Abstract
Rationale: Given the inconclusive science on the long-term effects of marijuana exposure on lung function, the increasing tetrahydrocannabinol composition of marijuana over time, and the increasing legal accessibility of the substance, continued investigation is needed.
Objectives: To determine the independent association between recent and chronic marijuana smoke exposure with spirometric parameters of lung function and symptoms of respiratory health in a large cohort of U.S. adults.
Methods: This is a cross-sectional study of U.S. adults who participated in the National Health and Nutrition Examination Survey cycles from 2007–2008 and 2009–2010, using the data from standardized spirometry and survey questions performed during these years.
Measurements and Main Results: In the combined 2007–2010 cohort, 59.1% replied that they had used marijuana at least once, and 12.2% had used in the past month. For each additional day of marijuana use in the prior month, there were no changes in percent predicted FEV1 (0.002 ± 0.04%; P = 0.9), but there was an associated increase in percent predicted FVC (0.13 ± 0.03%, P = 0.0001) and decrease in the FEV1/FVC ratio (−0.1 ± 0.04%; P < 0.0001). In multivariable regressions, 1–5 and 6–20 joint-years of marijuana use were not associated with an FEV1/FVC less than 70% (odds ratio [OR] = 1.1, 95% confidence interval [CI] = 0.7–1.6, P = 0.8, and OR = 1.2, 95% CI = 0.8–1.8, P = 0.4, respectively), whereas over 20 joint-years was associated with an FEV1/FVC less than 70% (OR = 2.1; 95% CI = 1.1–3.9; P = 0.02). For each additional marijuana joint–year smoked, there was no associated change in the mean percent predicted FEV1 (0.02 ± 0.02%; P = 1.00), an increase in percent predicted FVC (0.07 ± 0.02%; P = 0.004), and a decrease in FEV1/FVC (−0.03 ± 0.01%; P = 0.02).
Conclusions: In a large cross-section of U.S. adults, cumulative lifetime marijuana use, up to 20 joint-years, is not associated with adverse changes in spirometric measures of lung health. Although greater than 20 joint-years of cumulative marijuana exposure was associated with a twofold increased odds of a FEV1/FVC less than 70%, this was the result of an increase in FVC, rather than a disproportional decrease in FEV1 as is typically associated with obstructive lung diseases.
Keywords: cannabis, obstructive lung disease, marijuana smoking
Marijuana is a commonly used substance worldwide, with a United Nations report estimating that 3.9% of the global population uses the drug (1). There is also a growing acceptance of its use, with a recent poll finding that 54% of Americans support the legalization of marijuana, up from 17% in 1991 (2). Furthermore, a recent poll of medical professionals revealed that 76% supported the use of medical marijuana (3). U.S. states’ laws are following this popular opinion, with two states legalizing marijuana for adult use and 20 states legalizing its use for medicinal purposes (4). Given these changes, it is ever more relevant for continued research into the long-term health effects of marijuana use.
Although research on marijuana’s effects on respiratory health has been conducted since the 1970s, there is still considerable uncertainty and controversy. Although marijuana and tobacco have similar amounts of volatile and tar components, their pulmonary effects do not seem to be the same (5). Recent comprehensive reviews have summarized the findings to date: that marijuana exposure has an immediate and modest bronchodilator effect with a subsequent increase in airway inflammation and symptoms of chronic bronchitis, whereas the studies of its long-term effects demonstrate varying results that suggest either no effect or some decrement in lung function (5, 6). The acute bronchodilator effects appear to be due to tetrahydrocannabinol (THC), also the psychoactive ingredient in marijuana. Studies varying the THC content in marijuana cigarettes have demonstrated a dose-dependent effect on humans, whereas rodent studies have revealed that THC may inhibit acetylcholine release by binding to cannabinoid receptors on the axon terminals of respiratory nerves (7–9). This is of potential import regarding the respiratory effects of the drug, because the THC content of marijuana in the United States has been increasing over the past several decades, demonstrated at less than 2% in 1980, 4.6% in 1997, and 7.6% in 2006 (10, 11). This changing composition and the yet-unclear, long-term effects of marijuana on lung function warrant continued research in the field.
The aim of the current study is to examine the effects of current marijuana use on acute respiratory symptoms and lung function, and to determine the independent relationship of cumulative marijuana smoke exposure on spirometric measures of lung function in a current and representative U.S. sample. The last U.S. study to examine this question used data from the National Health and Nutrition Examination Survey (NHANES) III representing a large U.S. sample from 1988 to 1994 that responded to basic questions on marijuana use in the prior 30 days and whether or not participants had used more or less than 100 marijuana cigarettes in their lifetime. In contrast, recent iterations of the continuous NHANES from 2007 to 2010 have included detailed drug use questionnaires that allow investigators to calculate a more quantitative, cumulative lifetime exposure represented in joint-years.
Methods
This is a cross-sectional study of the NHANES cohort, combing survey years 2007–2008 and 2009–2010. It includes participants 18–59 years of age who underwent a standardized questionnaire regarding marijuana use and standardized spirometry.
From the survey data, we quantified the frequency of use in the past 30 days and the cumulative, lifetime exposure. The latter is measured in joint-years, with 1 joint-year equivalent to smoking one joint or pipe bowl of marijuana every day for 1 year. Of note, for the cumulative effects of chronic marijuana use, only the 2009–2010 data were used, as this was the only available survey cycle that included additional questions to allow for an estimation of each participant’s cumulative exposure. The other covariates of interest included tobacco exposure, age, race, sex, economic status, and history of asthma, emphysema, and chronic bronchitis. Race was dichotomized to white and nonwhite from the NHANES’ five-race classification. Economic status was determined as the poverty index ratio, which is a ratio of family income to the national poverty threshold.
