Highlights
-
•
Plastein is a gel-like product of protease-induced peptide aggregation.
-
•
Plastein is formed by peptide condensation, transpeptidation and physical interaction.
-
•
Plastein can be used in enhancing protein quality and debittering protein hydrolysates.
-
•
The peptide aggregate also possesses bioactivity related to health promotion.
-
•
Future directions toward industrial applications of plastein are suggested.
Keywords: Plastein, Peptide aggregation, Nutritional enrichment, Debittering, Bioactivity, Nutraceutical
Abstract
Plastein is a protease-induced peptide aggregate with prospective application in enhancing the nutritional quality of proteins and debittering protein hydrolysates. These properties are yet to be applied in product development possibly due to economic considerations (production cost vs. product yields). This paper reviews currently proposed mechanisms of plastein formation including condensation, transpeptidation and physical interaction of aggregating peptides. Emerging findings indicate that plastein possesses bioactivities, thereby expanding its prospective application. The role of proteases in inducing peptide interaction in plastein remains unclear. Understanding the protease function will facilitate the development of efficient proteases and scalable industrial processes for plastein production.
1. Introduction
Plastein is formed as gels or thixotropic products from concentrated peptide mixtures or protein hydrolysates in the presence of proteases under optimum conditions. The peptide aggregate was first observed by Danilewski in 1886 when stomach extract was added to concentrated peptic hydrolysates [50]. It was believed that active enzyme was responsible for precipitation since heat-inactivated extract did not induce precipitation. A decade later, Okunew suggested that the precipitates were newly synthesized products from protein fragments [50]. The term “plastein” was proposed in 1901 and its formation was believed to be a reversal of regular protease-induced protein hydrolysis through the reformation of peptide linkages [50], since the decreased free amino nitrogen content associated with plastein formation was regarded as the reversal of hydrolysis. Moreover, the aggregate was suggested to be similar to acid-denatured proteins [5], [49], [50], [51], although more recent evidence showed lack of defined structural pattern in plastein as deduced by differential scanning calorimetry [6]. It was also thought that plastein could be a mixture of substances, and that its insolubility and decreased free amino nitrogen content were due to cyclic anhydride formation [14], [15], [29]. Many other mechanisms have been proposed for plastein formation. The most recent mechanism involves aggregation through the formation of non-covalent bonds in interacting peptides [1], [38], [47], [59]. However, the exact role of the proteases in inducing aggregation remains unclear.
The value of plastein was not realized until the 1970s, several decades after its discovery. The first proposed application of plastein was focused on the production of high quality food proteins for nutritional uses. Plastein was explored as a vehicle for the delivery of essential amino acids and as means of debittering food protein hydrolysates. Despite the prospects, industrial application of plastein in food production was not popular possibly due to challenges associated with yield and cost of production, and these may have contributed to stagnation of interest on the peptide aggregates. However, recent efforts have rekindled interest in plastein research with the observation of its biological activity related to human health promotion. It is known that the chemical and physical properties of peptides are greatly altered after plastein reaction. Particularly, viscosity and molecular weight are increased in plastein [22], [53], and this can result in alteration of bioactivities associated with native molecular peptide structures.
2. Proposed mechanisms of plastein formation
The mechanism of plastein formation has not been completely elucidated. Plastein was first thought to be a resynthesized protein based on the decrease in free amino nitrogen content and absence of precipitate formation when inactivated protease was used [7], [48]. However, it was also thought that the precipitates are formed as a result of denaturation of high molecular weight substances in the hydrolysis product [7], [25]. Subsequently, a number of mechanisms have been proposed for plastein formation including peptide condensation, transpeptidation and aggregation induced by physical forces.
2.1. Peptide condensation
In a bid to debitter and increase the nutritional value of protein hydrolysates, Yamashita et al. [58] found that l-methionine derivatives can be incorporated into soy hydrolysates using papain to form plastein; the authors suggested the formation of peptide bonds during the reaction between the amino acids and protein hydrolysates. Condensation was not observed with free amino acids and was successful only with peptides or amino acid ethyl esters [58]. The peptide condensation mechanism can be supported by the decreased free amino nitrogen content, appearance of higher molecular weight complexes and increased viscosity observed in plastein compared to their protein hydrolysate precursors [22], [47], [57], [61]. A study with nuclear magnetic resonance spectroscopy and electrospray mass spectroscopy in a model system provided evidence of oligopeptide formation from condensation of smaller hydrophobic synthetic peptides, although the phenomenon could not be confirmed to occur to the same extent in complex protein hydrolysate system [37]. Furthermore, blocking the amino and carboxyl terminals of peptides was demonstrated to only slightly lower plastein yield [1], indicating the possibly minor role of peptide condensation and existence of other mechanisms of plastein formation.
