Supplemental Digital Content is available in the text
Keywords: disease activity, meta-analysis, rheumatic diseases, rheumatoid arthritis, systemic lupus erythematosus, vitamin D
Abstract
Background:
Vitamin D serum levels and the presence and activity of rheumatic conditions have been associated. However, many studies are merely observational, and the existent randomized clinical trials were never systematically analyzed. Therefore, this study aims to provide a systematic review and meta-analysis of such a topic.
Methods:
MEDLINE, EMBASE, LILACS, COCHRANE, and CINAHL were explored to identify randomized trials that investigated clinical repercussions of vitamin D (or analogs) supplementation for at least 3 months in rheumatic diseases. Standardized clinical and/or laboratorial outcomes related to disease activity were analyzed according to each disease before and after supplementation.
Results:
Database searches rendered 668 results; 9 were included—5 on rheumatoid arthritis, 3 on systemic lupus erythematosus, and 1 on systemic sclerosis. Seven of the studies were meta-analyzed. After vitamin D supplementation, rheumatoid arthritis recurrence decreased; however, not significantly (risk difference = −0.10, 95% CI = −0.21, 0.00, P = .05). No statistical significance was observed regarding visual analog scale (mean difference = 2.79, 95% CI = −1.87, 7.44, P = .24) and disease activity score28 (mean difference = −0.31, 95% CI = −0.86, 0.25, P = .28). Regarding systemic lupus erythematosus, anti-dsDNA positivity was significantly reduced (risk difference = −0.10, 95% CI = −0.18, −0.03; P = .005).
Conclusion:
Vitamin D supplementation reduced anti-dsDNA positivity on systemic lupus erythematosus and could possibly reduce rheumatoid arthritis recurrence, although novel randomized clinical trials are needed to confirm and extend the benefits of this hormone in immune-mediated rheumatic diseases.
1. Introduction
The association between vitamin D deficiency and rheumatic diseases has been described since a lower risk of developing autoimmune diseases was identified near the equator,[1,2] where people synthesize cholecalciferol on their skin for a longer period within the year.[3] Based on this finding, many researchers have investigated correlations between vitamin D serum levels and the presence/activity of rheumatic conditions.[1,4–6] With its increasing visibility, clinical repercussions of vitamin D supplementation have been studied in rheumatic diseases such as systemic lupus erythematosus (SLE)[1,4] and rheumatoid arthritis (RA)[7] and in non-rheumatic conditions such as tuberculosis[8] and psoriasis.[2] The results, then, prompted research on known and novel biomolecular and cellular functions of vitamin D.[2,3]
In addition to its classic action on the bone, kidneys and gastrointestinal tract, maintaining calcium and phosphorus homeostasis, the expression of the nuclear Vitamin D receptor was described in cells of the pituitary and parathyroid glands, kidneys, skin, gastrointestinal tract,[9] and the immune system.[10] Such discoveries have led to new research on fields that are not only related to bone metabolism[11] but also to the finding of new functions for vitamin D.[2] Pleiotropic actions of vitamin D on the immune system were described[11–14] and are of particular interest to this study because immune cells and cytokines participate directly in the pathobiology and activity of immune-mediated rheumatic diseases.
Recent in vitro studies have shown that vitamin D plays an important role in immune modulation by stimulating innate immunity, enhancing its activity, and decreasing adaptive immune activity.[15] This immunomodulatory action[16] is explained by a decreased production of cytokines such as interleukin-2 and interferon-γ, which is essential to the pro-inflammatory Th1 response,[17] as well as an increased interleukin-4 production, which is essential to Th2 response.[18] Vitamin D also stimulates regulatory T cells[19,20] while inhibiting Th17 and Th9 lymphocytes,[19] both involved in autoimmune disorders development as SLE,[21] RA,[22] and multiple sclerosis.[23] Following such findings, a therapeutic role for vitamin D in inflammatory and autoimmune conditions was theorized.
To assess this hypothesis, in vivo studies have used experimental models of autoimmune encephalomyelitis[24] and SLE.[25,26] Despite confirming the induction of regulatory T lymphocyte and double-positive CD4+/CD8+ T lymphocytes,[25] besides the inhibition of Th9 and Th17 lymphocytes by vitamin D, these studies still do not verify the clinical benefits of vitamin D supplementation as a treatment of autoimmune diseases.[3]
To this date, vitamin D supplementation is considered safe,[27] with a low risk of hypercalcemia[3] and urolithiasis[28] and with cardiovascular protection.[29] In rheumatology, vitamin D supplementation is well known to prevent glucocorticoid-induced osteoporosis[30,31] and to reduce fractures in elderly people with osteoporosis[32]; however, vitamin D supplementation is not well established in immune-mediated rheumatic diseases such as SLE, RA, systemic sclerosis (SSc), vasculitis and Sjögren Syndrome.[33]
These rheumatic conditions are prevalent[34] with decrease in quality of life and severe sequelae,[35,36] and they do not have accessible specific therapeutic targeting molecular structures.[37]
Despite scientific evidence of the possible therapeutic outcomes of vitamin D supplementation, many studies have been merely observational and have not led to precise conclusions.[33] The most trustworthy study designs are well-randomized and well-allocated experimental trials. However, the few existent randomized clinical trials that have investigated vitamin D and its analogs supplementation on the activity of immune-mediated rheumatic diseases were never analyzed systematically. No assertive conclusions exist on the clinical outcomes of vitamin D supplementation on such conditions.
Given this gap in the literature, this study aims to systematically analyze the results of such trials and to develop a meta-analysis in order to identify rheumatic conditions in which vitamin D supplementation could be an appropriate therapeutic strategy.
2. Materials and methods
2.1. Ethics disclosure
According to the policies of the authors’ institution, as no human patients or animal models were required in order to conceive this study, an ethics statement was not required. No funding bodies played a role in the design, writing, or decision to submit this work.
3. Eligibility criteria
3.1. Types of studies
Only randomized controlled trials, double-blinded or not, that studied the effect of vitamin D supplementation or its analogs were included.
3.2. Types of participants
Trials conducted on participants with any of the following diseases were eligible: Behçet Syndrome, Dermatomyositis, Juvenile Arthritis, Mixed Connective Tissue Disease, Polymyalgia Rheumatica, Rheumatic Fever, Rheumatoid Arthritis, Sjogren's Syndrome, Ankylosing Spondylitis, Systemic Lupus Erythematosus, Systemic Sclerosis, and Vasculitis.
