Abstract
Advanced glycation end products (AGEs), which accumulate in the body during the development of diabetes, may be one of the factors leading to pancreatic β-cell failure and reduced β-cell mass. However, the mechanisms responsible for AGE-induced apoptosis remain unclear. This study identified the role and mechanisms of action of tribbles homolog 3 (TRB3) in AGE-induced β-cell oxidative damage and apoptosis. Rat insulinoma cells (INS-1) were treated with 200 µg/ml AGEs for 48 h, and cell apoptosis was then detected by TUNEL staining and flow cytometry. The level of intracellular reactive oxygen species (ROS) was measured by a fluorescence assay. The expression levels of receptor of AGEs (RAGE), TRB3, protein kinase C β2 (PKCβ2) and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4 (NOX4) were evaluated by RT-qPCR and western blot analysis. siRNA was used to knockdown TRB3 expression through lipofection, followed by an analysis of the effects of TRB3 on PKCβ2 and NOX4. Furthermore, the PKCβ2-specific inhibitor, LY333531, was used to analyze the effects of PKCβ2 on ROS levels and apoptosis. We found that AGEs induced the apoptosis of INS-1 cells and upregulated RAGE and TRB3 expression. AGEs also increased ROS levels in β-cells. Following the knockdown of TRB3, the AGE-induced apoptosis and intracellular ROS levels were significantly decreased, suggesting that TRB3 mediated AGE-induced apoptosis. Further experiments demonstrated that the knockdown of TRB3 decreased the PKCβ2 and NOX4 expression levels. When TRB3 was knocked down, the cells expressed decreased levels of PKCβ2 and NOX4. The PKCβ2-specific inhibitor, LY333531, also reduced AGE-induced apoptosis and intracellular ROS levels. Taken together, our data suggest that TRB3 mediates AGE-induced oxidative injury in β-cells through the PKCβ2 pathway.
Keywords: advanced glycation end products, β-cell, apoptosis, tribbles homolog 3, protein kinase C β, reactive oxygen species
Introduction
Type 2 diabetes is one of the most prevalent chronic diseases worldwide and has serious social and health consequences, and poses a heavy economic burden. Its clinical characteristics include insulin resistance, pancreatic β-cell dysfunction and reduced β-cell numbers (1). In the pathogenesis of type 2 diabetes, high blood glucose, inflammatory cytokines, high free fatty acids (FFAs) and amyloid deposits are the important factors in the progression of diabetes, all of which lead to β-cell apoptosis (2). The identification of the mechanisms responsible for β-cell apoptosis are necessary in order to understand the pathogenesis and to aid in the development of effective treatments for patients with type 2 diabetes.
Recent studies have demonstrated that advanced glycation end products (AGEs) may promote β-cell apoptosis in the pathogenesis of type 2 diabetes (1,3–5). AGEs are irreversible, complex and ultimately form after a series of non-enzymatic reactions of proteins, lipids and reducing glucoses. The production is accelerated when blood glucose is high, thereby increasing the accumulation of AGEs in the body (6,7). Previous studies have demonstrated that AGEs are closely related to diabetic microangiopathy (7–10). After AGEs bind to the receptor for AGEs (RAGE) on endothelial cells, they activate the signaling pathways of glycogen synthase kinase 3β (GSK3β), p38 mitogen-activated protein kinase (p38 MAPK), extracellular signal-regulated kinase 1 and 2 (ERK1/2), c-Jun amino-terminal kinase (JNK) and nuclear factor-κB (NF-κB), all of which lead to endothelial cell dysfunction and diabetic vascular disease (11–15).
Recent studies have also demonstrated that AGEs play an important role in β-cell failure. The stimulation of AGEs in in vitro and in vivo models has been shown to directly cause the apoptosis of β-cells (3,6,16–18). AGEs stimulate reactive oxygen species (ROS) generation, and, mediated by their receptor (RAGE), induce β-cell apoptosis (3,16). However, these above-mentioned studies have not fully elucidated the molecular mechanisms of action of AGEs in β-cells. Therefore, the roles of AGEs in β-cell apoptosis and their mechanisms of action warrant further investigation.
Tribbles homolog 3 (TRB3) is one of the family members of tribble homologous proteins. It inhibits mitosis and is a regulatory factor of the protein kinase B (Akt) pathway (19). Through the inhibition of Akt activity, TRB3 negatively regulates the insulin-signaling pathway (20). Our previous studies demonstrated that TRBs play an important role in β-cell apoptosis. High blood glucose, high fat and endoplasmic reticulum (ER) stress upregulate TRB3 expression, which mediates β-cell apoptosis (21–23). The identification of TRB3 participation in AGE-induced β-cell apoptosis is worthy of investigation. Studies on cardiomyocytes, epithelial cells and retinal diabetic nephropathy have shown that the isoform of protein kinase C (PKC) and PKC β2 (PKCβ2) plays an important role in AGE-mediated cell damage and kidney damage. By increasing PKCβ2 expression, AGEs enhance PKCβ2 activity, as well as the effects and displacement of PKCβ2, increasing ROS formation, which ultimately causes oxidative damage (24–27). Our previous study demonstrated that TRB3 activated PKCδ and was involved in high-fat-mediated β-cell apoptosis (22). In this study, we focused on AGE-mediated β-cell apoptosis. We also determined whether TRB3 triggered the activation and isoform(s) of PKC, and whether it mediated the damaging effects of AGEs.
