Abstract
In this work, the production of fungal laccase was optimized from local isolate of Pleurotus ostreatus using solid state fermentation. Factorial design was used to study the effect of several nutrients on enzyme production. Purification and characterization of the enzyme and the effect of temperature, pH and gamma radiation on fungal growth and enzyme production was investigated.
Optimization of production conditions yielded an enzyme with activity over 32,450 IU/g of fermented substrate. Factorial design was capable of establishing the conditions that multiplied the activity of the enzyme several folds, consequently, reducing the cost of production. The enzyme was capable of decolorizing several dyes with over 80% reduction in color confirming the aromatic degrading capability of laccase. The enzyme was also used in the synthesis of gold nanoparticles, proving that laccase from Pleurotus ostreatus has a strong potential in several industrial applications.
Keywords: Laccase, Pleurotus ostreatus, Gold nanoparticles (GNPs)
1. Introduction
Enzyme production is an expanding field of biotechnology. Laccase (E.C. 1.10.3.2, p-benzenedial: oxygen oxidoreductase) is able to catalyze the oxidation of various aromatic compounds (particularly phenol) with the concomitant reduction of oxygen to water [1]. Although the enzyme is present in plants, insects and bacteria, the most important source are fungi and particularly basidiomycetes [1], [2]. The white-rot fungi are the most efficient microorganisms capable of extensive aerobic lignin degradation. Due to the higher redox potential of fungal laccase compared to plant or bacterial laccase, they are utilized in several biotechnological applications [3]. Fungal laccase is considered a key player in lignin degradation and/or the removal of potentially toxic phenols arising during morphogenesis, sporulation, or phytopathogenesis and fungal virulence [4]. The role of laccase in lignin and phenolic compound degradations has been evaluated in a large number of biotechnological applications such as dye degradation [5], [6], bioremediation of some toxic chemical wastes [5], [7], wastewater and soil treatments and also biosensor developments [8], [9]. In particular, the ability to biodegrade various types of dyes by white-rot fungi has proven to be effective, with their elimination being mediated through oxidoreduction reactions catalyzed by the lignin degrading enzymes they produce, such as lignin peroxidase, manganese peroxidase and laccase [10].
Most studies, dealing with ligninolytic enzyme production by white-rot fungi, have been carried out using the liquid culture conditions, in spite of the fact that these organisms grow in nature in solid-state conditions. Recent reviews on solid-state fermentation (SSF) point out the enormous potential of this culture technique for the development of different bioprocesses [11].
A lot of reports have emerged in the last few years describing the preparation and characterization of gold nanoparticles (GNPs), due to the extraordinary physicochemical characteristics and wide usages in different fields. Although preparation of nano-gold by physical procedures (such as laser ablation) provides GNPs with narrow range of particle size, it needs expensive equipment and has low yield [12]. Hazardous effects of organic solvents, reducing agents and toxic reagents applied for chemical synthesis of GNPs on the environment, has encouraged the development of eco-friendly methods for preparation of gold nanoparticles [13].
The aim of the present work is to optimize the production of laccase by Pleurotus ostreatus under SSF and to evaluate the industrial applications of laccase in the decolorization of several dyes and in the synthesis of GNPs.
2. Materials and methods
2.1. Fungal strains
Seven locally isolated fungal strains (Gliocladuim virens, Sclerotiam rolfsii, Penicilluim chrysogenum, Pleurotus ostreatus, Gliocladuim deliquescence, Rhizoctania solani and Penicilluim citrinum) were used in the study obtained from the culture collection in the Pharmaceutical Microbiology Laboratory Drug Radiation Research Department (NCRRT, Egypt). All strains were microscopically identified and kept on potato dextrose agar (PDA) at 4 °C and periodically sub-cultured to maintain viability. All strains were tested for production of laccase enzyme.
2.2. Fermentation medium
Fermentation was done in 250 ml Erlenmeyer flasks, where 8 ml of distilled water were added to 5 gm carbon source (66% moisture content) [14]. The chosen concentrations of inducers were then added (according to the experiment design) and autoclaved at 121 °C for 20 min. The fungus was added to the medium as a 2 ml spore suspension (∼8 × 106 spores/ml) and incubated at 29 °C statically in complete darkness.
2.3. Extraction of enzyme
After seven days, the whole contents of the flask were soaked in 100 ml, 1 mM citrate phosphate buffer (pH 5) for 2 h and put in a shaker at 200 rpm (LAB-Line R Orbit Environ, U.S.A) and then extracted in tincture press, centrifuged in cooling centrifuge (Hettich Universal 16 R, Germany) for 10 min at 6 °C and 2415 × g to remove particulate.
2.4. Enzyme assay
Laccase activity measurement was performed spectrophotometrically (JASCO V/560 UV/Vis, Japan) at wavelength of 525 nm in a reaction medium containing 1 mM syringaldazine (ϵ = 65 mM−1 cm−1), 50 mM phosphate buffer pH 5 and culture filtrate. Oxidation of syringaldazine was monitored by measuring the increase in absorbance for 4 min. Enzyme activity was expressed in units (U); one unit was defined as 1 μmol of syringaldazine oxidized per min [15].
2.5. Screening of carbon source and nitrogen source
Four agricultural wastes were screened as carbon sources for production of laccase. Banana peelings (dried in oven at 55 °C for 36 h), spent coffee ground (brought from local coffee factory), rice straw and wheat bran flakes (brought from local market) were all tested. Six nitrogen sources of natural and synthetic origin were screened which are yeast extract, tryptone, malt extract, ammonium sulphate, urea and ammonium chloride.
2.6. Experimental factorial design
The statistical software package (Minitab 16, U.S.A) was used for designing the experiment, regression analysis of experimental data and in plotting the relation between variables. The effects of the six variables in two level form namely: malt extract (1% nitrogen content or 2% nitrogen content), Tween-80 (0.01%(v/v) or 0.02%(v/v)), CuSO4 (0.625 mM or 1.25 mM), resorcinol (10 mg or 20 mg), dl-Methionine (5 mg or 10 mg) and tannic acid (2.5 mg or 5 mg) were assessed. The possible interactions between them were investigated using 32 experiments; the choice of the variables was based on the fact that the production of ligninolytic enzymes by fungi is highly regulated by nutrients [16]. The main effects of parameters on laccase production were estimated by subtracting the mean responses of parameters at their lower levels from their corresponding higher levels and divided by the total number of experimental runs. The adequacy of the model was tested and the parameters with statistically significant effects were identified using Fisher’s test for the analysis of variance (ANOVA).
2.7. Radiation of fungus
The process of irradiation was carried out using 60Co Gamma Chamber (4000-A-India) at a dose rate 10.28 kGy/h at the time of experiment. Seven days old Pleurotus ostreatus slant about (∼8 × 106 spores/ml) was irradiated at different doses (0.1, 0.25, 0.5, 0.75, 1, 1.5 and 2 kGy) then cultivated at optimized conditions for laccase production. Non-irradiated culture was used as control.
2.8. Laccase partial purification and characterization
Ammonium sulphate was added to the cell free filtrate obtained from Pleurotus ostreatus to attain 80% saturation and the flask was kept at 4 °C for 48 h. Content was centrifuged at 2415 g for 15 min at 4 °C and the supernatant was discarded. The pellet was dissolved in a 50 ml, 1 mM citrate phosphate buffer pH 5. The precipitate was desalted by dialysis bag to remove low molecular weight substances and other ions that interfere with the enzyme activity as previously described [17]. Protein concentration was quantified using the Bradford assay with bovine serum albumin as standard [18].
