Abstract
The fifth Jack Pepys Workshop on Asthma in the Workplace focused on the similarities and differences of work-related asthma (WRA) and non–work-related asthma (non-WRA). WRA includes occupational asthma (OA) and work-exacerbated asthma (WEA). There are few biological differences in the mechanisms of sensitization to environmental and occupational allergens. Non-WRA and OA, when due to high-molecular-weight agents, are both IgE mediated; it is uncertain whether OA due to low-molecular-weight agents is also IgE mediated. Risk factors for OA include female sex, a history of upper airway symptoms, and a history of bronchial hyperresponsiveness. Atopy is a risk factor for OA due to high-molecular-weight agents, and exposure to cleaning agents is a risk factor for both OA and non-WRA. WEA is important among workers with preexisting asthma and may overlap with irritant-induced asthma, a type of OA. Induced sputum cytology can confirm airway inflammation, but specific inhalation challenge is the reference standard diagnostic test. Inhalation challenges are relatively safe, with the most severe reactions occurring with low-molecular-weight agents. Indirect health care costs account for about 50% of total asthma costs. Workers with poor asthma control (WRA or non-WRA) are less likely to be employed. Income loss is a major contributor to the indirect costs of WRA. Overall, asthma outcomes probably are worse for adult-onset than for childhood-onset asthma but better for OA than adult-onset non-WRA. Important aspects of management of OA are rapid and proper confirmation of the diagnosis and reduction of exposure to sensitizers or irritants at work and home.
Overview
Introduction
Methods
Mechanisms and Pathophysiology
Epidemiology and Risk Factors
Allergens and Irritants
Allergens
Irritants
Diagnosis and Clinical Aspects
Nosological Aspects
Methods of Diagnosis
Psychosocial and Economic Impacts Outcomes and Management
Outcomes
Management
Pharmacologic and Other Treatment
Conclusions
Overview
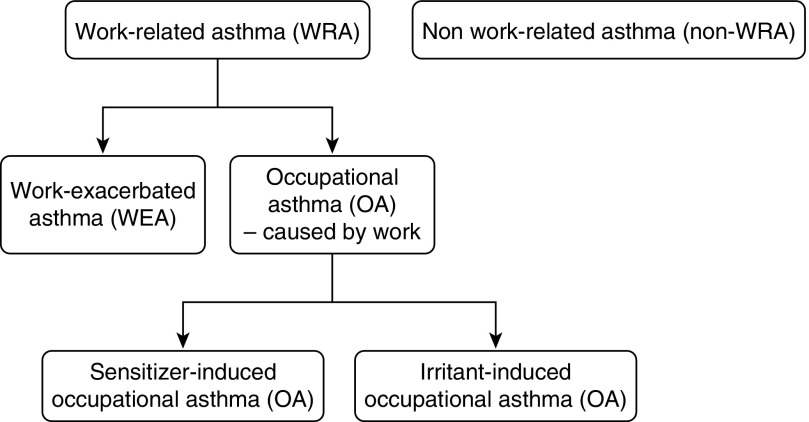
Work-related asthma (WRA) is a term that includes occupational asthma (i.e., asthma that is caused by an occupational exposure) and work-exacerbated asthma (WEA) (i.e., asthma that is worsened by an occupational exposure but not caused by an occupational exposure) (1) (Figure 1).
Figure 1.
Entities discussed in the Workshop.
The Jack Pepys Workshop is held every 3 years to discuss WRA (2). The fifth workshop was held in Montreal in 2013 and focused on the similarities and differences of WRA and non-WRA. Key conclusions were:
-
•
Occupational asthma (OA) is a useful model for studying non-WRA because the exposure that causes OA is often easily identified and workplace interventions facilitate studies of both the disease course and the impact of treatment.
-
•
The mechanisms and pathophysiology of WRA and non-WRA are similar. Both can be initiated by immunological responses or irritant exposures, and both can have predominantly eosinophilic or neutrophilic airway inflammatory responses.
-
•
There are important differences in the success of preventing WRA compared with non-WRA. These differences are likely related to the timing and feasibility of preventative interventions. Preventative measures for WRA that require work changes are often associated with negative socio-economic impacts; this is a deterrent to the implementation of preventative measures, especially if they lead to cessation of employment.
-
•
In both WRA and non-WRA, exacerbations can be due to multiple triggers. The severity of the exacerbation depends upon the exposure and the severity of the underlying asthma.
Introduction
Work-related asthma (WRA) is a term that includes occupational asthma (i.e., asthma that is caused by work) and work-exacerbated asthma (WEA) (i.e., asthma that is worsened by work but not initially caused by work) (1) (Figure 1). It has been estimated that at least 15% of adult asthma is work related. Some studies have reported that more than 25% of working adults with asthma have exacerbations of asthma at work.
A Jack Pepys Workshop on WRA has been held every 3 years since 2000, alternating between Toronto, Ontario and Montreal, Quebec, Canada. The format is a series of short presentations, each followed by extensive discussion. The fifth Jack Pepys Workshop was held in Montreal in 2013 and focused on the similarities and differences of WRA and non–work-related asthma (non-WRA).
Methods
Forty-five international experts from various disciplines were invited to the workshop. In addition, there was a small number of self-registered participants. Invitees were selected on the basis of publications in the area of WRA or general asthma. Represented specialties included occupational medicine, pulmonary medicine, and basic science research. Participants disclosed any potential conflicts of interest to the ATS and were managed in accordance with the policies and procedures of the ATS.
Topics for discussion at the workshop were selected by an International Advisory Committee (J.L.M., S.M.T., G.M., D.B., O.V., and P.C.). They included comparisons of WRA and non-WRA in each of the following domains: mechanisms and pathophysiology (J.S. and J.G.M.), epidemiology and risk factors (M.J. and N.L.M.), allergens and irritants (T.P.M. and D.H.), diagnosis and clinical aspects (F.D.B. and G.S.), psychosocial and economic aspects (P.D.B. and O.V.), and outcomes and management (J.L.M. and A.C.). The program was approved by the ATS’s Environmental and Occupational Health Assembly Planning Committee.
After a tribute to Professor Jack Pepys, short presentations were given by internationally acknowledged experts, who briefly reviewed the evidence. Each presentation was followed by extensive discussion. The content of each presentation is available online (www.asthma-workplace.com).
After the workshop, each presenter submitted a summary of his or her presentation and the accompanying discussion. The summaries were collated by the co-chairs (J.L.M. and S.M.T.) into a single workshop report. The presenters and co-chairs composed the writing committee. The full writing committee reviewed and approved the final workshop report.
Mechanisms and Pathophysiology
Occupational asthma (OA) and non-WRA are the result of interactions between environmental factors and host factors (Tables 1 and 2). Environmental factors relevant to OA include the route of exposure (skin or respiratory), the type of causative agent, and the causative agent’s airborne characteristics (particle size, structure, volatility), level (concentration, duration of exposure), and mode (gas or particle). Host factors relevant to OA include sex, atopy, nonspecific bronchial hyperresponsiveness, rhinitis, and genetic susceptibility (3). Potential genetic factors include glutathione S-transferase (GSTP1 and GSTM1) and N-acetyltransferase (4), antioxidant genes (5), catenins (6), and the Th2 response (7).
Table 1.
Aspects of mechanisms and pathophysiology
| Nonoccupational Asthma | Occupational Asthma |
|
|---|---|---|
| Immunological OA | Irritant-induced OA | |
| For some agents/some patients an IgE-dependent response, for others unknown initiating mechanisms. | IgE-dependent response for high-molecular-weight agents; variable or unknown for the rest of agents. | Acute phase: “toxic reaction”; chronic phase: inflammation and remodeling. |
| Th2 lymphocyte mechanism with or without participation of IgE. | Th2 lymphocyte mechanism with or without participation of IgE (low-molecular weight agents). | |
| Th17 activation associated with neutrophilic response. | Th17 activation associated with neutrophilic response. | Oxidative stress, role of neurogenic inflammation (substance P). |
| Inflammation and remodeling. | Inflammation and remodeling. | Mainly remodeling. |
| Genes related to atopy and antigenic recognition. | Genetic variants in antioxidant, catenin-control genes, genes related to Th2 and remodeling responses. Genes related to Th2 response. Genes related to remodeling. For diisocyanates, hypermethylation of IFN-γ gene promoter. | |
| Genes related to the epithelium. | ||
| Genes related to remodeling. | ||
| Non-IgE–related mechanisms are unclear, and hypothetically some may be similar to those of low-molecular-weight sensitizer–induced OA. | For low-molecular-weight agents, some may induce IgE-mediated responses. Others may resemble a delayed hypersensitivity response similar to contact dermatitis (e.g., persulfates). | Role of alarmins. Stimulation of epithelium with production of chemoattractant substances, toll-like receptors. General airway sensory hyperreactivity. |
Definition of abbreviations: OA = occupational asthma.
Similarities are printed in bold. Features that differ or have only been only evaluated for one condition are not bold.
Table 2.
Risk factors for non–work-related and sensitizer-induced occupational asthma*
| Non-WRA | Sensitizer-induced OA |
|---|---|
|
Host Risk Factors | |
| Younger age | Younger age |
| Female | Female (likely from sex distribution of higher risk exposures) |
| Family history of allergy/asthma | Family history of allergy/asthma |
| Atopy | Atopy (for high-molecular-weight agents) |
| Obesity | Obesity |
| Current/previous history of rhinitis or allergy | Current/previous history of work-related rhinitis |
| Previous bronchial hyperresponsiveness | Previous bronchial hyperresponsiveness |
| Some serious childhood respiratory diseases (e.g., cystic fibrosis) | |
|
Behavioral and Social Factors | |
| Active and passive smoking | Active smoking |
| Low household income | Low household income likely from association with greater risk of exposure at work to sensitizers |
|
Environmental Risk Factors | |
| Home environmental factors (e.g., fuel combustion, dampness and mold growth, environmental tobacco smoke) | High-molecular-weight or low-molecular-weight sensitizing agents at work |
| Exposure to cleaning sprays at home | Cleaning/disinfectant agents at work (likely several mechanisms) |
| Air pollution | |
Definition of abbreviations: OA = occupational asthma; WRA = work-related asthma.