For the primary spirometric outcomes, the raw values of the FEV1 and FVC were used to derive the FEV1/FVC ratio, the percent predicted FEV1, and the percent predicted FVC. We derived the latter two variables using standard reference equations that adjust for sex, age, height, and race (12). The FEV1/FVC is presented as a percent for consistency in presentation with the other values. The FEV1/FVC was also dichotomized at 70% for the multivariable logistic regression.
Given that there is a certain amount of nonresponse in any element of a national health survey, sensitivity analyses examining this were performed and are presented in more detail in the online supplement (13–15). In brief, there were differences in demographic characteristics among those with full data for every variable in the multivariable regressions and those with partial data. Given the low likelihood that these differences would confound a relationship between marijuana use and lung function, and the fact that our models control for many of these identified characteristics, we believe that these differences primarily affect the generalizability of the population estimates in the analysis, and were unlikely to confound the studied relationship between marijuana exposure and lung function in those participants with full data. Therefore, the original NHANES weights were not adjusted for the above differences.
Statistical Analysis
All statistical procedures were performed with SAS 9.3 (SAS Institute, Cary, NC), and statistical significance was set at P less than 0.05. Linear and logistic regression was used for all analyses of marijuana exposure on respiratory health. For the dichotomous variables of the respiratory symptoms, an answer of “yes” was given a value of 100, and an answer of “no” was given a value of 0, with the corresponding regression coefficient and confidence interval representing the proportion responding “yes.” This method was used because this is the only SAS SURVEY procedure that supplies confidence intervals for a proportion estimate; however, it does not allow for inference testing between multiple groups. For analyses of spirometric measures, we constructed the multivariable linear and logistic regression models in an a priori fashion, choosing covariates thought to be important confounders or to improve the precision of the estimates.
For readers who wish for more detail, there is an expanded version of this Methods section on the online supplement.
Results
Participants
For the combined 2007–2008 and 2009–2010 study group used to examine the effects of current marijuana exposure on respiratory symptoms and spirometry, there were 7,716 NHANES participants from ages 18 to 59 years who were eligible for the marijuana portion of the survey, and 5,657 (73.3%) with full data for all covariates in the multivariable regression models. For the 2009–2010 study group used to examine the effects of cumulative lifetime marijuana exposure on spirometry, there were 4,355 NHANES participants from ages 18 to 59 years who were eligible for the marijuana portion of the survey, and 2,956 (67.8%) with full data for all covariates in the multivariable regression model. Figures E1 and E2 in the online supplement contain a breakdown of the missing data in these cohorts.
In the overall 2007–2010 cohorts, 59.1% of those responding to the questionnaire replied that they had used marijuana or hashish at least once in their lifetime, and 12.2% were current users. Characteristics of current marijuana smokers are compared with current nonsmokers in Table 1. In summary, current marijuana smokers were more likely to be male, younger, of lower socioeconomic and education levels, to concurrently smoke tobacco, to have first tried marijuana at an earlier age, and have a history of a chronic respiratory illness. Among current marijuana smokers, average joint-years used was 15.8 and the average number days out of the prior 30 days that they had used marijuana was 12.0.
Table 1.
Demographic, smoking, and respiratory history characteristics of adults ages 18–59 years of the 2007–2010 National Health and Nutrition Examination Survey cohorts who acknowledge smoking marijuana in the previous 30 days
| |
Current Smokers |
Current Nonsmokers |
|---|---|---|
| Variable | (n = 855) | (n = 5,868) |
| Female, % | 36.1 (1.5) | 51.7 (0.7) |
| Age, yr, % | ||
| 20–29 | 42.3 (2.1) | 23.0 (0.9) |
| 30–39 | 22.0 (1.3) | 23.9 (0.8) |
| 40–49 | 23.8 (1.6) | 27.0 (0.8) |
| 50–59 | 11.8 (1.4) | 26.1 (1.0) |
| Race, % | ||
| Non-Hispanic white | 67.1 (2.8) | 66.5 (2.6) |
| Non-Hispanic black | 18.7 (2.1) | 10.8 (1.0) |
| Mexican American | 5.7 (1.2) | 10.4 (1.5) |
| Other Hispanic | 4.4 (1.0) | 5.6 (1.0) |
| Other | 4.0 (0.7) | 6.5 (0.7) |
| Poverty-index ratio < 1, % | 22.7 (1.7) | 13.1 (0.9) |
| Maximum education level, % | ||
| <Ninth grade | 2.0 (0.5) | 5.1 (0.5) |
| 9–11th grade | 18.9 (1.8) | 11.8 (0.7) |
| High school | 29.2 (2.1) | 22.4 (1.0) |
| Some college or associate degree | 34.0 (2.3) | 31.2 (0.7) |
| College degree | 15.9 (1.8) | 29.5 (1.4) |
| Marijuana smoking characteristics, mean | ||
| Age when first tried, yr | 15.7 (0.2) | 17.3 (0.1) |
| Age when first regularly used, yr | 17.5 (0.3) | 17.4 (0.1) |
| Age at last regular use, yr | NA | 25.8 (0.6) |
| Joint-years used | 15.8 (1.2) | 2.0 (0.2) |
| No. of days used in the previous month | 12.0 (0.5) | NA |
| Tobacco smoking characteristics | ||
| Currently smoking tobacco, % | 78.5 (2.3) | 50.7 (1.9) |
| Age when first regularly used, mean, yr | 16.1 (0.2) | 16.8 (0.1) |
| Pack-years used, mean | 10.1 (0.8) | 4.4 (0.4) |
| Respiratory health history, % | ||
| Asthma | 19.3 (1.7) | 13.7 (0.6) |
| Chronic bronchitis | 6.3 (1.1) | 4.3 (0.4) |
| Emphysema | 1.7 (0.5) | 0.9 (0.2) |
Definition of abbreviation: NA = not applicable.