2.2. Transpeptidation
Some studies have found that the amount of amino nitrogen remained unchanged with elevated hydrophobic amino acid residues during the formation of egg albumin and soy protein-derived plastein [23], [54]. Van Hofsten and Lalasidis [48] reported the absence of high molecular weight product in whey and fish protein-derived plastein, whereas other studies reported the formation of low- and high-molecular weight peptide complexes during plastein reaction [8]. Based on these findings, transpeptidation was proposed to be partially responsible for plastein formation [17], [20], [54]. Transpeptidation is a process involving peptide bond breakage and formation resulting in the rearrangement of amino acid residues. Therefore, amino acid residues are transferred from one peptide sequence to another leading to accumulation of hydrophobic amino acid residues in some peptides and subsequent formation of hydrophobic core and aggregation of the peptides. Transpeptidation was believed to occur with condensation during plastein synthesis [12] and this was supported by the observation of new and rearranged peptides in model systems [37]. However, the selectivity of transpeptidation in rearranging specific amino acid residues, and mechanism of protease-induced peptide bond breakage and formation (characteristic of transpeptidation) during plastein reaction are not apparent from the studies discussed above.
2.3. Physical forces in peptide aggregation
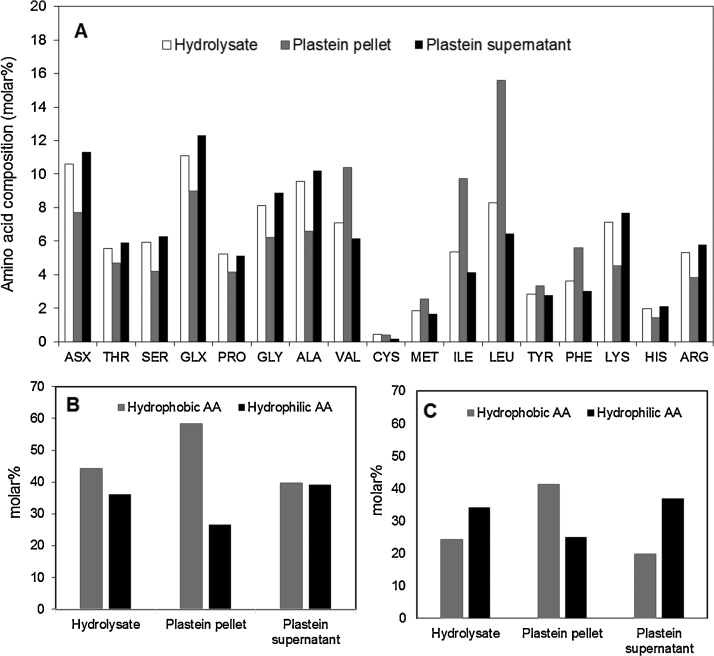
Andrews and Alichanidis observed that there was no substantial qualitative difference in peptide composition of resolubilized plastein and the starting hydrolysates. This finding indicated that new peptides may not be synthesized during plastein formation as suggested by gel electrophoresis and preliminary small-angle neutron scattering experiments [1]. Moreover, plastein formation was found in the study to proceed even after enzyme inactivation albeit at a slower rate. These evidences eliminate condensation and transpeptidation as the major mechanisms of plastein synthesis. It was then suggested that aggregation during plastein reaction could be entropy-driven as a result of non-covalent interaction of hydrophobic and ionic regions of the peptides [1], [37]. The hydrophobicity mechanism was supported by the observation of increased affinity of plastein for a hydrophobic probe, and over 98% decrease in turbidity of aqueous plastein suspension in the presence of a surfactant (2 mM sodium dodecyl sulfate), which would disrupt the hydrophobic bonds that hold the plastein structure together [3], [47]. High ionic strength solutions were found to have little or no effect on plastein turbidity, indicating the minor role of ionic interaction in the aggregate formation [40], [47], [48]. The hydrophobic interaction mechanism is highly plausible as isolated mycoprotein-derived plastein pellets were found to contain higher molar% of hydrophobic amino acids (Val, Leu, Met, Phe, Ile) and lower amounts of hydrophilic amino acids compared to its hydrolysates precursor and unincorporated peptides in the plastein supernatant [6], as shown in Fig. 1A–C. Therefore, selection of protein precursors rich in hydrophobic amino acid residues can be pursued for the production of high plastein yields. Although hydrophobicity-induced aggregation is the most currently accepted and plausible mechanism, there is still a knowledge gap on the specific role of proteases in inducing non-covalent peptide interactions during plastein formation.
Fig. 1.
(A) Amino acid composition, (B) total and (C) major hydrophobic and hydrophilic amino acid residues (molar%) of mycoprotein hydrolysates, its platein pellet and residual peptides in supernatant after plastein isolation (total hydrophobic: Pro, Ala, Val, Cys, Met, Ile, Leu, Tyr, Phe; total hydrophilic: Asx, Glx, Lys, His, Arg; major hydrophobic: Val, Ile, Leu, Phe; major hydrophilic: Asx, Glx, Lys, Arg); amino acid composition was obtained from Brownsell et al. [6].
