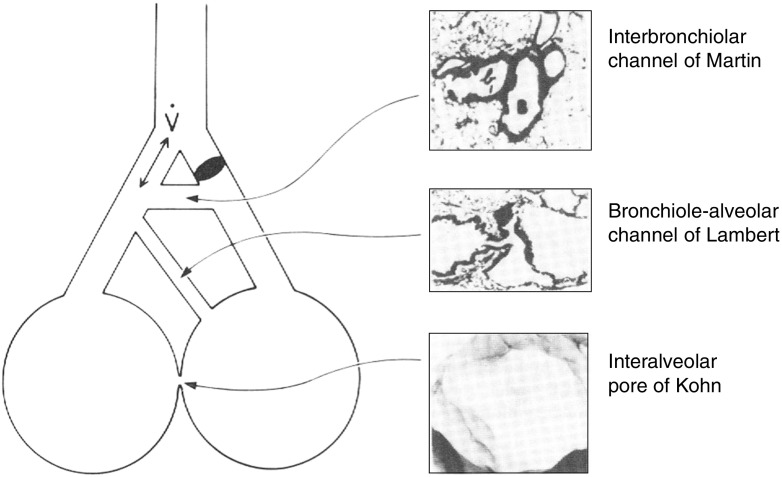
Collateral ventilation is thought to occur through alveolar pores of Kohn (3–13 μm diameter), interbronchiolar Martin’s channels (30 μm diameter) and bronchoalveolar Lambert’s channels (120 μm diameter) (4–6) (Figure 1). In addition, short, tubular, interlobular channels and intersegmental respiratory bronchioles (200 μm diameter) have also been described in humans (7, 8). Pores of Kohn, absent in newborns, develop at around 4 years of age, and are found in greatest numbers in the apical portions of upper and lower lobes, as well as in peribronchial, perivascular, and subpleural areas (9–13). In experiments in which sheep were studied at three different ages, high resistance to collateral airflow (RColl) in the young evolved into very low RColl in the aged, suggesting an increase and/or enlargement of collateral channels (14). The effect of age on the number of collateral channels in humans is not well studied, but available data suggest that RColl decreases with age, perhaps partially due to an observed increase in the size of pores (13, 15–17). In pathologic states, such as emphysema, much larger pathways develop as a result of alveolar wall destruction.
Figure 1.
Pathways for collateral ventilation. Upper insert reprinted by permission from Reference 6; middle insert reprinted by permission from Reference 5; lower insert reprinted by permission from Reference 4.
Collateral ventilation is affected by gross anatomy. RColl in humans is higher in the middle lobe than in all other areas of the lung that have been measured (3). Because there are only two segments within the middle lobe, it is hypothesized that the isolation of these by the surrounding pleura leads to a higher measured RColl between segments than occurs when three segments interface with each other. For the same reason of interfacing with one other segment, it is hypothesized that inferior lingula segment RColl is also high. All other lung segments, save the superior segment of the right lower lobe, which is rarely isolated by an accessory fissure, interface with at least two other lobar segments (18). Collateral ventilation does not occur between lobes when a complete visceral pleural envelope surrounds adjacent lobes, but does when the interlobar pleura is incomplete, estimated to occur in 50–83% of persons (19–21).
Physiology of Collateral Ventilation
In supine adult humans at functional residual capacity, RColl is about 50 times greater than airways resistance in the tracheobronchial tree (22). When humans inspire, both airways resistance and RColl decrease, but collateral resistance remains significantly greater than that of airways (22). In addition, studies show that, when a supine human has a segmental airway occluded, the partial pressure of oxygen in the obstructed segment rapidly falls to near mixed venous levels, but does not fall to the extent expected if atelectasis were occurring (23). Combined, these studies suggest that, in supine normal humans, there is little contribution of collateral ventilation to gas exchange.
Oxygen and CO2 effect collateral ventilation. When 5% CO2 is infused into an obstructed area of lung, RColl decreases significantly (46%) compared with when air flows into the area (24). A concentration of 10% CO2 causes a further minimal (9%) decrease in RColl. In addition, studies have shown that inhaled CO2 can cause airways dilation and active parenchymal tissue relaxation, which may then lead to increased lung volume and reduced RColl (25–27). These observations suggest that hypocapnic areas of lung with increased RColl, increased airways resistance, and reduced compliance may cause a homeostatic redistribution of ventilation to areas of the lung that are hypoventilating and where increased CO2 concentrations, airways dilation, parenchymal relaxation, and decreased RColl are present. Hypoxia (5%) increases RColl (36%), but its effect can be abolished by infusing CO2 simultaneously. This raises the question of whether the hypoxia is acting indirectly by inducing vascular constriction to reduce capillary blood flow, and therefore decreased regional CO2 delivery, with resultant lower expired CO2, as the actual cause of the increased RColl.
Limited physiologic studies have attempted to determine the effect of interdependence between normal and adjacent abnormal areas of lung on collateral ventilation. It is thought that the flow of air into a diseased or atelectatic area of lung through collateral channels is dependent on the pressure differential created by the interdependence of the adjacent areas of lung (28). Animal studies of bleomycin-induced fibrosis have shown that, although RColl is increased in fibrotic regions of lungs, the interdependence with adjacent areas of normal lung does not cause a disproportionate drop in collateral flow resistance in the fibrotic areas compared with adjacent areas of normal lung during inspiration (29). The effect of interdependence on collateral ventilation in humans with fibrotic lung disease is unknown.
