Abstract
Caenorhabditis elegans explores its environment by interrupting its forward movement with occasional turns and reversals. Turns and reversals occur at stable frequencies but irregular intervals, producing probabilistic exploratory behaviors. Here we dissect the roles of individual sensory neurons, interneurons, and motor neurons in exploratory behaviors under different conditions. After animals are removed from bacterial food, they initiate a local search behavior consisting of reversals and deep omega-shaped turns triggered by AWC olfactory neurons, ASK gustatory neurons, and AIB interneurons. Over the following 30 min, the animals disperse as reversals and omega turns are suppressed by ASI gustatory neurons and AIY interneurons. Interneurons and motor neurons downstream of AIB and AIY encode specific aspects of reversal and turn frequency, amplitude, and directionality. SMD motor neurons help encode the steep amplitude of omega turns, RIV motor neurons specify the ventral bias of turns that follow a reversal, and SMB motor neurons set the amplitude of sinusoidal movement. Many of these sensory neurons, interneurons, and motor neurons are also implicated in chemotaxis and thermotaxis. Thus, this circuit may represent a common substrate for multiple navigation behaviors.
Keywords: chemosensation, exploratory behavior, neural circuit
As an animal travels through its environment, its nervous system detects sensory cues, evaluates them based on context and the experience of the animal, and converts this information into adaptive movement. For simple behaviors, sensory neurons sometimes communicate directly with motor neurons, but, in more complex behavioral circuits, several layers of interneurons integrate sensory information and relay it to motor neurons. The path from sensory input to motor output has been defined in only a few cases, including circuits for crustacean feeding (1) and circuits for rapid escape in fish, flies, and nematodes (2–4). In the nematode Caenorhabditis elegans, the escape circuit was defined by using a complete synaptic wiring diagram of the 302 neurons in its nervous system (4, 5). Six mechanosensory neurons that detect noxious stimuli synapse onto four pairs of interneurons called forward and backward command neurons. The command neurons synapse in turn onto motor neurons responsible for forward and backward locomotion, leading to rapid withdrawal from the stimulus. The definition of the escape circuit has enabled analysis of its development, regulation, and modification by experience (6–11). The C. elegans wiring diagram provides an opportunity to define many complete neuronal paths from sensory stimulus to behavior.
In contrast with the escape circuit, the neuronal control of locomotion during exploratory behavior is poorly characterized. C. elegans navigates to favorable conditions by chemotaxis, thermotaxis, and aerotaxis. In these sensory behaviors and in exploratory behaviors in the absence of informative sensory cues, the animal moves forward and occasionally changes its direction of movement either by a transient reversal or by turning its head during forward movement. The largest change in direction is generated in a sharp omega turn during which the animal's body shape resembles the Greek letter Ω (12, 13) (Fig. 1A). Sinusoidal movement itself can also change during navigation, switching between shallow and deep bends. The neuronal pathways that convert sensory information into specific turning behaviors are incompletely defined. The command neurons are not required for the generation of sinusoidal forward movement (4, 11); the neurons that modulate forward movement to cause curving and omega turns are unknown.
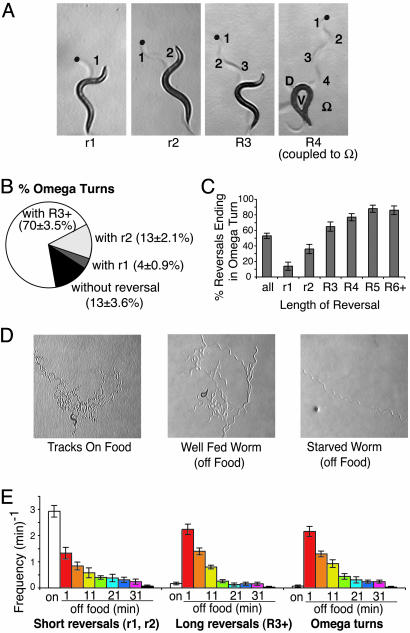
Fig. 1.
Regulation of reversals and omega turns during feeding, local search, and dispersal. (A) Four different examples of reversals of different lengths and degrees of reorientation. Tracks are visible as indentations in the agar. r1, reversal with a single head swing followed by an ≈40° change in direction; r2, reversal with two head swings and an ≈70° change in direction; R3, reversal with three head swings and an ≈90° change in direction; R4, reversal with four head swings followed by an omega turn, resulting in an ≈170° change in direction. Blue dots indicate position of the animal's head at the start of the reversal. Anterior is up. In R4, D indicates the dorsal side and V indicates the ventral side of the animal during the omega turn. (B) Some omega turns occur in the absence of a reversal, but most occur after a reversal. Most omega turns occur after a reversal of length R3+ (three head swings or greater). n = 285 omega turns. Animals were scored at 1–12 min off food. (C) The longer a reversal, the more likely it is to terminate in an omega turn. n = 249 omega turns. Animals were scored at 1–12 min off food. (D) Tracks of individual animals feeding on food, in the local search period during the first 12 min off food, and during dispersal after 40 min off food. The time intervals shown represent ≈10 min, 5 min, and 30 s, respectively. (E) Frequency of short reversals (r1 and r2), long reversals, and omega turns during 5-min intervals on food and at different intervals off food.
In C. elegans, taxis behaviors have features of a biased random walk (14–16) (J.M.G. and C.I.B., unpublished data), a strategy first described in bacterial chemotaxis (17). During a biased random walk, periods of relatively straight movement (“runs”) are occasionally interrupted by periods of rapid direction change (“tumbles” in bacteria and “pirouettes” in C. elegans). A pirouette is a period marked by reversals or sharp turns (14). In a biased random walk, individual trajectories are not predictable. However, when the environment is improving (e.g., when concentrations of attractant increase), pirouettes become less frequent. When the environment is declining, pirouettes become more frequent. This strategy biases the direction of travel toward favorable conditions.
We sought to understand the mechanisms by which sensory neurons modulate the frequency of reversals and turns in C. elegans and the downstream neuronal circuits that generate specific features of these behaviors. The presence of food affects many aspects of C. elegans locomotion (18–21). Here we use a systematic analysis of C. elegans neuroanatomy to dissect a circuit that uses sensory cues to modulate turning rates in two kinds of exploratory behavior: local search after animals are removed from food and long-range dispersal after prolonged food deprivation. These exploratory behaviors in the absence of directional cues share many features with a biased random walk. Our results delineate a behavioral circuit for navigation in C. elegans from sensory input to motor output.
