Abstract
The lung clearance index (LCI) is a lung function parameter derived from the multiple-breath washout (MBW) test. Although first developed 60 years ago, the technique was not widely used for many years. Recent technological advances in equipment design have produced gains in popularity for this test among cystic fibrosis (CF) researchers and clinicians, particularly for testing preschool-aged children. LCI has been shown to be feasible and sensitive to early CF lung disease in patients of all ages from infancy to adulthood. A workshop was convened in January 2014 by the North American Cystic Fibrosis Foundation to determine the readiness of the LCI for use in multicenter clinical trials as well as clinical care. The workshop concluded that the MBW text is a valuable potential outcome measure for CF clinical trials in preschool-aged patients and in older patients with FEV1 in the normal range. However, gaps in knowledge about the choice of device, gas, and standardization across systems are key issues precluding its use as a clinical trial end point in infants. Based on the current evidence, there are insufficient data to support the use of LCI or MBW parameters in the routine clinical management of patients with CF.
Keywords: multiple-breath washout, lung clearance index, cystic fibrosis, pulmonary function tests
Cystic fibrosis (CF) airway disease is characterized by airway surface liquid depletion, mucus plugging, and chronic infection and inflammation resulting in bronchiectasis. Although progressive airway obstruction as detected by spirometry is typical of CF, advances in multidisciplinary care over the last 2 decades have resulted in preservation of spirometric lung function within the normal range into young adulthood (1, 2). In contrast, there is mounting evidence that progression of bronchiectasis can go undetected by spirometry for many years (3, 4). Objective evaluation of lung function in early CF lung disease has been hampered by a lack of feasible sensitive outcome measures. On the other hand, early intervention before onset of irreversible lung damage requires assessment of infants and preschool children. The challenge is to find a feasible sensitive objective outcome measure to assess new interventions applied across all age groups.
Recently, an American Thoracic Society workshop report reviewed the literature supporting the use of preschool lung function tests in the diagnosis and monitoring of preschool lung disease, including CF (5). This report highlighted multiple-breath washout (MBW) for its ability to discriminate between health and disease in preschool children with CF and its potential use for monitoring preschool lung disease.
Due to the heightened interest in this lung function test for CF from both the clinical and research communities, the North American Cystic Fibrosis Foundation (CFF) and Therapeutics Development Network (TDN) hosted a workshop that brought together international leaders in the field to discuss the level of evidence and challenges in moving this outcome measure into clinical trials and practice. To better understand the degree of uptake of this new technology, a survey of the CF clinic directors was performed. Based on the survey and the goals of the workshop; this report was produced to give guidance to the North American CF community on the readiness of this tool for clinical trials and current limitations in knowledge that must be addressed before recommending implementation of this technology into clinical practice.
The workshop report is the cumulative efforts of all participants. Each of the participants was a member of one of the six working group based on their expertise. Each working group presented a summary of available evidence for their topic at the meeting. After the meeting, each group submitted a draft of their section for inclusion in the final report. The Chair (P.S.) and Co-Chairs (C.M., W.M.) of the workshop summarized the findings into an overall workshop report with extensive input from all participants. The final manuscript was approved by all workshop participants.
MBW Testing
An old technology that has been recently revisited, the MBW test is a gas washout technique. The technique was introduced by Ward S. Fowler in 1952. He described a method to measure nitrogen clearance curves of single breaths in healthy subjects and those with cardiorespiratory disease to quantify the “unevenness of gas distribution” (6). With advances in gas analyzers and computers to faster integration of gas and volume signals, the MBW method has been refined to allow the measurement of ventilation distribution and gas clearance curves during tidal breaths, thus permitting its use in very young patients. A recent American Thoracic Society/European Respiratory Society consensus statement was published on the measurement of MBW (7).
In any washout test, there is a wash-in and a washout phase. For inert extrinsic gases (i.e., sulfur hexafluoride, SF6; helium, He) or nonresident pulmonary gases, during the wash-in phase the test gas is delivered at a known concentration. Wash-in is complete when the expired gas concentration reaches the delivered gas concentration. For inert intrinsic gases (i.e., nitrogen, N2), no formal wash-in phase is required, and a few tidal breaths are measured to ensure that the measured resident N2 is stable (normally at 80%).
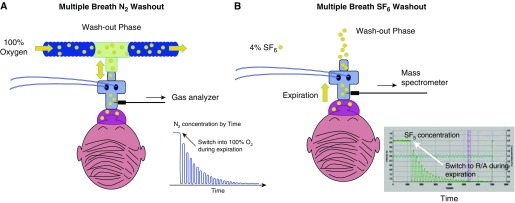
The two most commonly reported parameters from MBW tests are the lung clearance index (LCI) and moment ratios (MRs). Measurements of LCI and MR are taken during the washout period. During the washout phase, subjects inhale gases that do not contain the test gas of interest (i.e., room air for SF6 and 100% oxygen for N2). The principles of the washout are the same regardless of the test gas measured. The washout is stopped once the test gas reaches 1/40 of the initial gas concentration (Figure 1).
Figure 1.
Schematic representation of two washout phase tests using the two techniques. (A) N2-based setup. During the N2 washout, 100% oxygen is delivered using a bias flow. The blue tracing shows the decay in the N2 signal during expiration. (B) Extrinsic gas, SF6-based setup. The green tracing shows the decay in the SF6 concentration during the washout-phase. R/A = room air.
The LCI
The lung clearance index is a marker of overall lung ventilation inhomogeneity at the point that 1/40 of the concentration of the test gas remains in the exhaled breath. It is calculated as the number of lung volume turnovers required to clear the lung of the inert gas. Lung volume turnovers reflect the FRC, which is measured during the washout test.