Although the exact contribution of each of the possible anatomic pathways of collateral ventilation (pores of Kohn, Martin’s and Lambert’s channels) to gas exchange in both normal and disease states is unknown, the physiologic studies in which collateral ventilation was affected by a hypoxic and hypercarbic stimulus, as well as pharmacologic agents, suggest that Martin’s and Lambert’s channels and the intersegmental bronchioles are the effective collateral channels in normal lungs (30). These observations are supported by evidence on microscopy that no pores of Kohn exist in intersegmental septa (31).
Effects of Pharmacologic Agents on Collateral Ventilation
The effects of pharmacologic agents on collateral ventilation were first investigated by Alley and Lindskog in 1948 (32). After observing lower-lobe atelectasis in patients undergoing abdominal surgery, they hypothesized that surgery released a histamine-like substance compromising collateral ventilation and promoting atelectasis. In experiments in dogs, they demonstrated increased RColl after intravenous injection of histamine. Subsequent animal studies have shown that sympathomimetic drugs can increase collateral ventilation, whereas cholinergic drugs do the opposite (33, 34). The mediators of anaphylaxis prostaglandins D2 and F2 have been shown to increase RColl in dogs when given intravenously (35). Except for halothane and isoflurane, which reduce RColl, the effects of pharmacologic agents on collateral ventilation in humans is unknown (36).
Collateral Ventilation in Disease States
Asthma
Increased airways resistance, a hallmark of asthma, leads to increased residual and closing lung volumes, and hyperinflation (37). Although mucus plugging of small airways is characteristic of asthma, segmental and lobar atelectasis is rare in chronic asthma or during asthma attacks in adults (38). Inert gas exchange studies in asymptomatic subjects with asthma show that as many as one-half of airways may be completely closed and have areas of low ventilation–perfusion ratio, but do not show an increased shunt fraction, nor is the hypoxemia seen in mild and moderate asthma difficult to correct with supplemental oxygen (39, 40). It has been suggested that the absence of shunt is due to RColl to obstructed areas of lung when breathing at higher lung volumes (39, 40). During severe asthma requiring mechanical ventilation, virtually no shunt fraction is seen unless 100% oxygen is administered. In that case, shunt does occur, although as a small percentage of blood flow (41). It is hypothesized that this may be due to collateral ventilation becoming insufficient, leading to alveolar collapse in low ventilation–perfusion areas. Taken together, these observations suggest that collateral ventilation prevents atelectasis and promotes homogeneity of gas tensions in both obstructed and unobstructed areas during chronic and acute asthma in adults.
In contrast to adults and consistent with an increased RColl in the middle lobe and possibly the lingula, asthma in children is associated with middle lobe and lingula collapse (42). In a study of 3,528 subjects with asthma, 56 (1.62%) were found with middle lobe collapse, with one-half being less than 6 years of age (42). A study of 445 children showed that 11% of children without hypoxemia and 36% of children with hypoxemia with asthma exacerbations had evidence of partial or complete lobar atelectasis (43). Physiologic explanations for this include decreased peripheral airways conductance in younger children, fewer alveoli, and decreased collateral ventilation due to reduced numbers of alveolar pores (44, 45). In most children, this collapse is reversible; however, a few develop “middle lobe syndrome” and or chronic lingula collapse characterized by bronchiectasis and fibrosis, and require resection (46, 47).
Chronic Obstructive Pulmonary Disease
The Burrows type A emphysematous form of chronic obstructive pulmonary disease is characterized by hyperinflation, airway obstruction, little sputum, low diffusing capacity with hypoxemia easily corrected with small increases in FiO2, even with the most severe obstruction (48). It has been hypothesized that the resting PaO2 and ease with which hypoxemia is corrected are due to increased collateral ventilation (2, 15). Studies in excised emphysematous human lungs have shown RColl to be significantly lower than airways resistance (49). Studies in supine patients with emphysema have shown that RColl is significantly less than airways resistance (15). The contribution of reduced RColl to the maintenance of gas exchange in emphysema was shown by Morrell and colleagues (2) in studies measuring helium concentration in occluded lung segments.
In these studies, subjects breathed room air until bronchoscopically created airway occlusion was established. Subjects were then switched to breathe an oxygen–helium mixture and the rate of rise of helium concentration distal to the occlusion was measured. The rate of rise in helium concentration in an occluded segment of lung was 10 times faster in patients with emphysema, suggesting that RColl was significantly lower in patients with emphysema than in normal subjects. There was a positive correlation between the rate of rise in helium concentration and the final alveolar Po2 in the occluded segments, suggesting that increased collateral ventilation in these patients enhances arterial oxygenation. Inert gas exchange studies in similar patients reveal a ventilation–perfusion pattern showing no shunting of blood in spite of significant airways obstruction (50). Together, these studies show the importance of collateral ventilation in preserving gas exchange in the presence of severe airways obstruction.
Burrows’s type B bronchitic form of chronic obstructive pulmonary disease is characterized by sputum production, hypoxemia, hypercarbia, and relatively normal lung volumes. In contrast to the emphysematous form, inert gas studies show large areas of low ventilation to perfusion, partially explaining the hypoxemia seen (50). Even when high oxygen concentrations are inhaled by these individuals, shunt is not seen, thought to be at least partially prevented by collateral ventilation.
Restrictive Lung Diseases
Although there is much speculation, there is little objective evidence related to collateral ventilation in interstitial lung diseases. In a study of dogs in which bleomycin caused localized fibrosis, increased resistance to collateral ventilation was noted in the fibrotic areas of lung compared with control areas (29). There is a single study of two excised fibrotic human lungs showing that interlobar RColl was increased compared with other diseases (16). Gas exchange studies in interstitial lung diseases in humans suggest that different mechanisms of ventilation–perfusion balance occur in different diseases (51). When patients with idiopathic pulmonary fibrosis breathe 100% oxygen, the small shunt fraction does not change appreciably from that when breathing air, but more low ventilation–perfusion areas occur, suggesting that pulmonary vasoconstriction reduces gas exchange in these patients (52). This suggests that collateral ventilation was preventing atelectasis of poorly ventilated and perfused areas, but with increased inspired oxygen through collateral channels, the vasoconstriction is partially relieved, resulting in low ventilation–perfusion areas.