Estimates were derived accounting for National Health and Nutrition Examination Survey study design and weights. Numbers in parentheses represent SEM and SE of percent, accordingly.
Effects of Current Marijuana Use on Respiratory Health
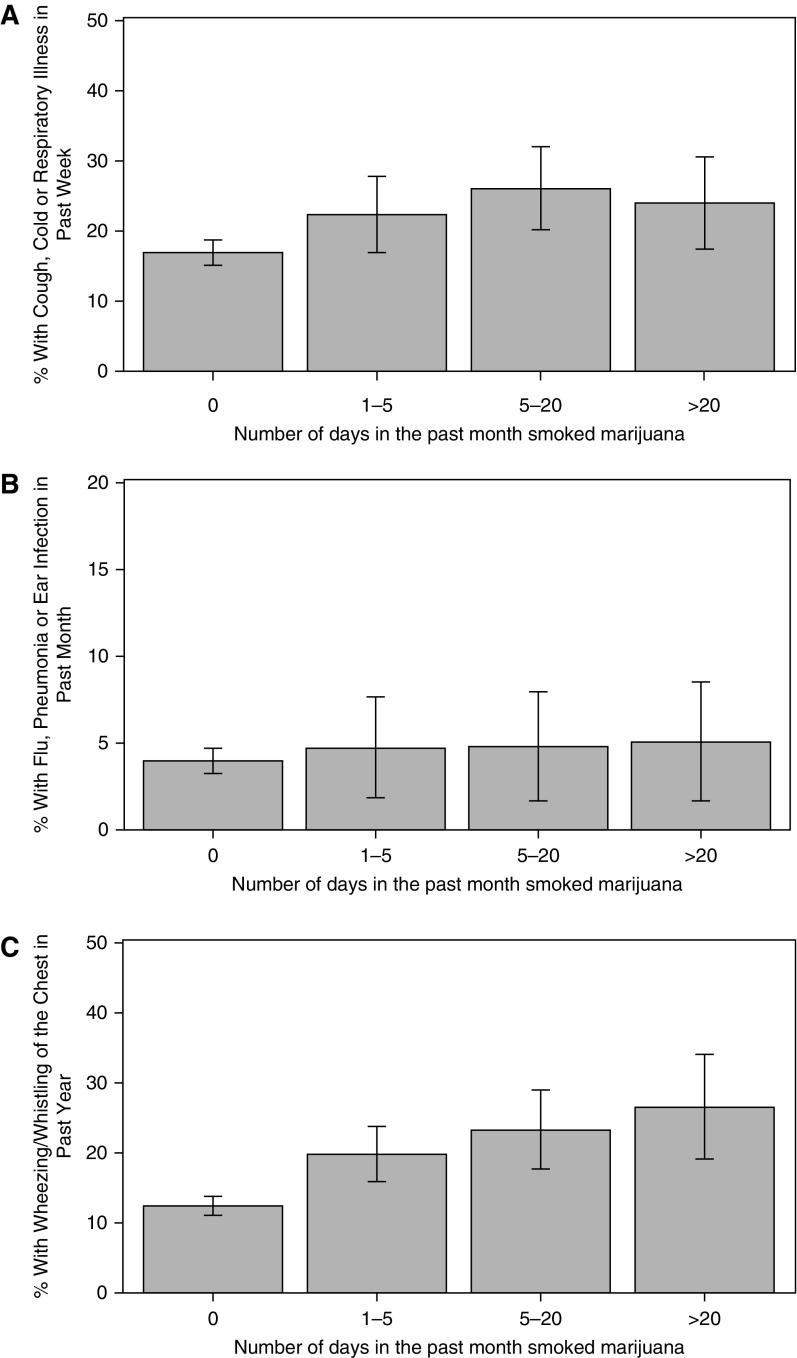
Among participants who reported smoking marijuana 0, 1–5, 6–20, or greater than 20 days out of the previous 30 days, there were trends toward increases in reported symptoms of bronchitis and respiratory illness. Specifically, among these groups, the proportions of each group reporting that they had a cough, cold, phlegm, or respiratory illness in the previous 7 days were 16.9%, 22.4%, 26.1%, and 24.0%, respectively. Those reporting having the flu, pneumonia, or an ear infection in the previous 30 days were 3.9%, 4.7%, 4.8%, and 5.1%, respectively. Finally, those reporting having whistling or wheezing in their chest during the past year were 12.4%, 19.8%, 23.3%, and 26.5%, respectively (Figure 1)
Figure 1.
Frequency of recent respiratory symptoms by frequency of current marijuana use in the previous 30 days in the National Health and Nutrition Examination Survey 2007–2010 cohorts. Among participants who reported smoking marijuana 0, 1–5, 6–20, or greater than 20 days out of the previous 30 days, the proportions of each group reporting that they had a (A) cough, cold, phlegm, or respiratory illness in the previous 7 days were 16.9% (95% confidence interval [CI] = 15.2–18.7), 22.4% (95% CI = 16.9–27.8), 26.1% (95% CI = 20.1–32.1), and 24.0% (95% CI = 17.4–30.6); (B) those reporting having the flu, pneumonia, or an ear infection in the previous 30 days were 3.9% (95% CI = 3.2–4.6), 4.7% (95% CI = 1.8–7.6), 4.8% (95% CI = 1.7–8.0), and 5.1% (95% CI = 1.6–8.5); and (C) those reporting having whistling or wheezing in their chest during the previous year were 12.4% (95% CI = 11.0–13.7), 19.8% (95% CI = 15.9–23.7), 23.3% (95% CI = 17.7–29.0), and 26.5% (95% CI = 19.0–34.0).
