Abstract
Nasal and oral exclusive breathing modes have benefits and drawbacks during submaximal exercise. It is unknown whether these responses would extend to anaerobic work performed at high intensity. Nine individuals (males N = 7, females N = 2) performed a standard Wingate Anaerobic cycle test on a cycle ergometer under nose (N) and mouth (M) only respiratory conditions, performed in a counterbalanced order. A 2 (condition: nose, mouth) × 6 (time: 0–5 sec, 5–10 sec, 10–15 sec, 15–20 sec, 20–25 sec, 25–30 sec) repeated measures ANOVA was used to analyze the data with significance accepted at the p<0.05 level. No differences between breathing mode were observed for any power output or performance measures associated with the Wingate Anaerobic cycle test. Respiratory exchange ratio (RER) was significantly higher in the oral respiration condition from 10 seconds to 25 seconds during the test (p<0.05). On the other hand, heart rate (HR) in the nasal condition was significantly greater during the final two time intervals (p<0.05). Nasal breathing was effective in reducing hyperventilation as RER remained below 1.0. However, elevated HR with nasal breathing indicates increased cardiovascular stress associated with this mode. As breathing mode does not affect power output or performance measures during completion of a high-intensity anaerobic test, preference of the participant should be the determining factor if a choice is available.
Keywords: Nose versus mouth, Wingate cycle test, maximal anaerobic capacity, metabolic measures
INTRODUCTION
One of the primary determinants of endurance performance is the maximum rate of oxygen uptake (VO2max), as it sets a ceiling on an individual’s ability to take in and consume O2 and has an effect on consequent energy production during exercise (13). When an individual exercises, O2 can be taken into the body through both nasal and/or oral passageways. Nasal breathing is innate to human respiration due to the important function of preparing inhaled air to reach structures of the respiratory system (16). Nasal inspiration has beneficial functions that include converting inspired air to temperatures near that of body temperature; increasing it’s humidity; and acting as a filter by extracting contaminants (including dust and bacteria) prior to air passing into the remaining respiratory system (6). If a higher concentration of nitric oxide is formed in the nasal passages and taken up in the lower respiratory tract, it is assumed that nasal nitric oxide will improve respiratory function (17). Therefore, there may be various benefits to nasal versus oral breathing while exercising.
In a study utilizing sport conditioning drills it was reported that restricting nasal breathing had no negative affects on physiological responses (8). It is a common observation that during exercise the mouth is more or less continuously open, and as ventilation increases one shifts from nasal to oronasal breathing (11). The switch from nasal to oronasal breathing during exercise is partly attributed to the relative high nasal airflow resistance and the resultant increased breathing effort sensation (15). This so-called switch is where nasal breathing becomes less optimal. One study reported that cycling at 60% of maximum heart rate with oral breathing resulted in higher levels of oxygen, ventilation volume and respiratory rate produced, as opposed to nasal breathing (3). As work rate and intensity increase, oral breathing seems better suited because it can deliver larger volumes of oxygen to the working body at a faster rate. The relative contributions and physiological determinants to nasal and oral breathing during exercise are still not well understood (1).
In a case study, a competitive triathlete who had adopted a nasal-only breathing strategy during training displayed greater maximal oxygen consumption and time to exhaustion during graded exercise tests performed in a nasal only condition and an oral only condition (4). As the energy contribution supplied at the end of a maximal exertion exercise test is significantly anaerobic, we wondered whether similar findings would be displayed during a classic test for anaerobic capacity. Therefore the purpose of this test was to analyze nasal breathing and oral breathing during completion of the Wingate anaerobic cycle test. Our primary hypothesis was that nasal breathing would be more advantageous than oral breathing, in regards to completing a thirty-second Wingate anaerobic test on a cycle ergometer. We believed nose-breathing to be more controlled as opposed to the mouth where an individual might tend to hyperventilate.
METHODS
Participants
Nine individuals (males N = 7, females N = 2) participated in the study (see table 1 for characteristics). All individuals were considered low risk according to the American College of Sports Medicine Stratification Screening Questionnaire. All participants provided informed consent and procedures were approved by the University of Nevada Las Vegas review board (protocol #885381-2).
Table 1.