Materials and methods
Cell culture
The rat insulinoma cell line, INS-1 (a gift from Dr Haiyan Wang, University of Geneva, Geneva, Switzerland), was maintained in RPMI-1640 containing 10% fetal bovine serum (FBS) (both from Life Technologies, Waltham, MA, USA), 10 mM HEPES, 2 mM glutamine and 1 mM sodium pyruvate (all from Sigma-Aldrich, St. Louis, MO, USA), 50 µM β-mercaptoethanol, 100 U/ml penicillin and 100 µg/ml streptomycin in an incubator containing 5% CO2 at 37°C. For the experiments, the INS-1 cells were cultured with or without 200 µg/ml AGEs (ab51995; Abcam, Cambridge, MA, USA) for 48 h and collected for further examination following treatment. INS-1 cells were pre-incubated with PKC inhibitor, LY-333531 (10 µM) (2362; Axon Medchem, Groningen, The Netherlands), for 30 min and then co-treated with AGEs for 48 h.
RNA interference
Lipofectamine 2000 (Invitrogen, Waltham, MA, USA) was used to transfect TRB3 small interfering RNA (siRNA siTRB3; purchased from GenePharma Co., Ltd., Shanghai, China) and the negative control small interference RNA (siNC) and into the INS-1 cells in accordance with the manufacturer's instructions. Target gene sequences were described in our previous study (23).
Detection of apoptosis
TUNEL staining and flow cytometry were used to detect the apoptosis of INS-1 cells. A TUNEL kit (Roche Diagnostics, Indianapolis, IN, USA) was used to detect the apoptosis of the INS-1 cells seeded in 96-well microplates following the individual treatments strictly according to the manufacturer's instructions. Positively stained cells were counted under a light microscope (DMI6000 B; Leica Microsystems, Wetzlar, Germany). The apoptotic rate of the INS-1 cells was examined by flow cytometry using an in situ cell apoptosis detection kit (BD Biosciences, San Diego, CA, USA), while strictly adhering to the manufacturer's instructions.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the INS-1 cells after the corresponding treatments using an RNA extraction kit (Qiagen, Hilden, Germany). Two micrograms of total RNA were used to synthesize the cDNA in a reverse transcription reaction (reverse transcriptase was purchased from Promega, Madison, WI, USA). The RT-PCR reaction and data were analyzed as previously described (28). The MyiQ real-time PCR thermal cycler and SYBR-Green PCR Master Mix kit (both from Bio-Rad Laboratories, Inc., Hercules, CA, USA) were used for the qPCR analyses. Target genes were quantified using MyiQ system software. The specific sequences of the primers used in this study were as follows: β-actin forward, 5′-GACATCCGTAAAGACCTCTATGCC-3′ and reverse, 5′-ATAGAGCCACCAATCCACACAGAG-3′; RAGE forward, 5′-GGAAGGACTGAAGCTTGGAAGG-3′ and reverse, 5′-TCCGATAGCTGGAAGGAGGAGT-3′; TRB3 forward, 5′-TGTCTTCAGCAACTGTGAGAGGACGAAG-3′ and reverse, 5′-GTAGGATGGCCGGGAGCTGAGTATC-3′; nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4 (NOX4 forward, 5′-TAGCTGCCCACTTGGTGAACG-3′ and reverse, 5′-TGTAACCATGAGGAACAATACCACC-3′.
Western blot analysis of protein expression
Following the corresponding treatments of the INS-1 cells, all cellular proteins were lysed in RIPA lysis buffer (Roche Diagnostics) containing protease inhibitors and the concentration was measured using a BCA protein assay kit (Beyotime Institute of Biotechnology, Shanghai, China). Total proteins (20–40 µg)were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were then transferred onto a PVDF membrane followed by blocking the non-specific antigen and incubating with the corresponding primary antibody overnight. The primary antibodies used in this study were: a mouse anti-rat β-actin antibody (A5316; 1:20,000) and a rabbit anti-rat RAGE antibody (R5278; 1:1,000) (both from Sigma-Aldrich); a rabbit anti-rat PKCβ2 antibody (07-873-I; 1:1,000) and a mouse anti-rat TRB3 antibody (ST1032; 1:1,000) (both from Calbiochem, Billerica, MA, USA), and a rabbit anti-rat NOX4 antibody (ab133303; 1:1,000; Abcam). The secondary antibodies used in this study were a goat anti-mouse IgG antibody (A3682) and a goat anti-rabbit IgG antibody (A0545) (1:20,000; both from Sigma-Aldrich). An analysis of the protein bands was performed using Quantity One gel analysis software (Bio-Rad Laboratories, Inc.).
Detection of ROS levels
ROS levels in the INS-1 cells cultured in 96-well microplates following the corresponding treatments were measured using a ROS detection assay kit (Shanghai Genmed Gene Pharmaceutical Technology Co., Ltd., Shanghai, China) with strict adherence to the manufacturer's instructions. A fluorescence detection microplate reader was used to measure the fluorescence intensity of the assay.
Statistical analysis
In this study, data are presented as the means ± standard error of the mean (means ± SEM). SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. A comparison between 2 groups was performed using the t-test. Comparisons among groups were performed using analysis of variance (ANOVA). A value of P<0.05 was considered to indicate a statistically significant difference.