3. Plastein production
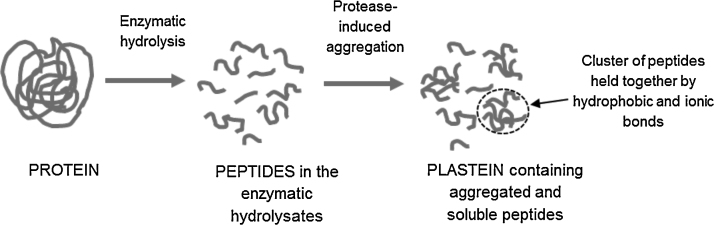
As shown in Fig. 2, the method of plastein production usually consists of three steps: (1) protein hydrolysis by proteases at low substrate concentration (3–5%, w/v); (2) concentration of protein hydrolysates by freeze-drying or evaporation, and (3) plastein formation by incubating high concentration of the protein hydrolysates with same or different protease [10]. Plastein reaction usually occurs at substrate concentrations of 30–50% (w/v), optimally at 40%, and at pH and temperature ranges from 3.0–7.0 to 37–50 °C, respectively. The optimum temperature and pH are not necessarily for the enzymatic activity during hydrolysis. It is recommended that the same protease be used for the protein hydrolysis and plastein formation steps in order to avoid further proteolysis during plastein reaction, which can decrease plastein yield [39]. Depending on conditions of incubation, the reaction time for plastein formation can range from 24 to 72 h [12], although the aggregates have also been produced during shorter durations [47], [61], [63]. Repeated washing was found to solubilize plastein in aqueous solution; therefore, plastein formation was considered to be reversible although the aggregate can be precipitated as insoluble material [1]. Proteases that are commonly used for protein hydrolysis and subsequent plastein reaction include papain, pepsin, α-chymotrypsin and trypsin; although there is currently no supporting mechanism, pepsin is considered the most effective for producing high yields of plastein with high gel strength especially near neutral pH [18], [52], [55]. Furthermore, inactivated proteases can catalyze plastein formation to some extent resulting in low product yield; thus, native active proteases are preferred for plastein production [1].
Fig. 2.
The reaction steps in protease induced-aggregation during plastein formation.
In general, proteinogenic amino acids have the potential to form plastein under optimum conditions but those with hydrophobic side chains are more likely to be easily incorporated into plastein [3], [12]. Several protein-containing agricultural and food materials have been utilized as substrates for plastein formation including soybean, plant leave proteins, zein, wheat, egg albumin, milk, corn, mycoprotein, squid, fish and fish silage [4], [11], [24], [30], [31], [34], [36], [39], [52].
4. Factors that influence plastein formation
The major factor that influences plastein formation is the substrate (protein hydrolysates or oligopeptide mixture) concentration [17], [44], [52]. For instance, plastein yield from mycoprotein hydrolysates produced with pepsin (enzyme–substrate ratio, E/S 1:100, pH 5.0, 37 °C) was found to increase exponentially from about 4 to 13% with respective peptide concentrations of 11–43% (w/w) [52]. Therefore, higher substrate content enhances peptide proximity, which encourages entropy-driven aggregation of the hydrophobic peptides [52]. In fact, low substrate concentration has been found to promote enzymatic hydrolysis instead of peptide aggregation [17], [44]. Moreover, the low-molecular weight of peptides has been shown to promote plastein formation. Fujimaki et al. [17] reported that increase in degree of hydrolysis resulted in higher yields of plastein produced from protein hydrolysates using microbial proteases. This was thought to be due to the requirement of free amino acids or small peptides for condensation and elongation [48], considering the plausible roles of these reactions in promoting the formation of plastein. However, the effect of increased hydrolysis on plastein yield was found to be independent of plastein reaction time, although substantial decrease in gel strength was observed during long (24 h) duration [11]. This suggests that higher molecular weight peptides can also form plastein but formation of stronger aggregate is favored with smaller peptide precursors. Sukan and Andrews [39] reported a peptide molecular size range of 400–800 Da for obtaining the highest plastein yield for pepsin–casein hydrolysates reaction, and Williams et al. [52] noted that further decrease in peptide size due to extensive hydrolysis (as observed with pronase) would likely not support plastein synthesis. It is possible that the ability of peptides to form plastein is related to the amount of their constituent hydrophobic amino acid residues, which are less likely to occur within a given peptide after extensive protein hydrolysis. The relationship between peptide molecular size and plastein yield still needs to be clearly defined.
Plastein reaction has been found to readily occur with peptides and less likely between peptides and free amino acids. Based on the condensation mechanism, the energy required to form a peptide bond for peptides (0.4 kcal/mol) is far less than that required for free amino acids (4 kcal/mol) [12], although the condensation mechanism in plastein formation appears thermodynamically unfavorable [1], [37], [48]. Based on energy requirements, earlier studies utilized amino acid ethyl esters for plastein formation with different proteins instead of free amino acids [4], [16], [57]. Moreover, hydrophobic amino acids appear more likely to be incorporated into plastein than hydrophilic amino acids, although highly hydrophobic amino acids present a challenge of precipitating before the occurrence of plastein reaction [3]. The incorporation of non-hydrophobic amino acids into plastein can be enhanced by alkylation. For instance, ethylation of the α- and γ-carboxyl groups of Glu enhanced its incorporation into soy globulin [59]. Furthermore, the type of protein starting material can also impact plastein yield. For instance, pepsin-induced plastein formation in mycoprotein and casein hydrolysates yielded 13% and 27% of aggregate products, respectively, under similar reaction conditions [39], [52]. This observation may be due to differences in the hydrophobicity of the proteins and the resulting enzymatic hydrolysates.
