Abstract
Rationale: The IFN-γ release assays and tuberculin skin tests are used to support the diagnosis of both latent and active tuberculosis. However, we previously demonstrated that a negative tuberculin test in active tuberculosis is associated with disseminated disease and death. It is unknown whether the same associations exist for IFN-γ release assays.
Objectives: To determine the association between these tests and site of tuberculosis and death among persons with active tuberculosis.
Methods: We analyzed IFN-γ release assays and tuberculin test results for all persons with culture-confirmed tuberculosis reported to the U.S. National Tuberculosis Surveillance System from 2010 to 2014. We used logistic regression to calculate the association between these tests and site of disease and death.
Measurements and Main Results: A total of 24,803 persons with culture-confirmed tuberculosis had either of these test results available for analysis. Persons with a positive tuberculin test had lower odds of disseminated disease (i.e., miliary or combined pulmonary and extrapulmonary disease), but there was no difference in the odds of disseminated disease with a positive IFN-γ release assay. However, persons who were positive to either of these tests had lower odds of death. An indeterminate IFN-γ release assay result was associated with greater odds of both disseminated disease and death.
Conclusions: Despite perceived equivalence in clinical practice, IFN-γ release assays and tuberculin test results have different associations with tuberculosis site, yet similar associations with the risk of death. Furthermore, an indeterminate IFN-γ release assay result in a person with active tuberculosis is not unimportant, and rather carries greater odds of disseminated disease and death. Prospective study may improve our understanding of the underlying mechanisms by which these tests are associated with disease localization and death.
Keywords: tuberculin test, Mycobacterium tuberculosis, public health surveillance
Although the tuberculin skin test (TST, tuberculin test) and IFN-γ release assays (IGRAs) can both be used to diagnose latent tuberculosis infection and to aid in the diagnosis of active tuberculosis, their significance as a prognostic indicator among patients with active tuberculosis has not been well explored. Current guidelines suggest that for the majority of patients these tests can be used interchangeably (1), yet there are important distinctions to keep in mind with these immunologic assays. A positive tuberculin test result represents an in vivo delayed-type hypersensitivity response of host memory T cells sensitized by prior mycobacterial exposure, whether to Mycobacterium tuberculosis complex (including bacillus Calmette-Guérin vaccination) or nontuberculous mycobacteria. The tuberculin test reaction involves multiple cell types, including lymphocytes, basophils, monocytes, and neutrophils, as well as local cytokine release, vasodilation, and edema (2–4). Meanwhile, a positive IGRA result, consisting of an in vitro IFN-γ release in response to specific antigens, detects prior sensitization to M. tuberculosis and will not cross-react with bacillus Calmette-Guérin or most nontuberculous mycobacteria (5). IGRA testing can also yield an indeterminate result if there is failure of either the positive control (i.e., lack of response to mitogen) or negative control (i.e., high baseline levels of IFN-γ). An indeterminate IGRA result is believed to represent an uncertain likelihood of M. tuberculosis infection.
There is increasing evidence that the immunologic processes underlying these assays may translate into biologically meaningful distinctions in the context of clinical disease. A study of isoniazid preventive therapy among people with HIV infection in Botswana found that extended prophylaxis was beneficial primarily for persons with a positive tuberculin test at baseline; isoniazid prophylaxis did not decrease the incidence of tuberculosis among those with a negative tuberculin test (6). Furthermore, among those given extended isoniazid, tuberculosis incidence was higher for tuberculin test–negative persons than for tuberculin test–positive persons. The reason for this difference in the protective effect of isoniazid preventive therapy by tuberculin test result is not clear but suggests that the natural history of tuberculosis infection and response to therapy may be different for persons with a positive tuberculin test as compared with a negative tuberculin test.
To investigate whether these reported differences in clinical disease by tuberculin test result were present for active tuberculosis, we previously analyzed the association between tuberculin test result and clinical characteristics of tuberculosis using U.S. national surveillance data. We found that persons with a positive tuberculin test had lower odds of disseminated disease, even after adjusting for HIV status, birthplace, age, and sex (7). We also found that persons with a positive tuberculin test had lower odds of death after initiating tuberculosis treatment (8). Taken together, these findings further reinforce that a positive tuberculin test may be more than simply a marker of infection and may be directly relevant to the clinical presentation and prognosis of active tuberculosis.
Since the publication of our previous analysis, IGRA results were added to the U.S. surveillance data and are now available for analysis. As there have been only limited reports to date on IGRA results among persons with active tuberculosis (9–11), we wanted to determine whether the significant associations that we previously observed between the tuberculin test result and active tuberculosis would hold true for the IGRAs. We also wanted to explore the significance of an indeterminate IGRA result in the setting of active tuberculosis, particularly as there is no analogous tuberculin test result category. Some of the results of these studies have been previously reported in the form of an abstract (12).
