ABSTRACT
Tuberculosis remains as the world’s biggest threat. In 2014, human tuberculosis ranked as a major infectious disease by the first time, overcoming HIV death rates. Bovine tuberculosis is a chronic disease of global distribution that affects animals and can be transmitted to humans by the consumption of raw milk, representing a serious public health concern. Despite the efforts of different countries to control and eradicate bovine tuberculosis, the high negative economic impact on meat and milk production chains remains, given the decreased production efficiency (approximately 25%), the high number of condemned carcasses, and increased animal culling rates. This scenario has motivated the establishment of official programs based on regulations and diagnostic procedures. Although Mycobacterium tuberculosis and Mycobacterium bovis are the major pathogenic species to humans and bovines, respectively, nontuberculous mycobacteria within the Mycobacterium genus have become increasingly important in recent decades due to human infections, including the ones that occur in immunocompetent people. Diagnosis of mycobacteria can be performed by microbiological culture from tissue samples (lymph nodes, lungs) and secretions (sputum, milk). In general, these pathogens demand special nutrient requirements for isolation/growth, and the use of selective and rich culture media. Indeed, within these genera, mycobacteria are classified as either fast- or slow-growth microorganisms. Regarding the latter ones, incubation times can vary from 45 to 90 days. Although microbiological culture is still considered the gold standard method for diagnosis, molecular approaches have been increasingly used. We describe here an overview of the diagnosis of Mycobacterium species in bovine milk.
Keywords: Milk, Mycobacteria, Bovine, Microbiology, Molecular
INTRODUCTION
The genus Mycobacterium includes a set of species with public health relevance, which has been divided into three groups according to clinical importance: (1) obligatory human and animal pathogens, (2) potentially pathogenic microorganisms (opportunistic) for animals and humans, and (3) saprophytes or ubiquitous microorganisms 1 - 3 . Obligatory pathogens include species belonging to the Mycobacterium tuberculosis complex (M. tuberculosis, M. bovis, M. africanum, M. caprae, M. microti, M. pinnipedii, M. leprae, M. canetti and recently described M. orygis, M. mungi and M. suricattae 4 - 6 ). The potentially pathogenic species are represented by the M. avium intracellulare complex (M. avium subspecies avium, M. avium paratuberculosis, M. avium hominissuis, M. avium silvaticum, M. colombiense and M. indicus pranii), M. marinum, M. fortuitum, M. scrofulaceum, M. kansasii, M. xenopi, among others. Saprophytic mycobacteria are represented by M. gordonae, M. terrae, M. smegmatis, and M. phlei, also called atypical, “nontuberculous”, or environmental mycobacteria 1 , 7 - 9 .
In the public health context, M. tuberculosis and M. bovis have the greatest impact, causing tuberculosis, an infectious disease of global distribution that shows chronic evolution and affects humans, domestic and wildlife animals 9 - 12 . The World Health Organization (WHO) considers tuberculosis a re-emerging disease and a major threat due to the increased number of reports in humans, particularly in people living with HIV/AIDS, as well as the rise of multidrug-resistant isolates 11 , 13 . In 2015, the WHO global report revealed about 9.6 million new cases of human tuberculosis, and 1.5 million deaths. The same organization reported that, in 2014, tuberculosis ranked by the first time as a major infectious disease 14 . It is estimated that M. bovis is the causal agent of human tuberculosis in about 10-15% of the cases in developing countries, and in about 1-2% of the cases in developed countries 11 , 15 . In Brazil, 70,000 new cases are officially reported each year, resulting in about 4,600 deaths 16 . Moreover, human cases caused by nontuberculous mycobacteria have increased 17 . The significance of these bacteria as primary pathogens have been considered after the 21st century, given the emergence of pulmonary infections in patients with pre-existing pulmonary disease 18 , and the increase in the number of people living with HIV/AIDS.
Mycobacterium species are transmitted to humans predominantly by the inhalation of contaminated aerosols and consumption of contaminated water 11 , 19 , as well as consumption of raw dairy products or those not heat-treated by pasteurization, boiling, or UHT (ultra-high temperature) processing. It is estimated that, in Brazil, about 20- 30% of cow’s milk is marketed without health inspection 20 . This fact enhances the potential zoonotic risk of the microorganism transmission through milk, especially pathogenic species of Mycobacterium 11 , 21 . Furthermore, Mycobacterium sp. can remain viable in cheese and yogurt made from raw milk for up to 14 days and up to 100 days in butter 22 . In humans, extrapulmonary manifestations (bone, joints, liver) are the most commonly reported in infections caused by M. bovis from cattle, whereas cases of pneumonia in rural and slaughterers workers have been reported, reflecting an occupational disease behavior 23 .
Currently, according to the Brazilian Institute of Geography and Statistic (IBGE), Brazil has the largest commercial cattle herd in the world, with about 212 million animals 24 . It is estimated that approximately 1.3% of the Brazilian cattle herd is affected by tuberculosis 25 , particularly dairy cattle 26 . In the public health context, mycobacterial infections in cattle cause substantial economic losses to owners, mainly involving the condemnation of carcasses in slaughterhouses, reduction in milk and meat yield, animals slaughtered because of positive tuberculin tests carried out in control/eradication programs, commercial value depreciation of tuberculin-positive animals and international embargoes on the export of products from cattle origin 27 .
In 2001, because of the negative impact of tuberculosis on the meat and milk production chain, the need to promote cattle product competitiveness in a highly demanding international market, as well as the zoonotic impact of the pathogen, Brazil created the National Program for the Control and Eradication of Brucellosis and Tuberculosis (PNCEBT), which established the rules and procedures for the diagnosis of the disease in cattle and buffaloes. In general, the program requires culling of reactor animals, standardized diagnostic tests and equipment (automatic antigen applicator and callipers), and training of veterinarians in diagnostic procedures 26 . In 2011, the Brazilian Normative Instruction (IN) 62 28 was established (replacing IN 51) standardizing procedures from production to the marketing of different types of milk in the country, with emphasis on the quality control of raw milk.
In raw milk, the identification of M. bovis and atypical mycobacteria has been described in Tanzania 29 , M. bovis and M. africanum in Nigeria 30 , M. bovis in Tunisia, 31 and atypical Mycobacterium species in Turkey 32 and Brazil 25 . Particularly in Brazil, Pardo et al. 33 in 52 cows showing positive or inconclusive results in Stormont test, identified M. bovis by phenotypic methods, and other atypical mycobacteria in 78 (10%) milk samples from 19 animals. In Tunisia, Ben Kahla et al. 31 , based on phenotypic methods and spoligotyping detected five (4.9%) isolates of M. bovis obtained from 306 milk samples from 102 cows that were positive in a single cervical tuberculin test. More recently, Pandey et al. 34 , in Zambia, investigated the presence of M. bovis in milk of 16 tuberculin-positive cows and identified three (18.7%) positive samples using phenotypic methods, which were confirmed by Polymerase Chain Reaction (PCR). All these studies have shown that cattle shed the pathogen into milk, displaying the public health impact of these findings, because of the risk of consumption of raw milk or milk products that did not undergo official health inspection and/or adequate heat treatment.
