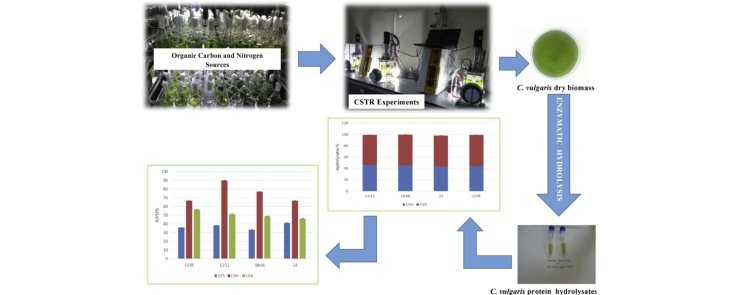
Graphical abstract
Key words: Bioreactors, Chlorella vulgaris, Bioprocess, FTIR, Microalgae, Pancreatin
Highlights
-
•
Microalgae cultivation in photomixotrophic conditions.
-
•
Proteolytic hydrolysis of crude microalgae cells.
-
•
Dietary supplement of microalgae and functional nutrition.
-
•
Utilization of FTIR spectroscopy to determine biochemical composition.
-
•
Enhancement of in vitro protein digestibility (IVPD).
Abstract
Chlorella vulgaris SAG 211-12, a green microalga, as model organism was cultivated photomixotrophically using various organic carbon and nitrogen sources at Erlenmeyer scale. The modified medium selected for the experiments was standard BG11 supplemented with 5 g l−1 glucose and 1 g l−1 proteose peptone (PP). To evaluate the effects of light/dark cycles, 12:12; 18:6 and 24:0 light/dark cycle conditions were examined on hourly basis. 24:0 continuous illumination condition was chosen to continue 2 l continuous stirred tank photobioreactor (CSTR) experiments under 1 vvm aeration, 120 rpm mixing time, 23 ± 2 °C, and 70 μE m−2 s−1 illumination conditions. The results showed significant effect of the culture conditions on the cellular composition. To enhance digestibility of the intact cell; dry biomass was digested with pancreatin enzyme solution and in vitro protein digestibility (IVPD) of crude biomass (UTS), cell debris (CVA) and protein hydrolysates (CVH) was measured. IVPD values of UTS, CVA and CVH were found to be 33–41%, 46–58%, 67–89%; respectively with no significant changes regarding culture conditions (p > 0,05). Results also showed the positive effect of the enzyme treatment for digestion which is a key advantage for nutritional characteristic of the algal biomass.
1. Introduction
Green microalgae are unicellular, eukaryotic, photosynthetic microorganisms living in fresh, saline or brackish water environments. They are composed of proteins, essential amino acids, fatty acids, antioxidant pigments, vitamins and other bioactive compounds that express unique features for the development of pharmaceutials, nutraceuticals, cosmetics and biofuel industry [33], [30]. The microalgal biotechnology showed a great progress in early 1950s with developments in processing technologies regarding the industrial applications of certain metabolites. The environmental conditions and nutritional modes alter the cell composition which can be used to produce diverse metabolites for various industries. The ease in cultivation, economically reliable features, high biomass efficiency and diversity of microalgal strains make the microalgae research even more attractive.
Among commercially important strains, Chlorella vulgaris has gained importance in terms of higher growth rates, high light to biomass conversion, ability to grow under phototrophic, photomixotrophic and heterotrophic conditions, high protein amount, essential amino acids and fatty acids [34]. Besides C. vulgaris has been accepted as a functional food source with anticancer, immunoregulator, immunostimulating, antioxidant, antimicrobial activities [23], [26], [12], [35].
C. vulgaris cells are surrounded by thick cellulosic wall composed of mostly hemicellulose fibrils and saccharides such as mannose, ramnose, xylose, galactose and glucose (Safi et al., 2014). Because of the thick cellulosic wall, the intact cell is poor in terms of digestibility [23]. There are several approaches to break down the cell integrity. However conventional methods such as acid or alkali hydrolysis damage the structure of free amino acids also low hydrolysis yield is another issue [15]. In that case; enzymatic digestion gives an opportunity to increase the yield of hydrolysis with increasing digestibility [23], [24], [4]. Enzymes like pepsin, papain, pancreatin, and trypsin are widely used to obtain enzymatic protein hydrolysates from conventional sources [24], [31], [10] other than bacterial proteases [3], [2] that offer a mild process conditions and ease in the operation.
Protein hydrolysates are commonly used as food or drink additives to supplement protein value but another important aspect is their possible utilization for individuals who suffer from digestibility problems such as gastrointestinal malfunction or cystic fibrosis [5]. There are various studies in the literature covering different aspects of enzymatic protein hydrolysates [27], [28] from conventional protein sources such as soybean or whey proteins [18], [13]. Today considering the need of alternative sustainable food sources for the growing demand of global population microalgal hydrolysates are also emerging as attractive functional protein nutrition products.