Methods
Study Population and Design
Our analysis was a retrospective cohort study including all persons with culture-confirmed tuberculosis with either a documented IGRA or tuberculin test result and disease site reported to the U.S. National Tuberculosis Surveillance System during January 1, 2010 through December 31, 2014. National Tuberculosis Surveillance System data include demographic, clinical, and risk factor information for all reported cases of tuberculosis. These data are collected by state and local tuberculosis programs and submitted electronically to the Centers for Disease Control and Prevention (CDC). The IGRA variable was added to the national tuberculosis case report forms in January 2009, and all states were required to report using the revised forms by 2010. Of note, reports from California from 2010 were excluded from this analysis, because HIV status was not routinely reported from that jurisdiction in that year (13). During the study period, there was U.S. Food and Drug Administration approval for two purified-protein derivatives, Tubersol and Aplisol, and four IGRAs, QuantiFERON-TB, QuantiFERON-TB GOLD, QuantiFERON-TB Gold In-Tube, and T-SPOT.TB.
Analysis
We first compared the sociodemographic and case characteristics for persons with and without documented IGRA or tuberculin test results. We next compared case characteristics by IGRA or tuberculin test results (i.e., positive, negative, indeterminate) and used Pearson chi-square statistic to assess differences in the distribution of IGRA or tuberculin test results by these characteristics.
We then examined the association between IGRA or tuberculin test result and site of tuberculosis disease using multinomial logistic regression. IGRA and tuberculin test results were the independent predictor variables, and site of disease was the dependent outcome variable. IGRA results were classified as negative, positive, or indeterminate. A tuberculin test result of 0 to 4 mm was considered negative, and a result greater than or equal to 5 mm was considered positive. (Although CDC guidelines for diagnosing latent infection stipulate differential interpretation of the tuberculin test result on the basis of patient risk factors [14], we previously determined that any result ≥5 mm could be classified as positive when analyzing active tuberculosis cases [7].) We also conducted a sensitivity analysis with tuberculin test cutoffs of 10 and 15 mm and found no appreciable change in the results (data not shown). Site of disease was defined as one of the following mutually exclusive categories: miliary disease, combined pulmonary and extrapulmonary disease, extrapulmonary-only disease, and pulmonary-only disease. Pulmonary-only disease was further divided into noncavitary pulmonary disease and cavitary pulmonary disease. Of note, on the standard case report forms, miliary disease is indicated on the basis of compatible chest radiographic findings.
For the multinomial analysis, noncavitary pulmonary disease, the largest disease site category, was selected as the referent outcome category, and a negative IGRA (or tuberculin test) was used as the referent predictor category. Thus, persons with noncavitary pulmonary disease with a negative IGRA (or tuberculin test) served as the comparison group to calculate odds ratios for each of the respective site of disease and IGRA (or tuberculin test) result category combinations (e.g., miliary disease with an indeterminate IGRA was compared with noncavitary pulmonary disease with a negative IGRA, and cavitary pulmonary disease with a positive IGRA was also compared with noncavitary pulmonary disease with a negative IGRA). On the basis of clinical relevance and our previous analyses, we included sex, age, HIV status, and birthplace in the models as covariates. We also analyzed IGRA result by the test type (i.e., any QuantiFERON assay versus T-SPOT.TB) and did not find evidence of confounding or interaction, and so test type was not included in the models.
We next determined the association between death and IGRA or tuberculin test results. We used multivariate logistic regression to determine the association between IGRA or tuberculin test results and (1) death at the time of tuberculosis diagnosis, and (2) death after starting tuberculosis treatment. We included site of disease as an additional covariate in both models and baseline drug susceptibility as an additional covariate in the model looking at death after starting tuberculosis treatment. Kaplan-Meier survival curves for the probability of survival after treatment initiation were constructed and compared using the Wilcoxon test. All analyses were conducted using R statistical software (version 3.1.2, nnet package) (15).
Ethics
Approval by an institutional review board was not required because data were collected and analyzed for this project as part of routine tuberculosis surveillance, and the project is therefore not considered research involving human subjects.