The two parameters are calculated as follows:
| , |
where CET is the end-tidal concentration of the gas measured at the start (CETstart) and end (CETend) of the washout. VCE is the cumulative net expired volume to reach 1/40 of the initial gas concentration (sum of tidal breath volumes minus the apparatus dead space) during the washout.
Thus, as lung ventilation worsens, the number of tidal breaths and expired volumes required to wash out the inert gas results in an increasing LCI value.
Moment Ratios
MRs are used to quantify the ventilation inhomogeneity present and are described in detail elsewhere (7–9). Calculation of MRs is more challenging, but it holds the potential advantage of describing the entire washout curve rather than LCI, which assesses one time point (final cumulative expired volume at 1/40 starting gas concentration). MRs calculate the area under the washout curve with emphasis on the inert gas release during latter portions of the washout curve, which represent delayed emptying. The curves generated by MR analysis are independent of changes in breathing frequency and Vt. This parameter has not been commonly reported in clinical trials and as such is not a focus of the current document, but it remains an area of research interest.
MBW Technical Considerations
Inert gas washout tests were described 60 years ago. Practical application required a series of important developments, including fast-responding inert gas analyzers, faster personal computers to process changes within breath, and standardization of test performance to produce robust accurate results. An international consensus statement (7) was published in early 2013 to guide both manufacturers and researchers in this technique. Standardization work is an ongoing process and is by no means complete. Available commercial devices are at different stages of development with respect to: degree of measurement validation, current suitability for different age ranges, affordability, regional regulatory agency approvals, and transparency of outcome (index) calculation (Table 1). There is no single device suitable for use across the entire pediatric and adult age range to date.
Table 1.
Current commercially available multiple-breath washout devices in North America
| Innovision Innocor | Eco Medics AG Exhalyzer D | Ndd EasyOne Pro | |
|---|---|---|---|
| Overall design of setup | Closed, rebreathing circuit wash-in designed with CO2 scrubber to prevent CO2 accumulation. Open circuit washout design. | Open circuit wash-in and washout design using bias flow. | Open circuit washout design using bias flow. |
| Self-contained, portable | Semiportable, requires computer | Self-contained, portable | |
| Inert gas assessed | SF6 | N2 and SF6 MBW options available | N2 |
| Bias flow gas mixture used | Wash-in gas available as licensed SF6 gas mixture supplied by Innovision. | Medical air and 100% O2 required for N2 based MBW. | Medical air and 100% O2 required for N2-based MBW. |
| Wash-in SF6/O2/N2 mixture for SF6-based MBW sourced by individual user from local supplier. | |||
| Suitability of commercial software for younger age groups | No online analysis available at present. | Online analysis and quality-control features limited. | Online analysis and quality-control features limited. |
| No incentive software at present. | Incentive software available. | No incentive software at present. | |
| Suitability of software and equipment for the preschool age range. | Applicable lung model FRC validation as yet not available. | Applicable lung model N2 MBW FRC validation published. | Applicable lung model FRC validation as yet not available. |
| Equipment Vd must be reduced for preschoolers. | Equipment Vd must be reduced for preschoolers. | Equipment Vd must be reduced for preschoolers. | |
| Current mainstream SF6 MBW method not validated beyond infancy. | |||
| Suitability of software and equipment for infant age range. | Current rebreathing setup not suitable for infants. | Lung model N2 MBW FRC validation not available for infant range. | Lung model N2 MBW FRC validation not available for infant range. |
| Effect of 100% O2 on MBW results in this age group needs to be addressed. | Effect of 100% O2 on MBW results in this age group needs to be addressed. | ||
| SF6 mainstream MBW method validated for FRC measurement in infants only. | |||
| Published reference data | Not available | Robust reference data not published to date.* | Robust reference data not published to date.* |
Definition of abbreviation: MBW = multiple-breathing washout.
At the time of publication.
The choice of tracer gas used is a key factor in selection of an MBW device. The most widely used inert gases are N2 and SF6. N2 MBW requires only 100% oxygen (O2) during the washout phase, which is readily available but may not be appropriate for use in infants due to effects of pure oxygen on tidal breathing parameters (10–12). Furthermore, the contribution of tissue N2 diffusion to washout characteristics, although believed to be negligible in healthy individuals, has not been rigorously studied. Although SF6 MBW seems physiologically preferable for use across all ages, critical issues preventing its continued use include cost and limited availability. Furthermore, SF6 was listed in the Kyoto protocol as one of the top six gases whose release should be limited (13); given that it is “the most potent greenhouse gas known” (14), its future continued sustained use is not feasible. N2 is becoming more widespread and is being used for all age groups except infants.
Recent standardization work in device development and close collaboration between experienced operators suggest that previously reported between-center differences (15) may be avoidable with the use of commercial MBW equipment (16, 17). Furthermore, efforts to standardize not only commercial equipment but also patient interfaces (face mask, tubing, etc.) are important features to optimize and standardize measurement conditions. The current status of different commercially available equipment is summarized in Table 1.
Currently, there are few commercially available licensed systems available to measure LCI in infants and young children. Furthermore, very few data are available comparing the measurements between systems and the algorithms used to calculate measurements. Measurements using different inert gases are not interchangeable, and their relationship in health and disease is different. For these reasons, normative data generated from one system should not be used for other devices. Thus, validation of equipment in young children and normative data for all age groups are key priority areas.
MBW Measurement Considerations
Quality control is an essential component during testing and during posttesting analysis. Recent work has produced the first in-depth standard operating procedure for a commercial MBW device. The current focus of the working groups within the MBW community is to produce standard operating procedures for validated commercial devices as they become available to aid in the transition toward more routine research and clinical use. Here we highlight key features affecting feasibility and quality control during MBW testing.
