Abstract
Rationale: Methicillin-resistant Staphylococcus aureus (MRSA) prevalence continues to increase in patients with cystic fibrosis (CF) in the United States, reaching 26.5% in 2012. Approximately 30% of strains are SCCmec (staphylococcal cassette chromosome mec) IV type, frequently USA300, which in the general population have different genotypic and phenotypic features than SCCmec II type.
Objectives: We hypothesized that risk factors for acquisition and outcomes in patients with CF differed for “health care–associated” (SCCmec II) versus “community-associated” (SCCmec IV) MRSA strains.
Methods: To determine the role of SCCmec type and Panton–Valentine leukocidin (PVL), MRSA isolates from patients not more than 18 years old at seven CF centers were typed and the association of potential risk factors and subsequent clinical course was assessed, using data provided by the CF Patient Registry.
Measurements and Main Results: Participants with chronic MRSA (295) had typeable isolates and clinical data; 205 (69.5%) had SCCmec II PVL(–), 39 (13.2%) had SCCmec IV PVL(–), and 51 (17.3%) had SCCmec IV PVL(+) strains. SCCmec IV, compared with SCCmec II, increased during the study period, 1996–2010 (P = 0.03). SCCmec II was associated with Pseudomonas aeruginosa–positive cultures and three or more clinic visits in the 6 months preceding the first positive MRSA culture (adjusted odds ratio, 2.05; 95% confidence interval, 1.13–3.74; P = 0.019). Lung function and anthropometrics remained unchanged in the 6 months after initial MRSA detection compared with the 6 months prior. Although CF care increased for participants in both groups in the 6 months after MRSA detection, inhaled antibiotics were prescribed more frequently in those with SCCmec II strains and increased hospitalizations occurred in those with SCCmec IV PVL(–) strains compared with those with PVL(+) strains (adjusted difference, 34.10%; 95% confidence interval, 7.58–60.61; P = 0.012). Participants in both groups had an increase in CF care in the 2 years after MRSA detection compared with the 2 years prior.
Conclusions: Increased exposure to CF clinics and P. aeruginosa may constitute risk factors for acquisition of SCCmec II MRSA strains. Clinical interventions increased 6 months and 2 years after initial MRSA detection regardless of SCCmec type.
Keywords: SCCmec type, Panton–Valentine leukocidin, risk factor, methicillin-resistant Staphylococcus aureus, clinic visits
Lung infections caused by methicillin-resistant Staphylococcus aureus (MRSA) have markedly increased in patients with cystic fibrosis (CF) (1), reaching 26.5% prevalence in the United States in 2012 (2). Chronic MRSA is associated with a more rapid decline in lung function and increased mortality (3, 4). These epidemiologic studies did not account for the genetic lineage of MRSA, which is warranted in patients with CF based on differences in risk factors, clinical presentations, and outcomes in the general population (5).
Methicillin resistance is conferred by the mecA gene located within the staphylococcal cassette chromosome (SCC) (6). Several SCCmec types have been described (7), of which SCCmec II is more commonly found in so-called hospital-associated (HA)-MRSA infections, whereas SCCmec IV is more commonly found in the community-associated (CA)-MRSA infections in the United States (8). Differences in the clinical presentations include the invasive/systemic disease typically seen in patients with comorbidities acquiring HA-MRSA (9–12) whereas CA-MRSA typically causes skin and soft tissue infections in previously healthy individuals (13). SCCmec IV strains may also carry the putative virulence factor Panton–Valentine leukocidin (PVL), which is commonly detected in USA300—the epidemic SCCmec IV strain in the United States (14). In some reports PVL status has been associated with more severe disease (15, 16), including in children with CF (17); however, the role of PVL remains debated (18).
Another key distinction between the SCCmec types is differential antibiotic resistance: SCCmec II encodes resistance to more antibiotic classes whereas the smaller SCCmec IV element encodes fewer antibiotic resistance genes. Thus, SCCmec IV strains are more likely to be susceptible to clindamycin, fluoroquinolone agents, and trimethoprim–sulfamethoxazole (TMP–SMX). We previously reported similar differences in the antibiotic susceptibilities of MRSA isolates from children with CF; SCCmec II isolates had higher frequencies of resistance to clindamycin and ciprofloxacin compared with SCCmec IV isolates (19).
Among U.S. patients with CF infected with MRSA, about two-thirds harbor SCCmec II strains (20–22). Several studies show that patients remain infected with the same strain for many years; thus, typing at one time point is indicative of past and future MRSA type (22, 23). In a single-center study, we reported that children and adults with CF infected with SCCmec II strains were older, had longer duration of MRSA infection, and were more frequently coinfected with Pseudomonas aeruginosa when compared with patients infected with SCCmec IV strains expressing PVL (24). In addition, in a multicenter study, we showed that pediatric patients with CF chronically infected with SCCmec II strains had a higher rate of treatment with oral antibiotics for protocol-defined pulmonary exacerbations than those with SCCmec IV strains (25). Thus, SCCmec type appears to be associated with different clinical outcomes in patients with CF.
In the current multicenter study, we evaluate risk factors for initial infection with the two MRSA SCCmec types (II vs. IV) among children with CF who remain persistently culture positive. We also evaluate risk factors for and outcomes associated with PVL among those children infected with SCCmec IV PVL(+) versus PVL(–) strains. We hypothesize that children with CF persistently infected with SCCmec II MRSA strains had more contact with the health care environment, before acquisition and/or more severe CF disease than those infected with SCCmec IV MRSA strains. In turn, we examine clinical outcomes after initial infection to evaluate whether SCCmec type and PVL status are related to subsequent changes in disease course.