Materials and Methods
Strains. C. elegans strains were maintained and grown according to standard procedures (22). The following strains were used: wild-type strain N2, PR811 osm-6 (p811) V, PR802 osm-3 (p802) IV, CB1033 che-2 (e1033) X, CB1112 cat-2 (e1112) II, GR1321 tph-1 (mg280) II, OH8 ttx-3 (mg158) X, CX3299 lin-15 (n765ts) X; kyIs50[odr-2b::gfp; lin-15(+)], CX3300 lin-15 (n765ts) X; kyIs51[odr-2b::gfp; lin-15(+)], CX6896 mgIs18[ttx-3::gfp] IV, and akIs3[nmr-1::gfp] (23).
Behavioral Assays. Young adult animals were first observed on OP50 food in covered plates for 5 min and then transferred onto a fresh, foodless nematode growth medium plate (22). Observation began 1 min after transfer (24). Reversals of three or more head swings were scored as long reversals. Omega turns were visually identified by the head nearly touching the tail or a reorientation of >135° within a single head swing. Animals were scored by an investigator blind to the genotype or ablation status of the animal.
To score postreversal turn angles, animals were video-recorded off food, and an observer blind to the animal's status drew lines denoting the worm's apparent heading immediately before and immediately after a turn. A detailed description of behavioral assays appears in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Laser Killing of Neurons. Individual neurons were identified either by using Normarski optics and a combination of position and morphological cues (25), or they were identified by using GFP-expressing transgenes. Cells from L1-stage worms were killed with a laser microbeam focused through the 100× Neofluor objective of a Zeiss Axioskop. Adults were assayed as young adults (not more than 72 h after the L4 adult molt), which allowed at least 2.5 days after laser surgery for the ablated cells to lose function. Ablated animals were tested with parallel controls of the same genotype (e.g., with the same transgenes, if relevant) on the same day.
Statistical Analysis. Although general features of the behavior patterns were reliable, the specific frequency of reversals or turns could vary from day to day and could be affected by transgenes used for cell identification. Therefore, laser-treated animals were always compared with approximately equal numbers of control animals tested on the same day under the same conditions. Comparisons between tested animals and matched controls were made by using Student's t test (statview). The results of statistical tests and the sample sizes are reported in Table 1, which is published as supporting information on the PNAS web site.
Results
Reversals and Omega Turns Are Coupled in Pirouettes. C. elegans crawls on its side, flexing in a dorsoventral direction to generate sinusoidal movement (Fig. 1 A). A turn occurs when the head swings are stronger in either the ventral or dorsal direction. One large head swing can cause a rapid turn, or a series of gently biased head swings can produce gradual curving. An extreme change of direction of ≈180° is generated by the omega turn, also called an omega wave or omega bend (12, 13).
To develop more specific assays for changes in the direction of movement, we categorized different types of reversals and turns by direct observation of freely moving animals. Reversals were scored as any perceptible backward movement of the entire animal, and reversal length was scored according to the number of head swings that took place during the reversal (Fig. 1 A). At the end of a reversal, the first forward head swing nearly always incorporated a turn that caused the animal to move forward in a direction different from its initial trajectory (Fig. 1 A). A subset of these postreversal turns were omega turns: sharp turns toward the ventral side of the animal (13). Omega turns were scored according to two criteria: the animal reoriented >135° over the course of a single head swing, or the head very nearly approached or touched the tail during the turn (Fig. 1 A, R4). Although omega turns could occur in isolation, they were often tightly coupled to reversals, such that the animal's head entered the omega turn just as the reversal ended (Fig. 1 A, R4; see also Fig. 1B). Omega turns were most commonly coupled to reversals of three or more head swings. In general, the longer the reversal, the more likely it was to end in an omega turns (Fig. 1C). These results are similar to those of Zhao et al. (24), who reported that reversals of longer temporal duration were more likely to terminate in omega turns.
The Nature and Frequency of Pirouettes Change in the Absence of Food. To understand how environmental cues regulate the frequency and character of pirouettes, we observed reversals and turns under different conditions. Individual animals were first observed while feeding in solitude. On a lawn of the bacterial food OP50, animals spend most of their time moving forward slowly and reversing frequently, a behavior pattern called dwelling (Fig. 1D) (19). We found that reversals in the presence of food were usually short, with only one or two head swings per reversal (Fig. 1E). These short reversals were followed by turns with relatively small turn angles (data not shown). Longer reversals and omega turns were rare on the bacterial lawn.
When animals were transferred to a bacteria-free agar plate, their pattern of locomotion changed dramatically. Immediately after removal from food, the frequency of short reversals declined, and continued to decline for the 40 min that the animals were monitored (Fig. 1E). By contrast, the frequency of long reversals (at least three head swings) increased 10- to 20-fold after removal from food, in parallel with a similar large increase in the frequency of omega turns. Thus, although overall reversal frequencies were similar on food and upon removal from food, the locomotion pattern changed to result in larger changes in direction. In addition, the average speed of forward movement upon removal from food was ≈10-fold higher than the average speed while on food (data not shown). These changes resulted in rapid exploration of a limited area, a behavior pattern that may represent a local search for food (Fig. 1D) (20). At longer times after removal from food, the speed of forward movement remained high, but the frequency of all reversals and omega turns decreased. As a result, animals moved in long, relatively straight paths, a strategy that may allow them to disperse to distant sources of food (Fig. 1 D and E).
These observations define three behavioral states in which the animals move with different patterns of reversals and omega turns: a feeding state on food, which was associated with many short reversals; a local search state shortly after removal from food, which was associated with many long reversals and omega turns; and a dispersal state after prolonged starvation, in which reversals and omega turns were rare. Aspects of these exploratory patterns have also been described by others (20, 26). For further analysis, we generally studied 5-min time periods representing the local search period (1–6 min and 7–12 min after removal from food) and the dispersal period after prolonged starvation (35–40 min after removal from food), as well as feeding behavior on the bacterial lawn.