Middle Lobe Syndrome
In 1937, Brock and colleagues (53) described a series of cases of recurrent or chronic atelectasis of the middle lobe. Graham and associates (54) coined the term “middle lobe syndrome,” and believed that tuberculous lymphadenopathy compressed the middle lobe bronchus, causing the atelectasis, leading to bronchiectasis and fibrosis. However, Culiner (55) and Bradham and colleagues (56) showed that, in many cases, the middle lobe airways were patent, and hypothesized that inadequate collateral ventilation was the explanation for the lobar collapse. In 1978, Inners and colleagues (3) showed that RColl in the middle lobe was five times higher than that of the upper lobe, consistent with Culiner’s hypothesis. This entity can be found in children and adults with a variety of lung infections, cancer, foreign bodies, and bronchiolitis.
Nodular Bronchiectasis—Lady Windermere’s Syndrome
A nodular bronchiectatic form of nontuberculous mycobacterial infection involving predominantly the middle lobe and lingula has been reported with increasing frequency in postmenopausal women who are thin, and may have pectus excavatum and or scoliosis (57, 58). Initial speculation was that this was due to unwillingness to cough and expectorate sputum ala the heroin of Oscar Wilde’s play, Lady Windermere’s Fan (59, 60). More recent studies have raised questions about the contributions to this syndrome by alterations in adipokines, IFN-γ, IL-10, fibrillin, ciliary dysfunction, and a form of Marfan’s syndrome (57, 58, 61). Together, these studies suggest a genetic predisposition to the syndrome causing impaired inflammatory responses and or aberrant mechanical and physiologic responses, coupled with poor collateral ventilation in these lobes, leading to poor secretion clearance, chronic inflammation, and bronchiectasis
Congenital Lesions
Bronchial cysts, pulmonary sequestrations, bronchial atresia, and the hyperlucent lung syndrome have all been shown to be accompanied by evidence of emphysema secondary to collateral ventilation distal to the occluded airways in each (62–64).
Foreign Bodies
Foreign body aspiration does not lead to lobar atelectasis when the foreign material obstructs distal to the first segmental bronchus, because air may enter the obstructed area via this proximal segmental bronchus. Lobar atelectasis does occur when there is complete lobar airway obstruction, unless the interlobar fissure is incomplete (65). In the latter case, overexpansion distal to the obstruction may occur.
Low Lung Volume States
Individuals who are obese, postoperative from major abdominal procedures, or with bilateral diaphragmatic paralysis or kyphoscoliosis may develop plate-like atelectasis and hypoxemia. The unifying characteristics are that they all have normal lung architecture and are breathing at low lung volumes. As demonstrated by Sinha and Bergofsky (66) in kyphoscoliotics, their atelectasis is reversible with lung inflation, as demonstrated by postinflation increased lung compliance. A likely, although unproven, partial explanation, is that, at low lung volumes, and with an inability to take sighs or deep breaths, collateral resistance is so high that air cannot enter poorly ventilated areas, and reabsorption atelectasis occurs.
Effect of Collateral Ventilation on Diagnostic Testing
Bronchoalveolar lavage is commonly performed to obtain diagnostic specimens. Studies have shown that the highest percentage fluid return is from the middle lobe and lingua (67). Although their nondependent nature may contribute to this, it is likely that an increased RColl prevents rapid fluid egress into adjacent segments, aiding in a higher percent return.
It is often necessary in diffuse interstitial lung disease to perform a more invasive lung biopsy when transbronchial and percutaneous approaches fail to make a diagnosis. The surgeon’s choice of biopsy site may be arbitrary or dependent on other clinical factors if the disease is diffuse. Lingula and middle lobe biopsies have commonly been performed because they are easily completed. However, in the past, it has been shown that these biopsy sites may be unreliable, because they have shown nonspecific inflammation and fibrosis not representative of the underlying disease (68, 69). Minimal collateral ventilation, leading to a propensity for the middle lobe and lingular to inadequately clear infections, is a likely contributor to these findings of nonspecific fibrosis and inflammation. A more recent study by Miller and colleagues (70) suggests that use of computed tomography may be helpful in guiding the surgeon to the appropriate biopsy site, especially in immunocompetent patients.
Collateral Ventilation and Treatment of Lung Disorders
Collateral ventilation has been a major determinant in the effectiveness of nonsurgical attempts to reduce lung volumes in patients with emphysema or those with giant bullae, thereby improving their quality of life (71–73). Most studies have used bronchoscopically placed one-way valves occluding segmental airways of target lobes. These valves allow air to leave, but not enter obstructed areas, so that lobar atelectasis can occur. These studies have shown variable outcomes, primarily dependent on the presence or absence of collateral ventilation between adjacent lobes (71, 72). When lobar fissures are incomplete, atelectasis of a lobe may not occur and the patient may not benefit from the procedure. In treated patients with no evidence of collateral ventilation and resultant lobar atelectasis, quality of life and exercise capacity improve (74).
Determination of the presence or absence of collateral ventilation is presently done using the Chartis system, which consists of a balloon-tipped catheter endobronchially placed to occlude a lobar bronchus (75). If air continues to flow from the obstructed lobe through the catheter, despite lobar bronchus occlusion, this indicates collateral ventilation from an adjacent lobe. Attempts are underway to develop noninvasive radiologic techniques to predict the presence or absence of interlobar collateral ventilation in potential candidates (76–78). Interestingly, some patients with relatively small areas of persistent interlobar collateral ventilation have benefitted, even though the obstructed lobes have not collapsed (79). This is due to reduced dynamic hyperinflation in the obstructed lobe during inspiration, leading to greater inflation of the adjacent lobe (80).
