Abstract
The circadian clock controls many aspects of mammalian physiology, including responses to cancer therapy. We find that wild-type and circadian mutant mice demonstrate striking differences in their response to the anticancer drug cyclophosphamide (CY). While the sensitivity of wild-type mice varies greatly, depending on the time of drug administration, Clock mutant and Bmal1 knockout mice are highly sensitive to treatment at all times tested. On the contrary, mice with loss-of-function mutations in Cryptochrome (Cry1-/-Cry2-/- double knockouts) were more resistant to CY compared with their wild-type littermates. Thus, both time-of-day and allelic-dependent variations in response to chemotherapy correlate with the functional status of the circadian CLOCK/BMAL1 transactivation complex. Pharmacokinetic analysis of plasma concentration of different CY metabolites shows that, in contrast to the traditional view, circadian variations in drug sensitivity cannot be attributed to the changes in the rates of CY metabolic activation and/or detoxification. At the same time, mice of different circadian genotypes demonstrate significant differences in B cell responses to toxic CY metabolites: B cell survival/recovery rate was directly correlated with the in vivo drug sensitivity. Based on these results, we propose that the CLOCK/BMAL1 transcriptional complex affects the lethality of chemotherapeutic agents by modulating the survival of the target cells necessary for the viability of the organism.
Keywords: chemotherapy, circadian clock, drug response
Abroad range of organisms from bacteria to animals display circadian oscillations in metabolism, cell proliferation, physiology, and behavior. Circadian rhythms are generated by an intracellular clock mechanism that involves a network of transcriptional feedback loops that drive rhythmic RNA and protein expression of key clock components (reviewed in refs. 1–3). The major loop is represented by two positive activators, CLOCK and BMAL1, which belong to the family of bHLH-PAS domain transcription factors. The CLOCK/BMAL1 complex regulates the expression of the Period (Per1, Per2, and Per3) and Cryptochrome (Cry1 and Cry2) genes that in turn act as repressors of their own transcription. The molecular mechanism of circadian oscillators is operative not only in the central pacemaker of the mammalian circadian system in the suprachiasmatic nucleus (SCN) but also in peripheral organs such as lung, liver, heart, and skeletal muscle (4). Both the central clock in the SCN and peripheral clocks in different tissues control output physiology by regulating the expression of multiple clock-controlled genes (5, 6). This circadian expression pattern demonstrates remarkable tissue specificity, and in many cases, involves rate-limiting steps of fundamental metabolic pathways in the cell (5, 7).
There has been substantial evidence that the circadian clock can modulate the morbidity and efficacy of anticancer therapy. Observations made >30 years ago demonstrated that the circadian pattern of arabinosyl cytosine administration determined both its toxicity and antitumor activity (8). More than 30 different anticancer drugs tested in mice and rats exhibit strong time-of-administration effects on drug-induced toxicity (9). These findings led to the idea of chronotherapy, wherein medication or other treatment can be optimized by delivery at appropriate times of the day. Although the chronotherapeutic approach has demonstrated encouraging results, it has not become routine in clinical practice, perhaps in part, because of the lack of a clear mechanistic basis (10).
To address the mechanistic aspects of the role of circadian clock in response to genotoxic stress induced by cancer therapy, we used three circadian mutant mouse models, Clock mutants, Bmal1-/- mice, and Cry1-/-Cry2-/- knockout mice, and compared their in vivo drug response to a widely used chemotherapeutic drug cyclophosphamide (CY). Here, we report that drug sensitivity is directly correlated with the functional status of the major circadian transactivation complex, suggesting that molecular determinants of sensitivity to CY may be directly regulated by CLOCK/BMAL1. We also present the evidence that this control is based on a CLOCK/BMAL1-dependent modulation of target B cell responses to drug-induced toxicity. These results provide an important mechanistic link between the circadian clock system and the response to genotoxic stress induced by cancer therapy.
Materials and Methods
Chemicals. CY was purchased from Sigma. The CY metabolites, 4-hydroperoxy-CY (4-OH), 3-dechloroehyl-CY (DCE), 4-keto-CY, Carboxyethyl-CY (CB), and phosphoroamide mustard (PM), were a generous gift from Asta Medica (Frankfurt, Germany). Collagenase was purchased from Crescent Chemicals (Islandia, New York).
Animals and CY Therapy. Control C57BL/6J male mice were purchased from The Jackson Laboratory. Clock mutant mice, originally obtained from the Takahashi laboratory at Northwestern University, were maintained on a C57BL/6J background (11). Bmal1-/- mice (12), obtained from C. Bradfield, and Cry1-/- and Cry2-/- knockout mice (13), provided by Dr. A. Sancar, were backcrossed to C57BL/6J (10 backcross generations). Animals were synchronized to a 12-h light:12-h dark cycle for at least 2 weeks before experiments. CY treatment (i.p. injections) was performed in a regimen described for tumor therapy (14). Mice received either a single injection at 300 mg/kg, or three injections at different doses that were performed every other day at different zeitgeber times (ZT; ZT0 corresponds to the beginning of the light phase of the daily cycle). Drug-induced toxicity was assessed by mortality and body weight loss. The loss of 20% of the original body weight was considered the end point of the experiment.