Test Duration
The duration of the MBW test is a key determinant of feasibility in clinical practice and interventional trials. Although MBW is a passive tidal breathing test, the length of time to perform one test takes longer than conventional lung function tests (on average, 2–5 min per maneuver in a healthy subject, but longer in subjects with airway obstruction).
The duration of MBW tests is determined in part by the number of trials that are performed and the cutoff values used for the lung clearance index.
Number of Trials
MBW is performed during tidal breathing; thus, criteria defining the “best attempt” are difficult to establish. For example, it is not known whether the lowest LCI reflects the most reliable MBW test. Currently, MBW analysis requires post hoc quality control assessment. Current standards require three repeatable tests after post hoc analysis, yet studies comparing the first two acceptable tests to this standard showed that the LCI value produced had comparable discriminative ability (18, 19). The impact of such a shortened protocol on research quality data for sensitivity to detect treatment responses is unknown.
Lung Clearance Index Cutoff Values
Recommendations to perform MBW tracer gas washout to 1/40 (2.5%) of the starting gas concentration are historically based on poor N2 sensor resolution and estimated N2 tissue contribution. One recent study showed that LCI from N2 MBW until 1/20 (5%) compared with LCI from standard MBW until 1/40 provided the same discriminative capacity between control subjects and children with CF (18). Total washout duration was abbreviated, and intratest variability was lower. Abbreviated MBW protocols potentially improve success rates, especially in patients with severe lung disease or patients with brief attention spans (20–22). However, there is a decrease in sensitivity to detect treatment differences associated with these abbreviated MBW protocol LCIs (23).
Effect of Dead Space
Currently, most studies in preschool children and infants have used respiratory mass spectrometers as gas analyzers for two reasons: (1) they allow sufficient gas sampling rates to allow for reliable gas concentration measurements in small Vt at higher respiratory rates, and (2) they have limited equipment dead space across all age groups (from infancy to adulthood).
Differences have been noted between respiratory mass spectrometer–based SF6 and commercial N2-based LCI measurements. N2 LCI measurements are reported as systematically higher than SF6-based mass spectrometer–based LCI measurements (24). These differences are larger in preschool children than older children and adults, appear to be due to the higher Vd/Vt ratio associated with N2 MBW equipment, and were independent of health status (patient with CF vs. healthy control subject) (25). Thus, every effort should be made to minimize equipment dead space in MBW systems.
Analysis of MBW Test Results
Offline data analysis using custom-made software has initially been used for MBW testing. This procedure is time consuming and would limit the clinical usefulness of these tests in a busy outpatient clinic. Further work is required to assess the impact of different software algorithms on MBW measures. This is especially relevant for preschool children, for whom nonuniform tidal breaths, small leaks, and other test artifacts (swallowing, talking, etc.) are an issue, and in infants, for whom rapid respiratory rates and smaller tidal breaths associated with sleep state can affect LCI measurements.
Use of MRs as an Alternative to Lung Clearance Ratios for Early Detection of Lung Disease
Compared with LCI, several other methods exist to mathematically describe the shape of the MBW washout curve. MR quantifies the area under the washout curve and has been shown to be sensitive in detecting CF lung disease (26, 27). Furthermore, MR seems to better reflect structural changes in infants with CF than LCI (28). Preliminary data suggest that MR tracks pulmonary function response to short-term interventions similarly to LCI and may be superior in detecting treatment effects for shortened washout period (23).
Normal Value Reference Data
Normative data generated from one system cannot be applied across other devices. Limited reference data are available and are device and gas specific (29, 30). LCI declines through infancy and early childhood, remains constant in early childhood to adulthood, and slowly increases in the elderly (29, 31). Age is an important predictor of other MBW indices (e.g., concentration-normalized phase III slope analysis indices, Scond and Sacin). These data taken together suggest that raw values will not be useful in pediatrics and thus must be converted to z-scores to aid in longitudinal data interpretation.
Gaps in Knowledge and Key Priority Areas for the Further Development of Measurement Techniques
Multiple technical issues still need to be clarified, especially in infants and preschool children. Device-specific reference data are required throughout all age groups. Key issues related to data analysis include standardization and validation of the online software quality control.
Usefulness of Lung Clearance Index: Evidence from Clinical Studies
Evidence supporting the usefulness of the LCI has derived from both observational studies and clinical trials.
Observational Studies
LCI Correlation with Other Pulmonary Parameters
The majority of studies performed in subjects with CF are cross-sectional, where LCI is related to other measures of lung function (26, 32), imaging (28, 33–35), or clinical status (36–38). These studies consistently demonstrate that, compared with healthy control subjects, LCI is abnormal in a significant proportion of preschool children with CF and in the majority of school-aged children and adults (32, 33, 35, 39).
In contrast to older groups, at least half of infants with CF have normal LCI (28, 40–42). Specifically, some infants appear to have normal LCI in the presence of abnormal infant spirometry (measured by the raised volume rapid thoracoabdominal compression technique) (40) or abnormal computed tomography (28). A multicenter study from London (43), tracking children from preschool years to early school age, demonstrated (1) that LCI is far more likely to be abnormal than spirometry in preschool years, and (2) abnormal LCI in preschool children predicts both abnormal LCI and abnormal spirometry during school-age years.
Change in LCI with Microbiological Status
Cross-sectional data in infants and children suggest that those with evidence of bacterial infection are more likely to have abnormal LCI (36, 38, 40). In a preschool cross-sectional study, children who had “ever cultured” Pseudomonas aeruginosa from airway secretions had higher LCI than those who had not (32). Repeated measures within the Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) study suggest that LCI increases more in infants with airway infection than in those without infection (44).
