Abstract
Jerusalem artichoke, a native plant to North America has recently been recognized as a promising biomass for bioeconomy development, with a number of advantages over conventional crops such as low input cultivation, high crop yield, wide adaptation to climatic and soil conditions and strong resistance to pests and plant diseases. A variety of bioproducts can be derived from Jerusalem artichoke, including inulin, fructose, natural fungicides, antioxidant and bioethanol. This paper provides an overview of the cultivation of Jerusalem artichoke, derivation of bioproducts and applicable production technologies, with an expectation to draw more attention on this valuable crop for its applications as biofuel, functional food and bioactive ingredient sources.
Keywords: Jerusalem artichoke, Functional food, Lactic acid, Bioactive ingredients, Bioethanol, Biobutanol
1. Introduction
Since the beginning of the 21st century, civilization has been facing two major problems: the steady decline of fossil fuels, and environmental problems caused by the extensive use of these fossil fuels for the production of energy and chemicals. One effective way to address these challenges is to use biomass instead of fossil fuels for the production of fuels and chemicals, an emerging area termed as “bioeconomy”. This type of economy undoubtedly contributes to environmental, social and economic sustainability if it is well designed and implemented [1], [2], [3]. A critical aspect of shifting from the current “petroeconomy” to a “bioeconomy” is to minimize the impact of new applications of biomass (i.e., fuels and chemicals) on traditional uses of biomass (i.e., food and feed), thereby preventing any resultant economic imbalance. Therefore, significant academic and industrial activities are focused on identifying abundant biomass sources and/or developing crops that are less competitive with conventional crops in terms of water, land and nutrient requirements. The availability and application of biomass sources are region-dependent and it is therefore essential to identify plant species suitable to local cultivation conditions to increase the economic viability of biomass production [4], [5], [6]. Two commonly cited examples of successful transition to a bioeconomy include bioethanol production from sugarcane in Brazil and biodiesel production from non-edible Jatropha oil in South Asia; however, these species cannot be applied readily to North America without considering the degree of climatic adaptation [7], [8], [9].
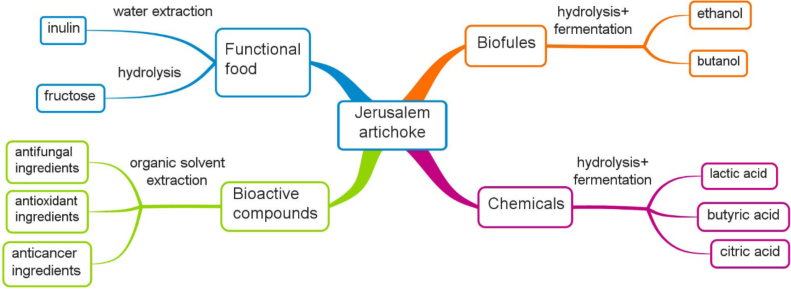
Jerusalem artichoke is a plant native to North America. It has a number of advantageous characteristics over traditionally agricultural crops, including high growth rate, good tolerance to frost, drought and poor soil, strong resistance to pests and plant diseases, with minimal to zero fertilizer requirements [10], [11]. Conventionally, Jerusalem artichoke has been used for food or animal feed [12], [13], and for the past two decades, alternative uses have been explored especially for the production of functional food ingredients such as inulin, oligofructose and fructose [14], [15]. It is also found that some bioactive ingredients can be extracted from its leaves and stems, which creates an opportunity for applications in the pharmaceutical sector [16], [17]. More recently, a renewed and rapidly growing interest is for the use of Jerusalem artichoke tubers, which are rich in inulin, as raw materials for bioethanol production [18], [19]. Multiple applications of Jerusalem artichoke are illustrated in Fig. 1. These diverse applications along with low-cost of plantation render Jerusalem artichoke a promising biomass for the development of a bioeconomy.
Fig. 1.
Multiple applications of Jerusalem artichoke.
This review is a comprehensive survey of the cultivation of Jerusalem artichoke, production of a variety of potential bioproducts and applicable production technologies. Considerable emphasis is placed on Jerusalem artichoke bioethanol production.
2. Characteristics of Jerusalem artichoke
Jerusalem artichoke (Helianthus tuberosus) is a perennial plant which consists of a stem about 1–3 m tall, small yellow flowers, hairy oval shaped leaves and an underground rhizome system which bears small tubers. It is an Angiosperm plant species of the Compositae family, which is commonly referred to as the sunflower or daisy family [10], [20], [21], [22]. The stems are stout and ridged which can become woody overtime. Its leaves alternate near the top of the stem, the lower leaves are larger and broader, and can grow up to 30 cm long while the higher ones are smaller and narrower. In terms of flower heads, each is 5–7.5 cm wide and formed by small, yellow, tubular disk flowers in the center and surrounded by florets, which occur separately or in groups at the end of alar branches and main stems. As for tubers, they are uneven and elongate varying from knobby to round clusters. The colors of tubers range from pale brown to white, red and purple [10], [23]. The morphology of Jerusalem artichoke plant and tubers are illustrated in Fig. 2.
Fig. 2.
Plant and tubers of Jerusalem artichoke.
The Jerusalem artichoke was first cultivated by Native Americans long before the arrival of the Europeans, and was called sunroots. Following its introduction to Europe, diverse Latin and common names were ascribed to Jerusalem artichoke. Kays and Nottingham [22] collected and reported nearly 100 common names used in different languages. Now some of the most commonly used English names include Jerusalem artichoke, sunchoke, topinambur, woodland sunflower or earth apple. Interestingly, the name “Jerusalem artichoke” is misleading as it is a type of sunflower in the same genus as the garden sunflower; however, it has no relation to Jerusalem, neither is it a type of artichoke [24].
3. Cultivation of Jerusalem artichoke
Jerusalem artichoke is native to temperate regions of North America and can tolerate an annual precipitation ranging from 31 to 282 cm, with suitable average temperature range of 6.3–26.6 °C, and pH of 4.5–8.2. Although it can adapt well to a wide range of soil types and pH levels in a sunny position, slightly alkaline soils are favorable for artichoke production. Generally the plant can tolerate sub-zero temperatures while the tubers can withstand freezing for several months even if the frost kills the stems and leaves. The cold-tolerant nature of the tubers allows them to be preserved in the ground during the cold winter until harvested as required [10], [22].
Several studies suggested that Jerusalem artichoke should be planted in early spring to a depth of 10–15 cm. Seed tubers should be spaced 30–60 cm apart in each row, with rows 45–120 cm apart. The optimal soil temperature for planting is between 6 and 7 °C due to the fact that tubers become dormant at temperatures lower than 5 °C. Ideally, it should be planted in well-drained soil with slight alkalinity. The suggested value of soil pH for growth ranges from 4.5 to 8.2. Irrigation is not normally needed and the plant is usually ready to be harvested in approximately 125 days. Yields are relatively high, typically 16–20 t/ha for tubers, and 18–28 t/ha green weight for foliage. It can be harvested using potato harvesting machinery. Once harvested, it needs to be handled carefully to prevent bruising. Recommended storage conditions are 0–2 °C at a relative humidity of 95% for 4–5 months [10], [13], [25], [26]. The Jerusalem artichoke has an aggressiveness nature and should be managed cautiously with tubers missed during harvest expected to grow aggressively in next season. However, this aggressive nature is advantageous and reduces the need for pest management since the plant grows so quickly [13], [27].
Due to the potential application of Jerusalem artichoke in the bioenergy sector, it is now increasingly grown as a specialty plant by many farmers. Although aggressive growth is expected, for commercial scale cultivation, it still may be necessary to identify regional pests and diseases that may affect crop growth. Unfortunately very little research is reported on this aspect. McCarter and Kays [28] found that rust and powdery mildew caused by Puccinia helianthi and Erysiphe cichoracearum, respectively reduced the tuber yield. Other potential issues such as slugs, birds, deer and rabbits may pose a threat to the plant, as do diseases such as powdery mildew and Sclerotinia rot [13], [29].
4. Bioproducts derived from Jerusalem artichoke
4.1. Functional foods
A functional food is defined as food that is demonstrated to affect at least one target function in the body beyond basic nutritional effects, in a way to either enhance stage of well-being and health and/or reduce the risk of disease. A functional food must remain in a normal food form rather than pills or capsules, and demonstrate their effects in amounts that can be consumed in the diet. A functional food can be a natural food, or a food which one or more components have been added to, or removed from, or a food where the nature/bioavailability of one or more components has been modified, or any combination of these possibilities [30], [31]. Jerusalem artichoke is a natural raw material for the derivation of a number of functional food ingredients such as inulin, oligofructose and fructose [10], [22], [31], [32], having both nutritional and functional attributes, particularly beneficial to individuals with Type 2 diabetes and obesity [33], [34], [35].
4.1.1. Inulin
Inulin is a polysaccharide, similar to starch, and exists as a white powder with neutral taste. Chemically, it is a linear biopolymer of d-fructose units connected by β (2,1) glycosidic linkages, and terminated with one d-glucose molecule linked to the fructose chain by an α (2,1) bond. The degree of polymerization of inulin generally ranges from 2 to 60. To date, inulin has been increasingly used as functional ingredients in processed foods due to its unique characteristics [10], [36], [37].
Inulin with the β (2,1) linkages between the fructose monomers cannot be digested by human intestinal enzymes, giving rise to important applications in functional foods suitable for management of Type 2 diabetes, obesity and other blood sugar-related health conditions [33], [36], [37], [38], [39]. When orally ingested, inulin passes through the mouth, stomach and small intestine without being metabolized, until it enters into the large intestine where it becomes fermented by the colonic microflora. Thus, consumption of inulin has no influence on blood sugar levels and stimulation of insulin secretion. The non-digestible nature of inulin inherently results in a caloric value that is significantly lower than other typical carbohydrates, since energy is only derived from the metabolism of fatty acids and lactate resulting from fermentation. Consequently, inulin can be used to replace fat, sugar and flour in dairy products, in cereals and in baked goods for calorie reduction [40], [41], [42]. Additionally, inulin is considered a form of soluble dietary fiber and is categorized as a prebiotic. Inulin influences intestinal functions by increasing stool frequency and stool amounts, particularly in constipated patients, along with decreasing fecal pH value. These effects help suppress the production of putrefactive substance in the colon [33], [37], [43], [44], [45]. Inulin as a prebiotic also simulates the growth of existing strains of beneficial bacteria in the colon which enhances the absorption of important mineral components like calcium and magnesium as well as the synthesis of B vitamins [33], [36], [46], [47], [48], [49]. It was also found that the incorporation of inulin in a diet reduced the lipid content of blood and liver in saturated fat-fed rats. However, similar effects have not yet been confirmed in humans [50], [51], [52], [53]. Recent studies observed that inulin played an important role in the prevention and inhibition of colorectal, colon and breast cancers [37], [54], [55], [56], [57], [58].
Oligofructose, another functional ingredient, is a short chain polysaccharide containing less fructose units (2–10). It can be derived from partial hydrolysis of inulin. Oligofructose has very similar functional and nutritional properties as inulin [31], [33], [37], [42], [44], [59], [60] and its applications as a functional food ingredient are not repeatedly stated in this paper.