The pH of reaction medium has been shown to impact plastein reaction. The optimum pH for enzymatic activity is needed for protein hydrolysis whereas the highest plastein yields are obtained at different pH ranging from 3.0 to 11.0, depending on the protease [12], [17]. Contrary observations have been reported where the yield of mycoprotein-derived plastein varied at pH 3.0–7.5 [52], and it appears that the substrate type, protease and reaction conditions may have contributed to the discrepancies. Moreover, protease immobilization has been demonstrated to impact plastein yield. For instance, immobilized α-chymotrypsin on hydrophobic agarose gels and chitin were found to produce higher plastein yields compared to the free enzyme [26], [32], [33], although there could be marked differences in the plastein amino acid composition compared to the traditionally produced aggregates [33]. Furthermore, plastein formation can be affected by water activity and matrix interactions of aggregating peptides with non-protein substances such as polysaccharides, lipids and salts [8], [21], [27], as well as the E/S ratio, protease mode of action (endo- vs. exoprotease activity) and surface property of peptides, e.g., hydrophobicity [37].
5. Proposed food applications of plastein
Plastein has been proposed for use in enhancing the nutritional profile of proteins, and in debittering protein hydrolysates (Table 1) although these applications are yet to be utilized in the food industry. Provided the cost of production is reasonable, these features (and the thixotropic property) of plastein can be utilized in enhancing food functionality during food product formulation.
Table 1.
Potential food and nutraceutical application of plastein.
| Potential applications | Notes | References |
|---|---|---|
| Improvement of the nutritional value of proteins | Essential amino acids can be incorporated into low-quality proteins by protease-induced plastein reaction | [4], [43], [56], [58], [59] |
| Enhancement of chemosensory properties of protein hydrolysates and peptides | Bitterness-contributing hydrophobic amino acid residues can be hidden in the plastein core thereby limiting interaction with bitter taste receptors | [37], [38], [43] |
| Biological activities | ||
| Antihypertensive | In vitro inhibition of angiotensin I-converting enzyme activity | [19], [41], [53] |
| Antioxidative | In vitro radical scavenging, metal chelating and lipid peroxidation inhibitory activities | [47], [63] |
| Cytoprotection against H2O2- and galactosamine-induced damage to rat hepatocyte (BRL) culture | [62] | |
| Antithrombotic | In vitro inhibition of platelet aggregation (anticoagulation) based on activated partial thromboplastin and prothrombin time | [60] |
5.1. Enhancement of nutritional quality of food proteins
Plastein reaction can be used to improve nutrient value of food proteins or unconventional protein sources such as plant or meat by-products and algae through the incorporation of essential amino acids. Soy proteins contain low amounts of sulfur-containing amino acids, Met and Cys and after plastein reaction with Met there was up to 6-fold higher content of the amino acid compared to the starting material [28], [58]. Feeding experiment indicated that protein efficiency ratio (PER) of the Met-modified protein product was 2-fold higher than that of the unmodified soy protein in rats [58]. Similar observations were reported in prawns with a suggestion that the Met in plastein was more bioavailable than crystalline Met [43]. The increased bioavailability could be due to the higher absorption rate (via dedicated peptide transporters) of plastein peptides containing the supplemental amino acid residues compared to free amino acids. However, Ashley et al. [2] reported similar PER for Met-modified plastein and free Met/protein combination in weanling rats. Other hydrophobic and hydrophilic amino acids including Trp, Lys, Glu and Thr have also been successfully incorporated into zein and soy globulin by plastein reaction [4], [56], [59]. Despite the prospects, there is limited interest in commercial utilization of plastein reaction in enhancing the nutritional quality of proteins, and this could be due to the cost of the enzymatic processes, low plastein product yield and availability of alternative proteins with the desired nutritional quality and functional properties.