Results
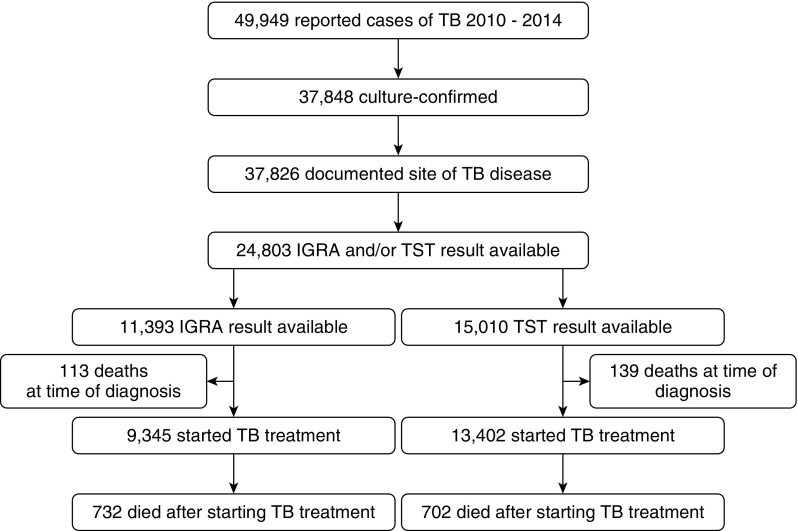
Of 49,949 tuberculosis cases reported in the United States during 2010 through 2014, 37,848 (76%) were culture confirmed, and 24,803 (66%) had either an IGRA or tuberculin test result reported and were eligible for this study (Figure 1). There were 11,393 persons with an IGRA result and 15,010 persons with a tuberculin test result reported; 3,397 persons had both an IGRA and a tuberculin test result reported, and these individuals were analyzed independently for each test result.
Figure 1.
Flow diagram for selection of U.S. tuberculosis (TB) cases reported to the Centers for Disease Control and Prevention during 2010 through 2014 for inclusion in the analysis. Note: Cases from California in 2010 were not included because of lack of HIV reporting in that year. IGRA = IFN-γ release assay; TB = tuberculosis; TST = tuberculin skin test.
Case Characteristics by IGRA Result
Among persons with a documented IGRA result, 9,232 (81%) had a positive IGRA, 1,520 (13%) had a negative IGRA, and 641 (6%) had an indeterminate IGRA (Table 1). Negative and indeterminate IGRAs were more common among those older than 65 years, infected with HIV or with unknown HIV status, and with either miliary or combined pulmonary and extrapulmonary disease. A positive IGRA was more common among those younger than 45 years, and a lower proportion of persons between the ages of 5 and 44 years had an indeterminate IGRA. Non-Hispanic white individuals and U.S.-born persons had lower proportions of a positive IGRA. There were no noteworthy differences in the distribution of IGRA results according to sex or baseline sputum smear result.
Table 1.
IFN-γ release assay result and characteristics of culture-confirmed tuberculosis cases
| Total | IGRA Positive n (%) | IGRA Negative n (%) | IGRA Indeterminate n (%) | |
|---|---|---|---|---|
| Total | 11,393 | 9,232 (81) | 1,520 (13) | 641 (6) |
| Age, yr | ||||
| 0–4 | 99 | 85 (86) | 8 (8) | 6 (6) |
| 5–14 | 114 | 102 (89) | 9 (8) | 3 (3) |
| 15–24 | 1,283 | 1,137 (89) | 110 (9) | 36 (3) |
| 25–44 | 3,662 | 3,100 (85) | 398 (11) | 164 (4) |
| 45–64 | 3,514 | 2,803 (80) | 491 (14) | 220 (6) |
| 65+ | 2,721 | 2,005 (74) | 504 (19) | 212 (8) |
| Sex | ||||
| Male | 6,942 | 3,693 (83) | 528 (12) | 230 (5) |
| Female | 4,451 | 5,539 (80) | 992 (14) | 411 (6) |
| Race/ethnicity* | ||||
| Hispanic | 3,150 | 2,627 (83) | 366 (12) | 157 (5) |
| American Indian | 89 | 72 (81) | 11 (12) | 6 (7) |
| Asian | 3,742 | 3,019 (81) | 515 (14) | 208 (6) |
| Black | 2,466 | 2,062 (84) | 260 (11) | 144 (6) |
| Native Hawaiian | 87 | 77 (89) | 6 (7) | 4 (5) |
| White | 1,661 | 1,224 (74) | 329 (20) | 108 (7) |
| Birthplace | ||||
| U.S. born | 3,620 | 2,825 (78) | 554 (15) | 241 (7) |
| Foreign born | 7,767 | 6,404 (82) | 964 (12) | 399 (5) |
| HIV status | ||||
| Negative | 9,664 | 7,955 (82) | 1,219 (13) | 490 (5) |
| Positive | 674 | 456 (68) | 148 (22) | 70 (10) |
| Unknown | 108 | 80 (74) | 18 (17) | 10 (9) |
| Site of disease | ||||
| Miliary | 549 | 413 (75) | 78 (14) | 58 (11) |
| Pulmonary/extrapulmonary | 1,333 | 1,035 (78) | 192 (14) | 106 (8) |
| Extrapulmonary | 2,190 | 1,791 (82) | 293 (13) | 106 (5) |
| Noncavitary pulmonary | 4,792 | 3,902 (81) | 647 (14) | 243 (5) |
| Cavitary pulmonary | 2,529 | 2,091 (83) | 310 (12) | 128 (5) |
| Sputum smear | ||||
| Negative | 5,049 | 4,127 (82) | 687 (14) | 235 (5) |
| Positive | 4,965 | 4,008 (81) | 634 (13) | 323 (7) |
| Death before treatment | ||||
| Survived | 11,277 | 9,178 (81) | 1,490 (13) | 609 (5) |
| Died | 113 | 52 (46) | 30 (27) | 31 (27) |
| Death during treatment | ||||
| Survived | 10,661 | 8,754 (82) | 1,365 (13) | 542 (5) |
| Died | 732 | 478 (65) | 155 (21) | 99 (14) |
Definition of abbreviations: IGRA = IFN-γ release assay.