For pharmacokinetic (PK) analysis, wild-type and Clock/Clock mice received 3 × 150 mg/kg i.p. injections of CY at ZT02 or ZT14. Blood samples obtained at different times after the last injection through the retroorbital bleeding, were placed into EDTA-treated tubes (Sarstedt); plasma was separated by centrifugation through Microtainer plasma separation tubes (Becton Dickinson) and stored at -80°C until analysis. All animal studies were conducted in accordance with the regulations of the Committee on Animal Care and Use at the Cleveland Clinic Foundation and Northwestern University.
Measurement of CY and Its Metabolites in Plasma. CY, 4-OH, DCE, CB, and PM were recovered from mouse plasma by using solid-phase extraction (Xpertek SPE C18 cartridge, P.J. Cobert Associates, St. Louis) according to the manufacturer's protocol. To recover for the loss of all tested compounds during sample preparation and extraction, ifosfamide was used as an internal standard. Reverse-phase HPLC with on-line electrospray ionization tandem MS was performed as described (15). A detailed description is provided in Supporting Methods, which is published as supporting information on the PNAS web site. Elimination half-life values (t1/2) were calculated by a linear regression analysis of semilog concentration-time graphs after Tmax (correlation coefficient r2 > 0.9 in all cases). Statistical significance was determined by using Student's t test.
RNA Isolation and Real-Time PCR Analysis. Control C57BL/6J and Clock/Clock mutant mice were maintained at a ratio of 12-h light:12-h dark. Tissue sampling, RNA extraction and Cyp3a13 mRNA quantitation were performed as described in ref. 5. Primers and probes used for real-time PCR analysis are described in Supporting Methods.
Hepatocyte Preparation and Coculture Experiments. Hepatocyte isolation from livers of wild-type and circadian mutant mice was performed as described in ref. 16 with some modifications (Supporting Methods). Isolated hepatocytes were resuspended in DMEM supplemented with 10% FCS and plated on collagen-treated 24-well plates at a density of 150,000 cells per well. C8 cells were plated on transwell inserts for 24-well plates at 20,000 cells per well. Cells were allowed to adhere for at least 2 h, after which CY in DMEM was added. After overnight incubation, the viability of both hepatocytes and C8 cells was tested by using the MTT assay (Sigma) according to the manufacturer's protocol.
Total Blood Cell Analysis. Hematological evaluation was performed either at day 5 (if 3 × 150 mg/kg injection protocol was used), or at day 3, when a single-dose (1 × 300 mg/kg) injection protocol was used. Peripheral blood obtained from the retroorbital sinus was collected into EDTA-treated tubes, and the complete blood counts with differentials were measured by using an Advia 120 hematology system (Bayer) and analyzed with the software applications for C57BL/6J mice. All control parameters were within the range previously described for this mouse strain (M. Justice clinical hematology parameters, accession number MPD:132, from the Mouse Phenome Database, The Jackson Laboratory, Bar Harbor, MA, which can be accessed at www.jax.org/phenome). A generalized linear model ANOVA (ncss, v.6.0, Kaysville, UT) was used to estimate the statistical significance of the effects of genotype and time of CY administration on WBC differentials. A Tukey's test was used for post hoc pairwise comparisons.
Flow Cytometry. Cell suspension preparation and flow cytometry analysis were carried out according standard protocols described in detail in Supporting Methods.
Results and Discussion
In Vivo Sensitivity to CY Depends on the Functional Status of the CLOCK/BMAL1 Transactivation Complex. It is well accepted that the circadian clock regulates multiple physiological and biochemical outputs through the periodic transcriptional activation and repression of target genes by the CLOCK/BMAL1 transactivation complex (3). To explore a possible molecular link between drug toxicity in vivo and the status of the circadian transactivation complex, we used several mouse models representing different states of CLOCK/BMAL1 functional activity and compared CY sensitivity of wild-type, Clock/Clock mutant, Bmal1-/-, and Cry1-/-Cry2-/- knockout animals. Phenotypically, all three mutant mouse models are characterized by disrupted circadian rhythmicity at the behavioral level (11, 12, 17). However, at the molecular level, these experimental models are correlated with different temporal states of circadian clock function and approximate two opposite extremes in relation to the activity of the CLOCK/BMAL1 transactivation complex. Clock/Clock and Bmal1-/- mice represent the minimal transcriptional activity of the complex because of the deficiency of transcriptional activators (12), whereas mice with targeted disruption of both Cry genes (Cry1-/-Cry2-/-) represent its maximally active state because of the absence of circadian repression (13, 18).