Given these health benefits of inulin, the development of more efficient processes for inulin production and feedstock identification have gained considerable attention. Jerusalem artichoke and Chicory are the two most commonly used sources for inulin production on an industrial scale [37]. Jerusalem artichoke tubers contain a high amount of medium-length chains of inulin that can be extracted by the processes similar to sugar extraction from sugarcane. A typical inulin production process includes three major steps, namely pretreatment, extraction and purification. In order to increase diffusion rate, Jerusalem artichoke tubers are first sliced and ground into small particles. Extraction usually is conducted in hot water. Following separation from solid residues, inulin and water solution is further purified by bleaching, activated carbon adsorption or ion-exchange approaches. Purified inulin in water is then concentrated and dried to give finished pure inulin powders [61]. Even though an inulin production process is not complicated, it is still challenging to obtain inulin with good quality and high yield. Therefore, efforts made to increase the economic viability of inulin production are hereby briefly reviewed.
Jerusalem artichoke tubers contain a kind of enzyme existing in their epidermal cells, called polyphenol oxidase (PPO). Once harvested, tubers are still engaged in a series of physiological and metabolic activities. PPO can oxidize the endogenous polyphenols into melanin in the presence of oxygen. This process is known as browning, which seriously affect the nutrient, flavor and appearance of the finished product. Li [62] systematically investigated the effects of temperature and pH value on the activity of PPO, and suggested that a pretreatment of Jerusalem artichoke in water with a neutral pH value at 80 °C for 2 min suppressed enzyme activity, and thus effectively eliminated the occurrence of browning. In a study conducted by Jiang [63], the effects of extraction operating parameters on the yield of inulin were evaluated, and optimal extraction conditions for achieving an inulin yield of 89.5% were provided: extraction temperature of 70 °C, ratio of water to Jerusalem artichoke solid of 15:1, extraction time of 90 min and two times of re-extraction of solid residues. Kierstan [64] attempted an enzymatic method to isolate inulin from Jerusalem artichoke, expecting an increased inulin yield. However their research indicated that enzyme treatment of crude Jerusalem artichoke had no significant improvement on the extraction efficiency and the best extraction method required the involvement of some mechanical means. Sonication is increasingly employed in solvent extraction of bioactive ingredients from vegetables as it is capable of enhancing mass transfer and solvent penetration [65]. Research [66] conducted regarding the performance comparison among conventional extraction, direct sonication extraction and indirect sonication extraction found that indirect sonication extraction was the most suitable method for inulin extraction from Jerusalem artichoke. Microwave-assisted extraction is another promising method to improve extraction efficiency [67]. Xiao et al. [68] applied a microwave-assisted extraction process in inulin isolation. They observed that with the aid of microwaves, the yield of inulin was increased from 10.8% to 12.2% on a wet basis while the extraction time was decreased from 100 min to just 6 min. Zhu’s group [69] employed a three-stage homogenate extraction for inulin preparation. In comparison with the conventional hot water extraction, this process had a number of advantages, including higher yields, less degradation of inulin, room temperature operation and much less amounts of water used.
4.1.2. Fructose
Inulin can be completely hydrolyzed to its monomer, fructose, which is widely used as sweetener instead of sucrose or glucose in functional foods, pharmaceuticals and beverages. The most significant difference between fructose and other monosaccharides is the difference in glycemic index (GI). GI was first introduced by Jenkins et al. [70] as a means to categorize carbohydrates according to their ability to raise blood glucose, and has been used to help individuals with Type 2 diabetes and obesity with their food selection. Based on this definition [70], GI is determined by measuring the blood glucose response caused by ingestion of a certain amount of carbohydrate with respect to a rise in blood glucose caused by the same amount of glucose intake. GI of glucose is assumed as 100, and GI of sucrose is 65 while GI of fructose is only 23 [71]. The significantly low GI of fructose makes it the most favorable sweetener for patients with diabetes or obesity [34], [35], [72]. More interestingly, the sweetness of fructose was reported to be 100–150% higher than that of sucrose, which indicates that using a smaller amount of fructose can provide the same texture and sweetness in food than obtained using other sugars [73], [74], [75], [76]. Less sugar intake definitely helps reduce the microbial decomposition and aggregation in the mouth leading to lower chance of tooth decay. Therefore it is extremely attractive as fructose additive, not only satisfies the taste buds, but also produces relevant health benefits.
Conventionally, fructose is produced from hydrolysis of starch, involving three enzymes amylases or alpha-amylase and glucoamylase in three steps while the resulting hydrolysate contains 45% fructose and 55% glucose [77], [78]. Inulin is a more advantageous feedstock than starch since hydrolysis of inulin needs only single enzymatic hydrolysis with inulinase as biocatalyst [79], with fructose yields reported as high as 95% [77]. The economy of a hydrolysis process is highly associated with the activity and stability of enzyme, operating mode and bioreactor configuration. Compared to most chemical reactions, biological reactions such as hydrolysis are relatively slow. It requires a long reaction time or the use of large bioreactors. In addition, large amounts of enzyme involved in hydrolysis remain in reaction broth after the reaction and are very difficult to recover. Therefore, significant research has been conducted to immobilize the enzyme to retain biocatalyst and at the same time to achieve continuous operation. Ricca et al. [80] have reviewed the main advancements achieved in the production of fructose from inulin enzymatic hydrolysis prior to 2007. Progress made in recent years or research not covered in Ricca's review is summarized here with a focus on new enzyme development, enzyme immobilization and innovative bioreactor design.
Research conducted by Sirisansaneeyakul, et al. [81] focused on the synergistic effect of a combined exo- and endo-inulinases system. They observed that an enzyme system consisted of mold and yeast inulinases with a mixing ratio of 5:1, was better at inulin hydrolysis than the mold and yeast alone. The resulting hydrolysate contained 78.2% of fructose. Yu et al. [82] developed a novel recombinant inulinase-secreting Saccharomyces cerevisiae strain to produce glucose-free fructose from Jerusalem artichoke. Such a recombinant biocatalyst was capable of hydrolyzing inulin into fructose and glucose, and subsequently metabolizing glucose. As a result, only fructose accumulated in the hydrolysate, which provided a promising one-step process to produce fructose with high purity. Guiraud et al. [83] attempted to immobilize inulinase on a DEAE-cellulose matrix by a simple adsorption method. As-prepared catalyst was tested in a continuous reactor at a flow rate of 10 mL/h, inulin solution of 5 g/L, at 40 °C for 3 weeks, achieving a conversion rate for inulin of 100%. In a study conducted by Santa et al. [84], a novel sol-gel immobilizing method was employed to deposit inulinase on a porous silica xerogel matrix. This biocatalyst presented a promising operational stability at 40 °C for 20 consecutive 24-h batch runs without noticeable decay in product yield. Singh et al. [85] developed a stable continuous flow reactor with inulinase immobilized on Duolite A568 to hydrolyze inulin. The reactor could run continuously at a flow rate of 4 mL/h, at 55 °C for 75 days and the experimental half-life was 72 days, which is a very encouraging advance in the continuous hydrolysis of inulin. Zhu’s group [86], [87] has conducted ongoing research on fructose production from Jerusalem artichoke since the early 1990s [86]. An innovative dynamic membrane separator was developed and was coupled to a hydrolysis reactor, which was able to perform reaction and separation at the same time. The enzyme was filtered by membrane spectator and recycled into the hydrolysis reactor providing a cost-effective process for fructose production. This unique design has been applied on an industrial scale.
4.2. Bioactive compounds
In addition to functional foods derived from Jerusalem artichoke tubers, the leaves also have important applications. Jerusalem artichoke leaves are traditionally used as a folk medicine for the treatment of bone fractures, skin wounds, swelling and pain [88], [89], [90]. A number of valuable bioactive compounds of medicinal significance have been isolated from the aerial parts of Jerusalem artichoke, demonstrating antifungal, antioxidant, anticancer activities and other medicinal effects [16], [17], [88], [91], [92], [93], [94], [95], [96], [97], [98].
In research conducted by Nakagawa et al. [92], two lectins were extracted from callus of Jerusalem artichoke, and then purified by chromatography and preparative electrophoresis. Both lectins showed a strong activity for hemagglutination. Pan et al. [16] successfully isolated nine bioactive compounds from the whole plant of Jerusalem artichoke, including ent-17-oxokaur-15(16)-en-19-oic acid, ent-17-hydroxykaur-15(16)-en-19-oic acid, ent-15β-hydroxykaur-16(17)-en-19-oic acid methyl ester, ent-15-nor-14-oxolabda-8(17), 12E-dien-18-oic acid, 4,15-isoatriplicolide angelate, 4,15-isoatriplicolide methylacrylate, (+)-pinoresinol, (−)-loliolide, and vanillin. The bioactivities of nine compounds were subsequently evaluated using the MCF-7 human breast cancer cell line and a soybean isoflavonoid defense activation bioassay. Two of the compounds were identified as cytotoxic agents, one of which was capable of stimulating defense metabolites, and four of which were able to block isoflavone accumulation in soybean. Continuous efforts on bioactive ingredient extraction from Jerusalem artichoke leaves in Xiao and Yuan’s team [17], [93], [94] have led to important advances. Six phenolic extracts from Jerusalem artichoke leaves exhibited free radical scavenging activities, rendering Jerusalem artichoke leaves a good source for natural antioxidants [17], [95]. Very recently, this group [94] further isolated a series of sesquiterpene lactones, exhibiting cytotoxicity against cancer cell lines, which is consistent with the results reported by Pan et al. [16].
The extracts from Jerusalem artichoke leaves also exhibited antifungal properties [96], [97], [98]. Liu et al. [96] used different solvents to extract Jerusalem artichoke leaves, and tested extracts’ antifungal properties in several fungi such as Rhizoctonia solani, Gibberella zeae, Alternaria solani and Botrytis cinerea. Their study indicated that Jerusalem artichoke leaf extracts from different solvents showed different antifungal activities. For example, the inhibitory effect of aqueous extracts was lower than those from organic solvents. The extracts from ethyl acetate showed the highest inhibitory activity to the four fungi employed in this research. While a study from Han et al. [97] found that the acetone extracts of Jerusalem artichoke leaves presented the strongest inhibitory ability for pepper gray mold within their experimental scope. In more recent research conducted by Chen et al. [98] six phenolic acids were isolated from Jerusalem artichoke leaves using n-butanol as a solvent, and among the six phenolic acids isolated, caffeic acid, 3,4-dicaffeoylquinic acid and 1,5-dicaffeoylquinic acid were identified to be responsible for G. zeae inhibition. This study sufficiently demonstrates that Jerusalem artichoke leaves are a potential source of natural fungicides.
Conventional solvent extraction has been a major extraction technology for the separation of bioactive ingredients from Jerusalem artichoke leaves. Since the leaves contain a wide variety of chemical compounds, extracts using different solvents without further purification showed varying bioactivities [96], [97], [98]. Therefore further work is needed to extract bioactive substances, subsequently separate extracts, identify and characterize the bioactivity of each compound. The release of bioactive compounds from Jerusalem artichoke can be affected by processing. For instance, puffing and extrusion processes were recently found to increase the total phenolic content, free radical scavenging activity and ferric reducing antioxidant power of Jerusalem artichoke tea infusion compared to processing by roasting and hot air drying [99]. Moreover, a previous study showed that a combination of high hydrostatic pressure (HPP) and enzymatic treatments and fermentation increased the phenolic content and in vitro antioxidant (radical scavenging and superoxide-like) activities of Jerusalem artichoke tuber extract compared to water extraction [100]. Therefore, for industry scale production of bioactive compounds from Jerusalem artichoke, processing and extraction technology should be critically evaluated and selected according to the physicochemical properties of targeted ingredients and the nature of the plant matrix. In addition to HPP, other non-conventional extraction techniques such as ultrasound-assisted, microwave-assisted and supercritical fluid extraction can be considered as alternative approaches to increase the yield and preserve the bioactivity of ingredients in Jerusalem artichoke [101], [102].
