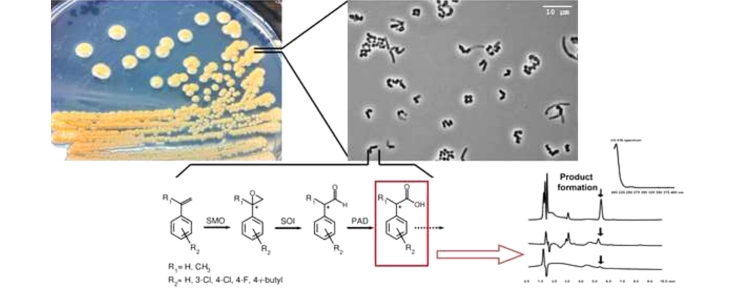
Graphical abstract
Keywords: Ibuprofen, Biocatalysis, Styrene degradation
Highlights
-
•
Styrene degradation via phenylacetic acid was shown for the strains described.
-
•
Co-metabolic transformation of substituted styrenes was shown.
-
•
Formation of several phenylacetic acids, e.g. ibuprofen, was reported.
-
•
α-Methylated substrates were transformed enantioselectively with an ee of up to 40%.
-
•
Pseud. fluorescens ST was identified as promising biocatalyst for phenylacetic acids.
Abstract
Some soil bacteria are able to metabolize styrene via initial side-chain oxygenation. This catabolic route is of potential biotechnological relevance due to the occurrence of phenylacetic acid as a central metabolite.
The styrene-degrading strains Rhodococcus opacus 1CP, Pseudomonas fluorescens ST, and the novel isolates Sphingopyxis sp. Kp5.2 and Gordonia sp. CWB2 were investigated with respect to their applicability to co-metabolically produce substituted phenylacetic acids. Isolates were found to differ significantly in substrate tolerance and biotransformation yields. Especially, P. fluorescens ST was identified as a promising candidate for the production of several phenylacetic acids. The biotransformation of 4-chlorostyrene with cells of strain ST was shown to be stable over a period of more than 200 days and yielded about 38 mmolproduct gcelldryweight−1 after nearly 350 days. Moreover, 4-chloro-α-methylstyrene was predominantly converted to the (S)-enantiomer of the acid with 40% enantiomeric excess.
1. Introduction
Phenylacetic acids represent an important class of compounds for several industries. They occur as natural ingredients in plants and fruits and are used as flavors and fragrances in cosmetics or food [14]. Additionally, these compounds have a high relevance for the pharmaceutical sector as precursors or drugs [29], [51]. For example, α-methylated phenylacetic acids are currently applied as starting materials for the production of virostatic agents [49] or for receptor agonists and antagonists, e.g. for the histamine H2 receptor [15], [19]. Phenylacetic acids also serve as precursors for analgesics like diclofenac [40], [41], [44] or show already an analgesic effect like 4-isobutyl‑α-methylphenylacetic acid which is also known as ibuprofen [8], [12]. Furthermore, antibiotics on the basis of penicillin can be obtained from 4-hydroxyphenylacetic acid or non-substituted phenylacetic acid [9], [11].
Because of the versatile applications of phenylacetic acids, different chemical strategies for their synthesis have been developed. The hydrolysis of phenylacetonitrile and its analogs in the presence of mineral acids at temperatures of up to 250 °C is one important way to produce phenylacetic acids [24]. Another important alternative is the carbonylation of benzyl chlorides in the presence of ruthenium(III) EDTA complexes [45], nickel catalysts [6], or rhodium-based catalysts [17]. Other chemical syntheses for compounds mentioned use α-hydroxynitriles [2], styrene and derivatives [8], or mandelic acid [29]. The α-methylated phenylacetic acids like ibuprofen are commonly obtained via corresponding phenylacetophenones from substituted- or non-substituted benzenes after initial Friedel–Crafts acylation [3], [12], [28], [43].
As an alternative to chemical syntheses biotechnological strategies have been investigated to obtain aromatic acids. Gilligan et al. [16] transformed racemic 2-phenylpropionitrile via an amide to (S)-2-phenylpropionic acid applying a nitrile hydratase (EC 4.2.1.84) and a stereoselective amidase (EC 3.5.1.4) from Rhodococcus equi TG328. A remarkable enantiomeric excess (ee) of about 99% was achieved. The amidase of Agrobacterium tumefaciens d3 is also able to convert racemic 2-phenylpropionamide into the corresponding acid with an ee of 95% [48]. Sosedov et al. [42] have reported the direct hydrolysis of arylacetonitriles to phenylacetic acids by a recombinant arylacetonitrilase (EC 3.5.5.1) from Pseudomonas fluorescens EBC191.
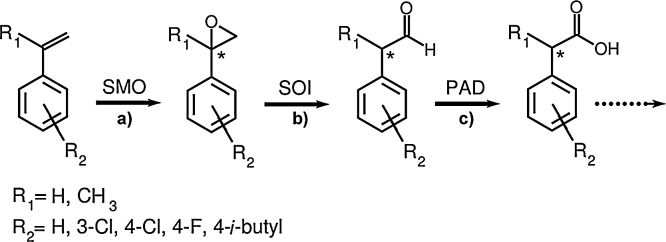
Another biotechnological route to phenylacetic acids seems feasible applying styrenes. These styrenes are partly available in large amounts from the polymer industry [23] and can be converted by soil bacteria harboring enzymes of the styrene-catabolic pathway of side-chain oxygenation [32], [36]. During side-chain oxygenation, the substrate styrene is initially oxidized into styrene oxide by styrene monooxygenase (SMO, EC 1.14.14.11, encoded by styA/styB) and subsequently transformed into phenylacetaldehyde by styrene oxide isomerase (SOI, EC 5.3.99.7, encoded by styC) (see Fig. 1). In a last step of this upper pathway, the aldehyde is oxidized to phenylacetic acid by phenylacetaldehyde dehydrogenase (PAD, EC 1.2.1.39, styD). The formed acid represents a substrate of the phenylacetyl-CoA ligase, which is the initial enzyme of the lower degradation pathway [7], [37], [38], [46]. A modification of this pathway by deletion or substitution of enzymes is possible as mentioned by Hartmans et al. [20] or Toda and Itoh [47] . Previous studies have elucidated enzymes involved in the side-chain oxygenation of styrene in, for example, representatives of the genera Corynebacterium, Rhodococcus, Pseudomonas, Sphingopyxis, or Xanthobacter [5], [20], [22], [33], [34], [35], [47].
Fig. 1.
Biotransformation of styrene and substituted analogs to phenylacetic acid(s).