3.3. Types of intervention
Selected manuscripts were required to include studies investigating the supplementation of vitamin D or its analogs for at least 3 months. This supplementation was compared with a matching placebo or no drugs. We considered that additional supplementation with calcium and/or use of other medications to control disease progression, if matched between groups, would not interfere with established outcomes.
3.4. Types of outcome measures
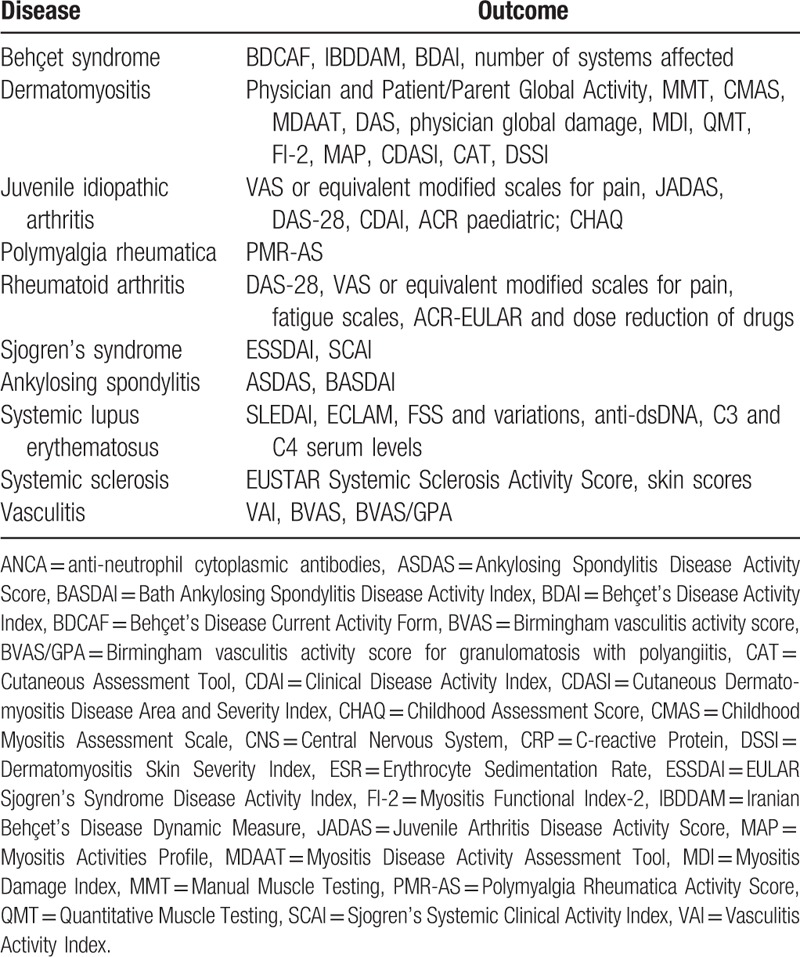
This review included trials that studied appropriate clinical and/or laboratory outcomes related to disease activity for each disease before and after vitamin D supplementation (Table 1).
Table 1.
Diseases included with its appropriate clinical and/or laboratorial outcomes.
3.5. Exclusion criteria
Trials with endpoints related solely to bone metabolism and/or cardiovascular events were excluded from the present study.
3.6. Information sources and search strategy
Manuscripts containing the following MeSH keywords and terms were identified by surveying MEDLINE, EMBASE, LILACS, COCHRANE, and CINAHL databases: rheumatic diseases, Behçet Syndrome, spondylitis, vasculitis, juvenile arthritis, rheumatoid arthritis, Sjogren's Syndrome, polymyalgia rheumatica, systemic lupus erythematosus, systemic sclerosis, dermatomyositis, vitamin D, cholecalciferol, hydroxycholecalciferols, ergocalciferols, 25-hydroxyvitamin D. Depending on the search mechanisms of each database, our strategy was adapted (supplementary File). Additional searches were performed using selected study references. There were no time or language restrictions, and the last search dates back to June/2016 for all databases.
3.7. Study selection
Identification, screening, and eligibility assessments were performed independently in an unblinded standardized manner by 2 reviewers. Disagreements between reviewers were discussed with a third researcher.
3.8. Data collection process
Two reviewers collectively developed a data extraction sheet. After extracting and checking the information, disagreements were resolved by discussion between the 2 review authors. If no agreement could be reached, a third author decided between the disagreement. After reading the full texts, 4 of the authors from the selected trials were contacted in order to retrieve further details. Only 1 author responded and provided numeric data that we previously could not access.
3.9. Data items
Initially, of all the data from the selected articles, we sought (I) the rheumatic disease(s) each of article studied; (II) the type of intervention; that is, what type of vitamin D was administered (cholecalciferol, calcitriol, alfacalcidol) and its corresponding doses and frequency of administration; (III) the comparison groups; that is, what type of treatment was compared to supplementation with vitamin D (other type or dose of vitamin D, placebo or no treatment); and (IV) the endpoints (described separately in the “types of outcome measures” section).