Similarities are printed in bold. Features that differ or have only been only evaluated for one condition are not bold.
Risk factors for irritant-induced asthma are not included here because they have not been sufficiently identified due to most case series being relatively small. Table reproduced and modified with permission from Reference 17.
Both OA and non-WRA due to high-molecular-weight agents (i.e., agents that generally contain proteins) have an IgE-mediated mechanism of sensitization. OA due to high-molecular-weight agents is an IgE-mediated response to an occupational allergen, whereas non-WRA can be an IgE-mediated response to an environmental allergen. It is uncertain whether OA due to low-molecular-weight agents (i.e., agents that are usually chemical products) is also IgE mediated (8). Arguing for an IgE-mediated mechanism are its clinical features, pattern of Th2 cytokines, and pathological features and the finding that treatment with anti-IgE humanized antibody was effective in some patients with OA due to low-molecular-weight agents (9). Arguing against an IgE-mediated mechanism are the failure to detect specific IgE antibodies against most of the low-molecular-weight agents and the shorter but overlapping latency periods for acquiring sensitization and disease.
Patients with OA may have an eosinophilic, neutrophilic, or mixed inflammatory cell pattern (10). They may also have cells that secrete both Th2 cytokines (IL-4 and IL-13) and a neutrophil chemoattractant (IL-17) (11). Taken together, these observations suggest that patients with OA may have different phenotypes depending upon the predominant cell pattern and cytokines, similar to patients with non-WRA.
Irritant-induced asthma is a type of OA that is considered nonimmunological. However, like non-WRA, inflammation plays an important pathogenic role. In irritant-induced asthma, inhaled irritants cause inflammation that injures the epithelial cells and other resident cells. The nature and severity of the inflammation depends upon the nature of the exposure, such as its intensity and physical properties (state, vapor pressure, solubility, and chemical reactivity). Secondary inflammation due to the cellular injuries then causes oxidative stress (12). This is supported by studies that showed that antioxidants and neutrophil depletion reduce airway hyperresponsiveness induced by chlorine inhalation (13). Several other mechanisms may also participate in the pathogenesis of irritant-induced asthma: the innate immune system may be activated by alarmins via Toll-like receptors (14); neurogenic inflammation may be promoted by transient receptor potential channels (irritant-sensing ion channels expressed in airway chemosensory nerves), which are activated by local changes in osmolarity and temperature and regulated by cellular redox status; innate lymphoid cells with a Th2 pattern of cytokines may be activated (15); and sensitization to allergens may occur. The end results are pathological findings that resemble a toxic mechanism during the acute phase and sensitizer-induced OA during the long-term phase (16).
Epidemiology and Risk Factors
OA and non-WRA are heterogeneous diseases with multiple phenotypes. Distinguishing phenotypes is important because it may help identify risk factors (17). As an example, adult-onset asthma is a phenotype of both OA and non-WRA that has been described using cluster analysis on sputum cells.
Adult-onset OA and adult-onset non-WRA are more prevalent among female patients (18) (Table 2). The former may be related to the distribution of various types of work across genders (19); women are more likely to be exposed to asthmagens (i.e., a substance that is causally related to asthma symptoms) at work, whereas men are more likely to be exposed to agents that do not cause asthma (17). Additional risk factors for both adult-onset OA and adult-onset non-WRA include a history of upper airway symptoms (20), a history of bronchial hyperresponsiveness (21), and atopy (18). Of note, the effect of atopy on adult-onset OA appears limited to OA due to high-molecular-weight agents. Although environmental exposures have a profound effect on adult-onset OA and childhood-onset non-WRA, they have a less certain effect on adult-onset non-WRA (17, 18).
Asthma prevalence has increased during the past decade in part due to changes in lifestyle (i.e., diet, smoking), socioeconomic status (i.e., income), and environmental factors (17). Regarding environmental factors, the increase in non-WRA prevalence has been attributed to domestic indoor exposures and ambient outdoor exposures, whereas the increase in WRA has been attributed to occupational exposure to cleaning agents (22) and other asthmagens. The most important occupational exposures include sensitizing agents (23), low-level irritants, and pollutants (e.g., diesel combustion fumes, spray cleaning agents). High-risk jobs (e.g., bakery workers, spray painters) and the mode, route, and level of exposure are also important factors (24). New-onset OA is often severe and uncontrolled (25, 26).
Further studies are needed to address the following issues: (1) epidemiological definitions of adult-onset non-WRA asthma; (2) new statistical approaches to identify asthma phenotypes; (3) risk factors for various adult-onset asthma phenotypes, especially with different airway inflammatory cell predominance; (4) the role of diet and obesity as a risk factor for all adult-onset asthma; (5) the frequency of systemic food-related allergic reactions associated with OA (among food processors); (6) novel/more precise approaches in the assessment of environmental exposures; (7) exposure–response relationships for common newly identified exposures in domestic and occupational settings (e.g., cleaning agents and cosmetics); and (8) gene–environment interactions and how these are modulated by exposure level.
Allergens and Irritants
Allergens
Exposure to occupational and nonoccupational allergens varies over time, affecting the prevalence of both OA and non-WRA (Table 3). As examples, (1) the number of people living on farms in Europe decreased 10-fold between 1945 and 1960, resulting in farmers’ lung becoming less common (27), and (2) the levels of dust mites in houses (28) were quantified after the discovery of dust mites in The Netherlands and have decreased 10-fold since 1980 due to public knowledge and resulting interventions.
Table 3.
Etiologic agents: allergens and irritants
| Non-WRA | Sensitizer-Induced OA | Irritant-Induced OA |
|---|---|---|
|
Allergens | ||
| Variable exposure over time. | Variable exposure over time. | |
| Inhalation of allergens on particles. | ||
| Absorption from skin exposure can cause sensitization but not asthma. | Absorption from skin exposure may cause sensitization but not asthma. | |
| Levels of exposure are an important risk factor. | Levels of exposure are an important risk factor. | |
| Highest levels of exposure can lead to decreased sensitivity. | Risk is present even at low levels of exposure to high-molecular-weight and low-molecular-weight agents: a pragmatic approach is to aim for an excess risk below a certain percentage. | |
|
Irritant Exposures Inducing Asthma | ||
| Acute irritant high-level exposure. | Acute irritant high-level exposure. | |
| Recurrent exposures to nonmassive levels. | Recurrent exposures to nonmassive levels. | |
| Long-term low exposures. | Long-term low exposures. | |
| Irritant exposures may have an adjuvant effect, increasing IgE-mediated responses (e.g., diesel exhaust). | Irritant exposures may have an adjuvant effect, increasing IgE-mediated responses (e.g., diesel exhaust). | Irritant exposures may have an adjuvant effect, increasing IgE-mediated responses (e.g., diesel exhaust). |
| Ascertaining exposures can be complex and can vary over time (e.g., cleaning agents). | Ascertaining exposures can be complex and can vary over time (e.g., cleaning agents). | |
| Gene–environment interactions can occur. | Gene–environment interactions can occur. | Gene–environment interactions can occur. |
|
Allergens and Irritants | ||
| Exposure controls may reduce risks of asthma onset and exacerbations. | Exposure controls may reduce risks of asthma onset and exacerbations. | Exposure controls may reduce risks of asthma onset and exacerbations. |
Definition of abbreviations: OA = occupational asthma; WRA = work-related asthma.
Similarities are printed in bold. Features that differ or have only been only evaluated for one condition are not bold.
Allergens have several components that are inhaled on particles. As an example, during tidal breathing, 5 to 10% of the fecal particles of mites reach the lung, which include mite DNA, bacterial DNA, endotoxins, chitins, Der p1, Der p2, and some other components. The quantity of particles inhaled, the particle size, and coexposures on particles are all important to the development of OA and non-WRA (29).
Absorption of allergens can also occur from skin exposure. As an example, very high levels of specific IgE to peanuts can be driven by skin exposure, and park rangers with tick bites can become allergic to an oligosaccharide, α-gal, resulting in a cross-reacting allergy to ingested meat. However, such sensitization without respiratory exposure is not related to asthma, suggesting that allergens must be inhaled to inflame the bronchi (30).
Overall levels of exposure to allergens appear to be the most important factor that contributes to the risk of sensitization (31). Reducing exposure is expected to reduce sensitization, although for most allergens there is still a small residual risk at low levels of exposure. A pragmatic approach is to aim for an associated risk below a certain percentage (the acceptable risk has been set at 1% in The Netherlands for standard setting purposes) of those exposed in occupational settings. A similar approach is reasonable for other allergens (e.g., dust mites). The highest levels of exposure may lead to decreased sensitivity (32), and owning a cat in early life does not appear to increase the risk of asthma among children (33). However, experience suggests that allowing individuals to remain exposed to high levels of allergens and hoping for decreased sensitization is not a prudent approach. In the occupational setting, the healthy worker effect may modify the exposure–risk relation.
Irritants
Irritants have been associated with the development of OA (i.e., irritant-induced asthma), WEA, and exacerbations of non-WRA. Each of these entities can occur at various levels of exposure. As an example, irritant-induced asthma may follow a single high-level exposure; recurrent exposures at lower levels; or long-term, low-level exposures (34). The propensity of irritants to cause asthma exacerbations reflects the observation that individuals with preexisting asthma react at lower levels to certain irritants (e.g., sulfur dioxide) compared with individuals without asthma.
