Abstract
Objective
The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) has been used extensively for clinical care and in research for patients with Mild Cognitive Impairment and Alzheimer’s disease (AD), however relatively few studies have evaluated the relationship between RBANS performance and AD imaging biomarkers. The purpose of the current study was to evaluate the association between a relatively new amyloid positron emission tomography imaging biomarker and performance on the RBANS.
Methods
Twenty-seven non-demented community-dwelling adults over the age of 65 underwent 18F-Flutemetamol amyloid- positron emission tomography imaging, along with cognitive testing using the RBANS and select behavioral measures. Partial correlation coefficients were used to identify relationships between the imaging and behavioral markers.
Results
After controlling for age and education, amyloid deposition and RBANS Indexes of Immediate Memory, Delayed Memory, and Total Scale score were significantly correlated (p’s <.001, r’s =−0.73 to −0.77, d’s =2.13–2.39), with greater amyloid burden being associated with lower RBANS scores. The Delayed Memory Index was particularly highly associated with 18F-Flutemetamol binding (r2 =.59, p <.001, d =2.39). Neither 18F-Flutemetamol binding nor RBANS performance was significantly correlated with levels of depression, subjective cognitive difficulties, or premorbid intellect.
Conclusions
Because of the limited use of amyloid imaging in clinical settings due to high cost and lack of reimbursement, these findings suggest that in particular RBANS Delayed Memory Index may be a cost-efficient tool to identify early signs of AD pathology, and its use may enlighten clinical decision making regarding potential progression to dementia due to AD.
INTRODUCTION
The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS)(Randolph, Tierney, Mohr, & Chase, 1998) has been used extensively since its creation almost two decades ago to assess cognition throughout clinical geriatric populations. Numerous research studies have been conducted regarding the ability of the measure to identify cognitive change in individuals with normal cognition (Andreotti & Hawkins, 2015; Cooley et al., 2015; Duff & Ramezani, 2015; Phillips et al., 2015; Thaler, Hill, Duff, Mold, & Scott, 2015), Mild Cognitive Impairment (MCI; Clark, Hobson, & O’Bryant, 2010; Duff, Hobson, Beglinger, & O’Bryant, 2010; Hobson, Hall, Humphreys-Clark, Schrimsher, & O’Bryant, 2010; Karantzoulis, Novitski, Gold, & Randolph, 2013; O’Mahar et al., 2012), and Alzheimer’s disease (AD; Burton, Enright, O’Connell, Lanting, & Morgan, 2015; Duff et al., 2008; Enright, O’Connell, MacKinnon, & Morgan, 2015; Heyanka, Scott, & Adams, 2015; McDermott & DeFilippis, 2010; Morgan, Linck, Scott, Adams, & Mold, 2010; Schmitt et al., 2010). Despite these efforts, however, there have been few studies examining the RBANS and its association with imaging biomarkers related to AD, such as decreased hippocampal volumes on magnetic resonance imaging (MRI; Choi et al., 2016; Jack et al., 2010), hypometabolism on fluoro-2-deoxyglucose (FDG) positron emission tomography (PET; Frings, Spehl, Hull, & Meyer, 2016; Jagust et al., 2010), and amyloid accumulations on amyloid PET (Beach, Thal, Zanette, Smith, & Buckley, 2016; L. Wu et al., 2012).
Currently, only a handful of studies have examined the relationship between RBANS performance and structural brain imaging through the use of MRI. Reduced RBANS Delayed Memory Index scores have consistently been associated with decreased hippocampal/medial temporal lobe volumes in both healthy older adults (Jiang et al., 2014) and individuals with MCI (England, Gillis, & Hampstead, 2014). The RBANS Total Scale Index has also been positively associated with total brain volume in non-clinical older adults (Paul et al., 2011). Lower RBANS performance has been shown to be associated with shorter white matter fiber bundle length in patients with MCI and high levels of cognitive reserve (L. M. Baker et al., 2016), as well as with greater white matter hyperintensities in patients with cerebral small vessel disease (J. G. Baker et al., 2012).
Even fewer studies have examined RBANS performance using non-MRI-based brain imaging. Baker and colleagues (2012) found that lower RBANS performances were associated with greater hypoperfusion on single-photon emission computed tomography (SPECT) imaging in their patients with small vessel disease. Forster and colleagues (2010) observed that performance on the RBANS Figure Copy test correlated with FDG uptake in the ventral visual stream and areas of higher-level visual processing for patients with AD. Additionally, reduced temporal lobe perfusion was associated with worse immediate memory performance on the RBANS using arterial spin labeling on MRI in healthy adults (Alosco et al., 2013). Finally, only one study to date has evaluated performance on the RBANS with amyloid-PET imaging modalities. Duff et al. (2013) identified that worse performances on RBANS Indexes of Delayed Memory, Visuospatial/Constructional, and Language were associated with increased amyloid burden using 18F-Flutemetamol-PET in non-demented participants (healthy controls and MCI), and this relationship was further strengthened when factoring in premorbid intellectual functioning.
While previous findings have shown an association between RBANS performance and various AD imaging biomarkers, these studies are relatively few in number and only one study to date has examined the relationship with amyloid-PET. With the increased emphasis of amyloid assessment in AD research, attempting to replicate previous findings is necessary to better characterize and validate the relationship between RBANS performance and amyloid deposition/accumulation. This is particularly true since at the time of the Duff (2013) study, 18F-Flutemetamol was still an experimental ligand; since that time, 18F-Flutemetamol has received Federal Drug Administration (FDA) approval (Vizamyl; GE Healthcare, Wauwatosa, WI) and the methodology for uptake quantification has undergone much greater scientific rigor than what was used previously. As a result, replication will be important to limit the potential for spurious findings based on a single study. Our hypothesis was that there would be a significant and negative relationship between RBANS Indexes and 18F-Flutemetamol uptake, particularly the Delayed Memory Index, such that worse RBANS performances were expected to be observed in non-demented individuals that possessed a greater degree of amyloid burden.