Methods
Study Design, Sites, and Participants
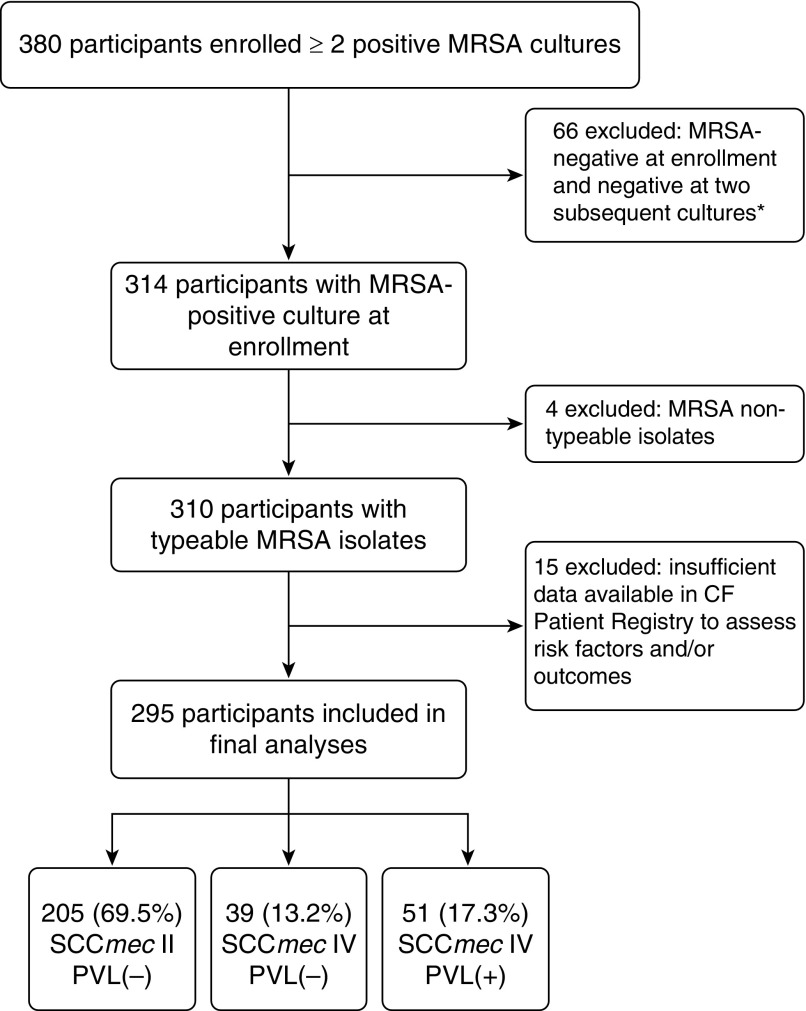
We performed an observational, multicenter study to assess clinical characteristics and potential risk factors associated with acquisition of various MRSA strains based on SCCmec typing and the presence of the PVL gene. Seven geographically diverse, Cystic Fibrosis Foundation (CFF)–accredited pediatric centers, each treating more than 200 children and adolescents and having at least 20% MRSA prevalence, participated in this study. Eligible participants with CF were not more than 18 years of age and had two or more cultures positive for MRSA within at least one of the 2 years preceding enrollment. Participants in whom no MRSA-positive culture could be obtained at enrollment/consent into this study and/or at two subsequent CF clinic visits were excluded from analyses (Figure 1). Patients who had undergone lung or liver transplantation were not eligible to participate. Each center’s institutional review board approved the study. Written consent was obtained from parents, and assent was obtained from children old enough to read.
Figure 1.
Flow diagram of participants included in final analyses. *Participants were excluded from further analysis as this study focused on those with persistently positive methicillin-resistant Staphylococcus aureus (MRSA) cultures. Participants who did not provide a positive MRSA culture did not differ from eligible participants for Pseudomonas aeruginosa or lung function, but had fewer MRSA-positive cultures overall (42).
Typing of MRSA Isolates
Participant respiratory cultures (deep pharyngeal swab, sputum, or bronchoalveolar lavage) were processed at each site’s clinical microbiology laboratory according to CFF guidelines (26). Three colonies of MRSA from the same culture were sent to the core laboratory at the University of North Carolina (Chapel Hill, NC), where the SCCmec type and presence of the PVL gene were determined by PCR methods as previously described (22, 27).
Data Collection
Data from the U.S. CFF Patient Registry were obtained from January 1996 through December 2012 and included demographic characteristics, cystic fibrosis transmembrane conductance regulator (CFTR) mutations, microbiology results, number of clinic visits, chronic CF medications, treatment with intravenous (IV) antibiotics, spirometry results, and growth parameters. Spirometry was ascertained in children at least 6 years old and expressed as FEV1 % predicted (28, 29). Weight for length in children less than 2 years of age, or body mass index (BMI) percentiles in children 2 years of age or older, were calculated on the basis of Centers for Disease Control and Prevention reference values (30, 31). Hospital admissions (for any reason) and treatment with IV antibiotics in the home have been available in the CFF Patient Registry since 2003.
Statistical Analyses
Year of MRSA acquisition during our study was stratified into three periods based on changes in MRSA prevalence and the emergence of USA300 (8, 14). Demographic and clinical characteristics were described using summary statistics and compared among participants with different SCCmec types and PVL status, using two-sided t tests for continuous variables or Fisher’s exact test for categorical variables. Values for FEV1% predicted and BMI (ages ≥2 yr) or weight for length (<2 yr of age) were averaged over 2 years. Microbiologic factors included detection of “any” methicillin-susceptible S. aureus (MSSA) or P. aeruginosa, and chronic infection with MSSA or P. aeruginosa defined as more than 50% of cultures positive in the year before MRSA detection (32).
To assess short-term (i.e., within 6 mo) risk factors for acquisition and clinical outcomes of various MRSA types, chronic CF medications, clinic visits, hospitalizations, and treatment with intravenous antibiotics were compared among participants with different SCCmec types and PVL status. Paired summaries of the change from 6 months before and the 6 months after MRSA acquisition, and 95% confidence intervals (CIs) were reported. To test whether changes differed by SCCmec type or PVL status, an analysis of covariance was performed, adjusted for sex, age, and year of MRSA acquisition, study site, and presence of any P. aeruginosa. To test our primary hypothesis, a multivariate logistic regression model was fit to estimate the odds of acquisition of SCCmec MRSA type by health care exposures, that is, number of CF clinic visits or hospitalizations, adjusted for the aforementioned confounding variables.
