Abstract
Telbivudine (LdT) is an orally l-nucleoside with potent and specific antihepatitis B virus (HBV) activity. The higher rate of hepatitis B e antigen (HBeAg) seroconversion of LdT treatment than other anti-HBV agents suggests a potential immunomodulatory effect. The aim of the study was to investigate the changes of regulatory T cell (Treg)/interleukin (IL)-17-producing CD4+T helper (Th17) balance during LdT treatment and to discuss the relationship of Treg/Th17 balance with HBeAg change in HBeAg-positive chronic hepatitis B (CHB) patients receiving LdT antiviral treatment. Twenty-seven HBeAg-positive CHB patients received LdT for 24 weeks and the percentages of Tregs and cells (Th17 cells) in peripheral blood as well as the serum TGF-β1 and IL-17 levels in these patients were longitudinally analyzed. We found that the frequencies of Tregs and Th17 cells in peripheral blood as well as the serum TGF-β1 and IL-17 levels increased significantly in CHB patients compared with healthy controls. During the LdT treatment, the Tregs frequency and TGF-β1 level tended to decrease, and Th17 cells frequency and IL-17 level showed a reverse “V”-type change. The frequency of Tregs and the ratio of Treg/Th17 were significantly lower in the HBeAg loss group than those in the HBeAg no-loss group at the baseline. More important, the Tregs frequency and TGF-β1 level were both positively correlated with HBeAg level during the LdT treatment for 24 weeks. Our data suggest that the lower Tregs frequency and Treg/Th17 ratio at the baseline of LdT treatment, the more likely to get the HBeAg loss. HBeAg negative can be predicted using changes in Tregs frequency and TGF-β1 level during LdT treatment in CHB patients. Maybe we could provide the immunology marker for exploring the mechanism of the higher HBeAg seroconversion rate of LdT therapy.
Keywords: chronic hepatitis B, HBeAg positive, telbivudine, Th17 cells, Tregs
1. Introduction
Hepatitis B virus (HBV) infection is a serious threat to human health. Approximately 2 billion people have been infected with HBV, and more than 370 million patients worldwide are chronically infected. Hepatitis B is a leading cause of chronic hepatitis, cirrhosis, and hepatocellular carcinoma and accounts for about 1 million deaths annually.[1]
HBV is a noncytopathic, hepatotropic deoxyribonucleic acid (DNA) virus that induces a series of host immune responses, resulting in progressive inflammatory liver damage.[2] The immune response between the host and virus can affect the clinical outcome of HBV infection and clinical antiviral therapy.[3] CD4+T cells play an important role in the induction and maintenance of specific T-cell immunity to clear viral infection.[4] Patients with chronic hepatitis B (CHB) exhibit a weak or undetectable virus-specific T-cell response.[5] This T-cell hyporesponsiveness is associated with high viral and/or antigen load in patients with CHB.[6,7] Nucleos(t)ide analogs can effectively suppress HBV replication, alleviate liver injury, and decelerate disease progression. Nevertheless, patients with CHB exhibit varied responses to these drugs, which is related to the immune status of the host.[3,8]
An immune disorder or imbalance exists in patients with CHB. Helper CD4+T cells can orchestrate host immune responses by releasing distinct cytokine profiles. Recent studies have described 2 additional subsets, namely, regulatory T cells (Tregs) and interleukin (IL)-17-producing CD4+T helper (Th17) cells.[9,10] The reciprocal relationships between T-helper subsets and the outcome of anti-HBV therapy have also been reported.[11,12] Tregs effectively inhibit other immune cells by secreting cytokines TGF-β1 and IL-10 to mediate immune tolerance and maintain immune balance.[9] Patients with hepatitis B e antigen (HBeAg) positive CHB exhibited a higher percentage of Tregs in their peripheral blood and liver, and the higher percentage of Tregs is positively correlated with HBV-DNA level.[13–15]. Furthermore, HBeAg-positive patients exhibit a higher percentage of Tregs in peripheral blood than HBeAg-negative patients.[13,16] Long-term treatment with adefovir dipivoxil or entecavir enhances HBV-specific T-cell immunity and reduces the frequency of Tregs in patients with CHB.[15,17,18] These findings suggest that Tregs actively participate in regulating anti-HBV responses. Th17 cells, a new discovery subtype of CD4+T cells, are characterized by secreting several kinds of cytokines, such as IL-17A (main effect cytokine), IL-17F, and IL-22. Th17 cells have been shown to play a role in a number of liver diseases, including alcoholic liver disease, primary biliary cirrhosis, and CHB.[19–22] Th17 cells are significantly increased in patients with CHB, and the frequency positively associated with the grade of liver inflammation in the patients, indicated by serum alanine aminotransferase (ALT) level.[19,20] Tregs and Th17 cells play a role in suppressing and promoting the inflammatory responses, respectively. Many studies have found that an imbalance between Tregs and Th17 cells is closely related to the development of chronic inflammation, autoimmune diseases, and cancer.[23–26] Su et al[26] found that the increase of Tregs was lower than that of Th17 cells in patients with low-to-moderate CHB, which led to a decreased ratio of Treg/Th17 cells. The frequency of Tregs significantly increased in patients with severe CHB, whereas that of Th17 cells slightly increased, resulting in increased ratio of Treg/Th17 cells. Zhang et al[18] also indicated that the Treg/Th17 ratio decreased during the first 3 months of entecavir treatment as the HBV DNA levels were inhibited. These results indicated that the balance of Treg/Th17 plays an important role in mediating the immune response of anti-HBV therapy.