METHOD
Sample and design
Twenty-seven non-demented older adults over the age of 65 were recruited from either a cognitive disorders clinic or through community presentations on brain health at independent living facilities and senior centers. Exclusion criteria included an ICD-10 diagnosis of dementia; medical comorbidities likely to affect cognition (e.g., history of major neurological disorders including strokes, brain lesions, head injury with loss of consciousness >30 minutes, radiation therapy to the brain, major psychiatric disorders [schizophrenia, bipolar disorder, severe major depressive disorder], or substance abuse); and use of anticonvulsant, antipsychotic, cognitive-enhancing, or cholinesterase-inhibiting medications. Of note, 19 of our 27 participants were also included in the Duff et al. (2013) study, though the results presented in the current study reflect unique and updated cognitive data and 18F-Flutemetamol amyloid-PET scans. This study followed an observational design, and sampling procedures were based on convenience sampling.
Neuropsychological and Behavioral Self-Report Measures
All participants were administered the following neuropsychological and behavioral tests:
The RBANS (Randolph et al., 1998) is a neuropsychological test battery comprising 12 subtests that are used to calculate index scores for domains of immediate memory, visuospatial/constructional, attention, language, delayed memory, and global neuropsychological functioning. Administration time for the RBANS is approximately 25–30 minutes. The index scores utilize age-corrected normative comparisons to generate standard scores (SS; M = 100, SD = 15), with higher scores indicating better cognition.
The Wide Range Achievement Test 4- Reading subtest (WRAT; Wilkinson, 2006) was used as an estimate of premorbid intellectual functioning. In this task, participants were presented a form that lists 55 irregular words and were asked to accurately pronounce each word out loud. The number of correct responses was summed and then normative comparisons were utilized to create age-matched standard scores (SS; M = 100, SD = 15), with higher scores indicating higher premorbid intellect.
The Geriatric Depression Scale (GDS; Yesavage et al., 1982) is a 30-item self-report questionnaire related to current depressive symptomology. Participants were asked to respond to questions in a ‘YES/NO’ format that best fit how they felt over the past week (e.g., “Are you basically satisfied with your life?”). Each positive endorsement of depressive symptoms was worth one point, for a range of scores from 0–30. Higher scores indicated more severe depression, with severity ranges being 0–9 for normal mood, 10–19 for moderate depression, and 20–30 for severe depression (Yesavage et al., 1982). This measure has been specifically validated for geriatric populations.
The Cognitive Failures Questionnaire (CFQ; Broadbent, Cooper, FitzGerald, & Parkes, 1982) is a 25-item self-report questionnaire related to current perceptions of cognitive functioning. Participants were asked to indicate the frequency that they experience commonly occurring events related to memory loss (e.g., “Do you find you forget appointments?”) using a scale of 0 (Never) to 4 (Very Often). Total scores ranged from 0–100, with higher scores indicating greater subjective complaints of cognitive functioning.
Amyloid Imaging
Participants received 18F-Flutemetamol imaging as described previously (Duff et al., 2013). 18F-Flutemetamol is a radioactive diagnostic agent indicated for PET imaging of the brain to estimate beta-amyloid neuritic plaque density in adult patients with cognitive impairment. In the current study, 18F-Flutemetamol was produced under PET cGMP standards and the studies were conducted under an approved FDA Investigational New Drug application. Imaging was performed 90 minutes after the injection of 185 mBq (5 mCi) of 18F-Flutemetamol. Emission imaging time was approximately 30 minutes. A GE ST PET/CT scanner (GE Healthcare) was used for 18F-Flutemetamol imaging in this study, which possessed the full width at half maximum spatial resolution at 5.0 mm. The field of view for reconstruction was set to 25.6 cm on the scanner to generate a pixel size of 2.0 mm X 2.0 mm (image matrix size 128 X 128). The native slice thickness was 3.27 mm. Volumes of interest were automatically generated by the CortexID Suite analysis software (GE Healthcare) with Z axis dimensions substantially larger than the slice thickness. 18F-Flutemetamol binding was analyzed using a regional semi-quantitative technique described by Vandenberghe et al. (2010) and refined by Thurfjell et al. (2014). In this technique, semi-quantitative regional (prefrontal, anterior cingulate, precuneus/posterior cingulate, parietal, mesial temporal, lateral temporal, occipital, sensorimotor, cerebellar grey matter, and whole cerebellum) and global composite standardized uptake value ratios (SUVRs) in the cerebral cortex were generated automatically and normalized to the pons using the CortexID Suite software (Lundqvist et al., 2013). For 18F-Flutemetamol amyloid imaging, there is no specific age-related “normal” level of binding in the CortexID Suite database to assess age-matched normality. Therefore, the study images were compared to the intrinsic software database control group as a whole to calculate the z scores (M = 0, SD = 1) compared to clinically negative amyloid scans.