Similarly, to assess longer term outcomes (i.e., within 2 yr) associated with SCCmec type and PVL status, the aforementioned variables were summarized as described above for participants in the 2 years before and after MRSA acquisition. In addition, changes in FEV1% predicted and BMI/weight-for-length percentiles were assessed. All analyses were performed with SAS version 9.2 (SAS, Cary, NC) and two-sided P values are reported.
Results
Study Population and MRSA Type
From October 2008 to April 2010, 380 potentially eligible participants with a history of MRSA infection were enrolled. After exclusion of 85 participants as detailed in Figure 1, 295 participants were included in this study. Distribution of SCCmec types and PVL status is shown in Table 1. The proportion of participants with SCCmec IV strains was lowest among those with initial MRSA detection from 1996 to 2002 and increased over time; 51% of all SCCmec IV strains were detected from 2007 to 2010 (Table 1). Similarly, the presence of the PVL gene among SCCmec IV strains increased over time; from 1996 to 2002, 3.9% of strains were PVL(+) whereas from 2007 to 2010, 60.8% of strains were PVL(+).
Table 1.
Year of initial positive isolate by methicillin-resistant Staphylococcus aureus SCCmec type and Panton–Valentine leukocidin status
| Year of Initial Detection | SCCmec II (n = 205) | SCCmec IV (n = 90) | P Value | IV PVL (–) (n = 39) | IV PVL(+) (n = 51) | P Value |
|---|---|---|---|---|---|---|
| 1996–2002 | 45 (22.0%) | 9 (10.0%) | 0.002* | 7 (18.0%) | 2 (3.9%) | 0.033* |
| 2003–2006 | 97 (47.3%) | 35 (38.9%) | 17 (43.6%) | 18 (35.3%) | ||
| 2007–2010 | 63 (30.7%) | 46 (51.1%) | 15 (38.5%) | 31 (60.8%) |
Definition of abbreviation: PVL = Panton–Valentine leukocidin; SCCmec = staphylococcal cassette chromosome mec.
Boldface indicates significance at the 0.05 level.
Proportion of cultures and their distribution over the years of study are shown.
P value for Fisher’s test of distributional differences between SCCmec types and PVL status.
Characteristics Associated with Acquisition of Various MRSA Types
The mean age for all participants at the time of their first MRSA-positive culture was 7.0 (SD 4.2) years and was similar among participants with different SCCmec types. The distributions of age, sex, and CFTR genotypes were also similar among those who acquired different SCCmec types; 56.1% of those with SCCmec II versus 47.8% of those with SCCmec IV MRSA strains were F508 del homozygote.
In the 2 years before the first MRSA-positive culture, anthropometric percentiles were similar for those who acquired SCCmec II versus SCCmec IV (Table 2). Lung function, available for 110 participants with SCCmec II and for 44 with SCCmec IV isolates, was also similar in the two groups.
Table 2.
Demographic and clinical characteristics associated with various methicillin-resistant Staphylococcus aureus types in children with cystic fibrosis
| Characteristic | SCCmec II (n = 205) | SCCmec IV (n = 90) | P Value |
|---|---|---|---|
| Age group, yr: n (%) | |||
| <3 | 42 (20.5%) | 16 (17.8%) | 0.86* |
| 3 to <6 | 42 (20.5%) | 21 (23.3%) | |
| 6 to <10 | 87 (42.4%) | 36 (40.0%) | |
| ≥10 to 18 | 34 (16.6%) | 17 (18.9%) | |
| Sex, n (%) | |||
| Female | 106 (51.7%) | 39 (43.3%) | 0.21 |
| Health status 24 mo before MRSA: n, mean (SD) | |||
| FEV1% predicted† | 110, 89.1 (16.8) | 44, 84.6 (20.4) | 0.16 |
| BMI or weight/height percentile‡ | 196, 44.4 (27.0) | 80, 44.9 (27.2) | 0.90 |
| Microbiology before MRSA acquisition, n (%) | |||
| MSSA any (6 mo prior) | 58 (28.3%) | 22 (24.4%) | 0.57 |
| MSSA chronic§ (1 yr prior) | 35 (17.1%) | 16 (17.8%) | 0.87 |
| P. aeruginosa any (6 mo prior) | 96 (46.8%) | 27 (30.0%) | 0.007 |
| P. aeruginosa chronic§ (1 yr prior) | 61 (29.8%) | 18 (20.0%) | 0.088 |
| CF medications 6 mo before MRSA acquisition | |||
| Chronic use of inhaled antibiotics, n (%) | 72 (35.1%) | 25 (27.8%) | 0.23 |
| Chronic use of DNase, n (%) | 67 (32.7%) | 30 (33.3%) | 0.99 |
| Chronic use of macrolides, n (%) | 34 (16.6%) | 12 (13.3%) | 0.60 |
| Days of IV antibiotics per IV course||¶**: n, mean (SD) | 47, 13.4 (8.1) | 21, 10.2 (6.7) | 0.12 |
| Health care exposure 6 mo before MRSA acquisition | |||
| CF clinic visits, n (%) | |||
| 0 | 20 (9.8%) | 18 (20.0%) | 0.007 |
| 1 or 2 | 108 (52.7%) | 52 (57.8%) | |
| ≥3 | 77 (37.5%) | 20 (22.2%) | |
| ≥1 hospitalization,|| n (%) | 44 (27.5%) | 20 (24.7%) | 0.76 |
| Hospital days per admission||**: mean (SD) | 9.0 (6.8) | 8.0 (5.5) | 0.57 |
Definition of abbreviations: BMI = body mass index; CF = cystic fibrosis; IV = intravenous; MRSA = methicillin-resistant Staphylococcus aureus; MSSA = methicillin-susceptible Staphylococcus aureus; P. aeruginosa = Pseudomonas aeruginosa; SCCmec = staphylococcal cassette chromosome mec.
Boldface indicates significance at the 0.05 level.
P value for Fisher’s test of distributional differences between SCCmec types.
For participants 6 years of age or older.
Weight/height percentiles in children less than 2 years of age and BMI percentiles in those 2 years of age and older.