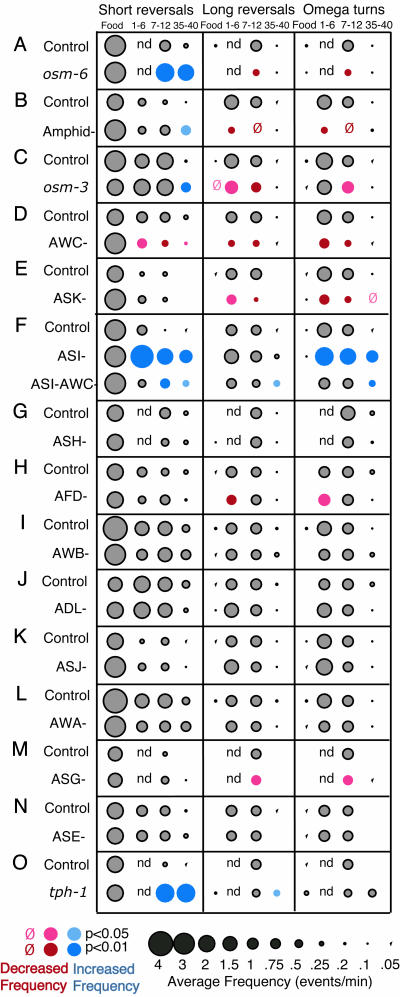
Chemosensory Neurons Regulate Exploratory Behavior After Removal from Food. Bacteria present a complex mixture of chemical, mechanical, and nutritional cues to C. elegans. To ask whether sensory input contributed to the behavioral changes observed after removal from food, we examined osm-6 mutants, which are defective in the development of all ciliated chemosensory and mechanosensory neurons (27). On food, osm-6 mutants exhibited normal feeding behavior, with reversal frequencies and reversal lengths comparable with those of wild-type animals (Fig. 2A). When removed from food, osm-6 mutants behaved as although food was still present: they failed to suppress short reversals and exhibited abnormally low frequencies of long reversals and omega turns (Fig. 2 A). After 35 min of starvation, osm-6 mutants continued to execute frequent short reversals, and even several hours of continued starvation did not completely suppress this behavior (data not shown). che-2 mutants, whose cilia are also defective, exhibited similar behavior (data not shown). These phenotypes suggest that the local search and dispersal behavior patterns are initiated by ciliated sensory neurons, probably those that detect sensory stimuli from food.
Fig. 2.
Sensory regulation of local search and dispersal behaviors. (A) osm-6 (p811) mutants exhibit dwelling-like behavior (feeding behavior) in the absence of food, with more short reversals and fewer long reversals than controls. (B) Animals with all amphid neurons killed have abnormal local search and dispersal behaviors. (C) osm-3 (p802) mutants exhibit milder defects in the absence of food. (D and E) Killing the AWC (D) or ASK (E) neurons blunts local search behavior, with fewer long reversals and omega bends at 1–6 and 7–12 min. (F) Killing ASI disrupts dispersal behavior. Killing ASI and AWC gives a mixed defect. (G–N) Killing ASH, AFD, AWB, ADL, ASJ, AWA, ASE, or ASG sensory neurons, respectively, has minor effects or no effect on feeding, local search, and dispersal behaviors. (O) tph-1 (mg280) mutants exhibit dwelling-like behavior off food, as indicated by a higher frequency of short reversals. For Figs. 2, 4, and 5, the food column refers to a 5-min interval on food, and the other columns refer to intervals after removal from food. The 1- to 6-min and 7- to 12-min intervals correspond to the local search state, and the 35- to 40-min interval corresponds to dispersal. For descriptions of short reversals or long reversals, see Fig. 1. For each data point, the circle size indicates the frequency of the behavior. Gray circles indicate controls or values not significantly different from controls. Colored circles denote statistical significance: blue, increases from control values; red, decreases from control values. The absence of a symbol indicates a value between 0 and 0.05; a red zero indicates a value in the same range that is statistically different from the control. n.d., not done. Sample sizes and P values are reported in Table 1.
osm-6 is expressed in many chemosensory and mechanosensory neurons. To narrow down the list of candidate neurons, we killed the neurons in the amphid chemosensory organs in wild-type animals by using a laser microbeam. Like osm-6 mutants, animals lacking amphid sensory neurons had defects in both local search and dispersal behavior, with reduced suppression of short reversals and little stimulation of long reversals and omega turns (Fig. 2B). An osm-3 mutation, which inactivates the gustatory but not olfactory neurons (28), had a similar but less severe defect compared with amphid neuron-ablated or osm-6 animals (Fig. 2C). These results suggest that gustatory and olfactory amphid neurons sense environmental changes to trigger local search and dispersal behaviors.
To identify specific cells with a role in local search and dispersal behaviors, individual sensory neurons were killed with a laser microbeam. Three classes of amphid chemosensory neurons had strong effects on the local search and dispersal behaviors. The two AWC neurons sense volatile olfactory attractants from food to direct chemotaxis (29). Killing the AWC neurons reduced reversals and omega turns during local search behavior but did not disrupt behavior on food or dispersal behavior (Fig. 2D). A similar effect was observed upon killing the two ASK neurons, which sense water-soluble attractants and repellents (Fig. 2E) (30, 31). These results suggest that AWC and ASK play a role in the local search state by stimulating long reversals and omega turns upon removal from food. By contrast, dispersal behavior required the ASI chemosensory neurons, which also regulate developmental responses to crowding and food deprivation (Fig. 2F). When ASI was killed, short reversals were not suppressed after removal from food, and omega turns were not suppressed at long times off food.
ASI makes several synapses onto AWC (5), so it seemed possible that ASI might suppress omega turns by inhibiting AWC directly. Animals lacking both ASI and AWC executed fewer short reversals and omega turns than animals lacking only ASI but turned and reversed more than AWC-ablated animals (Fig. 2F). Thus, ASI-mediated suppression of reversals and omega turns is partly dependent on and partly independent of AWC.