The isolation of Mycobacterium species has extremely demanding requirements, based on selective and rich medium (usually egg-based) 9 , 35 , 36 . Mycobacteria are classically divided into fast- and slow-growth species. Slow-growth species can take up to 90 days for isolation. Due to the long time required for microbial isolation combined with phenotypic diagnosis, the development of molecular techniques allowing fast, accurate and reliable diagnosis of Mycobacterium species was stimulated. Indeed, Polymerase Chain Reaction (PCR) techniques associated with restriction enzyme analysis pattern (PRA), Multiplex Chain Reaction (Multiplex PCR) and Nested Polymerase Chain Reaction (Nested PCR) have been used. Other studies have reported increased diagnostic sensitivity and specificity by combining phenotypic techniques and molecular markers for the diagnosis of Mycobacterium sp. in individual animals, the identification of mycobacterium species, the geographical distribution of allelic diversity in outbreaks, and for epidemiological surveillance studies of bovine tuberculosis 37 - 40 with spoligotyping (spacer oligonucleotide typing), and in the last decade, mycobacterial interspersed repetitive unit variable number of tandem repeats typing (MIRU-VNTR), and repeated identical sequences of DNA tandem (Exact tandem repeat-ETR) 41 .
Considering the threat bovine tuberculosis poses to animal health, the habit of consuming raw or non-heat-treated milk in Brazil, the Public Health concern in eliminating mycobacteria in dairy cows and the identification of mycobacteria genetic markers in molecular techniques that enable valuable diagnosis in short time, we describe here an overview of the diagnosis of Mycobacterium species in bovine milk.
BOVINE TUBERCULOSIS: GENERAL ASPECTS OF THE DISEASE
Tuberculosis is one of the most important zoonotic diseases in cattle, particularly in Latin America, Africa and Asia. The disease is caused by M. bovis, although other species of the genus Mycobacterium may affect cattle 11 , 12 , 42 .
Although some European countries have reached a tuberculosis-free status, such as Switzerland 43 , the increase in the number of human infections by M. bovis was noticed in the last decade, as a probable reflex of the endemic status of the disease in cattle. The increasing prevalence of bovine tuberculosis was recorded in the UK (3.27%) and Ireland (4.37%) 44 . In Africa, Sudan reported 0.18% prevalence of tuberculosis in slaughterhouse cattle 45 , whereas in Ethiopia the records on the occurrence of the disease have varied widely (1.5% to 78%) 46 . The US fights the disease since 1900 with a control program based on tuberculin testing and culling of positive animals. However, despite the significant reduction in prevalence in the last decades, the disease was observed to reemerge in the States of Texas (1995), Minnesota (2005), and more recently, California (2008) 27 .
In Latin America, a subdivision of the prevalence of bovine tuberculosis into categories was proposed: < 0.1%, from 0.1 to 1%, and > 1%; however, this classification is influenced by the number of animals raised in each country 47 . In Brazil, the disease is present throughout the country since 1920, but few actions with significant impact had been taken to fight the disease until 2001, when the PNCEBT 27 was instituted.
It is estimated that 1.3% of the Brazilian cattle herd is affected by tuberculosis 25 . A recent study (2012-2013) of bovine tuberculosis prevalence in Brazil was conducted by the Ministry of Agriculture, Livestock and Supply (MAPA), based on statistical estimates of samples. Data from this study - not officially published yet - were preliminarily reported by Ferreira Neto (personal communication) in the V Brazilian Milk Quality Congress, which took place from 10th to 12th June, 2013, in Águas de Lindóia, São Paulo. In the State of São Paulo, 2,009 farms were investigated and 173 (8.6%) tuberculin-positive animals were diagnosed. In these farms of the State of São Paulo, 20,278 animals were evaluated, from which 320 (1.6%) were positive. In the same study, in the State of Paraná, 1,419 farms were investigated, from which 33 (2.3%) animals were positive in the tuberculin test. In the farms from the State of Paraná, 15,982 animals were evaluated, from which 63 (0.39%) were positive. A summary of these data on the bovine tuberculosis prevalence in some States of Brazil were reported by Paes and Franco 48 (Table 1).
Table 1. Prevalence of bovine tuberculosis in some States of Brazil.
| State | Prevalence in the farms | Prevalence of positive animals |
|---|---|---|
| Rondônia | 2.3% | 0.1% |
| Bahia | 1.0% | 0.1% |
| Mato Grosso | 1.2% | 0.1% |
| Paraná | 2.32% | 0.39% |
| São Paulo | 8.61% | 1.57% |
Tuberculosis occurs in cattle regardless of age and gender, although due to the chronic evolution, adult animals are more often diagnosed and they are more commonly symptomatic. Bos taurus is usually more affected than Zebu cattle (Bos indicus) 42 . The introduction of infected animals in a herd is probably the main risk factor for the spread of the disease among dairy animals 49 . Mycobacteria are eliminated by respiratory tract secretions, milk, colostrum, feces, urine and, occasionally, semen. Cattle-to-cattle transmission occurs mainly by contact with aerosols or consumption of contaminated water, food and milk 49 - 51 .
After invasion by oral or respiratory routes, the pathogen is actively phagocytosed by macrophages and multiplies upon entry, but it is not eliminated in the phagolysosome due to the peculiar characteristics of the cell wall structure. Then, it is captured in regional lymph nodes and the response of CD4 + lymphocytes (6-8 weeks) starts, which is very important in the ante-mortem diagnosis, because these cells are recruited in the tuberculin test. The microorganism induces a typical granulomatous reaction, with caseous and/or necrotic lesions and a peculiar architecture formed by the free mycobacteria or the pathogen within macrophages, macrophages modulated in epithelioid cells and giant cells, and presence of caseum, all surrounded by a fibrous capsule. Chronic disease progression is characterized by formation of large caseous nodules, particularly in the lungs (airborne infection) and intestinal tract (oral infection). In immunocompromised or debilitated animals, early generalization may occur with spread of small nodules to several organs, including the mammary gland 27 , 52 - 54 .
Bovine tuberculosis causes production efficiency losses ranging from 10 to 25% in infected animals, leading to severe losses related to reduced milk and meat production, condemnation of carcasses in slaughterhouses, sanitary slaughter of tuberculin-positive animals, and restrictions on the export of animals and meat products of bovine origin 26 , 27 .
Bovine tuberculosis was fought in other countries and control or eradication of the disease was reached after systematic tuberculin testing, slaughtering of positive animals in officially inspected slaughterhouses, control of the animal movement, adoption of quarantine and testing of newly acquired animals, certification of farms free of the disease, health education, and heat treatment of milk 26 , 27 , 42 .
Brazilian regulations do not allow the treatment of cattle with tuberculosis and recommend the culling and slaughtering of animals with positive tuberculin tests in inspected abattoirs, or even mass killing in the farm of origin in the presence of large number of positive animals as well as other sanitary actions planned for positive herds 26 .
MYCOBACTERIA IN DAIRY CATTLE
Bovine tuberculosis affects various organs and tissues, including the mammary gland. It is usually caused by M. bovis, although other species can infect the udders, particularly atypical mycobacteria 42 , 55 . Infections with M. bovis in cattle are progressive 27 . In contrast, although M. tuberculosis can affect the mammary gland, infection is usually not progressive. Dairy cattle are more predisposed to tuberculosis probably due to the proximity of the animals in the milking and feeding environment 51 .
Mastitis
Unlike the most frequent agents of bovine mastitis, represented by staphylococci, streptococci and enterobacteria, which commonly cause an ascending infection of the teat canal, mycobacteria reach the mammary gland by systemic dissemination, probably after the spread of the primary infection by respiratory or digestive route 42 , 56 . Occasionally, infection can come from the environment of farms or by contamination of cannulas used in intramammary treatments. Mycobacteria as primary causes of bovine mastitis are considered of low occurrence, although prevalence can be high in farms endemic for tuberculosis or where outbreaks were observed 42 , 57 . In cattle, the disseminated form of tuberculosis occurs mainly in immunocompromised animals or those showing general weakness, with early generalization resulting in small nodules (granuloma) in the mammary gland and other organs 27 .