The effect of pH on the activity of partially purified enzyme was studied by incubating it with the following buffers for 7 min: citrate phosphate buffer for pH (3–5) and sodium phosphate for pH (6–8). The effect of temperature on activity was determined by incubating the enzyme in water bath in the range from 30 °C to 90 °C with 10 °C increments for (15 min). The effect of 5 doses of gamma radiation (2, 3, 4, 5 and 6 kGy) on the activity of laccase was studied. Also, the effect of several activators and inhibitors such as Cu2+, Zn2+ and Mg2+, used as sulphate salts and Ca2+, Cd2+, Co2+ and Ba2+ used as chloride salts and EDTA with the concentration of 1 mM. Laccase activity was monitored under standard assaying conditions.
The reaction assay mixture of laccase was incubated with activators or inhibitors, optimized buffer and syringaldazine and at respective optimum temperature. The change in absorbance was measured spectrophotometrically to evaluate the influence of these activators and inhibitors on enzyme activity. Results were expressed as percentage of the control (non-treated laccase).
2.9. Decolorization of dyes
Five dyes namely methyl orange, trypan blue, ramazol brilliant red, ramazol brilliant blue and ramazol brilliant yellow (Dye Star company, Germany) were chosen to test the enzyme’s ability to remove their color. A volume of 0.1 ml of the stock solution (20 ppm) was added to 2 ml distilled water and 2 ml of the partially purified enzyme extract with activity 417 U/ml respectively, the percentage reduction of color was monitored for 3 h and was determined spectrophotometrically (JASCO V/560 UV/Vis, Japan) by monitoring the absorbance at the characteristic wavelength of each dye. The decolorization efficiency (R%) was calculated as follows:Dye decolorization percentage = [(Initial absorbance − final absorbance)/(initial absorbance)] × 100
Initial absorbance indicated absorbance of the untreated dye at the characteristic peak and the final absorbance indicated absorbance of dye after treatment with laccase at the same peak after 3 hours.
2.10. Preparation and characterization of GNPs
GNPs were prepared as previously described [19], briefly, to 3 ml of laccase enzyme, containing 417 IU/mg, 0.1 ml of tetrachloroauric acid with concentration of (10 mg/1 ml) was added, (49% purity). The reaction mixture was stirred properly using magnetic stirrer, within 90 min the yellow colored solution started changing to pink then violet, detected visually and by UV/Visible spectrophotometer indicating the formation of GNPs. Average particle size and size distribution were determined by (PSS-NICOMP 380-ZLS) particle sizing system (St. Barbara, California, USA). UV/Visible Spectra of GNPs were recorded using a spectrophotometer (JASCO V-560UV/Vis, Japan) operated at a resolution of 1 nm from range of 200–700 nm and observed absorption peak at 550 nm due to excitation of surface plasmon vibration in GNPs solution or the SPR band. FT-IR measurements were carried out using a spectrophotometer (JASCO FT/IR-6300 infra-red) by employing KBr pellet technique. The size and morphology of synthesized GNPs were recorded using transmission electron microscope, TEM (JEM-100CX.TEM JEOL, Japan). TEM studies were carried by drop coating GNPs onto carbon-coated TEM grids. The film on the TEM grids were allowed to dry, the extra solution was removed using blotting paper.
2.11. Effect of temperature, gamma radiation and different volumes of HAuCl4 on GNPs synthesis
The effects of temperature (40°–100° with 10 °C increments), radiation (2, 2.5, 3, 3.5, 4, 5 and 6 kGy) and different concentrations of tetrachloroauric acid (0.1, 0.2, 0.3, 0.4 and 0.5 ml of 10 mg/ml solution) were assessed to study their effect on the formation of GNPs. Blank sample was done in case of radiation before mixing with HAuCl4.
3. Results
3.1. Screening for laccase producing fungi
After screening of the seven fungal strains, Pleurotus ostreatus was chosen due to its relatively high laccase activity (4610 U/gfs), (where gfs is defined as the number of units of enzyme produced from 5 g fermented substrate), compared to other fungi and consequently, Pleurotus ostreatus was used to optimize laccase production throughout the whole study.
3.2. Screening of carbon source and nitrogen source
The screening of the four agricultural wastes showed that wheat bran which is an abundant byproduct formed during wheat flour preparation was the highest in laccase activity 4610 (U/gfs) compared to other sources (Table 1). When screening different nitrogen sources, malt extract gave the highest laccase activity (8460 U/gfs) compared to yeast extract (7205 U/gfs) and tryptone (6500 U/gfs) (Table 2). Wheat bran and malt extract were both used to support growth of Pleurotus ostreatus.
Table 1.
Results of screening carbon sources.
| Carbon source | Laccase activity (U/gfs) |
|---|---|
| Wheat bran | 4610 |
| Banana peelings | 1280 |
| Rice straw | 569 |
| Spent coffee ground | 135 |
Table 2.
Screening of nitrogen sources.
| Nitrogen source | Laccase activity (U/gfs) |
|---|---|
| Malt extract | 8460 |
| Yeast extract | 7205 |
| Tryptone | 6500 |
| Ammonium sulphate | 5420 |
| Urea | 1789 |
| Ammonuim chloride | 819 |
3.3. Experimental factorial design
After carrying out 32 experiments, reflecting different combinations of the variables (Table shown in supplementary data), the results revealed that run number 28 gave the highest enzyme activity of (32,450 U/gfs) with all of the variables in their higher level except for tannic acid that is in its lower level.
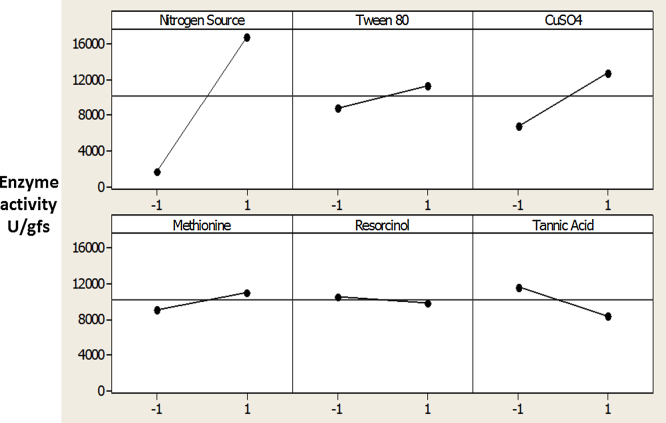
Main effects plotting showed the positive effect of nitrogen source, Tween-80, CuSO4 and methionine. In case of nitrogen source effect, a major difference between means was noticed. However, tannic acid had negative effect in its higher level as shown in Fig. 1.
Fig. 1.
Main effects of variables showing the positive effect of nitrogen source, Tween-80, CuSO4 and methionine.
The significance of the effect of the six variables was assessed using one way (ANOVA). Both the T-value and P-value statistical parameters were used to confirm the significance of factors studied as shown in Table 3. The results shown proved that nitrogen source, Tween-80 and tannic acid all had a significant effect with (P = <0.05).
Table 3.
Test of significance of variables.
| Term | T | P |
|---|---|---|
| Nitrogen source | 8.94 | 0.000* |
| Tween-80 | 2.61 | 0.015* |
| CuSO4 | 1.63 | 0.115 |
| Methionine | 1.99 | 0.057 |
| Resorcinol | 1.26 | 0.218 |
| Tannic acid | −2.59 | 0.016* |
Significant.