Results
AGEs induce the apoptosis of INS-1 cells
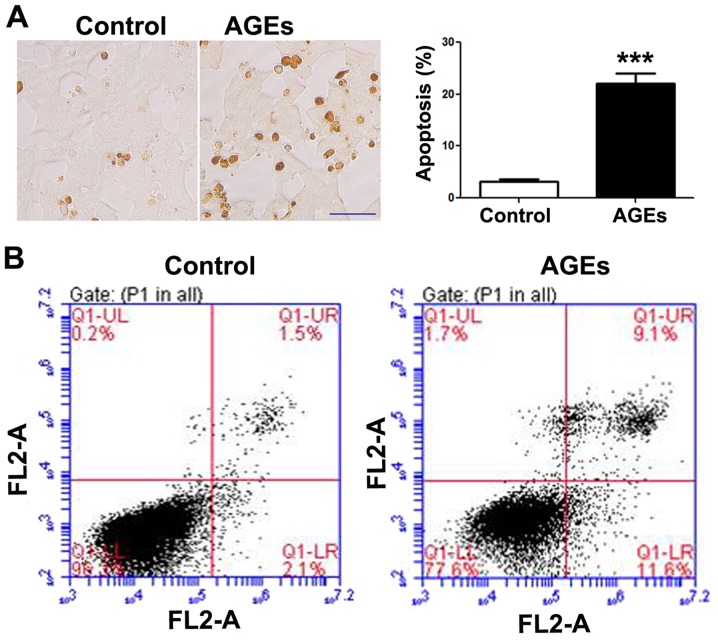
Following exposure to the AGEs (200 µg/ml) for 48 h, apoptosis was increased in the INS-1 cells as compared to the control group, as shown by TUNEL staining (Fig. 1A) and flow cytometry (Fig. 1B). A statistically significant difference in INS-1 cell apoptosis was observed between the AGE-treated group and the control group (untreated group).
Figure 1.
Advanced glycation end products (AGE) induce the apoptosis of INS-1 cells. (A) TUNEL staining detected the apoptosis of INS-1 cells 48 h following exposure to AGEs (200 µg/ml). Data are presented as the means ± SEM (n=3). ***P<0.001, compared with the control group. Scale bar, 50 µm. (B) Flow cytometry was used to detect the apoptosis of INS-1 cells. INS-1 cells were exposed to 200 µg/ml AGEs for 48 h, followed by Annexin V/PI staining assay to detect the apoptosis of INS-1 cells by flow cytometry. Top left quadrant, necrotic cells; bottom left quadrant, live cells; top right quadrant, late apoptotic cells; bottom right quadrant, early apoptotic cells.
AGEs upregulate intracellular TRB3 expression in INS-1 cells
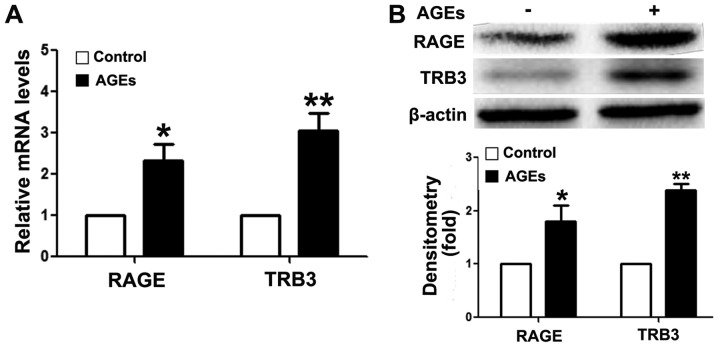
To analyze the mechanism of action of AGEs, we first detected RAGE expression in INS-1 cells following exposure to AGEs. As shown in Fig. 2, the mRNA (Fig. 2A) and protein expression (Fig. 2B) levels of RAGE were upregulated following exposure to AGEs, suggesting that AGEs mediated the apoptosis of INS-1 cells through RAGE. These findings further validate the results of previous studies (3,9). In addition, AGEs upregulated intracellular TRB3 expression levels at the mRNA and protein level (Fig. 2).
Figure 2.
Advanced glycation end products (AGE) increase intracellular tribbles homolog 3 (TRB3) expression of INS-1 cells. (A) RT-qPCR was used to detect the mRNA expression levels of receptor of AGEs (RAGE) and TRB3; (B) western blot analysis was used to measure the protein expression of RAGE and TRB3. β-actin was used as a loading control. Optical densitometry quantified the brightness of protein bands. Our findings demonstrated the fold difference compared to the control group. Data are presented as the means ± SEM (n=3). *P<0.05 and **P<0.01, compared with the control group.