Telbivudine (LdT), a nucleotide analog, has been used in antiviral treatment of CHB. Available data from clinical trials indicate that LdT is a potent inhibitor of HBV replication and a more efficient inducer of HBeAg seroconversion compared with other antiviral reagents in patients with CHB.[27,28] Chen et al[29] demonstrated that LdT treatment enhanced the reconstitution of CD4 response, but also showed a significant enhancement in stimulation of HBV-specific T cells activity and reduced HBV serum titers. LdT treatment also suppressed the programmed death ligand-1 expression in T cells and increased the secretion of IFN-γ, which may be attributed to HBeAg seroconversion.[30] Pan et al[31] found that patients with CHB, whose frequency of peripheral blood Tregs rapidly decreased at the early phase of LdT treatment, could achieve HBeAg seroconversion easily. These studies suggested that the higher HBeAg seroconversion rate of LdT antivirus therapy may be associated with the immunomodulatory activities of this regimen.
In this study, we performed a longitudinal study to investigate the changes of Treg/Th17 balance during LdT treatment and to discuss the relationship of Treg/Th17 balance with HBeAg change in HBeAg-positive CHB patients receiving LdT antiviral treatment. To provide the immunology marker for exploring the mechanism of the higher HBeAg seroconversion rate of LdT therapy.
2. Patients and methods
2.1. Patients
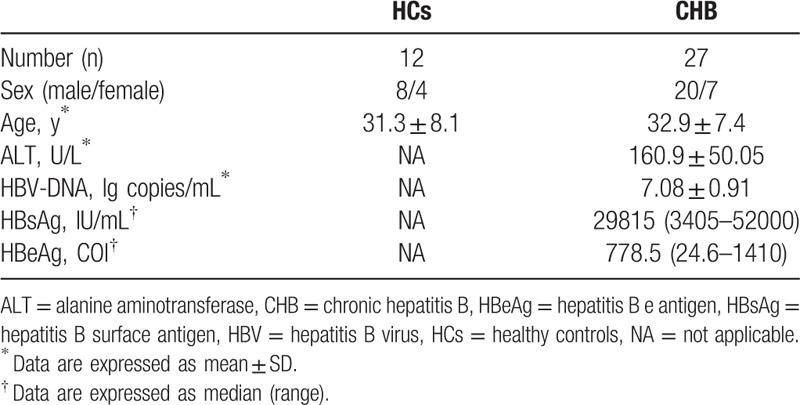
Twenty-seven treatment-naive patients with CHB (20 males and 7 females, aged 22–48 years) between September 2012 and September 2013 at the Department of Hepatology from Tianjin Second People's Hospital were enrolled in this study. All patients were consecutively followed up with protocol visits for 24 weeks during the course of the LdT antiviral treatment (600 mg orally per day). The CHB diagnostic criteria were described in detail in a previous study.[32] The screening criteria were as follows: all patients with seropositive for hepatitis B surface (HBsAg) for more than 6 months; seropositive for HBeAg; HBV DNA ≥ 105 copies/mL; serum ALT level ≥ twice the upper limit of the normal level; and has not received immunomodulatory agents or antivirus therapy. Patients coinfected with human immunodeficiency virus, hepatitis A virus, hepatitis C virus, or hepatitis D virus were excluded from this study. Patients with other possible causes of chronic liver damage, such as alcohol, drugs, autoimmune diseases, and congestive heart failure were also excluded. Another 12 gender-, age-, and ethnicity-matched healthy subjects were recruited as healthy controls (HCs). The clinical characteristics of these subjects are listed in Table 1. Peripheral venous blood samples were collected from each patient at baseline (0) and 4, 8, 12, 16, 20, and 24 weeks after LdT treatment. The samples were used to measure Tregs and Th17 cells frequencies and TGF-β1 and IL-17 levels. The study protocol was approved by the Ethics Committee of Tianjin Second People's Hospital, and written informed consents for the therapy and study were obtained from each patient.
Table 1.
Clinical characteristics of the populations enrolled in the study.
2.2. Flow cytometric analysis
For Treg cell examination, fresh heparinized periphera blood (100 μL) was firstly surface-stained with fluorescein isothiocyanate (FITC)-conjugated antihuman CD4 antibodies and allophycocyanin-conjugated antihuman CD25 antibodies for 30 minutes, then lysed with FACS lysing solution (BD PharMingen, San Diego, CA), and treated with eBioscience fix/perm mixture (eBiosciences, San Diego, CA) according to the manufacturer's instructions. Finally, cells were incubated with phycoerythrin (PE)-conjugated antihuman FoxP3 antibodies for 30 minutes fixed, and analyzed using FACSCalibur (BD Biosciences, New Jersey) and FlowJo software (Tristar, El Segundo, CA). For Th17 cell examination, fresh heparinized peripheral blood (200 μL) was incubated with 300-ng/ml phorbol-12-myristate-13-acetate and 1 mg/mL ionomycin (both from SigmaAldrich, St Louis, MO) in 800 mL of Roswell Park Memorial Institute 1640 medium supplemented with 10% fetal calf serum for 5 hours. Monensin (0.4 mM, BD PharMingen) was added during the first hour of incubation. The blood cells were then lysed with FACS lysing solution (BD PharMingen). Surface-stained with FITC-conjugated antihuman CD4 antibodies for 30 minutes, fixed and permeabilized with Perm/Fixsolution (BD PharMingen), and then stained intracellularly with PE-conjugated antihuman IL-17A. Isotope controls were used to ensure antibody specificity. All antibodies were purchased from BD PharMingen. Stained cells were analyzed by FACSCalibur (Becton Dickinson) and FlowJo software 7.6.1 (Tristar).