The occurrence of clinical or subclinical mastitis and the frequency of mycobacteria shedding into milk is controversial 58 - 60 . Cows with tuberculosis show reduced milk production, hardened masses in the udder, enlargement of regional lymph nodes and watery milk, although probably less than 1% of cows with tuberculosis show mammary signs. 42 Paes 27 reported that tuberculous mastitis causes hardening of mammary quarters and enlargement of supramammary lymph nodes. In the early stages of the disease, signs of clinical mastitis may not be seen in milk (subclinical mastitis). In chronic cases, milk can show fine lumps at the end of milking and color may be change to amber, with the elimination of bacilli in milk.
Raw milk
In raw milk, the risk of mycobacteria elimination in lactating cows was investigated in Tanzania using phenotypic and molecular methods. Among 805 milk samples, there was a predominance of atypical mycobacteria such as M. flavescens (n=13), M. terrae (n=7), M. smegmatis (n=4), M. fortuitum (n=2), M. gordonae (n=1), whereas only two isolates were identified as M. bovis. Despite the low prevalence of M. bovis, the presence of atypical mycobacteria in the milk of animals is a public health concern, because of the habit of consuming raw milk by these people, besides the high incidence of people living with HIV/AIDS 29 .
In Turkey, 35 samples of raw milk were evaluated for the presence of mycobacteria using phenotypic methods and confirmed by Polymerase Chain Reaction - Restriction Pattern Analysis (PCR-PRA), resulting in the detection of M. terrae, M. kansassii, M. agri, and M. haemophilum 32 . In another study in the same country, M. genavense, M. simiae, M. szulgai, M. bovis and M. fortuitum 61 were identified.
M. bovis (n=4) and M. africanum (n=1) were identified among 400 samples of unpasteurized cow milk in Nigeria using phenotypic and molecular methods 30 .
Tuberculin test
Few studies have focused on the identification of the presence of mycobacteria eliminated by dairy cows that were positive in tuberculin tests. In this context, the presence of mycobacteria was investigated in 306 samples of raw milk collected from 102 positive cows using the single cervical tuberculin test, from herds with tuberculosis history in Tunisia. Five isolates of M. bovis were identified by phenotypic methods and spoligotyping, underscoring the risks of raw milk consumption 31 . Pandey et al. 43 , in Zambia, investigated the presence of M. bovis in 16 tuberculin-positive dairy cows and three (18.7%) positive samples were detected using phenotypic methods, confirmed by PCR. This finding confirmed that the animals were eliminating the pathogen in milk and it reinforced the public health impact, given the high prevalence of people living with HIV/AIDS in the country, as well as all over the African continent.
In Egypt, 46 cows presenting clinical signs of tuberculosis, including low production, emaciation, anorexia, intermittent diarrhea (unresponsive to anthelmintic drugs), cough and labored breathing were subjected to the single cervical and caudal fold test. From 23 milk samples, one (4.35%) was positive for M. bovis using the Lowenstein-Jensen medium with sodium pyruvate, evidencing typical colonies and compatible biochemical results 62 . Likewise, Nasr et al. 63 collected samples from 50 cows reactors to the single cervical tuberculin test and 50 cows that were nonreactors. Results showed that samples from two reactor animals (4%) were suggestive of M. bovis based on growth in Lowenstein-Jensen medium with pyruvate; another sample was identified as Mycobacterium spp. by growth in Lowenstein-Jensen medium with glycerol.
Another study in India investigated mycobacteria in 181 raw milk samples, from which one sample was classified as M. tuberculosis by molecular methods. It is noteworthy that humans are the sources of M. tuberculosis infection in animals 64 . In Pakistan, Jalil et al. 65 analyzed 1,000 milk samples obtained from 185 bovines and 815 buffalos and reported 20 positive samples to M. bovis using Lowenstein-Jensen and Stonebrink media with subsequent inoculation in rabbits and Guinea pigs. Qamar and Ashar 66 , between January and June 2013, collected 210 bovine milk samples [UHT (n=35), pasteurized (n=33), retail (n=92), and bulk tank (n=60)], among which nine positive samples for Mycobacterium were identified, three in bulk tank milk. In Iraq, seven of 68 raw milk samples grown in mycobacteria medium showed colonies compatible with M. bovis, which were confirmed by biochemical and molecular methods 67 .
In Brazil, Pardo et al. 33 analyzed 52 cows with positive or inconclusive results to Stormont’s test, and identified M. bovis and atypical mycobacteria using phenotypic methods, in 78 (10%) milk samples from 19 animals. More recently, Franco et al. 25 in the same country, investigated the presence of mycobacteria in 300 milk samples, 100 from collective bulk tanks, 100 from bulk tanks of individual farms, and 100 samples marketed without sanitary inspection, popularly called “informal milk” in the State of São Paulo. These samples were assessed by phenotypic methods, confirmed by PCR-PRA. The following species were isolated in pure cultures: M. bovis, M. gordonae, M. fortuitum, M. intracellulare, M. flavescens, M. duvalii, M. haemophilum, M. immunogenum, M. lentiflavum, M. mucogenicum, M. novocastrense, M. parafortuitum, M. smegmatis, M. terrae, and M. vaccae. There were no significant differences between different types of milk, since mycobacteria were recovered and identified in 9% of the bulk tanks of individual farms, 7% of collective bulk tanks, and 8% of “informal” milk samples. The authors pointed out to the Public Health the threat posed by these results, because of the risk of milk consumption containing the pathogen, particularly from “informal milk”, which does not undergo any heat treatment and/or the official health inspection.
MICROBIOLOGICAL DIAGNOSIS
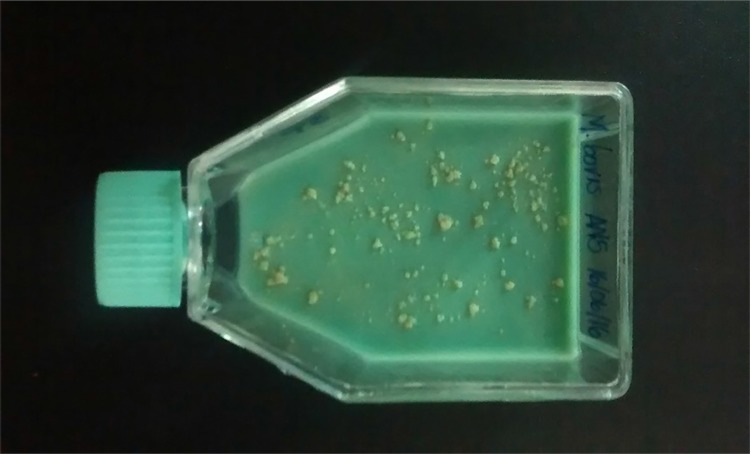
Microbiological culture remains the gold standard test for Mycobacterium sp. detection in clinical samples 68 , due to its sensitivity and specificity. After culture, other tests are carried out, such as species identification, antimicrobial susceptibility profile, genotyping, as well as monitoring of human patients response to treatment 69 . Rich selective media are required for mycobacteria species isolation, especially containing egg 35 , which is commonly used in veterinary microbiology 36 . Among these media, Lowenstein-Jensen, made with egg and glycerol 70 , 71 , favors the isolation of M. tuberculosis and M. avium, although glycerol inhibits the isolation of M. bovis. Lowenstein-Jensen medium has a green color due to the addition of malachite green to inhibit contaminants, and mycobacteria colonies show a yellowish hue with a creamy appearance (Figure 1). Stonebrink is another media, rich in pyruvate, favoring the multiplication of M. bovis (Figure 2), M. africanum and M. microti, since these species are unable to use glycerol as a source of carbon 48 , 72 , 73 .