4.3. Biofuels
4.3.1. Ethanol
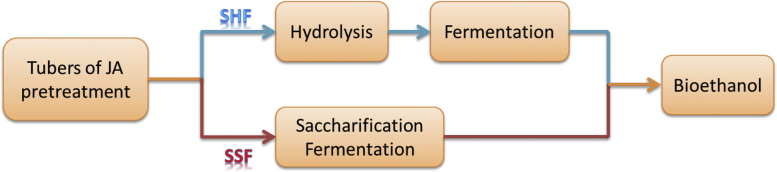
Jerusalem artichoke (JA) is currently recognized as an emerging energy crop for bioethanol production; however, ethanol production from Jerusalem artichoke has a long history. In the 19th century, a French chemist, Anselme Payen promoted Jerusalem artichoke tubers for beer production in France. Interestingly, the beer derived from Jerusalem artichoke tubers is sweet and has a fruity taste [103], [104]. Due to the unique aroma, Jerusalem artichoke ethanol has also been used to produce brandy in France and Germany as well as sake in Japan [105]. During and after World War I, extensive research was conducted by the British Department of Scientific and Industrial Research to develop fuel ethanol from Jerusalem artichoke. It was demonstrated that Jerusalem artichoke was able to produce the same amount of fermentable carbohydrate per acre for alcohol as sugar beet and more than the Irish potato. However, with the rise of the modern petroleum industry, interest in fuel ethanol declined [106], [107]. In the 1980s a renewed interest in Jerusalem artichoke ethanol was stimulated by the 1973 oil crisis [108], [109]; however this research was again interrupted with lower oil prices following the crisis. Renewed interest in Jerusalem artichoke as a source for ethanol production has occurred again and this time is largely driven by the rapid decline of fossil fuel reserves coupled with the negative effects of overconsumption of petroleum-based fuels on environment such as global warming, climate change, acid rain and ozone layer depletion. Much effort and significant progress have been made to develop biofuels, mainly bioethanol and biodiesel to substitute petro-fuels [110], [111]. Currently in North America, corn, wheat and barley are dominant feedstocks for commercial bioethanol production but they compete with food and feed supply, raising a heated debate on “fuel vs food”. As a consequence, efforts are reoriented to utilize lignocellulosic biomass (agricultural and forestry residues) and/or develop dedicated energy crops [1], [112], [113], [114], [115]. Undoubtedly, lignocellulose is the most economical and abundantly available feedstock, however the costly pretreatment of converting cellulose into fermentable sugar is the key technical barrier to economically competitive production. Bioethanol production from lignocellulosic biomass is still at the development stage [116]. Jerusalem artichoke tubers are rich in inulin, which can be easily hydrolyzed and then converted into ethanol using biocatalysts. The ethanol yield is equivalent to that of sugarcane and twice that obtained from corn. These characteristics make Jerusalem artichoke an outstanding substrate for ethanol production [18], [19], [117], [118], [119], and it has recently been listed as one of the most promising energy crops in China, Europe and New Zealand [4], [120], [121], [122]. Generally, there are two routes for bioethanol production from Jerusalem artichoke tubers: (1) separate hydrolysis and fermentation (SHF) and (2) simultaneous saccharification and fermentation (SSF) as shown in Fig. 3.
Fig. 3.
Two routes for bioethanol production from Jerusalem artichoke tubers.
4.4. Separate hydrolysis and fermentation
Separate hydrolysis and fermentation (SHF) method is characterized as inulin hydrolysis and sugar fermentation being conducted in two separate reactors. Typically, Jerusalem artichoke tubers are processed into pulpy mash and then hydrolyzed into fermentable sugars (fructose and glucose) using either dilute mineral acids or inulinase enzyme. Subsequently, fermentable sugar is separated from solid residues and transferred into a fermenter where sugar is fermented into ethanol employing yeasts such as Zymomonas mobilis, Kluyveromyces marxianus and Saccharomyces cerevisae.
The research on hydrolysis of inulin for fructose production has been stated in Section 4.1.2. [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87] Such-prepared fructose can be further converted into ethanol. For the ethanol production starting with tubers through SHF process, hydrolysis step has significant impacts on the following fermentation step. Complete hydrolysis of inulin produces a maximum amount of sugar that leads to high ethanol yield. However, hydrolysis process may generate some byproducts which could inhibit the activity of yeast in the fermentation step, consequently prolonging fermentation time. Fleming et al. [123] investigated the effectiveness of various mineral acids (hydrochloric, sulfuric, citric and phosphoric acids) in hydrolysis of Jerusalem artichoke tubers. They concluded that there was no significant difference among these acids in their influence on inulin hydrolysis. The research conducted by Toran-Diaz et al. [124] evaluated the effect of acid or enzymatic hydrolysis of inulin on the subsequent alcoholic fermentation. It was found that acid hydrolysis was faster than enzymatic hydrolysis while the byproducts from acid hydrolysis inhibited the growth of yeast in the subsequent fermentation step, which resulted in a low ethanol yield. It was also observed that Z. mobilis was able to ferment the Jerusalem artichoke juice into ethanol without adding any other nutrients, and the ethanol yield using Z. mobilis in fermentation was higher than found using K. marxianus. Kim and Hamdy [125] optimized hydrolysis conditions to obtain complete hydrolysis of inulin and minimum generation of yeast inhibitors. They proposed that Jerusalem artichoke slurry should be hydrolyzed in 0.1 M HCl at 97 °C for 15 min. Under such conditions, maximum reduction of sugar to 84.8% fructose equivalent was achieved while the concentration of inhibitor 5-hydroxymethylfurfural (HMF) was 0.07%, lower than 0.1%, the limit for yeast growth inhibition. Razmovski et al. [126] examined the effects of temperature, residence time and hydromodule on Jerusalem artichoke hydrolysis. The Jerusalem artichoke hydrolyzates obtained under various hydrolysis conditions were further tested in alcoholic fermentation with S. cerevisiae as a biocatalyst. They found that acid hydrolysis at high temperatures and long residence times increased the concentration of yeast inhibitor HMF and accelerated the degradation of fructan. Based on their experimental results, the highest ethanol yield of 7.6w/w was obtained when acid hydrolysis was carried out at 126 °C, 60 min of residence time and hydromodule ratio of 1:1. Zubr [127] investigated the effects of several enzymes on hydrolysis of Jerusalem artichoke tubers, including Novozym 188, Celluclast, Novo 230, Novo SP 249, and combinations of thereof. Their work indicated that cellulolytic enzymes like Novozym 188 and Cellules had very disappointing performance, generating a small amount of fructose and glucose. Novo 230 was the most efficient enzyme for inulin hydrolysis, giving the highest sugar yield under the authors’ experimental scope. At the same time, S. cerevisae, traditional brewing yeast, was identified as an efficient catalyst for the alcoholic fermentation of these fermentable sugars resulted from hydrolysis step.
4.5. Simultaneous saccharification and fermentation
Simultaneous saccharification and fermentation (SSF) is characterized as inulin hydrolysis and sugar fermentation being carried out in one bioreactor using combination biocatalysts. Obviously, such a direct conversion of soluble inulin into ethanol without a prior hydrolysis step is highly favorable from capital investment and operating cost perspectives. Moreover, a SSF process significantly reduces the fermentable sugar loss caused by separation and transfer of sugars from hydrolyzer into fermenter as in a SHF process. For Jerusalem artichoke ethanol production through SSF, the major technical barrier is the identification of the most efficient enzymes which are capable of facilitating both hydrolysis and fermentation.
One approach is to use a mixture of inulinase and yeast to convert ground Jerusalem artichoke tubers to ethanol. Kim and Rhee [128] co-immoblized inulinase and Z. moblilis in alginate beads to facilitate a one-step production of ethanol from Jerusalem artichoke tubers in a continuous mode. The maximum ethanol productivity was reported as 55.1 g/L/h and the yield was 95% of theoretical yield. In the study reported by Nakamura et al. [129], [130], simultaneous hydrolysis and fermentation of tubers was conducted in a batch operation mode using a mixture of Aspergillus niger 817 and S. cerevisiae 1200. The ethanol concentration was 10.4% (v/v) for 15 h fermentation time; the yield was 92% of theoretical yield. Szambelan et al. [131] compared the ethanol yields from different processes, using single yeast and a mixture culture of microorganisms. The mixed culture of Kluyveromyces fragilis and Z. mobilis or K. fragilis and S. cerevisiae gave the best results under the authors’ research scope. The yield of ethanol was 94% of maximum theoretical yield and the ethanol concentration was 9.9% (v/v). Ge et al. [132] attempted a newly isolated extroinulinase-hyperproducing strain, A. niger SL-09, coupled with S. cerevisiae Z-06 to ferment ground Jerusalem artichoke tubers into ethanol in a batch operation. The ethanol concentration was as high as 19.5% (v/v) for 48 h fermentation with the conversion efficiency of 90%. The result from this research is significant in that high ethanol concentration in the finished fermentation broth can dramatically reduce the cost of the subsequent distillation step, making the overall ethanol production more economically viable.