In multivariable regression controlling for age, sex, race, tobacco use, and the presence of asthma, emphysema, and chronic bronchitis, frequency of recent marijuana use was associated with changes in spirometric parameters (Table E4). For each 1-day increase in the number of days using marijuana in the previous 30 days, there were no changes in percent predicted FEV1 (0.002 ± 0.04%; P = 0.9), but there was an associated increase in percent predicted FVC (0.13 ± 0.03%; P = 0.0001) and decrease in the FEV1/FVC ratio (−0.1 ± 0.04%; P < 0.0001).
Effects of Cumulative Lifetime Marijuana Use on Spirometry
Table 2 presents a descriptive analysis of associated changes in spirometry by cumulative marijuana and tobacco exposure. The multivariable logistic regression analysis modeling for an FEV1/FVC less than 70% and controlling for age, sex, race, tobacco use, and the presence of asthma, emphysema, and chronic bronchitis is presented in Table 3. When compared with no marijuana exposure, greater than 20 joint-years of cumulative marijuana use showed an association with increased odds of a FEV1/FVC less than 70% (odds ratio = 2.1; 95% confidence interval = 1.1–3.9; P = 0.02). Lesser cumulative exposures to marijuana smoke showed no associations with a decreased FEV1/FVC ratio.
Table 2.
Spirometric measures by cumulative marijuana and tobacco exposures in the 2009–2010 National Health and Nutrition Examination Survey cohort (n = 2,956)
| Marijuana Joint-Years |
||||
|---|---|---|---|---|
| Tobacco Pack-Years | 0 | 1–5 | 5–20 | >20 |
| 0 | ||||
| Mean % predicted FEV1 ± SE | 97.7 ± 0.5 | 97.1 ± 1.1 | 93.7 ± 2.6 | 93.8 ± 3.1 |
| Mean % predicted FVC ± SE | 98.9 ± 0.6 | 100.2 ± 1.3 | 96.9 ± 1.8 | 96.5 ± 2.0 |
| Mean % FEV1/FVC ± SE | 80.6 ± 3.4 | 79.5 ± 5.5 | 77.4 ± 16.5 | 77.5 ± 16.8 |
| % with FEV1/FVC < 70% ± SE | 5.5 ± 1.1 | 8.0 ± 3.7 | 10.7 ± 8.9 | 25.1 ± 10.3 |
| 1–5 | ||||
| Mean % predicted FEV1 ± SE | 96.3 ± 0.9 | 96.4 ± 1.2 | 97.1 ± 1.3 | 95.3 ± 1.3 |
| Mean % predicted FVC ± SE | 99.3 ± 0.7 | 98.4 ± 1.1 | 101.7 ± 1.2 | 98.6 ± 0.8 |
| Mean % FEV1/FVC ± SE | 79.5 ± 5.7 | 80.3 ± 9.9 | 79.7 ± 8.2 | 79.4 ± 11.3 |
| % with FEV1/FVC < 70% ± SE | 12.2 ± 3.0 | 7.9 ± 3.7 | 5.0 ± 1.7 | 9.0 ± 3.3 |
| 5–20 | ||||
| Mean % predicted FEV1 ± SE | 94.8 ± 0.8 | 95.3 ± 2.6 | 95.6 ± 2.5 | 97.0 ± 1.9 |
| Mean % predicted FVC ± SE | 97.5 ± 0.9 | 100.0 ± 1.8 | 101.9 ± 2.3 | 101.5 ± 1.6 |
| Mean % FEV1/FVC ± SE | 78.4 ± 4.8 | 77.2 ± 10.9 | 76.3 ± 9.8 | 77.9 ± 14.6 |
| % with FEV1/FVC < 70% ± SE | 10.9 ± 2.2 | 12.4 ± 4.5 | 18.9 ± 2.7 | 14.8 ± 5.4 |
| >20 | ||||
| Mean % predicted FEV1 ± SE | 87.5 ± 1.3 | 93.0 ± 1.7 | 90.9 ± 2.5 | 91.3 ± 2.4 |
| Mean % predicted FVC ± SE | 93.4 ± 1.5 | 99.9 ± 1.7 | 96.3 ± 2.4 | 100.8 ± 3.1 |
| Mean % FEV1/FVC ± SE | 73.6 ± 5.7 | 73.6 ± 7.0 | 74.1 ± 10.4 | 71.4 ± 9.5 |
| % with FEV1/FVC < 70% ± SE | 26.8 ± 4.5 | 25.7 ± 7.6 | 27.2 ± 7.5 | 39.6 ± 7.3 |
Table 3.
Results from logistic regression modeling for an FEV1/FVC less than 70% for the 2009–2010 National Health and Nutrition Examination Survey cohort (n = 2,956)
| Variable | OR (95% CI) | P Value |
|---|---|---|
| Joint-years | ||
| >20 | 2.1 (1.1–3.9) | 0.02 |
| 5–20 | 1.2 (0.8–1.8) | 0.4 |
| 1–5 | 1.1 (0.7–1.6) | 0.8 |
| 0 | Reference | NA |
| Tobacco pack-years | ||
| >20 | 2.9 (1.9–4.3) | <0.0001 |
| 5–20 | 1.8 (1.3–2.7) | 0.001 |
| 1–5 | 1.9 (1.1–3.4) | 0.02 |
| 0 | Reference | NA |
| Female | 0.7 (0.5–1.0) | 0.07 |
| Increasing age, yr | 1.1 (1.0–1.1) | <0.0001 |
| White race | 1.5 (1.0–2.3) | 0.04 |
| History of asthma | 3.6 (2.5–5.3) | <0.0001 |
| History of chronic bronchitis | 0.5 (0.2–1.2) | 0.1 |
| History of emphysema | 2.9 (0.7–11.3) | 0.1 |
Definition of abbreviations: CI = confidence interval; NA = not applicable; OR = odds ratio.