The aim of this study is to cultivate C. vulgaris in photomixotrophic conditions and also see the effects of light/dark cycles to biochemical composition. As downstream process, the enzymatic hydrolysis is another key point of the study in order to enhance digestibility and gain a perspective in the field of microalgal dietary supplements. This can increase the preference of algal dietary supplements because when algal cells are disrupted the digestibility properties are enhanced besides the prevention of unwanted properties such as taste, fishy smell and green color can be avoided.
2. Materials and methods
2.1. Microalgae culturing and downstream processes
In this study; axenic cultures of C. vulgaris SAG 211-12 were used. Stock cultures of C. vulgaris were cultivated under continuous illumination of 40 μE m−2 s−1 in standard BG11 medium for 4–5 days. Cultures in mid-logarithmic phase were used as inoculum for photomixotrophic cultivation, light/dark cycles and continuous stirred tank photobioreactor (CSTR) experiments.
For photomixotrophic experiments; C. vulgaris cells were cultivated under continuous illumination of 70 μE m−2 s−1, 23 ± 2 °C, and 120 rpm agitation in 100 ml Erlenmeyer flasks. BG11 culture medium was modified with organic carbon sources (glucose (G), sucrose (S), fructose (F), glycerol or xylose); and organic nitrogen sources (yeast extract (YE), proteose peptone (PP) or urea) in various concentrations of 1 g l−1 and 5 g l−1, respectively. Cultures were maintained for 7 days and sampled daily basis.
For light/dark cycle experiments, selected organic carbon and nitrogen sources were added to BG11. C. vulgaris cells were cultivated photomixotrophically under 12:12, 18:6 and 24:0 h:h light/dark cycles in 1 l of Roux-type flat photobioreactors with 800 ml of working volume. Cultures were maintained for 7 days and sampled on daily basis.
2 l CSTR (Sartorious Biostat A-Plus, Germany) was used for bulk biomass cultivation under selected illumination strategy, at 23 ± 2 °C, and 120 rpm agitation conditions. Cells were centrifuged at 3000 × g for 8 min for harvesting and washed twice with distilled water. Harvested cells were freeze dried and kept at −20 °C until enzymatic hydrolysis.
2.2. Enzymatic hydrolysis
The enzymatic hydrolysis of crude microalgal biomass was done with pancreatin enzyme (Sigma–Aldrich, USA). The proteolitic activity of the enzyme was determined according to one proteolytic unit expressed as the amount of enzyme necessary to catalyze the release of 1nmol tyrosine from 6% denatured casein solution at 37 °C within 1 min (pH, 7.5).
1 g of dried C. vulgaris was mixed with 2.5% v/w distilled water (pH, 7.5) and incubated at 45 °C for 1 h in temperature controlled shaker. The pancreatic hydrolysis was done for 4 h at 45 °C with gentle shaking under selected E/S condition. The enzymatic reaction was stopped with heat treatment at 85 °C for 20 min [23]. The hydrolyzed mixtures were centrifuged at 3000 × g for 10 min. Supernatant (CVH) and cell debris (CVA) was freeze dried and kept at −20 °C for further analysis.
2.3. Analytical methods
For dry biomass analysis; 2 ml of samples were filtered and washed with distilled water twice. Total dry biomass (g l−1) was determined gravimetrically. Total soluble protein content was measured according to Lowry method [19]. Total gravimetric fatty acid was measured according to Bligh and Dyer method with some minor modifications [1].
In vitro protein digestibility (IVPD) was done according to Hsu multi-enzyme solution with some differences [9]. 5 mg of dried C. vulgaris biomass was mixed with 5 ml of distilled water (pH, 8). Trypsin (1.6 mg ml−1) and α-chymotrypsin (1.6 mg ml−1) enzyme mixture was dissolved in 1 ml distilled water (pH, 8). The pH drop is recorded for 10 min at 37 °C. Sodium caseinate was used as reference sample with 100% IVPD value.
Sephadex G100 and G25 columns were used to determine peptide fractions of the samples. 5 g of Sephadex G100 and Sephadex G25 was weighted and kept in phosphate buffer (pH, 6.8) for 5 h. Columns were filled with Sephadex G100 (2.5 cm × 25 cm) and G25 (2,5 cm × 15 cm) and waited for 24 h. 2 mg of CVH was dissolved in 1 ml phosphate buffer (pH, 6.8) and 200 μl sample was injected to column. Eluted volume was collected and absorbance was measured at 214 nm in UV–visible spectrophotometer (Optizen Pop Korea). Bovine serum albumin (67 kDa), peroxidase (44 kDa) were used for G100 column; approtinine (6.5 kDa) and cyanocobalamin (1.4 kDa) for G25 column were used as reference proteins.
Biochemical composition of dried UTS, CVH and CVA were characterized via FTIR spectroscopy (Perkin Elmer, USA). The samples were analyzed in between 4000 and 400 cm−1 wavelength and 2 cm−1 resolution with 4 parallel measurements.