There are many similarities in the clinical presentations of occupational irritant-induced asthma and WEA. For compensation purposes, the main challenge is to demonstrate that the asthma was caused by an occupational exposure. This begins with an extensive history to determine potential irritants in the work environment. This can be challenging because irritant exposures tend to change over time or location (e.g., chemicals in swimming pools, cosmetic use, volatile irritants) and because workers with WEA tend to have more exposures to ammonia, engine exhaust combustion by-products, aerosols, solvents, and other irritants than workers without WEA (35). Inhalation challenges can also be performed (36). However, although a careful history and inhalation challenges may support a relationship between the exposure and the asthma symptoms, neither a careful history nor inhalation challenges are capable of distinguishing irritant-induced asthma from WEA. Further complicating evaluations for compensation, there are different definitions of occupational irritant-induced asthma and WEA that are used by various medicolegal and compensation agencies, different requirements for compensation, and different extents of support (37).
Cleaning agents are among the most common irritants associated with irritant-induced asthma, WEA, and non-WRA exacerbations (22). However, it can be difficult to establish a causal relationship because the complexity of exposure is substantial (38). Cleaning agents contain mixtures of allergens and irritants, gene–environment interactions have been postulated (39), and exposure to irritants may enhance sensitization to common allergens due to an adjuvant effect (40). Research has failed to clarify many questions because the association of cleaning agent exposure with prior atopic sensitization is inconsistent (41) and because studies of individual exposures from different types of cleaning agents (42) have been limited by too few events per condition due to the vast number of chemicals.
The long-term prognosis of occupational irritant-induced asthma is similar to that of immunological OA (43). Initiatives are needed to improve education and labeling of chemical agents (particularly cleaning products) (22), and further research is needed to address many unanswered questions.
Diagnosis and Clinical Aspects
Nosological Aspects
WRA is typically divided into OA and WEA, with WEA arguably being more severe according to one study (35) (although the study may have been flawed by selection bias in the enrollment of patients with WEA [44]). OA can be further divided into OA due to high-molecular-weight agents or OA due to low-molecular-weight agents. There are no symptoms that definitively differentiate OA due to high-molecular-weight agents from OA due to low-molecular-weight agents (45). However, wheezing, nasal itching, and/or eye itching that are worse at work are more often seen in confirmed OA due to high-molecular-weight agents, whereas hoarseness at work is less common compared with suspected but unconfirmed cases. WRA can alternatively be divided according to triggers, disease severity, or type of inflammation, similar to non-WRA (46). As an example, WRA can be divided according to triggers as irritant-induced or irritant-exacerbated asthma, or sensitizer-induced asthma (47). Some circumstances expose an individual to irritants and sensitizers simultaneously, such as endotoxin exposures in animal exposures and dust exposures in bakery workers. Overlap between non-WRA and COPD has been demonstrated (48); however, it is unknown whether similar overlap exists between OA and COPD.
Asthma can also be classified by cell predominance. OA and non-WRA are more often eosinophilic than neutrophilic, according to studies that examined induced sputum (49). In OA, neutrophilic inflammation is associated with a poor prognosis (35), although the level of neutrophils that define this poor prognostic phenotype is unknown (50). In non-WRA, patients with neutrophilic inflammation are distinct and their symptoms and signs are more severe than patients with eosinophilic inflammation (51). Rhinitis contributes to the severity of asthma, including both OA and non-WRA (52) (Table 4).
Table 4.
Diagnosis and clinical aspects*
| Non-WRA | Sensitizer-induced OA |
|---|---|
|
Asthma Severity | |
| Greater severity in those with associated rhinitis | Greater severity in those with associated rhinitis |
| Neutrophilic vs. eosinophilic airway inflammation associated with increased severity, worse lung function, less steroid responsiveness, but not related to nonspecific airway hyperresponsiveness. | Neutrophilic vs. eosinophilic airway inflammation associated with OA due to low-molecular-weight agents, in a noneosinophilic variant of OA and WEA, and with a poor prognosis. |
|
Overlap with Chronic Obstructive Pulmonary Disease | |
| ∼10% overlap with chronic obstructive pulmonary disease. | Overlap to be evaluated in epidemiological studies of occupational chronic obstructive pulmonary disease. |
|
Symptoms | |
| Work-related wheeze, nose, eye itching, and lack of hoarseness are the most sensitive symptoms in cases of OA due to high-molecular-weight agents. | |
|
Diagnostic Tests | |
| Mannitol less sensitive than methacholine for challenges. | Mannitol challenge may differentiate subjects according to severity of disease. |
| FeNO is a surrogate marker of eosinophilic inflammation in asthma (rapid and less invasive). | FeNO may be useful in the interpretation of specific inhalation challenges in subjects who cannot produce satisfactory sputum samples. |
| Assessment of mediators in induced sputum can show aspects of airway inflammation and remodeling. | |
| Specific bronchial challenges are seldom used in non-WRA. | Specific bronchial challenges are reference tests for diagnosing OA, although not used in many centers. |
Definition of abbreviations: OA = occupational asthma; WRA = work-related asthma.
Similarities printed in bold. Features that differ or have only been only evaluated for one condition are not bold.
Irritant-induced asthma is not included here because these factors cannot be compared at present.
Methods of Diagnosis
A specific inhalation challenge (i.e., the subject inhales the agent of concern) is the reference standard for diagnosing sensitizer-induced OA. It is relatively safe (the most severe reactions can occur when challenging subjects with a low-molecular-weight agent [53]), but it is available in only a few centers, and false-positive or false-negative responses are common (54). Specific inhalation challenges may also be considered in the diagnosis of non-WRA (e.g., to identify the clinical relevance of dust mite sensitization) (55).
Bronchial hyperresponsiveness may be confirmed by direct challenges (e.g., with methacholine or histamine) or by indirect challenges (e.g., exercise, hypertonic saline, adenosine monophosphate, or mannitol) in the context of suspected WRA or non-WRA. Mannitol has been compared with methacholine (56) and appears to identify patients with more severe WRA (57). Airway inflammation is typically detected by induced sputum (49); however, it is time consuming and of limited availability. Different aspects of airway inflammation and remodeling may be assessed by the detection of mediators in the supernatant of induced sputum in non-WRA, but few similar data are available in WRA (58). The validity of the test has been questioned due to the possibility that sputum induction itself, or subsequent sputum processing, may activate airway inflammatory cells.
Exhaled nitric oxide (FeNO) is an alternative test used to evaluate airway inflammation. In non-WRA, an increase in FeNO is a surrogate of eosinophilic inflammation (59). In WRA, an increase in FeNO upon exposure to the suspected work trigger supports a diagnosis of WRA (49). However, there are conflicting data on what changes in FeNO over time are abnormal, and FeNO has been considered less discriminating than induced sputum for WRA (58).
Psychosocial and Economic Impacts
The potential psychosocial and economic impacts of sensitizer-induced OA and adult non-WRA are similar (Table 5). Regarding psychosocial outcomes, patients may experience disability, impaired health-related quality of life, and psychological morbidity. Employment disability is particularly important in WRA and can be quantified with multiple metrics beyond lost work days. These metrics include complete job loss, change in job duties, decreased productivity (negatively affected “presenteeism”), and early retirement. Assessment of health-related quality of life can be performed with generic or respiratory disease–specific instruments. The most studied psychological impact of asthma generally has been depression, which can be based on self-report or ascertained through clinical assessment.
Table 5.
Psychosocial and economic impacts*
| Non-WRA | Sensitizer-induced OA |
|---|---|
| Employment disability assessed by complete job loss, change in job duties, decreased productivity (presenteeism) and early retirement. | Employment disability assessed by complete job loss, change in job duties, decreased productivity (presenteeism) and early retirement. |
| Lost work days are common in asthma and increase with poorer control of disease. | Association with lower asthma-related quality of life, taking other factors into account. |
| Subjects with exposure to gas, dust, or fumes on the job are twice as likely to experience job loss. | WRA is associated with more frequent self-rated poor health. |
| Importance of anxiety as well as depression in the overall psychological morbidity of asthma. | Anxiety and depression common. |
| Direct health care costs increase with increasing severity and lower control of the disease. | Even higher direct healthcare utilization (i.e., visits to doctors and emergency departments as well as hospitalization). |
| Indirect health costs account for about 50% of the total asthma costs. | Higher indirect health costs, which account for up to 90% of the total asthma costs. |
| Rhinitis results in job loss intermediate between asthma and nonasthma referents. | Incremental impact of occupational rhinitis insufficiently examined. |
Definition of abbreviations: OA = occupational asthma; WRA = work-related asthma.
Similarities are printed in bold. Features that differ or have only been only evaluated for one condition are not bold.
Irritant-induced asthma is not included here because these factors cannot be compared at present.
WRA may play a significant role in work loss. Lost work days are twice as likely to occur in those with exposure to vapors, gas, dust, or fumes on the job compared with those without such exposures. Individuals with such exposures are also twice as likely to experience job loss (odds ratio, 1.96) according to a study that used data from the European Respiratory Health Survey (60). Non-WRA also contributes to work loss according to a large population-based study in Europe that found that individuals with poor asthma control were less likely to be employed (49 vs. 60%), more likely to have missed work (10 vs. 7%), and more likely to have experienced impairment (33 vs. 18%). The magnitude of these effects was similar to that seen in diabetes (61). These findings in non-WRA were supported by a comparison of patients with asthma and subjects without asthma in Finland that found that patients with asthma had nearly double the risk of long-term work disability (odds ratio, 1.8); this risk doubled with concomitant depression (62). Direct comparison of WRA with non-WRA found that WRA was associated with more frequent self-rated poor health (40 vs. 22%), impaired physical health (32 vs. 16%), and impaired mental health (26 vs. 14%) (63).