Procedure
All procedures were approved by the local Institutional Review Board before the study commenced. Participants provided informed consent before completing any procedures. As part of a larger study, participants completed a neuropsychological test battery to characterize their performances based on relevant cognitive domains. Participants performing below expectation on cognitive tasks based on premorbid estimates were classified as MCI (Winblad et al., 2004) and participants performing within or beyond expectation were classified as having normal cognition. The result of this classification procedure was that MCI participants were specifically of the MCI-Amnestic (single or multi-domain) subtype. Specifically, participants classified with MCI possessed a delayed-memory-to-premorbid-intelligence-discrepancy of 2.1SD based on a mean delayed memory composite of SS = 76.8 (SD 17.7) compared to a mean premorbid intellectual functioning of SS = 108.6 (SD 12.8), and participants classified as having normal cognition possessed a discrepancy of 0.1SD based on a mean delayed memory composite of SS = 112.0 (SD 7.5) compared to a mean premorbid intellectual functioning of SS = 111.2 (SD 4.6). All participants were additionally administered the RBANS, WRAT, CFQ, and GDS, for a total time of approximately 60 minutes of cognitive testing. All tests were administered using standard protocol and scored/normed based on their respective protocols. Participants were invited back for a separate appointment and completed 18F-Flutemetamol-PET imaging an average of 19.5 weeks (SD = 5.75) after completing the neuropsychological tests.
Data Analysis
For demographic analyses, continuous neuropsychological and behavioral self-report variables were compared based on diagnostic group categorization using analyses of variance (ANOVA). For categorical demographic variables (e.g. gender), Chi-Square analyses were calculated using diagnostic group as the independent variable. For the primary analyses, partial correlation coefficients were calculated comparing global composite of cerebral cortex SUVRs and age-corrected cognitive performances on RBANS, premorbid intellectual functioning, depression, and subjective memory complaints, controlling for age and education. Bonferroni corrections were utilized to counteract the impact of multiple comparisons and type I error on all primary analyses, resulting in an alpha of .006. Measures of effect size were expressed as Cohen’s d values.
RESULTS
Demographics
Of the 27 participants, 18 were classified as having MCI, and 9 were classified as having normal cognition. Eight participants were recruited from the clinic (100% classified with MCI), and 19 were recruited from the community (52% classified with MCI). Table 1 displays demographic, neuropsychological, and behavioral self-report data. There were no significant differences among the MCI and normal cognition groups with respect to age, education, gender, or premorbid intellectual functioning (all p’s > .49) with the mean age being 77.5 year old (SD = 6.37; range 66–87), mean education being 16.26 years (SD = 2.98; range 12–22), and 74% of the sample being female. The mean premorbid intellectual functioning was at the upper limit of the average range (M = 109.4, SD = 10.73). The MCI group performed worse than the normal cognition group on RBANS Indexes of Immediate Memory, F (1, 26) = 8.00, p = .009, d = 1.05, Language, F (1, 26) = 12.21, p = .002, d = 1.23, Delayed Memory, F (1, 26) = 7.12, p = .013, d = 1.01, and RBANS Total Score, F (1, 26) = 8.15, p = .009, d = 1.06. There were no differences on RBANS indices of Visuospatial/Constructional, F (1, 26) = 1.74, p = .20, or Attention, F (1, 26) = 2.69, p = .11, and no differences existed between diagnostic groups on levels of depression, F (1, 26) = 0.42, p = .52, or subjective report of cognitive difficulties, F (1, 26) = 0.07, p = .80.
Table 1.
Demographic, neuropsychological, and behavioral variables across the total sample (N = 27) and among the participants with normal cognition (n = 9) and Mild Cognitive Impairment (n = 18)
| Variable | Total Sample | Normal Cognition | Mild Cognitive Impairment |
|---|---|---|---|
| N | 27 | 9 | 18 |
| Age | 77.5 (6.4) | 77.9 (6.5) | 77.3 (6.5) |
| Education | 16.3 (3.0) | 17.2 (3.4) | 15.8 (2.7) |
| Gender (percent female) | 74% | 78% | 72% |
| GDS | 3.1 (3.8) | 2.2 (2.4) | 3.6 (4.3) |
| CFQ | 34.4 (11.7) | 34.3 (7.8) | 34.4 (13.5) |
| WRAT | 109.4 (10.7) | 111.2 (4.6) | 108.6 (12.3) |
| RBANS | |||
| Immediate Memory | 95.7 (22.1) | 113.3 (8.4) | 87.9 (21.5) |
| Visuospatial/Constructional | 95.5 (19.2) | 104.3 (17.2) | 91.1 (19.0) |
| Language | 98.1 (11.7) | 107.2 (9.1) | 93.6 (10.2) |
| Attention | 103.3 (20.1) | 113.3 (13.9) | 98.2 (21.2) |
| Delayed Memory | 91.9 (26.7) | 110.7 (7.8) | 82.4 (27.8) |
| Total Scale | 97.1 (23.4) | 115.0 (14.9) | 88.2 (21.5) |
Note: GDS = Geriatric Depression Scale, CFQ = Cognitive Failures Questionnaire, WRAT = Wide Range Achievement Test – 4, Reading Subtest, RBANS = Repeatable Battery for the Assessment of Neuropsychological Status. WRAT and RBANS mean scores listed as Standard Scores. Data presented as Mean (Standard Deviation).