AGEs increase intracellular ROS levels
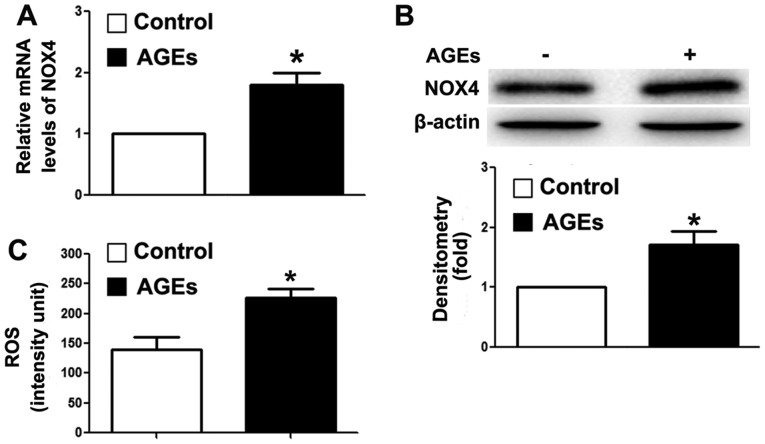
Our previous study demonstrated that the overexpression of TRB3 facilitated high-glucose-induced oxidative stress (21). Thus, in this study, we detected intracellular NOX4 expression and ROS levels. As shown in Fig. 3A and B, AGEs upregulated the mRNA and protein expression levels of NOX4. NOX4 is a major enzyme for the synthesis of intracellular ROS (39). In this study, we detected an increase in intracellular ROS levels in the cells following exposure to AGEs (Fig. 3C). Our findings indicated that AGEs promoted ROS synthesis, and further induced INS-1 cell damage and apoptosis through TRB3.
Figure 3.
Advanced glycation end products (AGEs) increase intracellular reactive oxygen species (ROS) synthesis in INS-1 cells. (A) RT-qPCR was used to detect the mRNA expression levels of nicotinamide adenine dinucleotide phosphate oxidase 4 (NOX4); (B) western blot analysis was used to detect the protein expression of NOX4. β-actin was used as a loading control measured the optical densitometry of the brightness of the protein bands. Our results represented the relative fold change compared to the control group; (C) a ROS detection assay was used to measure the intracellular ROS levels. Data are presented as the means ± SEM (n=3). *P<0.05 and **P<0.01, compared with the control group.
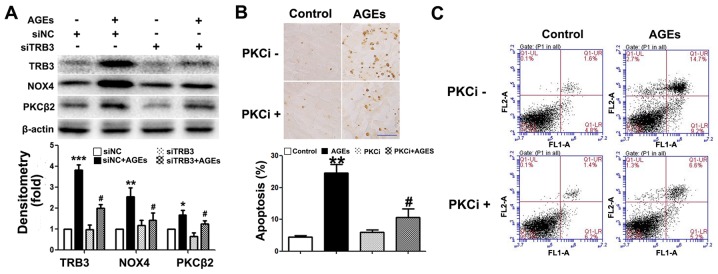
The silencing of TRB3 expression by siRNA suppresses AGE-induced ROS synthesis and the apoptosis of INS-1 cells
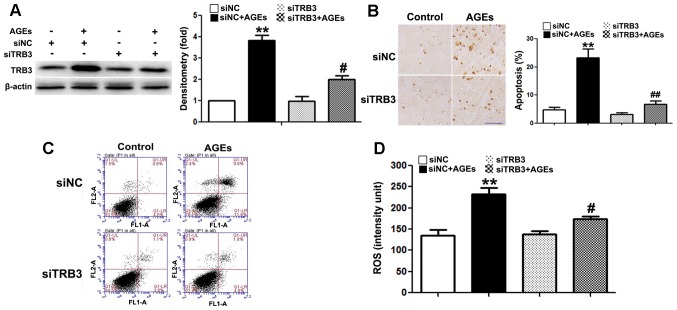
To further determine whether TRB3 participates in AGE-induced cell damage and apoptosis, we knocked down the expression of TRB3 in INS-1 cells using siRNA (Fig. 4A). Both AGE-induced cell apoptosis (Fig. 4B and C) and the intracellular ROS levels were significantly reduced in the cells in which TRB3 was knocked down (Fig. 4D). This result suggested that TRB3 is involved in AGE-induced oxidative damage and the apoptosis of INS-1 cells.
Figure 4.
Effect of the knockdown of tribbles homolog 3 (TRB3) on the advanced glycation end product (AGE)-induced apoptosis of INS-1 cells. (A) Western blot analysis confirmed the efficiency of intracellular TRB3 silencing in INS-1 cells; (B and C) TUNEL staining and flow cytometry were used to examine the effect of TRB3 silencing on the AGE-induced apoptosis of INS-1 cells. Scale bar, 50 µm. (D) Effect of TRB3 silencing on AGE-associated intracellular reactive oxygen species (ROS) levels in INS-1 cells. Data are presented as the means ± SEM (n=3). **P<0.01, compared with the siNC group; #P<0.05 and ##P<0.01, compared the siNC + AGEs group.
TRB3 regulates AGE-induced ROS synthesis and the apoptosis of INS-1 cells through the PKCβ2 pathway
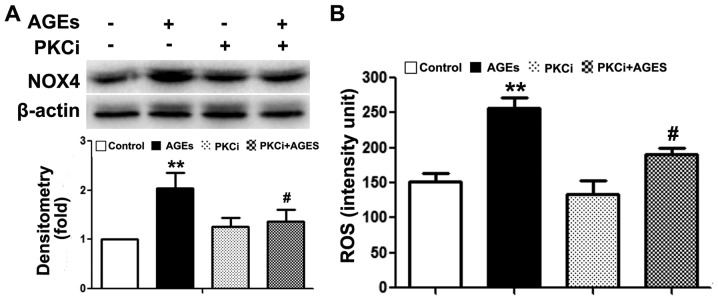
Previous studies have demonstrated that the PKCβ2 pathway plays a key role in AGE-induced oxidative damage to non-islet β-cells (26–29). However, its exact role in β-cells remains unclear. In this study, we observed an upregulated PKCβ2 expression in INS-1 cells following exposure to AGEs (Fig. 5A). Following the knockdown of TRB3 expression, PKCβ2 and NOX4 expression was downregulated (Fig. 5A). Furthermore, following pre-treatment with the PKCβ2 specific inhibitor, LY333531, AGE-induced INS-1 cell apoptosis, the activity of NOX4 and the intracellular ROS levels were all significantly decreased (Figs. 5B and C, and 6A and B). This result indicated that TRB3 was involved in AGE-induced oxidative damage and the apoptosis of INS-1 cells through the upregulation of PKCβ2 activity.