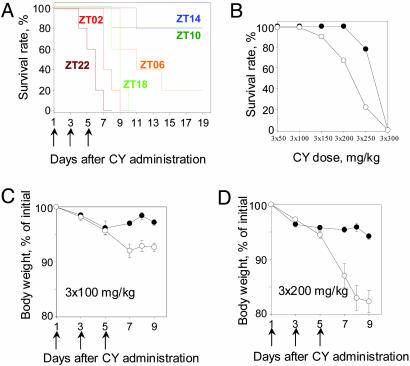
We first compared the in vivo drug response of control C57BL/6J mice treated with CY on a conventional schedule of the maximum tolerated dose (14) administered at different times of the day. As shown in Fig. 1A, the survival rate among different groups varied greatly, depending on the time of CY administration. Animals treated during the time of dark-to-light transition (ZT22 to ZT02) were more sensitive to the drug than mice injected at the time of light-to-dark transition (ZT10 to ZT14). This initial observation was confirmed by more detailed studies when different doses of CY were delivered at two time points, ZT02 and ZT14 (the most and the least sensitive times detected in our original experiment; Fig. 1B). Consistent with previous results, animals treated at ZT02 were more sensitive to the treatment; thus, at the dose of 3 × 250 mg/kg, survival rate in this group was 20% compared with 80% in the group that received the drug at ZT14. Only the highest dose used (3 × 300 mg/kg, 2-fold higher than maximum tolerated described for mice, resulted in 100% mortality in both groups. Even at doses that did not induce mortality (3 × 100 mg/kg), there were striking differences in morbidity, which was reflected in the extent of the body weight loss (Fig. 1 C and D). Thus, animals injected at ZT02 lost significantly more weight compared with the group that received the drug at ZT14, and these differences increased with the dose used. The two time windows, ZT02 to ZT22 and ZT10 to ZT14, which in our experiments represent maximal and minimal drug sensitivity in vivo, correlate with the peak and trough of CLOCK/BMAL1 transcriptional activity as judged by target Per1 and Per2 gene expression profiles in most peripheral tissues (3). Importantly, the time of the highest resistance to CY corresponds to the daily peak in the activity of the CLOCK/BMAL1 transactivation complex. This observation led us to speculate that the molecular determinants of sensitivity to CY could be directly regulated by CLOCK/BMAL1.
Fig. 1.
Effect of time of treatment on in vivo drug response in wild-type C57BL/6J mice. (A) Survival of C57BL/6J mice after 3 × 150 mg/kg i.p. injections of CY performed at different times of the day. Animals injected at ZT10 to ZT14 demonstrate higher survival rate compared with their littermates injected at ZT22 to ZT02. (B) Dose–response curve for the survival rate of C57BL/6J mice injected at ZT14 (•) or ZT02 (○). Animals injected at ZT14 can better tolerate higher doses of the drug. (C) Total body weight loss in C57BL/6J mice after 3 × 100 mg/kg CY i.p. injections administered at ZT14 (•) or ZT02 (○). (D) Results are the same as in C after 3 × 200 mg/kg i.p. injections. At both doses, percent of body weight loss is significantly higher if injections were performed at ZT02 (at day 9, P = 0.002). Arrows indicate the days of treatment. Values represent mean ± SEM.
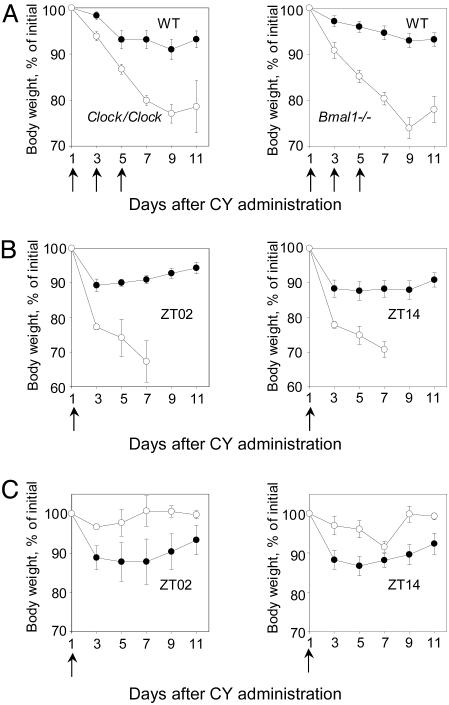
To test this hypothesis, we compared drug sensitivity of wild-type mice and mice with deficiencies in the CLOCK/BMAL1 transcriptional activators. As shown on Fig. 2A, control and circadian mutant mice demonstrated strikingly different drug responses, as assessed by body weight loss. Both Clock/Clock and Bmal1-/- lose up to 20% of their original body weight by days 9–11 after CY administration. In corresponding control groups, the body weight never went down >5–8% and was always followed by partial recovery and stabilization after the completion of injections. Thus, both Clock/Clock and Bmal1-/- animals, deficient in the CLOCK/BMAL1 transactivation function, demonstrated high levels of drug sensitivity. This effect did not depend on the drug administration schedule or on the time of CY treatment: when a single high dose of drug was delivered at ZT14 and ZT02, the least and the most sensitive times for control group, Clock/Clock mice always respond to the treatment by significantly higher body weight loss (Fig. 2B).
Fig. 2.
Effect of circadian mutations on in vivo drug response. (A) Body weight loss of control (•), Clock/Clock (Left, ○), and Bmal1-/- knockout mice (Right, ○). Ten animals of each genotype received 3 × 150 mg/kg CY at ZT10, the time of the highest resistance for control group. Both mutants respond to CY treatment by significantly higher total body weight loss as compared with their wild-type littermates. (B) Body weight loss of control (•) and Clock/Clock mutant (○) mice after a single 300 mg/kg CY injection performed at ZT02 (Left) or ZT14 (Right). At both times used, Clock/Clock mice demonstrate significantly higher drug sensitivity. (C) Body weight loss of control (•) and Cry1-/- Cry2-/- (○) knockout mice after a single 300 mg/kg CY dose administered at ZT02 (Left) or ZT14 (Right). Cry1-/-Cry2-/- double-knockout mice are more resistant to the treatment than their wild-type littermates (at day 9, P = 0.07 for ZT02 and P = 0.02 for ZT14).