2.4. Statistical analysis
The data analyzed by statistical tests using Microsoft Excel software. Statistical analysis consisted of summary statistics, including means, standard deviation and standard errors, where one way analysis of variance was done, and then comparison was conducted at the 0.05 level. All the experiments were done with 3 replicates.
3. Results and discussion
Microalgal biotechnology is an emerging area with several aspects. Even though it is not a new technology the developing strategies, study topics and perspectives are spreading very fast enabling new understandings. In this study, the effects of nutrients and environmental conditions were investigated in order to obtain enzymatic hydrolysates as dietary supplements with increased digestibility.
3.1. Photomixotrophic cultivation experiments
Microalgae are capable of using several organic carbon and nitrogen sources for biomass production. Under control conditions (BG11 medium, phototrophic production) the total dry biomass was 0.74 ± 0.03 g l−1. Cultures supplemented with xylose were died in the early period of growth, some literature data also support these findings [14] meanwhile in glycerol, cells were able to reproduce but in 5 g l−1 experiments the growth was inhibited. Cell survival and production could be seen in cultures supplemented with glucose, sucrose and fructose (Table 1). The selected carbon source for C. vulgaris cells was glucose which gave highest biomass value at 5 g l−1 experiment (2.06 ± 0.08 g l−1). Other carbon sources also increased biomass accumulation however compared to glucose the growth was lower. Glucose is known to be a suitable organic carbon source for microalgal growth both for photomixotrophic and heterotrophic cultivation because it can diffuse through the cell very fast and it has more ATP potential for the cell growth [32].
Table 1.
The dry biomass and protein values of photomixotrophic production.
| Component | Concentration (g l−1) | Dry biomass (g l−1) |
Protein (mg g−1) |
|---|---|---|---|
| Glucose | 1 | 1.58 ± 0.06 | 173.03 ± 9.84 |
| 5 | 2.06 ± 0.08 | 176.08 ± 9.85 | |
| Sucrose | 1 | 0.72 ± 0.03 | 184.42 ± 9,3 |
| 5 | 0.73 ± 0.03 | 160.25 ± 10.28 | |
| Fructose | 1 | 0.4 ± 0.02 | 152.61 ± 10.86 |
| 5 | 1.35 ± 0.05 | 204 ± 8.26 | |
| Proteose peptone | 1 | 0.92 ± 0.04 | 224.42 ± 8.22 |
| 5 | 1.0 ± 0.04 | 218.31 ± 8.38 | |
| Yeast extract | 1 | 0.6 ± 0.02 | 203.58 ± 8.94 |
| 5 | 0.5 ± 0.02 | 210.53 ± 7.91 |
The results showed that carbon sources did not have a significant effect on protein synthesis (Table 1). Some studies also suggest that carbon backbone of the organic carbon sources in culture media is used as precursors in starch or fatty acid metabolism in microalgae. Additional carbon input results as a dramatic increase in the biomass accumulation until the saturation point however does not stimulate protein accumulation.
In the case of organic nitrogen source experiments urea inhibited cell growth and increase in the urea concentration triggered cell death. But when PP and YE were used increase in the total protein was observed (Table 1) but did not significantly affect biomass accumulation. It is known that nitrogen sources are used for protein synthesis but increase in the concentration slightly decreased the protein amount, the reason is thought to be nitrogen accumulation in the culture media [32]. Other than the slight enhancement in the protein amount with PP compared to YE, the cultures grown in YE was contaminated in the later period of the growth lead the selection of the PP for the following experiments.
According to results; BG11 was supplemented with 5 g l−1 glucose and 1 g l−1 PP for light/dark cycle and CSTR experiments.
3.2. Light/dark cycles and CSTR experiments
Light is one of the major parameters affecting the regulation of microalgal metabolism. Some microalgae species can also grow in dark conditions but most of them are strict phototrophs. In this study, effect of light/dark cycles on hourly basis was investigated in photomixotrophic cultures.
The results briefly showed that, when illumination period was increased; the biomass accumulation is also increased. Biomass values of cultures were 1.76 ± 0.57, 2.56 ± 0.38; 2.75 ± 0.34 g l−1, respectively. It can be seen that illumination enhances biomass production. In dark period cells only use respiration metabolism and storage materials such as starch, lipid or protein as nutrients which results in the weight losses of overall biomass amount known as night loss in naturally illuminated cultures [37].
Light/dark cycles also affect the biochemical composition of the cells [11]. As it can be seen from Table 2, with the increase in the illumination period also fatty acid and protein content is increased, too. It can be stated according to the results that; C. vulgaris can survive in dark conditions but the overall biomass accumulation is highly related with the duration of illumination period [6], [7], [8].
Table 2.