Figure 5.
Inhibition of protein kinase C β2 (PKCβ2) pathway protects INS-1 cells from advanced glycation end product (AGE)-induced apoptosis. (A) Western blot analysis was used to detect the protein expression of tribbles homolog 3 (TRB3), PKCβ2 and NADPH. β-actin was the load control of the protein blot. Optical densitometry quantified the brightness of protein bands; (B and C) TUNEL staining and FACS analysis detected apoptosis of the 48 h AGEs-treated INS-1 cells with 10 nM LY333531 or without LY333531 invention. Scale bar, 50 µm. Data are as the means ± SEM (n=3). *P<0.05, **P<0.01 and ***P<0.001, compared with control or siNC group; #P<0.05, compared with the AGEs group.
Figure 6.
Inhibition of protein kinase C β2 (PKCβ2) pathway reduces advanced glycation end product (AGE)-induced NADPH oxidase activity and reactive oxygen species (ROS) synthesis. (A) Western blot analysis was used to examine the effect of PKCβ2 inhibitor on AGE-associated NAPDH protein expression. β-actin was used as the loading control of the protein blot. Optical densitometry was used to quantify the brightness of protein bands; (B) a ROS detection assay was used to examine the effect of PKCβ2 inhibitor on AGE-associated intracellular ROS level in the cells. Data are presented as the means ± SEM (n=3). **P<0.01, compared with the control group; #P<0.05, compared with the AGEs group.
Discussion
Studies using diabetic animal models and clinical specimens from diabetic patients have demonstrated that, with the progression of diabetes, the AGE levels in the body gradually increase (18,26). It has also been demonstrated that AGEs play an important role in diabetic retinopathy, kidney diseases, neuropathy and cardiomyopathy (29). Previous studies have shown that AGEs are the main factors which induce β-cell dysfunction and apoptosis (3,16,18). Thus, it is important to unravel the molecular mechanisms of action of AGEs in order to protect β-cells from injury. In this study, we found that AGEs upregulated TRB3 expression in INS-1 cells and mediated oxidative damage and the apoptosis of β-cells through PKCβ2.
AGEs bind with RAGE on cell membranes and trigger cellular functional response. RAGE is a multi-ligand cell surface receptor and belongs to the immunoglobulin super-family (30). RAGE can be activated by binding with different types of ligands, including AGEs, S100 proteins, HMGB1s and Aβ peptides (31–35). The activation of RAGE is associated with a number of chronic diseases, including different types of diabetic complications (e.g., neuropathy and nephropathy), microvascular disease and chronic inflammation (7). In this study, exposure to AGEs promoted the apoptosis of INS-1 cells and increased the expression of their receptor, RAGE; thus, RAGE mediates the damaging effects of AGEs on β-cells (3,16,18,36).
During the course of diabetes, oxidative stress and ER stress are the direct factors causing β-cell dysfunction and apoptosis (37), which results in insulin resistance in type 2 diabetes and β-cell dysfunction (38). Factors involved in oxidative stress include high blood glucose, FFAs and cytokines (38). In recent studies, AGEs have been shown to induce β-cell damage through oxidative stress (3,16,18). In this study, following exposure to AGEs, the ROS levels in INS-1 cells were significantly elevated. In addition, NOX expression was downregulated. This result indicated that AGEs induced oxidative stress in INS-1 cells. NADPH oxidases are the major sources for intracellular ROS synthesis and generally have NOX1, NOX2, NOX4 and NOX5 types. A notable feature of NOX4 is its constitutive activity and preferential generation of a hydrogen superoxide anion that acts as an oxygen sensor (39). In addition, NOX4 has been confirmed to play an important role in glucocorticoid-induced INS-1 cell injury (28). Our results indicated that the AGE-induced oxidative injury to INS-1 cells may be an important cause of the apoptosis of INS-1 cells.
Many pathways are involved in mediating oxidative damage in cells. Our previous study showed that TRB3 was associated with oxidative stress in high-glucose-induced β-cells failure (21). TRB3 is a homolog of Drosophila tribbles protein and mammalian protein. TRB3 is widely expressed in insulin targeted tissues and is closely associated with insulin resistance and glucose homeostasis (40). There is recent evidence to suggest that TRB3 plays an important role in apoptosis. However, its role remains controversial. Some studies have shown that TRB3 promotes the cytokine-induced apoptosis of pancreatic β-cells, as well as the ER stress-induced apoptosis of 293 cells, PC-12 cells (rat neuronal cell line) (41–43). Other studies have shown that TRB3 exerts an anti-apoptotic effect against the nutrient starvation-induced apoptosis of human prostate carcinoma PC-3 cells, and SaOS2 cells (44,45). These differences may be due to different cell types and stresses caused by different stimuli. Relevant studies on β-cell apoptosis have indicated that TRB3 plays a key role in high blood glucose, high fat, FFA and cytokine-induced apoptosis in β-cells (21,22,41). In this study, we found that AGEs stimulated INS-1 apoptosis and increased the expression of TRB3. The knockdown of TRB3 expression inhibited the apoptosis of INS-1 cells. Moreover, the NOX4 and ROS levels were also decreased, indicating that TRB3 plays an important role in the AGE-induced apoptosis of INS-1 cells by affecting ROS levels. The study by Gorasia et al demonstrated that β-cells were susceptible to injury caused by oxidative stress and ER stress (46) and an increased effect between oxidative damage and ER stress (47). TRB3 is an important regulatory molecule in the ER stress-induced apoptotic pathways (42). In this study, we also demonstrated that the knockdown of TRB3 expression affected AGE-induced ROS synthesis and provided evidence of the interaction between oxidative damage and ER stress in β-cells.
