Abstract
The in vitro antimicrobial activity of different fractions obtained from rhizome of Curcuma longa was investigated against standard strain and clinical isolates of Staphylococcus aureus. The clinical isolates were found more sensitive for different fractions, than the standard strain of S. aureus. Scanning electron microscopic observations revealed that test pathogen treated with C. longa extract showed morphological deformity, with partial lack of the cytoplasmic membrane, which leads to cell disruption The ability of rhizome of C. longa extracts to inhibit the growth of test pathogen is an indication of its broad spectrum antimicrobial potential which may be employed in the management of microbial infections.
Keywords: Curcuma longa, S. aureus, Antibacterial activity, Scanning electron microscopy
1. Introduction
Nosocomial infections are hospital acquired infections. Historically, staphylococci, pseudomonads, and Escherichia coli have been the nosocomial infection troika. It is estimated that in United States in 1995, nosocomial infections cost $4.5 billion and contributed to more than 88,000 deaths—one death every 6 min [24]. In the study from 1990 to 1996, the three most common gram-positive pathogens—Staphylococcus aureus, coagulase-negative staphylococci, and enterococci— accounted for 34% of nosocomial infections, and the four most common gram-negative pathogens—E. coli, Pseudomonas aeruginosa, Enterobacter spp., and Klebsiella pneumoniae—accounted for 32% of nosocomial infections [21].
Major force involved in nosocomial infections is indiscriminate antimicrobial use in hospitals and long-term care facilities. Widespread use of cephalosporin antibiotics is often cited as a cause of the emergence of MRSA, which became a major nosocomial threat. S. aureus has shown to exhibit resistance to wide range of commonly available antibiotics especially penicillin [14], [16]. Methicillin–resistant S. aureus (MRSA) is a major nosocomial pathogen [25]. According to a study, the MRSA prevalence has increased from 12% in 1992 to 80.83% in 1999 [30].
In addition to this problem, antibiotics are sometimes associated with adverse effects on the host including hypersensitivity, immune-suppression and allergic reactions [1]. Because of the side effects and the resistance that pathogenic microorganisms build against antibiotics, recently much attention has been paid to extracts and biologically active compounds isolated from plant species used in herbal medicine [13].
However, the potential of higher plants as sources for new drugs is still largely unexplored. India is the largest producer of medicinal herbs and appropriately called the botanical garden of the world [2]. Coincidentally, the last decade has also witnessed increasing intensive studies on extracts and biologically active compounds isolated from plant species used for natural therapies or herbal medicine [23].
Turmeric (Curcuma longa L.) belongs to the family Zingiberaceae, is a perennial rhizomatous shrub native to Southern Asia [20], [31], In India it is popularly known as “Haldi” and is extensively cultivated in all parts of India [7]. Its rhizomes are oblongonate, pyriform, often short branched and they are house hold remedy in Nepal [12]. As a powder called turmeric, it has been in continuous use for its flavoring, as a spice in both vegetarian and non-vegetarian food preparations and it also has digestive properties [15]. The objectives of this study was to evaluate the antimicrobial activity of the extracts from turmeric (C. longa L.) against common nosocominal pathogen S. aureus.
2. Material and methods
2.1. Plant material and extraction
The rhizome of C. longa (turmeric) was purchased from market and 25 gm of dry powder was packed in Soxhlet apparatus for extraction of respective soluble bioactive molecules from the rhizome by the use of different solvent (petroleum ether, benzene, chloroform, methanol and water). Fractions containing volatile solvents, were concentrated with the help of rotary evaporator (rota vapor) under reduce pressure. The concentrated extract was unloaded to sterilized collecting tube.
