Abstract
This study aimed to introduce a new stereoelectroencephalography (SEEG) system based on Leksell stereotactic frame (L-SEEG) as well as Neurotech operation planning software, and to investigate its safety, applicability, and reliability.
L-SEEG, without the help of navigation, includes SEEG operation planning software (Neurotech), Leksell stereotactic frame, and corresponding surgical instruments. Neurotech operation planning software can be used to display three-dimensional images of the cortex and cortical vessels and to plan the intracranial electrode implantation. In 44 refractory epilepsy patients, 364 intracranial electrodes were implanted through the L-SEEG system, and the postoperative complications such as bleeding, cerebral spinal fluid (CSF) leakage, infection, and electrode-related problems were also investigated.
All electrodes were implanted accurately as preoperatively planned shown by postoperative lamina computed tomography and preoperative lamina magnetic resonance imaging. There was no severe complication after intracranial electrode implantation through the L-SEEG system. There were no electrode-related problems, no CSF leakage and no infection after surgery. All the patients recovered favorably after SEEG electrode implantation, and only 1 patient had asymptomatic frontal lateral ventricle hematoma (3 mL).
The L-SEEG system with Neurotech operation planning software can be used for safe, accurate, and reliable intracranial electrode implantation for SEEG.
Keywords: intracranial electrodes, Leksell stereotactic frame, Neurotech operation planning software, refractory epilepsy, stereoelectroencephalography
1. Introduction
Surgery treatment should be considered for drug-resistant epilepsy. However, in some of them, the epileptic zone cannot be well localized with noninvasive methods.[1–3] Intracranial electrodes may directly record electroencephalogram (EEG) of the suspected brain regions, enabling direct mapping of epileptic networks as well as functional evaluation of patients with multifocal epilepsy.[4] Intracranial electrode can be implanted through craniotomy or stereoelectroencephalography (SEEG) technique. SEEG technique has its own advantages over craniotomy and has been proven to be safe in most cases.[5–7]
Different epilepsy centers have developed their own SEEG techniques.[8–12] Early SEEG technique based on digital subtraction angiography (DSA) and intraoperative x-ray was used in Saint Anne hospital in France;[13] SEEG technique based on navigation was used in Montreal Neurological Institute (MNI) in Canada.[14] Robot stereotactic-assisted (ROSA) SEEG technique, developed by Medtech Corporation, is also essentially based on navigation. Increasing epilepsy centers employed ROSA SEEG technique for the treatment of epilepsy. To facilitate the use of SEEG technique and to improve the clinical applicability of SEEG technique, the L-SEEG-based SEEG without the help of navigation was investigated.
The Leksell stereotactic frame was initially designed for the biopsy or deep brain stimulation in Parkinson's disease patients. Currently, it is widely used in the Department of Neurosurgery worldwide. We have developed an L-SEEG system based on the Leksell stereotactic frame, by using which 364 intracranial electrodes were implanted in 44 patients with refractory epilepsy between November 2013 and May 2015. Patients aging over 3 year old are suitable for L-SEEG. Their skull is hard enough to install the Leksell frame. Our results showed that this system could be used for safe, accurate, and reliable intracranial electrode implantation. Herein, we briefly introduced the L-SEEG system.
2. Methods
This study has been approved by the Ethics Committee of Aviation General Hospital (2015-24-1S). Informed patient consent was obtained from each patient or his/her guardian before the L-SEEG system implanted.