Another approach to improving the quality of life of patients with emphysema has been to attempt to create even more collateral ventilation by making airway openings between large airways and adjacent alveoli. In a large, well designed study of 315 patients, multiple proximal airway passages were created and up to 6 stents were placed in proximal airways of patients. Initial evaluation revealed improved physiologic parameters; however, at 6 months, there was no difference between the experimental and control groups (81). This was attributed to closure of the passages, and either expectoration or occlusion of the stents.
A more radical approach that takes advantage of the fact that airways resistance exceeds collateral resistance in patients with emphysema is to create transthoracic fistulae (spiracles), which connect subpleural alveolar spaces to the external environment. Air then enters the lungs through the trachea and spiracles, and may be expired through both. This solution, first proposed by Macklem (82), has been used in a pilot study of three patients with reported success (83). Recently, several patients with emphysema undergoing lung transplantation developed pleural tears in their native lungs, and were documented to be expiring through the pleural tears in preference to the endotracheal tube, demonstrating the preference for collateral ventilation over airways expiration (84).
Persistent air leaks postoperatively are not uncommon. When chest tube drainage, blood patches, and changes in suction pressure are not successful in stopping the leak and surgical treatment is contraindicated, pulmonologists have used endobronchial valves in an attempt to stop air leaks. Because of collateral ventilation, it is common to require that more than one segmental airway be occluded before airflow through the pleural tear ceases (85).
Physical Therapy
The principle benefits of chest physical therapy are to decrease atelectasis and aid in the clearance of secretions. Andersen and colleagues (86) showed that, in excised human lungs made atelectatic, a negative pressure surrounding the lungs to mimic deep breathing or normal tidal volumes combined with continuous positive airway pressure was more effective in opening atelectatic areas via collateral channels than mechanical ventilation techniques with or without positive end-expiratory pressure. They have shown in surgical patients that periodic continuous positive airway pressure by mask was superior to standard postoperative conventional therapy in decreasing atelectasis and improving gas exchange (87). The use of positive end-expiratory pressure maneuvers in patients with cystic fibrosis has been shown to increase lung volumes and promote peripheral collateral air movement distal to bronchi obstructed with mucus (88). Taken together, a reasonable conclusion is that chest physical therapy that includes maneuvers that increase lung volume works because enhanced collateral ventilation at higher lung volumes allows air to accumulate distal to airways narrowed or obstructed by inflammation and secretions, and that, during coughing and expiration, the resultant higher lung volumes and increased air pressure in alveoli distal to the airways obstruction promotes mucus movement to the more proximal, larger airways (89).
Future Directions
Our knowledge of the role of collateral ventilation is incomplete, especially in interstitial diseases. Measurements in various interstitial diseases need to be made to determine if there is variability dependent on the disease state. The demonstration that RColl is responsive to various gas and pharmacologic agents suggests that we need to explore how to exploit these observations. For example, knowing that subjects with asthma have a reduced arterial CO2 partial pressure, and knowing that increased inspired CO2 can reduce collateral resistance, suggest that having subjects with asthma inhale low concentrations of CO2 may be of benefit (90). Such a study was performed in subjects with asthma inhaling 6% CO2, and demonstrated a relaxation of central and peripheral airways and reduced total lung capacity (91). The authors suggested that collateral ventilation changes could explain some of the physiologic improvement.
Conclusions
In some circumstances, collateral ventilation, like collateral circulation, serves to maintain organ function by modulating the effects of disease that would otherwise result in a marked reduction in oxygen delivery to organs. In other circumstances, because of anatomic differences, an increased RColl predisposes to disease. Why, during evolution, such an anatomic difference should occur is unclear. Work needs to be done to discover and apply therapeutic agents that can improve collateral ventilation.
Footnotes