5.2. Debittering of protein hydrolysates
Protein hydrolysates are used as emulsifying agents in salad dressings, spreads, ice cream, coffee whitener and meat products, and as sources of bioactive peptides for health promotion [35], [46]. Hydrolyzed proteins can be bitter due to low-molecular peptides that are rich in hydrophobic amino acid residues such as Leu, Ile, Val, Phe, Pro, Tyr and Trp [35]. The hydrophobic amino acid residues are concentrated in the protein core where they are mostly inaccessible, but are exposed following enzymatic hydrolysis. A list of bitter peptides can be found in BIOPEP database under “sensory peptides and amino acids” at http://www.uwm.edu.pl/biochemia/index.php/en/biopep. Bitterness of protein hydrolysates can negatively impact consumer acceptability of food products, and can be eliminated by activated carbon treatment, alcohol extraction, precipitation and chromatography [35]. However, these methods can lead to loss of important peptides and functionality of the hydrolysates. Moreover, biotechnological methods have been applied in debittering protein hydrolysates, and this involves the use of endo- and exopeptidases to remove the amino acid (Pro) residues, that contribute to bitterness [13], [35]. Likewise, structural alterations will occur with enzymatic debittering potentially leading to loss of the hydrolysate function. In order to circumvent this limitation, a previous review discussed the possible use of peptide condensation during plastein reaction to eliminate bitterness [35], as demonstrated using a model synthetic peptide system [37]. Moreover, alcalase-induced plastein was reported to reduce the bitterness of protein hydrolysates by 50% [42]. Considering that condensation was found to rarely occur in complex protein hydrolysates that contain many peptides and possibly amino acids [37], peptide aggregation during plastein reaction may be responsible for hiding the hydrophobic amino acid residues of the hydrolysates from taste receptors during oral consumption. This may explain the “insoluble materials” identified by [37] that contributed to reduced bitterness. If confirmed, plastein reaction can become a means of debittering bioactive peptides possibly without loss of structure and function, which appears to be a challenge in formulating peptide-based functional foods [45].
6. Reemergence of plastein for potential nutraceutical application
Bioactive peptides derived from enzymatic hydrolysis of food proteins are widely pursued as functional food ingredients for human health promotion [46]. Plastein contains peptides of which some may be structurally modified especially if aggregation was induced by peptide condensation or transpeptidation. In recent years, the structural modifications and aggregation that occur during plastein reaction are thought to lead to alteration of bioactivity of peptides (Table 1). For instance, plastein reaction was reported to increase the angiotensin converting enzyme (ACE) inhibitory activity of enzymatic protein hydrolysates [19], [41], [53], [61]. Moreover, plastein has been reported to exhibit antioxidative activity [63], although contrasting observation has been reported where plastein reaction resulted in loss of metal chelating activity of casein hydrolysates and no substantial impact on reducing potential and inhibition of lipid peroxidation [47]. The antioxidative property of plastein was implicated in the cytoprotective activity of Phe- and Tyr-enriched plastein in H2O2- and galactosamine-induced damage to cultured rat hepatatocyte; the plastein increased cell viability and decreased malondialdehyde content and lactate dehydrogenase leakage associated with the hepatocyte damage [62]. Furthermore, plastein reaction was reported to improve the in vitro anticoagulation (inhibition of platelet aggregation) activity of soybean protein-derived plastein based on increased activated partial thromboplastin and prothrombin time [60]. These findings have added a new perspective to the application of plastein for the development of health-promoting products. Despite the prospects, there is a need for validation of the physiological benefits of plastein using animal models and human subjects, and to obtain further insight on the structural basis of its enhanced bioactivity relative to the hydrolysate precursors.
7. Plastein stability
Considering that non-covalent interactions hold the peptides within plastein, the use of the aggregates in food product formulation and as nutraceutical is faced with challenges such as possible instability during food processing and under physiological conditions. Plastein has been found to be stable over a wide range of acidic and alkaline conditions (pH 2–11) [6] and in boiling water at 100 °C where the aggregate turbidity and bioactivity were mostly retained [47]. The latter indicates that H-bonds are likely not involved in plastein stabilization. Therefore, the observed stability will promote the use of plastein in food formulation under these pH and temperature conditions. Moreover, Sun and Zhao [41] suggested that plastein is resistant to proteolysis after gastric protease treatment, but this conclusion was based on retention of bioactivity by the protease-treated aggregate. Subsequent evidence demonstrated that casein-derived plastein is disintegrated and further hydrolyzed by gastric enzymes (pepsin and pancreatin) based on HPLC profiles [47]. This observation potentially limits the application of plastein aggregates as physiologically relevant bioactive ingredients as gastric modification would lead to loss of structure and function, similar to the challenges generally faced in translating peptides into functional products intended for dietary consumption [45]. That notwithstanding, the conservation (and sometimes elevation) of bioactivity and elimination of bitterness observed in plastein compared to hydrolysate precursors are promising evidence to warrant further investigation into their application in the functional food industry.
8. Future direction
It is evident from recent literature that hydrophobic forces play a major role in peptide aggregation during plastein reaction. Moreover, this conclusion does not preclude the possible involvement of condensation and transpeptidation, which can contribute in rearranging amino acid residues in building “hydrophobic cores” prior to hydrophobicity-induced peptide aggregation. As highlighted in this review, the unresolved formation mechanisms, product heterogeneity and processing cost factors appear to hinder industrial application of the beneficial features of plastein in food product development. With the emerging properties of plastein as bioactive agents, it becomes imperative to reconsider the value of the peptide aggregates in food and health applications. In order to circumvent the limitations on the application of plastein, future work should consider the following:
-
1.
Understanding the particular role of the proteases during plastein formation. This information will help in designing more efficient proteases (by engineering specific functional regions of their structures) for production of high yields of plastein, which would potentially lower the cost associated with the enzymatic process.
-
2.