The model determination coefficient (R = 0.81) suggested that the fitted model could explain 81% of the total variation which implies a satisfactory representation of the process by the model. The coeffecient of determination (R-value) always lies between 0 and 1. As the closer the value of R is to 1.0, the stronger the model and the better it predicts the response. The analysis of variance for the selected factorial model showed that the model was significant with a model F-value of 17.75 (P = <0.05) as shown in Table 4, as the larger the magnitude of the T-value and smaller the P-value, the more significant is the corresponding coefficient [20].
Table 4.
Analysis of variance (ANOVA) results for experiments.
| Source | DFa | Sum of squares | Mean squares | Fb | Pc |
|---|---|---|---|---|---|
| Regression | 6 | 2138109479 | 356351580 | 17.75 | 0.000 |
| Residual error | 25 | 501854395 | 20074176 | ||
| Total | 31 | 2639963874 |
R2 = 81.0%, R2 (adjusted) = 76.4%.
DF: degree of freedom.
F: F-value.
P: probability.
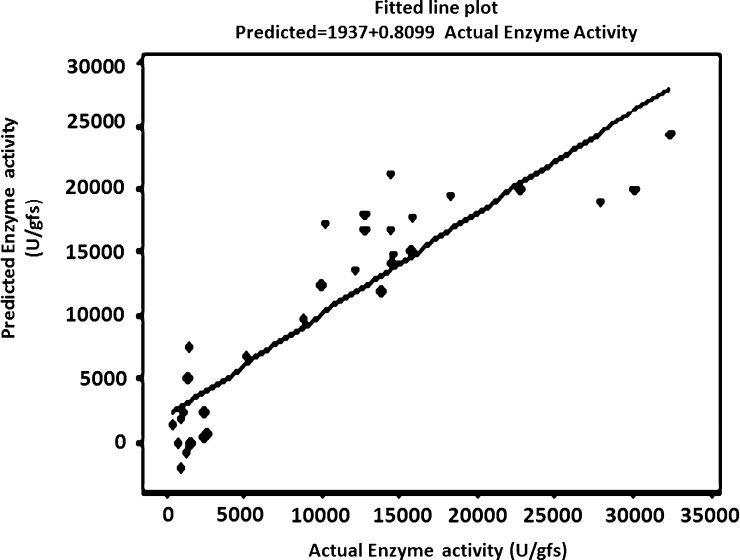
The mathematical expression for the relationship between the six variables for laccase production is given below in equation shown. The equation allows the prdection of the response in further future experiments where a relationship between tested factors is expressed based upon the experiments we did.The mathematical formula is originally: [Y = a + bx], where Y is the enzyme activity, a is the constant (slope of the line) and x is the concentration of the variable (we have six variables). The numbers before every factor are constants generated by the program based upon our results. The variables are as folows: malt extract (1% nitrogen content or 2% nitrogen content), Tween-80 (0.01%(v/v) or 0.02%(v/v)), CuSO4 (0.625 mM or 1.25 mM), resorcinol (10 mg or 20 mg), dl-methionine (5 mg or 10 mg) and tannic acid (2.5 mg or 5 mg). Substitution in the equation by the concentarions of the six variables will give the predicted enzyme activity.Predicted enzyme activity = 8511 + 7460 [nitrogen Source] + 2207 [Tween 80] + 1397 [CuSO4] + 1590 [methionine] + 1054 [resorcinol] − 2197 [tannic Acid]
Fig. 2 shows the actual enzyme activity and the predicted activity with an equation describing the relationship between them.
Fig. 2.
Actual enzyme activity versus predicted activity.
3.4. Effect of gamma irradiation on growth of Pleurotus ostreatus and laccase activity
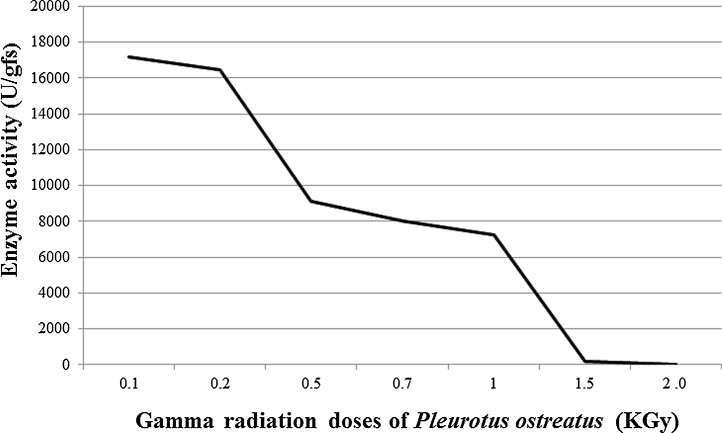
The results showed that as the radiation dose increased, the growth of Pleurotus ostreatus decreased gradually (Fig. 3), consequently, the production decreased. As for the activity of the produced laccase, it was highly affected by irradiating the fungus as it decreased to almost half (17,200 U/gfs), compared to the non-irradiated enzyme (32,450 U/gfs). The decrease in activity was directly proportional to the increase in the dose until complete loss in enzyme activity at 1.5 and 2 kGy.
Fig. 3.
Effect of Gamma radiation on the growth of the fungus and consequently the production of the enzyme. As radiation dose increased, production decreased.