Employment of a mixture of enzymes can realize simultaneous hydrolysis and fermentation of Jerusalem artichoke tubers for ethanol production. However, these processes involving two species with different culture conditions pose difficulties to process optimization. Ethanol productivity was compromised due to both biocatalysts working under sub-optimal conditions. Another effort is to use yeasts that possess inulinase activity to achieve simultaneous saccharification and fermentation. Guiraud et al. [133] first demonstrated that K. fragilis and K. fragilis with inulinase activity were able to convert Jerusalem artichoke tubers to ethanol directly. Following their pioneering research, numerous efforts were made to develop and/or screen more effective strains to facilitate SHF of Jerusalem artichoke. Duvnjak [134] evaluated the performance of several yeasts in hydrolysis and fermentation, including K. marxianus ATCC12708, K. marxianus ATCC 10,606, Kluyveromyces cerevisiae ATCC 22,295 and K. fragilis. Their work indicated that Jerusalem artichoke juice contained enough nutrients for both yeast growth and ethanol production. K. marxianus ATCC12708 was identified as the most suitable yeast, providing an ethanol yield of 87.5% of theoretical value within 25 h fermentation. From the early 1980s, Professor Margaritis’s team [108], [109], [135], [136], [137], [138], [139], [140], [141], [142], [143], [144], [145], [146], [147], [148], [149] have been devoting their research to ethanol production from Jerusalem artichoke. Extensive works were conducted ranging from yeast screening [135], enzyme immobilization method [136], [139], [140], [141], [142], batch and continuous operation modes [106], [141], [177], kinetics of ethanol production [136], [138], [142], [143] and hydrolysis of inulin [146], [147], [148], [149], which built a valuable foundation for the research on Jerusalem artichoke ethanol. In their early work, K. marxianus UCD (FST) 55-82 was demonstrated to be the most suitable yeast for the fermentation of Jerusalem artichoke tubers among eight yeasts tested in their investigation [135]. K. marxianus UCD (FST) 55-82 with inulinase activity was further employed in a continuous bioreactor to hydrolyze and ferment Jerusalem artichoke juice, producing ethanol with a yield of 90% of theoretical value [108]. The performance of this yeast was also tested in a batch operation for ethanol production [108]. The ethanol concentration was 44 g/L at the end of a fermentation time of 60 h, achieving a yield of 88% of the theoretical yield. Negro et al. [150] conducted a direct fermentation of inulin using K. marxianus CECT 10,875. The ethanol concentration was 19 g/L at the end of fermentation, with a yield of 96% of theoretical yield. Work by Lim et al. [151] identified S. cerevisiae KCCM50549 as another promising yeast for SFH process of Jerusalem artichoke tubers, giving a relatively high ethanol concentration of 36.2 g/L and a yield of 70% of theoretical value within 30 h. Ethanol produced from this yeast is 1.6 times higher than that from S. cerevisiae NCY625. Yu et al. [152] noticed that Kluyveromyces cicerisporus Y179 expressed a high level of inulinase activity, which was suitable for ethanol production by SSH method. The experimental results indicated that more ethanol was produced by this yeast at 30 °C than at 37 °C or 42 °C. After 144 h fermentation, ethanol with the concentration of 12.3% (v/v) was achieved, and the yield of ethanol was 86.9% of theoretical value. Dr. Bai’s group [153], [154], [155], [156] have conducted ongoing research on ethanol production from Jerusalem artichoke tubers using a consolidated bioprocessing (CBP) strategy which integrated inulinase production, hydrolysis of inulin and ethanol fermentation. In the early work conducted by Yuan et al. [153] K. marxianus ATCC8554 were proven to have a good alcoholic fermentation ability and high inulinase production capacity. Employment of this yeast converted Jerusalem artichoke tubers to ethanol directly, achieving an ethanol yield of 87% for fermentation time of 84 h. Yuan et al. [155] further developed a new recombinant yeast that contained commercially available S. cerevisiae 6525 integrated by inulinase gene cloned from K. marxianus. Using this new yeast, ethanol concentration in broth was significantly increased to 72.5 g/L at the end of 48 h fermentation, while using the host strain, S. cerevisiae 6525 alone, ethanol concentration was only 67.0 g/L in 60 h. Yuan et al. [156] also applied K. marxianus with overexpression of inulinase gene in the fermentation of Jerusalem artichoke tubers. Significant improvement of ethanol productivity was observed, detailed data of which are listed in Table 1. The research of Dr. Chi’s team [157], [158], [159] focused on the development and application of Saccharomyces sp W0 with inulinase gene expression in Jerusalem artichoke ethanol production. The exo-inulinase gene and endo-inulinase gene were integrated into Saccharomyces sp W0 respectively. Ethanol productivities using Saccharomyces sp W0 with and without inulinase gene expression are summarized in Table 1. Hu et al. [160] examined the activities of 21 newly isolated and 65 previously available S. cerevisiae strains in direct fermentation of Jerusalem artichoke through consolidated bioprocessing (CBP). Their work identified K. marxianus PT-1 and S. cerevisiae JZ1C as good thermo-tolerant strain candidates for Jerusalem artichoke ethanol production. Under their experimental conditions, these two strains gave 90% and 79.7% of theoretical ethanol yield respectively at 40 °C within 84 h fermentation. Very recently, Guo et al. [161] reported an improved CBP process in a helical ribbon stirring bioreactor using mutant yeast, S. cerevisiae DQ1. The ethanol concentration was as high as 128 g/L, which is very promising. Table 1 summarizes the biocatalysts, fermentation conditions and ethanol productivities of a variety of SSF processes for Jerusalem artichoke ethanol production reviewed in this paper.
Table 1.
SSF processes reported in literature.
| Biocatalysts | Fermentation conditions | Ethanol concentration | Yielda | Reference |
|---|---|---|---|---|
| Simultaneous saccharification and fermentation (SSF) (a mixture of various enzymes) | ||||
| Inulinase + Z. moblilis | 35 °C, continuous | 55.1 g/L/h | 95% | Kim [128] 1990 |
| A. niger 817 + S. cerevisiae 1200 | 30 °C, 15 h | 10.4% (v/v) | 92% | Nakamura et al. [130] 1996 |
|
K. fragilis + Z. moblilis 3881 |
30 °C, 72 h | 9.9% (v/v) | 94% | Szambelan et al. [131] 2005 |
|
K. fragilis + S. cerevisiae Bc16a |
30 °C, 72 h | 9.1% (v/v) | 86% | |
|
A. niger SL-09 + S. cerevisiae Z-06 |
30 °C, 48 h | 19.6% (v/v) | 90% | Ge and Zhang [132] 2005 |
| Simultaneous saccharification and fermentation (SSF) (yeast with inulinase activity) | ||||
| K. fragilis | 28 °C, 6 days | 11.1% (v/v) | 98% | Guiraud et al. [133] 1981 |
| K. marxianus | 28 °C, 6 days | 11.5% (v/v) | 98% | |
| K. marxianus ATCC12708 | 28 °C, 30 h | 14 g/L | 87% | Duvnjak et al. [134] 1981 |
| K. marxianus ATCC 10606 | 28 °C, 30 h | 12 g/L | 83% | |
| K. cicerisporus ATCC 22295 | 28 °C, 30 h | 13 g/L | 86% | |
| K. fragilis 105 | 28 °C, 30 h | 11 g/L | 79% | |
| K. marxianus UCD 55-82 | 35 °C, 60 h | 44 g/L | 88% | Bajpai and Margaritis [138], |
| K. marxianus UCD 55-82 | 35 °C, continuous | 7 g/L/h | 90% | Sachs et al. [106], 1982 |
| K. marxianus CECT 10875 | 28 °C, 30 h | 19 g/L | 96% | Negro [150], 2006 |
| K. cicerisporus Y179 | 30 °C, 144 h | 12.3%(v/v) | 86.9 | Yu et al. [152], 2010 |
| S. cerevisiae KCCM50549 | 30 °C, 36 h | 32.6 g/L | 70% | Lim et al. [151] 2011 |
| K. marxianus ATCC8554 | 35 °C, 84 h | 60.9 g/L | 87% | Yuan et al. [153], 2008 |
| S. cerevisiae 6525 with cloned inulinase gene | 35 °C, 48 h, | 72.5 g/L | 85% | Yuan et al. [155], 2013 |
| K. marxianus with overexpressed inulinase gene | 35 °C, 72 h, | 96.2 g/L | 93% | Yuan et al. [156] 2013 |
| Saccharomyces sp W0 with exo-inulinase gene | 28 °C, 144 h | 12.5%(v/v) | 62.5% | Zhang et al. [157] 2010 |
| Saccharomyces sp W0 with 18S rDNA integration of exo-inulinase gene | 28 °C,120 h | 12.6%(v/v) | 66% | Yuan et al. [156] 2011 |
| Saccharomyces sp W0 with endo-inulinase gene | 30 °C, 120 h | 12.6%(v/v) | 65% | Li et al. [159] 2013 |
| K. marxianus PT-1 | 40 °C, 84 h | 73.6 g/L | 90% | Hu et al. [160] 2012 |
| or S. cerevisiae JZ1C | 40 °C, 84 h | 65.2 g/L | 79.7% | |
| S. cerevisiae DQ1 | 30 °C, 72 h | 128.1 g/L | 73.5% | Guo et al. [161] 2013 |
Ethanol yield is the percentage of theoretical yield on the basis of total sugar in feedstock.
4.5.1. Butanol
Biobutanol as a new generation of biofuel has recently drawn increasing attention due to its higher heating value and low volatility in comparison with bioethanol. Explorative research on butanol production from Jerusalem artichoke has been conducted. Sarchami and Rehmann [162] optimized the enzymatic hydrolysis of inulin from Jerusalem artichoke, and obtained maximum inulin conversion of 94.5% under the optimal conditions(temperature of 48 °C, pH of 4.8, substrate concentration of 60 g/L, enzyme loading of 10 units/g substrate and fermentation time of 24 h), producing butanol of 9.6 g/L. Chen et al. [163] used Clostridium acetobutylicum L7 to hydrolyze Jerusalem artichoke juice. It was found that with a starting sugar concentration of 62.87 g/L, 11.2 g/L of butanol was produced for 60 h of fermentation, and the ratio of butanol, acetone and ethanol was 0.64:0.29:0.05.
4.6. Chemicals
Lactic acid is widely used in the food, pharmaceutical and chemical industries, and it is an important building block for synthesizing a variety of chemicals [164], [165]. In response to an increasing demand for lactic acid, Jerusalem artichoke stands out as a low cost raw material for lactic acid production. Ge et al. [165] first attempted to use mixed culture of A. niger SL-09 and Lactobacillus sp to produce lactic acid from JA. In a SSF process, the highest lactic acid concentration of 120.5 g/L was obtained in 36 h of the fed-batch fermentation with a high conversion efficiency of 94.5%. This group further enhanced the process by introducing Lactobacillus casei G-02, leading to an increased lactic acid concentration of 141.5 g/L [166]. It was reported by Choi et al. that a direct lactic acid fermentation could be realized using Lactobacillus paracasei without a pre-step of inulin hydrolysis. The lactic acid concentration was 92.5 g/L and the conversion efficiency of inulin-type sugars to lactic acid was 98% of the theoretical yield [167]. Dao et al. [168] developed a practical and economical way to produce lactic acid from JA. They used commercially available glucoamylase, glucoamylase GA-L New and Pediococcus acidilactici DQ 2 to hydrolyze and ferment JA tubes. The lactic acid concentration of 111.5 g/L was obtained. Another study on lactic acid production [169] conducted by Wang et al. used thermophilic Basillus coagulans strain. The obtained lactic acid was 134 g/L with a starting sugar concentration of 140 g/L. Research from Shi et al. [170] focused on the development of efficient bioreactors. A fibrous bed bioreactor with immobilized Lactococcus lactis significantly improve the overall production efficiency, resulting in lactic acid concentration of 142 g/L in a fed-batch mode operation.
Jerusalem artichoke also has potential for generating a variety of others chemicals [164], [171] such as butyric acid [172], citric acid [173], succinic acid [174], 2,3-butanediol [175], [176] and sorbitol [177]. A few studies have been reported, of which details are not stated in this paper.
5. Conclusions
Jerusalem artichoke is an economically important plant with advantages of low input cultivation, high crop yield and wide adaptation to climatic and soil conditions. In addition to its applications as functional food and bioactive ingredient sources, it is recognized as a sustainable feedstock for biofuel production. These diverse economic values identify it as a promising biomass for bioeconomy development. However, Jerusalem artichoke is currently underutilized. This paper provides a review of the considerable amount of research that has been already been conducted; however more research and development are necessary before the utilization of Jerusalem artichoke can become economically and technically viable. Further studies are expected to focus on: (1) optimization of the cultivation conditions to improve crop yield per input; (2) increasing inulin content in tubers by genetic modification of the species; (3) identification and development of enzymes with high activity and stability to facilitate more efficient bioprocesses for bioproduct production; and (4) exploring new enzyme immobilization technologies and designing advanced bioreactors for continuous operation to increase overall productivity. Outcomes of these research areas should establish Jerusalem artichoke as an essential natural resource in a developing bioeconomy.
Acknowledgement
This work was supported by National Research Council of Canada (NRC) Industrial Research Assistance Program (IRAP) program.