Evaluation of industrial scale-up of the use of immobilized proteases for protein hydrolysis and plastein formation. If successful, this process will facilitate easy recovery and repeated usage of the proteases, and potentially lower the cost of commercial production of plastein.
Furthermore, a number of opportunities are presented by plastein reaction and these can be explored in different applications including:
-
1.
Elimination of amino acid and peptide-induced bitterness associated with enzymatic processing or microbial fermentation of plant-based and meat products intended for oral consumption by humans and animals (e.g., pet foods, nutraceuticals);
-
2.
Entrapment of bioactive compounds and flavoring agents within the plastein matrix for efficient delivery in food products;
-
3.
Utilization of the gel-like properties of plastein as thickening factors in the formulation of food products with acceptable sensory properties.
Acknowledgement
Financial support from the Natural Sciences and Engineering Research Council of Canada (NSERC) is acknowledged.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Andrews A.T., Alichanidis E. The plastein reaction revisited: evidence for a purely aggregation reaction mechanism. Food Chem. 1990;35(4):243–261. [Google Scholar]
- 2.Ashley D.V.M., Temler R., Barclay D., Dormond C.A., Jost R. Amino acid-enriched plasteins: a source of limiting amino acids for the weanling rat. J. Nutr. 1983;113(1):21–27. doi: 10.1093/jn/113.1.21. [DOI] [PubMed] [Google Scholar]
- 3.Aso K., Yamashita M., Arai S., Fujimaki M. Hydrophobic force as a main factor contributing to plastein chain assembly. J. Biochem. 1974;76(2):341–347. doi: 10.1093/oxfordjournals.jbchem.a130575. [DOI] [PubMed] [Google Scholar]
- 4.Aso K., Yamashita M., Arai S., Fujimaki M. Tryptophan-, threonine-, and lysine-enriched plasteins from zein. Agric. Biol. Chem. 1974;38(3):679–680. [Google Scholar]
- 5.Beard H.H. The nutritive value of plastein. J. Biol. Chem. 1927;71(2):477–480. [Google Scholar]
- 6.Brownsell V.L., Williams R.J.H., Andrews A.T. Application of the plastein reaction to mycoprotein: II. Plastein properties. Food Chem. 2001;72(3):337–346. [Google Scholar]
- 7.Butler J.A.V., Dodds E.C., Phillips D.M.P., Stephen J.M.L. The action of pepsin on insulin and the plastein question. Biochem. J. 1948;42(1):122. [PMC free article] [PubMed] [Google Scholar]
- 8.Combes D., Lozano P. α-Chymotrypsin in plastein synthesis influence of water activity. Ann. N. Y. Acad. Sci. 1992;672(1):409–414. [Google Scholar]
- 10.Doucet D., Gauthier S.F., Otter D., Foegeding E.A. Enzyme-induced gelation of extensively hydrolyzed whey proteins by alcalase: comparison with the plastein reaction and characterization of interactions. J. Agric. Food Chem. 2003;51:6036–6042. doi: 10.1021/jf026041r. [DOI] [PubMed] [Google Scholar]
- 11.Edwards J.H., Shipe W.F. Characterization of plastein reaction products formed by pepsin, α-chymotrypsin, and papain treatment of egg albumin hydrolysates. J. Food Sci. 1978;43(4):1215–1218. [Google Scholar]
- 12.Eriksen S., Fagerson I.S. The plastein reaction and its applications: a review. J. Food Sci. 1976;41(3):490–493. [Google Scholar]
- 13.FitzGerald R.J., O'Cuinn G. Enzymatic debittering of food protein hydrolysates. Biotechnol. Adv. 2006;24(2):234–237. doi: 10.1016/j.biotechadv.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Flosdorf E.W., Mudd S., Flosdorf E.W. The apparent antigenicity of plastein. J. Immunol. 1937;32:441–450. [Google Scholar]
- 15.Folley S.J. The nature of plastein. Biochem. J. 1932;26:99–105. doi: 10.1042/bj0260099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujimaki M., Arai S., Yamashita M. Enzymatic protein degradation and synthesis for protein improvement. Adv. Chem. Ser. 1977;160:156–184. [Google Scholar]
- 17.Fujimaki M., Kato H., Arai S., Yamashita M. Application of microbial proteases to soybean and other materials to improve acceptability, especially through the formation of plastein. J. Appl. Bacteriol. 1971;34(1):119–131. doi: 10.1111/j.1365-2672.1971.tb02272.x. [DOI] [PubMed] [Google Scholar]
- 18.Fujimaki M., Yamashita M., Arai S., Kato H. Enzymic modification of proteins in foodstuffs. I. Enzymic proteolysis and plastein synthesis application for preparing bland protein-like substrance. Agric. Biol. Chem. 1970;34(9):1325–1332. [Google Scholar]