3.5. Laccase partial purification and characterization
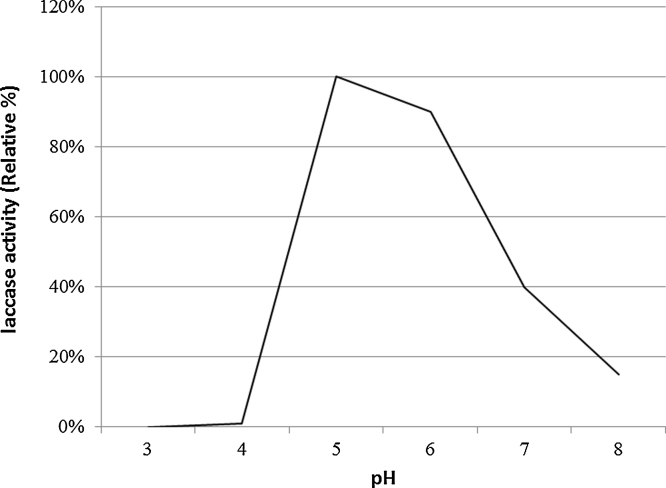
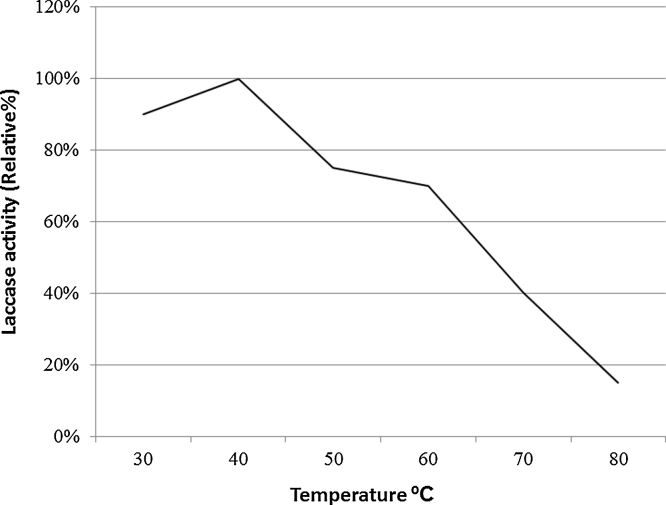
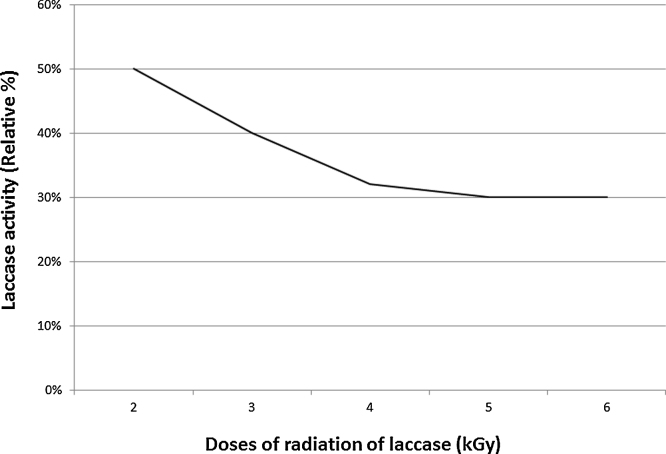
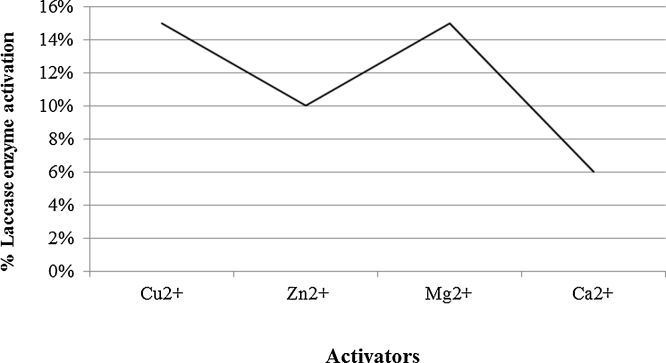
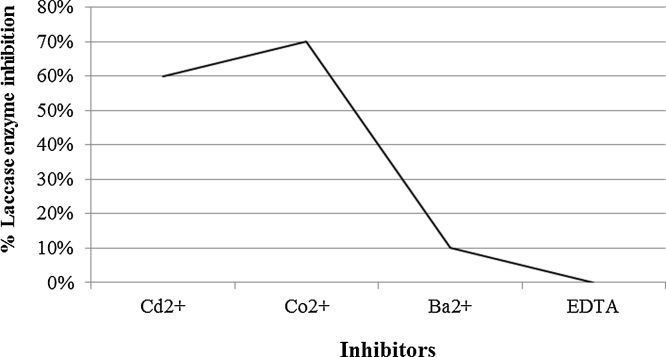
After precipitation of the enzyme using ammonium sulphate, total activity decreased from 675,000 to 622,000 U/1500 ml but the specific activity increased from 112.5 to 204 U/mg. The enzyme kept 90% of activity at pH 6 with abrupt decrease before and beyond that value (Fig. 4). Thermal stability of purified laccase showed that at temperature 40 °C, laccase exhibited the highest activity while above 60 °C laccase activity decreased sharply and at 80 °C only 10% of initial activity remained after 15 min incubation (Fig. 5). Gamma irradiation of the enzyme at 2 kGy decreased its activity to half, whereas up to 5 and 6 kGy, where 20% of the activity remained (Fig. 6). Activators including Cu2+, Zn2+, Mg2+ and Ca2+ had an enhancing effect on activity of laccase with different extents where Cu2+, and Mg2+ gave the highest activation (15%) at the concentration used (Fig. 7). Whereas, inhibitors as Cd2+, Co2+ and Ba2+, caused decrease in the activity of laccase, with Co2+ giving highest inhibition reaching 70% (Fig. 8). However, EDTA did not inhibit laccase activity at the concentration used.
Fig. 4.
Effect of pH on enzyme activity. The highest activity was observed at around pH 6.
Fig. 5.
Effect of temperature on enzyme activity. The highest activity is observed at 40 °C.
Fig. 6.
Effect of radiation on enzyme activity. Radiation decreased the activity of the laccase enzyme.
Fig. 7.
Activators and their effect on activity of the enzyme. Cu+2, and Mg+2 gave the highest activation.
Fig. 8.
Inhibitors and their effect on the activity of the enzyme. Co+2 gave the highest inhibition.
3.6. Dyes decolorization
Decolorization of four of the used dyes exceeded 50% within 3 h and was confirmed by the decrease in absorbance in the characteristic wavelength of every dye. The highest decolorization value was obtained in case of methyl orange and trypan blue, almost no decolorization was detected in case of ramazol yellow.
3.7. Preparation and characterization of GNPs
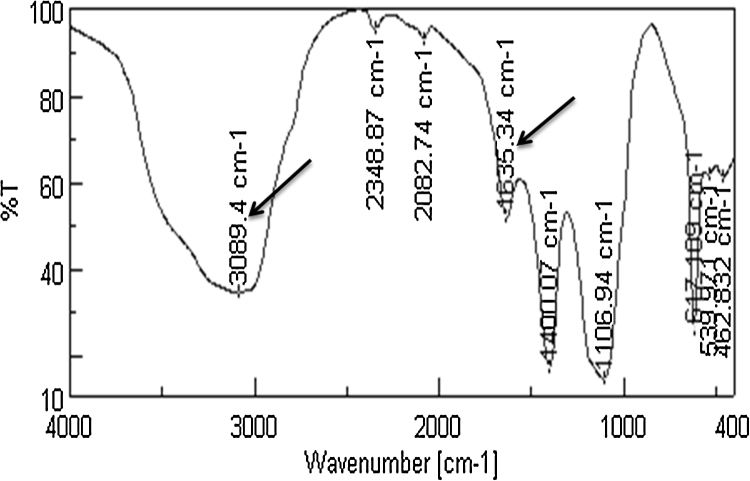
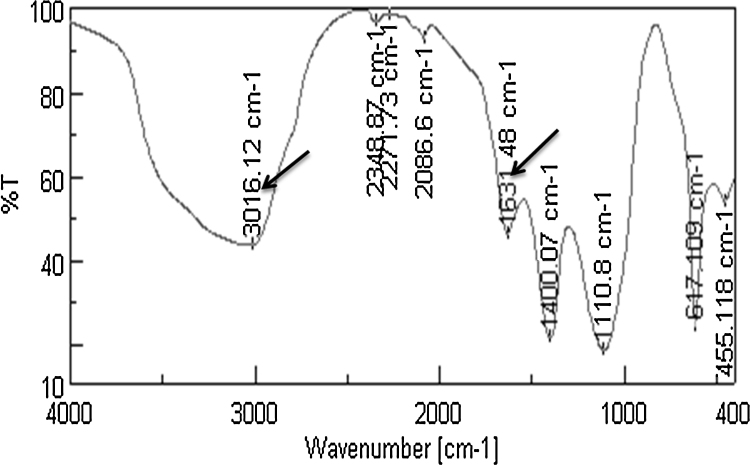
Formation of GNPs was confirmed by the formation of violet color after 90 min at room temperature that gave a significant peak at 550 nm. Size distribution of the formed GNPs using DLS and TEM imaging of GNPs showed highly mono dispersed GNPs with size range of 22–39 nm. The FTIR spectrum of laccase before and after formation of GNPs (Fig. 9, Fig. 10), showed the change in the corresponding peaks of functional groups before and after formation of GNPs, expressing change in intensity of the major peak at 3016 cm−1 that corresponds to OH and/or NH functional groups and the peak of 1631 cm−1 corresponds to carbonyl group, both could be ascribed to secondary amide structure.