Figure 1. Yellowish and creamy appearance of M. avium colonies in Lowenstein-Jensen medium after 20 days of incubation.
Figure 2. Colonies of M. bovis in Stonebrink media.
Nevertheless, clinical samples have to be decontaminated to allow Mycobacterium spp. isolation. The greatest difficulty in selecting a reagent and determining its concentration to decontaminate a sample is the adverse effect that most substances have on mycobacteria. The ideal reagent should have minimal toxicity to the genus Mycobacterium but maximum toxicity to other contaminating microorganisms 74 . The most commonly substances used are alkalis (8%) and acids (8%) (Petroff method) before microbiological culture 75 , 76 . The sample decontamination procedure consists of placing an aliquot of milk (about 8 mL), previously centrifuged at 7,280 g to concentrate the cells and fat, followed by the discard of serum, in a sodium hydroxide 8% (alkali) solution. The sample is homogenized by vortexing and incubated for 30 minutes at 37 ºC. Afterwards, sulfuric acid 8% is added to stabilize pH and, as a last step, the pellet (material resulting from the centrifugation) is washed two times to eliminate any sodium hydroxide or sulfuric acid residue 25 . Samples are grown under aerobic conditions at 37 °C and evaluated weekly for up to 90 days. Phenotypic diagnosis is classically based on isolation time, morphologic characteristics of the colonies and production of pigments in the presence or absence of light 77 , 78 .
MOLECULAR DIAGNOSIS
The long time required in microbial isolation (starting after six weeks) 27 and biochemical tests for mycobacteria phenotypical identification has stimulated the development of molecular techniques in the diagnosis of Mycobacterium species from human and animal origin. The advent of new molecular techniques has enabled the investigation of epidemiological data, diagnosis of outbreaks, virulence aspects, clonal structure of the pathogen population and distribution of species from different geographic areas. It has also enabled the investigation of pathogen evolution, taxonomic reclassification, and identification of new species. These data can support control measures, prophylaxis, and/or treatment of human and animal disease 37 - 40 , 79 .
M. tuberculosis complex species have genetic homology of 99.9% at nucleotide level and identical 16S rRNA sequences, which allows a certain adaptability of molecular techniques developed for the diagnosis. In this context, the genome of M. tuberculosis has more than 99.95% similarity with M. bovis genome. However, they have differences in virulence and host susceptibility 80 . Despite the low incidence of mutations, there are diverse loci among species that have been targets in the development of molecular techniques to be used in the diagnosis of the pathogen in different clinical specimens, leading to the recognition of new species and changes in mycobacterium taxonomy 37 .
Within the approaches used in molecular diagnosis, there are different techniques that can be applied to mycobacteria diagnosis with variable sensitivity and specificity. Table 2 shows studies around the world involving different methods of mycobacterial diagnosis in bovine milk samples, presented in chronological order, and Table 3 lists the non-tuberculous mycobacteria that have been identified in bovine milk.
Table 2. Chronological list of studies involving mycobacterial diagnosis in cow milk samples.
| Number of animals | Number of samples | Number of positive animals | Type tuberculin test | Culture media | Molecular assay | Serology | Staining method | NTM | M. bovis | M. tuberculosis | Country | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 805 | Non-related | LJ; ST | M-PCR | ZN | 27 | 2 | Tanzania | 29 | ||||
| 52 | 780 | Stormont | LJ; ST | ZN | 3 | 1 | Brazil | 33 | ||||
| 1000 | 73 | ST | ZN | 20 | Pakistan | 65 | ||||||
| 78 | Non-related | LJ; ST | PCR-RFLP | 10 | 1 | Brazil | 87 | |||||
| 144 | 753 | 37 | CSIT | LJ; ST | N-PCR | 20 | 19 | India | 86 | |||
| 35 | Non-related | LJ | PCR-PRA | ZN | 15 | 0 | Turkey | 32 | ||||
| 23 | CSIT; CFSIT | ST | ELISA | 0 | 1 | Egypt | 62 | |||||
| 68 | LJ; ST | PCR | ZN | 7 | Iraq | 67 | ||||||
| 102 | 306 | CSIT | LJ; Coletsos | Spoligotyping; VNTR | ZN | 6 | 5 | Tunisia | 31 | |||
| 145 | LJ | PCR | ZN | 11 | 1 | Turkey | 61 | |||||
| 1.025 | 16 | 21 | SICCT | LJ; ST | M-PCR; LAMP | 0 | 3 | Zambia | 34 | |||
| 100 | 100 | 50 | CSIT | LJ; ST | ELISA | ZN | 1 | 2 | Egypt | 63 | ||
| 60 | LJ; ST | ZN | 3 | Pakistan | 66 | |||||||
| 104 | 150 | PCR | 0 | Israel | 98 | |||||||
| 32 | Non-related | 13 | 0 | Brazil | 88 | |||||||
| 82 | PCR | ZN | 10 | Iraq | 90 | |||||||
| 150 | 150 | 150 | SICCT | LJ; ST | PCR | 75 | Brazil | 99 | ||||
| 50 | 50 | 34 | CSIT | M-PCR | 4 | 1 | Brazil | 100 | ||||
| 181 | LJ; ST | PCR; M-PCR | ZN; Auramin | 4 | India | 64 |
CSIT: Single Cervical Intradermal Test, CFSIT: Single Caudal Fold Intradermal Test, SICCT: Single Intradermal Cervical Comparative Test, LJ: Löwenstein-Jensen, ST: Stonebrink, PCR: Polymerase Chain Reaction, M-PCR: Multiplex Polymerase Chain Reaction, PCR-RFLP: Restriction Fragment Length Polymorphism, N-PCR: Nested Polymerase Chain Reaction, PCR-PRA: Polymerase Chain Reaction of Restriction Pattern Analysis, VNTR: Variable Number Tandem Repeat, LAMP: Loop-mediated Isothermal Amplification System, ELISA: Enzime-Linked ImmunoSorbent Assay; ZN: Ziehl- Neelsen, NTM: Nontuberculous Mycobacteria.
Table 3. Nontuberculous mycobacteria detected in milk samples by molecular and microbiological methods.
| Total number of isolates | M. terrae | M. peregrinum | M. smegmatis | M. Kansasii | M. flavescens | M. neoaurum | M. scrofulaceum | M. fortuitum | M. nonchromogenicum | M. gordonae | M. marinum | M. agri | M. haemophilum | M. genavense | M. simiae | M. szulgai | M. avium | M. paratuberculosis | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17 | 2 | 3 | 1 | 3 | 1 | 1 | 1 | 5 | 88 | ||||||||||
| 27 | 7 | 4 | 13 | 2 | 1 | 29 | |||||||||||||
| 6 | 1 | 31 | |||||||||||||||||
| 15 | 6 | 3 | 3 | 1 | 32 | ||||||||||||||
| 10 | 4 | 5 | 1 | 87 | |||||||||||||||
| 11 | 1 | 6 | 2 | 2 | 61 | ||||||||||||||
| 3 | 2 | 1 | 33 | ||||||||||||||||
| 265 | 80 | 102 | |||||||||||||||||
| 70 | 2 | 103 | |||||||||||||||||
| 220 | 63 | 104 |
Polymerase Chain Reaction (PCR)
Polymerase chain reaction (PCR) is a popular molecular biology technique in which DNA is replicated enzymatically without necessarily using a living organism, such as bacteria or yeasts. This technique enables the extensive amplification of a small amount of the DNA molecule in an exponential manner 81 .