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Van Allen CM, Lindskog GE, Richter HG. Collateral respiration: transfer of air collaterally between pulmonary lobules. J Clin Invest. 1931;10:559–590. doi: 10.1172/JCI100371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrell NW, Wignall BK, Biggs T, Seed WA. Collateral ventilation and gas exchange in emphysema. Am J Respir Crit Care Med. 1994;150:635–641. doi: 10.1164/ajrccm.150.3.8087331. [DOI] [PubMed] [Google Scholar]
- 3.Inners CR, Terry PB, Traystman RJ, Menkes HA. Collateral ventilation and the middle lobe syndrome. Am Rev Respir Dis. 1978;118:305–310. doi: 10.1164/arrd.1978.118.2.305. [DOI] [PubMed] [Google Scholar]
- 4.Kohn HN. Zur histologie der indurirenden fibrinosen pneumonie [in German] Munch Med Wochenschr. 1893;40:42–45. [Google Scholar]
- 5.Lambert MW. Accessory bronchiolealveolar communications. J Pathol Bacteriol. 1955;70:311–314. doi: 10.1002/path.1700700206. [DOI] [PubMed] [Google Scholar]
- 6.Martin HB. Respiratory bronchioles as the pathway for collateral ventilation. J Appl Physiol. 1966;21:1443–1447. doi: 10.1152/jappl.1966.21.5.1443. [DOI] [PubMed] [Google Scholar]
- 7.Raskin SP, Herman PG. Interacinar pathways in the human lung. Am Rev Respir Dis. 1975;111:489–495. doi: 10.1164/arrd.1975.111.4.489. [DOI] [PubMed] [Google Scholar]
- 8.Andersen JB, Jespersen W. Demonstration of intersegmental respiratory bronchioles in normal human lungs. Eur J Respir Dis. 1980;61:337–341. [PubMed] [Google Scholar]
- 9.Marchand Rl. Les pores des alveoles pulmonaires. Bibl Anat. 1912;22:57–71. [Google Scholar]
- 10.Muller J. Zur vergleichnden Histologie der Lungen unserer Haussaugetiere. Arch Mikr Anat. 1907;69:1. [Google Scholar]
- 11.Boyden EA. Notes on the development of the lung in infancy and early childhood. Am J Anat. 1967;121:749–761. doi: 10.1002/aja.1001210317. [DOI] [PubMed] [Google Scholar]
- 12.Merkus PJ, ten Have-Opbroek AA, Quanjer PH. Human lung growth: a review. Pediatr Pulmonol. 1996;21:383–397. doi: 10.1002/(SICI)1099-0496(199606)21:6<383::AID-PPUL6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 13.Macklin CC. Alveolar pores and their significance in the human lung. Arch Pathol (Chic) 1936;21:202–216. [Google Scholar]
- 14.Terry PB, Menkes HA, Traystman RJ. Effects of maturation and aging on collateral ventilation in sheep. J Appl Physiol (1985) 1987;62:1028–1032. doi: 10.1152/jappl.1987.62.3.1028. [DOI] [PubMed] [Google Scholar]
- 15.Terry PB, Traystman RJ, Newball HH, Batra G, Menkes HA. Collateral ventilation in man. N Engl J Med. 1978;298:10–15. doi: 10.1056/NEJM197801052980103. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg DE, Lyons HA. Collateral ventilation in excised human lungs. Respiration. 1979;37:125–134. doi: 10.1159/000194018. [DOI] [PubMed] [Google Scholar]
- 17.Woolcock AJ, Macklem PT. Mechanical factors influencing collateral ventilation in human, dog, and pig lungs. J Appl Physiol. 1971;30:99–115. doi: 10.1152/jappl.1971.30.1.99. [DOI] [PubMed] [Google Scholar]
- 18.Friedman PJ. Radiology of the superior segment of the lower lobe: a regional perspective introducing the B6 bronchus sign. Radiology. 1982;144:15–25. doi: 10.1148/radiology.144.1.7089247. [DOI] [PubMed] [Google Scholar]
- 19.Van Allen CM, Lindskog GE, Richter HG. Gaseous interchange between adjacent lung lobule. Yale J Biol Med. 1930;2:297–300. [PMC free article] [PubMed] [Google Scholar]
- 20.Raasch BN, Carsky EW, Lane EJ, O’Callaghan JP, Heitzman ER. Radiographic anatomy of the interlobar fissures: a study of 100 specimens. AJR Am J Roentgenol. 1982;138:1043–1049. doi: 10.2214/ajr.138.6.1043. [DOI] [PubMed] [Google Scholar]
- 21.Otsuji H, Uchida H, Maeda M, Iwasaki S, Yoshiya K, Hatakeyama M, Ohishi H, Iioka S, Kitamura S, Narita N. Incomplete interlobar fissures: bronchovascular analysis with CT. Radiology. 1993;187:541–546. doi: 10.1148/radiology.187.2.8475304. [DOI] [PubMed] [Google Scholar]
- 22.Inners CR, Terry PB, Traystman RJ, Menkes HA. Effects of lung volume on collateral and airways resistance in man. J Appl Physiol. 1979;46:67–73. doi: 10.1152/jappl.1979.46.1.67. [DOI] [PubMed] [Google Scholar]
- 23.Morrell NW, Roberts CM, Biggs T, Seed WA. Collateral ventilation and gas exchange during airway occlusion in the normal human lung. Am Rev Respir Dis. 1993;147:535–539. doi: 10.1164/ajrccm/147.3.535. [DOI] [PubMed] [Google Scholar]
- 24.Traystman RJ, Batra GK, Menkes HA. Local regulation of collateral ventilation by oxygen and carbon dioxide. J Appl Physiol. 1976;40:819–823. doi: 10.1152/jappl.1976.40.5.819. [DOI] [PubMed] [Google Scholar]
- 25.Swenson EW, Finley TN, Guzman SV. Unilateral hypoventilation in man during temporary occlusion of one pulmonary artery. J Clin Invest. 1961;40:828–835. doi: 10.1172/JCI104316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emery MJ, Eveland RL, Min JH, Hildebrandt J, Swenson ER. CO2 relaxation of the rat lung parenchymal strip. Respir Physiol Neurobiol. 2013;186:33–39. doi: 10.1016/j.resp.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Emery MJ, Eveland RL, Kim SS, Hildebrandt J, Swenson ER. CO2 relaxes parenchyma in the liquid-filled rat lung. J Appl Physiol (1985) 2007;103:710–716. doi: 10.1152/japplphysiol.00128.2006. [DOI] [PubMed] [Google Scholar]