Pearson chi-square test P value < 0.001 for all groups.
All categories other than “Hispanic” can be considered non-Hispanic.
There were also differences in sociodemographic and clinical characteristics according to which test was documented (i.e., IGRA, tuberculin test, or neither). Notably, a greater proportion of U.S.-born persons had only a tuberculin test result reported, and a greater proportion of foreign-born persons had only an IGRA result reported (see Table E1 in the online supplement). The distribution of tuberculin test results by sociodemographic and clinical characteristics is available in Table E2.
Association between IGRA Result, Tuberculin Test Result, and Site of Tuberculosis
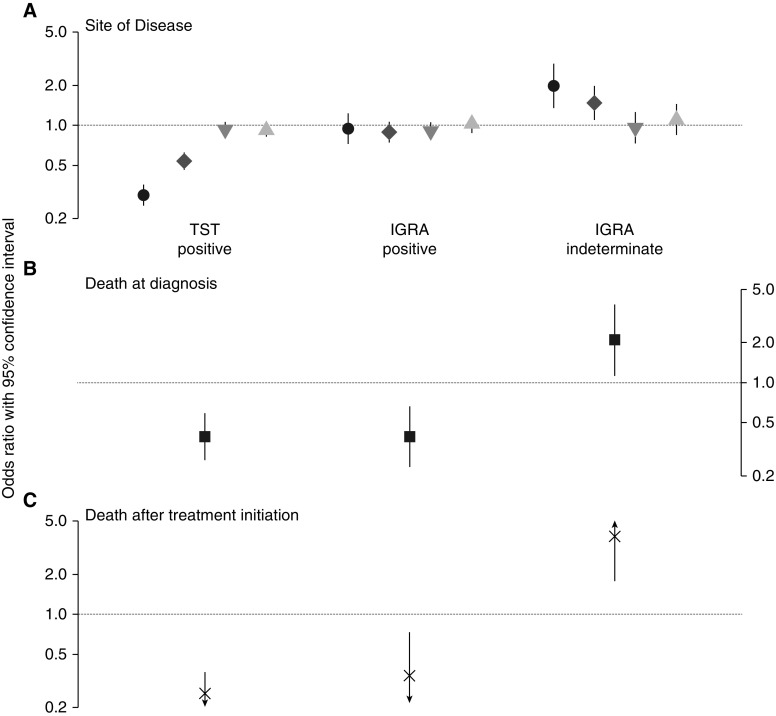
For the analysis of IGRA or tuberculin test and site of tuberculosis, we first confirmed the associations between tuberculin test and disseminated disease (i.e., miliary or combined pulmonary and extrapulmonary disease) identified in our previous analysis (7). We found that persons with a positive tuberculin test had 67% lower odds of miliary disease (adjusted odds ratio [aOR], 0.33; 95% CI, 0.28–0.39) and 41% lower odds of combined pulmonary and extrapulmonary disease (aOR, 0.59; 95% CI, 0.51–0.67) than those with a negative tuberculin test (Table 2 and Figure 2A). There was no association between a positive tuberculin test and either extrapulmonary-only disease or cavitary pulmonary disease. Next, in examining IGRA result and site of tuberculosis, we found no association between a positive IGRA and disease site when compared with a negative IGRA. However, an indeterminate IGRA was significantly associated with disease site, such that persons with an indeterminate IGRA had nearly twofold greater odds of miliary disease (aOR, 1.98; 95% CI, 1.36–2.87) and nearly 50% greater odds of combined pulmonary and extrapulmonary disease (aOR, 1.47; 95% CI, 1.11–1.96) than those with a negative IGRA.
Table 2.