- 19.Gao B., Zhao X.H. Modification of soybean protein hydrolysates by alcalase-catalyzed plastein reaction and the ACE-inhibitory activity of the modified product in vitro. Int. J. Food Prop. 2012;15(5):982–996. [Google Scholar]
- 20.Goepfert A., Lorenzen P.C., Schlimme E. Peptide synthesis during in vitro proteolysis-transpeptidation or condensation? Food/Nahrung. 1999;43(3):211–212. doi: 10.1002/(SICI)1521-3803(19990601)43:3<211::AID-FOOD211>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 21.Hagan R.C., Villota R. Effects of nonprotein substances on protein hydrolysis and plastein formation. Food Chem. 1987;23(4):277–294. [Google Scholar]
- 22.Hartnett E.K., Satterlee L.D. The formation of heat and enzyme induced (plastein) gels from pepsin-hydrolyzed soy protein isolate. J. Food Biochem. 1990;14(1):1–13. [Google Scholar]
- 23.Horowitz J., Haurowitz F. Mechanism of plastein formation. Biochim. Biophys. Acta. 1959;33(1):231–237. doi: 10.1016/0006-3002(59)90519-0. [DOI] [PubMed] [Google Scholar]
- 24.Hyung J.S., Lee H., Hong Y.C., Han C.Y. Production of protein hydrolysates and plastein from Alaska pollock. Korean Agric. Chem. Soc. 1992;35(5):339–345. [Google Scholar]
- 25.Kantola M., Virtanen A.I. X-ray diagram of plastein. Acta Chem. Scand. 1950;4(8):1314–1315. [Google Scholar]
- 26.Karube I., Yugeta Y., Suzuki S. Plastein synthesis by α-chymotrypsin immobilized on hydrophobic agarose gel. J. Mol. Catal. 1980;9(4):445–451. [Google Scholar]
- 27.Lozano P., Combes D. Effect of alkali halides on α-chymotrypsin activity in the plastein reaction. J. Sci. Food Agric. 1993;62(3):245–252. [Google Scholar]
- 28.Monti J.C., Jost R. Papain-catalyzed synthesis of methionine-enriched soy plasteins. Average chain length of the plastein peptides. J. Agric. Food Chem. 1979;27(6):1281–1285. [Google Scholar]
- 29.Northrop J.H. Plastein from pepsin and trypsin. J. Gen. Physiol. 1947;30:377–378. doi: 10.1085/jgp.30.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ono S., Kasai D., Sugano T., Ohba K., Takahashi K. Production of water soluble antioxidative plastein from squid hepatopancreas. J. Oleo Sci. 2004;53(5):267–273. [Google Scholar]
- 31.Onoue Y., Riddle V.M. Use of plastein reaction in recovering protein from fish waste. J. Fish. Board Can. 1973;30(11):1745–1747. [Google Scholar]
- 32.Pallavicini C., Finley J.W., Stanley W.L., Watters G.G. Plastein synthesis with α-chymotrypsin immobilised on chitin. J. Sci. Food Agric. 1980;31(3):273–278. [Google Scholar]
- 33.Pallavicini C., Peruffo A.D.B., Finley J.W. Comparative study of soybean plasteins synthesized with soluble and immobilized alpha-chymotrypsin. J. Agric. Food Chem. 1983;31(4):846–848. [Google Scholar]
- 34.Raghunath M.R., McCurdy A.R. Synthesis of plasteins from fish silage. J. Sci. Food Agric. 1991;54(4):655–658. [Google Scholar]
- 35.Saha B.C., Hayashi K. Debittering of protein hydrolyzates. Biotechnol. Adv. 2001;19(5):355–370. doi: 10.1016/s0734-9750(01)00070-2. [DOI] [PubMed] [Google Scholar]
- 36.Savangikar V.A., Joshi R.N. Modification of leaf protein concentrate by the use of plastein reaction. J. Sci. Food Agric. 1979;30(9):899–905. [Google Scholar]
- 37.Stevenson D.E., Morgan K.R., Fenton G.A., Moraes G. Use of NMR and mass spectrometry to detect and quantify protease-catalyzed peptide bond formation in complex mixtures. Enzyme Microb. Technol. 1999;25(3):357–363. [Google Scholar]
- 38.Stevenson D.E., Ofman D.J., Morgan K.R., Stanley R.A. Protease-catalyzed condensation of peptides as a potential means to reduce the bitter taste of hydrophobic peptides found in protein hydrolysates. Enzyme Microb. Technol. 1998;22:100–110. [Google Scholar]
- 39.Sukan G., Andrews A.T. Application of the plastein reaction to caseins and to skim-milk powder: I. Protein hydrolysis and plastein formation. J. Dairy Res. 1982;49(2):265–278. [Google Scholar]
- 40.Sukan G., Andrews A.T. Application of the plastein reaction to caseins and to skim-milk powder: II. Chemical and physical properties of the plasteins and the mechanism of plastein formation. J. Dairy Res. 1982;49:279–293. [Google Scholar]
- 41.Sun H., Zhao X.H. Angiotensin I converting enzyme inhibition and enzymatic resistance in vitro of casein hydrolysate treated by plastein reaction and fractionated with ethanol/water or methanol/water. Int. Dairy J. 2012;24(1):27–32. [Google Scholar]