In contrast to Clock/Clock and Bmal1-/- mice, animals with targeted disruption of both Cry genes are deficient in circadian repression, and express constantly high levels of CLOCK/BMAL1 transcriptional activity. Because high complex activity correlated with higher drug resistance in wild-type mice, we tested the drug response of Cry1-/-Cry2-/- double-knockout mice to determine whether they were also resistant. The time course of drug-induced body weight loss in the wild-type and Cry1-/-Cry2-/- mice are shown in Fig. 2C. Importantly, Cry1-/-Cry2-/- mice were more drug resistant compared with their wild-type littermates at both times tested. Taken together, these observations are consistent with the idea that both time-of-day and genotype-dependent variations in drug sensitivity correlate with the functional status of the major circadian CLOCK/BMAL1 transactivation complex: high-complex activity (either because of daily circadian variation or Cry deficiency) is associated with higher resistance to drug-induced toxicity, whereas low-complex activity (because of daily variation or Bmal1 or Clock deficiency) is correlated with higher sensitivity to drug-induced toxicity.
PK Analysis of Plasma Concentration of CY and Its Metabolites in Wild-Type and Clock Mutant Mice. Drug sensitivity of the organism results from a superposition of multiple levels of responses all contributing in various degrees to the final outcome, and each of these types of responses could be modulated by CLOCK/BMAL1 transcriptional activity. Thus, circadian control of drug sensitivity could occur through the regulation of the abundance and/or activity of drug-metabolizing enzymes involved in CY activation and/or detoxification. This view was supported recently by the results of the global analysis of the circadian transcriptional output using the microarray approach (5). Of 335 transcripts that demonstrated 24-h periodicities in their expression levels in the liver, many encode enzymes involved in drug activation/detoxification processes. Alternatively, the circadian clock could be involved in the control of sensitivity of target cells to CY-induced cytotoxicity through modulation of the cell response to genotoxic stress.
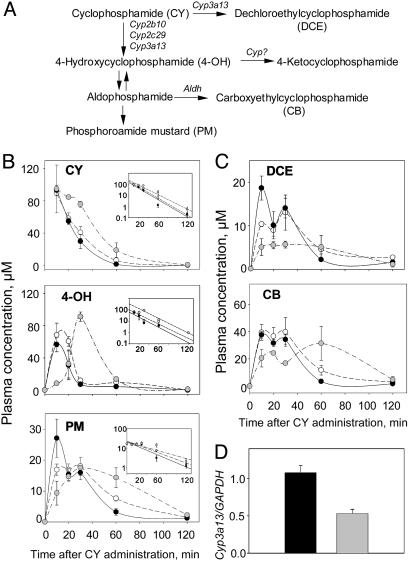
To discriminate between these two mechanisms, we performed a PK analysis of the plasma concentration of CY and its metabolites in wild-type mice injected at ZT02 or ZT14 and Clock/Clock mutant mice injected at a single time point at ZT14. CY is a prodrug that requires metabolic activation performed by a subset of hepatic P450 enzymes, followed by several alternative pathways, yielding ultimate alkylating metabolite PM and several inactive compounds (19) (Fig. 3A). The plasma concentration of each of CY metabolite at any given time point reflects the balance between the rate of its formation and the rate of elimination, which in turn, correlates with the relative activities of multiple drug metabolizing enzymes involved in CY metabolic transformation.
Fig. 3.
PK analysis of CY metabolites in the plasma of wild-type and Clock mutant mice. (A) Partial metabolic scheme for CY activation and detoxification. The initial activation step is mediated by the cytochrome P450 enzymes through two alternative pathways: the first pathway, leading to formation of 4-OH, and the second pathway, yielding biologically inactive DCE. 4-OH is a circulating metabolite whose spontaneous decomposition by means of aldophosphoamide yields the ultimate alkylating metabolite, PM, and acrolein. Alternatively, 4-OH may be enzymatically detoxified, yielding several inactive compounds. Cyp3a13, Cyp2b10, and Cyp2c29 are cytochrome P450 isoforms involved in the initial CY metabolic transformation. Aldh, aldehyde dehydrogenase. (B) Concentration versus time curves of CY and its toxic metabolites, 4-OH and PM, in the plasma of wild-type mice injected at ZT14 (black circles) and ZT02 (white circles) and Clock/Clock mice (gray circles). (Insets) Corresponding semilog plots used to calculate the t1/2 values for ZT02, ZT14, and Clock/Clock groups (CY: 11.85 ± 0.26, 12.74 ± 1.5, and 13.75 ± 1.77 min; 4-OH: 15.8 ± 5.4, 13.2 ± 1.6, and 13.9 ± 0.5 min; PM: 35.8 ± 8.4, 29.5 ± 7.8, and 28.45 ± 4.9 min). (C) Results are for the same curves for the inactive metabolites, DCE and CB. Three PK experiments performed with different dosing and administration schedule gave similar results. (D) Real-time PCR assay for Cyp3a13 mRNA abundance in the liver of control and Clock/Clock mice. Wild-type and Clock/Clock mice were entrained to a 12-h light:12-h dark cycle for 1 week before tissue collection. Three animals of each genotype were killed at ZT02, ZT06, ZT10, ZT14, ZT18, and ZT22; livers were removed, frozen on dry ice, and stored at -80°C until RNA isolation. Values on the bar graphs presented combined average values for all 18 animals of each genotype ± SEM.