DNA amplification via PCR techniques have become tools for epidemiological studies of bovine tuberculosis transmission, and have given prominence to a modern research field. The agility and speed in detecting mycobacteria can be decisive in the choice of these methods, which discriminate species and different isolates of the same species at the DNA level 82 . For example, a conventional PCR technique is used aiming at the amplification of a 500-base pair fragment of the RvD1-Rv2031c region, found only in M. bovis strains 83 , 84 .
Nested Polymerase Chain Reaction (Nested PCR)
Nested Polymerase Chain reaction is a modification of PCR with the purpose of reducing the binding of non-specific products due to non-expected amplification of primer binding sites. It runs with two sets of primer pairs used in two continuous PCRs. The second run targets the final product 81 .
In Egypt, the Nested PCR technique was used to investigate M. bovis in cattle and buffalo milk samples of animals that were positive (n=190) and negative (n=225) to the tuberculin skin test and in commercial milk samples (n=95). In that study, Mycobacterium was detected in 33 samples (6.35%), 22 belonging to the M. tuberculosis complex, whereas the Ziehl-Neelsen microscopic evaluation indicated the presence of BAAR in 23 samples, 14 positive samples in bacteriological culture, and 12 positive samples in mice inoculation 85 . In the same study, PCR was used to diagnose M. bovis, and 16 positive samples were obtained. Nested PCR performed better in terms of sensitivity and specificity, because it was able to identify a greater number of samples compared to traditional techniques, including conventional PCR 85 . In India 86 , the same technique was used to detected M. tuberculosis and M. bovis in different types of samples, including raw milk from cattle positive to the tuberculin test. The results on the correlation of the association of the standard technique (bacterial culture) with Nested PCR indicated a higher sensitivity (97.3%) compared to the cultures of the standard method (29.7%), but specificity was higher for culture (77.5%).
Multiplex Polymerase Chain Reaction (Mutiplex PCR)
Multiplex is a kind of PCR that uses several primer sets and amplifies many regions simultaneously. Bhanurekha et al. 64 used Multiplex PCR for the differentiation of M. tuberculosis and M. bovis in 181 raw milk samples from small farms to monitor the presence of zoonotic tuberculosis, identifying four positive samples for M. tuberculosis (n=3) and M. bovis (n=1).
Polymerase Chain Reaction with Pattern Restriction Analysis (PCR-PRA)
PCR-PRA is based on the segregation of DNA sections by restriction endonucleases, generating fragments used in the classification of mycobacteria species. This method has focused in the nucleotide sequence 79 of locus hsp65.
Particularly in Brazil, raw bovine milk and pasteurized milk were screened for the presence of mycobacteria by PCR-PRA 87 , 88 . The following species were reported: M. nonchromogenicum, M. peregrinum, M. smegmatis, M. fortuitum, M. marinum, and M kansasii. M. bovis was identified only in one raw milk sample 88 . Although most mycobacteria do not belong to the M. tuberculosis complex, it is important to identify failures in the process of milk pasteurization that pose risks to humans.
Restriction Fragment Length Polymorphism IS6110 (RFLP IS6110)
The insertion sequence IS6110 is considered specific to the members of the M. tuberculosis complex and the difference in location and number of copies of the insertion sequence is a source of polymorphism between isolates 80 . That polymorphism is detected by RFLP.
Vitale et al. 89 , in Italy, evaluated the possible application of PCR IS6110 (specific technique for the detection of M. tuberculosis complex) in the diagnosis of bovine tuberculosis. Milk, nasal swabs, and lymph nodes aspirates were sampled from animals subjected to skin test (n=100), and lymph nodes and lungs were sampled in animals with positive results (n=60). From the results of the analyses of all milk samples (n=54), 47 (87%) were positive in PCR. The sensitivity and specificity was 100% when compared with tissue samples. Other authors have also used IS6110 PCR in the diagnosis of bovine tuberculosis 90 .
A study carried out in Argentina by Zumárraga et al. 91 , searching for the presence of M. bovis in bulk milk from 117 tuberculosis-free certified farms and 80 non-certified farms using IS6110 PCR milk technique, found that 67 (38%) samples from certified-free herds were positive for M. bovis, and 35 (44%) samples from uncertified herds were positive for M. bovis. The study also compared the results of tuberculin tests in terms of sensitivity. It was found that in certified-free herds, 67 samples were positive in PCR IS6110 and only three were positive in the tuberculin test, while in uncertified farms, 17 of the 35 positive samples were negative in the tuberculin test. The authors attributed these results to the slaughtering of animals that were positive in the tuberculin test, before the official inspection could be able to issue the certificates on disease-free status.
GENETIC FINGERPRINTING
Spacer Oligonucleotide typing (Spoligotyping)
Typing of mycobacteria species can be performed by spoligotyping, which is based on DNA amplification of direct repeat (DR) loci, found only in the genome of the M. tuberculosis complex. The DR regions are repeated, with two types of continuous identical sequences (DR sequences) and different sequences (spacers). Polymorphism of mycobacteria is evaluated by the presence or absence of spacers at each locus, as well as by the number of DR regions in the isolates. The advantage of this technique is its speed, reliability, and species differentiation of M. tuberculosis and M. bovis. It also enables the molecular study of spoligotypes geographical dispersion 80 , 82 .
Variable number tandem repeat (VNTR) and mycobacterial interspersed repetitive units (MIRU) typing
Tandem repeats are allelic variations that occur as repetitions of sequences of nucleotides found in intergenic regions of the genome. These repeated sequences are known in eukaryotic cells. The hyper variability of these repetitions in human and animal cells generate the Variable number tandem repeat (VNTR). In the diagnosis of mycobacterial isolates, the polymorphism may be analyzed by repetitions of sequences of nucleotides, or even variation in the number of repeating units 39 , 40 . The identification of 24 VNTR loci of M. tuberculosis genome received the name Mycobacterial Interspersed Repetitive Units (MIRU) 92 , and it has been used as a molecular marker for diagnosis of mycobacterial infections. Identification and classification by MIRU-VNTR method has proven to be fast, sensitive and affordable, particularly for isolated mycobacteria 93 .
Spoligotyping and MIRU-VNTR were carried out in Tunisia by Kahla et al. 31 in milk samples (n=306) of cows (n=102), positive to the single cervical intradermal tuberculin test and with or without clinical signs of tuberculosis and mastitis. From 102 cows, five were positive to M. bovis. Spoligotyping and VNTR revealed the heterogeneity of the isolates, because the first technique determined three types, and the second one identified four M. bovis profiles.
DNA sequencing
DNA sequencing is the process of determining the precise order of nucleotides within a DNA molecule. It includes any method or technology that is used to determine the order of the four bases (adenine, guanine, cytosine and thymine) in a strand of DNA. The advent of rapid DNA sequencing methods has greatly accelerated biological and medical research and discovery. The knowledge of DNA sequences has become indispensable to basic biological research and to numerous applied fields, such as medical diagnosis, biotechnology, forensic biology, virology, and biological taxonomy. The high speed of sequencing achieved with modern DNA sequencing technology has been critical in the knowledge of complete DNA sequences, or genomes of numerous types and species, including the human genome and other complete DNA sequences of many animal, plant, and microbial species 94 .