LCI Change in Relation to Clinical Status
Among school-aged children, high LCI is consistently associated with increasing abnormality seen on chest CT, and this association has been demonstrated from infancy, through school age, to adulthood (28, 33–35). Increased LCI appears to predict those individuals who are more likely to have pulmonary exacerbations associated with infection (37), and LCI decreases when exacerbations are treated (45, 46).
Clinical Trials
The European CF Society Clinical Trial Network Standardization Committee has recently published a consensus document (47), which concludes that LCI has attractive features for its use in clinical trials involving young children and patients with mild lung disease. This assessment is based on several single-center and multicenter studies.
To date, one single-center study examined the usefulness of LCI (measured by respiratory mass spectrometry) in an interventional study in infants and preschool children (50). This add-on pilot study to the Infant Study of Inhaled Saline (ISIS) in CF trial (51) attempted MBW measurements in all 27 infant and preschool subjects participating in the Toronto center. Successful paired MBW measurements at baseline and end of therapy were obtained in 93% of participants. A significant treatment effect of hypertonic saline compared with isotonic saline was detected using LCI after adjustment for height. However, the pattern of LCI change with treatment differed between infants and preschool children. In preschool children, LCI was abnormal at baseline and improved with hypertonic saline treatment, whereas in infants, LCI was in the normal range at baseline and remained in the normal range after treatment with hypertonic saline. These different patterns of change in LCI have implications for sample size calculations for studies using LCI as an outcome measure for these different age groups.
Two randomized double-blind placebo-controlled single-center studies using an SF6 respiratory mass spectrometer have assessed the responsiveness of LCI in school-aged children with CF with normal spirometry measures. LCI improved after treatment with hypertonic saline (48) and with dornase alfa (49) over a 1-month period. Notably, FEV1 did not detect a treatment effect in these studies.
One multicenter interventional study enrolled patients, older than 8 years, with at least one copy of the G551D mutation and normal FEV1 (above 90% predicted at baseline) and assessed the ability of LCI to detect a treatment effect of ivacaftor (52). The magnitude of the treatment effect detected in this group of patients was twice as high when compared with the hypertonic saline and dornase alfa studies and provided evidence that LCI could be a responsive alternative outcome for pulmonary abnormalities in patients with mild lung disease. Notably, this study included centers with no previous experience in MBW measurements and implemented a training and qualification strategy that ensured high-quality data for the clinical trial. This type of strategy will be important to implement for future trials.
Gaps in Knowledge and Key Priority Areas for Future Clinical Studies
The majority of the observational studies cited above are cross-sectional studies. Given that more recent cohorts of young children with CF have less severe lung disease than those of a decade ago, previous observational studies may not provide valid predictions about change in LCI for current age groups. Longitudinal studies in health and disease are required to clarify, how LCI changes over time in CF.
Interventional studies to date have been performed with methodology that is not commercially available. Although these studies have found LCI sensitive at detecting a treatment effect, it is still unclear what magnitude of effect could be considered as clinically relevant. Also, no long-term interventional study with LCI as an outcome measure has been conducted to assess the sustainability of effects seen in shorter-term trials. Finally, it is unclear if noted improvements in LCI have predictive capacity for other known surrogate outcome factors for CF health status, such as rate of decline in FEV1 or pulmonary exacerbation rate.
Future work should focus on:
-
1.
Delineating the short-term and longer-term repeatability of LCI from commercial devices.
-
2.
Establishing the feasibility of performing MBW measurements in multicenter studies that include centers with limited experience.
-
3.
Establishing a minimal clinical important difference for clinical trial outcomes.
-
4.
Evaluating the role of LCI in interventional studies including patients with an FEV1 below the normal range
MBW Testing in the Clinical Setting
In an attempt to assess the current level of uptake and interest for LCI in the CF community and interest in the technology, a questionnaire was sent to all CF centers across the United States, Canada, and Europe. Eighty-four responses were received, of which 54 were from the United States, 6 from Canada, and 24 from Europe. Of these 84 centers, 31 (38%) are currently performing measurements of LCI, of which 21 reported its use primarily for research.
When asked “what were the most important issues to be considered prior to bringing this test into clinical practice,” 50% of the respondents cited a lack of evidence to support its role in clinical decision making as the most important issue. This was followed by: a need for a clinical practice guideline for interpretation, standardized training program for testing, and finally the generation of normative data.
Current Experience with Clinical Use of the LCI
To date, MBW is not a routine clinical test in U.S. and Canadian CF clinics. Many devices are not Food and Drug Administration approved, and cost recovery for the test (lack of billing code) is not possible in the United States.
A number of centers in Europe have experience using LCI in clinical care. At these centers, MBW is performed on at least an annual basis; however, MBW may be performed more often if the physician is unclear about symptomatology and/or if symptoms are being reported by the patient or parents (weight deviation, loss of appetite, fatigue, irritability). Currently, an increase of 1 unit in the LCI value is considered a sign of deterioration in these centers (36, 37). The medical history coupled with the LCI values is used as part of the clinical decision making for these patients. The overall goal is to keep LCI stable; patients with an increase in LCI values of more than 1 unit are often subjected to a modification in treatment or more aggressive investigations to understand the underlying cause for deterioration.
Current Challenges to Implementing MBW Testing in Routine Care
Implementation of MBW into routine clinical care presents several challenges, including equipment standardization, testing logistics in the clinical setting, staff training, cost, regulatory issues, and interpretation of data. These specific issues are highlighted below.