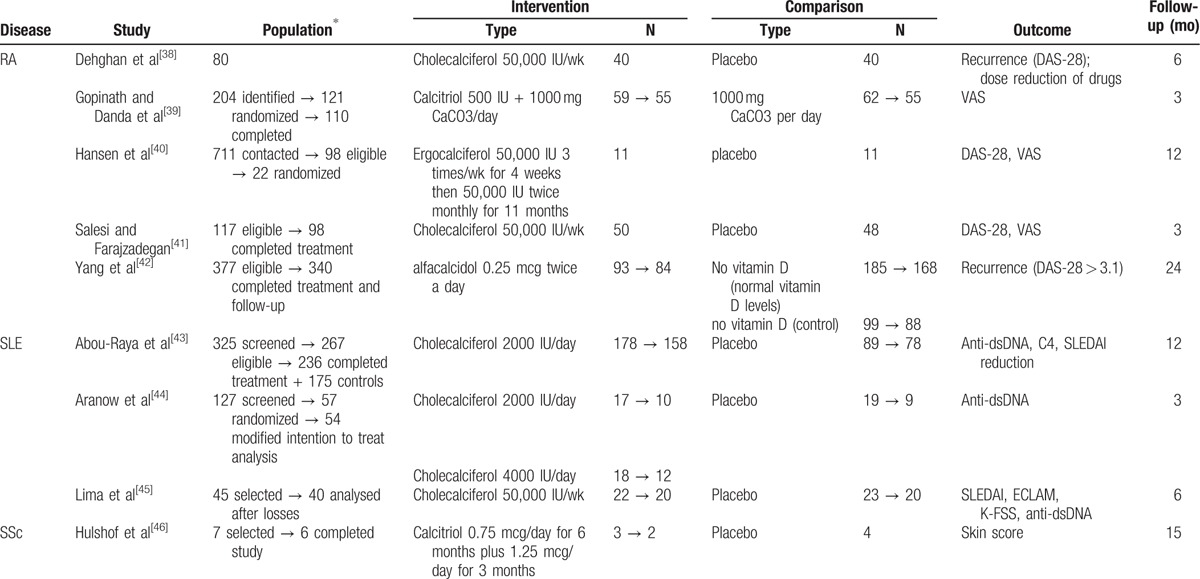
Subsequently, to simplify our further analyses, we expanded our data extraction to include numbers on (V) the total study population, (VI) the intervention and comparison group populations and (VII) the length of each trial (Table 2).
Table 2.
Study characteristics of the 9 studies included in the systematic review.
3.10. Risk of bias in individual studies
To ascertain the validity of eligible trials, 2 authors extracted and checked the adequacy of the (I) query of the study, (II) randomization, (III) concealment of allocation, (IV) blinding, (V) number of patients lost to follow-up, (VI) homogeneity between groups after randomization, (VII) outcome measures, and (VIII) an intention-to-treat analysis (Table 3).
Table 3.
Risk of bias in individual studies.
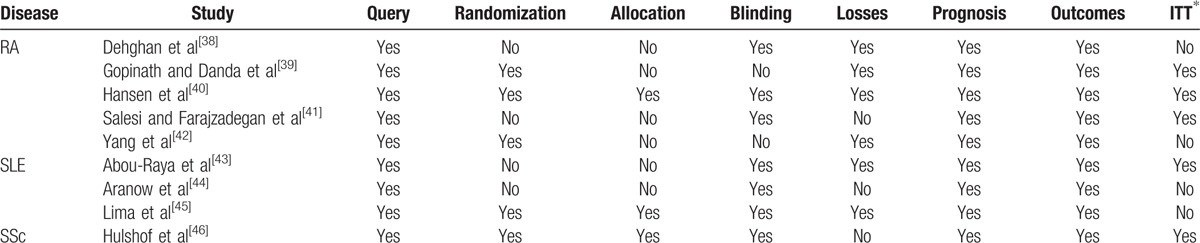
Regarding the study queries, we considered a study to have a low risk of bias whenever its objectives were clear. Randomization had to be generated by computer software or regulated by a pharmacy to be considered adequate. Unbiased allocation was defined as a sequence that was generated using computer random number generation or a random number table. Whenever the sequence generation method was not specified or was not random, allocation was considered inadequate (a high risk of bias). Double-blinding was recognized as the only adequate blinding. A high risk of bias was granted whenever more than 20% of any study branches were lost to follow-up. Homogeneity between groups after randomization was adequate if statistical significance between groups was not found. Whenever the proposed outcomes were adequate and the clinical and/or laboratory criteria used to assess the outcomes were relevant and followed up for at least 3 months, the outcomes were considered unbiased. Measurement of treatment effect was obtained using an intention-to-treat basis whenever possible. Whenever any of these items were poorly or not described, the said item was considered inadequate.
3.11. Summary measures
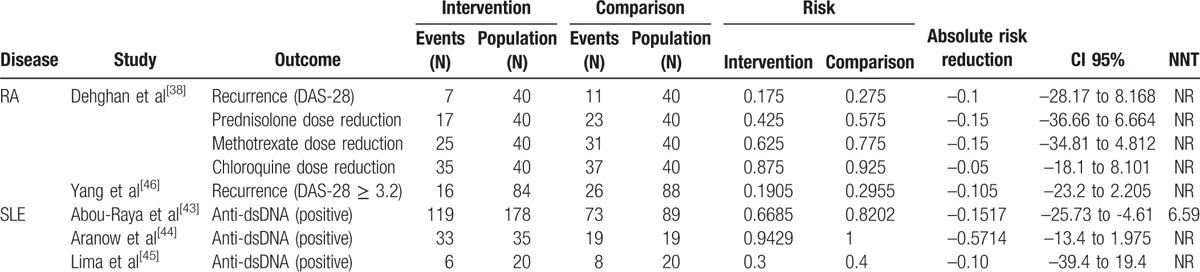
For every selected study, absolute risk reduction of clinical and/or laboratory parameters was the primary measure of treatment effect for each disease and outcome. Quantitative analyses were performed on an intention-to-treat basis and were confined to data derived from the period of end of treatment or follow-up. The number needed to treat (NNT), absolute risk reduction and 95% confidence intervals (95% CI) for each outcome measure were obtained from the articles when possible or were calculated using OpenEpi software.[47] When absolute risk reduction and 95%CI were not statistically significant, NNT was not calculated (shown as NA) (Table 4).
Table 4.
Study results sorted by disease (absolute variables).
Whenever studies presented continuous variables, we (I) depicted data as the mean or median, with its respective standard deviation (SD), (II) calculated a ratio between the means of each group, and (III) specified the significance of their differences (Table 5).
Table 5.
Study results sorted by disease (continuous variables).
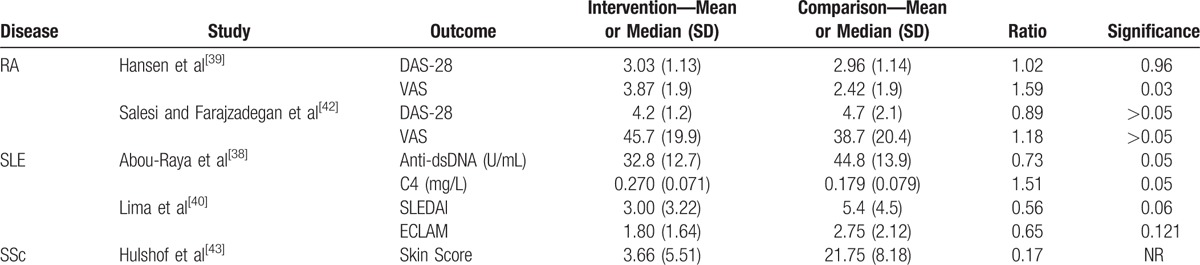
CI 95% = 95% confidence interval, DAS-28 = Disease Activity Score-28 for Rheumatoid Arthritis, N = absolute number, NNT = number needed to treat, NR = not reported, RA = rheumatoid arthritis, SLE = systemic lupus erythematosus.