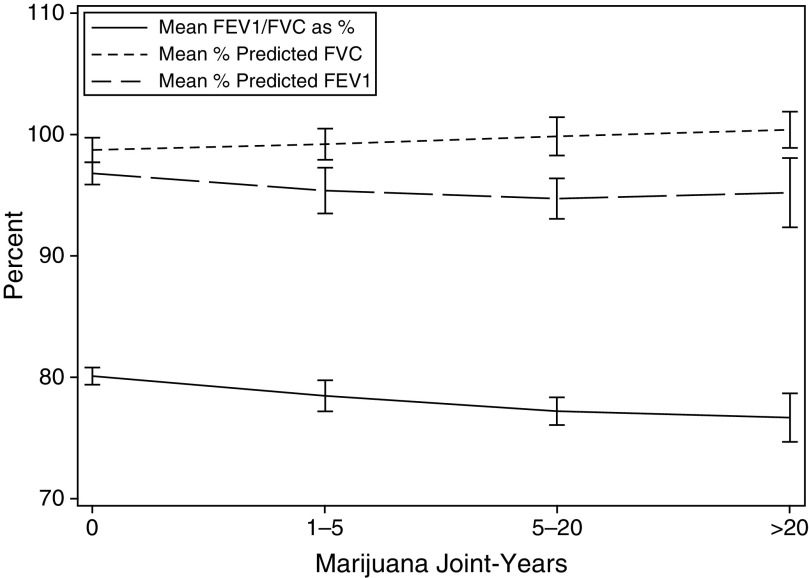
Looking at the components of the FEV1/FVC, a graphical representation of the changes in spirometric values of lung function by cumulative marijuana exposure is presented in Figure 2. In linear regressions, each controlling for age, sex, race, tobacco use, and the presence of asthma, emphysema, and chronic bronchitis, increasing cumulative marijuana use showed no associated changes in FEV1, with associated increases in FVC and decreases in FEV1/FVC (Table 4). Specifically, for each additional marijuana joint-year smoked, there was no change in the mean percent predicted FEV1 (0.02 ± 0.02%, P = 0.4), an increase in mean percent predicted FVC (0.07 ± 0.02%, P = 0.004), and a decrease in mean FEV1/FVC (−0.03 ± 0.01%; P = 0.02).
Figure 2.
Change in spirometric values by cumulative lifetime marijuana use among adult participants in the 2009–2010 National Health and Nutrition Examination Survey. Among participants reporting a joint-year usage of 0, 1–5, 6–20, or greater than 20 years, mean percent predicted FEV1s were 96.8% (95% confidence interval [CI] = 95.9–97.7), 95.4% (95% CI = 93.5–97.2), 94.7% (95% CI = 93.0–96.4), and 95.2% (95% CI = 92.3–98.0), respectively. Among these same groups, mean percent predicted FVCs were 98.7% (95% CI = 97.6–99.7), 99.2% (95% CI = 97.9–100.4), 99.8% (95% CI = 98.3–101.4), and 100.3% (95% CI = 98.8–101.8), respectively. Of these groups, mean FEV1/FVC ratios were 0.80 (95% CI = 0.79–0.81), 0.78 (95% CI = 0.77–0.80), 0.77 (95% CI = 0.76–0.78), and 0.77 (95% CI = 0.75–0.79), respectively.
Table 4.
Full results of three linear regression models for spirometric outcomes in the 2009–2010 National Health and Nutrition Examination Survey cohort (n = 2,956)
| Variable | Change in % Predicted FEV1 | P Value | Change in % Predicted FVC | P Value | Change in % FEV1/FVC | P Value |
|---|---|---|---|---|---|---|
| One additional joint-year | 0.02 ± 0.02 | 0.4 | 0.07 ± 0.02 | 0.004 | −0.03 ± 0.01 | 0.02 |
| One additional tobacco pack-year | −0.13 ± 0.02 | <0.0001 | −0.04 ± 0.03 | 0.2 | −0.1 ± 0.01 | <0.0001 |
| Increasing age, yr | −0.09 ± 0.002 | 0.001 | −0.11 ± 0.02 | <0.0001 | −0.2 ± 0.02 | <0.0001 |
| White race | 1.12 ± 0.66 | 0.1 | 1.8 ± 0.7 | 0.02 | −1.7 ± 0.3 | <0.0001 |
| Female | 1.80 ± 0.4 | 0.001 | 2.69 ± 0.53 | 0.0001 | 1.5 ± 0.3 | 0.0002 |
| History of asthma | −5.3 ± 1.11 | 0.0002 | −2.19 ± 1.06 | 0.06 | −3.0 ± 0.3 | <0.0001 |
| History of chronic bronchitis | −3.2 ± 1.65 | 0.07 | −3.25 ± 1.56 | 0.05 | −0.3 ± 0.6 | 0.6 |
| History of emphysema | −8.67 ± 6.91 | 0.2 | −1.86 ± 5.13 | 0.7 | −6.5 ± 2.9 | 0.04 |
Discussion
This study is the largest cross-sectional analysis examining the relationship between marijuana use and spirometric parameters of lung health to date. With the THC content of marijuana increasing in recent decades, it provides a relevant 20-year update on a similar study performed on the NHANES III cohort from 1988 to 1994. Furthermore, the more recent iterations of the NHANES have added new, more detailed questions regarding marijuana use, allowing us to more precisely quantitate cumulative lifetime marijuana exposure by way of joint-years, with 1 joint-year equal to smoking one joint daily for a year. Using these data, we asked the question “What are the effects of recent and cumulative lifetime marijuana use and respiratory symptoms and spirometric measures of lung health”? The study first shows that this is an important topic, as marijuana use is common among U.S. adults, with 59.1% reporting using marijuana in their lifetime and 12.2% reporting current use of marijuana in the previous 30 days. The study then demonstrates that current smokers are more likely to report recent symptoms of respiratory illness, but have little clinically significant associated changes in spirometry. Furthermore, it demonstrates that moderate cumulative lifetime marijuana use, up to 20 joint-years, is not associated with deleterious changes in spirometric measures of lung health. Although greater than 20 joint-years of cumulative marijuana exposure was associated with a twofold-increased odds of an FEV1/FVC less than 70%, this was the result of an increase in FVC, rather than a disproportional decrease in FEV1 as is typically associated with obstructive lung diseases.