Although Food and Drug Administration–approved devices are available that perform the MBW technique, validation has not been established to the same degree in the different systems (i.e., preschool age range). Furthermore, measurements from one system are not necessarily comparable to other systems. Also, offline analysis is still undertaken for some systems. These factors alone significantly limit the ability to routinely perform the test in the clinical setting.
No one device has addressed all of the necessary validation and quality-control issues to recommend its routine use for clinical practice. Table 1 summarizes the evidence available in the different systems. Furthermore, training is critical for implementation into routine clinical care. As reported previously for infant lung function testing (53), obtaining good-quality data for novel procedures is often challenging, especially in inexperienced centers, but can be effective with ongoing quality assurance (54).
Finally, and perhaps of greatest importance, no published data are available outlining the usefulness of MBW in clinical practice. Currently, few centers have incorporated MBW into the routine clinical setting, and therefore longitudinal data are limited. In particular, it is unclear what change in LCI constitutes a minimally important clinical difference. The absence of these data precludes specific recommendations for its use in the clinical setting.
Priorities for Future Research to Support Clinical Use
More evidence is needed regarding the usefulness of LCI for clinical decision making, and clinically meaningful changes in LCI need to be defined. MBW indices may be ideally suited for early detection of peripheral airway obstruction but may not be the ideal test when more central airway obstruction is evident. Further data are needed to understand the limitations in interpretation of LCI and its role in the armamentarium of clinical pulmonary function tests.
Conclusions
It was the unanimous consensus of the expert panel convened that advancements in technology and commercialization have launched MBW as a potential outcome measure for use in CF clinical trials in preschool-aged patients and in patients with FEV1 in the normal range. Gaps in knowledge about the choice of device, gas, and standardization across systems are key issues leading the committee to conclude that MBW is not ready for use as a clinical trial end point in infants. Furthermore, despite the level of evidence available, the panel also recommended further work must be done before recommending the use of LCI or MBW parameters in the management of clinical care of patients with CF. Table 2 provides a summary of the panel recommendations.
Table 2.
Summary of recommendations
| Infant | Preschool | Patients with CF with FEV1 in Normal Range | |
|---|---|---|---|
| Tracer gas | SF6-based only in specialized centers | N2-based commercial systems | N2-based commercial systems |
| Single-center trials | Yes (in specialized centers) | Yes | Yes |
| Multicenter trials | No | Yes (in specialized centers) | Yes |
| Clinical practice | No | No | No |
Definition of abbreviation: CF = cystic fibrosis.
There are still a few key areas outlined in this report that need further work. Ongoing collaborative efforts at a number of centers and organizations across the globe should in the near term provide the required information to produce a robust set of standard operating procedures, normative data, and recommendations for the implementation of MBW in the research setting. As future studies using commercial MBW in the research arena expand and more patients are studied, it is imperative that there is a systematic collection and analysis of clinical data to relate to MBW parameters. This type of concerted collaborative effort will enable the development of recommendations for the use of MBW in the clinical setting in the near future.
Acknowledgments
Workshop Participants: Chair: Padmaja Subbarao, M.D. M.Sc.; Co-Chairs: Carlos Milla, M.D.; and Wayne Morgan, M.D.; Participants: Frank Accurso, M.D., Ph.D.; Paul Aurora, M.R.C.P., Ph.D.; Preston Campbell, III, M.D.; Jane C. Davies, Ph.D.; Stephanie D. Davis, M.D.; Graham L. Hall, Ph.D.; Sonya Heltshe, Ph.D.; Jordana Hoppe; Jean Kirihara; Anders Lindblad, M.D.; Jessica E. Pittman, M.D., M.P.H.; Felix Ratjen, M.D., Ph.D.; Bonnie Ramsey, M.D.; Paul D. Robinson, M.D., Ph.D.; Margaret Rosenfeld, M.D., M.P.H.; Florian Singer, M.D.; Tim D. Starner, M.D., Ph.D.; Jill VanDelfsen; Lisya Van Housen; and Wayne Morgan, M.D.