CI 95% = 95% confidence interval, DAS-28 = Disease Activity Score-28 for Rheumatoid Arthritis, ECLAM = European Consensus Lupus Activity Measurement, NNT = number needed to treat, NR = not reported, RA = rheumatoid arthritis, SD = standard deviation, SLE = systemic lupus erythematosus, SLEDAI = Systemic Lupus Erythmatosus Disease Activity Index, SSc = systemic sclerosis, VAS = visual analog scale.
3.12. Planned methods of analysis
Meta-analyses were performed separately for each disease using Review Manager (version 5.3; The Nordic Cochrane Centre).[48] The results were expressed as a risk difference for categorical variables or as the mean difference for continuous variables. We measured the inconsistency (the percentage of total variation across studies due to heterogeneity) of effects across interventions using I2. In order to obtain adequate confidence intervals, we used a random-effects model if I2 > 40% and a fixed-effect model if I2 ≤ 40%. Statistically significant P values were considered as higher than P = .05.
3.13. Risk of bias across studies
The possibility of publication bias was assessed by evaluating a funnel plot of the trial mean differences for asymmetry, which can result from the non-publication of small trials with negative results. Nonetheless, other factors, such as differences in trial quality or true study heterogeneity, can produce asymmetry in funnel plots.
4. Results
4.1. Study selection
The MEDLINE, EMBASE, LILACS, COCHRANE, and CINAHL database searches identified 661 studies. An additional 7 studies that met the inclusion criteria were identified by reviewing the references of other relevant papers and by searching for studies that had cited these papers. Of these, 638 studies were discarded because after reviewing the abstracts, they clearly did not meet the inclusion criteria: other study types that did not involve clinical trials (reviews, commentary, letters, cross-sectional studies); interventions in nonrheumatic diseases or in non-autoimmune rheumatic conditions; interventions without vitamin D supplementation (calcium supplementation, bisphosphonates) or comparisons with other strategies as bisphosphonates or other vitamin D supplementation scheme; and cardiovascular, bone, genetic or molecular outcomes. Twenty-one studies were duplicated and therefore discarded. The full texts of the remaining 9 citations were examined in more detail and were included in this systematic review. Two studies did not have sufficient data and were excluded from the quantitative analysis. Therefore, 7 studies were meta-analyzed. No unpublished relevant studies were obtained (Fig. 1).
Figure 1.
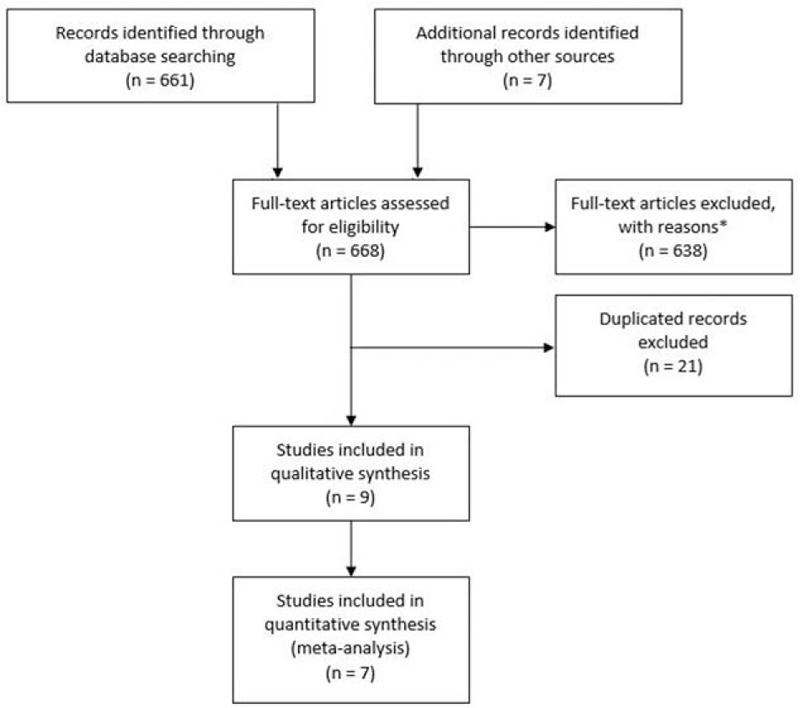
Flow diagram of study selection. ∗See the text for details.
4.2. Study characteristics
Because only studies conducted on patients with RA, SLE, and SSc met our eligibility criteria, only these 3 rheumatic diseases are reported in this systematic review and meta-analysis. Five trials were conducted on patients with RA, 3 trials on patients with SLE and only 1 trial on SSc. Characteristics of each study are summarized (Table 2) according to methods, participants, interventions, and outcomes.
4.3. Methods
All 9 studies selected for the review were randomized controlled trials published in English. However, 2of the trials,[39,42] both on RA, were not double-blind. The length of follow-up ranged from 3 to 24 months, in which 1 month was defined as 4 weeks when the period in months was not explicit.
4.4. Participants
The included studies involved 1161 participants, 640 with RA, 505 with SLE, and 6 with SSc.
The main inclusion criteria for patients with RA were its diagnosis according to the 2010 American College of Rheumatology and European League Against Rheumatism (ACR-EULAR) or the 1987 ACR criteria. For patients with SLE, the ACR criteria updated in 1997 were used by the 3 trials. For SSc, the diagnosis was “according to the criteria in the literature.”[46] All trials excluded patients with multiple comorbidities and polypharmacy.
4.5. Intervention
In RA studies, the intervention group used the following: cholecalciferol 50,000 IU/wk in 2 studies[38,41]; ergocalciferol 50,000 IU 3 times/wk for 4 weeks, then 50,000 IU twice monthly for 11 months in 1 study[40]; calcitriol 500 IU in 1 study[39]; and alfacalcidol 0.25 mcg twice a day in 1 study.[42] In all studies, the comparison group used placebo, and Gopinath and Danda[39] used calcium supplementation in both intervention and placebo groups.