Chronic defined as greater than 50% of cultures in 12 months positive for the pathogen.
Evaluable in n = 160 SCCmec II and n = 81 SCCmec IV with data after 2003.
Combined inpatient and outpatient intravenous therapy.
Mean and SD reported among those with event/hospitalization (excluding 0s).
In the 6 months before first MRSA-positive culture, more participants who acquired SCCmec II strains had at least one P. aeruginosa–positive culture (Table 2). However, the proportion of participants with chronic P. aeruginosa did not differ by SCCmec type. Differences in P. aeruginosa–positive cultures were not secondary to more frequent assessment as the number of cultures was similar for participants with SCCmec II versus SCCmec IV isolates (mean [SD], 3.1[1.7] vs. 3.0 [1.9]).
Among participants with SCCmec IV, none of the above-described characteristics were associated with the PVL gene (data not shown).
In the 6 months before first MRSA-positive cultures, the use of chronic CF medications and days of intravenous antibiotic treatment of pulmonary exacerbations were similar in the two groups (Table 2). However, a greater proportion of participants who acquired SCCmec II strains had three or more CF clinic visits compared with those who acquired SCCmec IV. This observation persisted when adjusted for the covariates (adjusted odds ratio, 2.05; 95% CI, 1.13–3.74; P = 0.019). The frequency of hospitalization and days of hospitalization were not associated with SCCmec type when adjusted for covariates (adjusted odds ratio, 0.99; 95% CI, 0.52–1.89; P = 0.97).
Changes in Clinical Parameters from 6 Months before to after Detection of Various MRSA Types
The association of SCCmec II versus SCCmec IV MRSA with changes in clinical care and outcomes from 6 months before to 6 months after initial MRSA detection is shown in Table 3. Differences between the two groups, adjusted for covariates, are also shown. The use of chronic CF medications increased within both groups, with the exception that the use of inhaled antibiotics remained unchanged in those with SCCmec IV strains but increased significantly in those with SCCmec II strains. The greatest percent increase occurred for use of DNase for both groups. The number of CF clinic visits increased regardless of SCCmec type. P. aeruginosa–positive cultures, hospitalizations, and duration of hospitalization were similar before and after MRSA detection and were similar by SCCmec type. There were no significant changes in lung function or anthropometric parameters.
Table 3.
Changes in clinical parameters 6 months before compared with after detection of various methicillin-resistant Staphylococcus aureus types
| Change within SCCmec II (n = 205) |
Change within SCCmec IV (n = 90) |
Adjusted* Difference between SCCmec Type (SCCmec II–SCCmec IV) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | 95% CI | n | Mean | 95% CI | Mean Difference | 95% CI | P Value | |
| Health status | |||||||||
| FEV1% predicted | 102 | −0.1 | –2.23, 2.04 | 34 | −1.45 | –5.15, 2.25 | 2.49 | –2.26, 7.25 | 0.30 |
| BMI or weight/stature percentile | 180 | −0.56 | –2.74, 1.62 | 68 | 2.87 | –0.68, 6.41 | −2.48 | –6.78, 1.82 | 0.257 |
| CF medications | 205 | 90 | |||||||
| Chronic inhaled antibiotics | 17.56% | 9.75, 25.37 | 4.44% | –1.76, 10.65 | 12.87% | –0.44, 26.19 | 0.058 | ||
| Chronic DNase | 18.05% | 11.35, 24.75 | 11.11% | 3.14, 19.08 | 7.50% | –4.62, 19.62 | 0.22 | ||
| Chronic azithromycin | 8.29% | 4.25, 12.34 | 7.78% | 2.14, 13.42 | 0.00% | –7.43, 7.31 | 0.98 | ||
| Days of IV antibiotics per IV course†‡ | 160 | 2.62 | 0.18, 5.06 | 81 | 2.06 | –0.51, 4.63 | 1.48 | –2.59, 5.54 | 0.48 |
| Microbiology | |||||||||
| Any P. aeruginosa | 205 | −1.46% | –7, 4.07 | 90 | −1.11% | –8.47, 6.25 | −4.75% | –14.09, 4.59 | 0.32 |
| Health care exposure | |||||||||
| CF clinic visits | 205 | 0.84 | 0.52, 1.16 | 90 | 1.00 | 0.44, 1.56 | −0.030 | –0.65, 0.60 | 0.94 |
| ≥1 hospitalization | 205 | 6.83% | –0.19, 13.85 | 90 | 2.22% | –9.52, 13.96 | 4.16% | –9.78, 18.11 | 0.56 |
| Days inpatient per hospitalization | 160 | 0.72 | –0.35, 1.78 | 81 | 1.02 | –0.87, 2.92 | −0.39 | –2.53, 1.75 | 0.72 |
Definition of abbreviations: BMI = body mass index; CF = cystic fibrosis; CI = confidence interval; IV = intravenous; MRSA = methicillin-resistant Staphylococcus aureus; P. aeruginosa = Pseudomonas aeruginosa; SCCmec = staphylococcal cassette chromosome mec.
Changes are shown for characteristics before compared with after MRSA by SCCmec types and adjusted changes. Boldface indicates significance at the two-sided 0.05 level.
Comparisons by SCCmec type are adjusted for site, sex, age, year of MRSA onset, and P. aeruginosa during the 6 months after MRSA acquisition.
Evaluable in n = 160 SCCmec II and n = 81 SCCmec IV with data after 2003.
Combined inpatient and outpatient intravenous therapy.
Among participants with SCCmec IV, those with PVL(–) strains had a significant increase in the use of DNase (15.38%; 95% CI, 1.4, 29.37) and the chronic macrolide agents azithromycin and clarithromycin (12.82%; 95% CI, 1.84, 23.8). In contrast, those with PVL(+) strains had a significant increase in the use of inhaled antibiotics (7.84%; 95% CI, 0.21, 15.48). However, none of these differences were significant between the groups when adjusted for covariates. Participants having at least one hospitalization significantly increased among those with PVL(–) (17.95%; 95% CI, –0.07, 35.97) compared with those with PVL(+) strains (–9.80%; 95% CI, –24.96, 5.35) and this difference remained significant after adjusting for covariates (adjusted difference, 34.10%; 95% CI, 7.58, 60.61; P = 0.012).