Our findings regarding the respiratory symptoms of habitual marijuana smokers corroborate the existing evidence. Many studies have demonstrated that habitual marijuana smoke increases symptoms of bronchitis, and our data similarly show an increase in recent self-reported respiratory illness, with trends toward increases in self-reported respiratory infections and symptoms of wheezing (5). Supporting these clinical findings, several studies of the respiratory epithelium of conducting airways and bronchoalveolar lavage fluid of habitual marijuana smokers have shown an increase macro- and microscopic signs of inflammation (16–21). Furthermore, studies have shown that marijuana smoke is associated with a decrease in airway conductance, consistent with large airway edema seen endoscopically (22–24). Despite this characterization of marijuana smoke as a large airway irritant, our data did not show an association between increasing exposure in the prior month and deleterious change in spirometric values of small airways disease. Rather, for each additional day of marijuana smoked in the previous month, there was no associated change in FEV1, with a 0.13% increase in predicted FVC.
Our data regarding the cumulative effects of marijuana on lung function are also consistent with existing clinical literature. A large cross-sectional study from New Zealand demonstrated that marijuana smokers had a higher proportion of participants with an FEV1/FVC less than 80% when compared with nonsmokers (25). In the study examining the NHANES III cohort, although marijuana smokers did not have an independent association with an FEV1/FVC less than 70% after controlling for asthma and tobacco use, this may have been confounded by the fact that marijuana use was categorized as having smoked more or less than 100 joints in a lifetime (26). Corroborating our lack of association with an FEV1 decline, in a study of nearly 400 Californians followed with serial spirometry, marijuana smokers did not show significant declines in FEV1 (27). Similarly, in a longitudinal cohort of over 1,000 young adults in New Zealand, cumulative exposure to marijuana smoke among nontobacco smokers was associated with increases in total lung capacity, but no changes in measurements of airflow (22). In the 779 with baseline spirometry in this study, cumulative marijuana exposure increased FVC, but had no effects on FEV1 or FEV1/FVC ratio when adjusting for tobacco exposure (22). Finally, a more recent analysis of a longitudinal cohort from four large U.S. metropolitan areas from 1984 to 2006 revealed that FEV1 and FVC, unadjusted for age, showed increases at low doses of chronic marijuana exposure that then trended downward for moderate and heavy smokers (28).
Although moderate use of marijuana seems to be associated with little effect on spirometry, the question still remains as to whether the decreased FEV1/FVC with heavier marijuana exposure represents airflow obstruction. On the one hand, the spirometric pattern that emerges from our study, one of a preserved FEV1 with a diminished FEV1/FVC, has been associated with early airflow obstruction (29, 30). Furthermore, the relatively smaller effect compared with tobacco may be an artifact of the units of measure for these two exposures. Specifically, the tobacco pack-year represents a significantly larger amount of smoke exposure than the joint-year, given that there are 20 tobacco cigarettes in a pack. However, the pattern of marijuana’s effects seems to be distinctly different when compared with that of tobacco. With marijuana, the decreased FEV1/FVC seems to be driven by the increasing FVC, whereas, for tobacco, it seems to be driven by the relatively larger decrease in FEV1 compared with FVC. Furthermore, close inspection of Table 2 suggests that, among tobacco smokers with greater than 20 pack-years, increasing cumulative exposure to marijuana seems to have a protective effect on FEV1, and, likewise among marijuana smokers with over 20 joint-years, the deleterious effects of increasing tobacco exposure on FEV1 seems to be diminished.
Overall, the data suggest that the decrease in FEV1/FVC seen in heavy marijuana smokers is distinctly different than that of heavy tobacco smokers, and may not necessarily represent obstructive lung disease. Although one may speculate that the preservation of FEV1 may be due to the aforementioned bronchodilator properties of THC, data from studies on the long-term use of bronchodilators has not shown that they alter airway remodeling (31, 32). Another hypothesis may be the fact that marijuana smoke does not seem to induce the same level of oxidant stress in the small airways as tobacco smoke, a mechanism postulated as a causative factor in the development of chronic obstructive pulmonary disease (5, 33). Furthermore, the increase in FVC may be due to the deep inhalation technique of marijuana smoking (5, 34). With one study showing that marijuana smokers inhale greater puff volumes and have longer smoke retention times than tobacco smokers, it is possible that this habitual inhalational exercising of the respiratory muscles could increase FVC over time (34). This is further supported by the New Zealand cohort study, showing an increase in 25 ml of total lung capacity for each additional marijuana joint-year smoked (22).