Multinomial associations between site of disease and tuberculin test result and IFN-γ release assay result
| Miliary Disease | Pulmonary and Extrapulmonary Disease | Extrapulmonary-Only Disease | Cavitary Pulmonary Disease | |
|---|---|---|---|---|
| Tuberculin test* | ||||
| Negative | Reference | Reference | Reference | Reference |
| Positive | 0.33 (0.28–0.39) | 0.59 (0.51–0.67) | 1.01 (0.89–1.14) | 0.91 (0.83–1.01) |
| IGRA | ||||
| Negative | Reference | Reference | Reference | Reference |
| Positive | 0.95 (0.73–1.23) | 0.89 (0.75–1.07) | 0.90 (0.78–1.05) | 1.03 (0.89–1.19) |
| Indeterminate | 1.98 (1.36–2.87) | 1.47 (1.11–1.96) | 0.96 (0.73–1.25) | 1.11 (0.86–1.43) |
Definition of abbreviation: IGRA = IFN-γ release assay.
Data presented as adjusted odds ratio (95% CI). Models are adjusted for age, sex, HIV status, and birthplace. Noncavitary pulmonary disease, with the largest number of cases, is the referent clinical category. Statistically significant results are represented in bold type.
Tuberculin test negative = 0–4 mm; tuberculin test positive = 5+ mm.
Figure 2.
Association between IFN-γ release assay (IGRA) result, tuberculin skin test (TST) result, and (A) site of disease, (B) death at the time of diagnosis, and (C) death after initiation of tuberculosis treatment. In A, black circles represent adjusted odds ratio for miliary disease, dark gray diamonds for combined pulmonary and extrapulmonary disease, gray downward triangles for extrapulmonary-only disease, light gray upward triangles for cavitary pulmonary disease. Odds modeled are those of each disease outcome category (the referent disease category is noncavitary pulmonary disease) for a positive (vs. negative) tuberculin test result or a positive or indeterminate (vs. negative) IGRA result. Lines represent the 95% confidence intervals for the point estimates. In B and C, odds modeled are those of death, and arrowheads represent a 95% confidence interval extending beyond the vertical axis.
Association between IGRA Result, Tuberculin Test Result, and Death
Among 11,393 persons with an IGRA result, 113 (1%) were dead at the time of tuberculosis diagnosis and 732 (8%) died after starting treatment (Figure 1). Similarly, among 15,010 persons with a tuberculin test result, 139 (1%) were dead at the time of diagnosis and 702 (6%) died after starting treatment. Persons with a positive tuberculin test had 75% lower odds of death at the time of diagnosis (aOR, 0.25; 95% CI, 0.17–0.36) (Table 3, Figure 2B) and 68% lower odds of death after initiating treatment than those with a negative tuberculin test (aOR, 0.32; 95% CI, 0.27–0.38). A similar pattern was observed with IGRA, where persons with a positive IGRA had 66% lower odds of death at diagnosis than those with a negative IGRA (aOR, 0.34; 95% CI, 0.17–0.73) and 34% lower odds of death than those with a negative IGRA (aOR, 0.66; 95% CI, 0.54–0.80). Persons with an indeterminate IGRA had an adjusted odds of death at diagnosis that was 3.85 (95% CI, 1.78–8.62) times greater than those with a negative IGRA and 11.18 (95% CI, 5.76–21.53) times greater than those with a positive IGRA.
Table 3.
Association between tuberculin test result, IFN-γ release assay result, and death
| Death at Time of Diagnosis | Death after Starting Tuberculosis Treatment* | |
|---|---|---|
| Tuberculin test† | ||
| Negative | Ref | Ref |
| Positive | 0.25 (0.17–0.36) | 0.32 (0.27–0.38) |
| IGRA | ||
| Negative | Ref | Ref |
| Positive | 0.34 (0.17–0.73) | 0.66 (0.54–0.80) |
| Indeterminate | 3.85 (1.78–8.62) | 1.46 (1.09–1.95) |
Definition of abbreviation: IGRA = IFN-γ release assay.
Data presented as adjusted odds ratio (95% CI). Models are adjusted for age, sex, HIV status, birthplace, and site of disease. Probability modeled is of death. Statistically significant results are represented in bold type.
Model is also adjusted for baseline drug susceptibility status.
Tuberculin test negative = 0–4 mm; tuberculin test positive = 5+ mm.
An indeterminate IGRA was also associated with a 46% increase in the odds of death after starting treatment as compared with a negative IGRA (aOR, 1.46; 95% CI, 1.09–1.95) (Table 3, Figure 2C). Kaplan-Meier survival curves depict worse survival for persons with a negative tuberculin test, negative IGRA, or indeterminate IGRA than persons with a positive test (P value < 0.001 for all comparisons) (Figure 3).
Figure 3.