2.3. Enzyme-linked immunosorbent assay
Serum concentrations of TGF-β1 and IL-17 were measured by commercially available Enzyme-linked immunosorbent assay Kits (R&D Systems, Minneapolis, MN) according to the protocols provided by the manufacturer. The minimal detectable concentration of TGF-β1 and IL-17 was 1.0 pg/mL. The data were read at 450 nm by a microplate reader (Alisei Quality System, SEAC, Italy). All samples were assessed in triplicate.
2.4. Virological and biochemical assessments
The serum HBV DNA level was measured by fluorescent quantitative PCR with commercially available kits (PG Biotech Company, Shenzhen, China) according to the manufacturer's instruction.
The threshold of the HBV DNA detection limit was 500 copies/mL, we defined it as the HBV DNA negativity if the HBV DNA load less than 500 copies/mL. The levels of HBsAg, anti-HBs, HBeAg, anti-HBe, anti-HBc, anti-hepatitis C virus, anti-HDV, anti-hepatitis G virus, and anti-human immunodeficiency virus were measured using commercially available kits (Abbot Laboratories, North Chicago, IL) in our clinical lab. The level of ALT was tested with a conventional auto analyzer using commercial reagents (Beckman, Brea, CA).
2.5. Statistical analysis
All data were analyzed using SPSS 17.0 (SPSS Inc, Chicago, IL). Data were presented as mean ± SD, or median with range. For comparison between the 2 groups by independent samples Student t test. Multiple comparisons at different time points between the 2 groups were carried out by analysis of variance for repeated measurement. The correlations between the variables were evaluated using the Spearman rank correlation test. For all tests, 2-sided P values less than .05 were considered significant.
3. Results
3.1. The comparison of Treg/Th17 frequencies and TGF-β1/IL-17 levels between CHB patients and HCs
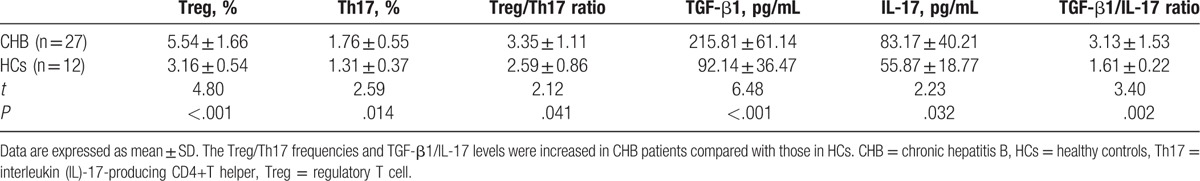
As shown in Table 2, the Tregs frequency, Th17 cell frequency and the ratio of Treg/Th17 were significantly increased in CHB patients compared with those in HCs (both P < .05). As well, the TGF-β1 level, IL-17 level, and the ratio of TGFβ1/IL-17 were higher in CHB patients compared with those in HCs (both P < .05).
Table 2.
The comparison of Treg/Th17 frequencies and TGF-β1/IL-17 levels between CHB patients and HCs (mean ± SD).
3.2. Correlation analysis of Tregs, Th17 cells, and their related cytokines and clinical parameters of patients with CHB at the baseline
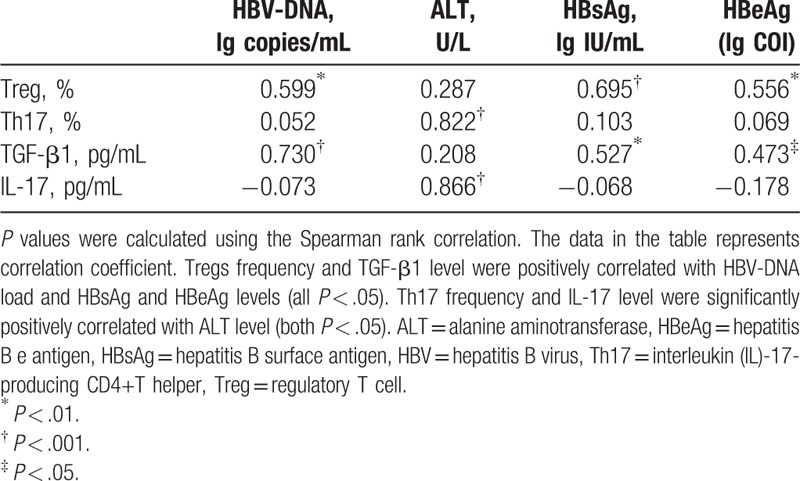
As shown in Table 3, Tregs frequency and TGF-β1 level were positively correlated with HBV-DNA load and HBsAg and HBeAg levels (all P < .05). Th17 cells frequency and IL-17 level were not correlated with HBV DNA load, HBsAg level, and HBeAg level (both P > .05). As well as there was no correlation among the Tregs frequency, TGF-β1 level, and ALT level (both P > .05), whereas Th17 frequency and IL-17 level were significantly positively correlated with ALT level (both P < .05).
Table 3.
The correlation between Treg/Th17 balance and clinic parameters.
3.3. Virological and biochemical response to LdT therapy in patients with CHB
During the entire 24-week therapy course, virological and biochemical parameters showed a decreasing trend from the 4-week. HBV-DNA load, the levels of HBsAg and HBeAg, and ALT levels remarkably reduced after 12 weeks of treatment (Fig. 1A–D). After 24 weeks, HBV-DNA negativity rate was 88.9%, and ALT level reduced to the normal levels (<40 U/L) in all patients. In addition, HBeAg negative was achieved in 6 patients, and seroconversion from HBeAg to anti-HBeAg was achieved in 2 patients. Loss of serum HBsAg during the treatment was not achieved in any patient.