Changes in Clinical Parameters from 2 Years before to after Detection of Various MRSA Types
Lung function, but not anthropometric measures, significantly declined in the 2 years after MRSA detection compared with the 2 years prior, regardless of SCCmec status (Table 4). Similarly, CF medication use and CF clinic visits also significantly increased in all participants compared with 2 years prior. In contrast, hospitalizations and IV antibiotic days per IV course significantly increased only for those with SCCmec II strains, and P. aeruginosa–positive cultures increased significantly in those with SCCmec IV strains. Whereas the number of cultures increased in each group, similar numbers of cultures were obtained before and after detection of MRSA; the mean (SD) cultures/participant/24 months was 5.4 (3.3) versus 5.3 (3.9) for those with SCCmec II versus SCCmec IV strains, respectively (P = 0.98) before MRSA detection and 8.6 (3.9) versus 9.1 (3.8) for those with SCCmec II versus SCCmec IV strains, respectively (P = 0.30) after MRSA acquisition. None of the changes that occurred within SCCmec type were significantly different between the two SCCmec types, adjusted for covariates.
Table 4.
Changes in clinical parameters 2 years before, compared with after, detection of various methicillin-resistant Staphylococcus aureus types
| Change within SCCmec II (n = 205) |
Change within SCCmec IV (n = 90) |
Adjusted* Difference between SCCmec Type (SCCmec II–SCCmec IV) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | 95% CI | n | Mean | 95% CI | Mean Difference | 95% CI | P Value | |
| Health status | |||||||||
| FEV1% predicted | 110 | −2.40 | –3.99, –0.72 | 43 | −2.64 | –5.29, –0.01 | −0.17 | –4.50, 4.17 | 0.94 |
| BMI or weight/stature percentile | 196 | 0.22 | –2.00, 2.43 | 80 | 1.32 | –2.19, 4.83 | −0.022 | –5.74, 5.69 | 0.99 |
| CF medications | 205 | 90 | |||||||
| Chronic inhaled antibiotics | 32.20% | 24.56, 39.83 | 14.44% | 2.88, 26.01 | 12.31% | –7.10, 31.72 | 0.21 | ||
| Chronic DNase | 27.32% | 21.02, 33.62 | 25.56% | 15.85, 35.26 | 11.16% | –3.62, 25.94 | 0.14 | ||
| Chronic macrolides | 24.88% | 18.76, 31.00 | 21.11% | 12.52, 29.71 | 14.27% | –1.51, 30.07 | 0.076 | ||
| Days of IV antibiotics per IV course†‡ | 160 | 1.66 | 0.09, 3.23 | 81 | 2.45 | –0.57, 5.47 | −0.60 | –4.53, 3.33 | 0.76 |
| Microbiology | 205 | 90 | |||||||
| Any P. aeruginosa | 1.00% | –7.09, 9.04 | 17.78% | 6.64, 28.91 | −12.25% | –27.76, 3.25 | 0.12 | ||
| Health care exposure | |||||||||
| CF clinic visits | 205 | 4.23 | 3.38, 5.08 | 90 | 5.44 | 3.56, 7.31 | −0.49 | –3.07, 2.10 | 0.71 |
| ≥1 Hospitalization | 205 | 12.7% | 4.8, 20.6 | 90 | 7.8% | –5.5, 20.0 | 3.2% | –11.7, 18.0 | 0.67 |
| Days inpatient per hospitalization | 160 | 0.92 | –0.23, 2.08 | 81 | 2.19 | –0.47, 4.85 | −1.82 | –4.90, 1.26 | 0.25 |
Definition of abbreviations: BMI = body mass index; CF = cystic fibrosis; CI = confidence interval; IV = intravenous; MRSA = methicillin-resistant Staphylococcus aureus; P. aeruginosa = Pseudomonas aeruginosa; SCCmec = staphylococcal cassette chromosome mec.
Changes are shown for characteristics 2 years before compared with 2 years after MRSA per SCCmec type. Boldface represents significance at the two-sided 0.05 level.
Comparisons by SCCmec type are adjusted for site, sex, age, year of MRSA onset, and P. aeruginosa during the 24 months after MRSA acquisition.
Evaluable in n = 160 SCCmec II and n = 81 SCCmec IV with data after 2003.
Combined inpatient and outpatient intravenous therapy.
In participants with SCCmec IV, changes in clinical parameters in the 2 years before versus 2 years after MRSA acquisition were similar in those with and without the PVL gene and remained similar when adjusted for covariates (data not shown). Whereas those with PVL(–) versus PVL(+) strains had a similar proportion of P. aeruginosa–positive cultures, those acquiring PVL(+) isolates had more cultures taken in the 2 years before MRSA detection; the mean (SD) cultures/participant/24 months for those with PVL(–) versus PVL(+) strains was 4.2 (2.9) versus 6.2 (4.5), respectively (P = 0.02). The number of cultures increased after MRSA detection in those with either PVL(–) and PVL(+) strains; the mean (SD) cultures/participant/24 months for those with PVL(–) versus PVL(+) strains was 8.9 (3.8) versus 9.2 (3.8), respectively (P = 0.67).
Discussion
In this multicenter study, initial detection of MRSA in children and adolescents with CF occurred at 7 years of age and was similar among those with different SCCmec types; 20% of participants were less than 3 years of age at the time of their first MRSA-positive culture. The proportion of SCCmec IV strains, both PVL(–) and PVL(+), increased from 1996 to 2010. The increase in SCCmec IV types during this time interval is consistent with the increase in community-associated (SCCmec IV) MRSA infections in the general U.S. population (8, 33). Our prior study in this CF study population has shown that the SCCmec IV isolates are usually USA300 (19). Further, despite some geographic variation of MRSA prevalence within the United States among non-CF patients (34), we found that the distribution of SCCmec II versus SCCmec IV strains did not differ by region (19).