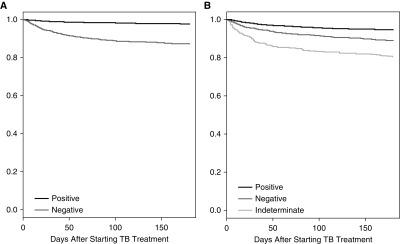
Kaplan-Meier survival curves for patients starting tuberculosis (TB) treatment by (A) tuberculin test or (B) IFN-γ release assay result.
Discussion
In this comprehensive cohort of all persons with culture-confirmed active tuberculosis reported in the United States between 2010 and 2014, we were surprised to find different associations for tuberculin test and IGRA results and tuberculosis. Although a positive tuberculin test was significantly protective against disseminated tuberculosis, there was no association between a positive IGRA and disease site. However, both a positive tuberculin test and a positive IGRA were associated with decreased odds of death. These findings contrasted with our initial hypothesis that the tuberculin test and IGRA would have similar patterns of association for persons with active tuberculosis. Additionally, an indeterminate IGRA result was significantly associated with both disease site and an increased odds of death as compared with a negative IGRA.
It is not clear why there would be inconsistent associations between these two assays and site of disease. Given that IGRA positivity is not associated with disease site, our findings suggest that the narrow, antigen-specific T-cell response represented by a positive IGRA is not important for disease localization. This finding should not be wholly unexpected, because the antigens included in the IGRAs, CFP-10, and ESAT-6 (and TB7.7 in the newest QuantiFERON-TB Gold In-Tube assay), were selected for their sensitivity and specificity for M. tuberculosis and not because a T-cell response to these antigens is necessarily relevant to disease pathogenesis (16). In contrast, the tuberculin test response includes many cell types responding to a broader mix of antigens that may represent a more physiologic and coordinated response comparable to an in vivo infection. A study of household contacts of persons with pulmonary tuberculosis found different regulatory T cell subsets for contacts with a positive tuberculin test and IGRA as opposed to persons with a positive tuberculin test and a negative IGRA (17). Such reports, in combination with our data, reinforce that the tuberculin test and IGRA measure distinct immunological processes, despite their relative equivalence in routine clinical practice (1).
We found that both a positive tuberculin test and a positive IGRA were associated with an approximately 70% lower odds of death at the time of diagnosis and 40 to 70% lower odds of death among persons who initiated tuberculosis treatment. An indeterminate IGRA was associated with the greatest odds of death, with more than a threefold increase in the odds of death at the time of diagnosis and a nearly 50% increase in the odds of death after starting treatment as compared with persons with a negative IGRA.
The reason for these increased odds of death seen with a negative tuberculin test or a negative or indeterminate IGRA cannot be discerned from these surveillance data. One possibility is that the inability to generate either an in vitro IFN-γ response or an in vivo tuberculin test response is associated with death. Another possibility is that negative or indeterminate results could contribute to diagnostic delays if they are mistakenly interpreted as definitive evidence against a tuberculosis diagnosis. Previous studies have found that atypical features of tuberculosis, such as smear-negative or extrapulmonary disease, are indeed associated with diagnostic delays and death before treatment (18, 19). It is plausible that a negative (or indeterminate) tuberculin test or IGRA result could have a similar effect.
In considering our findings about the associations for indeterminate IGRA results, it is important to keep in mind that an indeterminate IGRA result can come either from a failure of the positive control with lack of response to the mitogen or lymphopenia (positive control), or from a failure of the negative control due to high circulating levels of IFN-γ (1, 20). Additional reasons for an indeterminate test include test performance issues, such as underfilling of tubes and processing delays. Unfortunately, the IGRA results are reported in the national surveillance data with only the qualitative categories of positive, negative, and indeterminate, and the quantitative results for the negative control, mitogen, and tuberculosis response are not collected.
Thus, we were unable to determine what proportion of indeterminate results in this study population are due to failure of the positive versus negative controls. Failure of the positive control can be considered analogous to tuberculin test anergy seen among immunosuppressed patients and has been reported among persons infected with HIV, patients with lymphopenia, and elderly patients (10, 11, 21–26). Meanwhile, failure of the negative control attributable to elevated IFN-γ levels might be hypothesized to arise in the setting of overwhelming systemic inflammation that can be seen with disseminated disease. Failure of the negative control has been previously reported among patients with miliary disease, even in the presence of lymphopenia (27). Prospective studies with quantitative IGRA results could confirm whether failure of the positive control, negative control, or both are driving the associations with disseminated disease and death that we observed in our study.
Limitations
There are several limitations to our study. Although there were 49,949 cases of tuberculosis reported during the study period, an IGRA result was only documented for 34% of those cases. However, we were still able to analyze a very large study population of nearly 25,000 persons, including more than 11,000 persons with an IGRA result, which we believe is the largest cohort to date for IGRA results among persons with active tuberculosis. Additionally, although there were differences between the sociodemographic and clinical characteristics of those who had a tuberculin test or an IGRA test performed, most notably that U.S.-born persons were more likely to have tuberculin test and foreign-born persons were more likely to have an IGRA test, we attempted to address these differences in our statistical models.