Total protein and fatty acid values of UTS, CVH and CVA.
| Condition | Fatty Acid (%) | Protein (mg g−1) | |
|---|---|---|---|
| UTS | 12:12 | 10.38 ± 0.32 | 394.76 ± 6.10 |
| 18:6 | 11.7 ± 1.65 | 425.27 ± 4.2 | |
| 24:0 | 15.28 ± 1.52 | 499.8 ± 6.86 | |
| CSTR | 19.55 ± 2.6 | 545.47 ± 12.64 | |
| CVH | 12:12 | ND | 226.48 ± 4.31 |
| 18:6 | ND | 351.54 ± 4.5 | |
| 24:0 | ND | 447.09 ± 11.02 | |
| CSTR | ND | 443.85 ± 19.5 | |
| CVA | 12:12 | 2.56 ± 0.03 | 168.3 ± 6.15 |
| 18:6 | 3.045 ± 0.21 | 73.74 ± 5.2 | |
| 24:0 | 2.83 ± 0.17 | 52.72 ± 5.24 | |
| CSTR | 2.44 ± 0.05 | 101.61 ± 2.8 | |
Biomass value reached in the CSTR was 3.89 ± 0.26 g l−1 which is significantly higher than Roux-type experiment of continuous illumination other than the significant increase in the protein and fatty acid values. This enhancement in the amounts can be based on the design features of the CSTR which provides a more controlled environment in terms of illumination, agitation, aeration and contamination. In comparison with Roux type photobioreactors; even if the scale up was done keeping the mixing time constant, CSTR has special designs of mixers instead of magnetic bars that improved the micro environment of the cells to utilize the nutrients better [29].
3.3. Enzymatic hydrolysis
Before enzymatic hydrolysis, proteolytic activity of the enzyme was measured as 138 U/mg (4 USP, ≥100 U/mg proteolytic activity). Because the initial E/S (w/w) ratio is an important parameter for the success of the enzymatic hydrolysis, 1%, 2%, 5%, 8%, 10% and 20% values were examined. Morris et al. showed that the initial enzyme concentration is a selection parameter when enzymatic hydrolysis is aimed [22]. The results showed that, when the E/S ratio was increased, the yield of hydrolysis was also increased until the saturation point [10%]. After the limit of 10% the yield of hydrolysis was decreased (Fig. 1). Hydrolysis experiments were done according to the selected E/S ratio of 8%.
Fig. 1.
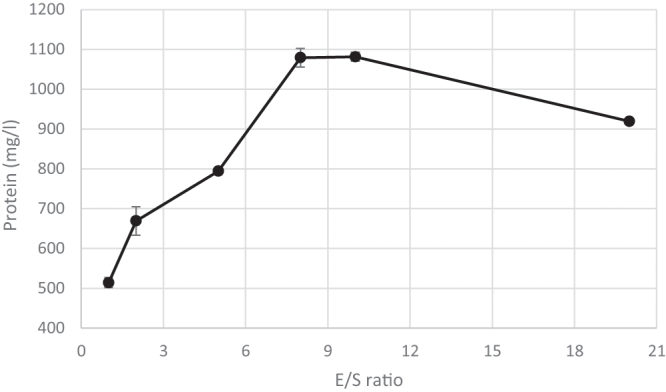
The initial enzyme concentration.
After enzymatic treatment of cell proteins, untreated sample (UTS) was divided into two phases; protein hydrolysates (CVH) and waste biomass after hydrolysis which still has important compounds left in (CVA). The results showed that hydrolysis yielded over 50% (Fig. 2). The degree of hydrolysis did not change according to the culture conditions (p > 0.05) which is an important point to mention. Incubation of cells at 45 °C for 1 h is thought to help cells to disrupt before enzyme treatment. The microscopic observations support that after 1 h cell integrity was damaged which helped enzyme to penetrate into cells. The other aspect is because pancreatin is an enzyme mixture of amylases, lipases and proteases, the combined activity of these enzymes increased the success of the hydrolysis. Morris et al. [22] suggested that ethanol, a GRAS solvent, treatment also helped the cells to break down and increased the yield. The thick cellulosic walls of the cells are the main challenge in front of the enzymatic hydrolysis, which some of the studies also suggests pretreatments [4], [28], [29]. The choice of the enzyme is another issue to be emphasized. Pancreatin as a gastrointestinal enzyme is a dietary supplement for patients suffering from digestion problems. In that case aiming the enhancement of digestibility, pancreatin is the most widely used enzyme both for algal or conventional protein products [13], [29], [17].
Fig. 2.
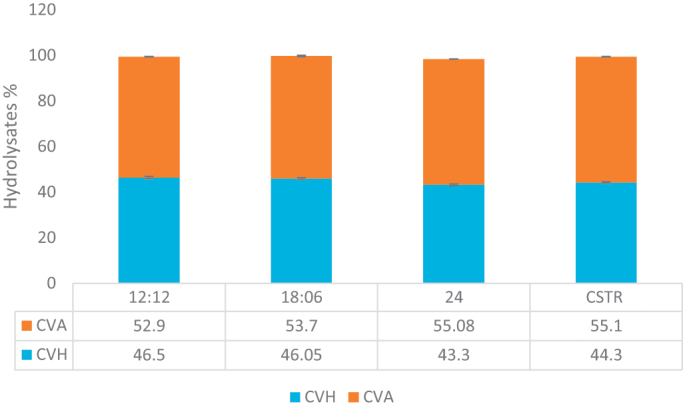
Enzymatic hydrolysis of intact microalgae cells.