Participant demographic characteristics (n = 9).
| Age (yr) | Height (cm) | Body mass (kg) | |
|---|---|---|---|
| Average ± SD | 24.44 ± 7.60 | 171.68 ± 6.94 | 74.27 ± 14.56 |
Protocol
All procedures were performed in the Exercise Physiology laboratory at the University of Nevada, Las Vegas. All descriptive characteristics were measured and recorded before testing was performed. Metabolic data was recorded from computerized software attached to the metabolic analysis system (Moxus, Applied Electrochemistry Incorporated, Pittsburgh, PA) as well as the cycle ergometer (Wattbike Ltd, Nottingham, UK). Prior to testing participants were outfitted with a heart rate monitor (H7, Polar, Oy, Findland) and respiratory mouthpiece.
Participants performed a standard Wingate Anaerobic Test on the cycle ergometer to which they chose a comfortable seat height with a predetermined resistance (7.5% of body mass). Standard procedures called for maximal effort while pedaling on the cycle ergometer for 30 seconds. Procedures were taken under two conditions, nose (N) and mouth (M), performed in a counterbalanced order. At least 30-min of rest was provided between bouts.
Under the N condition, participants had athletic tape placed over their mouth in order to prevent any oral breathing. For the M condition, the nose was plugged with a standard laboratory noseclip and respiratory headgear was attached to the head in order to secure metabolic data. The participant was allowed a warm up consisting of 3 minutes of unloaded pedaling. Immediately following the warm up participants performed as many cycle revolutions on the ergometer as possible. The sampling rate on both the metabolic analysis system and on the cycle ergometer was set to time intervals of 5 seconds.
Statistical Analysis
A 2 (condition: nose, mouth) × 6 (time: 0–5 sec, 5–10 sec, 10–15 sec, 15–20 sec, 20–25 sec, 25–30 sec) repeated measures ANOVA was used to analyze the data. If a significant main effect occurred for condition, a 2-tailed paired t-test was used to determine significance. If a main effect for time occurred, a one-way ANOVA was utilized to determine where differences occurred between time points. The statistical significance of this study was set at p < 0.05.
RESULTS
No differences between oral or nasal conditions were noted with respect to any power output parameters or performance measures associated with the Wingate Anaerobic cycle test (see table 2).
Table 2.
Power output and performance variables in subjects who completed Wingate cycle tests under conditions of mouth-only and nose-only respiration.
| Variable | Mouth | Nose | p-value |
|---|---|---|---|
| Peak Power (W) | 694.9±176.8 | 734.7±172.1 | 0.36 |
| Mean Power (W) | 503.1±125.1 | 513.6±122.6 | 0.58 |
| Estimated Energy (kcal) | 16.2±3.6 | 16.5±3.5 | 0.56 |
| Revolution count (#) | 64.1±4.4 | 63.7±3.0 | 0.74 |
| Mean Speed (km.h−1) | 51.4±5.1 | 51.7±4.8 | 0.66 |
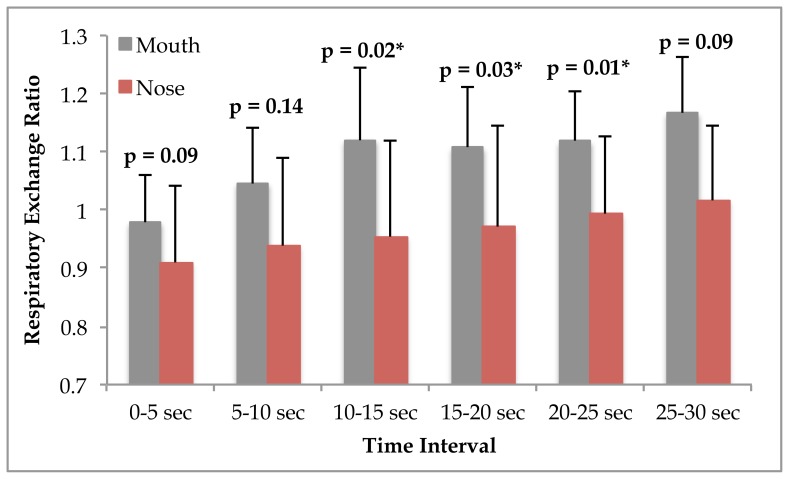
While no interaction between time and condition was observed for respiratory exchange ratio (RER), (p = 0.27), significant main effects for time (p = 0.01) and condition were found (p = 0.028). Post hoc analysis showed that RER was significantly higher in the mouth only respiration condition from 10 seconds to 25 seconds during the Wingate Anaerobic Cycle test (see figure 1).