The economic impact of WRA and non-WRA needs to be considered in terms of both direct and indirect health care costs. Direct and indirect health care costs are each estimated to account for approximately 50% of combined WRA and non-WRA total costs (64). Work exposures increase asthma severity (26) and asthma exacerbations, which increase the relative burden of indirect health care costs (65). In the United Kingdom, it was estimated that indirect and direct health care costs account for 90 and 10%, respectively, of the asthma-related costs of patients with OA. Nearly half of the costs were paid out-of-pocket, and only 3% of the costs were paid by employers (66). Data from Canada further confirm that income loss is a major factor in the costs of OA (67). The pattern of indirect costs is likely to be similar in WEA but has been less well studied.
It is important to recognize that the psychosocial and economic consequences of WRA and non-WRA are difficult to estimate because there are methodological challenges and related data limitations. For example, the interpretation of data on economic costs is complicated by differences in analytic approaches internationally and even within countries (68); there are limited asthma-specific quality of life data due, in part, to the inclusion of work-disability as a domain in some questionnaires; the potential importance of occupational rhinitis as an early indicator of disease has been understudied; there has been lack of “cross-talk” (e.g., using comparable assessment tools) among researchers studying psychosocial and economic outcomes in work-related dermatitis and those investigating the same in asthma; and there is a paucity of data regarding the importance of anxiety and depression on psychological morbidity in adult asthma.
Outcomes and Management
Outcomes
Childhood-onset asthma symptoms disappear or improve in the majority of cases (∼75%) (69) (Table 6). Symptomatic resolution of childhood asthma is associated with milder asthma, lack of sensitization or exposure to indoor allergens, higher prebronchodilator FEV1, and less airway hyperresponsiveness (70). Despite the improvement of symptoms, persistence of exposure to allergens results in an accelerated (i.e., more than the normal predicted) decline in the FEV1 that is associated with patient age and duration of asthma (71). In contrast, allergen avoidance leads to an improvement in lung function. Adult-onset non-WRA probably has a worse outcome than childhood-onset asthma because nonatopic asthma (previously called intrinsic asthma), which starts around age 40 to 50 years particularly in women, is more difficult to treat (72).
Table 6.
Outcome and management
| Non-WRA | Occupational Asthma |
|---|---|
|
Medical Outcomes | |
| Typical outcome of childhood onset asthma during transition to adult life: | Typical outcome of OA after removal from exposure: |
| Improvement (or disappearance) of symptoms | Improvement (or disappearance) of symptoms |
| Persistence of airway hyperresponsiveness | Persistence of airway hyperresponsiveness |
| Persistence of airway inflammation | Persistence of airway inflammation with more remodeling for IIA |
| Lesser response to bronchodilator for IIA | |
|
Factors Associated with a Worse Outcome | |
| More severe asthma, exposure or sensitization to indoor allergens, smaller airway caliber, more airway responsiveness, lack of allergen avoidance, older age, longer duration of asthma. | Long duration of symptomatic exposure, severe asthma at the time of removal from exposure, persistence of eosinophilic airway inflammation 10 yr or more after removal, low IFN-γ levels. |
| Adult-onset asthma: probably worse outcome compared with childhood-onset asthma or OA. | Probably better outcomes than adult-onset nonoccupational asthma. |
|
Management | |
| Proper confirmation of diagnosis. | Proper confirmation of diagnosis. |
| Identification of comorbidities. | Identification of comorbidities. |
| Verification of drug compliance and inhaler technique. | Verification of drug compliance and inhaler technique. |
| Education and action plans. | Education and action plans. |
| Monitoring of pulmonary function, and, possibly, airway responsiveness and inflammation. | Monitoring of pulmonary function, and, possibly, airway responsiveness and inflammation. |
| Elimination of work sensitizer and work irritants. | |
| Medical surveillance of other exposed workers. | |
| Use of predictive models to identify those who would benefit from closer surveillance for sensitizer-induced OA. | |
| Tertiary prevention should focus on accurate diagnosis: questionnaire is an inadequate means for sensitizer-induced OA. | |
| Once the diagnosis is made, emphasis on satisfactory work reentry programs. | |
| Assess the level of control. | Assess the level of control. |
| Treat as per guidelines (GINA). | Treat as for non-WRA. |
| Ensure sociopsychological and financial support. | |
Definition of abbreviations: IIA = irritant-induced asthma; OA = occupational asthma; WRA = work-related asthma.
Similarities are printed in bold. Features that differ or have only been only evaluated for one condition are not bold.
Outcomes due to OA are based on studies of sensitizer-induced OA, a type of allergic WRA that provides the unique opportunity to examine the patient before, during, and after the exposure (21). After removal from the exposure, symptoms typically improve, but there is persistence of airway hyperresponsiveness in most workers (73). Eosinophilic inflammation and structural changes may exist up to 10 years or more after ending the exposure in sensitizer-induced OA (74). Irritant-induced OA is also associated with improvement of symptoms but often has persistence of airway hyperresponsiveness following removal of the exposure.
When adult-onset non-WRA and sensitizer-induced OA are compared, the frequency of improvement is much higher among adults with sensitizer-induced OA (complete symptomatic recovery of 32%) (75) than among individuals with adult-onset non-WRA (complete symptomatic recovery of only 5–6%) (76). Factors associated with a poor outcome from sensitizer-induced OA are a long duration of symptomatic exposure and severe asthma at the time of removal from exposure (77). In the longest follow-up after cessation of exposure (17 yr), higher IFN-γ levels were associated with greater improvement (78). Structural changes may be more significant among individuals with occupational irritant-induced asthma than among those with sensitizer-induced OA or non-WRA (43).
Management
For non-WRA, typical management consists of (1) confirming the diagnosis; (2) identifying comorbidities (e.g., gastroesophageal reflux, rhinitis, and smoking); (3) verifying adherence with medication regimens and good inhaler technique; (4) controlling environmental triggers with allergen avoidance, often with difficulty due to multiple sensitization; and (5) providing education and action plans. Improving nonspecific bronchial hyperresponsiveness reduces mild exacerbations and improves FEV1 but may require higher doses of inhaled steroids (79). Monitoring induced sputum for eosinophilic inflammation and then intervening to minimize it reduces asthma exacerbations without requiring higher doses of inhaled steroids (80); monitoring FeNO does not have these same effects (81).
In contrast to non-WRA, optimal management of OA consists of primary prevention (i.e., eliminating or reducing exposures to prevent the development of OA) (31). It consists of environmental control and medical surveillance and is considered the best opportunity to reduce the incidence of OA (82). A self-administered questionnaire has been developed to facilitate screening (83). Prediction models for OA, work-related symptoms, and occupational sensitization have been proposed, which aim to identify those who would most benefit from closer surveillance (84).
Tertiary prevention consists of obtaining an accurate diagnosis and then removing individuals with confirmed OA from exposure. Regarding an accurate diagnosis, the medical history should be complemented by objective testing because the medical history alone is insufficient to diagnose OA. The value of FeNO in this context is controversial. Regarding removing individuals with confirmed OA from exposure, reduced exposure might be acceptable if the socioeconomic situation precludes total avoidance (85), but complete removal from exposure is preferable, particularly for individuals with immunological OA. A work reentry program has resulted in a reasonable percentage of unemployment (20%) 2 years after diagnosis (67). For WEA, workers who are unable to continue with the same employer are offered less support than those with OA, and WEA is often not accepted by workers’ compensation systems (86). The delay for referral is still much too long. The mean and median duration of symptoms before diagnosis of immunological OA are nearly 4 (87) and 1.4 years (88), respectively, in Quebec, Canada, which has one of the better assessment systems.
Pharmacologic and Other Treatment
Treatment of both WRA and non-WRA begins with a proper assessment of the level of control per guidelines (e.g., the Global Initiative for Asthma [GINA] guidelines). Preferred pharmacological therapies include inhaled bronchodilators and inhaled corticosteroids (89); for those failing such measures, additional possibilities include immunotherapy (if satisfactory extracts of specific occupational agents become available), anti-IgE therapy, and bronchial thermoplasty. Mood disorders are common, and treatment should be considered (88). Studies of anti–IL-5 and anti–IL-13 may provide insights for future therapeutic options. Social, psychological, and financial support should be offered.
Conclusions
WRA shares many features with non-WRA and can be considered a subtype of asthma. Non-WRA commonly starts in childhood but can begin at any age in adults. OA can also start at any age during working life. OA can be caused by occupational sensitizers (similar to environmental allergens in non-WRA) or irritant exposures (similar to irritant exposures that cause asthma in the nonoccupational setting). In those with asthma, exacerbations can occur from work-related exposures (i.e., WEA) or non–work-related exposures. Exposures causing WEA are common, occurring in 25% or more of working people with asthma.
The pathophysiology of WRA and non-WRA are similar but need further investigation. OA caused by sensitizers is a useful condition to study because the initiating agent can often be identified and because the natural history with and without subsequent exposure and treatment can be more readily assessed than in non-WRA. The potential for loss of working days and for significant socio-economic adverse effects is greater in WRA than in non-WRA. However, the potential for primary prevention is also greater.
Further research is needed to measure the potential for new chemicals to cause OA and to minimize adverse outcomes due to OA or WEA. Better early markers of sensitization to occupational agents are also needed. Irritant-induced asthma warrants further study, especially if caused by nonmassive exposures, because it may serve as a useful model to understand the effects of various exposures that occur in domestic or occupational settings.