References
- 1.Nigam P.S., Singh A. Production of liquid biofuels from renewable recourses. Prog. Energy Combust. Sci. 2011;37:52–68. [Google Scholar]
- 2.McCormick K., Kautto N. The bioeconomy in Europe: an overview. Sustainability. 2013;5:2589–2608. [Google Scholar]
- 3.Mathews J.A. From the petroeconomy to the bioeconomy: integrating bioenergy production with agricultural demands. Biofuels Bioprod. Biorefin. 2009:613–632. [Google Scholar]
- 4.Kerckhoffs H., Renquist R. Biofuel from plant biomass. Agron. Sustain. Dev. 2013;33:1–19. [Google Scholar]
- 5.Gao F., Zhao L., Wang X.Z. The Research Review about the effect of bio-fuel development on agricultural market and agriculture. Agric. Agric. Sci. Procedia. 2010:488–494. [Google Scholar]
- 6.Coulman B., Dalai A., Heaton E., Perez Lee C., Lefsrud M., Levin D., Lemaux P.G., Neale D., Shoemaker S.P., Singh J., Smith D.L., Whalen J.K. Developments in crops and management systems to improve lignocellulosic feedstock production. Biofuels Bioprod. Biorefin. 2013;7:582–601. [Google Scholar]
- 7.Matsuoka S., Ferro J., Arruda P. The Brazilian experience of sugarcane ethanol industry. In Vitro Cell. Dev. Biol.-Plant. 2009;45:372–381. [Google Scholar]
- 8.Meher L.C., Churamani C.P., Arif Md Ahmed Z., Naik S.N. Jatropha curcas as a renewable source for bio-fuels – a review. Renew. Sustain. Energy Rev. 2013;26:397–407. [Google Scholar]
- 9.Mofijur M., Masjuki H.H., Kalam M.A., Hazrat M.A., Liaquat A.M., Shahabuddin M., Varman M. Prospects of biodiesel from Jatropha in Malaysia. Renew. Sustain. Energy Rev. 2012;16:5007–5020. [Google Scholar]
- 10.Duke, J.A., 1983. Handbook of Energy Crops.
- 11.Slimestad R., Seljaasen R., Meijer K., Skar S.L. Norwegian-grown Jerusalem artichoke (Helianthus tuberosus L.): morphology and content of sugars and fructo-oligosaccharides in stems and tubers. J. Sci. Food Agric. 2010;90:956–964. doi: 10.1002/jsfa.3903. [DOI] [PubMed] [Google Scholar]
- 12.Ma X.Y., Zhang L.H., Shao H.B., Xu G., Zhang F., Ni F.T., Brestic M. Jerusalem artichoke (Helianthus tuberosus), a medicinal salt-resistant plant has high adaptability and multiple-use values. J. Med. Plants Res. 2011;5:1272–1279. [Google Scholar]
- 13.Swanton C.J., Hamill A.S. Ministry of Agriculture and Food; Ontario: 1994. Jerusalem Artichoke. Factsheet; pp. 077–94. [Google Scholar]
- 14.Panchev I., Delchev N., Kovacheva D., Slavov A. Physicochemical characteristics of inulins obtained from Jerusalem artichoke (Helianthus tuberosus L.) Eur. Food Res. Technol. 2011;233:889–896. [Google Scholar]
- 15.Praznik W., Cieślik E., Filipiak-Florkiewicz A. Soluble dietary fibers in Jerusalem artichoke powders: composition and application in bread. Nahrung. 2002;46:151–157. doi: 10.1002/1521-3803(20020501)46:3<151::AID-FOOD151>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Pan L., Sinden M.R., Kennedy A.H., Chai H., Watson L.E., Graham T.L., Kinghorn A.D. Bioactive constituents of Helianthus tuberosus (Jerusalem artichoke) Phytochem. Lett. 2009;2:15–18. [Google Scholar]
- 17.Yuan X.Y., Gao M.Z., Xiao H.B., Tan C.Y., Du Y.G. Free radical scavenging activities and bioactive substances of Jerusalem artichoke (Helianthus tuberosus L.) leaves. J. Food Chem. 2012;133:10–14. [Google Scholar]
- 18.Cepl J., Kasal P., Souckova H., Svobodova A., Bucher P. Non-food production of Jerusalem artichoke (Helianthus tuberosus) and possibilities of its energetic utilization. Actual Tasks Agric. Eng.-Zagreb. 2012;40:517–526. [Google Scholar]
- 19.Wieczorek A. The Jerusalem artichoke Helianthus tuberosus as potential energy source. Acta Aliment. Pol. 1988;14:115–122. [Google Scholar]
- 20.Monti A., Amaducci M.T., Venturi G. Growth response, leaf gas exchange and fructans accumulation of Jerusalem artichoke (Helianthus tuberosus L.) as affected by different water regimes. Eur. J. Agron. 2005;23:136–145. [Google Scholar]
- 21.Tassoni A., Bagni N., Ferri M., Franceschetti M., Khomutov A., Paula Marques M., Fiuza S.M., Simonian A.R., Serafini-Fracassini D. Helianthus tuberosus and polyamine research: past and recent applications of a classical growth model. Plant Physiol. Biochem. 2010;48:496–505. doi: 10.1016/j.plaphy.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 22.Kays S.J., Nottingham S.F. CRC press; Boca Raton, FL: 2007. Biology and Chemistry of Jerusalem artichoke: Helianthus tuberosus L. [Google Scholar]
- 23.Huxley A.J., Griffiths M., Levy M. Macmillan Publishers; London: 1992. The New Royal Horticultural Society Dictionary of Gardening. [Google Scholar]
- 24.Lindsayjean N. Jerusalem artichokes: nothing about this name is right. Food. 2013:52. [Google Scholar]
- 25.Grassi G., Gosse G. Commission of The European Communities; Luxembourg: 1989. Topinambour (Jerusalem Artichoke) [Google Scholar]
- 26.Chabbert, Braun P., Guiraud J.P., Arnoux M., Galzy P. Productivity and fermentability of Jerusulem artichoke according to harvesting date. Biomass. 1983;3:209–224. [Google Scholar]
- 27.Swanton C.J., Cavers P.B., Clements D.R., Moore M.J. The biology of Canadian weeds. 101. Helianthus tuberosus L. Can. J. Plant Sci. 1992;72:1367–1382. [Google Scholar]
- 28.McCarter S.M., Kays S.J. Diseases limiting production of Jerusalem artichoke in Georgia. Plant Dis. 1984;68:299–302. [Google Scholar]
- 29.Mckeown A., Todd J., Bakker C. Ministry of Agriculture and Food Ministry of Rural Affairs, Ministry of Agriculture and Food; Ontario: 2010. Jerusalem Artichoke. Specialty Opportunities, a Resource for Specialty Crop Growers. [Google Scholar]
- 30.Diplock A.T., Aggett P.J., Ashwell M., Bornet F., Fern E.B., Roberfroid M.B. Scientific concepts of functional foods in Europe: consensus document. Br. J. Nutr. 1999;81:S1–S28. [PubMed] [Google Scholar]
- 31.Roberfroid M.B. Functional food: concepts and application to inulin and oligofructose. Br. J. Nutr. 2002;87:S139–S143. doi: 10.1079/BJNBJN/2002529. [DOI] [PubMed] [Google Scholar]
- 32.Roberfroid M. Dietary fiber, inulin, oligofructose: a review comparing their physiological effects. Crit. Rev. Food Sci. Nutr. 1993;33:103–148. doi: 10.1080/10408399309527616. [DOI] [PubMed] [Google Scholar]
- 33.Niness K.R. Inulin and oligofructose: what are they? J. Nutr. 1999;129:1402S–1406S. doi: 10.1093/jn/129.7.1402S. [DOI] [PubMed] [Google Scholar]
- 34.Barta L., Rosta J. Effect of Jerusalem artichoke honey-containing isocaloric diet on the sugar metabolism of diabetic children. Gyermekgyogyaszat. 1958;9(8–9):280–283. [PubMed] [Google Scholar]
- 35.Bornet F.R. Undigestible sugars in food products. Am. J. Clin. Nutr. 1994;59:763S–769S. doi: 10.1093/ajcn/59.3.763S. [DOI] [PubMed] [Google Scholar]
- 36.Kelly G. Inulin type prebiotics – a review: Part I. Altern. Med. Rev. 2008;13:315–329. [PubMed] [Google Scholar]
- 37.Nair K.K., Kharb S., Thompkinson D.K. Inulin dietary fiber with functional and health attributes: a review. Food Rev. Int. 2010;26:189–203. [Google Scholar]
- 38.Root H.F., Baker M.L. Inulin and artichokes in the treatment of diabetes. Arch. Intern. Med. 1925;36:126–145. [Google Scholar]
- 39.Rumessen J.J., Bodé S., Hamberg O., Gudmand-Høyer E. Fructans of Jerusalem artichokes: intestinal transport, absorption, fermentation and influence on blood glucose, insulin and C-peptide responses in healthy subjects. Am. J. Clin. Nutr. 1990;52:675–681. doi: 10.1093/ajcn/52.4.675. [DOI] [PubMed] [Google Scholar]
- 40.Beringer A., Wenger R. Inulin in der ernährung des diabetikers. Dtsch. Z. Verdauungs Stofwechselkrankh. 1995;15:268–272. [PubMed] [Google Scholar]
- 41.Knudsen B.K.E., Hessov I. Recovery of inulin from Jerusalem artichoke (Helianthus tuberosus L.) in the small intestine of man. Br. J. Nutr. 1995;74:101–113. doi: 10.1079/bjn19950110. [DOI] [PubMed] [Google Scholar]
- 42.Franck A. Technological functionality of inulin and oligofructose. Br. J. Nutr. 2002;87:S287–S291. doi: 10.1079/BJNBJN/2002550. [DOI] [PubMed] [Google Scholar]
- 43.Trowell H., Burkitt D. Physiological role of dietary fiber: a ten-year review. ASDC J. Dent. Child. 1986;53:444–447. [PubMed] [Google Scholar]
- 44.Gibson G.R., Beatty E.R., Wang X., Cummings J.H. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995;108:975–982. doi: 10.1016/0016-5085(95)90192-2. [DOI] [PubMed] [Google Scholar]
- 45.Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonic microbiota—introducing the concept of prebiotics. J. Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 46.Coudray C., Demigné C., Rayssiguier Y. Effects of dietary fibers on magnesium absorption in animals and humans. J. Nutr. 2003;133:1–4. doi: 10.1093/jn/133.1.1. [DOI] [PubMed] [Google Scholar]