Conclusions
In summary, although the ability to mount a positive tuberculin test response or generate a positive IGRA result are both protective against death among persons with active tuberculosis, a positive tuberculin test is also protective against disseminated tuberculosis, but a positive IGRA is not. Thus, although the tuberculin test and IGRA are considered largely interchangeable for screening purposes, these indirect immune assays are not equivalent in their associations with disease site.
In addition, although an indeterminate IGRA does not help to confirm a diagnosis of either latent infection or active tuberculosis, our findings indicate that an indeterminate IGRA can be a very meaningful result. In the context of active tuberculosis, an indeterminate IGRA can identify persons with an increased risk of disseminated disease and death. It is important for clinicians to be aware of the negative prognostic significance of a negative tuberculin test, negative IGRA, or indeterminate IGRA, even if the underlying immune responses driving these different associations remain unclear. Future prospective studies of the tuberculin test and IGRA among patients with active tuberculosis could help to better define the immune response and biomarkers associated with these assays. Further understanding of the underlying biology represented by these different assays may lead to greater insight into the complex relationship between host and pathogen that results in tuberculosis infection, disease, localization, and either cure or death.
Footnotes
Supported by the Centers for Disease Control and Prevention, and in part by UL1 TR000454 from the Atlanta Clinical and Translational Science Institute. No direct funding was received for this study. No funding bodies had any role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript. The findings and conclusions of this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Author Contributions: All authors substantially contributed to the study conception and design. S.C.A. and S.H.L. performed the statistical analyses and S.C.A. wrote the first draft of the manuscript. All other authors critically revised the manuscript for important intellectual content. All authors have read and approved the final version of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K IGRA Expert Committee; Centers for Disease Control and Prevention (CDC) Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection: United States, 2010. MMWR Recomm Rep. 2010;59:1–25. [PubMed] [Google Scholar]
- 2.Huebner RE, Schein MF, Bass JB., Jr The tuberculin skin test. Clin Infect Dis. 1993;17:968–975. doi: 10.1093/clinids/17.6.968. [DOI] [PubMed] [Google Scholar]
- 3.Kuramoto Y, Tagami H. Histopathologic pattern analysis of human intracutaneous tuberculin reaction. Am J Dermatopathol. 1989;11:329–337. doi: 10.1097/00000372-198908000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Kuramoto Y, Sekita Y, Tagami H. Histoanalytical study of the cellular infiltrate in the tuberculin reaction. Clin Exp Dermatol. 1993;18:111–118. doi: 10.1111/j.1365-2230.1993.tb00989.x. [DOI] [PubMed] [Google Scholar]
- 5.Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet. 2000;356:1099–1104. doi: 10.1016/s0140-6736(00)02742-2. [DOI] [PubMed] [Google Scholar]
- 6.Samandari T, Agizew TB, Nyirenda S, Tedla Z, Sibanda T, Shang N, Mosimaneotsile B, Motsamai OI, Bozeman L, Davis MK, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:1588–1598. doi: 10.1016/S0140-6736(11)60204-3. [DOI] [PubMed] [Google Scholar]
- 7.Auld SC, Click ES, Heilig CM, Miramontes R, Cain KP, Bisson GP, Mac Kenzie WR. Association between tuberculin skin test result and clinical presentation of tuberculosis disease. BMC Infect Dis. 2013;13:460. doi: 10.1186/1471-2334-13-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auld SC, Click ES, Heilig CM, Miramontes R, Cain KP, Bisson GP, Mac Kenzie WR. Tuberculin skin test result and risk of death among persons with active TB. Plos One. 2013;8:e78779. doi: 10.1371/journal.pone.0078779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu F, Du FJ, Jia HY, Pan LP, Zhang X, Xing AY, Du BP, Sun Q, Nie LH, Li H, et al. Inadequate values from an interferon-gamma release assay for smear-negative tuberculosis in a high-burden setting. Int J Tuberc Lung Dis. 2014;18:1496–1501. doi: 10.5588/ijtld.14.0233. [DOI] [PubMed] [Google Scholar]
- 10.Cho K, Cho E, Kwon S, Im S, Sohn I, Song S, Kim H, Kim S. Factors associated with indeterminate and false negative results of QuantiFERON-TB Gold In-Tube test in active tuberculosis. Tuberc Respir Dis (Seoul) 2012;72:416–425. doi: 10.4046/trd.2012.72.5.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobashi Y, Mouri K, Miyashita N, Okimoto N, Matsushima T, Kageoka T, Oka M. QuantiFERON TB-2G test for patients with active tuberculosis stratified by age groups. Scand J Infect Dis. 2009;41:841–846. doi: 10.3109/00365540903186215. [DOI] [PubMed] [Google Scholar]
- 12. Auld SC, Lee SH, Click ES, Miramontes R, Day CL, Gandhi NR, Heilig CM. Interferon gamma release assay is associated with disease site and death in active TB [abstract]. Presented at the 47th Union World Conference on Lung Health, Oct. 27, 2016, Liverpool, UK.