No other amphid neuron had effects as substantial as those of AWC, ASK, and ASI. The nociceptive ASH sensory neurons trigger reversals and omega turns in response to many aversive stimuli (32), but killing ASH had no effect (Fig. 2G). The two AFD neurons are implicated in thermotaxis (33, 34), and killing AFD led to a small decrease in reversals and omega turns during local search in the first few minutes off food (Fig. 2H). Killing the AWB or ADL sensory neurons that sense volatile repellents, the ASE neurons that sense attractive water-soluble compounds, the AWA neurons that sense attractive odors, or the ASG and ASJ neurons that sense pheromones (35, 36) had little or no effect (Figs. 2 I–N).
The transition from local search to dispersal occurred after starvation. Two secreted neuronal signals that regulate other responses to starvation in C. elegans are TGF-β (daf-7), which is produced by ASI neurons (37) and serotonin (38). The serotonin-deficient mutant tph-1 was defective in exploratory behavior (Fig. 2O), as was daf-7 (data not shown), suggesting that these neuronal signaling molecules regulate exploratory behaviors in the absence of food.
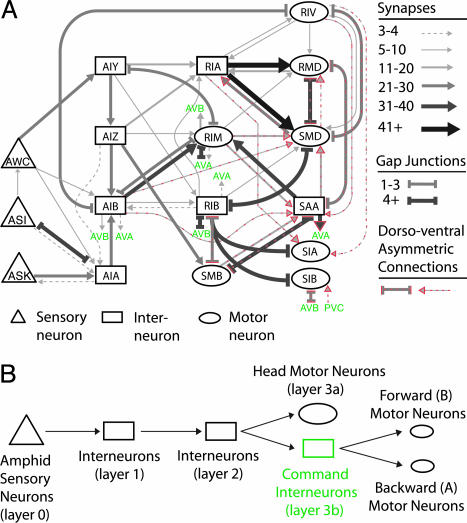
A Candidate Circuit for Navigation. To identify interneurons that function in navigation (Fig. 3), we traced the predominant synaptic output of sensory neurons by using two approaches (Fig. 3; see also Supporting Materials and Methods). In the first approach, we identified circuit paths from the amphid sensory neurons to any head motor neurons or command interneurons, focusing on the shortest paths with the most synapses or gap junctions. The command interneurons promote either forward or backward movement; head motor neurons comprise a partially independent motor system that can carry the animal forward even without the forward command neurons (4). In the second approach, we followed all synaptic output of the sensory neurons through four layers of downstream synapses, ignoring connections representing only a small fraction of the previous layer's output. This second approach (summarized in Table 2, which is published as supporting information on the PNAS web site) was unbiased with regard to the final output, but the majority of neurons and connections identified were the same as those identified in the first approach.
Fig. 3.
A predicted circuit for navigation. (A) Data from serial section reconstructions of electron micrographs (5) were used to assemble a circuit, as described in Supporting Methods. Each of the following neurons represents a bilaterally symmetric left–right pair: AWC, ASI, ASK, AIY, AIZ, AIB, AIA, RIA, RIM, RIB, and RIV. The head and neck motor neurons, SMD, SIA, SMB, and SIB, each have four members that innervate muscle quadrants (see Fig. 5). The interneuron SAA also has four members, a ventral and dorsal member on each side. RMD is a class of six radially arrayed neurons. Red dotted lines indicate connections that were asymmetric in the dorsoventral direction (e.g., seven of eight synapses from AIB to SMD are to the dorsal SMDs). The command interneurons are indicated in green. (B) A schematic showing information flow from sensory neurons to motor neurons.
Four groups of neurons emerged as major direct and indirect targets of the amphid sensory neurons (Fig. 3; see also Table 2 and Supporting Materials and Methods). Half of all synaptic output from the amphid was directed to the interneurons AIA, AIB, AIY, and AIZ (layer 1). These neurons in turn directed a large fraction of their output onto the RIA and RIB interneurons and the head motor neurons RIM and SMB (layer 2). In C. elegans, many motor neurons, including RIM and SMB, also synapse onto other neurons. Layer 2 had some synapses onto muscles, but more than half of its output was directed to layer 3, comprised of additional head interneurons and motor neurons (layer 3a: SAA, RIV, RMD, SMD, SIA, and SIB) and the command interneurons (layer 3b). Head motor neurons synapse mostly onto each other and onto muscles in the head.
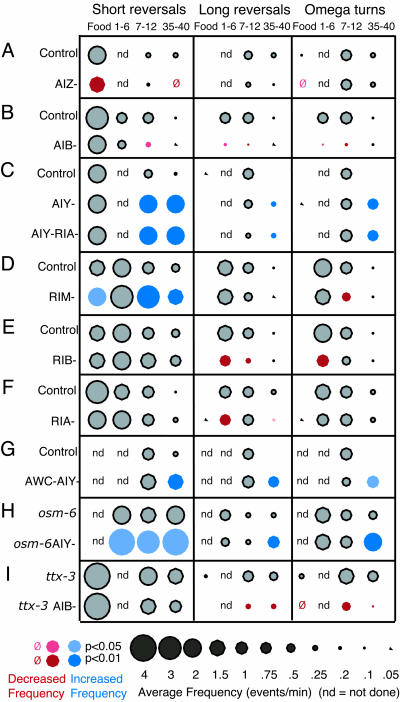
To test the role of interneurons and motor neurons, we ablated individual neuronal classes and examined spontaneous reversals and omega turns on food and off food during local search and dispersal behaviors. The three interneurons AIZ, AIB, and AIY each had preferential effects on one of the three behavioral states. On food, killing the AIZ neurons resulted in animals with a reduced frequency of short reversals (Fig. 4A). None of the other interneurons had strong effects on food. The local search behavior after removal from food was strongly affected by killing the AIB interneurons (Fig. 4B). Animals lacking these cells did not exhibit a strong stimulation in long reversals and omega turns upon removal from food. Instead, like AWC- or ASK-ablated animals, they exhibited premature dispersal behavior, with long runs of forward movement. Dispersal behavior was most strongly affected by killing the AIY interneurons (Fig. 4C). After 30 min off food, animals lacking AIY failed to suppress all classes of reversals and omega turns, instead exhibiting a behavior pattern reminiscent of the local search state.
Fig. 4.