- 47.Abrams S.A., Griffin I.J., Hawthorne K.M., Liang L.L., Gunn S.K., Darlington G., Ellis K.J. A combination of prebiotic short-and long-chain inulin-type fructans enhances calcium absorption and bone mineralization in young adolescents. Am. J. Clin. Nutr. 2005;82:471–476. doi: 10.1093/ajcn.82.2.471. [DOI] [PubMed] [Google Scholar]
- 48.Franck A. Prebiotics stimulate calcium absorption: a review. Food Aust. 2005;57:530–532. [Google Scholar]
- 49.Levrat M.A., Rémésy C., Demigné C. High propionic acid fermentations and mineral accumulation in the cecum of rats adapted to different level of inulin. J. Nutr. 1991;21:1730–1737. doi: 10.1093/jn/121.11.1730. [DOI] [PubMed] [Google Scholar]
- 50.Kaur N., Gupta A.K., Saijpaul B. Hypotriglyceridaemic effect of cichorium intybus roots in ethanol injected and saturated fat-fed rats. J. Ethnopharmacol. 1988;23:343. [Google Scholar]
- 51.Kaur H., Gupta A.K., Saijpal S., Indu Gupta P.P. Triglyceride and cholesterol lowering effect of chicory roots in the liver of dexamethosone-injected rat. Med. Sci. Res. 1989;17:1009–1010. [Google Scholar]
- 52.Pedersen A., Sandstrõm B., Van Amelsvoort J.M.M. The effect of ingestion of inulin on blood lipids and gastrointestinal symptoms in healthy females. Br. J. Nutr. 1997;78:215–222. doi: 10.1079/bjn19970141. [DOI] [PubMed] [Google Scholar]
- 53.Jackson K.G., Taylor G.R.J., Clohessy A.M., Williams C.M. The effect of the daily intake of inulin on fasting lipid, insulin and glucose concentrations in middle-aged men and women. Br. J. Nutr. 1999;82:23–30. doi: 10.1017/s0007114599001087. [DOI] [PubMed] [Google Scholar]
- 54.De Almeida Gualtieri K., Losi Guembarovski R., Oda J.M.M., Fiori-Lopes L., Ketelut Carneiro N., de Castro V.D., Soni Neto J., Angelica Ehara Watanabe M. Inulin: therapeutic potential, prebiotic properties and immunological aspects. Food Agric. Immunol. 2013;24:21–31. [Google Scholar]
- 55.Clark M.J., Robien K., Slavin J.L. Nutrition effect of prebiotics on biomarkers of colorectal cancer in humans: a systematic review. Nutr. Rev. 2012;70:436–443. doi: 10.1111/j.1753-4887.2012.00495.x. [DOI] [PubMed] [Google Scholar]
- 56.McIntosh G.H. Experimental studies of dietary fibre and colon cancer-an overview. In: van der Kamp J.W., Asp N.G., Miller Jones J., Schaafsma G., editors. Dietary Fibre: Bio-active Carbohydrates for Food and Feed. Wageningen Academic Press; Wageningen, The Netherlands: 2004. pp. 165–178. [Google Scholar]
- 57.Taper H.S., Roberfroid M. Influence of inulin and oligofructose on breast cancer and tumor growth. J. Nutr. 1999;129:1488S–1491S. doi: 10.1093/jn/129.7.1488S. [DOI] [PubMed] [Google Scholar]
- 58.Rumney C., Rowland I. Non-digestible oligosaccharides-potential anti-cancer agents. Nutr. Bull. 1995;20:194–203. [Google Scholar]
- 59.Weidmann M., Jager M. Synergistic sweeteners. Food Ingredients Anal. Int. 1997:51–56. [Google Scholar]
- 60.Kim D.H., Choi Y.J., Song S.K., Yun J.W. Production of inulooligosaccharides using endo-inulinase from a Pseudomonas sp. Biotechnol. Lett. 1997;19:369–371. [Google Scholar]
- 61.Abou-Arab A., Talaat H., Abu-Salem F. Physico-chemical properties of inulin produced from Jerusalem artichoke tubers on bench and pilot plant scale. Aust. J. Basic Appl. Sci. 2011;5:1297–1309. [Google Scholar]
- 62.Li J.Q. Polyphenol oxidase characteristics of Jerusalem artichoke. Acta Agriculturae Boreali-Occidentalis Sinica. 2010;8:038. [Google Scholar]
- 63.Jiang S.J. Research on extraction of inulin from Helianthus tuberosus L. Guangzhou Chem. Ind. 2012;40:82–84. [Google Scholar]
- 64.Kierstan M. Studies on enzymatic methods for extraction of inulin from Jerusalem artichoke. Enzyme Microbiol. Technol. 1983;5:445–448. [Google Scholar]
- 65.Toma M., Vinatoru M., Paniwnyk L., Mason T.J. Investigation of the effects of ultrasound on vegetal issues during solvent extraction. Utrason. Sonochem. 2001;8:137–142. doi: 10.1016/s1350-4177(00)00033-x. [DOI] [PubMed] [Google Scholar]
- 66.Wei L.Y., Wang J.H., Zheng X.D., Teng D., Yang Y.L., Cai C.G., Feng T.H., Zhang F. Studies on the extracting technical conditions of inulin from Jerusalem artichoke tubers. J. Food Eng. 2007;79:1087–1093. [Google Scholar]
- 67.Veggi P.C., Martinez J., Meireles M.A.A. Series 4. Springer; 2013. (Microwave-assisted Extraction for Bioactive Compounds: Theory and Practice, Food Engineering). [Google Scholar]
- 68.Xiao Z.J., Zhu D.H., Wang X.H., Zhang M.D. Study on extraction process of inulin from Helianthus tuberosus. Mod. Food Sci. Technol. 2013;29:02. [Google Scholar]
- 69.Li H.D., Zhu H.J., Qiao J.J., Du J.H., Zhang H. Optimization of the main liming process for inulin crude extract from Jerusalem artichoke tubers. Front. Chem. Sci. Eng. 2012;6:348–355. [Google Scholar]
- 70.Jenkins D.J., Wolever T.M., Taylor R.H., Barker H., Fielden H., Baldwin J.M., Bowling A.C., Newman H.C., Jenkins A.L., Goff D.V. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981;34:362–366. doi: 10.1093/ajcn/34.3.362. [DOI] [PubMed] [Google Scholar]
- 71.Foster-Powell K., Holt S.H.A., Brand-Miller J.C. International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- 72.Segal M.S., Johnson R.J. Is the fructose index more relevant with regards to cardiovascular disease than the glycemic index? Eur. J. Nutr. 2007;46:406–417. doi: 10.1007/s00394-007-0680-9. [DOI] [PubMed] [Google Scholar]
- 73.Fontvieille A.M., Faurion A., Helal I., Rizkalla S.W., Falgon S., Letanoux M., Tchobroutsky G., Slama G. Relative sweetness of fructose compared with sucrose in healthy and diabetic subjects. Diabetes Care. 1989;12:481–486. doi: 10.2337/diacare.12.7.481. [DOI] [PubMed] [Google Scholar]
- 74.Cameron A.T. The relative sweetness of sucrose, glucose, and fructose. Trans. R. Soc. Can. J. Biol. Sci. 1943;37:11–27. [Google Scholar]
- 75.Schallenberger R.S. Hydrogen bonding and the varying sweetness of the sugars. J. Food Sci. 1963;28:584–589. [Google Scholar]
- 76.Stone H., Oliver S.M. Measurement of the relative sweetness of selected sweeteners and sweetener mixtures. J. Food Sci. 1969;34:215–222. [Google Scholar]
- 77.Kim C.H., Rhee S.K. Fructose production from Jerusalem artichoke by inulinase immobilized on chitin. Biotechnol. Lett. 1989;11:201–206. [Google Scholar]
- 78.Kim W.Y., Byun S.M., Uhm T.B. Hydrolysis of inulin from Jerusalem artichoke by inulinase immobilized on aminoethylcellulose. Enzyme Microb. Technol. 1982;4:239–244. [Google Scholar]
- 79.Pandey A., Soccol C.R., Selvakumar P., Soccol V.T. Recent developments in microbial inulinases. Appl. Biochem. Biotechnol. 1999;81:129–145. doi: 10.1385/abab:81:1:35. [DOI] [PubMed] [Google Scholar]
- 80.Ricca E., Calabrò V., Curcio S., Lorio G. The state of the art in the production of fructose from inulin enzymatic hydrolysis. Crit. Rev. Biotechnol. 2007;27:129–145. doi: 10.1080/07388550701503477. [DOI] [PubMed] [Google Scholar]
- 81.Sirisansaneeyakul S., Worawuthiyanan N., Vanichsriratana W., Srinophakun P., Chisti Y. Production of fructose from inulin using mixed inulinases from Aspergillus niger and Candida guilliermondii. World J. Microbiol. Biotechnol. 2007;23:543–552. [Google Scholar]
- 82.Yu J., Jiang J.X., Ji W.M., Li Y.Y., Liu J.P. Glucose-free fructose production from Jerusalem artichoke using a recombinant inulinase-secreting Saccharomyces cerevisiae strain. Biotechnol. Lett. 2011;33:147–152. doi: 10.1007/s10529-010-0414-6. [DOI] [PubMed] [Google Scholar]
- 83.Guiraud J.P., Demeulle S., Galzy P. Inulin hydrolysis by the Debaryomyces phaffii inulinase immobilized on DEAE cellulose. Biotechnol. Lett. 1981;3:683–688. [Google Scholar]
- 84.Santa G.L.M., Bernardino S.M.S.A., Magalhães S., Mendes V., Marques M.P.C., Fonseca L.P., Fernandes P. From inulin to fructose syrups using sol-gel immobilization inulinase. Appl. Biochem. Biotechnol. 2011;165:1–12. doi: 10.1007/s12010-011-9228-9. [DOI] [PubMed] [Google Scholar]
- 85.Singh R.S., Dhaliwal R., Puri M. Development of a table continuous flow immobilized enzyme reactor for the hydrolysis of inulin. J. Indus. Microbiol. Biotechnol. 2008;35:777–782. doi: 10.1007/s10295-008-0348-3. [DOI] [PubMed] [Google Scholar]
- 86.Zhu H.J., Guo Q. The production and application of Jerusalem artichoke. Appl. Technol. Market. 2001:5. [Google Scholar]
- 87.Zhu H.J., Hu J.B., Zhao Z.X., Jin D.W., Nie Q.D. The study of producing fructose using dynamic membrane separation type enzymatic device. Chem. Indus. Eng. 1995;12:1–7. [Google Scholar]
- 88.Baba H., Yaoita Y., Kikuchi M. Sesquiterpenoids from the leaves of Helianthus tuberosus L. J. Tohoku Pharm. Univ. 2005;52:21–25. [Google Scholar]
- 89.Health Department and National Chinese Medicine Management Office. Chinese Herb Medicine. Shanghai Science Technology Press, 1998.