Footnotes
Supported by the North American CF Foundation.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Marshall BC, Nelson EC. Accelerating implementation of biomedical research advances: critical elements of a successful 10 year Cystic Fibrosis Foundation healthcare delivery improvement initiative. BMJ Qual Saf. 2014;23:i95–i103. doi: 10.1136/bmjqs-2013-002790. [DOI] [PubMed] [Google Scholar]
- 2.Bethesda, MD: 2012. Cystic Fibrosis Foundation. Patient registry 2012 annual data report. [Google Scholar]
- 3.Mott LS, Park J, Murray CP, Gangell CL, de Klerk NH, Robinson PJ, Robertson CF, Ranganathan SC, Sly PD, Stick SM AREST CF. Progression of early structural lung disease in young children with cystic fibrosis assessed using CT. Thorax. 2012;67:509–516. doi: 10.1136/thoraxjnl-2011-200912. [DOI] [PubMed] [Google Scholar]
- 4.Sanders DB, Li Z, Brody AS, Farrell PM. Chest computed tomography scores of severity are associated with future lung disease progression in children with cystic fibrosis. Am J Respir Crit Care Med. 2011;184:816–821. doi: 10.1164/rccm.201105-0816OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenfeld M, Allen J, Arets BH, Aurora P, Beydon N, Calogero C, Castile RG, Davis SD, Fuchs S, Gappa M, et al. American Thoracic Society Assembly on Pediatrics Working Group on Infant and Preschool Lung Function Testing. An official American Thoracic Society workshop report: optimal lung function tests for monitoring cystic fibrosis, bronchopulmonary dysplasia, and recurrent wheezing in children less than 6 years of age. Ann Am Thorac Soc. 2013;10:S1–S11. doi: 10.1513/AnnalsATS.201301-017ST. [DOI] [PubMed] [Google Scholar]
- 6.Fowler WS, Cornish ER, Jr, Kety SS. Lung function studies: VIII. Analysis of alveolar ventilation by pulmonary N2 clearance curves. J Clin Invest. 1952;31:40–50. doi: 10.1172/JCI102575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson PD, Latzin P, Verbanck S, Hall GL, Horsley A, Gappa M, Thamrin C, Arets HG, Aurora P, Fuchs SI, et al. Consensus statement for inert gas washout measurement using multiple- and single- breath tests. Eur Respir J. 2013;41:507–522. doi: 10.1183/09031936.00069712. [DOI] [PubMed] [Google Scholar]
- 8.Robinson PD, Goldman MD, Gustafsson PM. Inert gas washout: theoretical background and clinical utility in respiratory disease. Respiration. 2009;78:339–355. doi: 10.1159/000225373. [DOI] [PubMed] [Google Scholar]
- 9.Saidel GM, Salmon RB, Chester EH. Moment analysis of multibreath lung washout. J Appl Physiol. 1975;38:328–334. doi: 10.1152/jappl.1975.38.2.328. [DOI] [PubMed] [Google Scholar]
- 10.Singer F, Yammine S, Schmidt A, Proietti E, Kieninger E, Barben J, Casaulta C, Regamey N, Gustafsson P, Frey U, et al. Ventilatory response to nitrogen multiple-breath washout in infants. Pediatr Pulmonol. 2014;49:342–347. doi: 10.1002/ppul.22841. [DOI] [PubMed] [Google Scholar]
- 11.Cross KW, Warner P. The effect of inhalation of high and low oxygen concentrations on the respiration of the newborn infant. J Physiol. 1951;114:283–295. doi: 10.1113/jphysiol.1951.sp004620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller HC. Effect of high concentrations of carbon dioxide and oxygen on the respiration of fullterm infants. Pediatrics. 1954;14:104–113. [PubMed] [Google Scholar]
- 13.Betts KS. Worldwide effort underway to reduce SF6 greenhouse gas emissions. Environ Sci Technol. 1998;32:487A–488A. doi: 10.1021/es983788i. [DOI] [PubMed] [Google Scholar]
- 14.Maiss M, Brenninkmeijer CAM. Atmospheric SF6: trends, sources, and prospects. Environ Sci Technol. 1998;32:3077–3086. [Google Scholar]
- 15.Hülskamp G, Lum S, Stocks J, Wade A, Hoo AF, Costeloe K, Hawdon J, Deeptha K, Pillow JJ. Association of prematurity, lung disease and body size with lung volume and ventilation inhomogeneity in unsedated neonates: a multicentre study. Thorax. 2009;64:240–245. doi: 10.1136/thx.2008.101758. [DOI] [PubMed] [Google Scholar]
- 16.Fuchs SI, Ellemunter H, Eder J, Mellies U, Grosse-Onnebrink J, Tümmler B, Staab D, Jobst A, Griese M, Ripper J, et al. Feasibility and variability of measuring the Lung Clearance Index in a multi-center setting. Pediatr Pulmonol. 2012;47:649–657. doi: 10.1002/ppul.21610. [DOI] [PubMed] [Google Scholar]
- 17.Houltz B, Green K, Lindblad A, Singer F, Robinson P, Nielsen K, Gustafsson P. Tidal N2 washout ventilation inhomogeneity indices in a reference population aged 7–70 years. Eur Respir J. 2012;40:3797. [Google Scholar]
- 18.Yammine S, Singer F, Abbas C, Roos M, Latzin P. Multiple-breath washout measurements can be significantly shortened in children. Thorax. 2013;68:586–587. doi: 10.1136/thoraxjnl-2012-202345. [DOI] [PubMed] [Google Scholar]
- 19.Robinson PD, Stocks J, Aurora P, Lum S. Abbreviated multi-breath washout for calculation of lung clearance index. Pediatr Pulmonol. 2013;48:336–343. doi: 10.1002/ppul.22618. [DOI] [PubMed] [Google Scholar]