Styrene (R1, R2 = H) is transformed to phenylacetic acid through enzymes of side-chain oxygenation by the following steps: (a) initial epoxidation to styrene oxide by styrene monooxygenase (SMO), (b) isomerization to phenylacetaldehyde by styrene oxide isomerase (SOI), (c) oxidation by phenylacetaldehyde dehydrogenase (PAD) (reviewed by [32], [36]). A star indicates the formation of a stereocenter in case of R1 = CH3. The suitability of the catabolic route was tested for the formation of 3-chloro-, 4-chloro-, 4-fluoro-, α-methyl-, 4-chloro-α-methyl-, and 4-isobutyl-α-methylphenylacetic acid.
This study investigates the applicability of the styrene-degrading strains Rhodococcus opacus 1CP, P. fluorescens ST, Sphingopyxis sp. Kp5.2, and Gordonia sp. CWB2 as whole-cell biocatalysts for the co-metabolic production of substituted phenylacetic acids from corresponding styrenes.
2. Material and methods
2.1. Chemicals
Standard chemicals, substituted and non-substituted styrenes, styrene oxides, phenylacetaldehydes, and phenylacetic acids were purchased from Sigma–Aldrich (Steinheim, Germany), Merck KGaK (Darmstadt, Germany), AppliChem GmbH (Darmstadt, Germany), VWR International GmbH (Darmstadt, Germany), Riedel-de Haën (Seelze, Germany), Fisher Scientific (Loughborough, UK), Bio-Rad Laboratories GmbH (München, Germany), or Carl Roth (Karlsruhe, Germany) in highest purity available. The enantiomers of 4-chloro-α-methylphenylacetic acid were produced by the workgroup of Prof. Dr. Isamu Shiina (Tokyo University of Science) as described earlier [39].
4-Isobutyl-α-methylstyrene was obtained by Wittig-reaction of 4-isobutylacetophenone and in-situ-generated methylenetriphenylphosphorane according a protocol of [21]. The reaction product was purified by vacuum distillation and flash chromatography (silica gel, hexane) to yield the styrene as a colorless liquid. The retarded liquid contained 99% 4-isobutyl-α-methylstyrene (1.77 g, 10.2 mmol, 25.5% total yield). Purity was determined by silica gel chromatography and GC analysis while correct product formation was controlled via H NMR spectroscopy.
2.2. Bacterial strains and culture conditions
R. opacus 1CP (VKM Ac-2638 [18]), P. fluorescens ST (DSM 6290 [4], [5]) Sphingopyxis sp. Kp5.2 (DSM 28731 [35]), and Gordonia sp. CWB2 (DSM 46758 [35]) were cultivated on mineral medium plates [10] in the presence of 20 g l−1 glucose or in presence of gaseous styrene [34], [35] for preservation.
Fed-batch cultivation was initially performed in 500-ml baffled flasks containing 50 ml mineral medium with 0.05% (w/v) yeast extract at 30 °C under constant shaking (120 rpm). In total 0.5–0.75 mmol glucose were added to the precultures as 0.25-mmol aliquots every 2–5 days. Cell growth was determined by the optical density at 600 nm (OD600) and the cell dry weight.
The precultures were used to inoculate 1-l baffled flasks containing 200 ml mineral medium with 0.05% (w/v) yeast extract. Cultures were incubated at 30 °C and 120 rpm. In total 3–4 mmol glucose were added as 1-mmol aliquots during the first 3–4 days. In order to induce enzymes relevant for biotransformation, in total about 80 μmol styrene were added in 18–26-μmol portions through an evaporation adapter for further 5.5–6.5 days. The biomass obtained was applied immediately for biotransformation experiments. For this, biomass from two cultures of each strain was pooled and 200–400 ml of cells were harvested by centrifugation (5000 × g, 30 min, 4 °C). The cell pellet was washed twice with 50 ml of 25 mM phosphate buffer (pH 7.0) and centrifuged. The pellet obtained was suspended in 240–260 ml of fresh 25 mM phosphate buffer (pH 7.0) and the resulting cell suspension was used to investigate the substrate tolerance.
For long-time transformation of suitable substrates and strains, 1-l baffled flasks with 200 ml mineral medium containing 0.05–0.1% (w/v) yeast extract were inoculated with biomass from precultures, which was cultivated as described above, or directly by cells grown on solid medium or from cryo-cultures. Cultures were incubated at 30 °C and 120 rpm. In total 3.0–4.0 mmol glucose were added as 1.0-mmol aliquots during 10–11 days. Biomass obtained was, if appropriate, harvested as described above, pellet washed with sterile water, and cells subsequently suspended in 200 ml of fresh mineral medium without yeast extract. Cultures were initially incubated over 3–6 days in 1-l baffled flasks in presence of styrene (in total about 26–70 μmol, 18–26-μmol aliquots added through an evaporation adapter) for cell adaptation. Cells obtained were applied to investigate a fed-batch biotransformation in order to produce selected phenylacetic acids.
2.3. Investigation of substrate tolerance
20 ml of resuspended cells of each strain were distributed to 500-ml baffled flasks. 25 μmol of one of the following substrates were subsequently provided by means of an evaporation adapter: styrene, 3-chlorostyrene, 4-chlorostyrene, 4-fluorostyrene, α-methylstyrene, 4-chloro-α-methylstyrene, 4-isobutyl-α-methylstyrene. For the latter compound, also the direct addition to the culture medium was investigated because of its significantly reduced volatility compared to the other styrenes mentioned. Batches were cultivated at 30 °C and 120 rpm for 12 h. To determine product formation, samples of 750 μl were taken from the batches, centrifuged at 16.000 × g for 4 min, and supernatant analyzed by reversed-phase HPLC. To consider poor solubility of some products, especially of 4-chloro-α-methylphenylacetic acid and 4-isobutyl-α-methylphenylacetic acid in the culture medium, samples of 200 μl were diluted with 800 μl methanol. Diluted samples were mixed, centrifuged at 16.000 × g for 30 s, and supernatants analyzed by reversed-phase HPLC, too. The product yields determined after 12 h were normalized by the cell dry weight applied (μmolproduct gcelldryweight−1).
2.4. Fed-batch biotransformation for the production of selected phenylacetic acids
200 ml of a styrene-induced cell suspension of P. fluorescens ST were incubated with in total 3630 μmol 4-chlorostyrene which were added by 21–42-μmol aliquots via an evaporation adapter during 348 days. During the complete cultivation in total about 1750 μmol styrene were additionally supplied in portions of 19–20 μmol via the evaporation adapter to ensure cell adaptation.
A further culture of strain ST containing 200 ml of biomass was incubated in presence of in total 374 μmol 4-chloro-α-methylstyrene (added in 20–41-μmol aliquots via the evaporation adapter) for 25 days. Beside the halogenated substrate, also styrene was added in a total amount of about 196 μmol by 19–20-μmol portions via gas phase.