Primary Analysis of the Correlations between Neuropsychological and Self-Report Measures and 18F-Flutemetamol Imaging
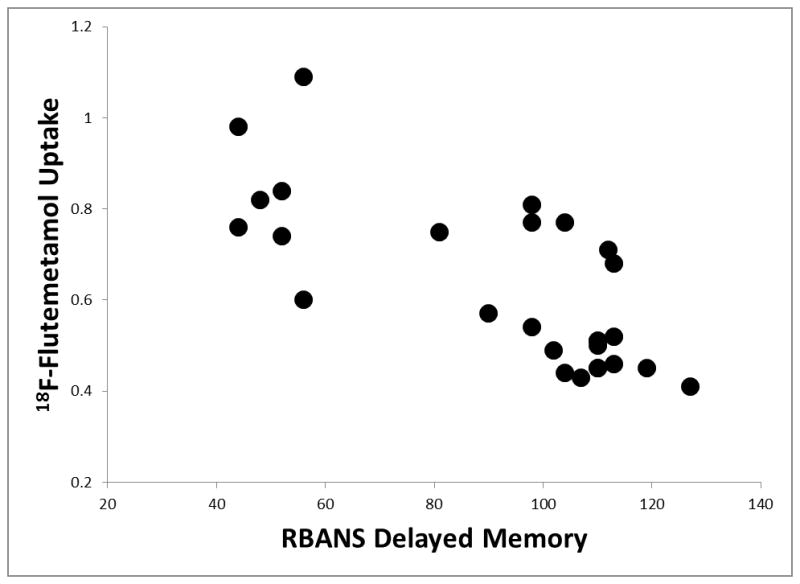
Significant partial correlations existed between amyloid deposition using 18F-Flutemetamol imaging and several of the RBANS Indexes when pooling the MCI and normal cognition participants, controlling for age and education. Please see Table 2. Specifically, significant negative correlations existed between 18F-Flutemetamol binding and RBANS Indexes of Immediate Memory, r = −0.76, p < .001, d = 2.32 (see Figure 1), Delayed Memory, r = −0.77, p < .001, d = 2.39 (see Figure 2), and Total Scale score, r = −0.73, p < .001, d = 2.13, suggesting that cognitive performance was significantly worse in those participants with higher 18F-Flutemetamol uptake. While trends were present, no significant associations were observed between 18F-Flutemetamol binding and the RBANS Indexes of Visuospatial/Constructional, r = −0.46, p = .020, Language, r = −0.53, p = .007, d = 1.23, or Attention, r = −0.44, p = .030, when using strict Bonferroni family-wise comparisons. Additionally, no association between 18F-Flutemetamol binding and an estimate of premorbid intellectual functioning, r = −0.18, p = .40, was observed. Interestingly, while 18F-Flutemetamol binding was not significantly correlated with depression levels, r = 0.01, p = .96, or subjective report of cognitive difficulties, r = 0.02, p = .93, these two behavioral measures were significantly positively correlated with each other, r = 0.54, p = .004, d = 1.28, such that more depression was associated with more cognitive complaints. Similarly, depression and subjective report of cognitive difficulties were not significantly correlated with any of the RBANS Indexes (e.g., r = 0.17, p = .41 for Delayed Memory and depression, and r = 0.08, p = .71 for Delayed Memory and subjective cognitive difficulties).
Table 2.
Partial correlations between 18F-Flutemetamol binding and RBANS or behavioral variables across the total sample (N = 27)
| Measure | r | Significance | Effect Size |
|---|---|---|---|
| WRAT | −0.18 | p = 0.40 | 0.37 |
| RBANS | |||
| Immediate Memory | −0.76* | p < 0.001 | 2.32 |
| Visual Construction | −0.46 | p = 0.02 | 1.04 |
| Language | −0.53 | p = 0.007 | 1.23 |
| Attention | −0.44 | p = 0.030 | 0.97 |
| Delayed Memory | −0.77* | p < 0.001 | 2.39 |
| Total Score | −0.73* | p < 0.001 | 2.13 |
| GDS | 0.01 | p = 0.96 | 0.02 |
| CFQ | 0.02 | p = 0.93 | 0.04 |
Note: WRAT = Wide Range Achievement Test – IV, Reading Subtest, RBANS = Repeatable Battery for the Assessment of Neuropsychological Status, GDS = Geriatric Depression Scale, CFQ = Cognitive Failures Questionnaire. Effect Sizes were measured using Cohen’s d.
Denotes significant correlation with 18F-Flutemetamol binding.
Figure 1.
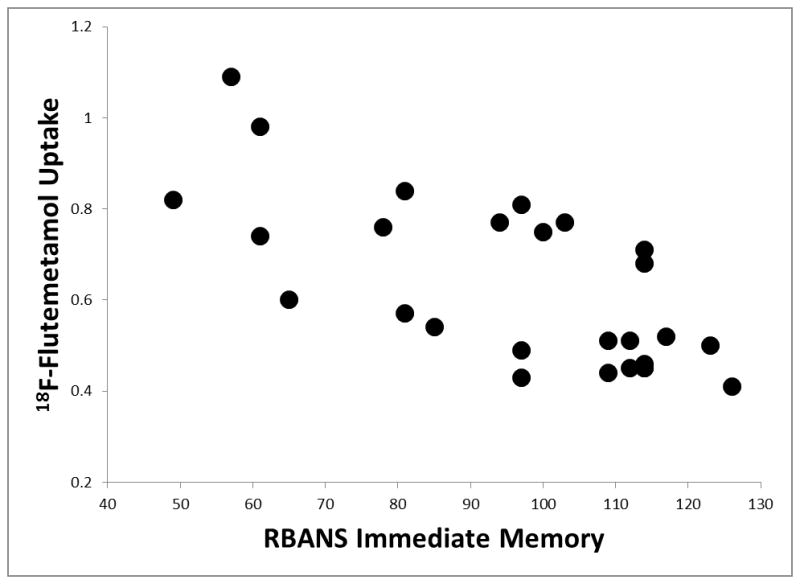
Figure 2.