2.2. Test microorganisms
The standard strains of S. aureus ATCC 6571 used as test organisms were obtained from All India Institute of Medical Sciences, New Delhi. While clinical isolates were obtained from clinical material submitted for diagnostic Microbiology, Department of Microbiology, S.N. Medical College, Agra. Cultures of bacteria were grown on nutrient broth (Hi Media, Mumbai) at 37 °C for 12–14 h and were maintained and preserved on nutrient agar slants (Hi Media, Mumbai) at 4 °C prior to use.
2.3. Phytochemical analysis of the plant extract
Preliminary phytochemical screening of plant was done following the standard procedures adapted by the various workers [11], [17], [18]. The extracts were subjected to phytochemical tests for determination of plant secondary metabolites such as tannins, saponins, steroid, alkaloids and glycosides in accordance with [17].
2.4. Antibacterial activity
To check the presence of antimicrobial substance, the antimicrobial susceptibility tests were performed by standard disc diffusion method [8]. In this method, antibiogram patterns were studied for different extracts of C. longa (rhizome) viz petroleum ether extract, benzene extract, chloroform extract, methanol extract and aqueous extract for comparing three concentrations 1250 μg/disc (50 mg/ml), 2500 μg/disc (100 mg/ml) and 5000 μg/disc (200 mg/ml) based on available literature [22], [9]. Empty sterile discs having a diameter of 6 mm were impregnated with 25 μl of each serial dilution of extract solution. These impregnated discs, now contain different concentration (1250, 2500, 5000 μg/disc) respectively of extract and then incubated for 15 min for proper diffusion of extract and placed onto nutrient agar surface spread with 0.1 ml of bacterial culture (standardized to 0.5 McFarland standards (106 cfu. ml−1). The plates were incubated at 37 °C for 12–14 h. The experiments were carried out in triplicate. The results (mean value n = 3) were recorded by measuring the zone of growth inhibition around the discs. Control discs contained DMSO only. For comparison, standard antibiotic gentamycin inhibiting bacterial cell wall biosynthesis was included in the assay. The antibacterial spectra showing zone of inhibition in millimeters and calculated as percentage by taking gentamycin as positive control with 100% inhibition.
2.5. Antibacterial activity of Curcuma longa studied by scanning electron microscopy
For SEM analysis bacterial cell treated with C. longa were collected by centrifugation of liquid sample at 1120 × g for 10 min. The cell biomass was fixed in the aluminum stubs and coated with thin layer of gold (ZEISS EVO 40 EP).
3. Results and discussion
3.1. Phytochemical analysis of Curcuma longa extract
Phytochemical analysis of C. longa extract showing antimicrobial activity revealed the presence of different active constituents in different extracts (Table 1). Curcuma longa extract contained alkaloids, tannin, flavonoid, glycoside and carbohydrate. There are reports showing that alkaloids and flavonoids are the responsible compounds for the antibacterial activities in higher plants [10].
Table 1.
Phytochemical analysis of various extracts of Curcuma longa (rhizome).