Interneurons regulate pirouette frequency. (A) Killing AIZ reduces reversals during feeding. (B) Killing AIB disrupts local search behavior. (C) Killing AIY disrupts dispersal behavior. (D) Killing RIM increases short reversals during feeding, local search, and dispersal. (E) Killing RIB reduces reversals during local search. (F) Killing RIA slightly decreases reversals during local search and dispersal. (G) AWC/AIY-double-ablated animals resemble AIY-ablated animals. (H) Killing AIY in an osm-6 (p811) background increases reversals and turns. (I) Killing AIB in a ttx-3 (mg280) background reduces reversals and turns.
To further understand the relationships between sensory neurons and interneurons, we examined animals in which multiple classes of neurons were inactivated. Guided by the neuroanatomy in Fig. 3, we assayed several compound lesions. AWC and AIY have opposite effects on reversals and omega turns, and AWC synapses onto AIY, which suggests that AWC might act by directly inhibiting AIY. Indeed, animals lacking both AWC and AIY resembled those lacking only AIY (Fig. 4G).
Both osm-6 sensory mutants and AIY-ablated animals exhibited persistent reversals and turns during the normal period for dispersal. Killing AIY in osm-6 mutants further increased reversals and turns both on and off food (Fig. 4H), indicating that AIY can suppress reversals and turns even when sensory neurons are defective.
Eliminating AIY (with a ttx-3 mutation that also eliminates several other cells) and AIB (by ablation) resulted in an animal distinct from either single manipulation (Fig. 4I): Short reversals were frequent (similar to when AIY was killed), long reversals and omega turns were decreased (as when AIB was killed), and, as a result, neither local search nor dispersal behavior was normal. Thus, the functions of the AIB and AIY interneurons may be additive, suggesting that these neurons act at least partly in parallel.
The interneurons and motor neurons that receive input from AIB, AIY, and AIZ (Fig. 3) were also analyzed systematically. Their functions were more selective than those of layer 1 interneurons, suggesting a transition from the coordinated regulation of exploratory behaviors (AIB and AIY) to a distributed regulation of smaller sets of behaviors at layer 2. Like animals lacking AIY, animals in which the RIM motor neurons were killed showed an inappropriate persistence of short reversals after removal from food (Fig. 4D); several gap junctions connect AIY and RIM. Like animals lacking AIB, animals in which RIB interneurons were killed had fewer long reversals and omega turns during local search (Fig. 4E); AIB synapses onto RIB. The RIA interneurons had a small effect on long reversals (Fig. 4F). The transition from coordinated control to individual behaviors became more marked in layers 3a and 3b, as described below.
The Navigation Circuit Regulates Reversal Frequency by Means of the AVA Command Neurons and Omega Turns by Means of SMD and RIV Head Motor Neurons. Information from the navigation interneurons can be transmitted to the muscles through either the command neurons and downstream ventral cord motor neurons or the motor neurons in the head, including RMD, SMD, SMB, SIA, SIB, RIM, and RIV (Fig. 3) (5). Head neurons belong to symmetrical groups of two (RIM and RIV), four (SMD, SMB, SIA, and SIB) or six (RMD) neurons (Fig. 5G). Three different sets of muscles are differentially innervated by ventral cord and head motor neurons (Fig. 5G) (5). The most anterior rows of muscles, the head muscles, are innervated only by head motor neurons. The next rows of muscles, the neck muscles, are innervated by head motor neurons and ventral cord motor neurons. The most posterior rows of muscles are innervated mainly by ventral cord motor neurons, with minor synapses from head motor neurons at the anterior end.
Fig. 5.
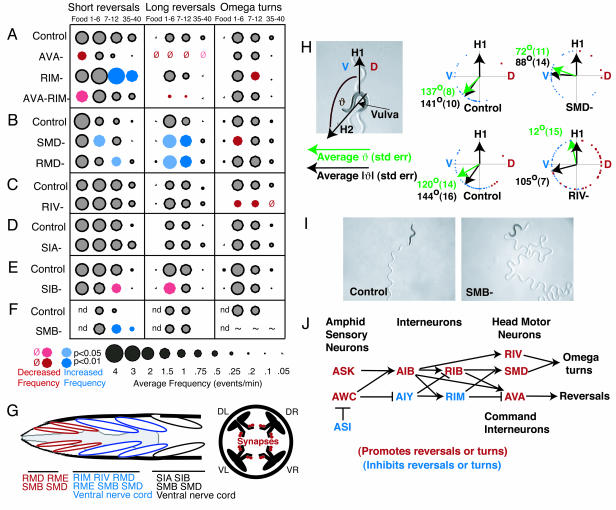
Motor neurons and the AVA command neuron have discrete functions in reversals, omega turns, and sinusoidal movement. (A) Killing AVA eliminates long reversals in local search and diminishes short reversals on food. Killing both RIM and AVA results in fewer reversals than a RIM ablation alone. (B) Killing SMD reduces the frequency of omega turns. Killing either SMD or RMD increases reversal frequency. (C) Killing RIV reduces the frequency of omega turns. (D and E) Killing SIA or SIB does not affect omega turns. (F) Killing SMB increases short reversal frequency. ∼, Because of loopy movement, omega turns could not be accurately scored. (G) Anatomy of head and neck motor neurons and muscles. (Left) Innervation of anterior muscle rows and approximate position of the muscle rows with respect to the pharynx (gray). Muscle groups are symmetric on the left and right sides; in this schematic, only dorsal left and ventral left muscles are shown. SIA and SIB were originally identified as interneurons but also have neuromuscular junctions (D. Hall, personal communication). IL1, URA, RMF, RMG, and RMH motor neurons are not shown. (Right) The four muscle quadrants, dorsal-left (DL), dorsal-right (DR), ventral-left (VL), and ventral-right (VR), and the location of head motor neuron synapses onto the muscles in the nerve ring. (H) Killing SMD reduces the amplitude of turns after a reversal, and killing RIV eliminates the ventral bias of postreversal turns. Turns were scored immediately after a reversal (Fig. 1 A). Green arrows and numbers indicate the average angle by which the direction of movement changed after the turn. A ventral turn was scored as positive (0–180°; blue dots), and a dorsal turn was scored as negative (0–180°; red dots). Black arrows and numbers indicate the average absolute angle by which the direction of movement changed after the turn. For this analysis, both ventral and dorsal turns were scored as positive (0–180°). Sample sizes are 30, 27, 41, and 68 for control (SMD), SMD, control (RIV), and RIV (at least three worms each). t test comparisons indicate statistical significance at P < 0.02 for SMD vs. control and RIV vs. control, comparing either the averages or the averages of the absolute values. (I) Killing SMB results in deeply flexed, loopy sinusoidal movement. Movement tracks are visible on the agar. (J) Neuronal functions in the navigation circuit from sensory input to motor output. ASI may act partly by inhibiting AWC. ASK and AWC may act by inhibiting AIY and stimulating AIB. Omega bends are generated by head motor neurons, and reversals are generated by the command interneurons.