Comparison of the plasma concentration versus time profiles of CY and its major metabolites between two groups of wild-type animals revealed that the time of drug administration had no effect on the rate of its metabolic transformation (Fig. 3 B and C). For all compounds tested, the concentration versus time profiles in both wild-type groups were indistinguishable. Consistently, for both groups the elimination half-time values (t1/2) for the parent compound (CY) and toxic metabolites (calculated from the semilog plots and presented in Fig. 3B Insets) were identical and very close to those previously reported in the literature (20). These results suggest that in wild-type mice, the rate of CY metabolic transformation by hepatic enzymes does not depend on the time of drug administration.
The comparison of the concentration profiles of the major CY metabolites in the plasma of wild-type and Clock mutant mice revealed that the Clock mutation results in the increase in time of the peak plasma concentration for 4-OH (up to 30 min after CY injection, as opposed to <10 min in both wild-type groups) and decreased the Cmax value for DCE (Fig. 3 B and C). However, no significant differences were detected in the elimination half-time for CY or either of the active metabolites (4-OH and PM). This finding suggests that although the rate of CY activation in Clock mutant mice is slightly delayed in time, the amount of active metabolites produced, and the extent of exposure to toxic agents, is similar to that observed in the wild-type groups.
Because the genotype-dependent differences in concentration profiles involve only the parent compound (CY) and both of its direct metabolic products, toxic 4-OH and biologically inactive DCE, we propose that the Clock mutation affects the initial step of CY activation. As reported previously, this initial step is carried out by a distinct set of cytochrome P450 enzymes: CYP2B10 and CYP2C29 are responsible for CY activation through 4-hydroxylation, whereas CYP3A13 is involved both in activation and inactivation through N-dechloroethylation (21, 22) (Fig. 3A). Thus, the delayed CY activation observed in Clock mutants could be explained through an impairment of CYP3A13 activity.
To test whether the Clock mutation affects Cyp3a13 at the transcriptional level, we compared the steady-state levels of Cyp3a13 mRNA in the livers of wild-type and Clock mutant mice at different times of the 24-h daily cycle by using real-time PCR. No temporal variations in Cyp3a13 mRNA were observed in the livers of wild-type mice, suggesting that, unlike some other P450 isoforms, CYP3a13 is not circadian-regulated. However, a comparison between wild-type and Clock mutant mice revealed ≈2-fold decrease in the average expression level in the mutant animals (Fig. 3D). Assuming that this decrease translates to the protein expression/activity level, it may explain the observed delayed CY activation kinetics in the livers of Clock mutant mice.
Clock and Bmal1 Deficiency Has No Effect on the Rate of CY Metabolism in Isolated Hepatocytes. To confirm independently that the deficiency in circadian transcription activators does not affect the rate of CY metabolic activation and the production of toxic metabolites, we compared the rate of drug activation in vitro by hepatocytes isolated from wild-type, Clock/Clock, and Bmal1-/- mice. It has been previously shown that during first few days of culturing, isolated hepatocytes retain their ability to metabolize different drugs, including CY (16, 23), which suggests that differences in CY metabolism in vivo are likely to be maintained in in vitro-cultured hepatocytes.
To compare CY activation rate by hepatocytes of different genotypes, we performed a series of experiments, in which CY-treated hepatocytes were cocultured with the drug-sensitive test cells. As test cells, we used mouse embryo fibroblasts transformed with E1a+ras, line C8, which undergo rapid p53-dependent apoptosis in response to variety of treatments (24). When CY was applied to hepatocytes or C8 cells separately, it was toxic to hepatocytes only at the highest concentrations. In contrast, it was completely nontoxic to C8 cells because they lack the mechanism of this drug activation (Fig. 5A, which is published as supporting information on the PNAS web site). However, in coculture experiments, CY undergoes metabolic transformations in hepatocytes, yielding toxic 4-OH and PM, which induces ≈60% cell death within 18 h of incubation. As demonstrated in Fig. 5 B and C, no significant differences in CY toxicity to C8 cells in the wide range of CY concentrations used were detected among the three genotypes. Thus, isolated hepatocytes of Clock/Clock and Bmal1-/- mutant mice metabolize CY at a rate similar to wild-type control animals. In summary, both in vivo PK analysis and in vitro assays did not reveal any significant time-of-administration- or genotype-dependent changes in the rate of CY metabolic activation/detoxification, which could reasonably explain the dramatic differences in drug sensitivity observed in our vivo experiments.
Differential Sensitivity to CY-Induced Toxicity Is Determined by CLOCK/BMAL1-Dependent Modulation of B Cell Survival. As has been demonstrated (25), the major target of CY-induced toxicity and the major dose-limiting factor of CY therapy is the hematopoietic system. In mice, the hematopoietic syndrome reaches its peak at days 3–5 after CY treatment, and is manifested by severe lymphopenia and neutropenia (followed later by a period of rebound neutrophilia), strong eosinophilia, and an altered lymphocyte/neutrophil ratio (26, 27).