Fig. 9.
FTIR spectrum for laccase before GNPs formation, arrows point to peak numbers that corresponds to OH and/or NH functional group at 3089 cm−1 and the carbonyl group at 1635 cm−1.
Fig. 10.
FTIR spectrum for laccase after GNPs formation, arrows point to peak numbers showing the change in intensity of the major peak at 3016 cm−1 that corresponds to OH and/or NH functional groups and the peak of 1631 cm−1 that corresponds to carbonyl group, both could be ascribed to secondary amide structure.
3.8. Effect of temperature, gamma radiation and different volumes of HAuCl4 on GNPs synthesis
Incubation of laccase enzyme in the presence of HAuCl4 at different temperatures showed that as temperature increased, absorbance increased which indicated higher concentration of formed GNPs. Testing the effect of gamma radiation on the production of GNPs showed that increasing the dose of radiation increased the production of GNPs; maximum GNPs production was noticed at 5 kGy. No color was detected in blank sample (radiation before mixing with HAuCl4). In case of effect of different concentrations of HAuCl4 on GNPs synthesis, the best volume of HAuCl4 was 0.3 ml as it gave the highest concentration of GNPs; further increase in gold volumes caused decrease in GNPs concentration
4. Discussion
The most efficient lignolytic fungi are the basidiomycetes. They could be either white or brown-rot fungi, both of which are taxonomically so close to each other that they sometimes appear in the same genus. Almost all species of white-rot fungi were reported to produce laccase to varying degree [21]. After screening seven fungal strains, Pleurotus ostreatus (a well-known white-rot fungus) was chosen due to its relatively high laccase activity compared to other laccase producing fungi. Pleurotus ostreatus is a common edible mushroom also known as Oyster mushroom. It was first cultivated in Germany as a subsistence measure during the World War I [22]. It is now grown commercially around the world for food.
Increasing the production of lignolytic enzymes may be achieved by modifying the source of carbon and nitrogen in the medium. Since the high cost of the enzyme is a major limitation in using laccase in an industrial scale; using agricultural wastes not only decreases the cost but also solves an environmental problem [23]. Wheat bran is an abundant byproduct formed during wheat flour preparation; it has been selected to perform the present study for its high yield of laccase. Wheat bran’s physical integrity serves as a supporting material and it provides the fungus an environment similar to its natural habitat, therefore encouraging growth of the fungus, also it is an abundant source for hydroxycinnamic acids, particularly ferulic and p-coumaric acids, which are known to stimulate laccase production and it was successfully used in laccase production by Trametes versicolor UAMH 8272 providing several fold increase in laccase activity [24], [25].
For the nitrogen source to be used, malt extract was selected as it gave significantly higher yield of laccase compared to other synthetic nitrogen sources. Malt extract served as nitrogen and also as carbon source in the growth of Cyathus bulleri where it resulted in a much higher yield of laccase than in mineral medium [26].
For laccase application in industrial processes, large amounts of enzyme are required. The major aim of the study was to find the optimized conditions for maximum laccase production. Reaching the optimized production conditions using the conventional one factor at a time technique would be quite laborious and time consuming. One of the currently available statistical designs to predict the behavior of a reaction is the factorial design. Such design of experiments completely explains the reaction and brings out the finer details by carrying out selected experiments. The variables chosen to assess their effects on laccase production were nutrients, surface active agents or possible inducers for enzyme production or activity. Their choice depended on previous studies done, in addition to the nature of the enzyme and its chemical structure.
Nitrogen source had always been an important nutrient for the growth of fungi and the production of enzymes. However, several fungi require the concentration of nitrogen to be in excess to produce laccase, while other fungi produce laccase only when induced by nitrogen starvation. Lentinula edodes and Phanerochaete chrysosporium provide examples of improved laccase production in nitrogen sufficient media [27], [28]. A nitrogen deficient medium was however required for high production of laccase in Pycnoporus sanguineus (cinnabarinus) [29]. Our results supported the first finding showing that laccase production was in excess with the higher concentration of malt extract (2% nitrogen content) as it was a significant variable (p = 0.000). This is probably due to the fact that fungi require nitrogen for their growth and their general metabolic processes and so providing nitrogen in excess subsequently increases enzyme production.
For the surfactant Tween-80, it was a significant variable (p = 0.015), as high concentration of the enzyme was usually accompanied by high concentration of Tween-80. The addition of the surfactant Tween-80 has resulted in higher yields of ligninolytic enzymes in certain fungi because there is evidence that these surface acting agents result in higher permeability of oxygen and extracellular enzyme transport through the cell membranes of fungi [29], [30].
Most common inducers used for laccase production included phenolic compounds, which are structurally related to lignin or lignin derivatives, considered to be the natural substrate for laccase, consequently, resorcinol and tannic acid were chosen. The effect of resorcinol was not significant but the optimized laccase production was observed at high concentration of resorcinol. Attempts were made to increase laccase production by the addition of the reported laccase inducer tannic acid to enhance the expression of laccase gene at the transcription level in the growth medium [31]. However, the optimized production condition required low concentration of tannic acid with significance of (p = 0.016) which might be due to the reaction between the produced laccase and tannic acid, which resulted in making laccase in an undetectable state by syringaldazine since tannic acid is one of the traditional screening reagents for laccase [32].
The effect of copper on laccase synthesis was studied in Trametes versicolor and Pleurotus ostreatus among several other white-rot fungi [33], [34]. As laccase is a multi-copper oxidase in its structure, the availability of copper in the medium might allow the synthesis of the enzyme. In addition, the presence of copper in Pleurotus ostreatus cultures decreases the activity of extracellular proteases which might degrade laccase [35]. However, copper present in high concentration was extremely toxic to microbial cells [36]. In the present study, copper was not a significant variable indicting that copper was not a critical component in both concentrations which was quite unexpected.
Gamma radiation was used in many cases to induce general metabolic processes and consequently increases enzymes production due to the well-known phenomena of “Hormesis”; which is the stimulation of any system by low doses of environmental, biotic and abiotic stress factors including pathogens, physical and chemical agents [37]. However, the reduction of growth and decrease of enzymes production by gamma radiation had also been recorded by other studies. The results obtained showed that, as the radiation dose increased, Pleurotus ostreatus growth decreased which was in agreement with other studies as in case of the strain Pleurotus sajor-caju [38]. The decrease in growth accompanying the increase in dose (up to 1.5 kGy) and subsequent decrease in laccase production, might be due to reduction in the viable count of fungi as a result of the over accumulation of free radicals that usually accompany the gamma irradiation process, when these rays interact with water molecules in an organism, they generate transient free radicals that can cause additional indirect damage to DNA and so causes injury in the microbial cells resulting in incomplete inhibition [39]. Complete inhibition of fungal growth and subsequent loss of enzyme activity were detected with 2 kGy, which might be due to break down of DNA structure of cells by that dose of gamma irradiation resulting in complete death [40].