Several studies in the past have indicated that the PKC path way is associated with oxidative stress induced by ROS synthesis (24–27,48,49). PKC regulates NADPH oxidase activity and induces ROS synthesis. In addition, PKC plays an important role in AGE-induced oxidative damage in cells. Studies using glomerular microvascular endothelial cells and cardiomyocytes have demonstrated that AGEs enhanced NADPH oxidase activity through PKCβ2 and increased ROS synthesis and cell damage (24–27). In this study, INS-1 cells exhibited an elevated expression of PKCβ2 following exposure to AGEs. Following the knockdown of TRB3, the expression of PKCβ2 was decreased and the activity of NADPH oxidase was also decreased. In addition, the application of specific inhibitors to suppress PKCβ2 activity significantly decreased the intracellular ROS levels and the apoptosis of INS-1 cells. TRB3 regulated NADPH oxidase and ROS levels which caused damage to INS-1 cells by affecting the activity of the PKCβ2 pathway. TRB3, as a related molecule of ER stress-induced apoptosis, regulates PKC. PKC is the important regulatory molecule in the pathway of ROS synthesis. Hence, this study provided a new direction in determining the mechanisms responsible behind the interaction between oxidative damage and ER stress.
In conclusion, this study demonstrated that AGE mediated oxidative stress through TRB3 to damage INS-1 cells and resulted in the apoptosis of INS-1 cells. TRB3 regulated NADPH oxidase activity, promoted ROS synthesis and resulted in oxidative stress in INS-1 cells through the PKCβ2 pathway. Our data provide a new understanding of the mechanisms responsible for AGE-induced oxidative injury to β-cells and a new direction for studies aiming to identify methods with which to protect β-cells from damage.
Acknowledgments
This study was supported by grants from the National Basic Research Program of China (2012CB966402 to Jinning Lou); and the National Nature Science Foundation of China (no. 81370873 to Wenjian Zhang and 81370918 to Xiuli Men).
References
- 1.Nowotny K, Jung T, Höhn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5:194–222. doi: 10.3390/biom5010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volchuk A, Ron D. The endoplasmic reticulum stress response in the pancreatic β-cell. Diabetes Obes Metab. 2010;12(Suppl 2):48–57. doi: 10.1111/j.1463-1326.2010.01271.x. [DOI] [PubMed] [Google Scholar]
- 3.Lim M, Park L, Shin G, Hong H, Kang I, Park Y. Induction of apoptosis of β cells of the pancreas by advanced glycation end-products, important mediators of chronic complications of diabetes mellitus. Ann N Y Acad Sci. 2008;1150:311–315. doi: 10.1196/annals.1447.011. [DOI] [PubMed] [Google Scholar]
- 4.Lee BW, Chae HY, Kwon SJ, Park SY, Ihm J, Ihm SH. RAGE ligands induce apoptotic cell death of pancreatic β-cells via oxidative stress. Int J Mol Med. 2010;26:813–818. [PubMed] [Google Scholar]
- 5.Jung H, Joo J, Jeon Y, Lee J, In J, Kim D, Kang E, Kim Y, Lim Y, Kang J, et al. Advanced glycation end products downregulate glucokinase in mice. Diabetes Metab Res Rev. 2011;27:557–563. doi: 10.1002/dmrr.1208. [DOI] [PubMed] [Google Scholar]
- 6.Puddu A, Storace D, Durante A, Odetti P, Viviani GL. Glucagon-like peptide-1 counteracts the detrimental effects of advanced glycation end-products in the pancreatic beta cell line HIT-T 15. Biochem Biophys Res Commun. 2010;398:462–466. doi: 10.1016/j.bbrc.2010.06.100. [DOI] [PubMed] [Google Scholar]
- 7.Jakus V, Rietbrock N. Advanced glycation end-products and the progress of diabetic vascular complications. Physiol Res. 2004;53:131–142. [PubMed] [Google Scholar]
- 8.Baumann M. Role of advanced glycation end products in hypertension and cardiovascular risk: human studies. J Am Soc Hypertens. 2012;6:427–435. doi: 10.1016/j.jash.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Chilelli NC, Burlina S, Lapolla A. AGEs, rather than hyperglycemia, are responsible for microvascular complications in diabetes: a 'glycoxidation-centric' point of view. Nutr Metab Cardiovasc Dis. 2013;23:913–919. doi: 10.1016/j.numecd.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Yamagishi S, Nakamura N, Suematsu M, Kaseda K, Matsui T. Advanced glycation end products: a molecular target for vascular complications in diabetes. Mol Med. 2015;21(Suppl 1):S32–S40. doi: 10.2119/molmed.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li BY, Li XL, Gao HQ, Zhang JH, Cai Q, Cheng M, Lu M. Grape seed procyanidin B2 inhibits advanced glycation end product-induced endothelial cell apoptosis through regulating GSK3β phosphorylation. Cell Biol Int. 2011;35:663–669. doi: 10.1042/CBI20100656. [DOI] [PubMed] [Google Scholar]