Figure 1.
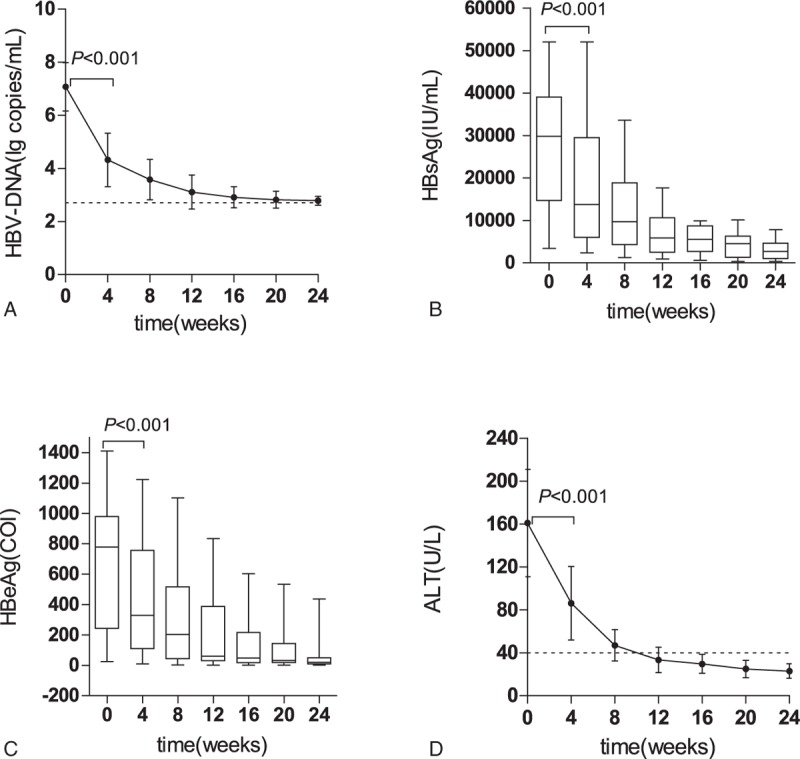
The change of clinical parameters of chronic hepatitis B patients undergoing telbivudine therapy. (A–C) The change of virological parameters during the treatment; (D) The change of serum alanine aminotransferase (ALT) level during the treatment. The hepatitis B virus DNA load, hepatitis B surface and hepatitis B e antigen levels, and ALT level at 4-week were significantly lower than those at the baseline (P < .001). Data were presented as mean ± SD in (A and D), data were presented as median with range in (B and C). ALT = alanine aminotransferase, DNA = deoxyribonucleic acid, HBeAg = hepatitis B e antigen, HBsAg = hepatitis B surface, HBV = hepatitis B virus.
3.4. Change in the frequencies of Tregs and Th17 cells and levels of TGF-β1 and IL-17 during LdT treatment
As show in Fig. 2, we found that the Tregs frequency and TGF-β1 level tended to decrease with the extension of antiviral therapy (Fig. 2A and B), which decreased obviously at week 8, respectively, compared with the baseline (both P < .05). At the week 24, Tregs frequency decreased obviously compared with the week 12 (P < .05, Fig. 2A), which reached close to the HCs (P > .05, Fig. 2A). However, no significant statistical difference was observed of TGF-β1 level between the weeks 24 and 12 (P > .05, Fig. 2B), and the TGF-β1 level was still higher than HCs at week 24 (P < .05, Fig. 2B). Interestingly, the frequencies of Th17 cells and IL-17 level showed a reverse “V”-type change (Fig. 2C and D). They increased significantly compared to the baseline at 4 and week 8, respectively (both P < .05), and reached to the maximal peak at week 8. Then they gradually decreased from week 12 to 24, with the levels reached close to the baseline level at week 24 (both P > .05), but they were still higher than HCs (both P < .05).
Figure 2.