- 28.Menkes HA, Traystman RJ. Collateral ventilation. Am Rev Respir Dis. 1977;116:287–309. doi: 10.1164/arrd.1977.116.2.287. [DOI] [PubMed] [Google Scholar]
- 29.Berzon DM, Menkes H, Dannenberg AM, Jr, Gertner A, Terry P, Plump D, Bromberger-Barnea B. Interstitial fibrosis and collateral ventilation. J Appl Physiol (1985) 1986;61:300–303. doi: 10.1152/jappl.1986.61.1.300. [DOI] [PubMed] [Google Scholar]
- 30.Traystman RJ, Terry PB, Menkes HA. Carbon dioxide—a major determinant of collateral ventilation. J Appl Physiol. 1978;45:69–74. doi: 10.1152/jappl.1978.45.1.69. [DOI] [PubMed] [Google Scholar]
- 31.Zuo Y, Li L, Liu S. Kohn’s pores are not responsible for collateral ventilation between inflated and deflated segments: a microscopic study of pulmonary intersegmental septa in the human lung. J Anat. 2015;226:381–385. doi: 10.1111/joa.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alley RD, Lindskog GE. Pharmacologic factors influencing collateral respiration: possible relation to the etiology of pulmonary complications. Ann Surg. 1948;128:497–508. [PubMed] [Google Scholar]
- 33.Weinmann GG, Spannhake EW, Bromberger-Barnea B, Menkes HA. Tonic beta-sympathetic activity in the lung periphery in anesthetized dogs. J Appl Physiol (1985) 1985;59:979–984. doi: 10.1152/jappl.1985.59.3.979. [DOI] [PubMed] [Google Scholar]
- 34.Delaunois L, Delaunois M. Mecholyl aerosolized in the surrounding lung increases the resistance of the collateral pathways. Eur Respir J. 1988;1:217–222. [PubMed] [Google Scholar]
- 35.Spannhake EW, Kadowitz PJ, Kleeberger SR. Influence of mediators of anaphylaxis on collateral ventilation and the lung periphery of the dog. J Pharmacol Exp Ther. 1985;234:491–497. [PubMed] [Google Scholar]
- 36.Alexander CM, Chen L, Ray R, Marshall BE. The influence of halothane and isoflurane on pulmonary collateral ventilation. Anesthesiology. 1985;62:135–140. doi: 10.1097/00000542-198502000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Irvin CG, Bates JH. Physiologic dysfunction of the asthmatic lung: what’s going on down there, anyway? Proc Am Thorac Soc. 2009;6:306–311. doi: 10.1513/pats.200808-091RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hogg JC. Varieties of airway narrowing in severe and fatal asthma. J Allergy Clin Immunol. 1987;80:417–419. doi: 10.1016/0091-6749(87)90065-0. [DOI] [PubMed] [Google Scholar]
- 39.Wagner PD, Hedenstierna G, Bylin G. Ventilation–perfusion inequality in chronic asthma. Am Rev Respir Dis. 1987;136:605–612. doi: 10.1164/ajrccm/136.3.605. [DOI] [PubMed] [Google Scholar]
- 40.Wagner PD, Dantzker DR, Iacovoni VE, Tomlin WC, West JB. Ventilation–perfusion inequality in asymptomatic asthma. Am Rev Respir Dis. 1978;118:511–524. doi: 10.1164/arrd.1978.118.3.511. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Roisin R, Ballester E, Roca J, Torres A, Wagner PD. Mechanisms of hypoxemia in patients with status asthmaticus requiring mechanical ventilation. Am Rev Respir Dis. 1989;139:732–739. doi: 10.1164/ajrccm/139.3.732. [DOI] [PubMed] [Google Scholar]
- 42.Sekerel BE, Nakipoglu F. Middle lobe syndrome in children with asthma: review of 56 cases. J Asthma. 2004;41:411–417. doi: 10.1081/jas-120033983. [DOI] [PubMed] [Google Scholar]
- 43.Tsai SL, Crain EF, Silver EJ, Goldman HS. What can we learn from chest radiographs in hypoxemic asthmatics? Pediatr Radiol. 2002;32:498–504. doi: 10.1007/s00247-001-0654-7. [DOI] [PubMed] [Google Scholar]
- 44.Hogg JC, Williams J, Richardson JB, Macklem PT, Thurlbeck WM. Age as a factor in the distribution of lower-airway conductance and in the pathologic anatomy of obstructive lung disease. N Engl J Med. 1970;282:1283–1287. doi: 10.1056/NEJM197006042822302. [DOI] [PubMed] [Google Scholar]
- 45.Dunnill MS. Postnatal growth of the lung. Thorax. 1962;17:329–333. [Google Scholar]
- 46.Soyer O, Ozen C, Cavkaytar O, Senyucel C, Dallar Y. Right middle lobe atelectasis in children with asthma and prognostic factors. Allergol Int. 2016;65:253–258. doi: 10.1016/j.alit.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Ayed AK. Resection of the right middle lobe and lingula in children for middle lobe/lingula syndrome. Chest. 2004;125:38–42. doi: 10.1378/chest.125.1.38. [DOI] [PubMed] [Google Scholar]
- 48.Burrows B, Niden AH, Fletcher CM, Jones NL. Clinical types of chronic obstructive lung disease in London and Chicago: a study of one hundred. Am Rev Respir Dis. 1964;90:14–27. doi: 10.1164/arrd.1964.90.1.14. [DOI] [PubMed] [Google Scholar]