The physical properties of CVH were yellow–white powder with no algae smell which is thought to be a good point for consumer’s choice. Besides CVH, CVA is also an economically important compound that can be used in the formulation of dietary supplements (Table 2). The enzymatic hydrolysis broke down the cellulosic wall structure; the main barrier in front of the digestibility, besides it still had proteins, carbohydrates, lipids and cellulosic material.
3.4. IVPD values
IVPD values are one of the major properties of dietary supplements. Microalgal nutritional supplements suffer from low digestibility even they are consisted of valuable products [20]. In this regard IVPD values can give an idea about digestibility value and criteria of a compound to be used as a dietary supplement which this study aims to improve.
The specific activity of enzymes used for multi-enzyme solution was found to be 98 U/mg and 115 U/mg for trypsin and α-chymotrypsin, respectively. The IVPD values differed according to the state of the sample dramatically. UTS had IVPD value around 30–40%; meanwhile CVH had 67–89% IVPD value. Also CVA had increased IVPD values compared to UTS (Fig. 3). The IVPD values were not significant according to culturing conditions but had a significant impact in between UTS, CVH and CVA [36]. According to results obtained from IVPD studies, CVH is a suitable protein supplement with high protein content and enhanced digestibility.
Fig. 3.
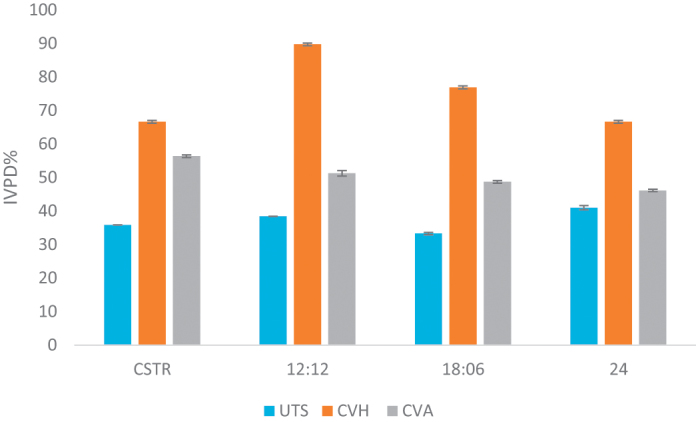
IVPD% values of UTS, CVH and CVA.
3.5. Protein fractioning
Molecular weight distribution of CVH was determined via gel filtration columns. According to the molecular weight analysis CVH phase was divided into 2 main classes; CVH-100 representing peptides fractioned with Sephadex G100 column and CVH-25 fractioned with Sephadex G25 column. Molecular weight distribution of CVH-100 for 12:12, 18:6 and 24:0 were in a broad range. These peptides were also divided into 3 major classes representing large peptides (>50 kDa), medium peptides (in between 30 and 50 kDa) and small peptides (<30 kDa). Peptide with 125 kDa molecular weight was observed in light/dark cycle experiments. In CSTR culture peptide distribution started with medium peptides. In CVH-25 molecular weights of the peptides were diverse with no significant common peptide (Table 3).
Table 3.
Peptide distribution of CVH in Sephadex G100 and Sephadex G25Column.
| Sephadex G100 |
Sephadex G25 |
|||
|---|---|---|---|---|
| Large (kDa) | Mid (kDa) | Small (kDa) | Da | |
| 12:12 | 125 | 48 | 28 | 1200 |
| 91 | 40 | 27 | 670 | |
| 72 | 35 | 25 | ||
| 59 | 32 | 23 | ||
| 18:6 | 125 | 48 | 30 | 2400 |
| 83 | 42 | 28 | 1300 | |
| 37 | 26 | 950 | ||
| 35 | 670 | |||
| 24:0 | 125 | 50 | 29 | 2100 |
| 100 | 42 | 27 | 1530 | |
| 59 | 36 | 24 | 1200 | |
| 22 | 1050 | |||
| 880 | ||||
| CSTR | – | 53 | 25 | 5600 |
| 44 | 2400 | |||
| 36 | 1120 | |||
| 31 | 730 | |||
The gel filtration column gave an idea about molecular weight patterns of the hydrolysates. The reason to have a broad spectrum of peptides is thought to be non-specific action of the pancreatin enzyme [21] because of the globular structure of the algal proteins within the cells was a limiting step for smaller fractioning [29].