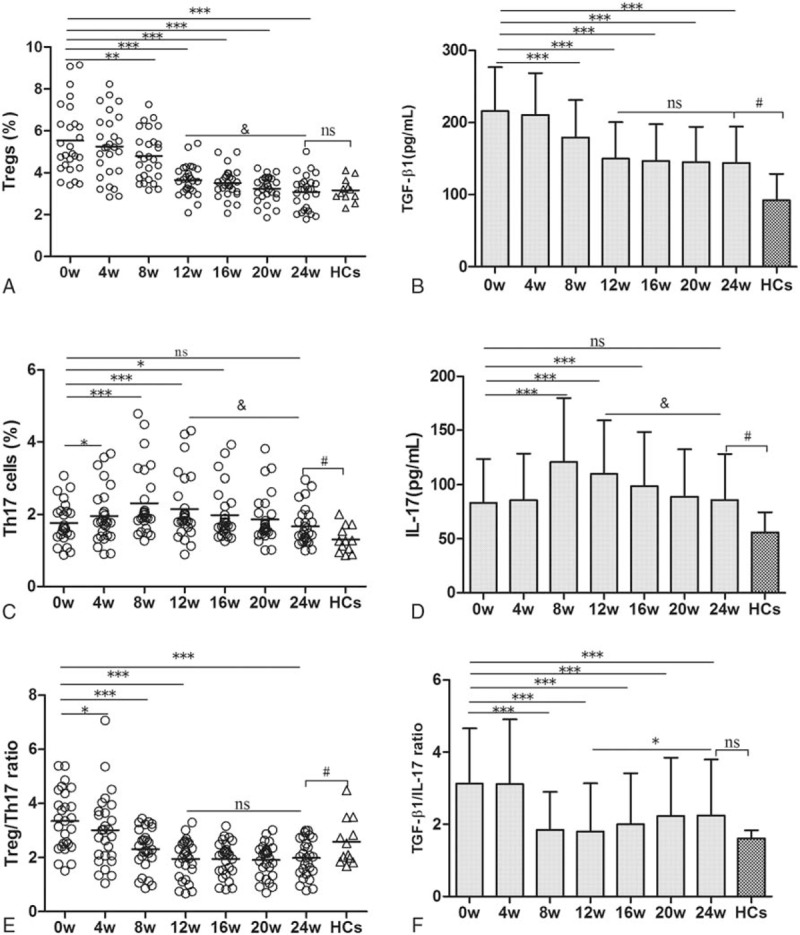
Change in the frequencies of regulatory T cells and interleukin (IL)-17-producing CD4+T helper cells and levels of TGF-β1 and IL-17 during telbivudine treatment. (A) ∗∗P < .01, ∗∗∗P < .001, compared with the baseline. &P < .001, compared between week 24 and 12. (B) ∗∗∗P < .001, compared with the baseline; #P < .01, compared between week 24 and healthy controls (HCs). (C) ∗P < .05, ∗∗∗P < .001, compared with the baseline; &P < .001, compared between week 24 and 12; #P < .05, compared between week 24 and HCs. (D) ∗∗∗P < .001, compared with the baseline; &P < .001, compared between week 24 and 12; #P < .05, compared between week 24 and HCs. (E) ∗P < .05, ∗∗∗P < .001, compared with the baseline; #P < .05, compared between week 24 and HCs. (F) ∗∗∗P < .001, compared with the baseline. ∗P < .05, compared between week 24 and 12. ns: no statistical significance. Data were presented as mean ± SD.
We also found the Treg/Th17 ratio decreased significantly at the week 4, 8, and 12 compared with the baseline (Fig. 2E, all P < .05). However, the Treg/Th17 ratio decreased steadily from week 12 to 24, and no significant statistical difference was observed between week 12 and 24 (P > .05, Fig. 2E). The TGFβ1/IL-17 ratio decreased significantly compared to the baseline at week 8 (Fig. 2F, P < .05) and reached to the lowest ratio at week 12. At the same time, we also found that the TGFβ1/IL-17 ratio showed slightly increase from week 12 onward to week 24, and then it reached close to the HCs at week 24 (P > .05, Fig. 2F).
3.5. Correlations between Treg/Th17 balance and HBeAg change
We divided all patients into HBeAg loss group and no-loss group according to HBeAg status at week 24 and then compared the difference of Treg/Th17 cell frequencies and TGF-β1/IL-17 levels between the 2 groups at the baseline. The frequency of Tregs and the ratio of Treg/Th17 were significantly lower in the HBeAg loss group than those in the HBeAg no-loss group at the baseline (both P < .05). In addition, the frequency of Th17 cells, the levels of TGF-β1 and IL-17 and the ratio of TGF-β1/IL-17 were not significantly statistically different between the 2 groups (both P > .05) (Table 4).
Table 4.
The comparison of Tregs/Th17 frequencies and TGF-β1/IL-17 ratio between HBeAg loss group and no-loss group at the baseline (mean ± SD).
Our results clearly indicated that the HBeAg level tended to decrease during the LDT treatment, and we also found the Treg/Th17 balance was changed during the treatment. So we further analyzed the correlation between changes in Treg/Th17 balance and HBeAg level in patients with CHB during the antiviral therapy. Results showed that the frequency of Tregs and the level of TGF-β1 were positively correlated with HBeAg level during the period from the baseline to week 24 (Fig. 3A and B; both P < .05), whereas no correlation were observed between the ratios of Treg/Th17 or TGF-β1/IL-17 and HBeAg level (Fig. 3C and D; both P > .05).
Figure 3.
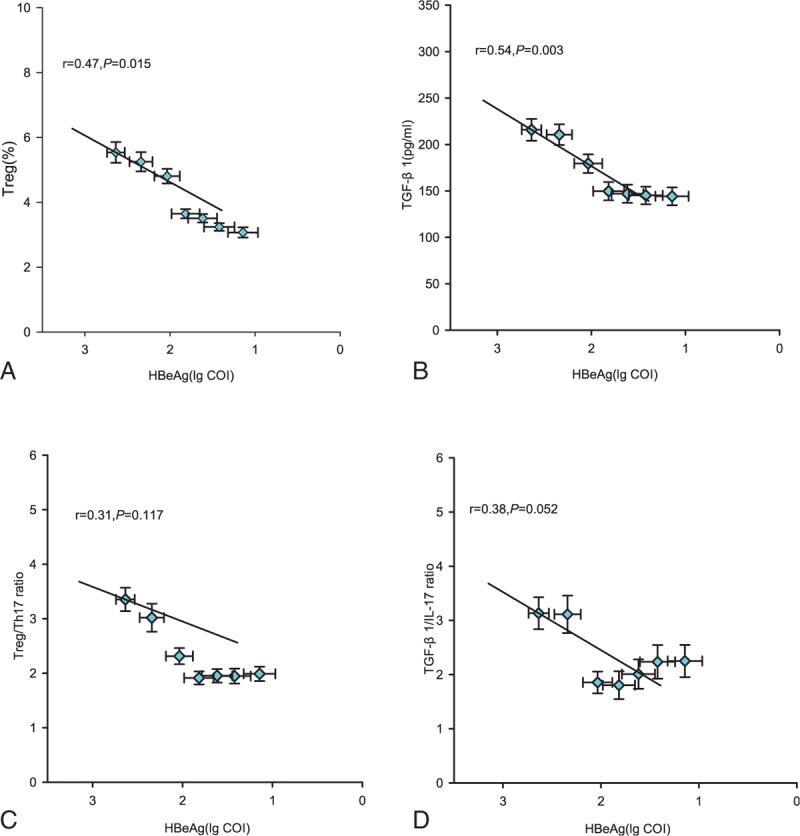
Correlation between the regulatory T cell (Treg)/interleukin (IL)-17-producing CD4+T helper (Th17) balance and hepatitis B e antigen (HBeAg) level. (A–D) The Tregs frequency, TGF-β1 level, the ratio of Tregs/Th17, the ratio of TGF-β1/IL-17, and HBeAg level at various time points of treatment (baseline and weeks 4, 8, 12, 16, 20, and 24) are indicated on the plots by diamonds. Error bars indicate standard error of mean.