- 20.Horsley AR, Macleod KA, Robson AG, Lenney J, Bell NJ, Cunningham S, Greening AP, Gustafsson PM, Innes JA. Effects of cystic fibrosis lung disease on gas mixing indices derived from alveolar slope analysis. Respir Physiol Neurobiol. 2008;162:197–203. doi: 10.1016/j.resp.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Verbanck S, Paiva M, Paeps E, Schuermans D, Malfroot A, Vincken W, Vanderhelst E. Lung clearance index in adult cystic fibrosis patients: the role of convection-dependent lung units. Eur Respir J. 2013;42:380–388. doi: 10.1183/09031936.00125312. [DOI] [PubMed] [Google Scholar]
- 22.Verbanck S, Paiva M, Schuermans D, Malfroot A, Vincken W, Vanderhelst E. Acinar and conductive ventilation heterogeneity in severe CF lung disease: back to the model. Respir Physiol Neurobiol. 2013;188:124–132. doi: 10.1016/j.resp.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Stanojevic S, Jensen R, Sundaralingam D, Salazar JG, Yammine S, Singer F, Latzin P, Amin R, Subbarao P, Gustafsson P, et al. Alternative outcomes for the multiple breath washout in children with CF. J Cyst Fibros [online ahead of print] 8 Jan 2015.; DOI: 10.1016/j.jcf.2014.12.008 [DOI] [PubMed]
- 24.Jensen R, Stanojevic S, Gibney K, Salazar JG, Gustafsson P, Subbarao P, Ratjen F. Multiple breath nitrogen washout: a feasible alternative to mass spectrometry. PLoS One. 2013;8:e56868. doi: 10.1371/journal.pone.0056868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benseler A, Stanojevic S, Jensen R, Gustafsson P, Ratjen F. Effect of equipment dead space on multiple breath washout measures. Respirology. 2015;20:459–466. doi: 10.1111/resp.12470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraemer R, Blum A, Schibler A, Ammann RA, Gallati S. Ventilation inhomogeneities in relation to standard lung function in patients with cystic fibrosis. Am J Respir Crit Care Med. 2005;171:371–378. doi: 10.1164/rccm.200407-948OC. [DOI] [PubMed] [Google Scholar]
- 27.Wall MA. Moment analysis of multibreath nitrogen washout in young children. J Appl Physiol (1985) 1985;59:274–279. doi: 10.1152/jappl.1985.59.1.274. [DOI] [PubMed] [Google Scholar]
- 28.Hall GL, Logie KM, Parsons F, Schulzke SM, Nolan G, Murray C, Ranganathan S, Robinson P, Sly PD, Stick SM, et al. AREST CF. Air trapping on chest CT is associated with worse ventilation distribution in infants with cystic fibrosis diagnosed following newborn screening. PLoS One. 2011;6:e23932. doi: 10.1371/journal.pone.0023932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lum S, Stocks J, Stanojevic S, Wade A, Robinson P, Gustafsson P, Brown M, Aurora P, Subbarao P, Hoo AF, et al. Age and height dependence of lung clearance index and functional residual capacity. Eur Respir J. 2013;41:1371–1377. doi: 10.1183/09031936.00005512. [DOI] [PubMed] [Google Scholar]
- 30.Fuchs O, Latzin P, Thamrin C, Stern G, Frischknecht P, Singer F, Kieninger E, Proietti E, Riedel T, Frey U. Normative data for lung function and exhaled nitric oxide in unsedated healthy infants. Eur Respir J. 2011;37:1208–1216. doi: 10.1183/09031936.00125510. [DOI] [PubMed] [Google Scholar]
- 31.Verbanck S, Thompson BR, Schuermans D, Kalsi H, Biddiscombe M, Stuart-Andrews C, Hanon S, Van Muylem A, Paiva M, Vincken W, et al. Ventilation heterogeneity in the acinar and conductive zones of the normal ageing lung. Thorax. 2012;67:789–795. doi: 10.1136/thoraxjnl-2011-201484. [DOI] [PubMed] [Google Scholar]
- 32.Aurora P, Bush A, Gustafsson P, Oliver C, Wallis C, Price J, Stroobant J, Carr S, Stocks J London Cystic Fibrosis Collaboration. Multiple-breath washout as a marker of lung disease in preschool children with cystic fibrosis. Am J Respir Crit Care Med. 2005;171:249–256. doi: 10.1164/rccm.200407-895OC. [DOI] [PubMed] [Google Scholar]
- 33.Ellemunter H, Fuchs SI, Unsinn KM, Freund MC, Waltner-Romen M, Steinkamp G, Gappa M. Sensitivity of Lung Clearance Index and chest computed tomography in early CF lung disease. Respir Med. 2010;104:1834–1842. doi: 10.1016/j.rmed.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Gustafsson PM, De Jong PA, Tiddens HA, Lindblad A. Multiple-breath inert gas washout and spirometry versus structural lung disease in cystic fibrosis. Thorax. 2008;63:129–134. doi: 10.1136/thx.2007.077784. [DOI] [PubMed] [Google Scholar]
- 35.Owens CM, Aurora P, Stanojevic S, Bush A, Wade A, Oliver C, Calder A, Price J, Carr SB, Shankar A, et al. London Cystic Fibrosis Collaboration. Lung Clearance Index and HRCT are complementary markers of lung abnormalities in young children with CF. Thorax. 2011;66:481–488. doi: 10.1136/thx.2010.150375. [DOI] [PubMed] [Google Scholar]
- 36.Singer F, Kieninger E, Abbas C, Yammine S, Fuchs O, Proietti E, Regamey N, Casaulta C, Frey U, Latzin P. Practicability of nitrogen multiple-breath washout measurements in a pediatric cystic fibrosis outpatient setting. Pediatr Pulmonol. 2013;48:739–746. doi: 10.1002/ppul.22651. [DOI] [PubMed] [Google Scholar]
- 37.Vermeulen F, Proesmans M, Boon M, Havermans T, De Boeck K. Lung clearance index predicts pulmonary exacerbations in young patients with cystic fibrosis. Thorax. 2014;69:39–45. doi: 10.1136/thoraxjnl-2013-203807. [DOI] [PubMed] [Google Scholar]
- 38.Belessis Y, Dixon B, Hawkins G, Pereira J, Peat J, MacDonald R, Field P, Numa A, Morton J, Lui K, et al. Early cystic fibrosis lung disease detected by bronchoalveolar lavage and lung clearance index. Am J Respir Crit Care Med. 2012;185:862–873. doi: 10.1164/rccm.201109-1631OC. [DOI] [PubMed] [Google Scholar]