We explored the association of various SCCmec types with CF health status within the 2 years before detection of MRSA and with health care exposures and antibiotic use within the 6 months before detection. These two time intervals were selected to assess various risk factors for MRSA (13, 35, 36); underlying health status often shows slower changes over time in contrast to the short-term health care exposures that could result in acquisition of HA-MRSA, that is, SCCmec II strains. Furthermore, both exposure and outcome can be measured in this shorter interval as patients with CF are seen at regular intervals and need to produce respiratory cultures frequently. Although CF health status was not associated with acquisition of a specific MRSA type, P. aeruginosa–positive cultures and an increased number of CF clinic visits were associated with SCCmec II. Acquisition of P. aeruginosa has been associated with more clinic visits in newborn screening studies (37, 38), and P. aeruginosa is transmissible from CF patient to CF patient (39). At present, it remains unclear whether CF clinic visits and/or P. aeruginosa are risks for acquiring SCCmec II MRSA.
Outcomes after MRSA acquisition showed a general intensification of care among all participants as evidenced by an increased number of clinic visits and use of CF medications. Of note, SCCmec type and PVL status were not known to the treating physicians in this study, and thus MRSA type did not influence treatment decisions. However, the use of inhaled antibiotics and more IV antibiotic days increased only in participants with SCCmec II strains within 6 months of MRSA acquisition. Both of these observations may reflect the higher prevalence of P. aeruginosa in this group, and the latter could be explained by the higher rate of antibiotic resistance genes in SCCmec II isolates requiring IV antibiotics. Overall, similar trends in care intensification were noted when the observation time was extended to 2 years. We can only speculate whether intensification of care reflects a response to progression of CF lung disease as evidenced in the decline in lung function observed in both groups, or is related to patient and clinician concerns after detection of MRSA. Others have reported initiation of CF therapies not directly targeting MRSA, use of inhaled tobramycin, was higher in subjects with MRSA compared with MSSA despite similar prevalence of P. aeruginosa (40, 41).
Our findings that participants with PVL(–) isolates had more hospital admissions within the 6 months after MRSA detection than those with PVL(+) isolates are somewhat at odds with prior reports of acute necrotizing pneumonia in children with CF (17) or previously healthy people (15, 16).
In the current analyses we lack information on the use of oral antibiotics targeting MRSA as these data have been available in the CF Patient Registry only since 2012. However, we previously demonstrated that those participants with longstanding SCCmec II MRSA infection had more exacerbations and increased use of oral antibiotics compared with those with SCCmec IV MRSA (25). We suggest that differences in study design may explain the seemingly different results in the current study compared with our prior study that prospectively evaluated exacerbations in the same participants. These differences include the duration of MRSA infections, that is, 3–4 years in the prior study versus the maximum 2 years in the current study and the outcome measures assessed, that is, symptoms and use of oral antibiotics versus the variables available in the CF Patient Registry. Future multicenter, prospective studies would be necessary to further evaluate the association of MRSA-specific factors, underlying disease severity, and clinical care practices with patient outcomes.
Although we cannot prove causality in this epidemiologic study, the higher rate of clinic visits and P. aeruginosa, which is also transmissible, could imply a health care–related source of infection for those with SCCmec II and thus argue for strict infection control policies. Combined with our prior observation of more exacerbations in subjects with chronic SCCmec II MRSA compared with those with SCCmec IV, knowledge of SCCmec status may be beneficial both regarding epidemiologic/risk assessment and subsequent treatment decisions. Again, firm conclusions should be made cautiously, based solely on epidemiologic studies; however, the higher use of antibiotics seen in our prior study and other risks may indicate that MRSA-specific therapies should be intensified in patients acquiring SCCmec II isolates.
There are limitations to this study. Although this is a multicenter study, it is unknown whether our findings are generalizable to all patients with CF, including adults, or to those with transient MRSA. Lung function is not reliably tested in children less than 6 years of age, but the proportion of participants without lung function data was similar across MRSA types. Hospitalizations and intravenous antibiotic treatment were not recorded before 2003. These missing data affect power, but are unlikely to cause bias. Although the ability to use data in the Registry since 1996 can enhance participant inclusion and follow-up, it is likely that changes in medical management that occurred over time can influence outcomes. Finally, being an exploratory study, adjustment for multiple testing was not performed, and therefore spurious findings cannot be ruled out.
In conclusion, we demonstrate that first detection of MRSA occurred in early childhood among those who go on to have persistent infection. Presence of any P. aeruginosa–positive culture and an increased number of CF clinic visits 6 months before initial MRSA detection adjusted for P. aeruginosa status were potential risk factors associated with acquisition of SCCmec II MRSA implicating patient and care-related risks. There was no increased morbidity in those with PVL(+) isolates. Short-term changes after initial MRSA detection showed increases in multiple chronic medications and clinic visits for all participants and, when adjusting for covariates, was similar among the various SCCmec types. To better delineate changes that occur during transition from early to chronic MRSA infection, translational and ideally prospective studies that study bacterial changes and host factors will be necessary.
Acknowledgments
Acknowledgment
The authors thank the Cystic Fibrosis Foundation for the use of CF Foundation Patient Registry data to conduct this study. In addition, the authors thank the patients, care providers, and clinic coordinators at CF centers throughout the United States for their contributions to the CF Foundation Patient Registry.
Footnotes
Supported by Cystic Fibrosis Foundation Therapeutics in the form of a grant (MUHLEB08A0).