| S. no. | Secondary metabolites | Name of test | Various extracts of Curcuma longa |
||||
|---|---|---|---|---|---|---|---|
| Petroleum ether | Benzene | Chloroform | Methanol | Water | |||
| 1 | Alkaloid | Mayer’s test | − | − | − | + | + |
| Hager’s test | − | − | − | − | − | ||
| 2 | Tannins and phenolic compounds | Ferric chloride test | + | + | + | + | + |
| Vanillin hydrochloride test | + | + | + | + | + | ||
| 3 | Protein | Ninhydrin test | − | − | − | − | − |
| Biuret test | − | − | − | − | − | ||
| 4 | Flavonoids | Shinoda test (magnesium hydrochloride reduction test) | + | + | + | + | + |
| Alkaline reagent test | + | + | + | + | + | ||
| 5 | Steroids and triterpenoids | Salkowski test | − | − | − | − | − |
| Libermann–Buchard test | − | − | − | − | − | ||
| 6 | Glycosides | Legal test | + | + | + | + | + |
| Sodium nitroprusside test | + | + | + | + | + | ||
| 7 | Carbohydrates | Benedit’s test | + | + | + | + | + |
| Fehling’s test | + | + | + | + | + | ||
3.2. In vitro analysis of Curcuma longa extracts
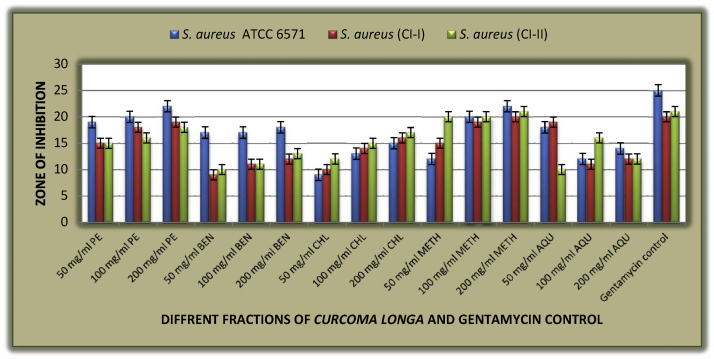
Antimicrobial susceptibility tests of different fractions of C. longa rhizome extract against S. aureus ATCC 6571 and clinical isolates show that all fractions of C. longa rhizome are highly active against standard and clinical isolates of S. aureus showing zone of inhibition ranges between 9 mm and 21 mm which was similar to the study of Negi et al. (1999) who reported the inhibitory effects of ethanol and hexane extract of turmeric against S. aureus. Further observations revealed that benzene extract was least active showing zone of inhibition of about 9 mm at the concentration of 50 mg/ml while methanolic extract was most active showing zone of inhibition of about 19 mm at the concentration of 50 mg/ml. while inhibitory activity of all other fractions ranges between the two. Similar observations has been reported for species such as C. longa, Curcuma zedoaria, Curcuma aromatic and Curcuma amada from the study of [34], [35], [33], [36] (Table 2, Fig. 1).
Table 2.
Antimicrobial susceptibility test of different fractions of Curcuma longa rhizome extract against S. aureus ATCC 6571 and clinical isolates.
| Test pathogens | Zone of inhibition (mm) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Petroleum ether |
Benzene |
Chloroform |
Methanol |
Aqueous |
Gentamycin control | |||||||||||
| 50 mg/ml | 100 mg/ml | 200 mg/ml | 50 mg/ml | 100 mg/ml | 200 mg/ml | 50 mg/ml | 100 mg/ml | 200 mg/ml | 50 mg/ml | 100 mg/ml | 200 mg/ml | 50 mg/ml | 100 mg/ml | 200 mg/ml | ||
| S. aureus ATCC 6571 | 19 | 20 | 22 | 17 | 17 | 18 | 9 | 13 | 15 | 12 | 20 | 22 | 18 | 12 | 14 | 25 |
| S. aureus (CI–I) | 15 | 18 | 19 | 9 | 11 | 12 | 10 | 14 | 16 | 15 | 19 | 20 | 19 | 11 | 12 | 20 |
| S. aureus (CI–II) | 15 | 16 | 18 | 10 | 11 | 13 | 12 | 15 | 17 | 20 | 20 | 21 | 10 | 16 | 12 | 21 |
Fig. 1.
Graphical representation of antimicrobial susceptibility test of different fractions of Curcuma longa rhizome extract against S. aureus ATCC 6571 and clinical isolates.