The transformation of in total 650 μmol 4-isobutyl-α-methylstyrene was investigated with 200 ml of a culture containing Gordonia sp. CWB2 over 28 days. The substrate was added in 50–100-μmol aliquots directly to the medium. Styrene was additionally fed to the culture through an evaporation adapter (in total about 314 μmol, 26-μmol aliquots).
Samples of 200 μl or 750 μl were frequently taken from the batches to determine product formation by both methods described above. Afterwards, samples were analyzed by reversed-phase HPLC. In all cases, viability of the cells and purity of the cultures was regularly proven by plating out cell suspension on solid LB medium or mineral medium containing 20 g l−1 glucose.
2.5. Product quantification and determination of stereoselectivity
Reversed-phase HPLC was performed on a Dionex instrument (P680 pump, UVD340S DAD detector, Gina 50 autosampler) using a vertex column (125 mm length × 4 mm i. d.) packed with Eurospher C18 (5 μm particle size, 100 Å pore size; Knauer, Berlin, Germany) as described previously [34], [35]. The mobile phase, which contained 50% or 65% (v/v) methanol and 0.1% (w/v) phosphoric acid, was used in an isocratic mode at a flow rate of 0.7 ml min−1. The following net retention volumes were obtained: phenylacetic acid, 2.8 ml (50% methanol); 3-chlorophenylacetic acid, 7.1 ml (50%); 4-chlorophenylacetic acid, 7.2 ml (50%); 4-fluorophenylacetic acid, 3.4 ml (50%); α-methylphenylacetic acid, 5.4 ml (50%); 4-chloro-α-methylphenylacetic acid, 13.7 ml (50%); 4-isobutyl-α-methylphenylacetic acid, 12.0 ml (65%). Peaks obtained were compared to authentic standards in respect of retention volume and UV-spectrum (200–300 nm).
For the chiral product of the 4-chloro-α-methylstyrene transformation, enantiomeric excess (ee) was determined with a column (200 mm × 4 mm) packed with Nucleodex α-PM (permethylated α-cyclodextrine) on Nucleosil silica (5 μm particle size, 100 Å pore size; Macherey-Nagel, Düren, Germany). 40% methanol with 0.06% (w/v) triethylammonium acetate (pH 4.1) served as mobile phase in an isocratic mode and at a flow rate of 0.7 ml min−1. Following net retention volumes were observed:
(R)-4-chloro-α-methylphenylacetic acid, 31.6 ml; (S)-4-chloro-α-methylphenylacetic acid, 33.3 ml.
3. Results and discussion
3.1. Detection of phenylacetic acid as an indicator for styrene catabolism via side-chain oxygenation
The ability of bacteria to convert styrene into phenylacetic acid could be an attractive basis for the conversion of substituted styrenes (Fig. 1), provided that the initial epoxidizing, isomerizing, and dehydrogenating enzyme activities are sufficiently unspecific. A restricted substrate spectrum of the following phenylacetate-converting enzyme then may lead to the accumulation of structural analogs as dead-end products. Such differences in substrate tolerance between the peripheral and central pathways of (chloro- and methyl-) aromatic degradation are well known and responsible for a number of co-metabolic transformations [25], [26], [27].
In order to investigate the suitability of the concept mentioned above, four strains were selected in that respect. The proteobacteria P. fluorescens ST and Sphingopyxis sp. Kp5.2 as well as the actinobacteria R. opacus 1CP and Gordonia sp. CWB2 are known to utilize styrene as the sole source of energy and carbon [5], [7], [35]. Although styrene-catabolic genes for side-chain oxygenation have been identified in strain ST [5], [7], strain Kp5.2 [35], and strain 1CP [35], their functional relevance for the degradation of styrene has been demonstrated only for the pseudomonad so far. Despite the proof of SOI activity in the case of strain 1CP and Kp5.2, it was not directly shown for the latter two strains if styrene is degraded by the pathway of side-chain oxygenation yielding phenylacetic acid as central intermediate. The styrene degradation pathway of strain CWB2 is even more speculative, since the genetic background has not yet been investigated. An alternative route could lead to 3-vinylcatechol which has been reported to be subject of a meta-cleavage pathway [50].
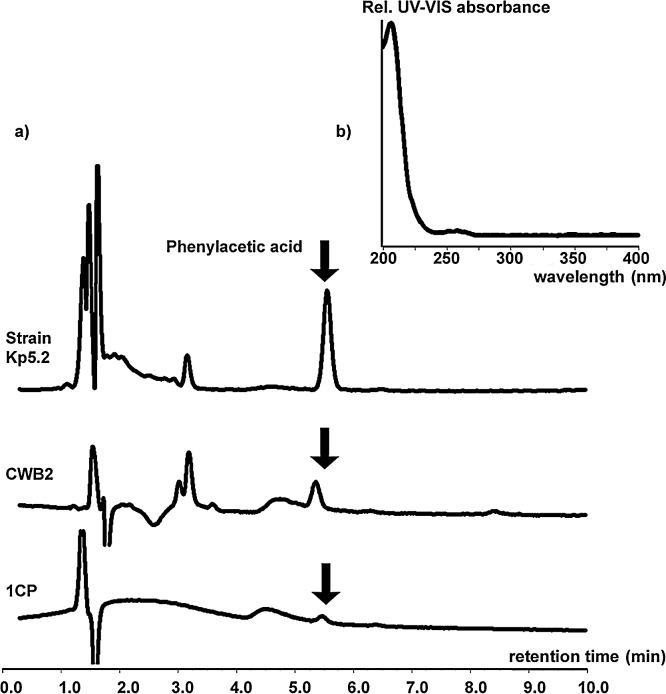
In order to establish the catabolic pathway of the above mentioned new isolates, styrene-grown biomass of each strain was incubated in the presence of this hydrocarbon and the culture medium was analysed for the occurrence of intermediates. Based on retention behavior and spectral data, phenylacetic acid could be detected in concentrations of 3.5–42.5 μM in cultures of strain 1CP, CWB2, and Kp5.2 after 12 h (Fig. 2). Surprisingly, no phenylacetic acid accumulated during growth of strain ST. But strain ST is known to produce this compound as an intermediate [4]. These results strongly confirm former studies [34], [35] and indicate that these strains mentioned degrade styrene via the route of site-chain oxygenation.
Fig. 2.
(a) Chromatographic and (b) spectral identification of the metabolite phenylacetic acid during styrene degradation of strains Kp5.2, CWB2, and 1CP.