4. Discussion
Previous studies showed that host immune status, HBV load, and HBeAg status are key predicting factors to decide the prognosis of patients with CHB. These patients exhibit varied responses to antiviral drugs, which could be due to different immune status of the host.[3] The balance between Tregs and Th17 cells may significantly influence the progress and prognosis of hepatic diseases.[25] LdT is an l-nucleoside that has been recently approved for use in patients with chronic HBV infections. Clinical trials have confirmed that LdT potently inhibits HBV replication and achieves the higher HBeAg seroconversion rate,[27,28] and exerts immune modulation effects on both the adaptive and innate immune systems.[29–31] Hence, this study aimed to observe changes in Treg/Th17 cells and their related cytokines under LdT therapy, analyzed the correlation between changes in Treg/Th17 balance and HBeAg negative or HBeAg level, and provided the immunological markers for exploring the mechanism of the higher HBeAg seroconversion rate of LdT therapy.
Previous studies showed that LdT treatment was superior to lamivudine in terms of the ability to reduce HBV load to undetectable levels, normalize serum ALT, and improve the rates of HBeAg seroconversion in patients with CHB.[27,28] In the present study, we observed that an early rapid viral load reduction and remarkably serum ALT level reduced after the LdT. This finding provided supporting data for the excellent anti-HBV effects of LdT.
To elucidate the roles of Tregs, Th17 cells, TGF-β1, and IL-17 on the immunopathology of chronic HBV infection, we analyzed the relationship between the frequencies of these cells and the levels of related cytokines and clinical parameters. We found that the peripheral blood Tregs frequency and TGF-β1 level were significantly higher in HBeAg-positive CHB patients than those in the HCs (P < .05). And Tregs frequency and TGF-β1 level were positively correlated with the level of HBV-DNA, HBsAg, and HBeAg (P < .05). This finding was consistent with the study of Xu et al[14] and Guo et al.[33] A previous study[12] showed that Tregs can secrete TGF-β1, which in turn induces Tregs proliferation in the absence of other inflammatory cytokines. The positive feedback effect between Tregs and TGF-β1 jointly mediate the immune tolerance status of HBeAg-positive CHB patients, resulting in HBV persistent infection. We also found that peripheral blood Th17 cells frequency and IL-17 level were significantly higher in HBeAg-positive CHB patients than those in the HCs (P < .05). Moreover, Th17 cells frequency and IL-17 level were positively correlated with the level of ALT (P < .05), which is consistent with the findings of previous studies.[19,20] These findings suggest that Th17 cells and their related cytokines were positively associated with the grade of liver inflammation and participated in liver injury process and HBV clearance. We also found that the ratio of Treg/Th17 and TGF-β1/IL-17 also increased in CHB patients compared with those in the HCs, the balance of Treg/Th17 is skewed toward Treg cells in HBeAg-positive CHB patients. Hence, observing changes in the Treg/Th17 ratio could provide reference for observing the immune status of host and antivirus therapy.
Previous study observed that Adefovir treatment can partially reduce the frequency of circulating Tregs, and this reduction occurs concomitantly with increase in HBV-specific T cell response[15]. Zhang et al[18] indicated that Treg/Th17 ratio decreased during the first 3 months of entecavir treatment along with the inhibition of HBV DNA level. In the present study, we found that Tregs frequency and TGF-β1 level tended to decrease with prolonged LdT antiviral therapy, which may contribute to the inhibition of HBV replication. We also found that Th17 cells frequency and IL-17 level showed a reverse “V”-type change, increased to their maximum levels at week 8 and then gradually decreased to the baseline. This trend may be related to the weakened immune suppression function to Th17 cells along with the reduction of Tregs frequency and TGF-β1 level at the early period of antiviral therapy. Thereby the damaged Th17 cells function was partially restored and IL-17 secretion increased, which may contribute to the HBV clearance. On the other hand, the previous study suggested that Th17 cell was positively associated with the grade of liver inflammation.[19,20] The HBV DNA decrease and ALT normalization at the early period of treatment may be adverse to the Th17 cell differentiation, resulting in the decline of Th17 cells frequency and IL-17 level after 12 weeks of treatment. The exact mechanism remains unclear. A previous study reported that the Treg/Th17 ratio declined in complete responders but was not significantly different in nonresponders during the antiviral therapy.[34] In our study, the Treg/Th17 ratio and TGF-β1/IL-17 ratio decreased after 12 weeks of the treatment, which was coincident with the decline of HBV DNA, HBsAg, and HBeAg. This suggested that changes in Treg/Th17 balance may be related to antiviral response. Hence, detection of Treg/Th17 balance has great clinical meaning for evaluating antiviral efficacy.