Although some authors have applied DNA sequencing to classify Mycobacterium species based on the hsp65 gene 95 - 97 , to date there is no evidence on the application of DNA sequencing for the diagnosis of Mycobacterium spp. in bovine milk.
Although there is a diversity of molecular methods for mycobacteria diagnosis, sequence analysis has been the most used method in clinical laboratories. The identification of specific signs in variable regions of highly conserved genes has simplified PCR protocols and the analysis of amplified products 97 - 102 . For NTM diagnosis, the hsp65 gene is the most widely target used to classify this group of mycobacteria. When the target is M. bovis or M. tuberculosis, the MIRU-VNTR is a highly discriminatory method and an important tool in epidemiological research 39 .
THE MYCOBACTERIUM BOVIS PUBLIC HEALTH RISK
Despite the fact that M. bovis is not the major cause of human tuberculosis, human infection by this species of mycobacteria has been increasingly reported. Humans become infected mainly by consumption of raw milk from infected cows 103 . In 2015, 149,000 cases of zoonotic TB occurred worldwide, with a global mortality rate of 13,400 cases104. Particularly in Brazil, despite the existence of a national eradication plan, milk is still marketed without sanitary inspection, which is a threat to public health 21 . Furthermore, there is a need to strengthen the surveillance of zoonotic TB in this country for better knowledge on the impact of the disease in dairy animals104.
CONCLUSIONS
Despite advances and control programs, tuberculosis remains as a major human and animal health threat. In Brazil, bovine tuberculosis is still a major infectious disease. Due to implications on public health and negative effects on milk production chain, demands on the research of bovine tuberculosis and other infections caused by mycobacteria have been increasing. The development and application of methods and more efficient techniques are critical tools in the diagnosis of the disease to determine control measures and effective eradication.
REFERENCES
- 1.Portaels F. Epidemiology of mycobacterial diseases. Clin Dermatol. 1995;13:207–222. doi: 10.1016/0738-081x(95)00004-y. [DOI] [PubMed] [Google Scholar]
- 2.Rastogi N, Legrand E, Sola C. The mycobacteria: an introduction to nomenclature and pathogenesis. Rev Sci Tech. 2001;20:21–54. doi: 10.20506/rst.20.1.1265. [DOI] [PubMed] [Google Scholar]
- 3.Stanford J, Stanford C. Mycobacteria and their world. Int J Mycobacteriol. 2012;1:3–12. doi: 10.1016/j.ijmyco.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Alexander KA, Laver PN, Michael AL, Williams M, van Helden PD, Warren RM, et al. Novel Mycobacterium tuberculosis complex pathogen, M. mungi. Emerg Infect Dis. 2010;16:1296–1299. doi: 10.3201/eid1608.100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Ingen J, Rahim Z, Mulder A, Boeree MJ, Simeone R, Brosch R, et al. Characterization of Mycobacterium orygis as M. tuberculosis complex subspecies. Emerg Infect Dis. 2012;18:653–655. doi: 10.3201/eid1804.110888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dippenaar A, Parsons SD, Sampson SL, van der Merwe RG, Drewe JA, Abdallah AM, et al. Whole genome sequence analysis of Mycobacterium suricattae. Tuberculosis (Edinb) 2015;95:682–688. doi: 10.1016/j.tube.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Songer JG, Post KW. Veterinary microbiology: bacterial and fungal agents of animal disease. St. Louis: Elsevier Saunders; 2005. [Google Scholar]
- 8.Ben Salah I, Adékambi T, Raoult D, Drancourt M. rpoB sequence-based identification of Mycobacterium avium complex species. Microbiology. 2008;54(Pt 12):3715–3723. doi: 10.1099/mic.0.2008/020164-0. [DOI] [PubMed] [Google Scholar]
- 9.Quinn PJ, Markey BK, Leonard FC, Fitzpatrick ES, Fanning S, Hartigan PJ. Veterinary microbiology and microbial disease. 2nd. Oxford: Wiley-Blackwell; 2011. [Google Scholar]
- 10.Fitzgerald JR, Musser JM. Evolutionary genomics of pathogenic bacteria. Trends Microbiol. 2001;9:547–553. doi: 10.1016/s0966-842x(01)02228-4. [DOI] [PubMed] [Google Scholar]
- 11.Acha PN, Szyfres B. Zoonosis y enfermidades transmissibles comunes al hombre y los animales. 3rd. Washington: Organización Panamericana de la Salud; 2001. [Google Scholar]
- 12.Verma AK, Tiwari R, Chakraborty S, Neha, Saminathan M, Dhama K, et al. Insights into bovine tuberculosis (bTB), various approaches for its diagnosis, control and its public health concerns: an update. Asian J Anim Vet Adv. 2014;9:323–344. [Google Scholar]
- 13.Holloway KL, Henneberg RJ, de Barros Lopes M, Henneberg M. Evolution of human tuberculosis: a systematic review and meta-analysis of paleopathological evidence. Homo. 2011;62:402–458. doi: 10.1016/j.jchb.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Ashford DA, Whitney E, Raghunathan P, Cosivi O. Epidemiology of selected mycobacteria that infect humans and other animals. Rev Sci Tech. 2001;20:325–337. doi: 10.20506/rst.20.1.1266. [DOI] [PubMed] [Google Scholar]
- 16.Brasil. Ministério da Saúde Portal da Saúde: Tuberculose. [cited 2017 Jan 16]. http://portalsaude.saude.gov.br/index.php?option=com_content&view=article&id=11045&Itemid=674.