Cell suspensions of Sphingopyxis sp. Kp5.2, Gordonia sp. CWB2, and Rhodococcus opacus 1CP were cultured and induced as described in the Section 2 and subsequently incubated for 12 h in the presence of 25 μmol gaseous styrene in 500-ml flasks. Culture supernatant was subjected to HPLC under conditions described in Section 2.
The considerable differences in concentration level likely result from strain-specific kinetics of phenylacetic acid formation and consumption. As shown later, these levels do not necessarily allow a conclusion to the suitability of an isolate to accumulate substituted analogs of phenylacetic acid.
3.2. Co-metabolic formation and accumulation of substituted phenylacetic acids
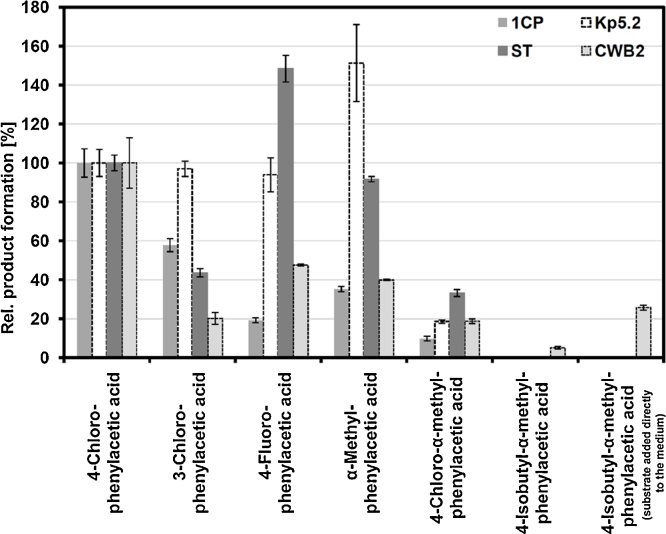
Glucose-grown and styrene-induced cell suspensions of P. fluorescens ST, Sphingopyxis sp. Kp5.2, R. opacus 1CP, and Gordonia sp. CWB2 (Supplemental materials Fig. S1, a–d) were treated as described in Section 2, in the course of this adjusted to an OD600 of about 1–6 (corresponding to 0.61–1.9 mgcelldryweight ml−1), and subsequently incubated in the presence of volatilized substituted styrenes. Analogs were either halogenated (3-chloro, 4-chloro-, 4-fluorostyrene), alkylated (α-methyl-, 4-isobutyl-α-methylstyrene), or both (4-chloro-α-methylstyrene) (see Fig. 1). During the course of 12 h the formation of corresponding phenylacetic acids was followed and product amounts obtained were referred to the biomass content. All four strains were found to convert most of the styrenes (Fig. 3, Fig. 4). However, specific conversion yields differed considerably ranging from 28 to 520 μmol gcdw−1 after 12 h for 4-chlorostyrene which was chosen as a reference compound in order to normalize conversion yields. P. fluorescens ST turned out to be the by far most active isolate for that substrate (520 ± 21 μmol gcdw−1 during 12 h) followed by strain Kp5.2 (98.2 ± 6.9 μmol gcdw−1), strain CWB2 (67.7 ± 8.8 μmol gcdw−1), and strain 1CP (27.9 ± 2.0 μmol gcdw−1).
Fig. 3.
Substrate specificities of strains ST, Kp5.2, 1CP, and CWB2 for the conversion of substituted styrenes into the corresponding phenylacetic acids.
Pseudomonas fluorescens ST (0.61 mgcdw ml−1), Sphingopyxis sp. Kp5.2 (1.0 mgcdw ml−1), Rhodococcus opacus 1CP (0.98 mgcdw ml−1), and Gordonia sp. CWB2 (1.9 mgcdw ml−1) were cultivated for 12 h in the presence of 25 μmol substrate as described in the Section 2. If not otherwise stated, the substrate was provided via gas phase. The product yields obtained during a period of 12 h were referred to the cell dry weight and normalized towards 4-chlorophenylacetic acid formation (=100%). Mean values and standard errors of three to four independent measurements are given. 100% rel. yields correspond to: strain ST: 520 ± 21 μmol gcdw−1; strain Kp5.2: 98.2 ± 6.9 μmol gcdw−1; strain CWB2: 67.7 ± 8.8 μmol gcdw−1; strain 1CP: 27.9 ± 2.0 μmol gcdw−1.
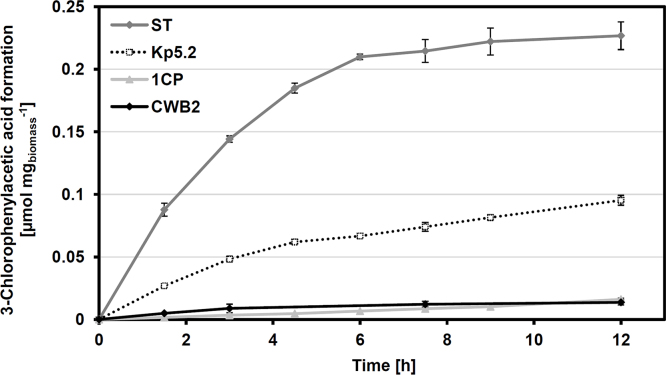
Fig. 4.
3-Chlorostyrene conversion by strains ST, Kp5.2, 1CP, and CWB2.
Pseudomonas fluorescens ST (0.61 mgcdw ml−1), Sphingopyxis sp. Kp5.2 (1.0 mgcdw ml−1), Rhodococcus opacus 1CP (0.98 mgcdw ml−1), and Gordonia sp. CWB2 (1.9 mgcdw ml−1) were incubated for 12 h in the presence of 25 μmol 3-chlorostyrene. 3-Chlorophenylacetic acid formation was followed and referred to cell dry weight. Mean values and standard errors of three to four independent measurements are given.
Significant differences were also observed for the substrate tolerance of isolates of which strain Kp5.2 probably showed the highest one (Fig. 3). 3-Chloro-, 4-chloro-, and 4-fluorophenylacetic acid were formed with relative yields of (nearly) 100% after 12 h and α-methylphenylacetic acid was accumulated even faster with 151% compared to the reference 4-chlorophenylacetic acid. However, the absolute transformation rates were considerably lower than in the case of strain ST.