- 12.Sun C, Liang C, Ren Y, Zhen Y, He Z, Wang H, Tan H, Pan X, Wu Z. Advanced glycation end products depress function of endothelial progenitor cells via p38 and ERK 1/2 mitogen-activated protein kinase pathways. Basic Res Cardiol. 2009;104:42–49. doi: 10.1007/s00395-008-0738-8. [DOI] [PubMed] [Google Scholar]
- 13.Adamopoulos C, Piperi C, Gargalionis AN, Dalagiorgou G, Spilioti E, Korkolopoulou P, Diamanti-Kandarakis E, Papavassiliou AG. Advanced glycation end products upregulate lysyl oxidase and endothelin-1 in human aortic endothelial cells via parallel activation of ERK1/2-NF-κB and JNK-AP-1 signaling pathways. Cell Mol Life Sci. 2016;73:1685–1698. doi: 10.1007/s00018-015-2091-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morita M, Yano S, Yamaguchi T, Sugimoto T. Advanced glycation end products-induced reactive oxygen species generation is partly through NF-kappa B activation in human aortic endothelial cells. J Diabetes Complications. 2013;27:11–15. doi: 10.1016/j.jdiacomp.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Sang HQ, Gu JF, Yuan JR, Zhang MH, Jia XB, Feng L. The protective effect of Smilax glabra extract on advanced glycation end products-induced endothelial dysfunction in HUVECs via RAGE-ERK1/2-NF-κB pathway. J Ethnopharmacol. 2014;155:785–795. doi: 10.1016/j.jep.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 16.Lin N, Zhang H, Su Q. Advanced glycation end-products induce injury to pancreatic beta cells through oxidative stress. Diabetes Metab. 2012;38:250–257. doi: 10.1016/j.diabet.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Zhu Y, Shu T, Lin Y, Wang H, Yang J, Shi Y, Han X. Inhibition of the receptor for advanced glycation endproducts (RAGE) protects pancreatic β-cells. Biochem Biophys Res Commun. 2011;404:159–165. doi: 10.1016/j.bbrc.2010.11.085. [DOI] [PubMed] [Google Scholar]
- 18.Coughlan MT, Yap FY, Tong DC, Andrikopoulos S, Gasser A, Thallas-Bonke V, Webster DE, Miyazaki J, Kay TW, Slattery RM, et al. Advanced glycation end products are direct modulators of β-cell function. Diabetes. 2011;60:2523–2532. doi: 10.2337/db10-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng WP, Wang BW, Lo HM, Shyu KG. Mechanical stretch induces apoptosis regulator TRB3 in cultured cardiomyocytes and volume-overloaded heart. PLoS One. 2015;10:e0123235. doi: 10.1371/journal.pone.0123235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300:1574–1577. doi: 10.1126/science.1079817. [DOI] [PubMed] [Google Scholar]
- 21.Qian B, Wang H, Men X, Zhang W, Cai H, Xu S, Xu Y, Ye L, Wollheim CB, Lou J. TRIB3 [corrected] is implicated in glucotoxicity- and endoplasmic reticulum-stress-induced [corrected] beta-cell apoptosis. J Endocrinol. 2008;199:407–416. doi: 10.1677/JOE-08-0331. [DOI] [PubMed] [Google Scholar]
- 22.Qin J, Fang N, Lou J, Zhang W, Xu S, Liu H, Fang Q, Wang Z, Liu J, Men X, et al. TRB3 is involved in free fatty acid-induced INS-1-derived cell apoptosis via the protein kinase C δ pathway. PLoS One. 2014;9:e96089. doi: 10.1371/journal.pone.0096089. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Fang N, Zhang W, Xu S, Lin H, Wang Z, Liu H, Fang Q, Li C, Peng L, Lou J. TRIB3 alters endoplasmic reticulum stress-induced β-cell apoptosis via the NF-κB pathway. Metabolism. 2014;63:822–830. doi: 10.1016/j.metabol.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Scivittaro V, Ganz MB, Weiss MF. AGEs induce oxidative stress and activate protein kinase C-β(II) in neonatal mesangial cells. Am J Physiol Renal Physiol. 2000;278:F676–F683. doi: 10.1152/ajprenal.2000.278.4.F676. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Renier G. Activation of nicotinamide adenine dinucleotide phosphate (reduced form) oxidase by advanced glycation end products links oxidative stress to altered retinal vascular endothelial growth factor expression. Metabolism. 2006;55:1516–1523. doi: 10.1016/j.metabol.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Jiang YW, Zhang WJ, Xu SQ, Liu HL, Yang WY, Lou JN. Differential activations of PKC/PKA related to microvasculopathy in diabetic GK rats. Am J Physiol Endocrinol Metab. 2012;302:E173–E182. doi: 10.1152/ajpendo.00184.2011. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Huang D, Shen D, Zhang C, Ma Y, Babcock SA, Chen B, Ren J. Inhibition of protein kinase C βII isoform ameliorates methylglyoxal advanced glycation endproduct-induced cardiomyocyte contractile dysfunction. Life Sci. 2014;94:83–91. doi: 10.1016/j.lfs.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Guo B, Zhang W, Xu S, Lou J, Wang S, Men X. GSK-3β mediates dexamethasone-induced pancreatic β cell apoptosis. Life Sci. 2016;144:1–7. doi: 10.1016/j.lfs.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol. 2014;18:1–14. doi: 10.4196/kjpp.2014.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med (Berl) 2005;83:876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- 31.Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, Elliston K, Stern D, Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998–15004. [PubMed] [Google Scholar]