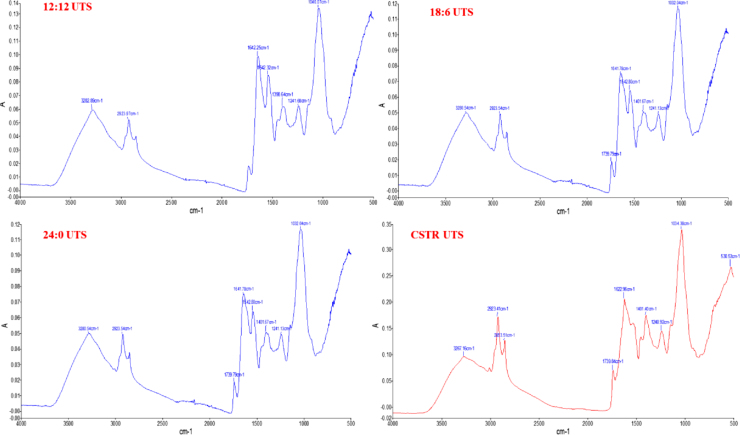
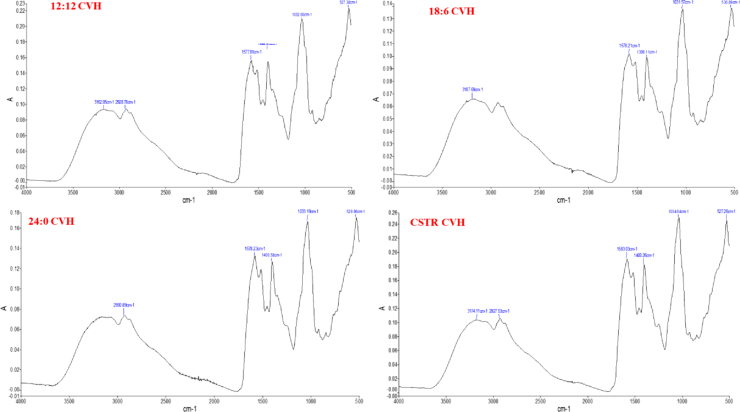
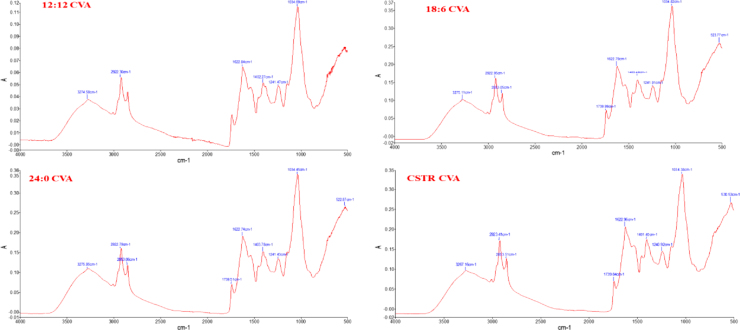
3.6. FTIR analysis
FTIR is a new and efficient technique which is helpful to analyze the biochemical composition of microalgal cells in a short period of time without complex sample preparation [25]. In this study FTIR is used to determine the biochemical composition of UTS (Fig. 4), CVH (Fig. 5) and CVA (Fig. 6). The UTS for light/dark cycle and CSTR experiments gave the same wavenumbers with no significant difference but it was seen that the peak areas were differed according to cultivation conditions of the cells. It was found that UTS was composed of proteins (3280 cm−1,1642 cm−1 amid 1, 1542 cm−1 amid II, 1398 cm−1 carboxylic group, lipids (2923 cm−1), carbohydrates (1040 cm−1), cellulose (1165-1000 cm−1) and nucleic acids (1241 cm−1). In CVH samples the major bands were 3162–3187 cm−1, 2928 cm−1, 1577–1583 cm−1, 1399–1402 cm−1,1031–1034 cm−1 and 527–529 cm−1. For CVA samples the FTIR bands are resulted as 3274 cm−1, 2922 cm−1, 1622 cm−1, 1402 cm−1, 1241 cm−1, 1034 cm−1 and 522 cm−1.
Fig. 4.
FTIR analysis of UTS.
Fig. 5.
FTIR analysis of CVH.
Fig. 6.
FTIR analysis of CVA.
The FTIR spectra has shown a very impotant result in terms of the efficiency of hydrolysis that the lipids, proteins, carbohydrates and cellulose bands were also found in CVA phase. The most important result obtained from FTIR analysis was the absence of the nucleic acids in CVH phase [29]. CVH was consisted of mainly proteins and carbohydrates. Also there were no relevant bands representing cellulose, a criteria for digestibility value.
4. Conclusion
Changes in the scientific perspectives also opened new insights in microalgal biotechnology leading researches related to innovative substances. In this study, aim was to see the effect of environmental conditions and nutritional mode on the biochemical composition of microalgae cells in the scope of protein synthesis. Also the emphasis was to increase the digestibility of intact algae cells in order to use for patients suffering from digestion problems especially patients with cystic fibrosis. A strong point for this study is the use of FTIR spectra as a tool to determine hydrolysates composition. Other than the importance of cystic fibrosis the relation of microalgal protein hydrolysates regarding the supplemental nutrition for cystic fibrosis patients were also wanted to be stressed to catch more attention for the possible improvement chances of the life quality of the patients. The results are promising in terms of increased digestibility and biochemical composition of CVH is a good motivation for the future studies focusing on cystic fibrosis cell lines [16].