2.1. Introduction of the L-SEEG system
The L-SEEG system includes Neurotech operation planning software, Leksell stereotactic frame, and corresponding surgical instruments designed for the Leksell stereotactic frame. Three-dimensional (3D) reconstruction of the cortex and cortical vessels from lamina magnetic resonance imaging (MRI) was performed through Neurotech operation planning software (Fig. 1). Intracranial electrode implantation (number of electrodes, cortical entry point, and target point for each electrode) was planned with Neurotech operation planning software (Fig. 1). The injury to the cortex vessels should be avoided during the planning (Fig. 1). The planning was dependent on preoperative evaluation. The alpha (α) angle was defined as the angle between the intracranial electrode and brain cross-section, and the beta (β) angle as the angle between the intracranial electrode and brain sagittal section (Fig. 2). The target point of an intracranial electrode was used as the center of the Leksell arc. Lamina MRI with the Leksell head frame was matched to preoperative MRI (with electrode implantation planning) with Neurotech operation planning software. Neurotech operation planning software can “feel” 6 contrast agent points in the frame on the axial lamina MRI for each target point (Fig. 3). If the target point and the cortical entry point of an electrode were set, the direction of this electrode was uniquely determined. The electrode implantation plan (target point and cortical entry point for each electrode) was matched with Neurotech operation planning software to lamina MRI with the Leksell frame. The spatial location (X, Y, and Z) of the target point and the cortical entry point of each electrode could be achieved with Neurotech operation planning software. Then, Neurotech operation planning software automatically calculated the α and β angles of each electrode (Fig. 1). Five parameters (X, Y, Z values of the target point and α and β angles) of an electrode could be read directly on the Leksell stereotactic frame (Fig. 2). Once the 5 parameters of an electrode were obtained, the electrode could be implanted correctly.
Figure 1.
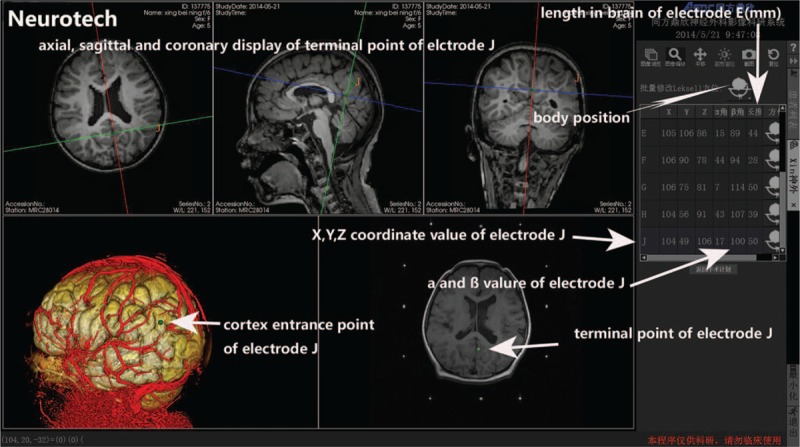
Neurotech operation planning software and preoperative electrode implantation (target point and cortex entry point for each electrode). Coordinate value of the target point of each electrode, α and β angles, and depth of each electrode in the brain are given by Neurotech operation planning software. Three-dimensional display of cortex and cortical vessels in Neurotech operation planning software.
Figure 2.
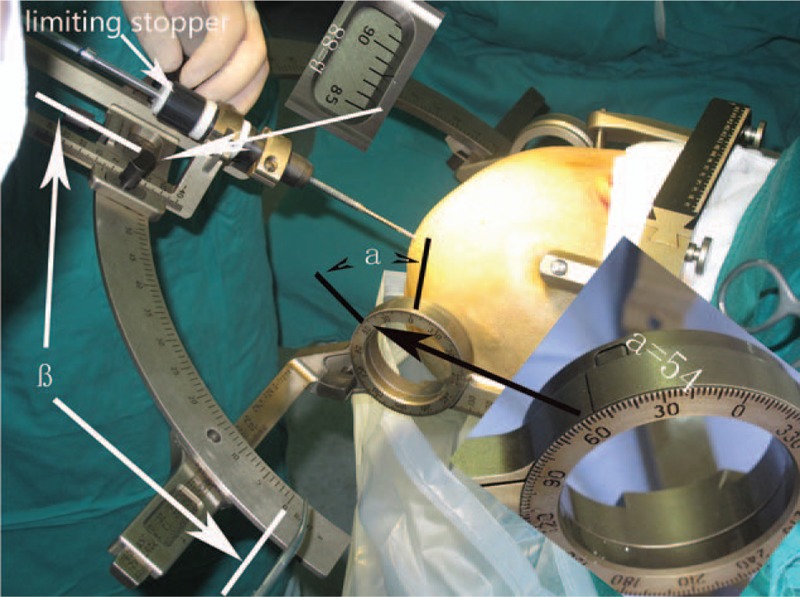
The α and β angles can be read directly on the Leksell stereotactic frame. Limiting stopper was designed for the safe drilling.