For SLE, all 3 studies used cholecalciferol in the intervention group and compared to placebo. Administered vitamin D doses were different: 2000 IU/day in 1 study[43]; 2000 IU/day and 4000 IU/day in 1 study[44]; and 50,000 IU/wk in another study.[45]
For SSc, the only study[46] used calcitriol 0.75 mcg/day for 6 months, then 1.25 mcg/day for 3 months, compared to the placebo.
4.6. Outcomes
The outcomes for RA were recurrence, based on DAS-28[38]; dose reduction of methotrexate, chloroquine and glucocorticoid[38]; reduction in Visual Analog Scale (VAS) and Disease Activity Score for RA (DAS-28)[39–41]; and recurrence of disease, defined as an increase of 3.6 in DAS-28.[42]
For SLE, the Disease Activity Index (SLEDAI),[43,45] European Consensus Lupus Activity Measurement (ECLAM),[45] K-FSS (Fatigue Severity Scale/children version),[45] positivity of anti-dsDNA[43–45] and serum C4 levels[43] were considered outcomes. Skin scores were the preferred outcome for SSc.[46]
All of these studies featured other outcomes that were considered unrelated or impertinent to our review and are not depicted here.
4.7. Risk of bias within studies
Risk of bias in all 9 works was analyzed. Every study was classified as having a high risk of bias for at least 1 parameter. The highest risk of bias was found in the trial on SLE by Aranow et al,[44] and the lowest was described in the trial on RA by Hansen et al.[40] The assessment of the different domains of risk of bias in each trial are shown in Table 3.
4.8. Results of individual studies
Outcomes considered for each study are summarized in Table 4 with data of each intervention group and intervention effect. One study had to be removed from this analysis because the data were insufficient; even though the author was contacted, we did not receive a response.[39]
In all studies, considering the 3 diseases, there was a risk reduction for all outcomes described as absolute variables, with no statistical significance. Nonetheless, Abou-Raya et al[43] reported that the reduction in anti-dsDNA positivity was statistically significant in SLE, with a NNT of 7.
Regarding continuous variables, VAS in RA[40] was found worse after vitamin D supplementation. Differently, fatigue in SLE was improved after 6 months of vitamin D supplementation using K-FSS for the following conditions: fatigue when performing exercise (P = .03), fatigue easily (P = .003), fatigue to medium efforts (P = .02), fatigue considered a problem (P = .03), and fatigue interfering with social life (P = .01).[45] Hulshof et al[46] did not find significant differences for skin scores after vitamin D supplementation for 9 months (Table 5), nor after another 6 months of follow-up after treatment (not shown).
4.9. Synthesis of results
Meta-analysis was performed for RA and SLE because we only found 1 study about SSc.[46] Therefore, 7 trials were included in this meta-analysis: 4 studies on RA and 3 studies on SLE.
For RA, 3 analyses were performed based on the following outcomes: VAS reduction, DAS-28 reduction, and recurrence (Fig. 2A–C). VAS reduction was meta-analyzed using 2 studies,[40,41] with 61 patients receiving vitamin D supplementation and 59 in the placebo group. Vitamin D was not associated with significant VAS reduction (mean difference = 2.79, 95%CI = –1.87, 7.44, P = .24), with a calculated medium heterogeneity between studies for this analysis (I2 = 44%). DAS-28 reduction was meta-analyzed using the same 2 studies[40,41] with low heterogeneity (I2 = 0% for this analysis), and vitamin D was not associated with significant DAS-28 reduction (mean difference = –0.31, 95%CI = –0.86, 0.25, P = .28). Recurrence was meta-analyzed using 2 other studies,[38,42] with 124 patients in the intervention group and 128 in the placebo group; vitamin D supplementation produced an insignificant reduction in recurrence (risk difference = –0.10, 95%CI = –0.21, 0.00, P = .05). Heterogeneity was, again, low (I2 = 0%).
Figure 2.
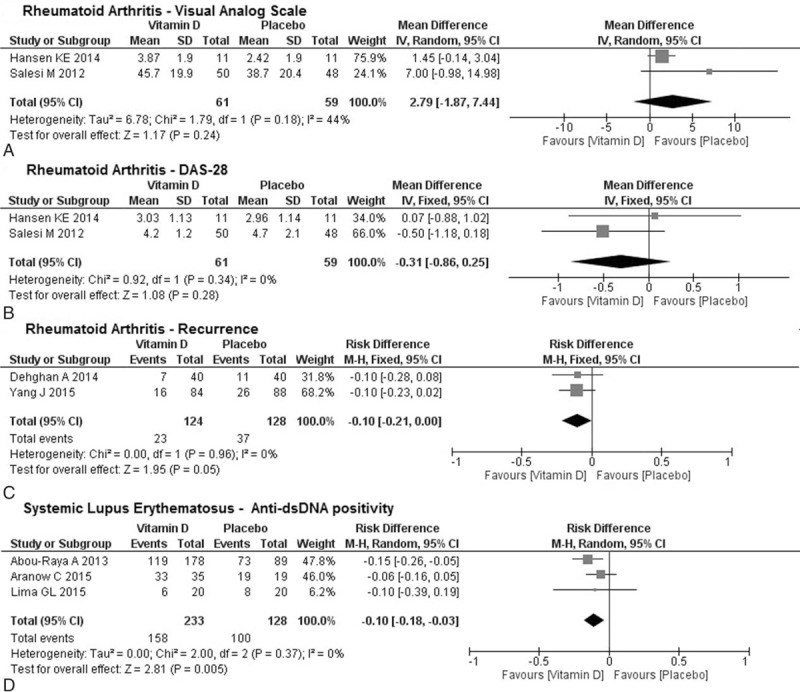
Mean difference of (A) VAS reduction and (B) DAS-28 reduction; and (C) risk difference of recurrence between studies on rheumatoid arthritis. No statistical significance was obtained. (D) Risk difference of anti-dsDNA positivity between studies on Systemic Lupus Erythematosus. Statistical significance was obtained (P = .005). DAS-28 = Disease Activity Score for Rheumatoid Arthritis, VAS = Visual Analog Scale.