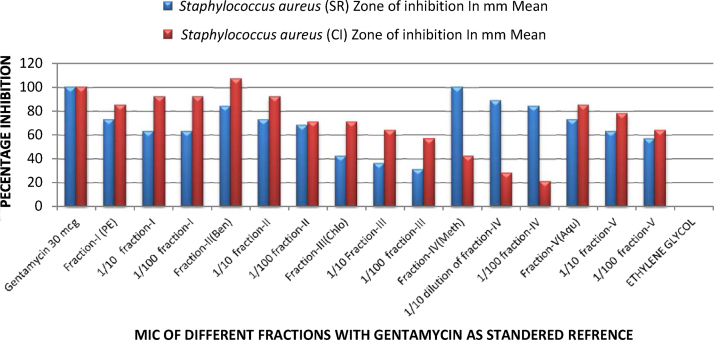
The minimum inhibitory concentration (MIC) was studied for different fractions (100 mg/ml i.e., 2500 μg/disc) at 1/10 (250 μg/disc) and 1/100 (25 μg/disc) dilutions respectively and results are compared with standard antibiotics as percentage. It was observed that dilution altered the activity gradually. In case of S. aureus ATCC 6571 percentage inhibition observed in first fraction of petroleum ether ranges from 73 to 63%, in case of fraction second of benzene it ranges from 84 to 68%, in case of fraction third of chloroform it ranges from 42 to 31%, in case of fraction fourth of methanol it ranges from 100 to 84% and in case of fraction five of aqueous it ranges from 73 to 57% and comparing it with clinical isolate of S. aureus in fraction first of petroleum ether ranges from 85 to 92%, in case of fraction second of benzene it ranges from 71 to 107%, in case of fraction third of chloroform it ranges from 57 to 71%, in case of fraction fourth of methanol it ranges from 21 to 42% and in case of fraction five of aqueous it ranges from 64 to 85%. (Table 3, Fig. 2).
Table 3.
Minimum inhibitory concentration of different fractions of Curcuma longa on gram-positive bacteria with gentamycin as a standard reference.
| Microorganism |
Staphylococcus aureus (SR) zone of inhibition |
Staphylococcus aureus (CI) zone of inhibition |
||
|---|---|---|---|---|
| Name of drug | In mm mean | As percentage | In mm mean | As percentage |
| Gentamycin 30 mcg | 25 | 100 | 20 | 100 |
| Fraction-I (petroleum ether) | 20 | 73 | 18 | 85 |
| 1/10 Dilution of fraction-I | 18 | 63 | 17 | 92 |
| 1/100 Dilution of fraction-I | 18 | 63 | 17 | 92 |
| Fraction-II(benzene) | 22 | 84 | 21 | 107 |
| 1/10 Dilution of fraction-II | 20 | 73 | 19 | 92 |
| 1/100 Dilution of fraction-II | 19 | 68 | 16 | 71 |
| Fraction-III(chloroform) | 14 | 42 | 16 | 71 |
| 1/10 Dilution of fraction-III | 13 | 36 | 15 | 64 |
| 1/100 Dilution of fraction-III | 12 | 31 | 14 | 57 |
| Fraction-IV(methanol) | 25 | 100 | 12 | 42 |
| 1/10 Dilution of fraction-IV | 23 | 89 | 10 | 28 |
| 1/100 Dilution of fraction-IV | 22 | 84 | 9 | 21 |
| Fraction-V(aqueous) | 20 | 73 | 18 | 85 |
| 1/10 Dilution of fraction-V | 18 | 63 | 17 | 78 |
| 1/100 Dilution of fraction-V | 17 | 57 | 15 | 64 |
| Ethylene glycol | 6 | 00 | 6 | 00 |
Fig. 2.
Graphical representation of minimum inhibitory concentration of different fractions of Curcuma longa on gram-positive bacteria with gentamycin as a standard reference.
Above observations was confirmed by the study of [32] who evaluated antimicrobial activity of extracts of C. zedoaria and Curcuma malabarica tubers against six bacterial strains. The MIC values for different strains and extracts ranged from 0.01 to 0.15 mg/ml in C. zedoaria and from 0.01 to 0.94 mg/ml in C. malabarica. S. aureus (Gram positive) was inhibited by C. malabarica but not by C. zedoaria. This study was the first report of the antimicrobial properties of C. malabarica. The findings also support the use of C.a zedoaria tubers in traditional medicine for the treatment of bacterial and fungal infections. While observations of [26] they evaluated the antibacterial activity of C. longa rhizome extracts showed that only the clinical isolate of S. aureus showed more sensitivity towards essential oil fraction than the standard strain. This was similar to sensitivity pattern of clinical and standard isolates in our study.