Figure 1.
Respiratory exchange ratio during the Wingate Anaerobic cycle test in participants (n = 9) who performed testing under oral and nasal conditions. Significance is shown in the figure and accepted at p≤0.05.
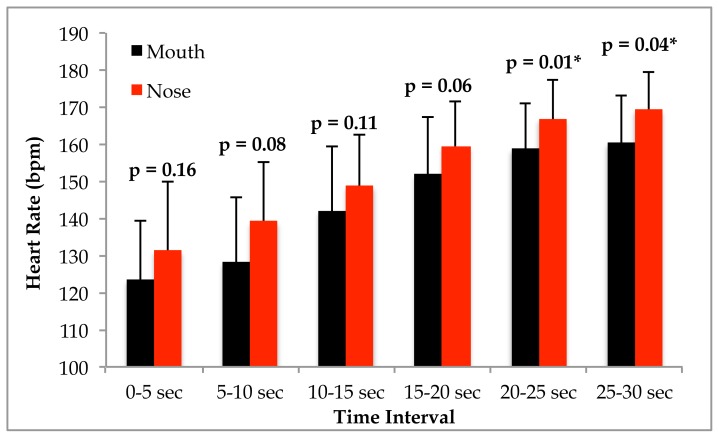
With respect to heart rate (HR), no interaction was evident (p = 0.74), but significant main effects were observed for time (p = 0.001) and condition (p = 0.028). Post hoc analysis revealed that HR in the nasal condition was significantly greater during the final two time intervals compared to respiring through the mouth (see figure 2).
Figure 2.
Heart rate response to the Wingate Anaerobic cycle test in participants (n = 9) who performed testing under mouth only and nasal only conditions. Data are displayed as mean and standard deviation. Significance is shown in the figure and accepted at p≤0.05.
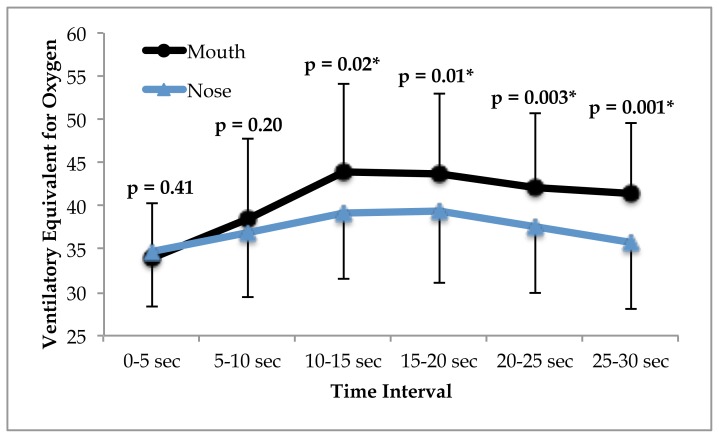
A significant time × condition interaction was observed for the ventilatory equivalent of oxygen (VeqO2) (p = 0.001). VeqO2 was significantly higher in the oral condition from 10 seconds through the remainder of the test (see figure 3).
Figure 3.
The ventilatory equivalent for oxygen response throughout the Wingate Anaerobic cycle test in subjects (n = 9) who performed under mouth and nose-only breathing conditions. Significance is shown in the figure and accepted at p≤0.05.
With respect to relative oxygen consumption, we observed no interaction (p = 0.57) or main effect for condition (p = 0.14). There was a main effect for time (p = 0.001), with oxygen consumption increasing throughout the duration of the test (see table 3). Additionally, no interaction (p = 0.11) or main effect for condition (p = 0.26) was noted for the ventilatory equivalent for carbon dioxide. While a significant main effect for time was initially noted (p = 0.04), pairwise comparisons revealed no significant differences at any time interval (p>0.05).
Table 3.
Oxygen uptake collapsed across oral and nasal conditions in participants who completed the Wingate Anaerobic test (n = 9).
| Time Interval (sec) | VO2 (ml.kg−1.min−1) |
|---|---|
| 0 – 5 | 16.0±4.3a |
| 5 – 10 | 20.6±5.0ab |
| 10 – 15 | 21.8±3.8b |
| 15 – 20 | 24.5±4.8bc |
| 20 – 25 | 27.9±5.6cd |
| 25 – 30 | 30.5±6.5d |
Time intervals with the same letter are statistically similar to each other.