- 49.Hogg JC, Macklem PT, Thurlbeck WM. The resistance of collateral channels in excised human lungs. J Clin Invest. 1969;48:421–431. doi: 10.1172/JCI105999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagner PD, Dantzker DR, Dueck R, Clausen JL, West JB. Ventilation–perfusion inequality in chronic obstructive pulmonary disease. J Clin Invest. 1977;59:203–216. doi: 10.1172/JCI108630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agusti AG, Roca J, Rodriguez-Roisin R, Xaubet A, Agusti-Vidal A. Different patterns of gas exchange response to exercise in asbestosis and idiopathic pulmonary fibrosis. Eur Respir J. 1988;1:510–516. [PubMed] [Google Scholar]
- 52.Agustí AG, Roca J, Gea J, Wagner PD, Xaubet A, Rodriguez-Roisin R. Mechanisms of gas-exchange impairment in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1991;143:219–225. doi: 10.1164/ajrccm/143.2.219. [DOI] [PubMed] [Google Scholar]
- 53.Brock RC, Cann RJ, Dickinson JR. Tuberculous mediastinal lymphadenitis in childhood: secondary effects on the lungs. Guys Hosp Rep. 1937;87:295. [Google Scholar]
- 54.Graham EA, Burford TH, Mayer JH. Middle lobe syndrome. Postgrad Med. 1948;4:29–34. doi: 10.1080/00325481.1948.11693655. [DOI] [PubMed] [Google Scholar]
- 55.Culiner MM. The right middle lobe syndrome, a non-obstructive complex. Dis Chest. 1966;50:57–66. doi: 10.1016/s0096-0217(15)33021-1. [DOI] [PubMed] [Google Scholar]
- 56.Bradham RR, Sealy WC, Young WG., Jr Chronic middle lobe infection: factors responsible for its development. Ann Thorac Surg. 1966;2:612–616. doi: 10.1016/s0003-4975(10)66625-8. [DOI] [PubMed] [Google Scholar]
- 57.Kartalija M, Ovrutsky AR, Bryan CL, Pott GB, Fantuzzi G, Thomas J, Strand MJ, Bai X, Ramamoorthy P, Rothman MS, et al. Patients with nontuberculous mycobacterial lung disease exhibit unique body and immune phenotypes. Am J Respir Crit Care Med. 2013;187:197–205. doi: 10.1164/rccm.201206-1035OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iseman MD, Buschman DL, Ackerson LM. Pectus excavatum and scoliosis: thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am Rev Respir Dis. 1991;144:914–916. doi: 10.1164/ajrccm/144.4.914. [DOI] [PubMed] [Google Scholar]
- 59.Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern: the Lady Windermere syndrome. Chest. 1992;101:1605–1609. doi: 10.1378/chest.101.6.1605. [DOI] [PubMed] [Google Scholar]
- 60.Reich JM. Cough suppression disorders spectrum. Respir Med. 2014;108:413–415. doi: 10.1016/j.rmed.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 61.Leung JM, Fowler C, Smith C, Adjemian J, Frein C, Claypool RJ, Holland SM, Prevots RD, Olivier K. A familial syndrome of pulmonary nontuberculous mycobacteria infections. Am J Respir Crit Care Med. 2013;188:1373–1376. doi: 10.1164/rccm.201306-1059LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Culiner MM, Wall CA. Collateral ventilation in “intralobar pulmonary sequestration”: report of a case. Dis Chest. 1965;47:118–122. doi: 10.1378/chest.47.1.118. [DOI] [PubMed] [Google Scholar]
- 63.Culiner MM. Bronchial cysts and collateral ventilation. Dis Chest. 1964;45:627–638. doi: 10.1378/chest.45.6.627. [DOI] [PubMed] [Google Scholar]
- 64.Culiner MM. Collateral ventilation and the hyperlucent lung. Am J Med. 1964;36:395–403. doi: 10.1016/0002-9343(64)90166-4. [DOI] [PubMed] [Google Scholar]
- 65.Baarsma PR, Dirken MN, Huizinga E. Collateral ventilation in man. J Thorac Surg. 1948;17:252–263. [PubMed] [Google Scholar]
- 66.Sinha R, Bergofsky EH. Prolonged alteration of lung mechanics in kyphoscoliosis by positive pressure hyperinflation. Am Rev Respir Dis. 1972;106:47–57. doi: 10.1164/arrd.1972.106.1.47. [DOI] [PubMed] [Google Scholar]
- 67.Pingleton SK, Harrison GF, Stechschulte DJ, Wesselius LJ, Kerby GR, Ruth WE. Effect of location, pH, and temperature of instillate in bronchoalveolar lavage in normal volunteers. Am Rev Respir Dis. 1983;128:1035–1037. doi: 10.1164/arrd.1983.128.6.1035. [DOI] [PubMed] [Google Scholar]
- 68.Ray JF, III, Lawton BR, Myers WO, Toyama WM, Reyes CN, Emanuel DA, Burns JL, Pederson DP, Dovenbarger WV, Wenzel FJ, et al. Open pulmonary biopsy: nineteen-year experience with 416 consecutive operations. Chest. 1976;69:43–47. doi: 10.1378/chest.69.1.43. [DOI] [PubMed] [Google Scholar]
- 69.Newman SL, Michel RP, Wang NS. Lingular lung biopsy: is it representative? Am Rev Respir Dis. 1985;132:1084–1086. doi: 10.1164/arrd.1985.132.5.1084. [DOI] [PubMed] [Google Scholar]
- 70.Miller RR, Nelems B, Müller NL, Evans KG, Ostrow DN. Lingular and right middle lobe biopsy in the assessment of diffuse lung disease. Ann Thorac Surg. 1987;44:269–273. doi: 10.1016/s0003-4975(10)62071-1. [DOI] [PubMed] [Google Scholar]
- 71.Sciurba FC, Ernst A, Herth FJ, Strange C, Criner GJ, Marquette CH, Kovitz KL, Chiacchierini RP, Goldin J, McLennan G VENT Study Research Group. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010;363:1233–1244. doi: 10.1056/NEJMoa0900928. [DOI] [PubMed] [Google Scholar]
- 72.Toma TP, Hopkinson NS, Hillier J, Hansell DM, Morgan C, Goldstraw PG, Polkey MI, Geddes DM. Bronchoscopic volume reduction with valve implants in patients with severe emphysema. Lancet. 2003;361:931–933. doi: 10.1016/S0140-6736(03)12762-6. [DOI] [PubMed] [Google Scholar]