- 42.Synowiecki J., Jagietka R., Shahidi F. Preparation of hydrolysates from bovine red blood cells and their debittering following plastein reaction. Food Chem. 1996;57(3):435–439. [Google Scholar]
- 43.Teshima S., Kanazawa A., Koshio S. Supplemental effects of methionine-enriched plastein in Penaeus japonicas diets. Aquaculture. 1992;101(1):85–93. [Google Scholar]
- 44.Tsai S.-J., Yamashita M., Arai S., Fujimaki M. Effect of substrate concentration on plastein productivity and some rheological properties of the products. Agr. Biol. Chem. 1972;36:1045. [Google Scholar]
- 45.Udenigwe C.C. Bioinformatics approaches, prospects and challenges of food bioactive peptide research. Trends Food Sci. Technol. 2014;36(2):137–143. [Google Scholar]
- 46.Udenigwe C.C., Aluko R.E. Food protein-derived bioactive peptides: production, processing, and potential health benefits. J. Food Sci. 2012;77(1):R11–R24. doi: 10.1111/j.1750-3841.2011.02455.x. [DOI] [PubMed] [Google Scholar]
- 47.Udenigwe C.C., Wu S., Drummond K., Gong M. Revisiting the prospects of plastein: thermal and simulated gastric stability in relation to the antioxidative capacity of casein plastein. J. Agric. Food Chem. 2014;62(1):130–135. doi: 10.1021/jf403405r. [DOI] [PubMed] [Google Scholar]
- 48.Van Hofsten B., Lalasidis G. Protease-catalyzed formation of plastein products and some of their properties. J. Agric. Food Chem. 1976;24:460–465. doi: 10.1021/jf60205a037. [DOI] [PubMed] [Google Scholar]
- 49.Virtanen A.I., Kerkkonen H.K., Laaksonen T. Obesrvations on the formation and structure of pla3. Acta Chem. Scand. 1948;2(10):933–935. [Google Scholar]
- 50.Wasteneys H., Borsook H. The enzymatic synthesis of protein. II. The effect of temperature on the synthesizing action of pepsin. J. Biol. Chem. 1924;62:15–29. [Google Scholar]
- 51.Wasteneys H., Borsook H. The enzymatic synthesis of protein. Physiol. Rev. 1930;10:110–145. doi: 10.1085/jgp.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams R.J.H., Brownsell V.L., Andrews A.T. Application of the plastein reaction to mycoprotein: I. Plastein synthesis. Food Chem. 2001;72(3):329–335. [Google Scholar]
- 53.Xu W., Kong B.H., Zhao X.H. Optimization of some conditions of neutrase-catalyzed plastein reaction to mediate ACE-inhibitory activity in vitro of casein hydrolysate prepared by Neutrase. Food Sci. Technol. 2014;51(2):276–284. doi: 10.1007/s13197-011-0503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamashita M., Arai S., Tanimoto S. Condensation reaction occurring during plastein formation by a-chymotrypsin. Agric. Biol. Chem. 1973;37(4):953–954. [Google Scholar]
- 55.Yamashita M., Arai S., Fujimaki M. Plastein reaction for food protein improvement. J. Agric. Food Chem. 1976;24(6):1100–1104. [Google Scholar]
- 56.Yamashita M., Arai S., Kokubo S., Aso K., Fujimaki M. Synthesis and characterization of a glutamic acid enriched plastein with greater solubility. J. Agric. Food Chem. 1975;23(1):27–30. [Google Scholar]
- 57.Yamashita M., Arai S., Tsai S.J., Fujimaki M. Supplementing sulfur-containing amino acids by plastein reaction. Agric. Biol. Chem. 1970;34(10):1593–1596. [Google Scholar]
- 58.Yamashita M., Arai S., Tsai S.-J., Fujimaki M. Plastein reaction as a method for enhancing the sulfur-containing amino acid level of soybean protein. J. Agric. Food Chem. 1971;19:1151T. doi: 10.1021/jf60178a029. [DOI] [PubMed] [Google Scholar]
- 59.Yamashita M., Arai S., Kokubo S., Aso K., Fujimaki M. A plastein with an extremely high amount of glutamic acid. Agric. Biol. Chem. 1974;38:1269. [Google Scholar]
- 60.Zhang M.L., Zhao X.H. In vitro calcium-chelating and platelet anti-aggregation activities of soy protein hydrolysate modified by the alcalase-catalyzed plastein reaction. J. Food Biochem. 2014;38(3):374–380. [Google Scholar]
- 61.Zhao X.H., Li Y.Y. An approach to improve ACE-inhibitory activity of casein hydrolysates with plastein reaction catalyzed by alcalase. Eur. Food Res. Technol. 2009;229(5):795–805. [Google Scholar]
- 62.Zhao X.H., Fu Y., Yue N. In vitro cytoprotection of modified casein hydrolysates by plastein reaction on rat hepatocyte cells. CyTA-J. Food. 2014;12(1):40–47. [Google Scholar]
- 63.Zhao X.H., Wu D., Li T.J. Preparation and radical scavenging activity of papain-catalyzed casein plasteins. Dairy Sci. Technol. 2010;90(5):521–535. [Google Scholar]