Author Contributions: All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. Additional contributions are shown by author: M.S.M.: Study design and inception, management of study progress, data collection, analyses, and writing. S.L.H.: Analyses and interpretation of data, writing and significant contribution to manuscript content. E.B.P.: Data acquisition, management of study progress, manuscript editing and writing. M.B.M.: Study design and inception, management of study progress, manuscript revision. V.T.: Analyses and interpretation of data, contribution to manuscript content. M.K.: Analyses and interpretation of data, contribution to manuscript content. T.F.: Study design and inception, management of study progress, critical input in data interpretation. W.C.H.: Data collection, management of study progress, critical input in data interpretation and manuscript revision. M.S.S.: Data collection, critical input in data interpretation and revision. L.S.: Study design and inception, data collection, analyses, and critical revision and editing of manuscript content.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Razvi S, Quittell L, Sewall A, Quinton H, Marshall B, Saiman L. Respiratory microbiology of patients with cystic fibrosis in the United States, 1995 to 2005. Chest. 2009;136:1554–1560. doi: 10.1378/chest.09-0132. [DOI] [PubMed] [Google Scholar]
- 2.Cystic Fibrosis FoundationPatient registry reports [accessed 2014 Dec]. Available from: http://www.cff.org/livingwithcf/qualityimprovement/patientregistryreport/
- 3.Dasenbrook EC, Checkley W, Merlo CA, Konstan MW, Lechtzin N, Boyle MP. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA. 2010;303:2386–2392. doi: 10.1001/jama.2010.791. [DOI] [PubMed] [Google Scholar]
- 4.Dasenbrook EC, Merlo CA, Diener-West M, Lechtzin N, Boyle MP. Persistent methicillin-resistant Staphylococcus aureus and rate of FEV1 decline in cystic fibrosis. Am J Respir Crit Care Med. 2008;178:814–821. doi: 10.1164/rccm.200802-327OC. [DOI] [PubMed] [Google Scholar]
- 5.Chen SY, Liao CH, Wang JL, Chiang WC, Lai MS, Chie WC, Chen WJ, Chang SC, Hsueh PR. Methicillin-resistant Staphylococcus aureus (MRSA) staphylococcal cassette chromosome mec genotype affects outcomes of patients with healthcare-associated MRSA bacteremia independently of vancomycin minimum inhibitory concentration. Clin Infect Dis. 2012;55:1329–1337. doi: 10.1093/cid/cis717. [DOI] [PubMed] [Google Scholar]
- 6.Ito T, Katayama Y, Asada K, Mori N, Tsutsumimoto K, Tiensasitorn C, Hiramatsu K. Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2001;45:1323–1336. doi: 10.1128/AAC.45.5.1323-1336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shore AC, Coleman DC. Staphylococcal cassette chromosome mec: recent advances and new insights. Int J Med Microbiol. 2013;303:350–359. doi: 10.1016/j.ijmm.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Tenover FC, McAllister S, Fosheim G, McDougal LK, Carey RB, Limbago B, Lonsway D, Patel JB, Kuehnert MJ, Gorwitz R. Characterization of Staphylococcus aureus isolates from nasal cultures collected from individuals in the United States in 2001 to 2004. J Clin Microbiol. 2008;46:2837–2841. doi: 10.1128/JCM.00480-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated methicillin-resistant Staphylococcus aureus. Lancet. 2010;375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deurenberg RH, Stobberingh EE. The molecular evolution of hospital- and community-associated methicillin-resistant Staphylococcus aureus. Curr Mol Med. 2009;9:100–115. doi: 10.2174/156652409787581637. [DOI] [PubMed] [Google Scholar]
- 11.Lowy FD. Methicillin-resistant Staphylococcus aureus: where is it coming from and where is it going? JAMA Intern Med. 2013;173:1978–1979. doi: 10.1001/jamainternmed.2013.8277. [DOI] [PubMed] [Google Scholar]
- 12.Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest. 2003;111:1265–1273. doi: 10.1172/JCI18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo SC, Chiang MC, Lee WS, Chen LY, Wu HS, Yu KW, Fung CP, Wang FD. Comparison of microbiological and clinical characteristics based on SCCmec typing in patients with community-onset meticillin-resistant Staphylococcus aureus (MRSA) bacteraemia. Int J Antimicrob Agents. 2011;39:22–26. doi: 10.1016/j.ijantimicag.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Klein E, Smith DL, Laxminarayan R. Community-associated methicillin-resistant Staphylococcus aureus in outpatients, United States, 1999–2006. Emerg Infect Dis. 2009;15:1925–1930. doi: 10.3201/eid1512.081341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francis JS, Doherty MC, Lopatin U, Johnston CP, Sinha G, Ross T, Cai M, Hansel NN, Perl T, Ticehurst JR, et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton–Valentine leukocidin genes. Clin Infect Dis. 2005;40:100–107. doi: 10.1086/427148. [DOI] [PubMed] [Google Scholar]
- 16.Tseng MH, Wei BH, Lin WJ, Lu JJ, Lee SY, Wang SR, Chen SJ, Wang CC. Fatal sepsis and necrotizing pneumonia in a child due to community-acquired methicillin-resistant Staphylococcus aureus: case report and literature review. Scand J Infect Dis. 2005;37:504–507. doi: 10.1080/00365540510037849. [DOI] [PubMed] [Google Scholar]
- 17.Elizur A, Orscheln RC, Ferkol TW, Atkinson JJ, Dunne WM, Jr, Buller RS, Armstrong JR, Mardis ER, Storch GA, Cannon CL. Panton–Valentine leukocidin-positive methicillin-resistant Staphylococcus aureus lung infection in patients with cystic fibrosis. Chest. 2007;131:1718–1725. doi: 10.1378/chest.06-2756. [DOI] [PubMed] [Google Scholar]
- 18.Voyich JM, Otto M, Mathema B, Braughton KR, Whitney AR, Welty D, Long RD, Dorward DW, Gardner DJ, Lina G, et al. Is Panton–Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis. 2006;194:1761–1770. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