- 17.Gentry CA. Pharmacotherapy Self-Assessment Program. 5th. Lexena: American College of Clinical Pharmacy; 2008. Atypical mycobacteria. [Google Scholar]
- 18.Wagner D, Young LS. Nontuberculous mycobacterial infections: a clinical review. Infection. 2004;32:257–270. doi: 10.1007/s15010-004-4001-4. [DOI] [PubMed] [Google Scholar]
- 19.Dailloux M, Laurain C, Weber M, Hartemann P. Water and nontuberculous mycobacteria. Water Res. 1999;33:2219–2228. [Google Scholar]
- 20.Motta RG, Silva AV, Giuffrida R, Siqueira AK, Paes AC, Motta IG, et al. Indicadores de qualidade e composição de leite informal comercializado na região sudeste do Estado de São Paulo. Pesq Vet Bras. 2015;35:417–423. [Google Scholar]
- 21.Abrahão RM. Tuberculose humana causada pelo Mycobacterium bovis: considerações gerais e a importância dos reservatórios animais. Arch Vet Scienc. 1999;4:5–15. [Google Scholar]
- 22.de la Rua-Domenech R. Human Mycobacterium bovis infection in the United Kingdom: incidence, risks, control measures, and review of the zoonotic aspects of bovine tuberculosis. Tuberculosis (Edinb) 2006;86:77–109. doi: 10.1016/j.tube.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention . Mycobacterium bovis (bovine tuberculosis) in humans. Atlanta: CDC; 2012. [cited 2016 Jul 03]. http://www.cdc.gov/tb/publications/factsheets/general/mbovis.htm. [Google Scholar]
- 24.Instituto Brasileiro de Geografia e Estatística . Produção da pecuária municipal 2014. Rio de Janeiro: IBGE; 2014. [Google Scholar]
- 25.Franco MM, Paes AC, Ribeiro MG, Pantoja JC, Santos AC, Miyata M, et al. Occurrence of mycobacteria in bovine milk samples from both individual and collective bulk tanks at farms and informal markets in the southeast region of Sao Paulo, Brazil. 85BMC Vet Res. 2013;9 doi: 10.1186/1746-6148-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brasil. Ministério da Agricultura, Pecuária e Abastecimento . Programa nacional de controle e erradicação da brucelose e da tuberculose animal (PNCEBT): manual técnico. Brasília: Ministério da Agricultura, Pecuária e Abastecimento; 2006. [Google Scholar]
- 27.Paes AC. Tuberculose bovina. In: Pires AV, editor, editor. Bovinocultura de corte. Piracicaba: FEALQ; 2010. pp. 993–1015. [Google Scholar]
- 28.Brasil. Ministério da Agricultura, Pecuária e Abastecimento . Diário Oficial da União. Brasília: Dec 30, 2011. Instrução normativa nº 62, de 29 de dezembro de 2011. Aprova o Regulamento Técnico de Produção, Identidade e Qualidade do Leite tipo A, o Regulamento Técnico de Identidade e Qualidade de Leite Cru Refrigerado, o Regulamento Técnico de Identidade e Qualidade de Leite Pasteurizado e o Regulamento Técnico da Coleta de Leite Cru Refrigerado e seu Transporte a Granel. Seção 1. [Google Scholar]
- 29.Kazwala RR, Daborn CJ, Kusiluka LJ, Jiwa SF, Sharp JM, Kambarage DM. Isolation of Mycobacterium species from raw milk of pastoral cattle of the southern highlands of Tanzania. Trop Anim Health Prod. 1998;30:233–239. doi: 10.1023/a:1005075112393. [DOI] [PubMed] [Google Scholar]
- 30.Cadmus SI, Yakubu MK, Magaji AA, Jenkins AO, van Soolingen D. Mycobacterium bovis, but also M. africanum present in raw milk of pastoral cattle in north-central Nigeria. Trop Anim Health Prod. 2010;42:1047–1048. doi: 10.1007/s11250-010-9533-2. [DOI] [PubMed] [Google Scholar]
- 31.Ben IK, Boschiroli ML, Souissi F, Cherif N, Benzarti M, Boukadida J, et al. Isolation and molecular characterization of Mycobacterium bovis from raw milk in Tunisia. Afr Health Sci. 2011;11(Suppl 1):S2–S5. doi: 10.4314/ahs.v11i3.70032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konuk M, Korcan E, Dulgerbaki S, Altindis M. Isolation and identification of Mycobacteria from raw milk samples in Afyonkarahisar district of Turkey. Int J Food Microbiol. 2007;115:343–347. doi: 10.1016/j.ijfoodmicro.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 33.Pardo RB, Langoni H, Mendonça LJ, Chi KD. Isolation of Mycobacterium spp. in milk from cows suspected or positive to tuberculosis. Braz J Vet Res Anim Sci. 2001;38:284–287. [Google Scholar]
- 34.Pandey GS, Hang’ombe BM, Mushabati F, Kataba A. Prevalence of tuberculosis among southern Zambian cattle and isolation of Mycobacterium bovis in raw milk obtained from tuberculin positive cows. Vet World. 2013;6:986–991. [Google Scholar]
- 35.Corner LA. Post mortem diagnosis of Mycobacterium bovis infection in cattle. Vet Microbiol. 1994;40:53–63. doi: 10.1016/0378-1135(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 36.Markey BK, Leonard FC, Archambault M, Cullinane A, Maguire D. Clinical veterinary microbiology. 2nd. Edinburgh: Elsevier Mosby; 2013. [Google Scholar]
- 37.Supply P, Mazars E, Lesjean S, Vincent V, Gicquel B, Locht C. Variable human minisatellite-like regions in the Mycobacterium tuberculosis genome. Mol Microbiol. 2000;36:762–771. doi: 10.1046/j.1365-2958.2000.01905.x. [DOI] [PubMed] [Google Scholar]
- 38.Supply P, Allix C, Lesjean S, Cardoso-Oelemann M, Rüsch-Gerdes S, Willery E, et al. Proposal for standardization of optimized mycobacterial interspersed repetitive unit–variable-number tandem repeat typing of Mycobacterium tuberculosis. J Clin Microbiol. 2006;44:4498–4510. doi: 10.1128/JCM.01392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romero B, Aranaz A, de Juan L, Alvarez J, Bezos J, Mateos A, et al. Molecular epidemiology of multidrug-resistant Mycobacterium bovis isolates with the same spoligotyping profile as isolates from animals. J Clin Microbiol. 2006;44:3405–3408. doi: 10.1128/JCM.00730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rocha VC, Figueiredo SC, Rosales CA, Grisi JH, Filho, Keid LB, Soares RM, et al. Molecular discrimination of Mycobacterium bovis in São Paulo, Brazil. Vector Borne Zoonotic Dis. 2013;13:17–21. doi: 10.1089/vbz.2012.1035. [DOI] [PubMed] [Google Scholar]
- 41.Roring S, Scott AN, Hewinson RG, Neill SD, Skuce RA. Evaluation of variable number tandem repeat (VNTR) loci in molecular typing of Mycobacterium bovis isolates from Ireland. Vet Microbiol. 2004;101:65–73. doi: 10.1016/j.vetmic.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Radostits OM, Gay CC, Hinchcliff KW, Constable PD, editors, editors. Veterinary medicine : a textbook of the diseases of cattle, horses, sheep, pigs, and goats. 10th. Philadelphia: Saunders; 2007. [Google Scholar]
- 43.Schiller I, Waters WR, Vordermeier HM, Jemmi T, Welsh M, Keck N, et al. Bovine tuberculosis in europe from the perspective of an officially tuberculosis free country: trade, surveillance and diagnostics. Vet Microbiol. 2011;151:153–159. doi: 10.1016/j.vetmic.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 44.European Food Safety Authority . Trends and sources of zoonoses and zoonotic agents in the European Union in 2007. Parma: EFSA; 2009. [Google Scholar]
- 45.Asil El TA, El Sanousi SM, Gameel A, El Beir H, Fathelrahman M, Terab NM, et al. Bovine tuberculosis in South Darfur state, Sudan: an abattoir study based on microscopy and molecular detection methods. Trop Anim Health Prod. 2012;45:469–472. doi: 10.1007/s11250-012-0241-y. [DOI] [PubMed] [Google Scholar]