For a variety of industrial applications characterization of produced enzyme would be a crucial step in order to know the conditions (pH, temperature, gamma radiation and activating and inhibiting ions) that the enzyme could function. Optimum pH for laccase exhibited variation which may be due to changes in the reaction caused by the substrate (syringaldazine), oxygen or the enzyme itself. The highest activity of the produced laccase was at pH 5 with syringaldazine as a substrate in agreement with the previous work [41]. Relative high thermostability is an attractive and desirable characteristic of an enzyme. In general, the optimum temperature for laccase activities can differ from one strain to another, with a range for most fungal laccases being 50–70 °C [42], in our case, laccase had optimum temperature at 30–50 °C and rapidly lost activity at temperatures above 60 °C which might be due to breaking down the integrity of laccase protein structure and so losing much of its activity [43], [44].
In general, laccase responds similarly to several inhibitors of enzyme activity. Many ions such as azide and halides can bind to the type 2 and type 3 copper atoms, resulting in the interruption of internal electron transfer with the subsequent inhibition of activity [45]. EDTA did not inhibit laccase activity as was observed with the laccase obtained from an unidentified basidiomycete [46].
Some of the most toxic dyes are amino-substituted azo dyes, which are often mutagenic and carcinogenic. Current methods for dye-decolorization are chemically derived and include adsorption, chemical transformation, and incineration [47]. It has been suggested that enhanced microbial decolorization of dyes may provide a less expensive and more environmentally acceptable alternative to chemical treatment. An advantage of using fungal oxidative mechanisms to degrade azo dyes over other microorganisms is that it is possible to avoid the formation of hazardous breakdown products such as anilines formed by the reductive cleavage of azo dyes [48]. The laccase oxidative transformation of dyes depends on their chemical structure. The presence of ortho-hydroxy groups with respect to the azo link was found to enhance the decolorization rates of azo-dyes with laccase whereas nitro groups stabilized the dye molecules against laccase action [49].
Green synthesis of nanoparticles using microorganisms or enzymes provides advancement over chemical and physical method as it is cost effective, environment friendly, easily scaled up for large scale synthesis and in this method there is no need to use high pressure, energy, temperature and toxic chemicals [50]. Studies have shown that the secreted proteins/enzymes and reducing agents such as amino acids, peptides and organic acids in biological entities, are found to be responsible for nanoparticle production. Similarly, in this study, laccase from Pleurotus ostreatus served as a rich source for the proteins and free amino groups reducing gold into GNPs. These compounds contain functional groups that play a role in the reduction and hence the synthesis as well as the stabilization of nanoparticles [51], [52]. These proteins create electrostatic attraction of negatively charged carboxylic groups, therefore stabilizing these nanoparticles by “capping” to prevent their aggregation through the creation of repulsive forces [53]. Laccase was performing the reduction process as a protein and not as an active enzyme as laccase in its active form was actually catalyzing oxidation and upon exposure to increasing temperature or gamma radiation, laccase lost its activity as breaking down the integrity of its protein structure and exposing of various amino acids began.
FTIR measurements (Fig. 9, Fig. 10) were carried out to identify the possible interactions between gold ions and enzyme protein which acted as reducing agent to synthesize and stabilize gold nanoparticles. Enzyme protein contains three main functional groups, including the amino, carboxylic, and thiol group, which are easily used as active sites to modify the other molecules or nanomaterials. FTIR spectrum confirmed the presence of the functional groups, 3016 cm−1 peak corresponded to OH and/or NH functional groups and presence of carbonyl group could be ascribed to the peak of 1631 cm−1 [54].
Our finding was in agreement with previous studies [55], which characterized the GNPs produced by marine microalgal strain of Tetraselmis suesica and according to that study, these functional groups could be used in bioconjugation and/or immobilization of various compounds.
The broad band contour which appears in the range of 3000–3400 cm−1 is the summation of associated intermolecular hydrogen bonds arising from —NH2 and —OH groups in protein molecules which becomes much broader and more intense after the reaction with gold ions, indicating that the N—H vibration is affected due to the gold attachment and revealing that nitrogen atoms are the binding sites for gold on protein [56]. The peaks at 1637 cm−1 and 1151 cm−1 arise from a carbonyl stretching vibration and phenolic groups which shows the carbonyl stretching vibration from the carboxylate ions and the hydroxyl stretching vibration from the phenolic ions in the protein [57]. This spectrum indicates that the secondary structure of the protein of laccase is affected as a consequence of reaction with the gold ions or binding with the GNPs.
Based on previous studies [12], the key role of exposing thiol groups of α-amylase for GNPs formation is high temperature (70 °C) that destructs the appropriate folding of α-amylase and exposes hydrophobic and hidden groups with reductive ability and makes it possible to form nanometallic structures. The effect of temperature was determined by carrying out the reaction using (0.3 ml of 10 mg/ml) of HAuCl4 at different temperatures. It was found that as temperature increases, the GNPs synthesis rate increases and the time taken for color conversion was much reduced. At room temperature, there was an initial lag period for the formation of GNPs and the synthesis time was longer, reaching 90 min. At 100 °C, only 10 min were required to get the highest absorbance (3.2) indicating the highest productivity of GNPs, this result was in agreement with previous studies [13]. The radiation-induced synthesis is one of the most promising strategies because it is simple, clean and has harmless feature [58]. During radiation, when aqueous solution is exposed to gamma radiation, it creates solvated electrons which are able to reduce metal ions forming nano metals. Exposure of the extract to different doses of radiation was performed after addition of HAuCl4, surface plasmon resonance (SPR) band was noted for all doses, maximum absorbance (4) was found at a dose of 5 kGy, after which further increase in radiation dose resulted in decrease in absorbance at 550 nm, while no peak was recorded in blank sample (radiation before mixing with HAuCl4).
The formation of GNPs can be attributed to the radiolytic reduction which generally involves radiolysis of aqueous solutions that provides an efficient method to reduce metal ions. In the radiolytic method, when aqueous solutions are exposed to gamma rays, they create solvated electrons, which reduce the metal ions and the metal atoms eventually coalesce to form aggregates [59]. Exposure of water or aqueous solutions to ionizing radiation leads to formation of primary species H3O+, H•, OH•, H2O2. These free radicals have major importance in radiolytic chemical reactions. The combined effect of both radiolytic reduction and presence of peptide resulted in formation of GNPs by radiolytic reactions and stabilization by prevention of aggregates formation by “capping”. The higher concentration of GNPs indicated by in absorbance value of 4 with radiation compared to a value of 3.2 with temperature, gave an obvious advantage for radiation over temperature in the production of GNPs. The volume of HAuCl4 added strongly affects the reaction. Absorbance increased with increase in volume HAuCl4, the best volume of HAuCl4 was 0.3 ml (10 mg/ml), which indicates increased rate of reaction by increasing the volume of HAuCl4 used, and further increase as reported causes decrease in formation of GNPs used due to aggregation as reported previously [60].