HBeAg was considered a toleragen in HBV infection. HBeAg seroconversion indicates that the immune status of HBeAg-positive CHB patients changed from immune tolerance to immune activation and which has been considered the key marker of treatment success. Previous studies[13,35]found that HBeAg-positive patients exhibited a higher percentage of Tregs in peripheral blood or hepatic tissues compared with HBeAg-negative patients. This finding suggested that HBeAg may be involved in the induction of Tregs. We compared the difference in Treg/Th17 cell frequencies and TGF-β1/IL-17 levels at the baseline between the HBeAg loss group and HBeAg no-loss group at week 24. The results showed that the frequency of Tregs and the ratio of Treg/Th17 were significantly lower in the HBeAg loss group compared with those in the HBeAg no-loss group at the baseline (P < .05). Nevertheless, no significant statistical difference was observed between the 2 groups in Th17 cell frequency, TGF-β1 and IL-17 levels and TGF-β1/IL-17 ratio. Our data suggest that the lower Tregs frequency and Treg/Th17 ratio at the baseline of antiviral therapy, the more likely to get the HBeAg loss, and vice versa, which further suggested that the balance of Treg/Th17 may influence HBeAg seroconversion. Zhang et al[18] highlighted the existing direct relationship between Treg/Th17 ratio and HBV DNA levels. We dissected the relationship between changes in Treg/Th17 balance and HBeAg level in these patients with CHB during antiviral therapy. Results showed that the frequency of Tregs and the level of TGF-β1 were both positively correlated with HBeAg level during the period from the baseline up to week 24. Hence, HBeAg negative can be predicted using changes in Treg cell frequency and TGF-β1 level undergoing LdT treatment.
In conclusion, LdT therapy not only rapidly suppresses HBV DNA replication but also exerts immunomodulatory effect on Treg/Th17 balance. The decrease in Treg cell frequency and TGF-β1 level could disrupt the immune tolerance, and the increase in Th17 cell frequency and IL-17 level could contribute to immune injury and HBV DNA clearance. Our data also suggest HBeAg negative can be predicted using changes in Tregs frequency and TGF-β1 level during LdT treatment in CHB patients. Maybe we could provide the immunological markers for exploring the higher HBeAg seroconversion rate of LdT therapy.
We recognize that our study has some limitations, such as a relatively small sample size, a single-center study, and short observation period. The bias may exist in the study. Only 2 patients achieved HBeAg seroconversion, and we could not analyze the relationship between the Treg/Th17 balance and HBeAg seroconversion rate, just through the change of HBeAg level to predict HBeAg negative or seroconversion. Therefore, we will perform a large sample, multicenter, and a longer observation period study to obtain a reliable immunological index for forecasting HBeAg seroconversion of LdT treatment and explore the immunomodulatory mechanism of LdT antiviral therapy.
Footnotes
Abbreviations: ALT = alanine aminotransferase, CHB = chronic hepatitis B, HBeAg = hepatitis B e antigen, HBsAg = hepatitis B surface, HBV = hepatitis B virus, HC = healthy control, LdT = telbivudine, Th17 cell = interleukin (IL)-17-producing CD4+T helper cell, Treg = regulatory T cell.
Funding/support: The present study was supported by grants for The Key Research and Development Project of Tianjin Health Industry from Tianjin Municipal Health and Family Planning Committee (Project No:13KG126). JL and WL designed the study. XY, JL, MG, and LZ conducted the study, collected the data, and drafted the manuscript. JL and WL finalized the manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Liaw YF, Chu CM. Hepatitis B virus infection. Lancet 2009;373:582–92. [DOI] [PubMed] [Google Scholar]
- [2].Maini MK, Boni C, Ogg GS, et al. Direct ex vivo analysis of hepatitis B virus specific CD8+T cells associated with the control of infection. Gastroenterology 1999;117:1386–96. [DOI] [PubMed] [Google Scholar]
- [3].Wang FS, Zhang Z. Host immunity influences disease progression and antiviral efficacy in humans infected with hepatitis B virus. Expert Rev Gastroenterol Hepatol 2009;3:499–512. [DOI] [PubMed] [Google Scholar]
- [4].Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol 2005;5:215–29. [DOI] [PubMed] [Google Scholar]
- [5].Bertoletti A, Naoumov NV. Translation of immunological knowledge into better treatments of chronic hepatitis B. J Hepatol 2003;39:115–24. [DOI] [PubMed] [Google Scholar]
- [6].Dunn C, Brunetto M, Reynolds G, et al. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J Exp Med 2007;204:667–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhang Z, Chen DW, Yao JX, et al. Increased infiltration of intrahepatic DC subsets closely correlate with viral control and liver injury in immune active pediatric patients with chronic hepatitis B. Clin Immunol 2007;122:173–80. [DOI] [PubMed] [Google Scholar]
- [8].Huang YW, Chayama K, Tsuge M, et al. Differential effects of interferon and lamivudine on serum HBV DNA inhibition in patients with chronic hepatitis B. Antivir Ther 2010;15:177–84. [DOI] [PubMed] [Google Scholar]
- [9].Sakaguchi S. Naturally arising CD4+regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 2004;22:531–62. [DOI] [PubMed] [Google Scholar]