In summary, this study adds to the literature on the effects of the marijuana on lung function in that it uses spirometric outcomes adjusted for age, uses a more precise estimation of cumulative marijuana exposure, and allows for more direct comparison to the effects of tobacco. The surveys and spirometry were conducted in a systematic and validated fashion by the NHANES, limiting data variability. Furthermore, the extensive information gathered in the surveys allowed for controlling of important variables. Despite these strengths, the study has several limitations given its cross-sectional design. As with many elements of a NHANES survey, there is a fair amount of missing data (13–15). Given that the original NHANES survey weights do not account for nonresponse to each questionnaire item, this may limit the generalizability of the estimates to the overall U.S. adult population, but are unlikely to add additional confounding to the studied relationships between marijuana use and lung function. Furthermore, as with any survey, there may be recall bias in the reporting of marijuana usage, impairing the precision of smoke exposure. There may also be a healthy survivor effect in that those participants who were eligible for the spirometric exam had to be alive and not using long-term oxygen, potentially overrepresenting those individuals who are not as affected by chronic smoke exposure. Finally, given the cross-sectional design, the study can only describe the association of lung function and chronic exposure, and not measure the longitudinal effect of that exposure in individuals over time. These limitations would tend to bias the result toward a null value.
In conclusion, in a large representative sample of U.S. adults, ongoing use of marijuana is associated with increased respiratory symptoms of bronchitis without a significant functional abnormality in spirometry, and cumulative marijuana use under 20 joint-years is not associated with significant effects on lung function. With current marijuana smokers reporting a mean joint-year exposure of 15.8 joint-years, these data have important public health implications. With the shifting political climate in the United States, these are important public health concerns that necessitate further inquiry into this growing field. Future research directions may potentially target study populations in those states in the United States where marijuana is now legally consumed, and it will now be more feasible to longitudinally follow users’ consumption patterns, pulmonary function, and symptoms.
Footnotes
Supported by National Institutes of Health grants T32 AA013528 (J.A.K.), R21 HL110044 (G.S.M.), P50 AA013757 (G.S.M.), UL1 TR000454 (J.A.K. and G.S.M.), and UL1 TR000455 (J.A.K. and G.S.M.).
Author Contributions: all authors revised the manuscript; J.A.K. contributed to the data acquisition, analysis, and writing of the manuscript; E.G.H. contributed to study concept and design; G.S.M. contributed to study concept, design, and analysis.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374:1383–1391. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- 2.Dimock M, Doherty C, Motel S. Washington, DC: Pew Research Center for the People and the Press; 2014. Majority now supports legalizing marijuana. [Google Scholar]
- 3.Adler JN, Colbert JA. Clinical decisions: medicinal use of marijuana—polling results. N Engl J Med. 2013;368:e30. doi: 10.1056/NEJMclde1305159. [DOI] [PubMed] [Google Scholar]
- 4.Marijuana Policy Project. Map of State Marijuana Laws 2014 [accessed 2014 Jul 14]. Available from: http://www.mpp.org/assets/pdfs/library/Map-of-State-Marijuana-Laws.jpg
- 5.Tashkin DP. Effects of marijuana smoking on the lung. Ann Am Thorac Soc. 2013;10:239–247. doi: 10.1513/AnnalsATS.201212-127FR. [DOI] [PubMed] [Google Scholar]
- 6.Tetrault JM, Crothers K, Moore BA, Mehra R, Concato J, Fiellin DA. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Arch Intern Med. 2007;167:221–228. doi: 10.1001/archinte.167.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tashkin DP, Shapiro BJ, Frank IM. Acute pulmonary physiologic effects of smoked marijuana and oral 9-tetrahydrocannabinol in healthy young men. N Engl J Med. 1973;289:336–341. doi: 10.1056/NEJM197308162890702. [DOI] [PubMed] [Google Scholar]
- 8.Calignano A, Katona I, Desarnaud F, Giuffrida A, La Rana G, Mackie K, Freund TF, Piomelli D. Bidirectional control of airway responsiveness by endogenous cannabinoids. Nature. 2000;408:96–101. doi: 10.1038/35040576. [DOI] [PubMed] [Google Scholar]
- 9.Vachon L, FitzGerald MX, Solliday NH, Gould IA, Gaensler EA. Single-dose effects of marihuana smoke: bronchial dynamics and respiratory-center sensitivity in normal subjects. N Engl J Med. 1973;288:985–989. doi: 10.1056/NEJM197305102881902. [DOI] [PubMed] [Google Scholar]
- 10.Mehmedic Z, Chandra S, Slade D, Denham H, Foster S, Patel AS, Ross SA, Khan IA, ElSohly MA. Potency trends of Delta9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci. 2010;55:1209–1217. doi: 10.1111/j.1556-4029.2010.01441.x. [DOI] [PubMed] [Google Scholar]
- 11.ElSohly MA, Ross SA, Mehmedic Z, Arafat R, Yi B, Banahan BF., III Potency trends of delta9-THC and other cannabinoids in confiscated marijuana from 1980–1997. J Forensic Sci. 2000;45:24–30. [PubMed] [Google Scholar]