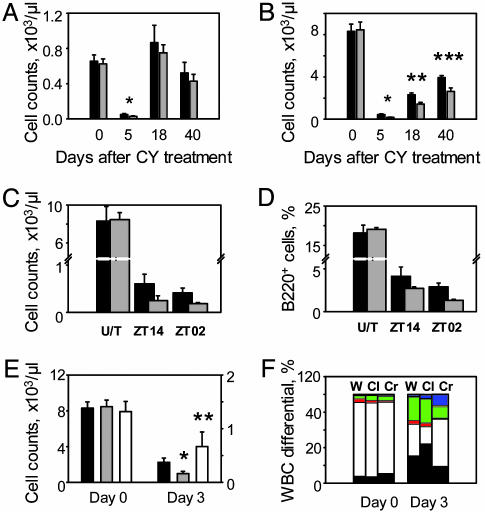
To test whether the higher sensitivity of Clock mutant mice to CY can be explained by an effect on hematopoietic cells, we first measured basic hematological parameters (WBC counts with differentials) of wild-type and Clock/Clock mice at days 0, 5, 18, and 40 after receiving a 3 × 150 mg/kg dose of CY at ZT14. Untreated mice of both genotypes showed no difference in WBC parameters (Table 1, which is published as supporting information on the PNAS web site). As expected, at day 5 after CY administration, both groups demonstrate severe reduction in the neutrophil and lymphocyte counts, which was more pronounced in Clock mutant mice. While the neutrophil counts did not reach a statistically significant difference (Fig. 4A), reduction in the number of circulating lymphocytes was more severe in Clock/Clock mice both at the peak time of bone marrow suppression (day 5) and during the recovery period (days 18 and 40) (Fig. 4B and Table 1). These data suggest that the time-of-administration- and genotype-related differences in response to CY-induced toxicity may reflect CLOCK/BMAL1-dependent modulation of lymphocyte survival/recovery rate.
Fig. 4.
The functional status of CLOCK/BMAL1 transcriptional complex modulates the sensitivity of hematopoietic cells to CY-induced toxicity. (A) Neutrophil counts in peripheral blood of wild-type (black bars) and Clock/Clock mice (gray bars) measured at different times after 3 × 150 mg/kg CY injected at ZT14. At day 5 after treatment, the amount of neutrophils in Clock/Clock mice is slightly lower (P = 0.1). (B) Lymphocyte counts in the same blood samples. At all times tested, Clock mutant mice show significant lower number of circulating lymphocytes (*, P = 0.02; **, P = 0.004; ***, P = 0.01) when compared with wild-type controls. (C) Lymphocyte counts in peripheral blood of wild-type and Clock/Clock mice at day 3 after 1 × 300 mg/kg CY injected at ZT14 or ZT02. A generalized linear model ANOVA detects significant effect of genotype (df = 1, F = 20.83, P = 0.000115) and time of injection (df = 1, F = 4.28, P = 0.049). No significant interaction of two parameters was detected. (D) B220-positive cells in the bone marrow of wild-type and Clock/Clock mice at day 3 after 1 × 300 mg/kg CY injection at ZT14 and ZT02. A generalized linear model ANOVA detects significant effect of genotype (df = 1, F = 31.55, P = 0.000039) and time of injection (df = 1, F = 24.37, P = 0.000149). No significant interaction was detected. (E) Lymphocyte counts in peripheral blood of mice of different circadian genotypes after 1 × 300 mg/kg CY administration at ZT11.5. No differences detected in untreated mice of all three genotypes. However, at day 3, Clock/Clock mice show significantly more severe reduction in lymphocyte survival rate compared with wild-type animals (*, P = 0.03), whereas Cry1-/-Cry2-/- mice retain higher lymphocyte levels (**, P = 0.05). (F) WBC composition of wild-type, Clock/Clock, and Cry1-/-Cry2-/- mice at days 0 and 3 after 1 × 300 mg/kg CY administration. Each stack in the bar represents cell type percentage: neutrophils are black, lymphocytes are white, monocytes are red, eosinophils are green, basophils are yellow, and the large unstained cell population is blue. W, wild-type; Cl, Clock/Clock; Cr, Cry1-/-Cry2-/-.
To test this hypothesis, we measured the number of circulating lymphocytes in the blood of control and Clock mutant mice at day 3 after a single CY injection performed at ZT02 or ZT14 (schedule is presented in Fig. 2A). Consistent with the in vivo response, the number of circulating lymphocytes was significantly lower in wild-type mice injected at ZT02 compared with ones injected at ZT14, whereas Clock mutant mice showed the reduced number of circulating lymphocytes at both injection times (Fig. 4C).
The reduction in WBC counts during the course of drug therapy reflects the suppression of hematopoietic activity of the bone marrow. To test whether the observed differences in the number of circulating lymphocytes reflect similar differences in the sensitivity of the bone marrow cells, we performed flow cytometry analysis of the bone marrow cells stained with FITC-labeled B220 antibodies. CY treatment results in severe reduction in the amount of B cells that, similar to circulating lymphocytes, was significantly more pronounced in Clock/Clock mice and in wild-type mice injected at ZT02 (Fig. 4D).