Electron microscopy determined the morphology of the bacteria after treatment with C. longa extract. The cells that are placed in solution containing 0 μl of C. longa extract for 24 h showed normal morphology of S. aureus with a multilayered surface consisting of the outer membrane (Fig. 3). In contrast, the cells exposed to a 55 μl of (0.5 g/ml) concentration of C. longa extract for 24 h showed various phases in the process of cell death (Fig. 4). In Fig. 4, Fig. 5 which correspond to an intermediate from stage of cell disruption, plasmolysis and partial disappearance of the cytoplasmic membrane are observed. The structure of the outer membrane is apparently unaffected at this stage, and the cell takes on a deformed morphology, with partial lack of the cytoplasmic membrane. In Fig. 6 which obviously corresponds to the final stage of cell disruption, the outer membrane is progressively lost and the cytoplasm tends to spill out of the cell and finally cell death.
Fig. 3.
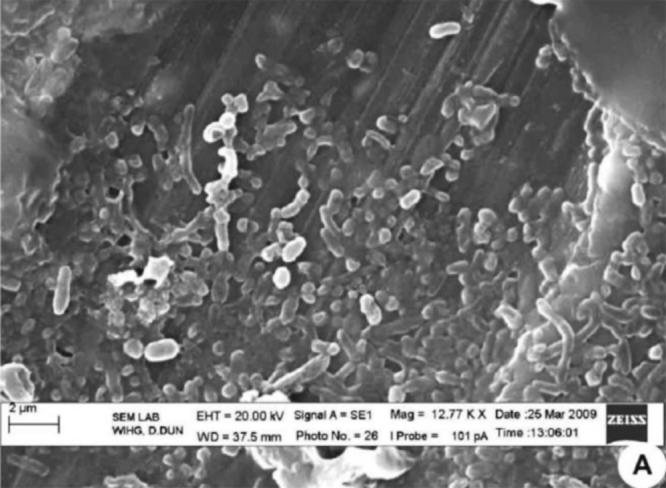
Untreated Staphylococcus aureus.
Fig. 4.

Staphylococcus aureus supplemented with Curcuma longa active compound indicates partial membrane damage.
Fig. 5.
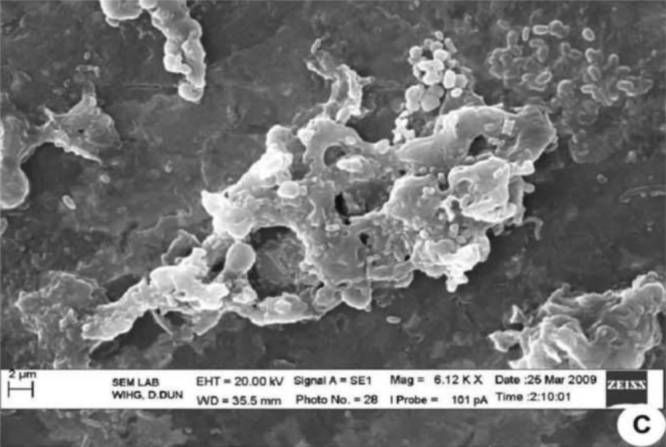
Staphylococcus aureus supplemented with Curcuma longa active compound indicates the cytoplasm tends to spill out of the bacterial cells.
Fig. 6.