Reversals and backward movement require the backward command neurons AVA and AVD (4), with minor cross-talk from the forward command neurons AVB and PVC (11). AVA and AVB receive input from the navigation circuit (AIB, RIB, RIM, and SMB) (Fig. 3). Animals lacking the AVA neurons were unable to generate long reversals under any conditions and generated abnormally few short reversals on food (Fig. 5A). However, omega turns were present at approximately normal frequencies. These results suggest that the navigation circuit stimulates long reversals by means of the command neuron AVA but can generate short reversals and omega turns by using other motor pathways.
Several classes of head motor neurons inhibited reversals. Killing RIM increased the frequency of short reversals; killing both RIM and AVA neurons suppressed the effect of eliminating RIM (Fig. 5A). RIM forms synapses and gap junctions onto the command interneurons, suggesting that it may inhibit short reversals through these connections. SMB motor neurons also synapse onto the command neurons, and SMB inhibited short reversals to a lesser extent (Fig. 5F). Neither RMD nor SMD synapse directly onto the command neurons, but killing either cell increased the frequency of reversals (Fig. 5B).
Head motor neurons could also affect forward locomotion. Killing the SMB neurons led to a dramatic increase in the amplitude of dorsoventral head swings and resulting loopy sinusoidal movement (Fig. 5I). These head bends were so steep that it was not possible to score omega turns accurately when SMB was killed.
Omega turns were stimulated by SMD and RIV motor neurons. Killing SMD led to a small but significant decrease in the frequency of omega turns (Fig. 5B). Because of the concomitant increase in reversal frequency, animals ablated for SMD executed many reversals without omega turns (data not shown). Killing RIV neurons also led to a decrease in the frequency of omega turns. Neither SMD nor RIV was required for reversals, indicating that the final motor pathways for executing reversals (command/ventral cord neurons) and omega turns (SMD and RIV) are largely distinct.
SMD Encodes Omega Turn Amplitude, and RIV Underlies the Ventral Asymmetry of Omega Turns. We next analyzed specific features of the omega turn, a steep asymmetric bend that usually occurs in the ventral direction (13). In intact animals, omega turns occurred with high probability immediately after long reversals (Fig. 1C). In animals in which SMD was killed, long reversals were followed by more shallow turns. The average angle of postreversal turns was 137° ± 8° in intact animals and 72° ± 11° in animals in which SMD was killed (Fig. 5H). These results suggest that SMD neurons control the steepness of turning to generate omega turns. RIV motor neurons had a smaller effect on the steepness of turns but a dramatic effect on their ventral bias (Fig. 5H). In intact animals, the first head swing after a reversal was highly biased in the ventral direction, even though not all turns in the ventral direction are omega turns (Fig. 5H). This ventral bias was lost in animals lacking RIV. Killing SMD did not alter the ventral bias (Fig. 5H). These results indicate that RIV biases both omega turns and other postreversal head swings in the ventral direction.
Among head motor neurons, the RIV motor neuron class is unique in innervating ventral but not dorsal neck muscles (5). To ask whether the effect of killing RIV could be attributed simply to a generic decrease in ventral muscle innervation in the head, we killed ventral members of several other classes of head motor neurons (SMD, RMD, and SMB) while sparing dorsal members. These ablations did not affect the frequency of omega bends but did, in some cases, result in gentle curvature of forward movement over the course of several head bends (data not shown).
Discussion
Sensory Neurons and Interneurons Regulate the Transition Between Exploratory Behavioral States. C. elegans has distinct exploratory states in which it changes its overall pattern of locomotion based on its recent experience with food (this work and refs. 20, 21, and 26). The animal's behavior upon removal from food moves from an initial local search state, with many reversals and omega turns, to a subsequent dispersal state with few reversals and omega turns. These two behavioral states require distinct sets of sensory neurons and interneurons (Fig. 5J). The AWC, ASK, and AFD sensory neurons and the AIB and RIB interneurons increase the probability of reversals and turns in the local search state. The ASI sensory neurons and the AIY interneurons decrease the probability of reversals and omega turns and are required for the dispersal state.
In some respects, these exploratory behaviors echo the biased random walk, or pirouette, model of chemotaxis. A high frequency of pirouettes is observed after an unfavorable change in the environment (in this case, the disappearance of food). However, in a constant environment, pirouettes eventually decrease to a low baseline level, leading to dispersal. During chemotaxis, the frequency of pirouettes adapts quickly to a change in the stimulus, returning to baseline within a few minutes. By contrast, in exploratory behavior after removal from food, the rate of turning falls much more slowly. These results suggest that pirouette rates are regulated by sensory experience across a variety of time scales.
When combined with synaptic connectivity data (5), the effects of laser ablations on turning frequency can be used to make predictions about the nature of neurotransmission within the circuit. For example, AWC synapses heavily onto AIY, but these two neurons have opposite effects on turning frequency. A simple hypothesis is that release of neurotransmitter from AWC inhibits AIY activity. Based on this logic, many of the synapses in the navigation circuit may be inhibitory.
The high level of pirouettes during local search behavior reflects a sensory memory of food that is expressed by the navigation circuit. Because ASK and AWC are required for local search, it is possible that the memory of food is partially encoded in the activity of these neurons. The transition from local search to dispersal requires the ASI sensory neurons. Dispersal may be caused by an increase in ASI activity, a decrease in AWC or ASK activity, inputs that have yet to be identified, or some combination thereof. Serotonin and the TGF-β molecule DAF-7, which is produced by ASI, contribute to this transition. Serotonin and TGF-β affect a variety of other neuronal responses associated with starvation (21, 37).