Figure 3.
Neurotech operation planning software can “feel” the 6 contrast agent points (A, B, C, D, E, and F) on the Leksell frame at the level of each target point in axial lamina MRI. These 6 contrast points should be manually matched (arrows on keyboard) as shown in the green circle (zoom in a contrast agent point). The cross should be located at the center of contrast agent (white in green circle). Once 6 contrast points are accurately matched, the location (X, Y, Z) and direction (α and β angles) of an electrode are calculated with Neurotech operation planning software. MRI = magnetic resonance imaging.
2.2. Procedures for SEEG with L-SEEG technique
2.2.1. Imaging preparation
MRI scan included 3D-3T brain voxel (1 mm3), 3D-3T (1 mm3) flair sequence, and TWIST angiography sequence. All the images were in digital imaging and communications in medicine (DICOM) format.
2.2.2. Image processing with Neurotech operation planning software
The 3D reconstruction of all above images was processed with Neurotech operation planning software. Different kinds of imaging layer can be added or deleted. computed tomography (CT) image of the scalp and skull could be transparentized to different degrees (0–100%). Neurotech operation planning software could clearly display the 3D images of the cortex and cortical vessels (Fig. 4).
Figure 4.
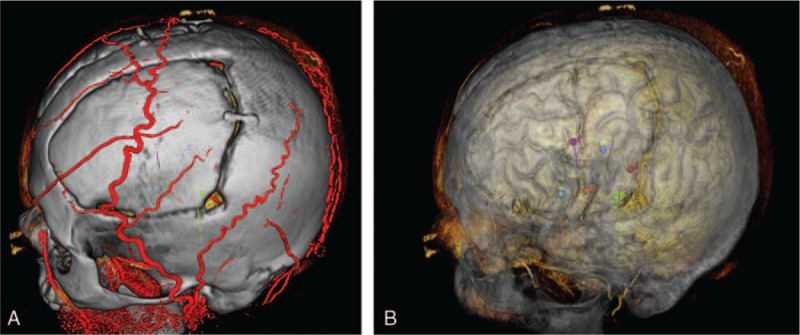
Preoperative SEEG planning and intraoperative implantation according to the electrode parameters (X, Y, Z, α, and β). In the SEEG planning, what you see is what you get. SEEG = stereoelectroencephalography.
2.2.3. Intracranial electrode implantation planning with Neurotech operation planning software
3D images of the cortex and cortical vessels were opened in Neurotech operation planning software. Intracranial electrode parameters (number of electrodes, location of electrodes [target point on the brain], and entry point on the cortex) were set according to preoperatively evaluated. If an entry point was set on the brain and a target point within the brain, the unique direction of this depth electrode was determined with Neurotech operation planning software. The injury to vessels should be avoided in the electrode planning (Fig. 4). Depth of each electrode in the brain was automatically given by Neurotech operation planning software (Fig. 1). Electrode implantation plan could be saved and edited in Neurotech operation planning software.
2.2.4. Preoperative processing on the day of operation
The Leksell head frame was fixed on the head on the day of operation under local anesthesia. The lamina MRI (1 mm) scan with the Leksell head frame was matched to the preoperative MRI with the electrode implantation plan. The electrode implantation plan and Leksell coordinate system were matched with Neurotech operation planning software. These 6 contrast agent points at the level of target point should be manually matched one by one (Fig. 3), so that Neurotech operation planning software could “feel” their spatial position and automatically calculate 5 parameters (X, Y, Z, α, and β values) of each electrode.