Acknowledgments
Acknowledgment
The co-chairs express their deepest gratitude to the additional members of the Scientific Committee (Gianna Moscato, David Bernstein, Paul Cullinan, Olivier Vandenplas). They also thank the Ontario Workplace Safety and Insurance Board, the Commission de la santé et sécurité du travail du Québec and the Institut de recherche Robert-Sauvé en santé et sécurité du travail, the Fonds de recherche Québec-Santé (Réseau en santé respiratoire and Réseau de Recherche en santé et sécurité du travail), the Center for Asthma in the Workplace, the Centre for Research Excellence in Occupational Diseases, and the American Thoracic Society, for their support of this as a project of the Environmental and Occupational Health Assembly and Dr. Kevin Wilson, ATS Documents Editor, for his extremely helpful editing of this document.
This Workshop Report was prepared by an ad hoc committee of the ATS Assembly on Environmental, Occupational, and Population Health
Members of the writing committee:
Jean-Luc Malo, M.D.
Susan M. Tarlo, M.B., B.S.
Joaquin Sastre, M.D., Ph.D.
James Martin, M.D.
Mohamed F. Jeebhay, M.B., Ch.B., Ph.D.
Nicole Le Moual, Ph.D.
Dick Heederik, Ph.D.
Thomas Platts-Mills, M.D., Ph.D.
Paul D. Blanc, M.D., M.S.P.H.
Olivier Vandenplas, M.D.
Gianna Moscato, M.D.
Frédéric de Blay, M.D.
André Cartier, M.D.
List of speakers, discussants, and other formal attendees:
J. Beach, M. Becklake, D. Bernstein, P. D. Blanc, P.S. Burge, C. Carlsten, R. Castano, A. Cartier, M. Cruz, F. de Blay, E. Fixman, I. Folletti, D. Gautrin, P. Harber, D. Heederik, P. Henneberger, D.L. Holness, R. Hoy, M. S. Jaakkola, M. F. Jeebhay, M. Labrecque, A. M. Lauzon, C. Lemière, G. M. Liss, D. Lougheed, P. Maestrelli, J.-L. Malo, J. G. Martin, A. McIvor, D. Miedinger, R. Merget, G. Moscato, X. Munoz, T. A. Platts-Mills, M. Raulf-Heimsoth, C. Redlich, M. Ribeiro, J. Sastre, T. Sigsgaard, A. Siracusa, E. Suarthana, S. M. Tarlo, O. Vandenplas, A. Wisnewski
List of additional participants:
S. Aubin, P. Auger, M. Baillargeon, M. Barrette, C. Beauregard, S. D. Betschel, R. Boileau, N. Bourdeau, P. Brown, S. Chaboilez, Y. Cloutier, M. Dansereau, M. Debia, L. DeGuire, G. Denis, J. Dumont, M. N. Fernandez, L. Fontaine, N. Germain-Lacroix, P. Gomez, M. Isler, I. Kudla, L. Jacques, F. Lussier, N. Murgia, S. Paquette, L. Patry, P. Phenix, B. Pouliot, F. Sava, A. A. Simard, M. Soligon, A. Thomson, C. Trudeau, D. Vizcaya
All Workshop participants with degrees and affiliations:
S. Aubin, M.Sc., IRSST, Montréal, Québec, Canada
P. Auger, M.D., Québec, Canada
M. Baillargeon, Montréal, Québec, Canada
M. Barrette, M.D., Boucherville, Québec, Canada
C. Beauregard, Boucherville, Québec, Canada
J. Beach, M.B., B.S., M.D., University of Alberta, Edmonton, Alberta, Canada
M. Becklake, M.D., McGill University, Montréal, Québec, Canada
D. I. Bernstein, M.D., University of Cincinnati, Cincinnati, Ohio
S. Betschel, M.D., University of Toronto, Toronto, Ontario, Canada
P. D. Blanc, M.D., M.S.P.H., University of California San Francisco, San Francisco, California
R. Boileau, M.D., University de Sherbrooke, Sherbrooke, Québec, Canada
N. Bourdeau, St-Eustache, Québec, Canada
P. Brown, M.D., Gatineau, Québec, Canada
P. S. Burge, M.B., B.S., University of Birmingham, Birmingham, United Kingdom
C. Carlsten, M.D., University of British Columbia, Vancouver, British Columbia, Canada
A. Cartier, M.D., Université de Montréal, Montréal, Québec, Canada
R. Castano, M.D., Université de Montréal, Montréal, Québec, Canada
S. Chaboillez, Hôpital du Sacré-Coeur de Montréal, Montréal, Québec, Canada
Y. Cloutier, B.Eng., IRSST, Montreal, Québec, Canada
M. Cruz, M.D., Servei de Pneumologia Hospital Vall d'Hebron, Barcelona, Spain
M. Dansereau, St-Jérôme, Québec, Canada
M. Debia, Hôpital du Sacré-Coeur de Montréal, Montréal, Québec, Canada
F. de Blay, M.D., University of Strasbourg, Strasbourg, France
L. DeGuire, M.D., Institut national de santé publique du Québec, Montréal, Québec, Canada
G. Denis, M.D., CSSS de la Pointe-de-l’Ile, Montréal, Québec, Canada
J. Dumont, CSST, Montréal, Québec, Canada
M. M. Fernandez, M.D., Fundación Jimenez Diaz, Madrid, Spain
E. Fixman, Ph.D., McGill University, Montréal, Québec, Canada
L. Fontaine, CSST, Montréal, Québec, Canada
I. Folletti, M.D., University of Perugia, Perugia, Italy
D. Gautrin, Ph.D., Hôpital du Sacré-Coeur de Montréal, Montréal, Québec, Canada
N. Germain-Lacroix, M.D., Val d’Or, Québec, Canada
P. Gomez, St Michael's Hospital, Toronto, Ontario, Canada
P. Harber, M.D., UCLA, Los Angeles, California
D. Heederik, Ph.D., Institute for Risk Assessment Sciences, Utrecht University, Utrecht, The Netherlands
P. K. Henneberger, M.P.H. Sc.D., NIOSH, Atlanta, Georgia
D.L. Holness, M.D., M.H.Sc., University of Toronto, Toronto, Ontario, Canada
R. Hoy, M.B., B.S., University of Melbourne, Melbourne, Australia
M. Isler, M.D., Institut national de santé publique du Québec, Montréal, Québec, Canada
M. S. Jaakkola, M.D., Ph.D., University of Oulu, Oulu, Finland
L. Jacques, M.D., Direction de la santé publique, Montréal, Québec, Canada
M. F. Jeebhay, M.B., Ch.B., Ph.D., University of Cape Town, Cape Town, South Africa
I. Kudla, H.B.Sc., M.H.Sc., C.I.H., St Michael's Hospital, Toronto, Ontario, Canada
M. Labrecque, M.D., M.Sc., Université de Montréal, Montréal, Québec, Canada
A. M. Lauzon, Ph.D., McGill University, Montréal, Québec, Canada
C. Lemière, M.D., M.Sc., Université de Montréal, Montréal, Québec, Canada
N. Le Moual, Ph.D., Inserm CESP U1018, Université Paris Sud 11, Villejuif, France
G. M. Liss, M.D., University of Toronto, Toronto, Ontario, Canada
D. Lougheed, M.D., Queens University, Kingston, Ontario, Canada
F. Lussier, DSP de Lanaudière, Joliette, Québec, Canada
P. Maestrelli, M.D., University of Padova, Padova, Italy
J. L. Malo, M.D., Université de Montréal, Québec, Canada
J. G. Martin, M.D., McGill University, Montréal, Québec, Canada
A. McIvor, M.D., McMaster University, Hamilton, Ontario, Canada
D. Meidinger, M.D., Lucerne, Switzerland
R. Merget, M.D., University of Bochum, Bochum, Germany
G. Moscato, M.D., University of Pavia, Pavia, Italy
X. Muñoz, M.D., Ph.D., Servei de Pneumologia Hospital Vall d'Hebron, Barcelona, Spain
N. Murgia, M.D., Sezione di Medicina del Lavoro, Perugia, Italy
S. Paquette, St-Eustache, Québec, Canada
L. Patry, M.D., Direction de la santé publique, Montréal, Québec, Canada
L. Perfetti, M.D., Fondazione Salvatore Maugeri IRCCS Servizio Autonomo di Allergologia e Immunologia Clinica Centro di Riferimento Regione Lombardia, Italy
P. Phénix, M.D., Direction de la santé publique, Montréal, Québec, Canada
T. A. Platts-Mills, M.D., Ph.D., University of Virginia, Charlottesville, Virginia
B. Pouliot, M.D., Direction de la santé au travail, Rivière-du-Loup, Québec, Canada
M. Raulf, Ph.D., Institut für Prävention und Arbeitsmedizin der Deutschen Gesetzlichen Unfallversicherung Institut der Ruhr-Universität Bochum (IPA), Bochum, Germany
C. Redlich, M.D., M.P.H., Yale University, New Haven, Connecticut
M. Ribeiro, M.D., Ph.D., University of Toronto, Toronto, Ontario, Canada
J. Sastre, M.D., Ph.D., Fundacion Jimenez Diaz, University of Madrid, Madrid, Spain
F. Sava, M.D., Montréal, Québec, Canada
T. Sigsgaard, M.D., Ph.D., Aarhus University, Aarhus, Denmark
A. A. Simard, Boucherville, Québec, Canada
A. Siracusa, M.D., Ph.D., University of Perugia, Perugia, Italy
M. Soligon, Hôpital du Sacré-Coeur de Montréal, Montréal, Québec, Canada
E. Suarthana, Ph.D., Hôpital du Sacré-Coeur de Montréal, Montréal, Québec, Canada
S. M. Tarlo, M.B., B.S., University of Toronto, Toronto, Ontario, Canada
A. M. S. Thompson, M.D., University of Toronto, Toronto, Ontario, Canada
C. Trudeau, Hôpital du Sacré-Coeur de Montréal, Montréal, Québec, Canada
O. Vandenplas, M.D., Université de Louvain, Louvain-La-Neuve, Belgium
D. Vizcaya, Ph.D., Université de Montréal, Montréal, Québec, Canada
A. Wisnewski, Ph.D., Yale University, New Haven, Connecticut
Footnotes
Author disclosures: J.S. was a consultant for FAES Farma, Genentech, GlaxoSmithKline, Merck, Novartis, Roche, Sanofi, Schering-Plough, and Thermo Fisher Scientific. He was a speaker for FAES Farma, GlaxoSmithKline, Novartis, Stallergenes and UCB, and received research support from ALK-Abelló, GlaxoSmithKline and Thermo Fisher Scientific. F.d.B. was a consultant for ALK-Abelló, Mundipharma, Novartis, Stallergenes. He served on advisory committees of ALK-Abelló, Mundipharma, Novartis, Same and Stallergenes, and participated in review activities of ALK-Abelló and Stallergenes. He received research support from Chiesi and Stallergenes. A.C. was a consultant for Merck and a speaker for Merck, Novartis and Takeda. He received travel support from Merck and Novartis. J.-L.M., S.M.T., J.M., M.F.J., N.L.M., D.H., T.P.-M., P.D.B., O.V. and G.M. reported no relevant commercial interests.