- 32.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/S0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 33.Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270:25752–25761. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- 34.Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, et al. RAGE mediates amyloid-β peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 35.Mackic JB, Stins M, McComb JG, Calero M, Ghiso J, Kim KS, Yan SD, Stern D, Schmidt AM, Frangione B, et al. Human blood-brain barrier receptors for Alzheimer's amyloid-beta 1–;40. Asymmetrical binding, endocytosis, and transcytosis at the apical side of brain microvascular endothelial cell monolayer. J Clin Invest. 1998;102:734–743. doi: 10.1172/JCI2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lo MC, Chen MH, Lee WS, Lu CI, Chang CR, Kao SH, Lee HM. Nε-(carboxymethyl) lysine-induced mitochondrial fission and mitophagy cause decreased insulin secretion from β-cells. Am J Physiol Endocrinol Metab. 2015;309:E829–E839. doi: 10.1152/ajpendo.00151.2015. [DOI] [PubMed] [Google Scholar]
- 37.Hasnain SZ, Prins JB, McGuckin MA. Oxidative and endoplasmic reticulum stress in β-cell dysfunction in diabetes. J Mol Endocrinol. 2016;56:R33–R54. doi: 10.1530/JME-15-0232. [DOI] [PubMed] [Google Scholar]
- 38.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Nisimoto Y, Diebold BA, Cosentino-Gomes D, Lambeth JD. Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry. 2014;53:5111–5120. doi: 10.1021/bi500331y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prudente S, Sesti G, Pandolfi A, Andreozzi F, Consoli A, Trischitta V. The mammalian tribbles homolog TRIB3, glucose homeostasis, and cardiovascular diseases. Endocr Rev. 2012;33:526–546. doi: 10.1210/er.2011-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Humphrey RK, Newcomb CJ, Yu SM, Hao E, Yu D, Krajewski S, Du K, Jhala US. Mixed lineage kinase-3 stabilizes and functionally cooperates with TRIBBLES-3 to compromise mitochondrial integrity in cytokine-induced death of pancreatic beta cells. J Biol Chem. 2010;285:22426–22436. doi: 10.1074/jbc.M110.123786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J. 2005;24:1243–1255. doi: 10.1038/sj.emboj.7600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zou CG, Cao XZ, Zhao YS, Gao SY, Li SD, Liu XY, Zhang Y, Zhang KQ. The molecular mechanism of endoplasmic reticulum stress-induced apoptosis in PC-12 neuronal cells: the protective effect of insulin-like growth factor I. Endocrinology. 2009;150:277–285. doi: 10.1210/en.2008-0794. [DOI] [PubMed] [Google Scholar]
- 44.Schwarzer R, Dames S, Tondera D, Klippel A, Kaufmann J. TRB3 is a PI 3-kinase dependent indicator for nutrient starvation. Cell Signal. 2006;18:899–909. doi: 10.1016/j.cellsig.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Ord D, Meerits K, Ord T. TRB3 protects cells against the growth inhibitory and cytotoxic effect of ATF4. Exp Cell Res. 2007;313:3556–3567. doi: 10.1016/j.yexcr.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 46.Gorasia DG, Dudek L, Veith PD, Shankar R, Safavi-Hemami H, Williamson NA, Reynolds EC, Hubbard MJ, Purcell AW. Pancreatic beta cells are highly susceptible to oxidative and ER stresses during the development of diabetes. J Proteome Res. 2015;14:688–699. doi: 10.1021/pr500643h. [DOI] [PubMed] [Google Scholar]
- 47.Zhang K. Integration of ER stress, oxidative stress and the inflammatory response in health and disease. Int J Clin Exp Med. 2010;3:33–40. [PMC free article] [PubMed] [Google Scholar]
- 48.Pérez LM, Milkiewicz P, Ahmed-Choudhury J, Elias E, Ochoa JE, Sánchez Pozzi EJ, Coleman R, Roma MG. Oxidative stress induces actin-cytoskeletal and tight-junctional alterations in hepatocytes by a Ca2+-dependent, PKC-mediated mechanism: protective effect of PKA. Free Radic Biol Med. 2006;40:2005–2017. doi: 10.1016/j.freeradbiomed.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 49.Pérez LM, Milkiewicz P, Elias E, Coleman R, Sánchez Pozzi EJ, Roma MG. Oxidative stress induces internalization of the bile salt export pump, Bsep, and bile salt secretory failure in isolated rat hepatocyte couplets: a role for protein kinase C and prevention by protein kinase A. Toxicol Sci. 2006;91:150–158. doi: 10.1093/toxsci/kfj113. [DOI] [PubMed] [Google Scholar]