5. Conclusion
From the above mentioned results we can conclude that laccase production by Pleurotus ostreatus has been shown to depend markedly on the composition of the culture medium, carbon, nitrogen content and inducer compounds. We were able to reach the conditions that helped in multiplying the enzyme concentration to almost 10 folds (compared to fermentation of wheat bran alone) indicating that factorial design can be a practical useful tool for optimizing the reaction parameters for enhancing the activity of laccase. Using the partially purified enzyme in the decolorization of five different reactive azo dyes, we were able to get relatively high percentage of decolorization, which underscores the importance of dye decolorization using fungal enzymes. The decolorization of model dyes is a simple method to assess the aromatic degrading capability of laccase which opens a door towards using of laccase in further biotechnological processes.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
This work is a part of the Project “Nutraceuticals and Functional Foods Production by using Nano/Biotechnological and Irradiation Processes” and Nanotechnology Research Unit (P.I. Prof.Dr. Ahmed El-Batal) at Pharmaceutical Microbiology Laboratory in Drug Radiation Research Department and the financial support was provided by NCRRT.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.btre.2014.11.001.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Abd el-Raheem A., Shearer C.A. Extracellular enzyme production by fresh water ascomycetes. Fungal Divers. 2002;11:1–19. [Google Scholar]
- 2.Urairuj C., Khanongnuch C., Lumyong S. Ligninolytic enzymes from tropical endophytic xylariaceae. Fungal Divers. 2003;13:209–219. [Google Scholar]
- 3.Chawachart N., Khanongnuch C., Watanabe T., Lumyong S. Rice bran as an efficient substrate for laccase production from thermotolerant basidiomycete Coriolus versicolor strain RC3. Fungal Divers. 2004;15:23–32. [Google Scholar]
- 4.Gianfreda L., Xu F., Bollag J.M. Laccases: a useful group of oxidoreductive enzymes. Biorem. J. 1999;3:1–26. [Google Scholar]
- 5.Wong Y., Yu J. Laccase-catalyzed decoiorization of synthetic dyes. Water Res. 1999;33:3512–3520. [Google Scholar]
- 6.Mendoza L., Jonstrup M., Hatti-Kaul R., Mattiasson B. Azo dye decolorization by a laccase/mediator system in a membrane reactor: enzyme and mediator reusability. Enzyme Microb. Technol. 2011;49(5):478–484. doi: 10.1016/j.enzmictec.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Mayer A.M., Staples R.C. Laccase: new functions for an old enzyme. Phytochemistry. 2002;60:551–565. doi: 10.1016/s0031-9422(02)00171-1. [DOI] [PubMed] [Google Scholar]
- 8.Nelson D., Elisa E. Potential applications of oxidative enzymes and phenoloxidase-like compounds in wastewater and soil treatment. Appl. Catal. B-Environ. 2000;28:83–99. [Google Scholar]
- 9.Nelson D., Maria A.R., Alessandro D., Liliana G. Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports. Enzyme Microb. Tech. 2002;31:907–931. [Google Scholar]
- 10.Toh Y.C., Yen J.J.L., Obbard J.P., Ting Y.P. Decolourization of azo dyes by white-rot fungi (WRF) isolated in Singapore. Enzyme Microb. Technol. 2003;33:569–575. [Google Scholar]
- 11.Pandey A. Recent developments in solid state fermentation. Process Biochem. 1992;27:109–117. [Google Scholar]
- 12.Kalishwaralal K., Gopalram S., Vaidyanathan R., Deepak V., Pandian S.R.K., Gurunathan G. Optimization of α-amylase production for the green synthesis of gold nanoparticles. Colloid Surf. B. 2010;77:174–180. doi: 10.1016/j.colsurfb.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Faramarzi M.A., Forootanfar H. Biosynthesis and characterization of gold nanoparticles produced by laccase from Paraconiothyrium variabile. Colloid Surf. B. 2011;87:23–27. doi: 10.1016/j.colsurfb.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 14.Patel H., Gupte A., Gupte S. Effect of different culture conditions and inducers on production of laccase by a basidiomycete fungal isolate Pleurotus ostreatus HP-1 under solid state fermentation. Bioresour. Technol. 2009;4:268–284. [Google Scholar]
- 15.Leonowicz A., Grzywnowicz K. Quantitative estimation of laccase forms in some white-rot fungi using syringaldazine as a substrate. Enzyme Microb. Technol. 1981;3:55–58. [Google Scholar]
- 16.Ben Hamman O., De la Rubia T., Martinez J. The effect of manganese on the production of phanerochaete flavido-alba ligninolytic peroxidases in nitrogen limited cultures. FEMS Microbiol. Lett. 1999;177:137–142. [Google Scholar]
- 17.Janani L.K., Gaurav Kumar K.V., Rao B. Screening of pectinase producing microorganisms from agricultural waste dump soil. Asian J. Biochem. Pharm. Res. 2011;2:2231–2560. [Google Scholar]
- 18.Bradford M.M. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 19.El-Batal A.I., Abd-Algawad M.H., Abdelbaky N.M. Enhancement of some natural antioxidants activity via microbial bioconversion process using gamma irradiation and incorporation into gold nanoparticles. World Appl. Sci. J. 2012;19(1):1–11. [Google Scholar]
- 20.Myers R.H., Montgomery D.C. Process and Product Optimization Using Designed Experiments. 2nd ed. Wiley; New York: 2002. Response surface methodology. [Google Scholar]
- 21.Hatakka A. Lignin-modifying enzymes from selected white-rot fungi: production and role in lignin degradation. FEMS Microbiol. Rev. 1994;13:125–135. [Google Scholar]
- 22.Eger G., Eden G., Wissig E. Pleurotus ostreatus breeding potential of a new cultivated mushroom. Theor. Appl. Genet. 1976;47(1976):55–163. doi: 10.1007/BF00278373. [DOI] [PubMed] [Google Scholar]
- 23.Gómez J., Pazos M., Couto S.R., Sanromán R.A. Chestnut shell and barley bran as potential substrates for laccase production by Coriolopsis rigida under solid-state conditions. J. Food Eng. 2005;68:315–319. [Google Scholar]
- 24.Neifar M., Jaouani A., Ellouze-Ghorbel R., Ellouze-Chaabouni S., Penninckx M.J. Effect of culturing processes and copper addition on laccase production by the white-rot fungus Fomes fomentarius MUCL 35117. Lett. Appl. Microbiol. 2009;49:73–78. doi: 10.1111/j.1472-765X.2009.02621.x. [DOI] [PubMed] [Google Scholar]
- 25.Osma J.F., Toca Herrera J.L., Rodríguez Couto S. Laccase production by Trametes pubescens grown on wheat bran under solid-state conditions, (2006) 6th European Symposium on Biochemical Engineering Science, Salzburg (Austria).