In general, strains exhibited low activities towards 4-chloro-α-methylstyrene. The corresponding acid was formed by strain ST in a relative yield of about 33%. With an additional isobutyl-group in para-position of the phenyl moiety, the substrate tolerance of most isolates was exhausted. The corresponding 4-isobutyl-α-methylphenylacetic acid (ibuprofen) was only detected from conversions of strain CWB2, a fact that might emphasize this isolate for the production of this drug. Although ibuprofen was detected with only 3.45 ± 0.48 μmol gcdw−1 after 12 h from CWB2-catalyzed biotransformation, minor yields are to some extent caused by the low bioavailability of 4-isobutyl-α-methylstyrene. The direct addition of the pure compound into the medium increased the product formation by the factor five to 17.4 ± 0.9 μmol gcdw−1 after 12 h (Fig. 3). The remarkable ability of strain CWB2 to produce ibuprofen indicates substantial differences of at least one of the enzymes involved in the biotransformation. Whereas for strain Kp5.2, 1CP, and ST the styrene-catabolic genes for initial styrene degradation have been identified and corresponding SOI activity has been determined during previous studies [5], [7], [35], attempts to detect the styC-encoded styrene oxide isomerase in strain CWB2 failed because no SOI activity was found [35].
The results obtained suggest that the enzymes of the upper styrene degradation are able to catalyze the transformation of substituted styrenes to the corresponding phenylacetic acids. Moreover, substitution obviously impairs ability of phenylacetyl-CoA ligase or ring 1,2-phenylacetyl-CoA epoxidase (as described by [46] to metabolize these artificial structure analogs. This leads to an accumulation of these phenylacetic acids in the culture medium. Since the specific conversion yields of strain ST surpassed the yields of the other ones (e.g. demonstrated by Fig. 4) and since strain CWB2 was able to produce ibuprofen (Fig. 3), both isolates are of special interest.
3.3. Stereoselective conversion of 4-chloro-α-methylstyrene
As illustrated in Fig. 1 the conversion of an α-substituted styrene to the corresponding phenylacetic acid results in the creation of an asymmetric center at C-2. Chiral HPLC was performed in order to investigate the potential of the four strains for enantioselective production of 4-chloro-α-methylphenylacetic acid and to determine the overall behavior of initial enzymes. An appreciable enantioselectivity was obtained for P. fluorescens ST which yielded about 43% ee of the (S)-enantiomer. Enantiopreference of strain 1CP (20% ee of (S)-isomer) and strain CWB2 (15% ee of (R)-isomer) was considerably lower and almost absent for strain Kp5.2.
The two initial enzymes of side-chain oxygenation have been shown to be responsible for the formation of an asymmetric center and determine its absolute stereochemistry. At first, styrene monooxygenases (SMOs) introduce the oxygen atom highly enantioselective and most representatives investigated in that respect strongly favor the formation of the (S)-enantiomer of styrene oxide [31]. At second, the few styrene oxide isomerases characterized clearly prefer the conversion of this (S)-enantiomer of styrene oxide into phenylacetaldehyde [30], [22], [35]. In the case of an α-substitution SOI would again affect the absolute configuration of the product. The final enzyme phenylacetaldehyde dehydrogenase of the metabolic cascade proposed does not act on the chiral carbon and thus should not affect stereochemistry.
Highly enantioselective biotechnological synthesis of α-methylphenylacetic acid has been reported by Gilligan et al. [16] using (R,S)-2-phenylpropionic nitrile. However, biotechnological enantioselective production of α-substituted phenylacetic acids from corresponding styrenes has not been described before and indicates that some SOIs effectively affect the absolute configuration of the product.
3.4. Microbial production of substituted phenylacetic acids
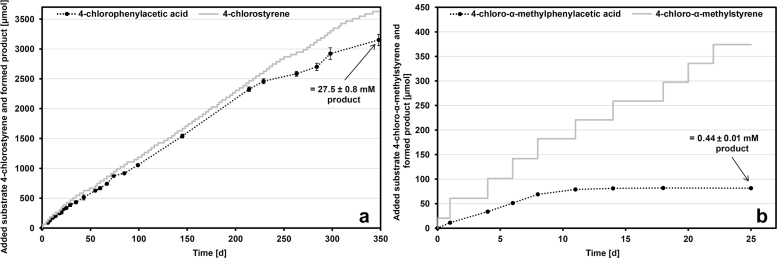
The co-metabolic formation of 4-chloro-α-methyl- and 4-chlorophenylacetic acid by P. fluorescens ST and of 4-isobutyl-α-methylphenylacetic acid (ibuprofen) by Gordonia sp. CWB2 was extended to longer periods of time in order to evaluate process stability and to maximize yield. Additionally supplemented styrene served as an energy source for the supply of reduction equivalents and as an inducer for relevant enzyme activities. Glucose-grown and styrene-induced biomass of strain ST was adjusted to an OD600 of about 0.8 which corresponds to a biomass titer of 0.41 mgcdw ml−1. During the course of 348 days, 3630 μmol 4-chlorostyrene and 1750 μmol styrene were in parallel spiked through the gas phase. Conversion of 4-chlorostyrene was shown to be nearly quantitative (96% theory yield) over a period of 214 days (Fig. 5a), but decreased in efficiency for the following 134 days (87% theory yield). About 3150 ± 90 μmol (=537 ± 15 mg) 4-chlorophenylacetic acid were obtained referred to the initial biomass amount of 82 mgcdw. Phenylacetic acid, which might occur as a metabolite from the natural substrate styrene, was not detected at significant levels and thus did not affect product recovery and purity.
Fig. 5.
Applicability of strain ST for the formation of 4-chloro- and 4-chloro-α-methylphenylacetic acid.
Pseudomonas fluorescens ST (0.41 mgcdw ml−1) was incubated for 348 days in the presence of in total 3630 μmol 4-chlorostyrene (added in 21–42-μmol portions) (a) or for 25 days with overall 374 μmol 4-chloro-α-methylstyrene (added in 20–41-μmol portions) (b) as described in Section 2. Product formation was quantified by HPLC (see Section 2). Mean values and standard errors of four independent measurements are given.
Another aliquot of the cell suspension of strain ST mentioned before was applied in the transformation of 374 μmol 4-chloro-α-methylstyrene. Again, styrene was supplemented in approximately half of this molar amount. A linear correlation between substrate addition and product formation was restricted here for 8 days and conversion completely stagnated after 11–14 days to yield 81.4 ± 1.9 μmol (=15.0 ± 0.4 mg) 4-chloro-α-methylphenylacetic acid (Fig. 5b). Compared to the conversion of 4-chlorostyrene, process stability and transformation efficiency is considerably limited.