The AIB interneurons associated with local search and the AIY interneurons associated with dispersal have been identified in several recent papers that examined spontaneous reversal behaviors off food (none of the other studies examined omega turns and different kinds of reversals separately). Wakabayashi et al. (26) described a local search-like behavior shortly after removal from food, which they call pivoting, and a later dispersal behavior, or traveling. Pivoting required AIB interneurons, like local search, and the suppression of reversals in traveling required AIY neurons. AIY also suppresses reversals in other sensory paradigms and has a smaller role suppressing reversals during local search and on food (this work and refs. 15, 16, and 39).
Perhaps surprisingly, the sensory neurons identified in different exploratory paradigms were not identical. After removal from food, pivoting requires ASK and AWC sensory neurons, like local search behavior, but is also regulated by AWA and ADL, which did not affect local search (26). A different shortterm reversal assay scored between 1 and 4 min off food was affected by ASE, AWA, and AFD sensory neurons but not AWC (39). Long-term traveling behavior was sensitive to ADF, ASH, and AWA sensory neurons, as well as ASI (26), but dispersal required only ASI. Behavior in another assay that compared reversals at short and long time periods was regulated by dopamine signaling (cat-2, which encodes a putative tyrosine hydroxylase), which did not have strong effects in our assays (data not shown) (20). We suggest that these differences may be due to the complete set of sensory changes that animals experience when they are removed from food. Our assays in the absence of food were conducted on nematode growth medium agar plates, the same plates used for cultivation, whereas others transferred the animals from food to low osmotic-strength assay plates (20) or agarose plates that differed from nematode growth medium agar in their mechanical properties, pH, and cholesterol content (26). Dopamine mechanosensory signaling is strongly enhanced at low osmotic strength (40), and traveling varies depending on the chemical composition of the assay plate (26). One interpretation of the different results is that animals respond rapidly to changes in food, chemicals, and osmolarity by regulating reversals and turns; over time, they adapt slowly to all sensory differences between the new condition and their previous condition, leading to a slow transition to the dispersal state. The exact conditions of the assay would define the dominant sensory neurons.
The AIY interneurons have roles in at least four behaviors: dispersal, thermotaxis, regulation of swimming behavior in response to chemical and thermal cues, and behavioral plasticity in paradigms in which starvation is paired with a thermal or chemical cue (33, 39, 41). The roles of AIY in thermotaxis and swimming regulation are rapid and can be explained by direct inputs from sensory neurons onto AIY. However, some of the effects of AIY on plasticity could be indirect effects of its general function in suppressing turns and reversals. The frequent reversals in animals lacking AIY could alter locomotion and navigation under many conditions.
Head and Neck Motor Neurons Control Omega Turns and Sinusoidal Movement. The predicted connectivity of C. elegans suggests that information from sensory neurons is ultimately directed to head and neck motor neurons that mediate sinusoidal movement and omega turns and to forward and backward command interneurons. Long reversals are normally tightly coupled to omega turns, but animals lacking the AVA backward command neurons had normal omega turns in the near-complete absence of reversals. Conversely, SMD and RIV were important in omega turns but were not required for reversals. Thus, reversal and omega turn behaviors have distinct final motor pathways.
We found at least four distinct functions for different classes of head motor neurons: regulation of omega turn amplitude (SMD), regulation of omega turn ventral bias (RIV), suppression of reversals (RIM and others), and regulation of sinusoidal amplitude (SMB). The RIV motor neurons may provide a ventral bias to omega turns by making synapses only onto ventral muscles; most other head motor neuron classes innervate both ventral and dorsal muscles. The steepest part of the omega bend originates in the neck region (Fig. 1 A), where SMD neurons form abundant synapses. Excitatory synapses from SMD to this region may shape the specific properties of the omega turn.
Killing the SMB head motor neurons resulted in high-amplitude sinusoidal movement. This result was surprising, because sinusoidal movement was thought to be generated primarily by body motor neurons. A different kind of loopy behavior, restricted to the head, is observed when RME motor neurons are killed (42). RME forms neuromuscular junctions only onto the most anterior head muscles, whereas SMB innervates head and neck muscles and innervates RME (Fig. 5G). These results suggest that neck muscles can regulate the overall amplitude of sinusoidal forward movement.
Navigation and Probabilistic Behavior. Many features of the navigation circuit remain to be defined. Chief among these is the relationship between this circuit and chemotaxis and thermotaxis behaviors. The sensory neurons involved in chemotaxis and thermotaxis, AWC, AFD, AWA, and ASE, synapse primarily onto the circuit described here, and the interneurons AIY, AIZ, and RIA are required for thermotaxis (33). The regulation of reversals and turns by the circuit described here may play a role in the biased random walk component of taxis.
The well studied escape behaviors in C. elegans are deterministic: A mechanical or chemical repellent leads to a rapid and reliable reversal. By contrast, navigation is probabilistic: It can be described as a set of frequencies, but the exact timing of a particular reversal or turn is unpredictable. Different behavioral states were associated with characteristic frequencies of reversals and omega turns. Most sensory neurons and interneurons regulated the probabilities of several behaviors in a behavioral state. By contrast, motor neurons were more specialized and could even affect individual features of movements. The mechanisms for generating probabilistic behavior are likely to reside in the transformation of information between the sensory neurons, interneurons, and motor neurons in this circuit.
Supplementary Material
Acknowledgments
We thank Villu Maricq, Ikue Mori, and Jon Pierce-Shimomura for comments on the manuscript; Shawn Lockery, Thomas Hills, Sreekanth Chalasani, and Tim Yu for valuable discussions; and Takeshi Ishihara and the Caenorhabditis Genetics Center for strains. This work was supported by funding from the Howard Hughes Medical Institute. J.M.G was supported by a Howard Hughes Medical Institute Predoctoral Fellowship. C.I.B. is an Investigator of the Howard Hughes Medical Institute.
Author contributions: J.M.G. and C.I.B. designed research; J.M.G. and J.J.H. performed research; J.M.G. and C.I.B. analyzed data; and J.M.G. and C.I.B. wrote the paper.
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 29, 2003.
See accompanying Biography on page 3181.
References
- 1.Nusbaum, M. P. & Beenhakker, M. P. (2002) Nature 417, 343-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faber, D. S. & Korn, H. (1978) Neurobiology of the Mauthner Cell (Raven, New York).