- 26.Vasev K., Kuhad R.C. Induction of Laccase production in Cyathus bulleri under shaking and static conditions. Folia. Microbiol. 1994;39(4):326–330. [Google Scholar]
- 27.Buswell J.A., Cai Y., Chang S.T. Effect of nutrient nitrogen and manganese on manganese peroxidase and laccase production by Lentinula (Lentinus) edodes. FEMS Microbiol. Lett. 1995;128:81–88. [Google Scholar]
- 28.Dittmer J.K., Patel S.W., Dhawale S.W., Dhawale S.S. Production of multiple laccase isoforms by Phanerochaete chrysosporium grown under nutrient sufficiency. FEMS Microbiol. Lett. 1997;149:65–70. [Google Scholar]
- 29.Eggert C., Temp U., Dean J.F.D., Eriksson K.E.L. The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl. Environ. Microbiol. 1996;62:1151–1158. doi: 10.1128/aem.62.4.1151-1158.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leštan D., Leštan M., Perdih A. Physiological aspects of biosynthesis of lignin peroxidases by Phanerochaete chrysosporium. Appl. Environ. Microbiol. 1994;60:606–612. doi: 10.1128/aem.60.2.606-612.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rothschild N., Hadar Y., Dosoretz C. Ligninolytic system formation by Phanerochaete chrysosporium in air. Appl. Environ. Microbiol. 1995;61:1833–1838. doi: 10.1128/aem.61.5.1833-1838.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bourbonnais R., Paice M.G., Leech D., Freiermuth B. In Proceedings of the TAPPI Biological Sciences Symposium. TAPPI Press; Atlanta, GA: 1997. Reactivity and mechanism of laccase mediators for pulp delignification; pp. 335–338. [Google Scholar]
- 33.Harkin J.M., Obst J.R. Syringaldazine, an effective reagent for detecting laccase and peroxidase in fungi. Experientia. 1973;39:381–387. [Google Scholar]
- 34.Collins P.J., Dobson A.D.W. Regulation of laccase gene transcription in Trametes versicolor. Appl. Environ. Microbiol. 1997;63:3444–3450. doi: 10.1128/aem.63.9.3444-3450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmieri G., Giardina P., Bianco C., Fontanella B., Sannia G. Copper induction of laccase isoenzymes in the ligninolytic fungus Pleurotus ostreatus. Appl. Environ. Microbiol. 2000;66:920–924. doi: 10.1128/aem.66.3.920-924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Labbé S., Thiele D.J. Pipes and wiring: the regulation of copper uptake and distribution in yeast. Trends Microbiol. 1997;7:500–505. doi: 10.1016/s0966-842x(99)01638-8. [DOI] [PubMed] [Google Scholar]
- 37.Luckey T.D. Low-dose irradiation reduces cancer deaths. Radiat. Protect. Manag. 1997;1:58–64. [Google Scholar]
- 38.Abo-State M.A.M., Khatab O., Abo-E.L Nasar A., Mahmoud B. Factors affecting laccase production by Pleurotus ostreatus and Pleurotus sajor-caju. World Appl. Sci. J. 2011;14(11):1607–1619. [Google Scholar]
- 39.Aubrey F.M. Control of Food Borne Microorganisms. Marcel Dekker, Inc.; 2002. Inactivation by Irradiation; p. 535. [Google Scholar]
- 40.Smith J.C., Pillai S. Irradiation and food safety. Food Technol. 2004;58(11):48–54. [Google Scholar]
- 41.Manole A., Herea D., Chiriac H., Melnig V. Laccase activity determination. Scientific Annals of Alexandru Ioan Cuza din Iaşi University, Tom IV, Biomaterials in Biophysics Medical Physics and Ecology, (2008) 17–24.
- 42.Luisa M., Goncalves F.C., Steiner W. Purification and characterisation of laccase from a newly isolated wood-decaying fungus. Am. Chem. Soc. 1996;20:258–263. [Google Scholar]
- 43.Galhaup C., Wagner H., Hinterstoisser B., Haltrich D. Increased production of laccase by the wood-degrading basidiomycete Trametes pubescens. Enzyme Microb. Technol. 2002;30:529–536. [Google Scholar]
- 44.Palonen H., Saloheimo M., Viikari L., Kruus K. Purification,characterization and sequence analysis of a laccase from the ascomycete Mauginiella sp. Enzyme Microb. Technol. 2003;33:854–862. [Google Scholar]
- 45.Kunamneni A., Ballesteros A., Plou F.J., Alcalde M. Fungal laccase – a versatile enzyme for biotechnological applications. In: Méndez-Vilas A., editor. Vol. 1. Formex, Badajoz; Spain: 2007. pp. 233–245. (Communicating Current Research and Educational Topics and Trends in Applied Microbiology). [Google Scholar]
- 46.Jordaan J., Pletschke B.I., Leukes W.D. Purification and partial characterization of a thermostable laccase from an unidentified basidiomycete. Enzyme Microb. Technol. 2004;34:635–641. [Google Scholar]
- 47.Selvam K., Swaminathan K., Chae K.S. Decolourization of azo dyes and a dye industry effluent by a white rot fungus Thelephora sp. Bioresour. Technol. 2003;88:115–119. doi: 10.1016/s0960-8524(02)00280-8. [DOI] [PubMed] [Google Scholar]
- 48.Zilly A., Souza C.G.M., Barbosa-Tessmann I.P., Peralta R.M. Decolorization of industrial dyes by a brazilian strain of Pleurotus pulmonarius producing laccase as the sole phenol-oxidizing enzyme. Folia Microbiol. 2002;47(3):273–277. doi: 10.1007/BF02817651. [DOI] [PubMed] [Google Scholar]
- 49.Martins M.A.M., Lima N., Silvestre A.J.D., Queiroz M.J. Comparative studies of fungal degradation of single or mixed bioaccessible reactive azo dyes. Chemosphere. 2003;52:967–973. doi: 10.1016/S0045-6535(03)00286-8. [DOI] [PubMed] [Google Scholar]
- 50.Kandelbauer A., Maute O., Kessler R.W., Erlacher A., Gübitz G.M. Study of dye decolourization in an immobilized laccase enzyme-reactor using online spectroscopy. Biotechnol. Bioeng. 2004;87:552–563. doi: 10.1002/bit.20162. [DOI] [PubMed] [Google Scholar]
- 51.Safaepour M., Shahverdi A.R., Shahverdi H.R., Khorramizadeh M.R., Gohari A.R. Green synthesis of small silver nanoparticles using geraniol and its cytotoxicity against fibrosarcoma-wehi 164. Avicenna J. Med. Biotechnol. 2009;1:111–115. [PMC free article] [PubMed] [Google Scholar]
- 52.Gole A., Dash C., Ramakrishnan V., Sainkar S.R., Mandale A.B., Rao M., Sastry M. Pepsin-gold colloid conjugates: preparation, characterization, and enzymatic activity. Langmuir. 2001;17:1674–1679. [Google Scholar]
- 53.Tortora G.J., Funke B.R., Case L. 10th ed. Pearson Education Private Limited; Singapore: 2004. Microbiology an Introduction; p. 115. [Google Scholar]
- 54.Rastogi L., Arunachalam J. Sunlight base irradiation strategy for rapid green synthesis of the highly stable silver nanoparticles using aqueous garlic (Allium sativum) extract and their antibacterial potential. J. Mater. Chem. Phys. 2011;129:558–563. [Google Scholar]
- 55.Noruzi M., Zare D., Khoshnevisan K., Davoodi D. Rapid green synthesis of gold nanoparticles using Rosa hybrida petal extract at room temperature. J. Spectrochim. Acta Part A. 2011;791:46–1465. doi: 10.1016/j.saa.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 56.Shakibaie M., Forootanfar H., Mollazadeh-Moghaddam K., Bagherzadeh Z., Nafissi-Varcheh N., Shahverdi A.R., Faramarzi M.A. Green synthesis of gold nanoparticles by the marine microalga Tetraselmis suecica. Biotechnol. Appl. Biochem. 2010;57(2):71–75. doi: 10.1042/BA20100196. [DOI] [PubMed] [Google Scholar]
- 57.Sanghi R., Verma P. 15 Microbes as green and eco-friendly nanofactories. In: Sharma S., editor. Green Chemistry for Environmental Sustainability. CRC Press; 2010. p. 315. [Google Scholar]
- 58.Mandal S., Phadtare S., Sastry M. Use of aqueous foams for the synthesis of gold nanoparticles of variable morphology. Curr. Appl. Phys. 2005;5:118–127. [Google Scholar]
- 59.Mostafavi M., Delcourt M.O., Picq G. Study of the interaction between polyacrylate and silver oligomer clusters. J. Radiat. Phys. Chem. 1993;41:453–459. [Google Scholar]
- 60.Marignier J.L., Belloni J., Delcourt M.O., Chevalier J.P. Microaggregates of non-noble metals and bimetallic alloys prepared by radiation-induced reduction. Nature. 1985;317:344–345. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.