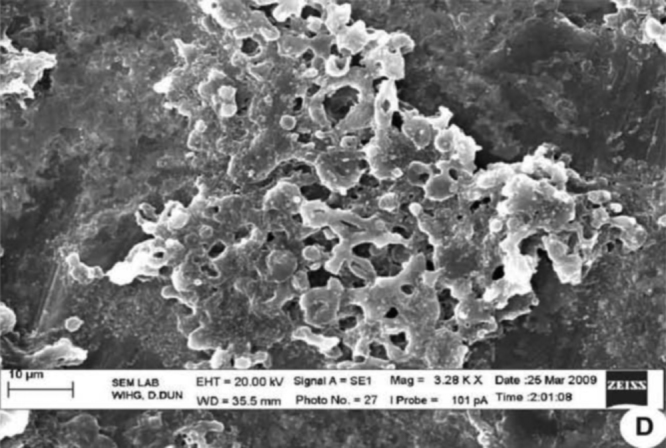
Staphylococcus aureus supplemented with Curcuma longa active compound indicates complete membrane damage.
4. Conclusions
Thus, in this study, the efficiency of turmeric fractions, such as petroleum ether, chloroform, benzene, methanol and aqueous were evaluated for their inhibitory effect on clinical and standard strains of pathogenic bacteria S. aureus. The methanolic fraction of C. longa rhizome had high potential to inhibit some pathogenic bacteria S. aureus to a greater degree than other fractions of C. longa. In our study the results show that the different fractions (petroleum ether, methanol etc.) of C. longa rhizome was more effective antimicrobial agents than the crude extract of C. longa.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Ahmad I., Mehmood Z., Mohammad F. Screening of some Indian medicinal plants for their antimicrobial properties. J. Ethnopharmacol. 1998;62:183–193. doi: 10.1016/s0378-8741(98)00055-5. [DOI] [PubMed] [Google Scholar]
- 2.Ahmedulla M., Nayar M.P. Botanical Survey of India; Calcutta: 1999. Red Data Book of Indian Plants; p. 4. [Google Scholar]
- 7.Ayurvedic Pharmacopoeia of India (API), New Delhi: Government of India Ministry of Health and Family Welfare—Department of Health. 45–46, (1989).
- 8.Bauer A.W., Kirby W.M.M., Sherris J.C., Turke M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- 9.Chairandy C.M., Seaforth C., Phelps R.H., Pollard G.V., Khambey B.P.S. Screening of medicinal plants from Trinidad and Tobago for antimicrobial and insecticidal properties. J. Ethnopharmacol. 1999;64:265–270. doi: 10.1016/s0378-8741(98)00130-5. [DOI] [PubMed] [Google Scholar]
- 10.Cordell G.A., Quinn-Beattie M.L., Farnsworth N.R. The potential of alkaloids in drug discovery. Phytother. Res. 2001;15:183–205. doi: 10.1002/ptr.890. [DOI] [PubMed] [Google Scholar]
- 11.Daniel M. Kalyani publishers; New Delhi, India: 1991. Method in Plant Chemistry and Economic Botany. [Google Scholar]
- 12.Eigner D., Scholz D. Ferula asa-foetida and Curcuma longa in traditional medicinal treatment and diet in Nepal. J. Ethnopharmacol. 1999;67:1–6. doi: 10.1016/s0378-8741(98)00234-7. [DOI] [PubMed] [Google Scholar]
- 13.Essawi T., Srour M. Screening of some Palestinian medicinal plants for antibacterial activity. J. Ethnopharmacol. 2000;70:343–349. doi: 10.1016/s0378-8741(99)00187-7. [DOI] [PubMed] [Google Scholar]
- 14.Ghobashy A.A., Chiori C.O., Azubike C.O. Susceptibilty of Staphylococcus aureus to penicillin’s and other antibiotics. Niger. J. Pharm. 1984;15:24–26. [Google Scholar]
- 15.Govindarajan V.S. Turmeric—chemistry, technology, and quality. Crit. Rev. Food Sci. Nutr. 1980;12(3):199–301. doi: 10.1080/10408398009527278. [DOI] [PubMed] [Google Scholar]
- 16.Haldane E.V., Affias S. Penicillin tolerance in Staphylococcus aureus. The Lancet. 1981;2:39. doi: 10.1016/s0140-6736(77)90035-6. [DOI] [PubMed] [Google Scholar]
- 17.Harborne J.B. Chapman and Hall; London: 1998. Phytochemical Methods – A Guide to Modern Techniques of Plant Analysis; pp. 182–190. [Google Scholar]
- 18.Kokate C.K., Purohit A.P., Gokhale S.B. second ed. Vallabh Prakashan; Delhi: 2004. Practical Pharmacognasy. [Google Scholar]
- 20.Leung A.Y., Foster S. second ed. John Wiley & Sons Inc.; New York: 1996. Encyclopedia of Common Natural Ingredients Used in Food, Drugs and Cosmetics; pp. 499–501. [Google Scholar]
- 21.New York Times, March 12: Sect. (1998) A12.