For SLE, only 1 analysis was performed based on the reduction of anti-dsDNA positivity that was reported by 3 studies[43–45] (Fig. 2D). Overall, 233 patients in the intervention group and 128 patients in the placebo group were analyzed, and vitamin D supplementation was significantly associated with a reduction in anti-dsDNA positivity (risk difference = –0.10, 95%CI = –0.18, –0.03; P = .005), and no evidence of heterogeneity was found (I2 = 0%).
4.10. Risk of bias across studies
Even though all meta-analyses that were performed had no evidence of heterogeneity, a funnel plot was drawn for every outcome analyzed. No considerable asymmetry was observed in any of the plots (data not shown).
5. Discussion
5.1. Summary of evidence
Few randomized clinical trials have investigated the clinical benefits of vitamin D supplementation on rheumatic diseases. RA, SLE, SSc were the only 3 rheumatic diseases studied by eligible clinical trials. Therefore, the evidence is, overall, not sufficiently robust to determine the effectiveness of vitamin D supplementation on immune-mediated rheumatic diseases.
Five randomized trials on RA were eligible for systematic review, and 4 were meta-analyzed. However, as vitamin D supplementation schemes and clinical or laboratory outcomes were different across studies, few conclusions could be drawn from the meta-analysis.
Despite analyzing only 2 studies[38,42] that used different methods to assess recurrence in remitted patients, this meta-analysis showed a tendency of reduction in recurrence (P = .06) after vitamin D supplementation. Individually, Yang et al[42] concluded that low vitamin D levels are a risk factor for RA recurrence but that treatment with alfacalcidol for 24 months did not change recurrence rates significantly (P = .11). Dehghan et al[38] also described non-significant differences in frequency of recurrence after cholecalciferol supplementation for 6 months (P = .42). Notably, the study[42] with the bigger population showed results that were closer to statistical significance. A pathophysiological explanation for this finding is that active vitamin D decreases IL-17-expressing CD4+ T cells, and consequently reduces proinflammatory cytokines such as IL-1β, IL-6, and TNF. It also decreases Th17-induced osteoclast activity and RA-associated bone resorption by inducing expression of RANK ligand on fibroblast-like synoviocytes and osteoblasts.[7] Articular damage is further prevented by vitamin D because it inhibits interleukin 1A-mediated production of matrix metalloproteinases.[49] Moreover, vitamin D also increases regulatory T cells, wich are impaired in RA.[7]
Furthermore, a recent meta-analysis that evaluated only serum vitamin D levels in RA showed a negative relationship between 25-hydroxyvitamin D serum concentrations and C-reactive protein and DAS-28, further increasing evidence that vitamin D deficiency is correlated to inflammatory biomarkers and disease activity.[50]
Regarding SLE, all 3 studies also had different supplementation schemes but shared 1 outcome of anti-dsDNA positivity, which rendered a reduction on the autoantibody positivity. Two studies[43,45] reported a statistically significant reduction in anti-dsDNA levels after 12 months[43] (P = .05) and after 6 months[45] (P = .03) of supplementation. However, 1 trial[44] reported fairly stable anti-dsDNA levels throughout 3 months of follow-up, and this difference may be explained by study length. Additionally, Lima et al[45] administered higher cholecalciferol doses than the other 2 studies.
Vitamin D deficiency has been associated with higher antinuclear antibodies levels in healthy subjects and in treatment-naive SLE patients, suggesting it might be a trigger for autoantibody production.[51,52] Moreover, elevated anti-dsDNA titers have been associated with moderate-to-severe SLE flares,[53] especially when its clinical presentation depends pathophysiologically on anti-dsDNA immune complex deposition as in renal impairment.[54–56] However, this correlation is controversial and other studies describe, with more consistent results, increases in anti-dsDNA titers as predictive biomarkers of clinical SLE flare.[57,58] Moreover, in patients with more than a 50% increase in anti-dsDNA titers, precautionary treatment prevents flares.[57] Thus, vitamin D supplementation may be beneficial to patients with high anti-dsDNA positivity, possibly reducing clinical flares.
Other less robust conclusions such as improvements to fatigue severity[45] can be drawn based solely on single trials due to the lack of comparable outcomes between studies.
Evidence on SSc is even poorer because the only identifiable trial was also not able to draw significant conclusions on its own, despite not finding any difference in skin scores after vitamin D supplementation.
6. Limitations
6.1. Outcome level
This meta-analysis, as any other, combines data from studies and estimates treatment effects with more precision than is possible with 1 study only. Thus, its main limitation, as with any overview, is that the patient population, the vitamin D supplementation schemes and the outcome definitions are not the same across studies. Moreover, a limited number of randomized controlled trials on vitamin D supplementation have been conducted in rheumatic diseases, and 2 of these studies were excluded because important data could not be extracted.[39,46]
Notably, despite having positive statistical significance between groups after supplementation, several outcomes were not associated with clinical results such as anti-dsDNA in SLE. Additionally, new clinical trials should have a follow-up longer than 6 or 12 months, as shorter times may be insufficient to determine this correlation.
Studies on RA were highly heterogeneous primarily regarding intervention schemes and comparison groups. Also, 1 study[40] had a small sample size (N = 22).
6.2. Study and review level
This review also has several limitations as the quality of trials varied. Randomization was inadequate in 4 of 9 trials, and allocation was inadequate in 6 of the trials, compromising the reliability of these data. Four of the trials did not analyze the data according to the intention-to-treat principle, which could lead to overestimation of the treatment effect in these trials.
7. Conclusion
Few randomized clinical trials investigated vitamin D supplementation on the activity of immune-mediated rheumatic diseases, and no assertive conclusions were drawn regarding its clinical outcomes. This work demonstrated a trend of reduction in rheumatic disease activity using vitamin D supplementation in RA, with a possible reduction in its recurrence, and in SLE, with a significant reduction in anti-dsDNA positivity, which is a biomarker of clinical flares. Nonetheless, novel randomized clinical trials are needed in order to increase the evidence level on vitamin D supplementation for immune-mediated rheumatic diseases, especially SLE and RA.