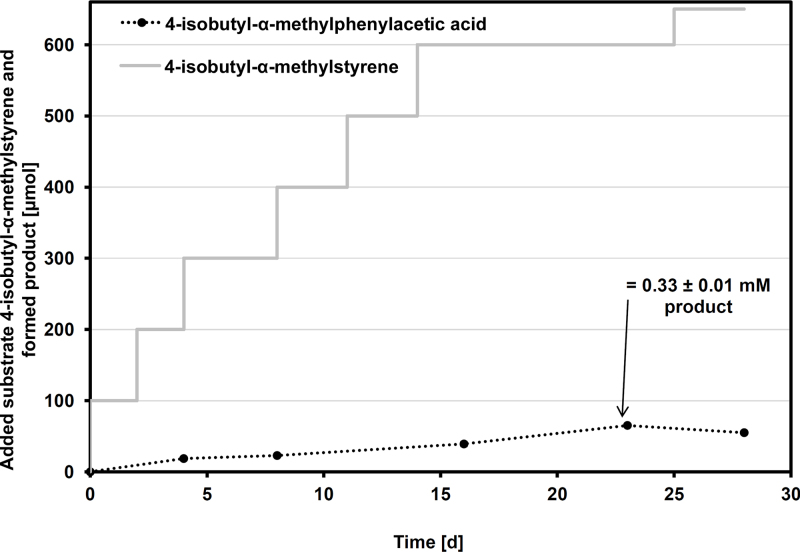
A similar effect was observed during the conversion of the sterically most demanding substrate 4-isobutyl-α-methylstyrene. In the presence of 600 μmol of this compound and in the course of 23 days, the biomass of Gordonia sp. CWB2 (OD600 of about 6.0; 1.9 mgcdw ml−1) accumulated up to 65.2 ± 2.1 μmol (=13,4 ± 0.4 mg) ibuprofen (Fig. 6). Again, the kinetics obtained clearly pointed at an inactivation event. A second experiment with a more limited substrate supply yielded similar results (data not shown) which excludes reversible substrate inhibition to be the reason for process stagnation.
Fig. 6.
Utilization of strain CWB2 for the formation of 4-isobutyl-α-methylphenylacetic acid (ibuprofen).
A cell suspension of Gordonia sp. CWB2 (1.9 mgcdw ml−1) was incubated for 28 days in the presence of in total 650 μmol 4-isobutyl-α-methylstyrene (50–100-μmol portions) as described in the Section 2. Product formation was determined by HPLC (see Section 2). Mean values and standard errors of two to three independent measurements are given.
Several studies have reported on an antimicrobial activity of this anti-inflammatory compound especially towards Gram-positive bacteria. Depending on the pH, growth suppression was observed at levels below 150 μg ml−1 [13] which are in the range of this biotransformation approach. According a more recent study, minimal inhibition concentration towards the Gram-positive isolate Staphylococcus aureus has been reported to be one order of magnitude higher (minimal inhibition concentration = 1.25 mg ml−1) [1]. However, the strains investigated showed different susceptibilities and it has still to be examined, to what extent strain CWB2 is susceptible towards this drug.
4. Conclusion
In summary, the study has revealed that substituted phenylacetic acids can in principal be produced from correspondingly substituted styrenes by means of suitable styrene-degrading bacterial isolates which harbor enzymes of the side-chain oxygenation. 4-chlorostyrene turned out to be a well suited co-metabolic substrate and in particular, it was most efficiently converted by P. fluorescens ST. Considerable differences were also observed in the enantioselective potential of the initial degradation pathway relevant in the conversion of α-substituted styrene analogs. Again, strain ST showed highest specificity and an enantiomeric excess of 40% was determined for the (S)-4-chloro-α-methylphenylacetic acid. The remarkable ability of a Gordonia-isolate to convert 4-isobutyl-α-methylstyrene into ibuprofen demonstrates a novel route towards this drug. However, a biotechnological application of these strains requires further optimization approaches to improve the product yields.
Acknowledgments
Michel Oelschlägel and Juliane Zimmerling were supported by a pre-doctoral fellowship from the Deutsche Bundesstiftung Umwelt and Dirk Tischler by a grant of the European Social Fund and Saxonian Government (GETGEOWEB: 100101363). We thank Prof. Dr. Isamu Shiina and his workgroup (Department of Applied Chemistry, Faculty of Science, Tokyo University of Science) for the synthesis and provision of the (S)- and (R)-enantiomers of 4-chloro-α-methylphenylacetic acid.
Footnotes
Available online 21 January 2015
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.btre.2015.01.003.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Al-Janabi A.A.H.S. In vitro antibacterial activity of ibuprofen and acetaminophen. J. Glob. Infect. Dis. 2010;2:105–108. doi: 10.4103/0974-777X.62880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aramini A., Sablone M.R., Bianchini G., Amore A., Fanì M., Perrone P., Dolce A., Allegretti M. Facile one-pot preparation of 2-arylpropionic acids from cyanohydrins by treatment with aqueous HI. Tetrahedron. 2009;65:2015–2021. [Google Scholar]
- 3.Baddeley G., Wrench E. The interaction of alkylbenzenes with excess of Friedel-Crafts acetylating agent. J. Chem. Soc. 1956:4943–4945. [Google Scholar]
- 4.Baggi G., Boga M.M., Catelani D., Galli E., Treccani V. Styrene catabolism by a strain of Pseudomonas fluorescens. Syst. Appl. Microbiol. 1983;4:141–147. doi: 10.1016/S0723-2020(83)80042-3. [DOI] [PubMed] [Google Scholar]
- 5.Beltrametti F., Marconi A.M., Bestetti G., Colombo C., Galli E., Ruzzi M., Zennaro E. Sequencing and functional analysis of styrene catabolism genes from Pseudomonas fluorescens ST. Appl. Environ. Microbiol. 1997;63:2232–2239. doi: 10.1128/aem.63.6.2232-2239.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertleff W., editor. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH; Weinheim, Germany: 2005. Carbonylation. [Google Scholar]
- 7.Bestetti G., Gennaro P.D., Colmegna A., Ronco I., Galli E., Sello G. Characterization of styrene catabolic pathway in Pseudomonas fluorescens ST. Int. Biodeterior. Biodegr. 2004;54:183–187. [Google Scholar]
- 8.Chen A., Ren L., Crudden C.M. Catalytic asymmetric hydrocarboxylation and hydrohydroxymethylation: a two-step approach to the enantioselective functionalization of vinylarenes. J. Org. Chem. 1999;64:9704–9710. [Google Scholar]
- 9.Corse J.W., Jones R.G., Soper Q.F., Whitehead C.W., Behrens O.K. Biosynthesis of penicillins. V. Substituted phenylacetic acid derivatives as penicillin precursors. J. Am. Chem. Soc. 1948;70:2837–2843. doi: 10.1021/ja01189a001. [DOI] [PubMed] [Google Scholar]
- 10.Dorn E., Hellwig M., Reineke W., Knackmuss H.-J. Isolation and characterization of a 3-chlorobenzoate-degrading pseudomonad. Arch. Microbiol. 1974;99:61–70. doi: 10.1007/BF00696222. [DOI] [PubMed] [Google Scholar]
- 11.Douma R.D., Deshmukh A.T., de Jonge L.P., de Jong B.W., Seifar R.M., Heijnen J.J., van Gulik W.M. Novel insights in transport mechanisms and kinetics of phenylacetic acid and penicillin-G in Penicillium chrysogenum. Biotechnol. Prog. 2012;28:337–348. doi: 10.1002/btpr.1503. [DOI] [PubMed] [Google Scholar]
- 12.V. Elango, M.A. Murphy, B.L. Smith, K.G. Davenport, G.N. Mott, E.G. Zey, G.L. Moss, Method for producing ibuprofen. US patent 4981995 (BHC Company) 1991.