DISCUSSION
Although research has suggested an association between cognitive performance and AD imaging biomarkers (Jack et al., 2013), there exists a paucity of research specifically examining cognitive functioning using the RBANS (Randolph et al., 1998) and amyloid deposition/accumulation. The results of the current study suggest that amyloid deposition using 18F-Flutemetamol imaging was negatively correlated with RBANS Indexes of Immediate Memory, Delayed Memory, and Total Scale score, meaning that participants with higher levels of cerebral amyloid deposition performed worse across cognitive tasks of memory on the RBANS. Although trends existed for tasks of visuospatial, language, and attention skills, these negative correlations did not remain significant after using a strict correction for multiple comparisons. These results are consistent with prior studies using MRI (England et al., 2014; Jiang et al., 2014), arterial spin labeling (Alosco et al., 2013), FDG-PET (Forster et al., 2010) and SPECT (J. G. Baker et al., 2012) showing poor RBANS performances being associated with more brain pathology. Similarly, our current results are consistent with Duff et al. (2013), with greater amyloid burden being correlated with poorer RBANS scores. This association between amyloid accumulation and cognition is especially relevant because amyloid deposition is proposed to occur early in the development of AD pathology, suggesting that poor RBANS performance may reflect early AD pathology.
The magnitude of the relationship observed between 18F-Flutemetamol uptake and RBANS performance in the current study appears to be clinically meaningful as well. For example, the Delayed Memory Index and 18F-Flutemetamol uptake shared approximately 59% of their variance. This is notably higher than relationships between RBANS performance and other imaging modalities (e.g., 7–16% variance between MRI whole brain volume or white matter fiber bundle length and Total Scale score, respectively (L. M. Baker et al., 2016; Paul et al., 2011), and 17–29% variance between MRI hippocampal volumes and Delayed Memory Index (England et al., 2014; Jiang et al., 2014)). Similarly, memory and other demographic variables have tended to share smaller relationships (13–16% variance for education and age, respectively (Nyberg, Backman, Erngrund, Olofsson, & Nilsson, 1996)). The magnitude of the variance observed in our study suggests that the Delayed Memory Index from the RBANS appears to be particularly sensitive to amyloid deposition, compared to the smaller variances observed for the other non-memory RBANS Indexes (19–27%). As such, in older patients presenting with a memory problem, a low performance on the RBANS Delayed Memory Index may lead the clinician to suspect that AD pathology may be present after common comorbidities are excluded. Since the high cost of amyloid-PET imaging and constraints related to its appropriate use (Johnson et al., 2013) have led to it being either cost prohibitive or restricted in many clinical settings, these results suggest that RBANS Delayed Memory Index may be a cost-efficient tool to identify early signs of AD pathology, and its use may enlighten clinical decision making regarding potential progression to dementia due to AD.
Whereas Duff et al. (2013) observed that the relationship between 18F-Flutemetamol uptake and cognition was mediated by premorbid intellectual functioning, such that higher premorbid IQ led to a stronger positive relationship, the current study found no association between premorbid intellect and amyloid burden. This discrepancy can likely be accounted for by differences in the severity of cognitive difficulty between the two sample populations. Duff et al. (2013) utilized participants with memory abilities firmly in the average range (SS = 103.5), while the current sample tended to display lower memory skills (SS = 91.9; see Table 1). Although cognitive reserve is theorized to reduce susceptibility to cognitive decline (Stern, 2002) and possible dementia (Rentz et al., 2010; Stern, 2006), there exists a limit to reserve’s protective effects. As such, our current study’s more cognitively compromised sample may have possessed more individuals whose memory difficulties were beyond the threshold of receiving a benefit from cognitive reserve, thus reducing the mediational effect observed in Duff’s study.
The lack of association between either RBANS Index scores or 18F-Flutemetamol binding and subjective memory experience observed in our study is consistent with other findings in the literature, including studies examining these relationships among the RBANS specifically (Fyock & Hampstead, 2015), episodic memory (Zwan et al., 2015), or in vivo amyloid burden (Amariglio et al., 2012; Snitz et al., 2015). Similarly, our findings of no association between RBANS Index scores or amyloid burden and depression is consistent with the lack of relationship consistently observed between RBANS Index scores and depression (Andreotti & Hawkins, 2015; Han et al., 2015) or amyloid burden and level of depression (Madsen et al., 2012; Pietrzak et al., 2015; K. Y. Wu et al., 2014) in the literature.
As 19 of our participants were also included in the Duff et al. (2013) study, we were able to conduct a supplementary assessment of the relationship between the change in cognition between 2011 and 2015 and the degree of current 18F-Flutemetamol uptake and subsequent amyloid pathology. In order to appropriately quantify change over time on the RBANS and to control for the effects of practice due to repeated exposure of test materials, complex standardized regression-based (SRB) prediction formulas were implemented from a prior study that established normative standards for practice effects on RBANS performance with repetition over one year (Duff et al., 2004). A modest, but non-significant negative relationship was observed in the correlation between the change over time in the Delayed Memory Index and the degree of 18F-Flutemetamol uptake, r = −.28, p = .24. While the small sample size of this group receiving a repeated RBANS administration may have limited our ability to detect significance in this relationship, these results are suggestive of a trend towards declining RBANS performance being associated with increased 18F-Flutemetamol uptake and subsequent amyloid pathology.