DISCUSSION
The main purpose of this study was to investigate how the different breathing mechanisms of the nose and mouth affected power output and metabolic measures during performance of an anaerobic capacity test. It was hypothesized that nasal breathing would be more advantageous than oral breathing, due to its effect on respiratory control. Based on RER data we found that nasal breathing did significantly reduce hyperventilation, however this made relatively little difference on power output and performance measures.
Previous literature has suggested that oral breathing during submaximal exercise allows for greater ventilation to meet the oxygen consumption demands of physical activity (3). Hall asked participants to perform exercise on a cycle ergometer at 60% of their maximum heart rate and concluded that oral breathing resulted in a higher ventilation volume and uptake of oxygen in comparison to nasal breathing (3). Conversely, oral-only breathing during speaking tasks has been shown to cause vocal cord dehydration that results in increased pressure needed for verbal communication and increased vocal effort (12). While not directly associated with the exercise task in the current investigation, it is possible that some of these mechanisms (i.e. vocal cord dehydration, increased pressure and effort) could adversely affect mouth-only breathing during exercise.
On the other hand, nasal breathing filters large particles from the surrounding environment and may help to deliver air with fewer contaminants to the respiratory system (2). Additionally, nasal breathing increases the humidity of the inspired air and may make it more pleasurable to the lungs for utilization (6). Finally, because of its effect as a potent vasodilator, NO produced during nasal breathing could be beneficial during exercise (7). Yasuda et al. showed that while NO increases two-fold during cycle exercise at 60W performed in a nasal breathing condition compared to mouth only respiration, no differences were observed with respect to cardiorespiratory measures (17).
It should be noted that we determined some limitations with the current study. A convenience sampling of the student population was obtained, and therefore the results may not be generalizable to individuals outside of this setting. Another drawback that we noted upon completion of the study was a relatively wide range of fitness levels among the participants. Because of this some participants anecdotally reported muscle soreness due to the activity and this may have affected the subsequent trial. Another limitation is that nitric oxide production was not evaluated, reducing our ability to mechanistically explain respiratory results.
Given the limitations noted above, future investigations are warranted. Subsequent studies should measure and report nitric oxide produced during oral and nasal exercise bouts. Additionally, extending sampling times into the recovery period may provide additional insight into the differences between oral and nasal breathing. Also, controlling for fitness or training level, or testing specific populations (such as athletes from sports that tend to prefer only mouth breathing, or only nasal respiration) could provide to be beneficial.
The results of the current investigation provide mixed results with respect to which mode of breathing is the most advantageous during an anaerobic capacity test. While research indicates that psychological stress could increase hyperventilation (14), we feel that the very short nature of the anaerobic bout somewhat limits this influence. However, as we did not obtain respiratory measures immediately prior to the tests, this opens an interesting area for future study. Nevertheless, from a respiratory control perspective, we believe that nasal breathing was effective in reducing hyperventilation as RER remained below 1.0, whereas the oral breathing condition returned RER values that are similar to those found at the end of maximal exertion (VO2max) treadmill tests (9). Additionally, the ventilatory equivalent for oxygen data supports nasal breathing as a more efficient mode given that the same amount of mechanical work could be completed at a lower metabolic cost compared to oral respiration. However, heart rate during the later stages of the Wingate Anaerobic cycle test was significantly higher during nose-only breathing. Previous investigations utilizing lower intensity exercise have found no difference with respect to the heart rate response between breathing modes (3, 5, 17). It is possible that as the exercise intensity increases to maximal or near-maximal, that exclusively nasal breathing results in greater cardiovascular stress. This possibility warrants further investigation.
To our knowledge, cardiorespiratory measures under the conditions of oral-only, or nasal-only breathing have not been reported for an anaerobic exercise bout. Therefore, the results presented here represent novel data in this relatively limited area of research. As there are benefits and drawbacks associated with either type of breathing mode, the ultimate preference is at the discretion of the participant (10). As breathing mode does not affect power output or performance measures in completion of a high-intensity anaerobic exercise test, the preference of participant should be the determining factor.