- 13.Centers for Disease Control and PreventionReported tuberculosis in the United States, 2008. Atlanta, GA: Centers for Disease Control and Prevention; 2009 [Google Scholar]
- 14.Centers for Disease Control and Prevention.Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Recomm Rep 2000. 49:1–51 [PubMed]
- 15.R Core TeamR: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013 [Google Scholar]
- 16.Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med. 2007;146:340–354. doi: 10.7326/0003-4819-146-5-200703060-00006. [DOI] [PubMed] [Google Scholar]
- 17.Serrano CJ, Castañeda-Delgado JE, Trujillo-Ochoa JL, González-Amaro R, García-Hernández MH, Enciso-Moreno JA. Regulatory T-cell subsets in response to specific Mycobacterium tuberculosis antigens in vitro distinguish among individuals with different QTF and TST reactivity. Clin Immunol. 2015;157:145–155. doi: 10.1016/j.clim.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen LT, Hamilton CD, Xia Q, Stout JE. Mortality before or during treatment among tuberculosis patients in North Carolina, 1993-2003. Int J Tuberc Lung Dis. 2011;15:257–262, i. [PMC free article] [PubMed] [Google Scholar]
- 19.Greenaway C, Menzies D, Fanning A, Grewal R, Yuan L, FitzGerald JM Canadian Collaborative Group in nosocomial Transmission of Tuberculosis. Delay in diagnosis among hospitalized patients with active tuberculosis: predictors and outcomes. Am J Respir Crit Care Med. 2002;165:927–933. doi: 10.1164/ajrccm.165.7.2107040. [DOI] [PubMed] [Google Scholar]
- 20.Pai M, Lewinsohn DM. Interferon-gamma assays for tuberculosis: is anergy the Achilles’ heel? Am J Respir Crit Care Med. 2005;172:519–521. doi: 10.1164/rccm.2506003. [DOI] [PubMed] [Google Scholar]
- 21.Nei T, Fujisawa Y, Izumi Y, Tetsuka A, Arita Y, Murata H, Sawai K, Kitamura M, Miyachi H, Hosokawa Y, et al. Miliary tuberculosis with indeterminate interferon gamma release assay results. Intern Med. 2013;52:2583–2585. doi: 10.2169/internalmedicine.52.0708. [DOI] [PubMed] [Google Scholar]
- 22.Kobashi Y, Fukuda M, Yoshida K, Oka M. An indeterminate QuantiFERON TB-2G response for miliary tuberculosis, due to severe pancytopenia. J Infect Chemother. 2007;13:414–417. doi: 10.1007/s10156-007-0559-y. [DOI] [PubMed] [Google Scholar]
- 23.Kobashi Y, Sugiu T, Mouri K, Obase Y, Miyashita N, Oka M. Indeterminate results of QuantiFERON TB-2G test performed in routine clinical practice. Eur Respir J. 2009;33:812–815. doi: 10.1183/09031936.00075008. [DOI] [PubMed] [Google Scholar]
- 24.Miranda C, Yen-Lieberman B, Terpeluk P, Tomford JW, Gordon S. Reducing the rates of indeterminate results of the QuantiFERON-TB Gold In-Tube test during routine preemployment screening for latent tuberculosis infection among healthcare personnel. Infect Control Hosp Epidemiol. 2009;30:296–298. doi: 10.1086/595732. [DOI] [PubMed] [Google Scholar]
- 25.Brock I, Ruhwald M, Lundgren B, Westh H, Mathiesen LR, Ravn P. Latent tuberculosis in HIV positive, diagnosed by the M. tuberculosis specific interferon-gamma test. Respir Res. 2006;7:56. doi: 10.1186/1465-9921-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cattamanchi A, Smith R, Steingart KR, Metcalfe JZ, Date A, Coleman C, Marston BJ, Huang L, Hopewell PC, Pai M. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: a systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2011;56:230–238. doi: 10.1097/QAI.0b013e31820b07ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hangai S, Yoshimi A, Hosoi A, Matsusaka K, Ichikawa M, Fukayama M, Kurokawa M. An indeterminate result of QuantiFERON-TB Gold In-Tube for miliary tuberculosis due to a high level of IFN-γ production. Int J Hematol. 2014;99:523–526. doi: 10.1007/s12185-014-1504-3. [DOI] [PubMed] [Google Scholar]