The current study is not without limitations. First, although our sample size is generally comparable to other studies assessing amyloid PET imaging and either cognition (Duff et al., 2013) or depression (Butters et al., 2008; Kumar et al., 2011; Madsen et al., 2012; K. Y. Wu et al., 2014), future research on this topic using larger samples is encouraged to aid in generalizability of the findings. However, the elevated cost of 18F-Flutemetamol imaging may be a barrier in many studies. Second, while our results coincide with literature suggesting that depression may not be a major risk factor for amyloid burden in later life, a more thorough examination of 18F-Flutemetamol uptake and depression should be undertaken with participants possessing a wider range of depression scores before any definitive conclusions can be made. Third, it is unclear if the current results would be similarly observed in a study utilizing more heterogeneous participants in regards to race, education, and health. Finally, while the RBANS is a common clinical tool, it fails to assess cognitive domains related to executive functioning, which are known to be affected in older adults with cognitive decline or neurodegenerative disease. Consequently, future studies should be undertaken to examine this relationship using measures more sensitive to executive dysfunction.
Despite these limitations, the current study is noteworthy in showing that non-demented community-dwelling adults with higher levels of cerebral amyloid deposition assessed with 18F-Flutemetamol performed worse on cognitive tasks of memory on the RBANS, regardless of their premorbid intellectual skills or endorsements of depression. Given how early in the development of AD pathology amyloid accumulation is proposed to occur, these results suggest that poor RBANS performance may reflect early signs of AD pathology.
References
- Alosco ML, Gunstad J, Jerskey BA, Xu X, Clark US, Hassenstab J, … Sweet LH. The adverse effects of reduced cerebral perfusion on cognition and brain structure in older adults with cardiovascular disease. Brain Behav. 2013;3(6):626–636. doi: 10.1002/brb3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amariglio RE, Becker JA, Carmasin J, Wadsworth LP, Lorius N, Sullivan C, … Rentz DM. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50(12):2880–2886. doi: 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreotti C, Hawkins KA. RBANS Norms based on the Relationship of Age, Gender, Education, and WRAT-3 Reading to Performance within an Older African American Sample. Clin Neuropsychol. 2015;29(4):442–465. doi: 10.1080/13854046.2015.1039589. [DOI] [PubMed] [Google Scholar]
- Baker JG, Williams AJ, Ionita CC, Lee-Kwen P, Ching M, Miletich RS. Cerebral small vessel disease: cognition, mood, daily functioning, and imaging findings from a small pilot sample. Dement Geriatr Cogn Dis Extra. 2012;2:169–179. doi: 10.1159/000333482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LM, Laidlaw DH, Cabeen R, Akbudak E, Conturo TE, Correia S, … Paul RH. Cognitive reserve moderates the relationship between neuropsychological performance and white matter fiber bundle length in healthy older adults. Brain Imaging Behav. 2016 doi: 10.1007/s11682-016-9540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, Thal DR, Zanette M, Smith A, Buckley C. Detection of Striatal Amyloid Plaques with [18F]flutemetamol: Validation with Postmortem Histopathology. J Alzheimers Dis. 2016 doi: 10.3233/JAD-150732. [DOI] [PubMed] [Google Scholar]
- Broadbent DE, Cooper PF, FitzGerald P, Parkes KR. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol. 1982;21(Pt 1):1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- Burton RL, Enright J, O’Connell ME, Lanting S, Morgan D. RBANS embedded measures of suboptimal effort in dementia: effort scale has a lower failure rate than the effort index. Arch Clin Neuropsychol. 2015;30(1):1–6. doi: 10.1093/arclin/acu070. [DOI] [PubMed] [Google Scholar]
- Butters MA, Klunk WE, Mathis CA, Price JC, Ziolko SK, Hoge JA, … Meltzer CC. Imaging Alzheimer pathology in late-life depression with PET and Pittsburgh Compound-B. Alzheimer Dis Assoc Disord. 2008;22(3):261–268. doi: 10.1097/WAD.0b013e31816c92bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi MH, Kim HS, Gim SY, Kim WR, Mun KR, Tack GR, … Chung SC. Differences in cognitive ability and hippocampal volume between Alzheimer’s disease, amnestic mild cognitive impairment, and healthy control groups, and their correlation. Neurosci Lett. 2016 doi: 10.1016/j.neulet.2016.03.044. [DOI] [PubMed] [Google Scholar]
- Clark JH, Hobson VL, O’Bryant SE. Diagnostic accuracy of percent retention scores on RBANS verbal memory subtests for the diagnosis of Alzheimer’s disease and mild cognitive impairment. Arch Clin Neuropsychol. 2010;25(4):318–326. doi: 10.1093/arclin/acq023. [DOI] [PubMed] [Google Scholar]