- 13.Elvers K.T., Wright S.J. Antibacterial activity of the anti-inflammatory compound ibuprofen. Lett. Appl. Microbiol. 1995;20:82–84. doi: 10.1111/j.1472-765x.1995.tb01291.x. [DOI] [PubMed] [Google Scholar]
- 14.Fahlbusch K.-G., Hammerschmidt F.-J., Panten J., Pickenhagen W., Schatkowski D., Bauer K., Garbe D., Surburg H., editors. Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH; Weinheim, Germany: 2012. Flavors and fragrances. [Google Scholar]
- 15.Ghorai P., Kraus A., Keller M., Götte C., Igel P., Schneider E., Schnell D., Bernhardt G., Dove S., Zabel M., Elz S., Seifert S., Buschauer A. Acylguanidines as bioisosteres of guanidines: NG-acylated imidazolylpropylguanidines, a new class of histamine H2 receptor agonists. J. Med. Chem. 2008;51:7193–7204. doi: 10.1021/jm800841w. [DOI] [PubMed] [Google Scholar]
- 16.Gilligan T., Yamada H., Nagasawa T. Production of S-(+)-2-phenylpropionic acid from (RS)-2-phenylpropionitrile by the combination of nitrile hydratase and stereoselective amidase in Rhodococcus equi TG328. Appl. Microbiol. Biotechnol. 1993;39:720–725. doi: 10.1007/BF00164456. [DOI] [PubMed] [Google Scholar]
- 17.Giroux A., Nadeau C., Han Y. Synthesis of phenylacetic acids under rhodium-catalyzed carbonylation conditions. Tetrahedron Lett. 2000;41:7601–7604. [Google Scholar]
- 18.Gorlatov S.N., Maltseva O.V., Shevchenko V.I., Golovleva L.A. Degradation of chlorophenols by a culture of Rhodococcus erythropolis. Mikrobiologiya. 1989;58:802–806. (Microbiology 58: 647–651) [Google Scholar]
- 19.Gualtieri F., Conti G., Dei S., Giovannoni M.P., Nannucci F., Romanelli M.N., Scapecchi S., Teodori E., Fanfani L., Ghelardini C., Giotti A., Bartolini A. Presynaptic cholinergic modulators as potent cognition enhancers and analgestic drugs: 1 Tropic and 2-phenylpropionic acid esters. J. Med. Chem. 1994;37:1704–1711. doi: 10.1021/jm00037a022. [DOI] [PubMed] [Google Scholar]
- 20.Hartmans S., Smits J.P., van der Werf M.J., Volkering F., de Bont J.A.M. Metabolism of styrene oxide and 2-phenylethanol in the styrene-degrading Xanthobacter strain 124X. Microbiology. 1989;55:2850–2855. doi: 10.1128/aem.55.11.2850-2855.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishibashi H., Maeki M., Yagi J., Ohba M., Kanai T. A modification of the asymmetric dihydroxylation approach to the synthesis of (S)-2-arylpropanoic acids. Tetrahedron. 1999;55:6075–6080. [Google Scholar]
- 22.Itoh N., Hayashi K., Okada K., Ito T., Mizuguchi N. Characterization of styrene oxide isomerase: a key enzyme of styrene and styrene oxide metabolism in Corynebacterium sp. Biosci. Biotechnol. Biochem. 1997;61:2058–2062. doi: 10.1271/bbb.61.2058. [DOI] [PubMed] [Google Scholar]
- 23.James D.H., Castor W.M., editors. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH; Weinheim, Germany: 2012. Styrene. [Google Scholar]
- 24.K. Kagawa, N. Kanda, F. Masuko, H. Nakanishi, Synthesis of substituted phenylacetic acid. US patent 4220592 (Sumitomo Chemical Company) 1980.
- 25.Kim M.H., Hao O.J. Cometabolic degradation of chlorophenols by Acinetobacter species. Water Res. 1999;33:562–574. [Google Scholar]
- 26.Knackmuss H.-J., Hellwig M., Lackner H., Otting W. Cometabolism of 3-methylbenzoate and methylcatechols by a 3-chlorobenzoate utilizing Pseudomonas: accumulation of (+)-2,5-dihydro-4-methyl-and (+)-2,5-dihydro-2-methyl-5-oxo-furan-2-acetic acid. Eur. J. Appl. Microbiol. Biotechnol. 1976;2:267–276. [Google Scholar]
- 27.Kohler H.-P.E., Kohler-Staub D., Focht D.D. Cometabolism of polychlorinated biphenyls: enhanced transformation of Arochlor 1254 by growing bacterial cells. Appl. Environ. Microbiol. 1988;54:1940–1945. doi: 10.1128/aem.54.8.1940-1945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D.D. Lindley, T.A. Curtis, T.R. Ryan, E.M. de la Garza, C.B. Hilton, T.M. Kenesson, Process for the production of 4'-isobutylacetophenone US patent 5068448 A (Hoechst Celanese Corporation, BHC Company) 1991.