If the response to CY indeed correlates with B cell sensitivity, then, based on in vivo data (Fig. 2), one might expect that lymphocytes of Cry1-/-Cry2-/- double-knockout mice would also be more resistant to CY. To test this prediction, we compared the WBC parameters of wild-type, Clock/Clock, and Cry1-/-Cry2-/- mice before and after (on day 3) CY administration. There was no difference in circulating lymphocyte counts or in the lymphocyte/neutrophil ratio in untreated mice from the three groups (Fig. 4 E and F and Table 1); however, after exposure to CY, Cry1-/-Cry2-/- mice still retain significantly higher lymphocyte levels even compared with wild-type controls, and their overall WBC parameters were not dramatically distorted as a result of treatment (Fig. 4F). Similar results were obtained during the analysis of the survival of splenic B cells obtained from the same animals (Fig. 6, which is published as supporting information on the PNAS web site).
In summary, these data conclusively demonstrate that circadian control of drug response to CY in vivo is mediated through a CLOCK/BMAL1-dependent modulation of B cell survival/recovery. The exact mechanism of this control and the direct molecular targets however remains to be identified. Thus, similar to what has been reported for a different circadian model, Per2 mutant mice (28), CLOCK/BMAL1 may be directly involved in the regulation of expression of cell-cycle- and apoptosis-related genes, in which case daily and genotype-dependent variations in balance of their products will determine response to stress at the individual cell level. On the other hand, circadian regulation of the CY response could be modulated by CLOCK/BMAL1-dependent control of extracellular signaling pathways. It is well known that treatment with different growth factors and cytokines can prevent cell death induced by a variety of toxic agents. Some of these agents, such as basic fibroblast growth factor, epidermal growth factor, and transforming growth factor, are known to display daily variations in plasma concentrations (29–31). In this case, time-of-day and genotype-dependent changes in drug response might be explained by variations in plasma concentrations of growth factors and cytokines important for B cell survival.
Although the exact mechanism of circadian control of CY-induced toxicity remains to be determined, the striking differences in response of different circadian mutant mice to CY treatment and the direct correlation of this response with the functional status of major circadian transactivation complex not only provides compelling evidence for circadian control of cancer therapy but also highlights a cellular target of this regulation. CLOCK/BMAL1-dependent modulation of the lymphocyte survival/recovery rate may be an important factor determining in vivo drug response and survival in clinical therapy. Thus, the reduction of the lymphocytes and late recovery may imply that, besides the overall increased infection risk due to neutropenia, patients treated with chemotherapy are at risk for developing bacterial infection for a considerable period after treatment, extending beyond the period of bone marrow depression.
The elucidation of the molecular mechanisms underlying the circadian control of B cell survival will provide a rationale not only for adjusting the timing of chemotherapeutic treatment to be less toxic but also for providing a basis for a search for pharmacological modulators of drug toxicity acting through circadian system regulators. This result may significantly increase the therapeutic index and reduce morbidity associated with anticancer treatment.
Supplementary Material
Acknowledgments
We thank Dr. Merrill Egorin and Dr. Ernest Borden for helpful discussions on the PK data analysis, Dr. Anatoly Gleiberman for assistance in hepatocyte culture preparation, and Dr. Martha Vitaterna for assistance with animal experiments. We also thank Mass Spectrometry II Core in the Lerner Research Institute of the Cleveland Clinic Foundation for analysis of CY and metabolites. J.S.T. is an Investigator in the Howard Hughes Medical Institute. This work was supported by National Institutes of Health Grants CA102522 (to M.P.A.) and CA88071 (to A.V.G.).
Author contributions: V.Y.G., A.V.G., J.S.T., and M.P.A. designed research; V.Y.G., R.V.K., R.Z., S.C., and M.P.A. performed research; V.Y.G., R.V.K., A.V.G., and M.P.A. analyzed data; and J.S.T. and M.P.A. wrote the paper.
Abbreviations: CY, cyclophosphamide; 4-OH. 4-hydroperoxy-CY; DCE, 3-dechloroehyl-CY; CB, carboxyethyl-CY; PM, phosphoroamide mustard; ZT, zeitgeber time; PK, pharmacokinetic.
References
- 1.Reppert, S. M. & Weaver, D. R. (2002) Nature 418, 935-941. [DOI] [PubMed] [Google Scholar]
- 2.Panda, S., Hogenesch, J. B. & Kay, S. A. (2002) Nature 417, 329-335. [DOI] [PubMed] [Google Scholar]
- 3.Lowrey, P. L. & Takahashi, J. S. (2004) Annu. Rev. Genomics Hum. Genet. 5, 407-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoo, S. H., Yamazaki, S., Lowrey, P. L., Shimomura, K., Ko, C. H., Buhr, E. D., Siepka, S. M., Hong, H. K., Oh, W. J., Yoo, O. J., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 5339-5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panda, S., Antoch, M. P., Miller, B. H., Su, A. I., Schook, A. B., Straume, M., Schultz, P. G., Kay, S. A., Takahashi, J. S. & Hogenesch, J. B. (2002) Cell 109, 307-320. [DOI] [PubMed] [Google Scholar]
- 6.Storch, K. F., Lipan, O., Leykin, I., Viswanathan, N., Davis, F. C., Wong, W. H. & Weitz, C. J. (2002) Nature 417, 78-83. [DOI] [PubMed] [Google Scholar]
- 7.Akhtar, R. A., Reddy, A. B., Maywood, E. S., Clayton, J. D., King, V. M., Smith, A. G., Gant, T. W., Hastings, M. H. & Kyriacou, C. P. (2002) Curr. Biol. 12, 540-550. [DOI] [PubMed] [Google Scholar]
- 8.Haus, E., Halberg, F., Pauly, J. E., Cardoso, S., Kuhl, J. F., Sothern, R. B., Shiotsuka, R. N. & Hwang, D. S. (1972) Science 177, 80-82. [DOI] [PubMed] [Google Scholar]
- 9.Levi, F. (1997) in Handbook of Experimental Pharmacology: Physiology and Pharmacology of Biological Rhythms (Springer, Berlin), Chapter 11, pp. 299-331.