- 22.Paech K., Tracey M.V. Vol. 3. Springer Verlag; Berlin: 1995. pp. 626–654. (Modern Methods of Plant Analysis). [Google Scholar]
- 23.Rıos J., Recio M. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005;100:80–84. doi: 10.1016/j.jep.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 24.Robert A.W. Nosocomial infection update. Emerg. Infect. Dis. 1998;4:3. doi: 10.3201/eid0403.980320. (special issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sachdev D., Amladi S., Natraj G., Baveja S., Kharkar V., Mahajan S., Khopkar U. An outbreak of methicillin-resistant Staphylococcus aureus (MRSA) infection in dermatology indoor patients. Indian J. Dermatol. Venereol. Leprol. 2003;69:377–380. [PubMed] [Google Scholar]
- 26.Singh R., Chandra R., Bose M., Luthra P.M. Antibacterial activity of Curcuma longa rhizome extract on pathogenic bacteria research communications. Curr. Sci. 2002;83(6):738. [Google Scholar]
- 30.Verma S., Joshi S., Chitnis V., Hemwani N., Chitnis D. Growing problem of methicillin-resistant staphylococci – Indian scenario. Indian J. Med. Sci. 2000;54:535–540. [PubMed] [Google Scholar]
- 31.Wichtl M., Bisset N.G. Medpharm Scientific Publishers; Stuttgart: 1994. Herbal Drugs and Phytopharmaceuticals; pp. 173–175. [Google Scholar]
- 32.Wilson B., Abraham G., Manju S., Mathew M., Vimala B., Sundaresan S., Nambisa B. Antimicrobial activity of Curcuma zedoaria and Curcuma malabarica tubers. J. Ethnophamacol. 2005;99:147–151. doi: 10.1016/j.jep.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Negi P.S., Jayaprakasha G.K., Jagan M.R.L., Sarariah K.K. Antibacterial activity of turmeric oil: a byproduct from curcumin manufacture. J. Agric Food Chem. 1999;47:4297–4300. doi: 10.1021/jf990308d. [DOI] [PubMed] [Google Scholar]
- 34.Apisariyakul A., Vamittanakom N., Buddhasukh D. J. Ethnopharmacol. 1995;49:163–169. doi: 10.1016/0378-8741(95)01320-2. [DOI] [PubMed] [Google Scholar]
- 35.Yoshioka T., Fujii E., Endo M., Wada K., Tokunage Y., Shiba N., Hohsho H., Shibuya H., Muraki T. Antiflammatory potency of dehydrocurdione, a zedoary-derived sesquiterpene. Inflamm. Res. 1998;47:476–481. doi: 10.1007/s000110050361. [DOI] [PubMed] [Google Scholar]
- 36.Majumdar A.M., Naik D.G., Dandge C.N., Puntambekar H.M. Antiflammatory activity of Curcuma amadain albino rats. Indian J. Pharmacol. 2000;32:375–377. [Google Scholar]