References
- 1.Tarlo SM, Lemiere C. Occupational asthma. N Engl J Med. 2014;370:640–649. doi: 10.1056/NEJMra1301758. [DOI] [PubMed] [Google Scholar]
- 2.Chan-Yeung M, Malo JL, Tarlo SM, Bernstein L, Gautrin D, Mapp C, Newman-Taylor A, Swanson MC, Perrault G, Jaques L, et al. American Thoracic Society. Proceedings of the first Jack Pepys Occupational Asthma Symposium. Am J Respir Crit Care Med. 2003;167:450–471. doi: 10.1164/rccm.167.3.450. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein DI. Genetics of occupational asthma. Curr Opin Allergy Clin Immunol. 2011;11:86–89. doi: 10.1097/ACI.0b013e3283449fc9. [DOI] [PubMed] [Google Scholar]
- 4.Mapp CE, Fryer AA, De Marzo N, Pozzato V, Padoan M, Boschetto P, Strange RC, Hemmingsen A, Spiteri MA. Glutathione S-transferase GSTP1 is a susceptibility gene for occupational asthma induced by isocyanates. J Allergy Clin Immunol. 2002;109:867–872. doi: 10.1067/mai.2002.123234. [DOI] [PubMed] [Google Scholar]
- 5.Yucesoy B, Johnson VJ, Lummus ZL, Kissling GE, Fluharty K, Gautrin D, Malo JL, Cartier A, Boulet LP, Sastre J, et al. Genetic variants in antioxidant genes are associated with diisocyanate-induced asthma. Toxicol Sci. 2012;129:166–173. doi: 10.1093/toxsci/kfs183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SH, Cho BY, Park CS, Shin ES, Cho EY, Yang EM, Kim CW, Hong CS, Lee JE, Park HS. Alpha-T-catenin (CTNNA3) gene was identified as a risk variant for toluene diisocyanate-induced asthma by genome-wide association analysis. Clin Exp Allergy. 2009;39:203–212. doi: 10.1111/j.1365-2222.2008.03117.x. [DOI] [PubMed] [Google Scholar]
- 7.Bernstein DI, Wang N, Campo P, Chakraborty R, Smith A, Cartier A, Boulet LP, Malo JL, Yucesoy B, Luster M, et al. Diisocyanate asthma and gene-environment interactions with IL4RA, CD-14, and IL-13 genes. Ann Allergy Asthma Immunol. 2006;97:800–806. doi: 10.1016/S1081-1206(10)60972-6. [DOI] [PubMed] [Google Scholar]
- 8.Maestrelli P, Boschetto P, Fabbri LM, Mapp CE. Mechanisms of occupational asthma. J Allergy Clin Immunol. 2009;123:531–542, quiz 543–544. doi: 10.1016/j.jaci.2009.01.057. [DOI] [PubMed] [Google Scholar]
- 9.Lavaud F, Bonniaud P, Dalphin JC, Leroyer C, Muller D, Tannous R, Mangiapan G, De Blay F. Usefulness of omalizumab in ten patients with severe occupational asthma. Allergy. 2013;68:813–815. doi: 10.1111/all.12149. [DOI] [PubMed] [Google Scholar]
- 10.Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006;11:54–61. doi: 10.1111/j.1440-1843.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- 11.Cosmi L, Liotta F, Maggi E, Romagnani S, Annunziato F. Th17 cells: new players in asthma pathogenesis. Allergy. 2011;66:989–998. doi: 10.1111/j.1398-9995.2011.02576.x. [DOI] [PubMed] [Google Scholar]
- 12.Auerbach A, Hernandez ML. The effect of environmental oxidative stress on airway inflammation. Curr Opin Allergy Clin Immunol. 2012;12:133–139. doi: 10.1097/ACI.0b013e32835113d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGovern T, Day BJ, White CW, Powell WS, Martin JG. AEOL10150: a novel therapeutic for rescue treatment after toxic gas lung injury. Free Radic Biol Med. 2011;50:602–608. doi: 10.1016/j.freeradbiomed.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massin N, Hecht G, Ambroise D, Héry M, Toamain JP, Hubert G, Dorotte M, Bianchi B. Respiratory symptoms and bronchial responsiveness among cleaning and disinfecting workers in the food industry. Occup Environ Med. 2007;64:75–81. doi: 10.1136/oem.2005.026203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang YJ, DeKruyff RH, Umetsu DT. The role of type 2 innate lymphoid cells in asthma. J Leukoc Biol. 2013;94:933–940. doi: 10.1189/jlb.0313127. [DOI] [PubMed] [Google Scholar]
- 16.Lemière C, Malo JL, Boutet M. Reactive airways dysfunction syndrome due to chlorine: sequential bronchial biopsies and functional assessment. Eur Respir J. 1997;10:241–244. doi: 10.1183/09031936.97.10010241. [DOI] [PubMed] [Google Scholar]
- 17.Jeebhay MF, Ngajilo D, le Moual N. Risk factors for nonwork-related adult-onset asthma and occupational asthma: a comparative review. Curr Opin Allergy Clin Immunol. 2014;14:84–94. doi: 10.1097/ACI.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 18.Antó JM, Sunyer J, Basagaña X, Garcia-Esteban R, Cerveri I, de Marco R, Heinrich J, Janson C, Jarvis D, Kogevinas M, et al. Risk factors of new-onset asthma in adults: a population-based international cohort study. Allergy. 2010;65:1021–1030. doi: 10.1111/j.1398-9995.2009.02301.x. [DOI] [PubMed] [Google Scholar]
- 19.Dimich-Ward H, Camp PG, Kennedy SM. Gender differences in respiratory symptoms: does occupation matter? Environ Res. 2006;101:175–183. doi: 10.1016/j.envres.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Malo JL, Lemière C, Desjardins A, Cartier A. Prevalence and intensity of rhinoconjunctivitis in subjects with occupational asthma. Eur Respir J. 1997;10:1513–1515. doi: 10.1183/09031936.97.10071513. [DOI] [PubMed] [Google Scholar]
- 21.Gautrin D, Ghezzo H, Infante-Rivard C, Malo JL. Natural history of sensitization, symptoms and occupational diseases in apprentices exposed to laboratory animals. Eur Respir J. 2001;17:904–908. doi: 10.1183/09031936.01.17509040. [DOI] [PubMed] [Google Scholar]
- 22.Siracusa A, De Blay F, Folletti I, Moscato G, Olivieri M, Quirce S, Raulf-Heimsoth M, Sastre J, Tarlo SM, Walusiak-Skorupa J, et al. Asthma and exposure to cleaning products: a European Academy of Allergy and Clinical Immunology task force consensus statement. Allergy. 2013;68:1532–1545. doi: 10.1111/all.12279. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy SM, Le Moual N, Choudat D, Kauffmann F. Development of an asthma specific job exposure matrix and its application in the epidemiological study of genetics and environment in asthma (EGEA) Occup Environ Med. 2000;57:635–641. doi: 10.1136/oem.57.9.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vandenplas O. Occupational asthma: etiologies and risk factors. Allergy Asthma Immunol Res. 2011;3:157–167. doi: 10.4168/aair.2011.3.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Moual N, Carsin AE, Siroux V, Radon K, Norback D, Torén K, Olivieri M, Urrutia I, Cazzoletti L, Jacquemin B, et al. Occupational exposures and uncontrolled adult-onset asthma in the European Community Respiratory Health Survey II. Eur Respir J. 2014;43:374–386. doi: 10.1183/09031936.00034913. [DOI] [PubMed] [Google Scholar]
- 26.Le Moual N, Siroux V, Pin I, Kauffmann F, Kennedy SM Epidemiological study on the genetics and environment of asthma. Asthma severity and exposure to occupational asthmogens. Am J Respir Crit Care Med. 2005;172:440–445. doi: 10.1164/rccm.200501-111OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King T.Epidemiology and causes of hypersensitivity pneumonitis (extrinsic allergic alveolitis) UptoDate 2014. [accessed 2015 Jun 26]. Available from: http://www.uptodate.com [Google Scholar]
- 28.Platts-Mills J.Allergen avoidance in the treatment of asthma and allergic rhinitis UptoDate 2014. [accessed 2015 Jun 26]. Available from: http://www.uptodate.com [Google Scholar]
- 29.Platts-Mills TA, Woodfolk JA. Allergens and their role in the allergic immune response. Immunol Rev. 2011;242:51–68. doi: 10.1111/j.1600-065X.2011.01021.x. [DOI] [PubMed] [Google Scholar]
- 30.Commins SP, Kelly LA, Rönmark E, James HR, Pochan SL, Peters EJ, Lundbäck B, Nganga LW, Cooper PJ, Hoskins JM, et al. Galactose-α-1,3-galactose-specific IgE is associated with anaphylaxis but not asthma. Am J Respir Crit Care Med. 2012;185:723–730. doi: 10.1164/rccm.201111-2017OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heederik D, Henneberger PK, Redlich CA ERS Task Force on the Management of Work-related Asthma. Primary prevention: exposure reduction, skin exposure and respiratory protection. Eur Respir Rev. 2012;21:112–124. doi: 10.1183/09059180.00005111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Platts-Mills T, Vaughan J, Squillace S, Woodfolk J, Sporik R. Sensitisation, asthma, and a modified Th2 response in children exposed to cat allergen: a population-based cross-sectional study. Lancet. 2001;357:752–756. doi: 10.1016/S0140-6736(00)04168-4. [DOI] [PubMed] [Google Scholar]