2.2.5. Electrode implantation
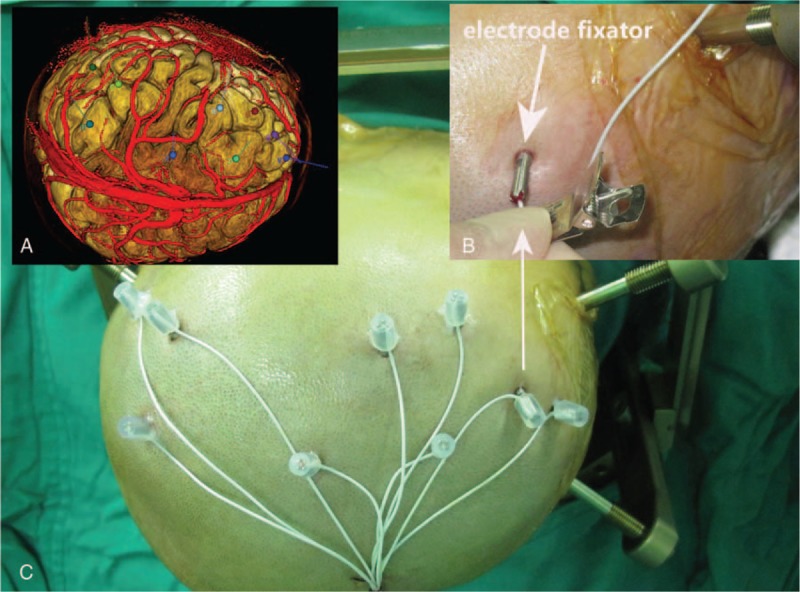
An electrode could be rapidly implanted according to its 5 parameters which were directly read on the Leksell frame with the help of corresponding surgical instruments. Parameters of each electrode could be converted based on the position (supine, lateral, or semireclining position) in Neurotech operation planning software. The hindrance from the ring of the Leksell frame could occur in some temporal lobe electrode implantation in the supine position. This can be resolved by changing the location of rings from the bilateral temporal area to the anterior and posterior head in the semireclining position (X and Y values should be read as in lateral position). Neurotech operation planning software could display different parameters of each electrode at different positions (Fig. 1). With regulation of body position, electrodes could be implanted in bilateral frontal, temporal, parietal, and occipital lobes without difficulty.
2.2.6. Actual location of intracranial electrodes
The postoperative lamina (1 mm) CT was matched to the preoperative MRI with Neurotech operation planning software and whether the location of intracranial electrodes was consistent with that of preoperative plan was evaluated.
3. Results
A total of 364 electrodes were implanted in 44 refractory epilepsy patients with the L-SEEG system. There were 22 males and 20 females. The average age was 19 ± 7.4 years (range: 5–38 years). The L-SEEG system can provide direct view of the cortex and cortical vessels (Fig. 4). All electrodes were accurately implanted as preoperatively planned in Neurotech operation planning software as shown by the preoperative lamina MRI and postoperative lamina CT and intraoperative finding during craniotomy. The average time of monitoring was 9 ± 7 days. There was no severe complication after intracranial electrode implantation. There were no electrode problems (pulling out and fragment left), no CSF leakage, and no infection during intracranial EEG monitoring. Forty-four patients recovered favorably after electrode implantation, and among whom only 1 patient had asymptomatic frontal lateral ventricle hematoma (3 mL). There were no other complications during intracranial EEG monitoring.