- 90.Talipova Lipids of Helianthus tuberosus L. Chem. Nat. Compd. 2001;37:213–215. [Google Scholar]
- 91.Ahmed M.S., El-Sakhawy F.S., Soliman S.N., Abou H.D.M.R. Phytochemical and biological study of Helianthus tuberosus L. Egypt. J. Biomed. Sci. 2005;18:134–147. [Google Scholar]
- 92.Nakagawa R., Yasokawa D., lkeda T., Nagashima K. Purification and characterization of two lectins from callus of Helianthus tuberosus. Biosci. Biotechnol. Biochem. 1996;60:259–262. doi: 10.1271/bbb.60.259. [DOI] [PubMed] [Google Scholar]
- 93.Yuan X., Gao M., Wang K., Xiao H.B., Tan C., Du Y. Analysis of chlorogenic acids in Helianthus tuberosus Linn leaves using high performance liquid chromatography–mass spectrometry. Chin. J. Chromatogr. 2008;26:335–338. [PubMed] [Google Scholar]
- 94.Yuan X.Y., Cheng M.C., Gao M.Z., Zhuo R.J., Zhang L.X., Xiao H.B. Cytotoxic constituents from the leaves of Jerusalem artichoke (Helianthus tuberosus L.) and their structure–activity relationships. Phytochem. Lett. 2013;6:21–25. [Google Scholar]
- 95.Moskovitz J., Yim M.B., Chock P.B. Free radicals and disease. Arch. Biochem. Biophys. 2002;397:354–359. doi: 10.1006/abbi.2001.2692. [DOI] [PubMed] [Google Scholar]
- 96.Liu H.W., Liu Z.P., Liu L., Zhao G.M. Studies on the antifungal activities and chemical components of extracts from Helianthus tuberosus leaves. Nat. Prod. Res. Dev. 2007;19:405–409. [Google Scholar]
- 97.Han R., Wang L.H., Zhong Q.W., Sun K., Li Y. Study on antifungal activity of the extract from the leaves of Helianthus tuberosus. Mod. Agric. Sci. Technol. 2010:5. [Google Scholar]
- 98.Chen F.J., Long X.H., Yu M.N., Liu Z.P., Liu L., Shao H.B. Phenolics and antifungal activities analysis in industrial crop Jerusalem artichoke (Helianthus tuberosus L.) leaves. Indus. Crops Prod. 2013;47:339–345. [Google Scholar]
- 99.Lee Y.J., Lee M., Yu S.Y. Changes in physicochemical characteristics and antioxidant activities of Jerusalem artichoke tea infusions resulting from different production processes. Food Sci. Biotechnol. 2014;23:1885–1892. [Google Scholar]
- 100.Kim D., Fan J.P., Chung H.C. Changes in extractability and antioxidant activities of Jerusalem artichoke (Helianthus tuberosus L.) tubers by various high hydrostatic pressure treatments. Food Sci. Biotechnol. 2010;19:1365–1367. [Google Scholar]
- 101.Azmir J., Zaidul I.S.M., Rahman M.M., Sharif K.M., Mohamed A., Sahena F., Jahurul M.H.A., Ghafoor K., Norulaini N.A.N., Omar A.K.M. Techniques for extraction of bioactive compounds from plant materials: a review. J. Food Process. Eng. 2013;117:426–436. [Google Scholar]
- 102.Fornari T., Vicente G., Vázquez E., García-Risco M.R., Reglero G. Isolation of essential oil from different plants and herbs by supercritical fluid. J. Chromatogr. A. 2012;1250:34–48. doi: 10.1016/j.chroma.2012.04.051. [DOI] [PubMed] [Google Scholar]
- 103.Hu J.F., Qiu S.Y. Research process in ethanol production by the fermentation of Jerusalem artichoke. Liq.-Mark. Sci. Technol. 2009;182:100–104. [Google Scholar]
- 104.Zelenkov, V.N., 2000. Method of Beer Brewing Using Jerusalem Artichoke. Russian federation patent 149894.
- 105.Hui, Y.H., 1991. Data Source Book for Food Scientists and Technologist.
- 106.Sachs R.M., Low C.B., Vasavada A., Sully M.J., Williams L.A., Ziobro G.C. Fuel alcohol from Jerusalem artichoke. Calif. Agric. 1981;35:4–6. [Google Scholar]
- 107.Biofuel.org, UK, 2014 History of Biofuels http://biofuel.org.uk/history-of-biofuels.html (Retrieved on 03.02.14).
- 108.Margaritis A., Bajpai P. Continuous ethanol production from Jerusalem artichoke tubers. I. Use of free cells of Kluyveromyces marxianus. Biotechnol. Bioeng. 1982;24:1473–1482. doi: 10.1002/bit.260240702. [DOI] [PubMed] [Google Scholar]
- 109.Margaritis A., Bajpai P. Continuous ethanol production from Jerusalem artichoke tubers. II. Use of immobilized cells of Kluyveromyces marxianus. Biotechnol. Bioeng. 1982;24:1483–1493. doi: 10.1002/bit.260240703. [DOI] [PubMed] [Google Scholar]
- 110.Zhang G.J., Long W.D. A key review on energy analysis and assessment of biomass resources for a sustainable future. Energy Policy. 2010;38:2948–2955. [Google Scholar]
- 111.Hill J. Environmental cost and benefits of transportation biofuel production from food- and lignocellulose-based energy crops. A review. Agron. Sustain. Dev. 2007;27:1–12. [Google Scholar]
- 112.Sanchez O.J., Cardona C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008;99:5270–5295. doi: 10.1016/j.biortech.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 113.Naylor R.L., Liska A., Burke M.B., Falcon W.P., Gaskell J.C. The ripple effect: biofuels, food security, and the environment. Environment. 2007;49:30–43. [Google Scholar]
- 114.Demirbas A. Political, economic and environmental impacts of biofuels: a review. Appl. Energy. 2009;86:S108–S117. [Google Scholar]
- 115.Brown R.C., Brown T.R. Wiley-Blackwell Publishing; 2013. Biorenewable Resources Engineering New Products from Agriculture. [Google Scholar]
- 116.Limayem A., Ricke S.C. Lignocellulosic biomass for bioethanol production: current perspective, potential issues and prospects. Prog. Energy Combust. Sci. 2012;38:449–467. [Google Scholar]
- 117.Li L.L., Li L., Wang Y.P., Du Y.G., Qin S. Bio-refinery products from the inulin-containing crop Jerusalem artichoke. Biotechnol. Lett. 2013;35:471–477. doi: 10.1007/s10529-012-1104-3. [DOI] [PubMed] [Google Scholar]
- 118.Matías J., González J., Cabanillas J., Royano L. Influence of NKP fertilization and harvest data on agronomic performance of Jerusalem artichoke crop in the Guadiana Basin (Southwestern Spain) Indus. Crops Prod. 2013;48:191–197. [Google Scholar]
- 119.Kosarik N., Cosentino G.P., Wieczorek A. The Jerusalem artichoke as an agricultural crop. Biomass. 1984;5:1–36. [Google Scholar]
- 120.Li X.F., Hou S.L., Su M., Yang M.F., Shen S.H., Jiang G.M., Qi D.M., Chen S.Y., Liu G.S. Major energy plant and their potential for bioenergy development in China. Environ. Manage. 2010;46:579–589. doi: 10.1007/s00267-010-9443-0. [DOI] [PubMed] [Google Scholar]
- 121.Goor F., Dubuisson X., Jossart J.M. Suitability, environmental impact and energy balance of some energy crops in Belgium. Cahiers d’Etudes et de Recherches Francophones Agricultures. 2000;9:59–64. [Google Scholar]
- 122.Fernández J., Curt M.D. New energy crops for bioethanol production in Mediterranean region. Int. Sugar J. 2005;107:622–627. [Google Scholar]
- 123.Fleming S.E., GrootWassink J.W.D., Murray E.D. Preparation of high-fructose syrup from the tubers of the Jerusalem artichoke (Helianthus tuberosus L.) Crit. Rev. Food Sci. Nutr. 1979;12:1–28. doi: 10.1080/10408397909527271. [DOI] [PubMed] [Google Scholar]
- 124.Toran-diaz I., Jain V.K., Allais J.J., Baratti J. Effect of acid or enzymatic hydrolysis on ethanol production by Zymomonas mobilis growing on Jerusalem artichoke juice. Biotechnol. Lett. 1985;7:527–530. [Google Scholar]
- 125.Kim K., Hamdy M.K. Acid hydrolysis of Jerusalem artichoke for ethanol fermentation. Biotechnol. Bioeng. 1986;28:138–141. doi: 10.1002/bit.260280124. [DOI] [PubMed] [Google Scholar]
- 126.Razmovski R.N., Šcban M.B., Vučurović V.M. Bioethanol production from Jerusalem artichoke by acid hydrolysis. Rom. Biotechnol. Lett. 2011;16:6497–6503. [Google Scholar]
- 127.Zubr J. In: Fuels from Jerusalem artichoke, in Topinambour (Jerusalem artichoke) [M]. Report EUR 11855. Grassi G., Gosse G., editors. CEC; Luxembourg: 1988. pp. 165–175. [Google Scholar]
- 128.Kim C.H., Rhee S.K. Ethanol production from Jerusalem artichoke by inulinase and Zymomonas mobilis. Appl. Biochem. Biotechnol. 1990;23:171–180. [Google Scholar]
- 129.Ohta K., Hamada S., Nakamura T. Production of high concentration of ethanol from inulin by simultaneous saccharification and fermentation using Aspergillus niger and Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1993;59:729–733. doi: 10.1128/aem.59.3.729-733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nakamura T., Ogata Y., Hamada S., Ohta K. Ethanol production from Jerusalem artichoke tubers by Aspergillus niger and Saccharomyces cerevisiae. J. Ferment. Bioeng. 1996;81:564–566. [Google Scholar]
- 131.Szambelan K., Nowak J., Czarnecki Z. Use of Zymomonas mobilis and Saccharomyces cerevisiae mixed with Kluyveromyces fragilis for improved ethanol production from Jerusalem artichoke tubers. Biotechnol. Lett. 2004;26:845–848. doi: 10.1023/b:bile.0000025889.25364.4b. [DOI] [PubMed] [Google Scholar]
- 132.Ge X.Y., Zhang W.G. A shortcut to the production of high ethanol concentration from Jerusalem artichoke tubers. Food Technol. Biotechnol. 2005;43:241–246. [Google Scholar]
- 133.Guiraud J.P., Daurelles J., Galzy P. Alcohol production from Jerusalem artichoke using yeast with inulinase activity. Biotechnol. Bioeng. 1981;23:1461–1465. [Google Scholar]
- 134.Duvnjak Z., Kosaric N., Hayes R.D. Kinetics of ethanol production from Jerusalem artichoke juice with some Kluyveromyces species. Biotechnol. Lett. 1981;3:589–594. [Google Scholar]
- 135.Margaritis A., Bajpai P., Cannell E. Optimization studies for the bioconversion of Jerusalem artichoke tubers to ethanol and microbial biomass. Biotechnol. Lett. 1981;3:595–599. [Google Scholar]
- 136.Margaritis A., Bajpai P. Repeated batch-production of ethanol from Jerusalem artichoke tubers using recycled immobilized cells of Kluyveromyces fragilis. Biotechnol. Lett. 1981;3:679–682. [Google Scholar]
- 137.Margaritis A., Bajpai P. Ethanol production from Jerusalem artichoke tubers (Helianthus tuberosus L.) using Kluyveromyces marxianus and Saccharomyces rosei. Biotechnol. Bioeng. 1982;24:941–953. doi: 10.1002/bit.260240414. [DOI] [PubMed] [Google Scholar]
- 138.Bajpai P., Margaritis A. Ethanol inhibition-kinetics of Kuyveromyces marxianus grown on Jerusalem artichoke juice. Appl. Environ. Microbiol. 1982;44:1325–1329. doi: 10.1128/aem.44.6.1325-1329.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Margaritis A., Bajpai P., Lachance M.A. The use of free and immobilized cells of Debaryomyces-polymorphus to produce ethanol from Jerusalem artichoke tubers. J. Ferment. Technol. 1983;61:533–537. [Google Scholar]