- 33.Lødrup Carlsen KC, Roll S, Carlsen KH, Mowinckel P, Wijga AH, Brunekreef B, Torrent M, Roberts G, Arshad SH, Kull I, et al. GALEN WP 1.5 ‘Birth Cohorts’ working group. Does pet ownership in infancy lead to asthma or allergy at school age? Pooled analysis of individual participant data from 11 European birth cohorts. PLoS ONE. 2012;7:e43214. doi: 10.1371/journal.pone.0043214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandenplas O, Wiszniewska M, Raulf M, de Blay F, Gerth van Wijk R, Moscato G, Nemery B, Pala G, Quirce S, Sastre J, et al. European Academy of Allergy and Clinical Immunology. EAACI position paper: irritant-induced asthma. Allergy. 2014;69:1141–1153. doi: 10.1111/all.12448. [DOI] [PubMed] [Google Scholar]
- 35.Lemière C, Boulet LP, Chaboillez S, Forget A, Chiry S, Villeneuve H, Prince P, Maghni K, Kennedy WA, Blais L. Work-exacerbated asthma and occupational asthma: do they really differ? J Allergy Clin Immunol. 2013;131:704–710. doi: 10.1016/j.jaci.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 36.Vandenplas O, D’Alpaos V, Evrard G, Jamart J, Thimpont J, Huaux F, Renauld JC. Asthma related to cleaning agents: a clinical insight. BMJ Open. 2013;3:e003568. doi: 10.1136/bmjopen-2013-003568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tarlo SM, Balmes J, Balkissoon R, Beach J, Beckett W, Bernstein D, Blanc PD, Brooks SM, Cowl CT, Daroowalla F, et al. Diagnosis and management of work-related asthma: American College Of Chest Physicians Consensus Statement. Chest. 2008;134(3):1S–41S. doi: 10.1378/chest.08-0201. [DOI] [PubMed] [Google Scholar]
- 38.Dodson RE, Nishioka M, Standley LJ, Perovich LJ, Brody JG, Rudel RA. Endocrine disruptors and asthma-associated chemicals in consumer products. Environ Health Perspect. 2012;120:935–943. doi: 10.1289/ehp.1104052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smit LA, Kogevinas M, Antó JM, Bouzigon E, González JR, Le Moual N, Kromhout H, Carsin AE, Pin I, Jarvis D, et al. Transient receptor potential genes, smoking, occupational exposures and cough in adults. Respir Res. 2012;13:26. doi: 10.1186/1465-9921-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Preller L, Doekes G, Heederik D, Vermeulen R, Vogelzang PF, Boleij JS. Disinfectant use as a risk factor for atopic sensitization and symptoms consistent with asthma: an epidemiological study. Eur Respir J. 1996;9:1407–1413. doi: 10.1183/09031936.96.09071407. [DOI] [PubMed] [Google Scholar]
- 41.Zock J, Plana E, Anto J, Benke G, Blanc P, Carosso A, Dahlman-Hoglund A, Heinrich J, Jarvis D, Kromhout H, et al. Domestic use of hypochlorite bleach, atopic sensitization, and respiratory symptoms in adults. J Allergy Clin Immunol. 2009;124:731–738, e731. doi: 10.1016/j.jaci.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 42.Vizcaya D, Mirabelli MC, Orriols R, Antó JM, Barreiro E, Burgos F, Arjona L, Gomez F, Zock JP. Functional and biological characteristics of asthma in cleaning workers. Respir Med. 2013;107:673–683. doi: 10.1016/j.rmed.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 43.Malo JL, L’archevêque J, Castellanos L, Lavoie K, Ghezzo H, Maghni K. Long-term outcomes of acute irritant-induced asthma. Am J Respir Crit Care Med. 2009;179:923–928. doi: 10.1164/rccm.200810-1550OC. [DOI] [PubMed] [Google Scholar]
- 44.Henneberger P, Liang X, Lemière C. A comparison of work-exacerbated asthma cases from clinical and epidemiological settings. Can Respir J. 2013;20:159–164. doi: 10.1155/2013/495767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vandenplas O, Ghezzo H, Munoz X, Moscato G, Perfetti L, Lemière C, Labrecque M, L’Archevêque J, Malo JL. What are the questionnaire items most useful in identifying subjects with occupational asthma? Eur Respir J. 2005;26:1056–1063. doi: 10.1183/09031936.05.00024705. [DOI] [PubMed] [Google Scholar]
- 46.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368:804–813. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 47.Moscato G, Pala G, Barnig C, De Blay F, Del Giacco SR, Folletti I, Heffler E, Maestrelli P, Pauli G, Perfetti L, et al. European Academy of Allergy and Clinical Immunology. EAACI consensus statement for investigation of work-related asthma in non-specialized centres. Allergy. 2012;67:491–501. doi: 10.1111/j.1398-9995.2011.02784.x. [DOI] [PubMed] [Google Scholar]
- 48.Carolan BJ, Sutherland ER. Clinical phenotypes of chronic obstructive pulmonary disease and asthma: recent advances. J Allergy Clin Immunol. 2013;131:627–634, quiz 635. doi: 10.1016/j.jaci.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 49.Quirce S, Lemière C, de Blay F, del Pozo V, Gerth Van Wijk R, Maestrelli P, Pauli G, Pignatti P, Raulf-Heimsoth M, Sastre J, et al. Noninvasive methods for assessment of airway inflammation in occupational settings. Allergy. 2010;65:445–458. doi: 10.1111/j.1398-9995.2009.02274.x. [DOI] [PubMed] [Google Scholar]
- 50.Anees W, Huggins V, Pavord ID, Robertson AS, Burge PS. Occupational asthma due to low molecular weight agents: eosinophilic and non-eosinophilic variants. Thorax. 2002;57:231–236. doi: 10.1136/thorax.57.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Sutherland ER, Chinchilli VM, Fahy JV Asthma Clinical Research Network of the National Heart, Lung, and Blood Institute. A large subgroup of mild-to-moderate asthma is persistently noneosinophilic. Am J Respir Crit Care Med. 2012;185:612–619. doi: 10.1164/rccm.201109-1640OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moscato G, Vandenplas O, Gerth Van Wijk R, Malo JL, Quirce S, Walusiak J, Castano R, De Groot H, Folletti I, Gautrin D, et al. EAACI Task Force on Occupational Rhinitis. Occupational rhinitis. Allergy. 2008;63:969–980. doi: 10.1111/j.1398-9995.2008.01801.x. [DOI] [PubMed] [Google Scholar]
- 53.Vandenplas O, D’Alpaos V, Evrard G, Jamart J. Incidence of severe asthmatic reactions after challenge exposure to occupational agents. Chest. 2013;143:1261–1268. doi: 10.1378/chest.12-1983. [DOI] [PubMed] [Google Scholar]
- 54.Vandenplas O, Sherwood Burge S, Moscato G, Malo J. Functional assessment. In: Malo J-L, Chan-Yeung M, Bernstein DI, editors. Asthma in the workplace. 4th ed. Boca Raton, FL: CRC Press; 2013. pp. 1113–1132. [Google Scholar]
- 55.Rønborg SM, Mosbech H, Poulsen LK. Exposure chamber for allergen challenge: a placebo-controlled, double-blind trial in house-dust-mite asthma. Allergy. 1997;52:821–828. doi: 10.1111/j.1398-9995.1997.tb02153.x. [DOI] [PubMed] [Google Scholar]
- 56.Cockcroft D, Davis B. Direct and indirect challenges in the clinical assessment of asthma. Ann Allergy Asthma Immunol. 2009;103:363–369, quiz 369–372, 400. doi: 10.1016/S1081-1206(10)60353-5. [DOI] [PubMed] [Google Scholar]
- 57.Lemiere C, Miedinger D, Jacob V, Chaboillez S, Tremblay C, Brannan JD. Comparison of methacholine and mannitol bronchial provocation tests in workers with occupational asthma. J Allergy Clin Immunol. 2012;129:555–556. doi: 10.1016/j.jaci.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 58.Vandenplas O, Suojalehto H, Aasen TB, Baur X, Burge PS, de Blay F, Fishwick D, Hoyle J, Maestrelli P, Muñoz X, et al. ERS Task Force on Specific Inhalation Challenges with Occupational Agents. Specific inhalation challenge in the diagnosis of occupational asthma: consensus statement. Eur Respir J. 2014;43:1573–1587. doi: 10.1183/09031936.00180313. [DOI] [PubMed] [Google Scholar]