- Cooley SA, Heaps JM, Bolzenius JD, Salminen LE, Baker LM, Scott SE, Paul RH. Longitudinal Change in Performance on the Montreal Cognitive Assessment in Older Adults. Clin Neuropsychol. 2015;29(6):824–835. doi: 10.1080/13854046.2015.1087596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Foster NL, Dennett K, Hammers DB, Zollinger LV, Christian PE, … Hoffman JM. Amyloid deposition and cognition in older adults: the effects of premorbid intellect. Arch Clin Neuropsychol. 2013;28(7):665–671. doi: 10.1093/arclin/act047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Hobson VL, Beglinger LJ, O’Bryant SE. Diagnostic accuracy of the RBANS in mild cognitive impairment: limitations on assessing milder impairments. Arch Clin Neuropsychol. 2010;25(5):429–441. doi: 10.1093/arclin/acq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Humphreys Clark JD, O’Bryant SE, Mold JW, Schiffer RB, Sutker PB. Utility of the RBANS in detecting cognitive impairment associated with Alzheimer’s disease: sensitivity, specificity, and positive and negative predictive powers. Arch Clin Neuropsychol. 2008;23(5):603–612. doi: 10.1016/j.acn.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Ramezani A. Regression-Based Normative Formulae for the Repeatable Battery for the Assessment of Neuropsychological Status for Older Adults. Arch Clin Neuropsychol. 2015;30(7):600–604. doi: 10.1093/arclin/acv052. [DOI] [PubMed] [Google Scholar]
- Duff K, Schoenberg MR, Patton D, Mold J, Scott JG, Adams RL. Predicting change with the RBANS in a community dwelling elderly sample. J Int Neuropsychol Soc. 2004;10(6):828–834. doi: 10.1017/s1355617704106048. [DOI] [PubMed] [Google Scholar]
- England HB, Gillis MM, Hampstead BM. RBANS memory indices are related to medial temporal lobe volumetrics in healthy older adults and those with mild cognitive impairment. Arch Clin Neuropsychol. 2014;29(4):322–328. doi: 10.1093/arclin/acu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enright J, O’Connell ME, MacKinnon S, Morgan DG. Predictors of Completion of Executive-Functioning Tasks in a Memory Clinic Dementia Sample. Appl Neuropsychol Adult. 2015;22(6):459–464. doi: 10.1080/23279095.2014.992070. [DOI] [PubMed] [Google Scholar]
- Forster S, Teipel S, Zach C, Rominger A, Cumming P, Fougere C, … Burger K. FDG-PET mapping the brain substrates of visuo-constructive processing in Alzheimer’s disease. J Psychiatr Res. 2010;44(7):462–469. doi: 10.1016/j.jpsychires.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Frings L, Spehl TS, Hull M, Meyer PT. Left Anterior Temporal Glucose Metabolism and not Amyloid-beta Load Predicts Naming Impairment in Alzheimer’s Disease. Curr Alzheimer Res. 2016 doi: 10.2174/1567205013666160322141955. [DOI] [PubMed] [Google Scholar]
- Fyock CA, Hampstead BM. Comparing the relationship between subjective memory complaints, objective memory performance, and medial temporal lobe volumes in patients with mild cognitive impairment. Alzheimers Dement (Amst) 2015;1(2):242–248. doi: 10.1016/j.dadm.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Page EE, Stewart LM, Deford CC, Scott JG, Schwartz LH, … George JN. Depression and cognitive impairment following recovery from thrombotic thrombocytopenic purpura. Am J Hematol. 2015;90(8):709–714. doi: 10.1002/ajh.24060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyanka DJ, Scott JG, Adams RL. Improving the diagnostic accuracy of the RBANS in mild cognitive impairment with construct-consistent measures. Appl Neuropsychol Adult. 2015;22(1):32–41. doi: 10.1080/23279095.2013.827574. [DOI] [PubMed] [Google Scholar]
- Hobson VL, Hall JR, Humphreys-Clark JD, Schrimsher GW, O’Bryant SE. Identifying functional impairment with scores from the repeatable battery for the assessment of neuropsychological status (RBANS) Int J Geriatr Psychiatry. 2010;25(5):525–530. doi: 10.1002/gps.2382. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr, Bernstein MA, Borowski BJ, Gunter JL, Fox NC, Thompson PM, … Weiner MW. Update on the magnetic resonance imaging core of the Alzheimer’s disease neuroimaging initiative. Alzheimers Dement. 2010;6(3):212–220. doi: 10.1016/j.jalz.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, … Trojanowski JQ. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust WJ, Bandy D, Chen K, Foster NL, Landau SM, Mathis CA, … Koeppe RA. The Alzheimer’s Disease Neuroimaging Initiative positron emission tomography core. Alzheimers Dement. 2010;6(3):221–229. doi: 10.1016/j.jalz.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Cheng Y, Li Q, Tang Y, Shen Y, Li T, … Li C. Cross-sectional study of the association of cognitive function and hippocampal volume among healthy elderly adults. Shanghai Arch Psychiatry. 2014;26(5):280–287. doi: 10.11919/j.issn.1002-0829.214036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Minoshima S, Bohnen NI, Donohoe KJ, Foster NL, Herscovitch P, … Thies WH. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. J Nucl Med. 2013;54(3):476–490. doi: 10.2967/jnumed.113.120618. [DOI] [PubMed] [Google Scholar]
- Karantzoulis S, Novitski J, Gold M, Randolph C. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Utility in detection and characterization of mild cognitive impairment due to Alzheimer’s disease. Arch Clin Neuropsychol. 2013;28(8):837–844. doi: 10.1093/arclin/act057. [DOI] [PubMed] [Google Scholar]
- Kumar A, Kepe V, Barrio JR, Siddarth P, Manoukian V, Elderkin-Thompson V, Small GW. Protein binding in patients with late-life depression. Arch Gen Psychiatry. 2011;68(11):1143–1150. doi: 10.1001/archgenpsychiatry.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist R, Lilja J, Thomas BA, Lotjonen J, Villemagne VL, Rowe CC, Thurfjell L. Implementation and validation of an adaptive template registration method for 18F-flutemetamol imaging data. J Nucl Med. 2013;54(8):1472–1478. doi: 10.2967/jnumed.112.115006. [DOI] [PubMed] [Google Scholar]