4. Discussion
SEEG technique is microinvasive and has advantages over craniotomy for intracranial electrode implantation in most conditions.[3,13–15] SEEG is especially suitable for the treatment of tuberculous sclerosis, traumatic epilepsy, and epilepsy patients with intracranial adhesion.[16]
In our group, intracranial bleeding (small lateral ventricular hematoma, 3 mL) occurred in 1 patient without any symptom. In the MNI group, the incidence of intracranial bleeding was 0.8% (4/ 521).[16,17] The procedures of bone hole drilling and 3D display of the cortex and cortical vessels in the L-SEEG system are similar to those in MNI SEEG technique. Although, the sample size was small in the present study, we speculate that the incidence of complications was similar to that with MNI SEEG technique.
The most important feature of the L-SEEG system is that it can be used without the help of navigation to guide the electrode implantation, which facilitates the clinical SEEG implantation. If the location (X, Y, Z) of 2 points (target point in the brain and entry point of the cortex) in the Leksell frame was obtained, the direction (α and β values) of this electrode could be determined in Neurotech operation planning software. The actual location of electrode was uniquely determined by the direction (α and β) and target point (X, Y, Z).
Although the incidence of complications of L-SEEG technique is similar to that of other SEEG techniques,[12] it has several advantages over other SEEG techniques. Firstly, the L-SEEG system does not need the help of navigation. As we know, most of other SEEG techniques need the help of navigation or DSA. ROSA technique is essentially based on navigation. Secondly, it might be more accurate for electrode implantation than other SEEG techniques. As we know, the Leksell stereotactic frame can be applied in deep brain stimulation (DBS) for Parkinson's disease. Navigation is hard to be used for DBS because it is more like to have systemic error. Thirdly, the Leksell frame is the main hardware of the L-SEEG system. It does not need heavy equipment like DSA, navigation equipment, and ROSA equipment. It might be the smallest SEEG equipment. Fourthly, the L-SEEG system is as convenient as the ROSA system in the electrode implantation. Five parameters of each electrode can be read directly on the Leksell frame. It is not needed to look for the direction of each electrode during operation. Finally, L-SEEG technique is much cheaper than other SEEG technique. The Leksell frame is commonly used in the Department of Neurosurgery and expensive equipments like ROSA are not needed.
Neurotech operation planning software, specially designed for the Leksell frame, is a useful software program for MRI reconstruction and electrode implantation planning. All intracranial electrodes in our group were accurately implanted as preoperatively planned according to the preoperative lamina MRI and postoperative lamina CT. A visualized 3D display of the cortex and cortical vessels is also an advantage of L-SEEG technique. Clinicians have a clear view of the electrode implantation plan in Neurotech operation planning software (Fig. 4). It means that what you see is what you get. The damage to important cortical vessels can be avoided when the electrodes implantation is planned. A visualized 3D display of the cortex and cortical vessels is also helpful for the planning of epileptic zone resection after intracranial monitoring.
As for the limitations of this study, child patients with less hard skull cannot be installed with the Leksell frame and thus are not suitable for the L-SEEG method. So, L-SEEG method might not be applied in patients younger than 3 year old.
Taken together, the L-SEEG system with Neurotech operation planning software can be used for safe, accurate, and reliable intracranial electrode implantation for SEEG. Although the complication incidence of L-SEEG technique is similar to that of other SEEG techniques, it facilitates the SEEG procedure. Thus, the L-SEEG system can be applied in the surgical intervention of epilepsy.
Footnotes
Abbreviations: DBS = deep brain stimulation, DICOM = digital imaging and communications in medicine, DSA = digital subtraction angiography, EEG = electroencephalogram, MNI = Montreal Neurological Institute, ROSA = Robot stereotactic assisted, SEEG = stereoelectroencephalography.
This study was supported by the National Natural Science Foundation of China (No. 51472068) and key discipline construction of clinical medicine of China Aviation Industry.
The authors have no conflicts of interest to disclose.