- 29.Milne J.E., Storz T., Colyer J.T., Thiel O.R., Dilmeghani Seran M., Larsen R.D., Murry J.A. Iodide-catalyzed reductions: development of a synthesis of phenylacetic acids. J. Org. Chem. 2011;76:9519–9524. doi: 10.1021/jo2018087. [DOI] [PubMed] [Google Scholar]
- 30.Miyamoto K., Okuro K., Ohta H. Substrate specificity and reaction mechanism of recombinant styrene oxide isomerase from Pseudomonas putida S12. Tetrahedron Lett. 2007;48:3255–3257. [Google Scholar]
- 31.Montersino S., Tischler D., Gassner G.T., van Berkel W.J.H. Catalytic and structural features of flavoprotein hydroxylases and epoxidases. Adv. Synth. Catal. 2011;353:2301–2319. [Google Scholar]
- 32.Mooney A., Ward P.G., ÓConnor K.E. Microbial degradation of styrene: biochemistry, molecular genetics, and perspectives for biotechnological applications. Appl. Microbiol. Biotechnol. 2006;72:1–10. doi: 10.1007/s00253-006-0443-1. [DOI] [PubMed] [Google Scholar]
- 33.ÓConnor K., Buckley C.M., Hartmans S., Dobson A.D.W. Possible regulatory role for nonaromatic carbon sources in styrene degradation by Pseudomonas putida CA-3. Appl. Environ. Microbiol. 1995;61:544–548. doi: 10.1128/aem.61.2.544-548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oelschlägel M., Gröning J.A.D., Tischler D., Kaschabek S.R., Schlömann M. Styrene oxide isomerase of Rhodococcus opacus 1CP, a highly stable and considerably active enzyme. Appl. Environ. Microbiol. 2012;78:4330–4337. doi: 10.1128/AEM.07641-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oelschlägel M., Zimmerling J., Schlömann M., Tischler D. Styrene oxide isomerase of Sphingopyxis species Kp5.2. Microbiology. 2014;160:2481–2491. doi: 10.1099/mic.0.080259-0. [DOI] [PubMed] [Google Scholar]
- 36.ÓLeary N.D., ÓConnor K.E., Dobson A.D.W. Biochemistry, genetics and physiology of microbial styrene degradation. FEMS Microbiol. Rev. 2002;26:403–417. doi: 10.1111/j.1574-6976.2002.tb00622.x. [DOI] [PubMed] [Google Scholar]
- 37.Olivera E.R., Reglero A., Martínez-Blanco H., Fernández-Medarde A., Moreno M.A., Luengo J.M. Catabolism of aromatics in Pseudomonas putida U. Formal demonstration that phenylacetic acid and 4-hydroxyphenylacetic acid are catabolized by two unrelated pathways. Eur. J. Biochem. 1994;221:375–381. doi: 10.1111/j.1432-1033.1994.tb18749.x. [DOI] [PubMed] [Google Scholar]
- 38.Olivera E.R., Miñambres García B.B., Muñiz C., Moreno M.A., Ferrández A., Díaz E., García J.L., Luengo J.M. Molecular characterization of the phenylacetic acid catabolic pathway in Pseudomonas putida U: the phenylacetyl-CoA catabolon. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6419–6424. doi: 10.1073/pnas.95.11.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shiina I., Nakata K., Ono K., Onda Y., Itagaki M. Kinetic resolution of racemic α-arylalkanoic acids with achiral alcohols via the asymmetric esterification using carboxylic anhydrides and acyl-transfer catalysts. J. Am. Chem. Soc. 2010;132:11629–11641. doi: 10.1021/ja103490h. [DOI] [PubMed] [Google Scholar]
- 40.Skoutakis V.A., Carter C.A., Mickle T.R., Smith V.H., Arkin C.R., Alissandratros J., Petty D.A. Review of diclofenac and evaluation of its place in therapy as a nonsteroidal antiinflammatory agent. Drug Intel. Clin. Pharm. 1988;22:850–859. doi: 10.1177/106002808802201102. [DOI] [PubMed] [Google Scholar]
- 41.Small R.E. Diclofenac sodium. Clin. Pharm. 1989;8:545–558. [PubMed] [Google Scholar]
- 42.Sosedov O., Baum S., Bürger S., Matzer K., Kiziak C., Stolz A. Construction and application of variants of the Pseudomonas fluorescens EBC191 arylacetonitrilase for increased production of acids or amides. Appl. Environ. Microbiol. 2010;76:3668–3674. doi: 10.1128/AEM.00341-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.N.J. Stuart, A.S. Sanders, Phenyl propionic acids U.S. patent 3385886 (Boots Pure Drug Co., Ltd.) 1968.
- 44.M. Takase, T. Nakamura, K. Kamiya, T. Takezawa, H. Yamazaki, T. Iwaki, Process for production of o-(2,6-dichloroanilino)-phenylacetic acid U.S. patent 4410724 (Zenyaku Kogyo Kabushiki Kaisha) 1983.
- 45.Taqui Khan M.M., Halligudi S.B., Abdi H.R. Carbonylation of benzyl chloride to phenylacetic acid and its ester using water-soluble Ru(III)-EDTA complex catalyst. J. Mol. Catal. 1988;44:179–181. [Google Scholar]
- 46.Teufel R., Mascaraque V., Ismail W., Voss M., Perera J., Eisenreich W., Haehnel W., Fuchs G. Bacterial phenylalanine and phenylacetate catabolic pathway revealed. Proc. Natl. Acad. Sci. U.S.A. 2010;107:14390–14395. doi: 10.1073/pnas.1005399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toda H., Itoh N. Isolation and characterization of styrene metabolism genes from styrene-assimilating soil bacteria Rhodococcus sp. ST-5 and ST-10. J. Biosci. Bioeng. 2012;113:12–19. doi: 10.1016/j.jbiosc.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 48.Trott S., Bauer R., Knackmuss H.-J., Stolz A. Genetic and biochemical characterization of an enantioselective amidase Agrobacterium tumefaciens strain d3. Microbiology. 2001;147:1815–1824. doi: 10.1099/00221287-147-7-1815. [DOI] [PubMed] [Google Scholar]
- 49.Wagner R., Larson D.P., Beno D.W.A., Bosse T.D., Darbyshire J.F., Gao Y., Gates B.D., He W., Henry R.F., Hernandez L.E., Hutchinson D.K., Jiang W.W., Kati W.M., Klein L.L., Koev G., Kohlbrenner W., Krueger A.C., Liu J., Liu Y., Long M.A., Maring C.J., Masse S.V., Middleton T., Montgomery D.A., Pratt J.K., Stuart P., Molla A., Kempf D.J. Inhibitors of hepatitis C virus polymerase: synthesis and biological characterization of unsymmetrical dialkyl-hydroxynaphthalenoyl-benzothiadiazines. J. Med. Chem. 2009;52:1659–1669. doi: 10.1021/jm8010965. [DOI] [PubMed] [Google Scholar]
- 50.Warhurst A.M., Clarke K.F., Hill R.A., Holt R.A., Fewson C.A. Metabolism of styrene by Rhodococcus rhodochrous NCIMB 13259. Appl. Environ. Microbiol. 1994;60:1137–1145. doi: 10.1128/aem.60.4.1137-1145.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu Y.-J., Zhou H.-T., Hu Y.-H., Tang J.-Y., Su M.-X., Guo Y.-J., Chen Q.-X., Liu B. Antityrosinase and antimicrobial activities of 2-phenylethanol: 2-phenylacetaldehyde and 2-phenylacetic acid. Food Chem. 2011;124:298–302. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.