- 140.Margaritis A., Bajpal P. Effect of sugar concentration in Jerusalem artichoke extract on Kluyveromyces marxianus growth and ethanol-production. Appl. Environ. Microbiol. 1983;45:723–725. doi: 10.1128/aem.45.2.723-725.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Margaritis A., Bajpai P. Novel immobilized-cell system for the production of ethanol from Jerusalem artichoke. Ann. N.Y. Acad. Sci. 1983;413:479–482. [Google Scholar]
- 142.Bajpal P., Margaritis A. Ethanol-production from Jerusalem artichoke juice using flocculant cells of Kluyveromyces marxianus. Biotechnol. Lett. 1986;8:361–364. [Google Scholar]
- 143.Bajpai P., Margaritis A. Continuous ethanol-production from Jerusalem artichoke using immobilized cells of Klyveromyces marxianus. Process Biochem. 1986;21:86–89. [Google Scholar]
- 144.Bajpal P., Margaritis A. Kinetics of ethanol-production by immobilized Klyveromyces marxianus cells at varying sugar concentrations of Jerusalem artichoke juice. Appl. Microbiol. Biotechnol. 1987;26:447–449. [Google Scholar]
- 145.Bajpal P., Margaritis A. The effect of temperature and pH on ethanol-production by free and immobilized cells of Klyveromyces marxianus grown on Jerusalem artichoke extract. Biotechnol. Bioeng. 1987;30:306–313. doi: 10.1002/bit.260300222. [DOI] [PubMed] [Google Scholar]
- 146.Parekh S.R., Margaritis A. Application of immobilized cells of Kluyveromyces marxianus for continuos hydrolysis to fructose of fructans in Jerusalem artichoke extracts. J. Food Technol. 1986;21:509–515. [Google Scholar]
- 147.Parekh S.R., Margaritis A. Continuous hydrolysis of fructans in Jerusalem artichoke extracts using immobilized nonviable cells of Kluyveromyces marxianus. J. Food Sci. 1986;51:854–855. [Google Scholar]
- 148.Bajpai P., Margaritis A. Optimization studies for production of high fructose syrup from Jerusalem artichoke using calcium alginate immobilized cells of Kluyveromyces marxianus. Process Biochem. 1986;21:16–18. [Google Scholar]
- 149.Bajpai P., Margaritis A. Production of high fructose syrup from Jerusalem artichoke tubers using Kluyveromyces marxianus cells immobilized in agar-gel. J. Gen. Appl. Microbiol. 1985;31:305–311. [Google Scholar]
- 150.Negro M.J., Ballesteros I., Manzanares P., Oliva J.M., Sáez F., Ballesteros M. Inulin-containing biomass for ethanol production: carbohydrate extraction and ethanol fermentation. Appl. Biochem. Biotechnol. 2006 doi: 10.1385/abab:132:1:922. 129-132:922-932. [DOI] [PubMed] [Google Scholar]
- 151.Lim S.H., Ryu J.M., Lee H., Jeon J.H., Sok D.E., Choi E.S. Ethanol fermentation from Jerusalem artichoke powder using Saccharomyces cerevisiae KCCM50549 without pretreatment for inulin hydrolysis. Bioresour. Technol. 2011;102:2109–2111. doi: 10.1016/j.biortech.2010.08.044. [DOI] [PubMed] [Google Scholar]
- 152.Yu J., Jiang J., Zhang Y., Lu H., Li Y., Liu J. Simultaneous saccharification and fermentation of Jerusalem artichoke tubers to ethanol with an inulinase-hyperproducing yeast Kluyveromyces cicerisporus. Chin. J. Biotechnol. 2010;26:982–990. [PubMed] [Google Scholar]
- 153.Yuan W.J., Zhao X.Q., Ge X.M., Bai F.W. Ethanol fermentation with Kluyveromyces marxianus from Jerusalem artichoke grown in salina and irrigated with a mixture of seawater and freshwater. J. Appl. Microbiol. 2008;105:2076–2083. doi: 10.1111/j.1365-2672.2008.03903.x. [DOI] [PubMed] [Google Scholar]
- 154.Yuan W.J., Chang B.L., Ren J.G., Liu J.P., Bai F.W., Li Y.Y. Consolidated bioprocessing strategy for ethanol production from Jerusalem artichoke tubers by Kluyveromyces marxianus under high gravity conditions. J. Appl. Microbiol. 2012;112:38–44. doi: 10.1111/j.1365-2672.2011.05171.x. [DOI] [PubMed] [Google Scholar]
- 155.Yuan W., Li N.N., Zhao X.Q., Chen L.J., Kong L., Bai F.W. Engineering an industrial Saccharomyces cerevisiae strain with the inulinase gene for more efficient ethanol production from Jerusalem artichoke tubers. Engin. Life Sci. 2013;13:472–478. [Google Scholar]
- 156.Yuan W.J., Zhao X.Q., Chen L.J., Bai F.W. Improved ethanol production in Jerusalem artichoke tubers by overexpression of inulinase gene in Kluyveromyces marxianus. Biotechnol. Bioprocess Eng. 2013;18:721–727. [Google Scholar]
- 157.Zhang T., Chi Z., Zhao C.H., Chi Z.M., Gong F. Bioethanol production from hydrolysates of inulin and the tuber meal of Jerusalem artichoke by Saccharomyces sp W0. Bioresour. Technol. 2010;101:8166–8170. doi: 10.1016/j.biortech.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 158.Wang J.M., Zhang T., Chi Liu Z.G.L., Chi Z.M. 18S rDNA integration of the exo-inulinase gene into chromosomes of the high ethanol producing yeast Saccharomyces sp. W0 for direct conversion of inulin to bioethanol. Biomass Biotechnol. 2011;35:3032–3039. [Google Scholar]
- 159.Li Y., Liu G.L., Chi Z.M. Ethanol production from inulin and unsterilized meal of Jerusalem artichoke tubers by Saccharomyces sp. W0 expressing the endo-inulinase gene from Arthrobacter sp. Bioresour. Technol. 2013;147:254–259. doi: 10.1016/j.biortech.2013.08.043. [DOI] [PubMed] [Google Scholar]
- 160.Hu N., Yuan B., Sun J., Wang S.A., Li F.L. Thermotolerant Kluyveromyces marxianus and Saccharomyces cerevisiae strains representing potentials for bioethanol production from Jerusalem artichoke by consolidated bioprocessing. Appl. Microbiol. Biotechnol. 2012;95:1359–1368. doi: 10.1007/s00253-012-4240-8. [DOI] [PubMed] [Google Scholar]
- 161.Guo L.H., Zhang J., Hu F.X., Ryu D.D., Bao J. Consolidated bioprocessing of highly concentrated Jerusalem artichoke tubers for simultaneous saccharification and ethanol fermentation. Biotechnol. Bioeng. 2014;2103(110):2606–2614. doi: 10.1002/bit.24929. [DOI] [PubMed] [Google Scholar]
- 162.Sarchami T., Rehmann L. Optimizing enzymatic hydrolysis of inulin from Jerusalem artichoke tubers for fermentative butanol production. Biomass Bioenergy. 2014;69:175–182. [Google Scholar]
- 163.Chen L.J., Xin C., Deng P. Butanol production from hydrolysate of Jerusalem artichoke juice by Clostridium acetobutylicum L7. Sheng Wu Gong Cheng Xue Bao. 2010;26:991–996. [PubMed] [Google Scholar]
- 164.Chi Z.M., Zhang T., Cao T.S. Biotechnological potential of inulin for bioprocesses. Bioresour. Technol. 2011;102:4295–4303. doi: 10.1016/j.biortech.2010.12.086. [DOI] [PubMed] [Google Scholar]
- 165.Ge X.Y., Qian H., Zhang W.G. Improvement of l-lactic acid production from Jerusalem artichoke tubers by mixed culture of Aspergillus niger and Lactobacillus sp. Bioresour. Technol. 2009;100:1872–1874. doi: 10.1016/j.biortech.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 166.Ge X.Y., Qian H., Zhang W.G. Enhancement of l-lactic acid production in Lactobacillus casei from Jerusalem artichoke tubers by kinetic optimization and citrate metabolism. J. Ind. Microbiol. Biotechnol. 2010;20:101–109. [PubMed] [Google Scholar]
- 167.Choi H.Y., Ryu H.K., Park K.M. Direct lactic acid fermentation of Jerusalem artichoke tuber extract using Lactobacillus paracasei without acidic or enzymatic inulin hydrolysis. Bioresour. Technol. 2012;114:745–747. doi: 10.1016/j.biortech.2012.03.075. [DOI] [PubMed] [Google Scholar]
- 168.Dao T.H., Zhang J., Bao J. Characterization of inulin hydrolyzing enzyme(s) in commercial glucoamylases and its application in lactic acid production from Jerusalem artichoke tubers (Jat) Bioresour. Technol. 2013;148:157–162. doi: 10.1016/j.biortech.2013.08.123. [DOI] [PubMed] [Google Scholar]
- 169.Wang L.M., Xue Z.W., Zhao B. Jerusalem artichoke powder: a useful material in producing high-optical-purity l-lactate using an efficient sugar-utilizing thermophilic Bacillus coagulans strain. Bioresour. Technol. 2013;130:174–180. doi: 10.1016/j.biortech.2012.11.144. [DOI] [PubMed] [Google Scholar]
- 170.Shi Z.M., Zhu Wei P.L. Efficient production of l-lactic acid from hydrolysate of Jerusalem artichoke with immobilized cells of Lactococcus lactis in fibrous bed bioreactors. Enzyme Microb. Technol. 2012;51:263–268. doi: 10.1016/j.enzmictec.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 171.Li L.L., Wang Y.P., Du Y.G. Biorefinery products from the inulin-containing crop Jerusalem artichoke. Biotechnol. Lett. 2013;35:471–477. doi: 10.1007/s10529-012-1104-3. [DOI] [PubMed] [Google Scholar]
- 172.Huang J., Cai J., Wang J. Efficient production of butyric acid from Jerusalem artichoke by immobilized Clostridium tyrobutyricum in a fibrous-bed bioreactor. Bioresour. Technol. 2011;102:3923–3926. doi: 10.1016/j.biortech.2010.11.112. [DOI] [PubMed] [Google Scholar]
- 173.Liu X.Y., Chi Z., Liu G.L. Inulin hydrolysis and citric acid production from inulin using the surface-engineered Yarrowia lipolytica displaying inulinase. Metab. Eng. 2010;12:469–476. doi: 10.1016/j.ymben.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 174.Sridhar J., Eiteman M.A. Influence of redox potential on product distribution in Clostridium thermosuccinogenes. Appl. Biochem. Biotechnol. 1999;82:91–101. doi: 10.1385/abab:94:1:51. [DOI] [PubMed] [Google Scholar]
- 175.Gao J., Xu H., Li Q.J. Optimization of medium for one-step fermentation of inulin extract from Jerusalem artichoke tubers using Paenibacillus polymyxa ZJ-9 to produce R,R-2,3-butanediol. Bioresour. Technol. 2010;101:7076–7082. doi: 10.1016/j.biortech.2010.03.143. [DOI] [PubMed] [Google Scholar]
- 176.Li D., Dai J.Y., Xiu Z.L. A novel strategy for integrated utilization of Jerusalem artichoke stalk and tuber for production of 2,3-butanediol by Klebsiella pneumonia. Bioresour. Technol. 2010;101:8342–8347. doi: 10.1016/j.biortech.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 177.Wei W., Wu K., Qin Y. Intergeneric protoplast fusion between Kluyveromyces and Saccharomyces cerevisiae to produce sorbitol from Jerusalem artichokes. Biotechnol. Lett. 2001;23:799–803. [Google Scholar]