- [10].Weaver CT, Hatton RD, Mangan PR, et al. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 2007;25:821–52. [DOI] [PubMed] [Google Scholar]
- [11].Boni C, Penna A, Ogg GS, et al. Lamivudine treatment can overcome cytotoxic T-cell hyporesponsiveness in chronic hepatitis B: new perspectives for immune therapy. Hepatology 2001;33:963–71. [DOI] [PubMed] [Google Scholar]
- [12].Bettlli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature 2006;441:235–8. [DOI] [PubMed] [Google Scholar]
- [13].Stoop JN, van der Molen RG, Baan CC, et al. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology 2005;41:771–8. [DOI] [PubMed] [Google Scholar]
- [14].Xu D, Fu J, Jin L, et al. Circulating and liver resident CD4+CD25+regulatory T cells actively influence the antiviral immune response and disease progression in patients with hepatitis B. J Immunol 2006;177:739–47. [DOI] [PubMed] [Google Scholar]
- [15].Stoop JN, van der Molen RG, Kuipers EJ, et al. Inhibition of viral replication reduces regulatory T cells and enhances the antiviral immune response in chronic hepatitis B. Virology 2007;361:141–8. [DOI] [PubMed] [Google Scholar]
- [16].El-Badawy O, Sayed D, Badary MS, et al. Relations of regulatory T cells with hepatitis markers in chronic hepatitis B virus infection. Hum Immunol 2012;73:335–41. [DOI] [PubMed] [Google Scholar]
- [17].Jiang Y, Li W, Yu L, et al. Enhancing the antihepatitis B virus immune response by adefovir dipivoxil and entecavir therapies. Cell Mol Immunol 2011;8:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhang JY, Song CH, Shi F, et al. Decreased ration of Treg cells to Th17 cells correlates with HBV DNA suppression in chronic hepatitis B patients undergoing entecavir treatment. PLoS One 2010;5:e13869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ge J, Wang K, Meng QH, et al. Implication of Th17 and Th1 cells in patients with chronic active hepatitis B. J Clin Immunol 2010;30:60–7. [DOI] [PubMed] [Google Scholar]
- [20].Zhang JY, Zhang Z, Lin F, et al. IL-17-producing CD4+T cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology 2010;51:81–91. [DOI] [PubMed] [Google Scholar]
- [21].Lan RY, Salunga TL, Tsuneyama K, et al. Hepatic IL-17 responses in human and murine primary biliary cirrhosis. J Autoimmun 2009;32:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lemmers A, Moreno C, Gustot T, et al. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology 2009;49:646–57. [DOI] [PubMed] [Google Scholar]
- [23].Li J, Qiu SJ, She WM, et al. Significance of the balance between regulatory T (Treg)and T helper 17 (Th17) cells during hepatitis B virus related liver fibrosis. PLoS One 2012;7:e39307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang GL, Xie DY, Lin BL, et al. Imbalance of interleukin-17-producing CD4 T cells/regulatory T cells axis occurs in remission stage of patients with hepatitis B virus-related acute-on-chronic liver failure. J Gastroen Heptaol 2013;28:513–21. [DOI] [PubMed] [Google Scholar]
- [25].Amedei A, Munari F, Bella CD, et al. Helicobacter pylori secreted peptidyl prolyl cis, trans-isomerase drives Th17 inflammation in gastric adenocarcinoma. Intern Emerg Med 2014;9:303–9. [DOI] [PubMed] [Google Scholar]
- [26].Su ZJ, Yu XP, Guo RY, et al. Changes in the balance between Treg and Th17 cells in patients with chronic hepatitis B. Diagn Micr Infec Dis 2013;76:437–44. [DOI] [PubMed] [Google Scholar]
- [27].Lai CL, Gane E, Liaw YF, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med 2007;357:2576–88. [DOI] [PubMed] [Google Scholar]
- [28].Jiang H, Wang J, Zhao W. Lamivudine versus telbivudine in the treatment of chronic hepatitis B: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis 2013;32:11–8. [DOI] [PubMed] [Google Scholar]
- [29].Chen Y, Li X, Ye B, et al. Effect of telbivudine therapy on the cellular immune response in chronic hepatitis B. Antiviral Res 2011;91:23–31. [DOI] [PubMed] [Google Scholar]
- [30].Xie DY, Lin BL, Chen FJ, et al. Programmed death-1 (PD-1) and PD-L1 expression during antiviral treatment of chronic hepatitis B. Chin J Hepatol 2010;18:646–50. [DOI] [PubMed] [Google Scholar]
- [31].Pan XC, Yang F, Chen M, et al. The effect of telbivudine on peripheral blood CD4+CD25+ regulatory T cells and its significance in patients with chronic hepatitis B. Chin J Hepatol 2008;16:885–8. [PubMed] [Google Scholar]
- [32].Chinese Society of Hepatology and Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B (2010 version). Chin J Hepatol 2011;19:13–24. [DOI] [PubMed] [Google Scholar]
- [33].Guo PP, Li SP, Wu W, et al. Circulating CD4+CD25+regulatory T cells correlate with chronic hepatitis B infection. Immunology 2008;123:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yu XP, Guo RY, Su ML, et al. Dynamic changes of Treg and Th17 cells and related cytokines closely correlate with the virological and biochemical response in chronic hepatitis b patients undergoing nucleos(t)ide analogues treatment. Hepat Mon 2013;13:e15332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yang GL, Liu AL, Xie Q, et al. Association of CD4+CD25+Foxp3+regulatory T cells with chronic activity and viral clearance in patients with hepatitis B. Int Immunol 2007;19:133–40. [DOI] [PubMed] [Google Scholar]