- 39.Gustafsson PM, Aurora P, Lindblad A. Evaluation of ventilation maldistribution as an early indicator of lung disease in children with cystic fibrosis. Eur Respir J. 2003;22:972–979. doi: 10.1183/09031936.03.00049502. [DOI] [PubMed] [Google Scholar]
- 40.Hoo AF, Thia LP, Nguyen TT, Bush A, Chudleigh J, Lum S, Ahmed D, Balfour Lynn I, Carr SB, Chavasse RJ, et al. London Cystic Fibrosis Collaboration. Lung function is abnormal in 3-month-old infants with cystic fibrosis diagnosed by newborn screening. Thorax. 2012;67:874–881. doi: 10.1136/thoraxjnl-2012-201747. [DOI] [PubMed] [Google Scholar]
- 41.Lum S, Gustafsson P, Ljungberg H, Hülskamp G, Bush A, Carr SB, Castle R, Hoo AF, Price J, Ranganathan S, et al. London Cystic Fibrosis Collaboration. Early detection of cystic fibrosis lung disease: multiple-breath washout versus raised volume tests. Thorax. 2007;62:341–347. doi: 10.1136/thx.2006.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen TT, Thia LP, Hoo AF, Bush A, Aurora P, Wade A, et al. Evolution of lung function during the first year of life in newborn screened cystic fibrosis infants. Thorax. 2014;69:910–917. doi: 10.1136/thoraxjnl-2013-204023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aurora P, Stanojevic S, Wade A, Oliver C, Kozlowska W, Lum S, Bush A, Price J, Carr SB, Shankar A, et al. London Cystic Fibrosis Collaboration. Lung clearance index at 4 years predicts subsequent lung function in children with cystic fibrosis. Am J Respir Crit Care Med. 2011;183:752–758. doi: 10.1164/rccm.200911-1646OC. [DOI] [PubMed] [Google Scholar]
- 44.Ramsey KA, Rosenow T, Skoric B, Adams A, Gallagher CM, Banton GL, Murray C, Ranganathan S, Stick SM, Hall GL. The ability of the Lung Clearance Index to detect bronchiectasis on chest computed tomography in infants and children with cystic fibrosis [abstract] Pediatr Pulmonol. 2014;49:359. [Google Scholar]
- 45.Horsley AR, Davies JC, Gray RD, Macleod KA, Donovan J, Aziz ZA, Bell NJ, Rainer M, Mt-Isa S, Voase N, et al. Changes in physiological, functional and structural markers of cystic fibrosis lung disease with treatment of a pulmonary exacerbation. Thorax. 2013;68:532–539. doi: 10.1136/thoraxjnl-2012-202538. [DOI] [PubMed] [Google Scholar]
- 46.Robinson PD, Cooper P, Van Asperen P, Fitzgerald D, Selvadurai H. Using index of ventilation to assess response to treatment for acute pulmonary exacerbation in children with cystic fibrosis. Pediatr Pulmonol. 2009;44:733–742. doi: 10.1002/ppul.20956. [DOI] [PubMed] [Google Scholar]
- 47.Kent L, Reix P, Innes JA, Zielen S, Le Bourgeois M, Braggion C, Lever S, Arets HG, Brownlee K, Bradley JM, et al. European Cystic Fibrosis Society Clinical Trial Network (ECFS-CTN) Standardisation Committee. Lung clearance index: evidence for use in clinical trials in cystic fibrosis. J Cyst Fibros. 2014;13:123–138. doi: 10.1016/j.jcf.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Amin R, Subbarao P, Jabar A, Balkovec S, Jensen R, Kerrigan S, Gustafsson P, Ratjen F. Hypertonic saline improves the LCI in paediatric patients with CF with normal lung function. Thorax. 2010;65:379–383. doi: 10.1136/thx.2009.125831. [DOI] [PubMed] [Google Scholar]
- 49.Amin R, Subbarao P, Lou W, Jabar A, Balkovec S, Jensen R, Kerrigan S, Gustafsson P, Ratjen F. The effect of dornase alfa on ventilation inhomogeneity in patients with cystic fibrosis. Eur Respir J. 2011;37:806–812. doi: 10.1183/09031936.00072510. [DOI] [PubMed] [Google Scholar]
- 50.Subbarao P, Stanojevic S, Brown M, Jensen R, Rosenfeld M, Davis S, Brumback L, Gustafsson P, Ratjen F. Lung clearance index as an outcome measure for clinical trials in young children with cystic fibrosis: a pilot study using inhaled hypertonic saline. Am J Respir Crit Care Med. 2013;188:456–460. doi: 10.1164/rccm.201302-0219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenfeld M, Ratjen F, Brumback L, Daniel S, Rowbotham R, McNamara S, Johnson R, Kronmal R, Davis SD ISIS Study Group. Inhaled hypertonic saline in infants and children younger than 6 years with cystic fibrosis: the ISIS randomized controlled trial. JAMA. 2012;307:2269–2277. doi: 10.1001/jama.2012.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davies J, Sheridan H, Bell N, Cunningham S, Davis SD, Elborn JS, Milla CE, Starner TD, Weiner DJ, Lee PS, et al. Assessment of clinical response to ivacaftor with lung clearance index in cystic fibrosis patients with a G551D-CFTR mutation and preserved spirometry: a randomised controlled trial. Lancet Respir Med. 2013;1:630–638. doi: 10.1016/S2213-2600(13)70182-6. [DOI] [PubMed] [Google Scholar]
- 53.Davis SD, Rosenfeld M, Kerby GS, Brumback L, Kloster MH, Acton JD, Colin AA, Conrad CK, Hart MA, Hiatt PW, et al. Multicenter evaluation of infant lung function tests as cystic fibrosis clinical trial endpoints. Am J Respir Crit Care Med. 2010;182:1387–1397. doi: 10.1164/rccm.200908-1236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gray DM, Willemse L, Alberts A, Simpson S, Sly PD, Hall GL, Zar HJ. Lung function in African infants: a pilot study. Pediatr Pulmonol. 2015;50:49–54. doi: 10.1002/ppul.22965. [DOI] [PMC free article] [PubMed] [Google Scholar]