References
- [1].Cossu M, Cardinale F, Colombo N, et al. Stereoelectroencephalography in the presurgical evaluation of children with drug-resistant focal epilepsy. J Neurosurg 2005;103(4 suppl):333–43. [DOI] [PubMed] [Google Scholar]
- [2].Alomar S, Jones J, Maldonado A, et al. The stereo-electroencephalography methodology. Neurosurg Clin N Am 2016;27:83–95. [DOI] [PubMed] [Google Scholar]
- [3].Cardinale F, Pero G, Quilici L, et al. Cerebral angiography for multimodal surgical planning in epilepsy surgery: description of a new three-dimensional technique and literature review. World Neurosurg 2015;84:358–67. [DOI] [PubMed] [Google Scholar]
- [4].Serletis D, Bulacio J, Bingaman W, et al. The stereotactic approach for mapping epileptic networks: a prospective study of 200 patients. J Neurosurg 2014;121:1239–46. [DOI] [PubMed] [Google Scholar]
- [5].Burneo JG, Steven DA, McLachlan RS, et al. Morbidity associated with the use of intracranial electrodes for epilepsy surgery. Canadian J Neurol Sci 2006;33:223–7. [DOI] [PubMed] [Google Scholar]
- [6].Cardinale F, Cossu M, Castana L, et al. Stereoelectroencephalography: surgical methodology, safety, and stereotactic application accuracy in 500 procedures. Neurosurgery 2013;72:353–66. discussion 366. [DOI] [PubMed] [Google Scholar]
- [7].Balanescu B, Franklin R, Ciurea J, et al. A personalized stereotactic fixture for implantation of depth electrodes in stereoelectroencephalography. Stereotact Funct Neurosurg 2014;92:117–25. [DOI] [PubMed] [Google Scholar]
- [8].Dorfer C, Minchev G, Czech T, et al. A novel miniature robotic device for frameless implantation of depth electrodes in refractory epilepsy. J Neurosurg 2016;126:1–7. [DOI] [PubMed] [Google Scholar]
- [9].Roessler K, Sommer B, Merkel A, et al. A frameless stereotactic implantation technique for depth electrodes in refractory epilepsy utilizing intraoperative MR imaging. World Neurosurg 2016;94:206–10. [DOI] [PubMed] [Google Scholar]
- [10].Gonzalez-Martinez J, Bulacio J, Thompson S, et al. Technique, results, and complications related to robot-assisted stereoelectroencephalography. Neurosurgery 2016;78:169–80. [DOI] [PubMed] [Google Scholar]
- [11].Guenot M, Isnard J, Ryvlin P, et al. Neurophysiological monitoring for epilepsy surgery: the Talairach SEEG method. StereoElectroEncephaloGraphy. Indications, results, complications and therapeutic applications in a series of 100 consecutive cases. Stereotact Funct Neurosurg 2001;77:29–32. [DOI] [PubMed] [Google Scholar]
- [12].Gonzalez-Martinez J, Mullin J, Vadera S, et al. Stereotactic placement of depth electrodes in medically intractable epilepsy. J Neurosurg 2014;120:639–44. [DOI] [PubMed] [Google Scholar]
- [13].Scarabin JM. Sterotaxy and Epilepsy Surgery. Montrouge: Cambridge University Publishing; 2012. [Google Scholar]
- [14].Olivier A. Techniques in Epilepsy Surgery. Cambridge: Cambridge University Publishing; 2012. [Google Scholar]
- [15].Nowell M, Rodionov R, Diehl B, et al. A novel method for implementation of frameless StereoEEG in epilepsy surgery. Neurosurgery 2014;10(suppl 4):525–33. discussion 533–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].De Almeida AN, Olivier A, Quesney F, et al. Efficacy of and morbidity associated with stereoelectroencephalography using computerized tomography—or magnetic resonance imaging-guided electrode implantation. J Neurosurg 2006;104:483–7. [DOI] [PubMed] [Google Scholar]
- [17].Sansur CA, Frysinger RC, Pouratian N, et al. Incidence of symptomatic hemorrhage after stereotactic electrode placement. J Neurosurg 2007;107:998–1003. [DOI] [PubMed] [Google Scholar]