- 59.Schneider A, Schwarzbach J, Faderl B, Welker L, Karsch-Völk M, Jörres RA. FENO measurement and sputum analysis for diagnosing asthma in clinical practice. Respir Med. 2013;107:209–216. doi: 10.1016/j.rmed.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 60.Kim JL, Blanc PD, Villani S, Olivieri M, Urrutia I, van Sprundel M, Storaas T, Le Moual N, Zock JP, Torén K. Predictors of respiratory sickness absence: an international population-based study. Am J Ind Med. 2013;56:541–549. doi: 10.1002/ajim.22178. [DOI] [PubMed] [Google Scholar]
- 61.Demoly P, Annunziata K, Gubba E, Adamek L. Repeated cross-sectional survey of patient-reported asthma control in Europe in the past 5 years. Eur Respir Rev. 2012;21:66–74. doi: 10.1183/09059180.00008111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hakola R, Kauppi P, Leino T, Ojajärvi A, Pentti J, Oksanen T, Haahtela T, Kivimäki M, Vahtera J. Persistent asthma, comorbid conditions and the risk of work disability: a prospective cohort study. Allergy. 2011;66:1598–1603. doi: 10.1111/j.1398-9995.2011.02729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knoeller GE, Mazurek JM, Moorman JE. Health-related quality of life among adults with work-related asthma in the United States. Qual Life Res. 2013;22:771–780. doi: 10.1007/s11136-012-0206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Birnbaum HG, Berger WE, Greenberg PE, Holland M, Auerbach R, Atkins KM, Wanke LA. Direct and indirect costs of asthma to an employer. J Allergy Clin Immunol. 2002;109:264–270. doi: 10.1067/mai.2002.121310. [DOI] [PubMed] [Google Scholar]
- 65.Henneberger PK, Mirabelli MC, Kogevinas M, Antó JM, Plana E, Dahlman-Höglund A, Jarvis DL, Kromhout H, Lillienberg L, Norbäck D, et al. The occupational contribution to severe exacerbation of asthma. Eur Respir J. 2010;36:743–750. doi: 10.1183/09031936.00135109. [DOI] [PubMed] [Google Scholar]
- 66.Ayres JG, Boyd R, Cowie H, Hurley JF. Costs of occupational asthma in the UK. Thorax. 2011;66:128–133. doi: 10.1136/thx.2010.136762. [DOI] [PubMed] [Google Scholar]
- 67.Miedinger D, Malo JL, Ghezzo H, L’Archevêque J, Zunzunegui MV. Factors influencing duration of exposure with symptoms and costs of occupational asthma. Eur Respir J. 2010;36:728–734. doi: 10.1183/09031936.00198209. [DOI] [PubMed] [Google Scholar]
- 68.Akinbami LJ, Sullivan SD, Campbell JD, Grundmeier RW, Hartert TV, Lee TA, Smith RA. Asthma outcomes: healthcare utilization and costs. J Allergy Clin Immunol. 2012;129(Suppl):S49–S64. doi: 10.1016/j.jaci.2011.12.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bisgaard H, Bønnelykke K. Long-term studies of the natural history of asthma in childhood. J Allergy Clin Immunol. 2010;126:187–197, quiz 198–199. doi: 10.1016/j.jaci.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 70.Covar RA, Strunk R, Zeiger RS, Wilson LA, Liu AH, Weiss S, Tonascia J, Spahn JD, Szefler SJ Childhood Asthma Management Program Research Group. Predictors of remitting, periodic, and persistent childhood asthma. J Allergy Clin Immunol. 2010;125:359–366, e3. doi: 10.1016/j.jaci.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339:1194–1200. doi: 10.1056/NEJM199810223391703. [DOI] [PubMed] [Google Scholar]
- 72.Rackemann FM. A working classification of asthma. Am J Med. 1947;3:601–606. doi: 10.1016/0002-9343(47)90204-0. [DOI] [PubMed] [Google Scholar]
- 73.de Groene G, Pal T, Beach J, Tarlo S, Spreeuwers D, Frings-Dresen M, Mattioli S, Verbeek J. Workplace interventions for treatment of occupational asthma. Cochrane Database Syst Rev. 2011:5. doi: 10.1002/14651858.CD006308.pub3. [DOI] [PubMed] [Google Scholar]
- 74.Sumi Y, Foley S, Daigle S, L’Archevêque J, Olivenstein R, Letuvé S, Malo JL, Hamid Q. Structural changes and airway remodelling in occupational asthma at a mean interval of 14 years after cessation of exposure. Clin Exp Allergy. 2007;37:1781–1787. doi: 10.1111/j.1365-2222.2007.02828.x. [DOI] [PubMed] [Google Scholar]
- 75.Rachiotis G, Savani R, Brant A, MacNeill SJ, Newman Taylor A, Cullinan P. Outcome of occupational asthma after cessation of exposure: a systematic review. Thorax. 2007;62:147–152. doi: 10.1136/thx.2006.061952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maestrelli P, Schlünssen V, Mason P, Sigsgaard T ERS Task Force on the Management of Work-related Asthma. Contribution of host factors and workplace exposure to the outcome of occupational asthma. Eur Respir Rev. 2012;21:88–96. doi: 10.1183/09059180.00004811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Perfetti L, Cartier A, Ghezzo H, Gautrin D, Malo JL. Follow-up of occupational asthma after removal from or diminution of exposure to the responsible agent: relevance of the length of the interval from cessation of exposure. Chest. 1998;114:398–403. doi: 10.1378/chest.114.2.398. [DOI] [PubMed] [Google Scholar]
- 78.Carlsten C, Dybuncio A, Pui MM, Chan-Yeung M. Respiratory impairment and systemic inflammation in cedar asthmatics removed from exposure. PLoS One. 2013;8:e57166. doi: 10.1371/journal.pone.0057166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sont JK, Willems LN, Bel EH, van Krieken JH, Vandenbroucke JP, Sterk PJ The AMPUL Study Group. Clinical control and histopathologic outcome of asthma when using airway hyperresponsiveness as an additional guide to long-term treatment. Am J Respir Crit Care Med. 1999;159:1043–1051. doi: 10.1164/ajrccm.159.4.9806052. [DOI] [PubMed] [Google Scholar]
- 80.Jayaram L, Parameswaran K, Sears MR, Hargreave FE. Induced sputum cell counts: their usefulness in clinical practice. Eur Respir J. 2000;16:150–158. doi: 10.1034/j.1399-3003.2000.16a27.x. [DOI] [PubMed] [Google Scholar]
- 81.Petsky HL, Cates CJ, Lasserson TJ, Li AM, Turner C, Kynaston JA, Chang AB. A systematic review and meta-analysis: tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils) Thorax. 2012;67:199–208. doi: 10.1136/thx.2010.135574. [DOI] [PubMed] [Google Scholar]
- 82.Liss G, Tarlo S, Labrecque M, Malo J. Prevention and surveillance. In: Malo J-L, Chan-Yeung M, Bernstein DI, editors. Asthma in the workplace. 4th ed. Boca Raton, FL: CRC Press; 2013. pp. 150–162. [Google Scholar]
- 83.Pralong JA, Moullec G, Suarthana E, Gérin M, Gautrin D, Archevêque JL, Labrecque M. Screening for occupational asthma by using a self-administered questionnaire in a clinical setting. J Occup Environ Med. 2013;55:527–531. doi: 10.1097/JOM.0b013e3182851790. [DOI] [PubMed] [Google Scholar]
- 84.Suarthana E, Malo JL, Heederik D, Ghezzo H, L’Archevêque J, Gautrin D. Which tools best predict the incidence of work-related sensitisation and symptoms. Occup Environ Med. 2009;66:111–117. doi: 10.1136/oem.2008.041079. [DOI] [PubMed] [Google Scholar]
- 85.Vandenplas O, Dressel H, Nowak D, Jamart J ERS Task Force on the Management of Work-related Asthma. What is the optimal management option for occupational asthma? Eur Respir Rev. 2012;21:97–104. doi: 10.1183/09059180.00004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moullec G, Lavoie K, Malo J, Gautrin D, L'Archevêque J, Labrecque M. Long-term socioprofessional and psychological status in workers investigated for occupational asthma in Québec. J Occup Environ Med. 2013;55:1052–1064. doi: 10.1097/JOM.0b013e31829904ab. [DOI] [PubMed] [Google Scholar]
- 87.Maghni K, Lemière C, Ghezzo H, Yuquan W, Malo JL. Airway inflammation after cessation of exposure to agents causing occupational asthma. Am J Respir Crit Care Med. 2004;169:367–372. doi: 10.1164/rccm.200309-1238OC. [DOI] [PubMed] [Google Scholar]
- 88.Miedinger D, Lavoie KL, L’Archevêque J, Ghezzo H, Zunzunuegui MV, Malo JL. Quality-of-life, psychological, and cost outcomes 2 years after diagnosis of occupational asthma. J Occup Environ Med. 2011;53:231–238. doi: 10.1097/JOM.0b013e31820d1338. [DOI] [PubMed] [Google Scholar]
- 89.Malo JL, Cartier A, Côté J, Milot J, Leblanc C, Paquette L, Ghezzo H, Boulet LP. Influence of inhaled steroids on recovery from occupational asthma after cessation of exposure: an 18-month double-blind crossover study. Am J Respir Crit Care Med. 1996;153:953–960. doi: 10.1164/ajrccm.153.3.8630579. [DOI] [PubMed] [Google Scholar]