- 10.Fu, L. & Lee, C. C. (2003) Nat. Rev. Cancer 3, 350-361. [DOI] [PubMed] [Google Scholar]
- 11.Vitaterna, M. H., King, D. P., Chang, A. M., Kornhauser, J. M., Lowrey, P. L., McDonald, J. D., Dove, W. F., Pinto, L. H., Turek, F. W. & Takahashi, J. S. (1994) Science 264, 719-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bunger, M. K., Wilsbacher, L. D., Moran, S. M., Clendenin, C., Radcliffe, L. A., Hogenesch, J. B., Simon, M. C., Takahashi, J. S. & Bradfield, C. A. (2000) Cell 103, 1009-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vitaterna, M. H., Selby, C. P., Todo, T., Niwa, H., Thompson, C., Fruechte, E. M., Hitomi, K., Thresher, R. J., Ishikawa, T., Miyazaki, J., et al. (1999) Proc. Natl. Acad. Sci. USA 96, 12114-12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Browder, T., Butterfield, C. E., Kraling, B. M., Shi, B., Marshall, B., O'Reilly, M. S. & Folkman, J. (2000) Cancer Res. 60, 1878-1886. [PubMed] [Google Scholar]
- 15.Sottani, C., Turci, R., Perbellini, L. & Minoia, C. (1998) Rapid Commun. Mass Spectrom. 12, 1063-1068. [DOI] [PubMed] [Google Scholar]
- 16.DeLeve, L. D. (1996) Hepatology 24, 830-837. [DOI] [PubMed] [Google Scholar]
- 17.van der Horst, G. T., Muijtjens, M., Kobayashi, K., Takano, R., Kanno, S., Takao, M., de Wit, J., Verkerk, A., Eker, A. P., van Leenen, D., et al. (1999) Nature 398, 627-630. [DOI] [PubMed] [Google Scholar]
- 18.Okamura, H., Miyake, S., Sumi, Y., Yamaguchi, S., Yasui, A., Muijtjens, M., Hoeijmakers, J. H. & van der Horst, G. T. (1999) Science 286, 2531-2534. [DOI] [PubMed] [Google Scholar]
- 19.Boddy, A. V. & Yule, S. M. (2000) Clin. Pharmacokinet. 38, 291-304. [DOI] [PubMed] [Google Scholar]
- 20.Sladek, N. E. (1988) Pharmacol. Ther. 37, 301-355. [DOI] [PubMed] [Google Scholar]
- 21.Roy, P., Yu, L. J., Crespi, C. L. & Waxman, D. J. (1999) Drug Metab. Dispos. 27, 655-666. [PubMed] [Google Scholar]
- 22.Huang, Z., Roy, P. & Waxman, D. J. (2000) Biochem. Pharmacol. 59, 961-972. [DOI] [PubMed] [Google Scholar]
- 23.DeLeve, L. D., Wang, X., Kuhlenkamp, J. F. & Kaplowitz, N. (1996) Hepatology 23, 589-599. [DOI] [PubMed] [Google Scholar]
- 24.Lowe, S. W., Ruley, H. E., Jacks, T. & Housman, D. E. (1993) Cell 74, 957-967. [DOI] [PubMed] [Google Scholar]
- 25.Colvin, O. M. (1999) Cur. Pharm. Des. 5, 555-560. [PubMed] [Google Scholar]
- 26.Watters, J. W., Kloss, E. F., Link, D. C., Graubert, T. A. & McLeod, H. L. (2003) J. Appl. Physiol. 95, 1352-1360. [DOI] [PubMed] [Google Scholar]
- 27.Artym, J., Zimecki, M. & Kruzel, M. (2004) Med. Sci. Monit. 10, BR84-BR89. [PubMed] [Google Scholar]
- 28.Fu, L., Pelicano, H., Liu, J., Huang, P. & Lee, C. (2002) Cell 111, 41-50. [DOI] [PubMed] [Google Scholar]
- 29.Haus, E., Dumitriu, L., Nicolau, G. Y., Bologa, S. & Sackett-Lundeen, L. (2001) Chronobiol. Int. 18, 709-727. [DOI] [PubMed] [Google Scholar]
- 30.Holzheimer, R. G., Curley, P., Saporoschetz, I. B., Doherty, J. M., Mannick, J. A. & Rodrick, M. L. (2002) Shock 17, 527-529. [DOI] [PubMed] [Google Scholar]
- 31.Liu, J. H. (2002) Curr. Eye Res. 24, 75-80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.