- 73.Tian Q, An Y, Xiao BB, Chen L-A. Treatment of giant emphysamous bulla with endobronchial valves in patients with chronic obstructive pulmonary disease: a case series. J Thorac Dis. 2014;6:1674–1680. doi: 10.3978/j.issn.2072-1439.2014.11.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klooster K, ten Hacken NHT, Hartman JE, Kerstjens HAM, van Rikxoort EM, Slebos DJ. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med. 2015;373:2325–2335. doi: 10.1056/NEJMoa1507807. [DOI] [PubMed] [Google Scholar]
- 75.Gompelmann D, Eberhardt R, Slebos DJ, Ficker J, Reichenberger F, Schmidt B, Elk L, Herth F.Study of the use of Chartis pulmonary assessment system to optimize subject selection for endobronchial lung volume reduction (ELVR) - results and subgroup analysis [abstract] Chest 2011140(4_MeetingAbstracts)46A [Google Scholar]
- 76.Reymond E, Jankowski A, Pison C, Bosson JL, Prieur M, Aniwidyaningsih W, Ferretti GR. Prediction of lobar collateral ventilation in 25 patients with severe emphysema by fissure analysis with CT. AJR Am J Roentgenol. 2013;201:W571–W575. doi: 10.2214/AJR.12.9843. [DOI] [PubMed] [Google Scholar]
- 77.Diso D, Anile M, Carillo C, Ruberto F, Patella M, Russo E, Fraioli F, De Giacomo T, Mantovani S, Rendina E, et al. Correlation between collateral ventilation and interlobar lung fissures. Respiration. 2014;88:315–319. doi: 10.1159/000363538. [DOI] [PubMed] [Google Scholar]
- 78.Schuhmann M, Raffy P, Yin Y, Gompelmann D, Oguz I, Eberhardt R, Hornberg D, Heussel CP, Wood S, Herth FJ. Computed tomography predictors of response to endobronchial valve lung reduction treatment: comparison with Chartis. Am J Respir Crit Care Med. 2015;191:767–774. doi: 10.1164/rccm.201407-1205OC. [DOI] [PubMed] [Google Scholar]
- 79.Hopkinson NS, Toma TP, Hansell DM, Goldstraw P, Moxham J, Geddes DM, Polkey MI. Effect of bronchoscopic lung volume reduction on dynamic hyperinflation and exercise in emphysema. Am J Respir Crit Care Med. 2005;171:453–460. doi: 10.1164/rccm.200407-961OC. [DOI] [PubMed] [Google Scholar]
- 80.Fessler HE. Collateral ventilation, the bane of bronchoscopic volume reduction. Am J Respir Crit Care Med. 2005;171:423–424. doi: 10.1164/rccm.2412005. [DOI] [PubMed] [Google Scholar]
- 81.Shah PL, Slebos DJ, Cardoso PFG, Cetti E, Voelker K, Levine B, Russell ME, Goldin J, Brown M, Cooper JD, et al. EASE Trial Study Group. Bronchoscopic lung-volume reduction with Exhale airway stents for emphysema (EASE trial): randomised, sham-controlled, multicentre trial. Lancet. 2011;378:997–1005. doi: 10.1016/S0140-6736(11)61050-7. [DOI] [PubMed] [Google Scholar]
- 82.Macklem PT. Collateral ventilation. N Engl J Med. 1978;298:49–50. doi: 10.1056/NEJM197801052980112. [DOI] [PubMed] [Google Scholar]
- 83.Saad Jr R, Dorgan Neto V, Botter M, Stirbulov R, Rivaben JH, Gonçalves R. Therapeutic application of collateral ventilation with pulmonary drainage in the treatment of diffuse emphysema: report of three cases [in English, Portuguese] J Bras Pneumol. 2009;35:14–19. doi: 10.1590/s1806-37132009000100003. [DOI] [PubMed] [Google Scholar]
- 84.Chahla M, Larson CD, Parekh KR, Reed RM, Terry P, Schmidt GA, Eberlein M. Transpleural ventilation via spiracles in severe emphysema increases alveolar ventilation. Chest. 2016;149:e161–e167. doi: 10.1016/j.chest.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 85.Gkegkes ID, Mourtarakos S, Gakidis I. Endobronchial valves in treatment of persistent air leaks: a systematic review of clinical evidence. Med Sci Monit. 2015;21:432–438. doi: 10.12659/MSM.891320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Andersen JB, Qvist J, Kann T. Recruiting collapsed lung through collateral channels with positive end-expiratory pressure. Scand J Respir Dis. 1979;60:260–266. [PubMed] [Google Scholar]
- 87.Andersen JB, Olesen P, Eikard B, Gansen E, Qvist J. Periodic continuous positive airway pressure, CPAP, by mask in the treatment of atelectasis. Eur J Respir Dis. 1980;61:20–25. [Google Scholar]
- 88.Darbee JC, Ohtake PJ, Grant BJ, Cerny FJ. Physiologic evidence for the efficacy of positive expiratory pressure as an airway clearance technique in patients with cystic fibrosis. Phys Ther. 2004;84:524–537. [PubMed] [Google Scholar]
- 89.Hristara-Papadopoulou A, Tsanakas J, Diomou G, Papadopoulou O. Current devices of respiratory physiotherapy. Hippokratia. 2008;12:211–220. [PMC free article] [PubMed] [Google Scholar]
- 90.McFadden ER, Jr, Lyons HA. Arterial-blood gas tension in asthma. N Engl J Med. 1968;278:1027–1032. doi: 10.1056/NEJM196805092781901. [DOI] [PubMed] [Google Scholar]
- 91.Fisher HK, Hansen TA. Site of action of inhaled 6 per cent carbon dioxide in the lungs of asthmatic subjects before and after exercise. Am Rev Respir Dis. 1976;114:861–870. doi: 10.1164/arrd.1976.114.5.861. [DOI] [PubMed] [Google Scholar]