Supplementary Material
Footnotes
Abbreviations: 95% CI = 95% confidence interval, ACR = American College of Rheumatology, DAS-28 = Disease Activity Score for Rheumatoid Arthritis, ECLAM = European Consensus Lupus Activity Measurement, EULAR = European League Against Rheumatism, K-FSS = Fatigue Severity Scale/children version, NNT = number needed to treat, RA = rheumatoid arthritis, SD = standard deviation, SLE = systemic lupus erythematosus, SLEDAI = Systemic Lupus Erythematosus Disease Activity Index, SSc = systemic sclerosis, VAS = visual analog scale.
ASF and TQF equally contributed for this study.
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo [2016/08530–7 to T.Q.F.]; Conselho Nacional de Desenvolvimento Científico e Tecnológico [301805/2013–0 to R.M.R.P.]; and Federico Foundation [to R.M.R.P.].
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Pelajo CF, Lopez-Benitez JM, Miller LC. Vitamin D and autoimmune rheumatologic disorders. Autoimmun Rev 2010;9:507–10. [DOI] [PubMed] [Google Scholar]
- [2].Holick MF. Vitamin D: a millenium perspective. J Cell Biochem 2003;88:296–307. [DOI] [PubMed] [Google Scholar]
- [3].Christakos S, Dhawan P, Verstuyf A, et al. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev 2016;96:365–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kamen D, Aranow C. Vitamin D in systemic lupus erythematosus. Curr Opin Rheumatol 2010;20:532–7. [DOI] [PubMed] [Google Scholar]
- [5].Rossini M, Maddali Bongi S, La Montagna G, et al. Vitamin D deficiency in rheumatoid arthritis: prevalence, determinants and associations with disease activity and disability. Arthritis Res Ther 2010;12:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Eloi M, Horvath DV, Ortega JC, et al. 25-Hydroxivitamin D serum concentration, not free and bioavailable vitamin D, is associated with disease activity in systemic lupus erythematosus patients. PLoS One 2017;12:e0170323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jeffery LE, Raza K, Hewison M. Vitamin D in rheumatoid arthritis-towards clinical application. Nat Rev Rheumatol 2015;12:201–10. [DOI] [PubMed] [Google Scholar]
- [8].Rook GA, Steele J, Fraher L, et al. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology 1986;57:159–63. [PMC free article] [PubMed] [Google Scholar]
- [9].Stumpf W, Sar M, Reid F, et al. Target cells for 1,25-dihydroxyvitamin D3 in intestinal tract, stomach, kidney, skin, pituitary, and parathyroid. Science 1979;206:1188–90. [DOI] [PubMed] [Google Scholar]
- [10].Adams JS, Hewison M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch Biochem Biophys 2012;523:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gatenby P, Lucas R, Swaminathan A. Vitamin D deficiency and risk for rheumatic diseases: an update. Curr Opin Rheumatol 2017;25:184–91. [DOI] [PubMed] [Google Scholar]
- [12].Marinho A, Taveira M, Vasconcelos C. Topics on vitamin D in systemic lupus erythematosus: analysis of evidence and critical literature review. Immunol Res 2017;65:495–511. [DOI] [PubMed] [Google Scholar]
- [13].He XJ, Ding Y, Xiang W, et al. Roles of 1,25(OH)2D3 and vitamin D receptor in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus by regulating the activation of CD4 + T cells and the PKCδ /ERK signaling pathway. Physiology C 2017;40:743–56. [DOI] [PubMed] [Google Scholar]
- [14].Vasile M, Corinaldesi C, Antinozzi C, et al. Vitamin D in autoimmune rheumatic diseases: a view inside gender differences. Pharmacol Res 2016;117:228–41. [DOI] [PubMed] [Google Scholar]
- [15].Cantorna MT. Mechanisms underlying the effect of vitamin D on the immune system. Proc Nutr Soc 2010;69:286–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Baeke F, Takiishi T, Korf H, et al. Vitamin D: modulator of the immune system. Curr Opin Pharmacol 2010;10:482–96. [DOI] [PubMed] [Google Scholar]
- [17].Lemire JM, Adams JS, Kermani-Arab V, et al. 1,25-Dihydroxyvitamin D3 suppresses human T helper/inducer lymphocyte activity in vitro. J Immunol 1985;134:3032–5. [PubMed] [Google Scholar]
- [18].Mahon BD, Wittke A, Weaver V, et al. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem 2003;89:922–32. [DOI] [PubMed] [Google Scholar]
- [19].Joshi S, Pantalena L-C, Liu XK, et al. 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol Cell Biol 2011;31:3653–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Unger WWJ, Laban S, Kleijwegt FS, et al. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. Eur J Immunol 2009;39:3147–59. [DOI] [PubMed] [Google Scholar]
- [21].Garrett-Sinha LA, John S, Gaffen SL. IL-17 and the Th17 lineage in systemic lupus erythematosus. Curr Opin Rheumatol 2008;20:519–25. [DOI] [PubMed] [Google Scholar]
- [22].Hsu H-C, Yang P, Wang J, et al. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol 2008;9:166–75. [DOI] [PubMed] [Google Scholar]
- [23].Matusevicius D, Kivisäkk P, He B, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler 1999;5:101–4. [DOI] [PubMed] [Google Scholar]
- [24].Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci U S A 1996;93:7861–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ding Y, Liao W, He X-J, et al. Effects of 1,25(OH)2 D3 and vitamin D receptor on peripheral CD4+/CD8+ double-positive T lymphocytes in a mouse model of systemic lupus erythematosus. J Cell Mol Med 2016;21:975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lemire JM, Ince A, Takashima M. 1,25-Dihydroxyvitamin D3 attenuates the expression of experimental murine lupus of MRL/l mice. Autoimmunity 1992;12:143–8. [DOI] [PubMed] [Google Scholar]
- [27].Cranney A, Horsley T, O’Donnell S, et al. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep) 2007;158:1–235. [PMC free article] [PubMed] [Google Scholar]
- [28].Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 2006;354:669–83. [DOI] [PubMed] [Google Scholar]
- [29].Abrahamsen B, Sahota O. Do calcium plus vitamin D supplements increase cardiovascular risk? BMJ 2011;342:d2080. [DOI] [PubMed] [Google Scholar]