- Madsen K, Hasselbalch BJ, Frederiksen KS, Haahr ME, Gade A, Law I, … Hasselbalch SG. Lack of association between prior depressive episodes and cerebral [11C]PiB binding. Neurobiol Aging. 2012;33(10):2334–2342. doi: 10.1016/j.neurobiolaging.2011.11.021. [DOI] [PubMed] [Google Scholar]
- McDermott AT, DeFilippis NA. Are the indices of the RBANS sufficient for differentiating Alzheimer’s disease and subcortical vascular dementia? Arch Clin Neuropsychol. 2010;25(4):327–334. doi: 10.1093/arclin/acq028. [DOI] [PubMed] [Google Scholar]
- Morgan DR, Linck J, Scott J, Adams R, Mold J. Assessment of the RBANS Visual and Verbal Indices in a sample of neurologically impaired elderly participants. Clin Neuropsychol. 2010;24(8):1365–1378. doi: 10.1080/13854046.2010.516769. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Backman L, Erngrund K, Olofsson U, Nilsson LG. Age differences in episodic memory, semantic memory, and priming: relationships to demographic, intellectual, and biological factors. J Gerontol B Psychol Sci Soc Sci. 1996;51(4):234–240. doi: 10.1093/geronb/51b.4.p234. [DOI] [PubMed] [Google Scholar]
- O’Mahar KM, Duff K, Scott JG, Linck JF, Adams RL, Mold JW. Brief report: the temporal stability of the Repeatable Battery for the Assessment of Neuropsychological Status Effort Index in geriatric samples. Arch Clin Neuropsychol. 2012;27(1):114–118. doi: 10.1093/arclin/acr072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Lane EM, Tate DF, Heaps J, Romo DM, Akbudak E, … Conturo TE. Neuroimaging signatures and cognitive correlates of the montreal cognitive assessment screen in a nonclinical elderly sample. Arch Clin Neuropsychol. 2011;26(5):454–460. doi: 10.1093/arclin/acr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips R, Qi G, Collinson SL, Ling A, Feng L, Cheung YB, Ng TP. The Minimum Clinically Important Difference in the Repeatable Battery for the Assessment of Neuropsychological Status. Clin Neuropsychol. 2015;29(7):905–923. doi: 10.1080/13854046.2015.1107137. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Lim YY, Neumeister A, Ames D, Ellis KA, Harrington K, … Maruff P. Amyloid-beta, anxiety, and cognitive decline in preclinical Alzheimer disease: a multicenter, prospective cohort study. JAMA Psychiatry. 2015;72(3):284–291. doi: 10.1001/jamapsychiatry.2014.2476. [DOI] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Rentz DM, Locascio JJ, Becker JA, Moran EK, Eng E, Buckner RL, … Johnson KA. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol. 2010;67(3):353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt AL, Livingston RB, Smernoff EN, Reese EM, Hafer DG, Harris JB. Factor analysis of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) in a large sample of patients suspected of dementia. Appl Neuropsychol. 2010;17(1):8–17. doi: 10.1080/09084280903297719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snitz BE, Weissfeld LA, Cohen AD, Lopez OL, Nebes RD, Aizenstein HJ, … Klunk WE. Subjective Cognitive Complaints, Personality and Brain Amyloid-beta in Cognitively Normal Older Adults. Am J Geriatr Psychiatry. 2015;23(9):985–993. doi: 10.1016/j.jagp.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448–460. [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(2):112–117. doi: 10.1097/01.wad.0000213815.20177.19. [DOI] [PubMed] [Google Scholar]
- Thaler NS, Hill BD, Duff K, Mold J, Scott JG. Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) intraindividual variability in older adults: Associations with disease and mortality. J Clin Exp Neuropsychol. 2015;37(6):622–629. doi: 10.1080/13803395.2015.1039962. [DOI] [PubMed] [Google Scholar]
- Thurfjell L, Lilja J, Lundqvist R, Buckley C, Smith A, Vandenberghe R, Sherwin P. Automated quantification of 18F-flutemetamol PET activity for categorizing scans as negative or positive for brain amyloid: concordance with visual image reads. J Nucl Med. 2014;55(10):1623–1628. doi: 10.2967/jnumed.114.142109. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Van Laere K, Ivanoiu A, Salmon E, Bastin C, Triau E, … Brooks DJ. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol. 2010;68(3):319–329. doi: 10.1002/ana.22068. [DOI] [PubMed] [Google Scholar]
- Wilkinson GSR, GJ . WRAT 4: Wide Range Achievement Test: Professional Manual. Psychological Assessment Resources, Incorportated; 2006. [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, … Petersen RC. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Wu KY, Hsiao IT, Chen CS, Chen CH, Hsieh CJ, Wai YY, … Lin KJ. Increased brain amyloid deposition in patients with a lifetime history of major depression: evidenced on 18F-florbetapir (AV-45/Amyvid) positron emission tomography. Eur J Nucl Med Mol Imaging. 2014;41(4):714–722. doi: 10.1007/s00259-013-2627-0. [DOI] [PubMed] [Google Scholar]
- Wu L, Rowley J, Mohades S, Leuzy A, Dauar MT, Shin M, … Rosa-Neto P. Dissociation between brain amyloid deposition and metabolism in early mild cognitive impairment. PLoS One. 2012;7(10):e47905. doi: 10.1371/journal.pone.0047905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Zwan MD, Villemagne VL, Dore V, Buckley R, Bourgeat P, Veljanoski R, … Rowe CC. Subjective Memory Complaints in APOEvarepsilon4 Carriers are Associated with High Amyloid-beta Burden. J Alzheimers Dis. 2015;49(4):1115–1122. doi: 10.3233/JAD-150446. [DOI] [PubMed] [Google Scholar]