- 19.Champion EA, Miller MB, Popowitch EB, Hobbs MM, Saiman L, Muhlebach MS STAR-CF Study Team. Antimicrobial susceptibility and molecular typing of MRSA in cystic fibrosis. Pediatr Pulmonol. 2014;49:230–237. doi: 10.1002/ppul.22815. [DOI] [PubMed] [Google Scholar]
- 20.Stone A, Quittell L, Zhou J, Alba L, Bhat M, DeCelie-Germana J, Rajan S, Bonitz L, Welter JJ, Dozor AJ, et al. Staphylococcus aureus nasal colonization among pediatric cystic fibrosis patients and their household contacts. Pediatr Infect Dis J. 2009;28:895–899. doi: 10.1097/inf.0b013e3181a3ad0a. [DOI] [PubMed] [Google Scholar]
- 21.Glikman D, Siegel JD, David MZ, Okoro NM, Boyle-Vavra S, Dowell ML, Daum RS. Complex molecular epidemiology of methicillin-resistant Staphylococcus aureus isolates from children with cystic fibrosis in the era of epidemic community-associated methicillin-resistant S. aureus. Chest. 2008;133:1381–1387. doi: 10.1378/chest.07-2437. [DOI] [PubMed] [Google Scholar]
- 22.Goodrich JS, Sutton-Shields TN, Kerr A, Wedd JP, Miller MB, Gilligan PH. Prevalence of community-associated methicillin-resistant Staphylococcus aureus in patients with cystic fibrosis. J Clin Microbiol. 2009;47:1231–1233. doi: 10.1128/JCM.00255-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Zubeidi D, Hogan PG, Boyle M, Burnham CA, Fritz SA. Molecular epidemiology of methicillin-resistant Staphylococcus aureus isolated in serial cultures from the respiratory tract of children with cystic fibrosis. Pediatr Infect Dis J. 2014;33:549–553. doi: 10.1097/INF.0000000000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muhlebach MS, Miller M, LaVange LM, Mayhew G, Goodrich JS, Miller MB. Treatment intensity and characteristics of MRSA infection in CF. J Cyst Fibros. 2011;10:201–206. doi: 10.1016/j.jcf.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Heltshe SL, Saiman L, Popowitch EB, Miller MB, Kloster M, Thompson V, Ferkol TW, Hoover WC, Schechter MS, Muhlebach MS. Outcomes and treatment of chronic methicillin-resistant Staphylococcus aureus differs by staphylococcal cassette chromosome mec (SCCmec) type in children with cystic fibrosis. J Pediatr Infect Dis Soc. 2014. pp. 1–7. [DOI] [PMC free article] [PubMed]
- 26.Miller MB, Gilligan PH. Laboratory aspects of management of chronic pulmonary infections in patients with cystic fibrosis. J Clin Microbiol. 2003;41:4009–4015. doi: 10.1128/JCM.41.9.4009-4015.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliveira DC, de Lencastre H. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2002;46:2155–2161. doi: 10.1128/AAC.46.7.2155-2161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol. 1993;15:75–88. doi: 10.1002/ppul.1950150204. [DOI] [PubMed] [Google Scholar]
- 29.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 30.Flegal KM, Wei R, Ogden C. Weight-for-stature compared with body mass index-for-age growth charts for the United States from the Centers for Disease Control and Prevention. Am J Clin Nutr. 2002;75:761–766. doi: 10.1093/ajcn/75.4.761. [DOI] [PubMed] [Google Scholar]
- 31.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 32.Lee TW, Brownlee KG, Conway SP, Denton M, Littlewood JM. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros. 2003;2:29–34. doi: 10.1016/S1569-1993(02)00141-8. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and PreventionABCs report: methicillin-resistant Staphylococcus aureus, 2011 [Emerging Infections Program Network] [accessed 2014 Dec]. Available from: http://www.cdc.gov/abcs/reports-findings/survreports/mrsa11.html
- 34.Sun L, Klein EY, Laxminarayan R. Seasonality and temporal correlation between community antibiotic use and resistance in the United States. Clin Infect Dis. 2012;55:687–694. doi: 10.1093/cid/cis509. [DOI] [PubMed] [Google Scholar]
- 35.Robicsek A, Beaumont JL, Thomson RB, Jr, Govindarajan G, Peterson LR. Topical therapy for methicillin-resistant Staphylococcus aureus colonization: impact on infection risk. Infect Control Hosp Epidemiol. 2009;30:623–632. doi: 10.1086/597550. [DOI] [PubMed] [Google Scholar]
- 36.Robicsek A, Beaumont JL, Wright MO, Thomson RB, Jr, Kaul KL, Peterson LR. Electronic prediction rules for methicillin-resistant Staphylococcus aureus colonization. Infect Control Hosp Epidemiol. 2011;32:9–19. doi: 10.1086/657631. [DOI] [PubMed] [Google Scholar]
- 37.Farrell PM, Shen G, Splaingard M, Colby CE, Laxova A, Kosorok MR, Rock MJ, Mischler EH. Acquisition of Pseudomonas aeruginosa in children with cystic fibrosis. Pediatrics. 1997;100:E2. doi: 10.1542/peds.100.5.e2. [DOI] [PubMed] [Google Scholar]
- 38.Kosorok MR, Jalaluddin M, Farrell PM, Shen G, Colby CE, Laxova A, Rock MJ, Splaingard M. Comprehensive analysis of risk factors for acquisition of Pseudomonas aeruginosa in young children with cystic fibrosis. Pediatr Pulmonol. 1998;26:81–88. doi: 10.1002/(sici)1099-0496(199808)26:2<81::aid-ppul2>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 39.Fothergill JL, Walshaw MJ, Winstanley C. Transmissible strains of Pseudomonas aeruginosa in cystic fibrosis lung infections. Eur Respir J. 2012;40:227–238. doi: 10.1183/09031936.00204411. [DOI] [PubMed] [Google Scholar]
- 40.Ren CL, Morgan WJ, Konstan MW, Schechter MS, Wagener JS, Fisher KA, Regelmann WE Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Presence of methicillin resistant Staphylococcus aureus in respiratory cultures from cystic fibrosis patients is associated with lower lung function. Pediatr Pulmonol. 2007;42:513–518. doi: 10.1002/ppul.20604. [DOI] [PubMed] [Google Scholar]
- 41.Taccetti G, Neri AS, Festini F, Galici V, Cocchi P, Campana S. Methicillin resistant Staphylococcus aureus in cystic fibrosis. Pediatr Pulmonol. 2008;43 doi: 10.1002/ppul.20774. 309, author reply 310. [DOI] [PubMed] [Google Scholar]
- 42.Muhlebach MS, Popwitch E, Miller MB, Peng A, LaVange L, Saiman L STAR-CF Study Team. Risk factors for MRSA subtypes in CF. Pediatr Pulmonol. 2012;33(Suppl):304S. [Google Scholar]