REFERENCES
- 1.Bennett WD, Zeman KL, Jarabek AM. Nasal contribution to breathing with exercise: effect of race and gender. J Appl Physiol. 2003;95(2):497–503. doi: 10.1152/japplphysiol.00718.2002. [DOI] [PubMed] [Google Scholar]
- 2.Carlisle AJ, Sharp NCC. Exercise and outdoor ambient air pollution. Br J Sports Med. 2001;35(4):214–222. doi: 10.1136/bjsm.35.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall RL. Energetics of nose and mouth breathing, body size, body composition, and nose volume in young adult males and females. Am J Human Biol. 2005;17(3):321–330. doi: 10.1002/ajhb.20122. [DOI] [PubMed] [Google Scholar]
- 4.Hostetter K, McClaran SR, Cox DG, Dallam G. Triathlete adapts to breathing restricted to the nasal passage without loss in VO2max or VVo2max. J Sport Human Performance. 2016;4(1):1–7. [Google Scholar]
- 5.LaComb CO, Tandy RD, Lee SP, Young JC, Navalta JW. Oral versus nasal breathing during moderate to high intensity submaximal aerobic exercise. Int J Kinesiology Sports Sci. 2017;5(1):9–16. [Google Scholar]
- 6.Lester RA, Hoit JD. Nasal and oral inspiration during natural speech breathing. J Speech Lang Hear R. 2014;57(3):734–42. doi: 10.1044/1092-4388(2013/13-0096). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumoto A, Hirata Y, Momomura S, Fujita H, Yao A, Sata M, Serizawa T. Increased nitric oxide production during exercise. Lancet. 1994;343(8901):849–850. doi: 10.1016/s0140-6736(94)92047-8. [DOI] [PubMed] [Google Scholar]
- 8.Meir R, Zhao GG, Zhou S, Beavers R, Davie A. The acute effect of mouth only breathing on time to completion, heart rate, rate of perceived exertion, blood lactate, and ventilatory measures during a high-intensity shuttle run sequence. J Strength Cond Res. 2014;28(4):950–957. doi: 10.1519/JSC.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 9.Navalta JW, Tibana RA, Fedor EA, Vieira A, Prestes J. Three consecutive days of interval runs to exhaustion affects lymphocyte subset apoptosis and migration. BioMed Res Int. 2014;2014:694801. doi: 10.1155/2014/694801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niinimaa V. Oronasal airway choice during running. Respir Physiol. 1983;53(1):129–133. doi: 10.1016/0034-5687(83)90021-x. [DOI] [PubMed] [Google Scholar]
- 11.Saibene F, Mognoni P, Lafortuna CL, Mostardi R. Oronasal breathing during exercise. Eur J Physiol. 1978;378(1):65–69. doi: 10.1007/BF00581959. [DOI] [PubMed] [Google Scholar]
- 12.Sivasankar M, Fisher KV. Oral breathing increases Pth and vocal effort by superficial drying of vocal fold mucosa. J Voice. 2002;16(2):172–181. doi: 10.1016/s0892-1997(02)00087-5. [DOI] [PubMed] [Google Scholar]
- 13.Sperlich PF, Holmberg HC, Reed JL, Zinner C, Mester J, Sperlich B. Individual versus standardized running protocols in the determination of VO2max. J Sport Sci Med. 2015;14(2):386–393. [PMC free article] [PubMed] [Google Scholar]
- 14.Suess WM, Alexander AB, Smith DD, Sweeney HW, Marion RJ. The effects of psychological stress on respiration: a preliminary study of anxiety and hyperventilation. Psychophysiology. 1980;17(6):535–540. doi: 10.1111/j.1469-8986.1980.tb02293.x. [DOI] [PubMed] [Google Scholar]
- 15.Tong TK, Fu FH, Chow BC. Nostril dilatation increases capacity to sustain moderate exercise under nasal breathing condition. J Sports Med Phys Fitness. 2001;41(4):470–478. [PubMed] [Google Scholar]
- 16.Trevisan ME, Boufleur J, Soares JC, Haygert CJP, Ries LGK, Correa ECR. Diaphragmatic amplitude and accessory inspiratory muscle activity in nasal and mouth-breathing adults: A cross-sectional study. J Electromyogr Kines. 2015;25(3):463–468. doi: 10.1016/j.jelekin.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Yasuda Y, Itoh T, Miyamura M, Nishino H. Comparison of exhaled nitric oxide and cardiorespiratory indices between nasal and oral breathing during submaximal exercise in humans. Jap J Physiol. 1997;47(5):465–470. doi: 10.2170/jjphysiol.47.465. [DOI] [PubMed] [Google Scholar]