Acknowledgements
The authors would like to thank Ege University for the financial support. The authors would also like to thank Prof. Dr. Fazilet Vardar Sukan, Prof. Dr. Murat Elibol and Prof. Dr. Figen Zihnioglu of Ege University for their valuable comments and support during the study.
Footnotes
Available online 21 February 2015
References
- 1.Bligh E.G., Dyer W.J. A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 2.Chen R., Yue Z., Deitz L., Liu Y., Mulbry W., Liao W. Use of an algal hydrolysate to improve enzymatic hydrolysis of lignocellulose. Bioresour. Technol. 2012;108:149–154. doi: 10.1016/j.biortech.2011.12.143. [DOI] [PubMed] [Google Scholar]
- 3.Chiang W.D., Shih C.J., Chu Y.H. Functional properties of soy protein hydrolysate produced from a continuous membrane reactor system. Food Chem. 1999;65:189–194. [Google Scholar]
- 4.Cian R.E., Martiner-Augustin O., Drago S.R. Bioactive properties of peptides obtained by enzymatic hydrolysis from protein byproducts of Porphyra columbina. Food Res. Int. 2012;49:364–372. [Google Scholar]
- 5.Clemente A. Enzymatic protein hydrolysates in human nutrition. Trends Food Sci. Tech. 2000;11:254–262. [Google Scholar]
- 6.Fabregas J., Maseda A., Sominguez A., Ferreira M., Otero A. Changes in the cell composition of the marine microalga Nannochloropsis gaditana, during a light: dark cycle. Biotechnol. Lett. 2002;24:1699–1703. [Google Scholar]
- 7.Foy R.H., Gibson C.E., Smith R.V. The influence of daylength, light intensity and temperature on the growth rates of planktonic blue–green algae. Br. Phycol. J. 1976;11(2):151–163. [Google Scholar]
- 8.Han F., Wang W., Li Y., Shen G., Wana M., Wang J. Changes of biomass, lipid content and fatty acids composition under a light–dark cyclic culture of Chlorella pyrenoidosa in response to different temperature. Bioresour. Technol. 2013;132:182–189. doi: 10.1016/j.biortech.2012.12.175. [DOI] [PubMed] [Google Scholar]
- 9.Hsu H.W., Vavak D.L., Satterlee L.D., Miller G.A. A multienzyme technique for estimating protein digestibility. J. Food Sci. 1977;42:1269–1273. [Google Scholar]
- 10.Kang K.-H., Qian Z.-J., Ryu B., Karadeniz F., Kim D., Kim S.-K. Antioxidant peptides from protein hydrolysate of microalgae Navicula incerta and their protective effects in Hepg2/CYP2E1 cells induced by ethanol. Phytother. Res. 2012;26:1555–1563. doi: 10.1002/ptr.4603. [DOI] [PubMed] [Google Scholar]
- 11.Khoeyi Z.A., Seyfabadi J., Ramezanpour Z. Effect of light intensity and photoperiod on biomass and fatty acid composition of the microalgae, Chlorella vulgaris. Aquacult. Int. 2012;20:41–49pp. [Google Scholar]
- 12.Kim S.-K., Wijesekara I. Development and biological activities of marine-derived bioactive peptides: a review. J. Funct. Foods. 2010;2:1–9. [Google Scholar]
- 13.Kong X., Zhou H., Quian H. Enzymatic preparation and functional properties of wheat gluten hydrolysates. Food Chem. 2007;101:615–620. [Google Scholar]
- 14.Kratz A.W., Myers J. Nutrition and growth of several blue–green algae. Am. J. Bot. 1955;42(3):282–287. [Google Scholar]
- 15.Kristinsson H.G., Rasco B.A. Biochemical and functional properties of atlantic salmon (Salmo salar) muscle proteins hydrolyzed with various alkaline proteases. J. Agric. Food Chem. 2000;48:657–666. doi: 10.1021/jf990447v. [DOI] [PubMed] [Google Scholar]
- 16.Lloyd-Still J.D., Powers C.A., Hoffman D.R., Boyd-Trull K., Lester L.A., Benisek D.C., Arterburn L.M. Bioavailability and safety of a high dose of docosahexaenoic acid triacylglycerol of algal origin in cystic fibrosis patients: a randomized controlled study. Nutrition. 2006;22:36–46. doi: 10.1016/j.nut.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Lo W.M.Y., Li-Chan E.C.Y. Angiotensin I converting enzyme inhibitory peptides from in vitro pepsin–pancreatin digestion of soy protein. J. Agric. Food Chem. 2005;53:3369–3376. doi: 10.1021/jf048174d. [DOI] [PubMed] [Google Scholar]
- 18.Lourenco da Costa E., Antonio da Rocha Gontijo J., Netto F.M. Effect of heat and enzymatic treatment on the antihypertensive activity of whey protein hydrolysates. Int. Dairy J. 2007;17:632–640. [Google Scholar]