- [30].Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2010;62:1515–26. [DOI] [PubMed] [Google Scholar]
- [31].Pereira RMR, de Carvalho JF, Paula AP, et al. Guidelines for the prevention and treatment of glucocorticoid-induced osteoporosis. Rev Bras Reumatol 2012;52:580–93. [PubMed] [Google Scholar]
- [32].Tang BMP, Eslick GD, Nowson C, et al. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet (London, England) 2007;370:657–66. [DOI] [PubMed] [Google Scholar]
- [33].Abrahamsen B, Harvey NC. The role of vitamin D supplementation in patients with rheumatic diseases. Nat Rev Rheumatol 2013;9:411–22. [DOI] [PubMed] [Google Scholar]
- [34].Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum 2008;58:15–25. [DOI] [PubMed] [Google Scholar]
- [35].Radner H, Yoshida K, Smolen JS, et al. Multimorbidity and rheumatic conditions-enhancing the concept of comorbidity. Nat Rev Rheumatol 2014;10:252–6. [DOI] [PubMed] [Google Scholar]
- [36].Marsico A, Atzeni F, Piroddi A, et al. Costs of pain in rheumatology. Reumatismo 2014;66:103–7. [DOI] [PubMed] [Google Scholar]
- [37].Péntek M, Poór G, Wiland P, et al. Biological therapy in inflammatory rheumatic diseases: issues in Central and Eastern European countries. Eur J Health Econ 2014;15(suppl 1):S35–43. [DOI] [PubMed] [Google Scholar]
- [38].Dehghan A, Rahimpour S, Soleymani-Salehabadi H, et al. Role of vitamin D in flare ups of rheumatoid arthritis. Z Rheumatol 2014;73:461–4. [DOI] [PubMed] [Google Scholar]
- [39].Gopinath K, Danda D. Supplementation of 1,25 dihydroxy vitamin D3 in patients with treatment naive early rheumatoid arthritis: A randomised controlled trial. Int J Rheum Dis 2011;14:332–9. [DOI] [PubMed] [Google Scholar]
- [40].Hansen KE, Bartels CM, Gangnon RE, et al. An evaluation of high-dose vitamin D for rheumatoid arthritis. J Clin Rheumatol 2014;20:112–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Salesi M, Farajzadegan Z. Efficacy of Vitamin D in patients with active rheumatoid arthritis receiving methotrexate therapy. Rheumatol Int 2012;32:2129–33. [DOI] [PubMed] [Google Scholar]
- [42].Yang J, Liu L, Zhang Q, et al. Effect of vitamin D on the recurrence rate of rheumatoid arthritis. Exp Ther Med 2015;10:1812–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Abou-Raya A, Abou-Raya S, Helmii M. The effect of vitamin D supplementation on inflammatory and hemostatic markers and disease activity in patients with systemic lupus erythematosus: a randomized placebo-controlled trial. J Rheumatol 2013;40:265–72. [DOI] [PubMed] [Google Scholar]
- [44].Aranow C, Kamen DL, Dall’Era M, et al. Randomized, double-blind, placebo-controlled trial of the effect of vitamin D3 on the interferon signature in patients with systemic lupus erythematosus. Arthritis Rheumatol (Hoboken, NJ) 2015;67:1848–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lima GL, Paupitz J, Aikawa NE, et al. Vitamin D supplementation in adolescents and young adults with juvenile systemic lupus erythematosus for improvement in disease activity and fatigue scores: a randomized, double-blind, placebo-controlled trial. Arthritis Care Res (Hoboken) 2016;68:91–8. [DOI] [PubMed] [Google Scholar]
- [46].Hulshof MM, Bavinck JNB, Bergman W, et al. Double-blind, placebo-controlled study of oral calcitriol for the treatment of localized and systemic scleroderma. J Am Acad Dermatol 2000;43:1017–23. [DOI] [PubMed] [Google Scholar]
- [47].Dean AG, Sullivan KM, Soe MM. OpenEpi: Open Source Epidemiologic Statistics for Public Health. Available at: www.OpenEpi.com. Accessed January 1, 2015. [Google Scholar]
- [48].The Nordic Cochrane Centre TCC. Review Manager (RevMan). 2014. [Google Scholar]
- [49].Tetlow LC, Woolley DE. The effects of 1 alpha,25-dihydroxyvitamin D(3) on matrix metalloproteinase and prostaglandin E(2) production by cells of the rheumatoid lesion. Arthritis Res 1999;1:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lin J, Liu J, Davies ML, et al. Serum Vitamin D level and rheumatoid arthritis disease activity: review and meta-analysis. PLoS One 2016;11:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Shahin D, El-farahaty RM, Houssen ME, et al. Serum 25-OH vitamin D level in treatment-naive systemic lupus erythematosus patients: Relation to disease activity. IL-23 and IL-17 2016;23:1–0. [DOI] [PubMed] [Google Scholar]
- [52].Ritterhouse LL, Crowe SR, Niewold TB, et al. Vitamin D deficiency is associated with an increased autoimmune response in healthy individuals and in patients with systemic lupus erythematosus. Ann Rheum Dis 2011;70:1569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Petri MA, Van Vollenhoven RF, Buyon J, et al. Baseline predictors of systemic lupus erythematosus flares: data from the combined placebo groups in the phase III belimumab trials. Arthritis Rheum 2013;65:2143–53. [DOI] [PubMed] [Google Scholar]
- [54].Petri M, Singh S, Tesfasyone H, et al. Prevalence of flare and influence of demographic and serologic factors on flare risk in systemic lupus erythematosus: a prospective study. J Rheumatol 2009;36:2476–80. [DOI] [PubMed] [Google Scholar]
- [55].Nikpour M, Urowitz MB, Ibañez D, et al. Frequency and determinants of flare and persistently active disease in systemic lupus erythematosus. Arthritis Care Res 2009;61:1152–8. [DOI] [PubMed] [Google Scholar]
- [56].Durcan L, Petri M. Immunomodulators in SLE: clinical evidence and immunologic actions. J Autoimmun 2016;74:73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Floris A, Piga M, Cauli A, et al. Predictors of flares in Systemic Lupus Erythematosus: preventive therapeutic intervention based on serial anti-dsDNA antibodies assessment. Analysis of a monocentric cohort and literature review. Autoimmun Rev 2016;15:656–63. [DOI] [PubMed] [Google Scholar]
- [58].Pan N, Amigues I, Lyman S, et al. A surge in anti-dsDNA titer predicts a severe lupus flare within six months. Lupus 2014;23:293–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