- 12.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 13.Sternberg M, Hadgu A. Alexandria, VA: American Statistical Association; 2007. A comparison of methods to further adjust for non-response due to missing lab data in National Health and Nutrition Examination Survey (NHANES) [Google Scholar]
- 14.Mohadjer L, Bell B, Waksberg J. Rockville, MD: Westat, Inc; 1994. National Health and Nutrition Examination Survey III: accounting for item nonresponse bias. [Google Scholar]
- 15.Ezzati T, Khare M. Alexandria, VA: American Statistical Association; 1992. Nonresponse adjustments in a national health survey. [Google Scholar]
- 16.Barbers RG, Gong H, Jr, Tashkin DP, Oishi J, Wallace JM. Differential examination of bronchoalveolar lavage cells in tobacco cigarette and marijuana smokers. Am Rev Respir Dis. 1987;135:1271–1275. doi: 10.1164/arrd.1987.135.6.1271. [DOI] [PubMed] [Google Scholar]
- 17.Fligiel SE, Roth MD, Kleerup EC, Barsky SH, Simmons MS, Tashkin DP. Tracheobronchial histopathology in habitual smokers of cocaine, marijuana, and/or tobacco. Chest. 1997;112:319–326. doi: 10.1378/chest.112.2.319. [DOI] [PubMed] [Google Scholar]
- 18.Sherman MP, Campbell LA, Gong H, Jr, Roth MD, Tashkin DP. Antimicrobial and respiratory burst characteristics of pulmonary alveolar macrophages recovered from smokers of marijuana alone, smokers of tobacco alone, smokers of marijuana and tobacco, and nonsmokers. Am Rev Respir Dis. 1991;144:1351–1356. doi: 10.1164/ajrccm/144.6.1351. [DOI] [PubMed] [Google Scholar]
- 19.Baldwin GC, Tashkin DP, Buckley DM, Park AN, Dubinett SM, Roth MD. Marijuana and cocaine impair alveolar macrophage function and cytokine production. Am J Respir Crit Care Med. 1997;156:1606–1613. doi: 10.1164/ajrccm.156.5.9704146. [DOI] [PubMed] [Google Scholar]
- 20.Shay AH, Choi R, Whittaker K, Salehi K, Kitchen CM, Tashkin DP, Roth MD, Baldwin GC. Impairment of antimicrobial activity and nitric oxide production in alveolar macrophages from smokers of marijuana and cocaine. J Infect Dis. 2003;187:700–704. doi: 10.1086/368370. [DOI] [PubMed] [Google Scholar]
- 21.Roth MD, Arora A, Barsky SH, Kleerup EC, Simmons M, Tashkin DP. Airway inflammation in young marijuana and tobacco smokers. Am J Respir Crit Care Med. 1998;157:928–937. doi: 10.1164/ajrccm.157.3.9701026. [DOI] [PubMed] [Google Scholar]
- 22.Hancox RJ, Poulton R, Ely M, Welch D, Taylor DR, McLachlan CR, Greene JM, Moffitt TE, Caspi A, Sears MR. Effects of cannabis on lung function: a population-based cohort study. Eur Respir J. 2010;35:42–47. doi: 10.1183/09031936.00065009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tashkin DP, Calvarese BM, Simmons MS, Shapiro BJ. Respiratory status of seventy-four habitual marijuana smokers. Chest. 1980;78:699–706. doi: 10.1378/chest.78.5.699. [DOI] [PubMed] [Google Scholar]
- 24.Tashkin DP, Coulson AH, Clark VA, Simmons M, Bourque LB, Duann S, Spivey GH, Gong H. Respiratory symptoms and lung function in habitual heavy smokers of marijuana alone, smokers of marijuana and tobacco, smokers of tobacco alone, and nonsmokers. Am Rev Respir Dis. 1987;135:209–216. doi: 10.1164/arrd.1987.135.1.209. [DOI] [PubMed] [Google Scholar]
- 25.Taylor DR, Poulton R, Moffitt TE, Ramankutty P, Sears MR. The respiratory effects of cannabis dependence in young adults. Addiction. 2000;95:1669–1677. doi: 10.1046/j.1360-0443.2000.951116697.x. [DOI] [PubMed] [Google Scholar]
- 26.Moore BA, Augustson EM, Moser RP, Budney AJ. Respiratory effects of marijuana and tobacco use in a US sample. J Gen Intern Med. 2005;20:33–37. doi: 10.1111/j.1525-1497.2004.40081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tashkin DP, Simmons MS, Sherrill DL, Coulson AH. Heavy habitual marijuana smoking does not cause an accelerated decline in FEV1 with age. Am J Respir Crit Care Med. 1997;155:141–148. doi: 10.1164/ajrccm.155.1.9001303. [DOI] [PubMed] [Google Scholar]
- 28.Pletcher MJ, Vittinghoff E, Kalhan R, Richman J, Safford M, Sidney S, Lin F, Kertesz S. Association between marijuana exposure and pulmonary function over 20 years. JAMA. 2012;307:173–181. doi: 10.1001/jama.2011.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barisione G, Crimi E, Bartolini S, Saporiti R, Copello F, Pellegrino R, Brusasco V. How to interpret reduced forced expiratory volume in 1 s (FEV1)/vital capacity ratio with normal FEV1. Eur Respir J. 2009;33:1396–1402. doi: 10.1183/09031936.00183708. [DOI] [PubMed] [Google Scholar]
- 30.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 31.Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, Conway WA, Jr, Enright PL, Kanner RE, O'Hara P, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: the Lung Health Study. JAMA. 1994;272:1497–1505. [PubMed] [Google Scholar]
- 32.Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M, Investigators US. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 33.Sherman MP, Roth MD, Gong H, Jr, Tashkin DP. Marijuana smoking, pulmonary function, and lung macrophage oxidant release. Pharmacol Biochem Behav. 1991;40:663–669. doi: 10.1016/0091-3057(91)90379-g. [DOI] [PubMed] [Google Scholar]
- 34.Wu TC, Tashkin DP, Djahed B, Rose JE. Pulmonary hazards of smoking marijuana as compared with tobacco. N Engl J Med. 1988;318:347–351. doi: 10.1056/NEJM198802113180603. [DOI] [PubMed] [Google Scholar]