- 19.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with Folin-phenol reagent. Biochem. Eng. J. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Misurcova L. Seaweed digestibility and methods used for digestibility determination. In: Kim S.K., editor. Handbook of Marine Macroalgae, Biotechnology and Applied Phycology. Wiley-Blackwell; 2012. pp. 285–301. [Google Scholar]
- 21.Morais A.H., Silvestre M.P.C., Silveira J.N., Silva C.S., Silva V.D., Silva M.R. Action of a pancreatin and an Aspergillus oryzae protease on whey proteins: correlation among the methods of analysis of the enzymatic hydrolysates. Braz. Arch. Biol. Tech. 2013;56(6):985–995. [Google Scholar]
- 22.Morris H.J., Almarales A., Carrillo O., Mermudez R.C. Utilisation of Chlorella vulgaris cell biomass for the production of enzymatic protein hydrolysates. Bioresour. Tech. 2008;99:7723–7729. doi: 10.1016/j.biortech.2008.01.080. [DOI] [PubMed] [Google Scholar]
- 23.Morris H.J., Carrillo O., Almarales A., Bermudez R.C., Lebequee Y., Fontaine R., Llaurad G., Beltran Y. Immunostimulant activity of an enzymatic protein hydrolysate from green microalga Chlorella vulgaris on undernourished mice. Enzyme Microb. Tech. 2007;40:456–460. [Google Scholar]
- 24.Morris H.J., Carrillo O.V., Alonso M., Bermudez R.C., Almarales A., Llaurado G., Lebequee Y., Fontaine R. Oral Administration of an enzymatic protein hydrolysate from the green microalga Chlorella vulgaris enhances the nutritional recovery of malnourished mice. J. Med. Food. 2011:1–7. doi: 10.1089/jmf.2010.0283. [DOI] [PubMed] [Google Scholar]
- 25.Murdock J.N., Wetzel D.L. FT–IR microspectroscopy enhances biological and ecological analysis of algae. Appl. Spectrosc. Rev. 2009;44(4):335–361. 26. [Google Scholar]
- 26.Nasirpour A., Scher J., Desorby S. Baby foods: formulations and interactions (a review) Crit. Rev. Food Sci. 2007;46(8):665–681. doi: 10.1080/10408390500511896. [DOI] [PubMed] [Google Scholar]
- 27.Nongonierma A.B., Fitzgerald R.J. Dipeptidyl peptidase IV inhibitory and antioxidative properties of milk protein-derived dipeptides and hydrolysates. Peptides. 2013;39:157–163. doi: 10.1016/j.peptides.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 28.O'Keeffe M.B., Fitzgerald R.J. Antioxidant effects of enzymatic hydrolysates of whey protein concentrate on cultured human endothelial cells. Int. Dairy J. 2014;36:128–135. [Google Scholar]
- 29.Oncel S., Kose A. Comparison of tubular and panel type photobioreactors for biohydrogen production utilizing Chlamydomonas reinhardtii considering mixing time and light intensity. Bioresour. Technol. 2014;151:265–270. doi: 10.1016/j.biortech.2013.10.076. [DOI] [PubMed] [Google Scholar]
- 30.Oncel S. Microalgae for a macro energy World. Renew. Sust. Energ. Rev. 2013;26:241–264. [Google Scholar]
- 31.Otte J., Shalabya S.M., Zakora M., Prippa A.H., El-Shabrawy S.A. Angiotensin-converting enzyme inhibitory activity of milk protein hydrolysates: effect of substrate, enzyme and time of hydrolysis. Int. Dairy J. 2007;17:488–503. [Google Scholar]
- 32.Perez-Garcia O., Escalante F.M.E., de-Bashan L.E., Bashan Y. Heterotrophic cultures of microalgae: metabolism and potential products. Water Res. 2011;45:11–36. doi: 10.1016/j.watres.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 33.Pulz O., Gross W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004;65:635–648. doi: 10.1007/s00253-004-1647-x. [DOI] [PubMed] [Google Scholar]
- 34.Safi C., Zebib B., Merah O., Pontalier P.Y., Vaca-Garcia C. Morphology, composition, production, processing and applications of Chlorella vulgaris: a review. Renew. Sust. Energ. Rev. 2014;35:265–278. [Google Scholar]
- 35.Samarakoon K.W., Ko J.Y., Shah M.R., Lee J.H., Kang M.C., Nam K.O., Lee J.B., Jeon Y.J. In vitro studies of anti-inflammatory and anticancer activities of organic solvent extracts from cultured marine microalgae. Algae. 2013;28(1):111–119. [Google Scholar]
- 36.Samek D., Misurcova L., Machu L., Ambrozova J.V., Fisera M. Enzymatic and mechanical disruption method of algal cellulotic cell valls as a factor of influencing their in vitro digestibility. Potravinarstvo. 2013;7 [Google Scholar]
- 37.Yang C., Hua Q., Shimizu K. Integration of the information from gene expression and metabolic fluxes for the analysis of the regulatory mechanisms in Synechocystis. Appl. Microbiol. Biotechnol. 2002;58:813–822. doi: 10.1007/s00253-002-0949-0. [DOI] [PubMed] [Google Scholar]