- 3.Tanouye, M. A. & Wyman, R. J. (1980) J. Neurophysiol. 44, 405-421. [DOI] [PubMed] [Google Scholar]
- 4.Chalfie, M., Sulston, J. E., White, J. G., Southgate, E., Thomson, J. N. & Brenner, S. (1985) J. Neurosci. 5, 956-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White, J., Southgate, E., Thomson, J.N., and Brenner, S. (1986) Philos. Trans. R. Soc. London B 314, 1-340. [DOI] [PubMed] [Google Scholar]
- 6.Lee, R. Y., Sawin, E. R., Chalfie, M., Horvitz, H. R. & Avery, L. (1999) J. Neurosci. 19, 159-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rankin, C. H. (1991) J. Comp. Physiol. A 169, 59-67. [DOI] [PubMed] [Google Scholar]
- 8.Rankin, C. H., Gannon, T. & Wicks, S. R. (2000) Dev. Psychobiol. 36, 261-270. [PubMed] [Google Scholar]
- 9.Rankin, C. H. & Wicks, S. R. (2000) J. Neurosci. 20, 4337-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wicks, S. R., Roehrig, C. J. & Rankin, C. H. (1996) J. Neurosci. 16, 4017-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng, Y., Brockie, P. J., Mellem, J. E., Madsen, D. M. & Maricq, A. V. (1999) Neuron 24, 347-361. [DOI] [PubMed] [Google Scholar]
- 12.Wallace, H. R. (1969) Nematologica 15, 75-89. [Google Scholar]
- 13.Croll, N. A. (1975) J. Zool. (London) 176, 159-176. [Google Scholar]
- 14.Pierce-Shimomura, J. T., Morse, T. M. & Lockery, S. R. (1999) J. Neurosci. 19, 9557-9569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryu, W. S. & Samuel, A. D. (2002) J. Neurosci. 22, 5727-5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zariwala, H. A., Miller, A. C., Faumont, S. & Lockery, S. R. (2003) J. Neurosci. 23, 4369-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berg, H. C. & Brown, D. A. (1972) Nature 239, 500-504. [DOI] [PubMed] [Google Scholar]
- 18.de Bono, M. & Bargmann, C. I. (1998) Cell 94, 679-689. [DOI] [PubMed] [Google Scholar]
- 19.Fujiwara, M., Sengupta, P. & McIntire, S. L. (2002) Neuron 36, 1091-1102. [DOI] [PubMed] [Google Scholar]
- 20.Hills, T., Brockie, P. J. & Maricq, A. V. (2004) J. Neurosci. 24, 1217-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawin, E. R., Ranganathan, R. & Horvitz, H. R. (2000) Neuron 26, 619-631. [DOI] [PubMed] [Google Scholar]
- 22.Brenner, S. (1974) Genetics 77, 71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brockie, P. J., Mellem, J. E., Hills, T., Madsen, D. M. & Maricq, A. V. (2001) Neuron 31, 617-630. [DOI] [PubMed] [Google Scholar]
- 24.Zhao, B., Khare, P., Feldman, L. & Dent, J. A. (2003) J. Neurosci. 23, 5319-5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bargmann, C. I. & Avery, L. (1995) Methods Cell Biol. 48, 225-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wakabayashi, T., Kitagawa, I. & Shingai, R. (2004) Neurosci. Res. 50, 103-111. [DOI] [PubMed] [Google Scholar]
- 27.Perkins, L. A., Hedgecock, E. M., Thomson, J. N. & Culotti, J. G. (1986) Dev. Biol. 117, 456-487. [DOI] [PubMed] [Google Scholar]
- 28.Tabish, M., Siddiqui, Z. K., Nishikawa, K. & Siddiqui, S. S. (1995) J. Mol. Biol. 247, 377-389. [DOI] [PubMed] [Google Scholar]
- 29.Bargmann, C. I., Hartwieg, E. & Horvitz, H. R. (1993) Cell 74, 515-527. [DOI] [PubMed] [Google Scholar]
- 30.Bargmann, C. I. & Horvitz, H. R. (1991) Neuron 7, 729-742. [DOI] [PubMed] [Google Scholar]
- 31.Hilliard, M. A., Bargmann, C. I. & Bazzicalupo, P. (2002) Curr. Biol. 12, 730-734. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan, J. M. & Horvitz, H. R. (1993) Proc. Natl. Acad. Sci. USA 90, 2227-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori, I. & Ohshima, Y. (1995) Nature 376, 344-348. [DOI] [PubMed] [Google Scholar]
- 34.Gomez, M., De Castro, E., Guarin, E., Sasakura, H., Kuhara, A., Mori, I., Bartfai, T., Bargmann, C. I. & Nef, P. (2001) Neuron 30, 241-248. [DOI] [PubMed] [Google Scholar]
- 35.Schackwitz, W. S., Inoue, T. & Thomas, J. H. (1996) Neuron 17, 719-728. [DOI] [PubMed] [Google Scholar]
- 36.Bargmann, C. I. & Horvitz, H. R. (1991) Science 251, 1243-1246. [DOI] [PubMed] [Google Scholar]
- 37.Ren, P., Lim, C. S., Johnsen, R., Albert, P. S., Pilgrim, D. & Riddle, D. L. (1996) Science 274, 1389-1391. [DOI] [PubMed] [Google Scholar]
- 38.Sze, J. Y., Victor, M., Loer, C., Shi, Y. & Ruvkun, G. (2000) Nature 403, 560-564. [DOI] [PubMed] [Google Scholar]
- 39.Tsalik, E. L. & Hobert, O. (2003) J. Neurobiol. 56, 178-197. [DOI] [PubMed] [Google Scholar]
- 40.Schafer, W. R., Sanchez, B. M. & Kenyon, C. J. (1996) Genetics 143, 1219-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishihara, T., Iino, Y., Mohri, A., Mori, I., Gengyo-Ando, K., Mitani, S. & Katsura, I. (2002) Cell 109, 639-649. [DOI] [PubMed] [Google Scholar]
- 42.McIntire, S. L., Jorgensen, E., Kaplan, J. & Horvitz, H. R. (1993) Nature 364, 337-341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.