- 46.Demelash B, Inangolet F, Oloya J, Asseged B, Badaso M, Yilkal A, et al. Prevalence of bovine tuberculosis in Ethiopian slaughter cattle based on post-mortem examination. Trop Anim Health Prod. 2009;41:755–765. doi: 10.1007/s11250-008-9248-9. [DOI] [PubMed] [Google Scholar]
- 47.de Kantor IN, Ritacco V. An update in bovine tuberculosis programs in Latin America and Caribbean countries. Vet Microbiol. 2006;112:111–118. doi: 10.1016/j.vetmic.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 48.Paes AC, Franco MM. Megid J, Ribeiro MG, Paes AC.organizadores . Doenças infecciosas em animais de produção e companhia. Rio de Janeiro: Roca; 2016. Tuberculose em animais de produção; pp. 512–542. [Google Scholar]
- 49.Corrêa WM, Corrêa CN. Enfermidades infecciosas dos mamíferos domésticos. 2ª. Rio de Janeiro: MEDSI; 1992. [Google Scholar]
- 50.Neill SD, Pollock JM, Bryson DB, Hanna J. Pathogenesis of Mycobacterium bovis infection in cattle. Vet Microbiol. 1994;40:41–52. doi: 10.1016/0378-1135(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 51.Menzies FD, Neill SD. Cattle-to-cattle transmission of bovine tuberculosis. Vet J. 2000;160:92–106. doi: 10.1053/tvjl.2000.0482. [DOI] [PubMed] [Google Scholar]
- 52.Neill SD, Bryson DG, Pollock JM. Pathogenesis of tuberculosis in cattle. Tuberculosis (Edinb) 2001;81:79–86. doi: 10.1054/tube.2000.0279. [DOI] [PubMed] [Google Scholar]
- 53.Wilsmore T, Taylor N. Bovine tuberculosis: an update. Reading: University of Reading; 2008. [Google Scholar]
- 54.Domingo M, Vidal E, Marco A. Pathology of bovine tuberculosis. Res Vet Sci. 2014;97(Supp):S20–S29. doi: 10.1016/j.rvsc.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 55.Watts JL. Etiological agents of bovine mastitis. Vet Microbiol. 1988;16:41–66. doi: 10.1016/0378-1135(88)90126-5. [DOI] [PubMed] [Google Scholar]
- 56.Ribeiro MG. Andrade SF. Manual de terapêutica veterinária. 3ª. São Paulo: Roca; 2008. Princípios terapêuticos na mastite em animais de produção e de companhia; pp. 759–771. [Google Scholar]
- 57.Philpot WN, Nickerson SC. Vencendo a luta contra a mastite. Jaguariúna: Westfalia Landtechnik do Brasil; 2002. [Google Scholar]
- 58.Goodchild AV, Clifton-Hadley RS. Cattle-to-cattle Transmission of Mycobacterium bovis. Tuberculosis (Edinb) 2001;81:23–41. doi: 10.1054/tube.2000.0256. [DOI] [PubMed] [Google Scholar]
- 59.Beals T. The risk of bovine TB from raw milk consumption with a focus on Michigan. Washington: Weston A. Price Foundation; 2008. [cited 2015 Aug 15]. http://www.realmilk.com/safety/risk-of-bovine-tb-from-raw-milk-consumption/ [Google Scholar]
- 60.Pérez A, Reniero A, Forteis S, Meregalli B, López B, Ritacco V. Study of Mycobacterium bovis in milk using bacteriological methods and polymerase chain reaction. Rev Argent Microbiol. 2002;34:45–51. [PubMed] [Google Scholar]
- 61.Aydin FE, Ülger M, Emekdaş G, Aslan G, Günal S. Isolation and identification of Mycobacterium bovis and non-tuberculous Mycobacteria in raw milk samples in Mersin Province. Mikrobiyol Bul. 2012;46:283–289. [PubMed] [Google Scholar]
- 62.Hassanain NA, Hassanain MA, Soliman YA, Ghazy AA, Ghazyi YA. Bovine tuberculosis in a dairy cattle farm as a threat to public health. Afr J Microbiol Res. 2009;3:446–450. [Google Scholar]
- 63.Nasr SE, Saad NM, Nasr EA, Wahba NM, Elsherif WM. Detection of bovine tuberculosis in milk and serum of tuberculin reactors dairy farm animals in Assiut City, Egypt. Basic Res J Anim Sci. 2013;1:1–6. [Google Scholar]
- 64.Bhanurekha V, Gunaseelan L, Pawar G, Nassiri R, Bharathy S. Molecular detection of Mycobacterium tuberculosis from bovine milk samples. J Adv Vet Anim Res. 2015;2:80–83. [Google Scholar]
- 65.Jalil H, Das P, Suleman A. Bovine tuberculosis in dairy animals at Lahore, threat to the public health. Devon: Priory Lodge Education; 2003. [cited 2015 Oct 12]. http://www.priory.com/vet/bovinetb.htm. [Google Scholar]
- 66.Qamar MF, Azhar T. Detection of mycobacterium from bovine milk in Lahore. Pakistan. Sci Int (Lahore) 2013;25:353–357. [Google Scholar]
- 67.Al-Saqur IM, Al-Thwani AN, Al-Attar IM. Detection of Mycobacteria spp. in cows milk using conventional methods and PCR. Iraqi J Vet Sci. 2009;23(Suppl 2):259–262. [Google Scholar]
- 68.de la Rua-Domenech R, Goodchild AT, Vordermeier HM, Hewinson RG, Christiansen KH, Clifton-Hadley RS. Ante mortem diagnosis of tuberculosis in cattle: a review of the tuberculin tests, c-interferon assay and other ancillary diagnostic techniques. Res Vet Sci. 2006;81:190–210. doi: 10.1016/j.rvsc.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 69.Association of Public Health Laboratories Mycobacterial culture. [cited 2017 Jan 16]. https://www.aphl.org/programs/infectious_disease/tuberculosis/TBCore/MycobacterialCulture_FINAL.pdf.
- 70.Cheng AF, Li MS, Chan CY, Chan CH, Lyon D, Wise R, et al. Evaluation of three culture media and their combinations for the isolation of Mycobacterium tuberculosis from pleural aspirates of patiens with tuberculous pleurisy. J Trop Med Hyg. 1994;97:249–253. [PubMed] [Google Scholar]
- 71.Laymon CW. Culture of tubercle bacilli by the Löwenstein Method. Arch Derm Syphilol. 1933;28:35–41. [Google Scholar]
- 72.Stonebrink B. The use of a pyruvate containing egg medium in the culture of isoniazid resistant strains of Mycobacterium tuberculosis var. Hominis Acta Tuberc Scand. 1958;35:67–80. [Google Scholar]
- 73.Lesslie IW. A comparison of biological and some cultural methods for the primary isolation of Mycobacterium tuberculosis. J Comp Pathol. 1959;69:1–10. doi: 10.1016/s0368-1742(59)80001-1. [DOI] [PubMed] [Google Scholar]
- 74.Corner LA, Trajstman AC, Lund K. Determination of the optimum concentration of decontaminants for the primary isolation of Mycobacterium bovis. N Z Vet J. 1995;43:129–133. doi: 10.1080/00480169.1995.35871. [DOI] [PubMed] [Google Scholar]
- 75.Balian SC, Pinheiro SR, Guerra JL, Morais ZM, Ferreira F, Ferreira JS., Neto Estudo comparativo de dois métodos de descontaminação na pesquisa de micobactérias. Arq Inst Biol. 2002;69:11–14. [Google Scholar]
- 76.Ambrosio SR, Oliveira EM, Rodriguez CA, Ferreira JS, Neto, Amaku M. Comparison of three decontamination methods for Mycobacterium bovis isolation. Braz J Microbiol. 2008;39:241–244. doi: 10.1590/S1517-83822008000200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Runyon EH. Anonymous mycobacteria in pulmonary disease. Med Clin North Am. 1959;43:273–290. doi: 10.1016/s0025-7125(16)34193-1. [DOI] [PubMed] [Google Scholar]
- 78.Brasil. Ministério da Saúde. Centro de Referência Professor Hélio Fraga . Manual de bacteriologia da tuberculose. 3ª. Rio de Janeiro: Ministério da Saúde; 2005. [Google Scholar]
- 79.Telenti A, Marchesi F, Balz M, Bally F, Böttger EC, Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol. 1993;31:175–178. doi: 10.1128/jcm.31.2.175-178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haddad N, Masselot M, Durand B. Molecular differentiation of Mycobacterium bovis isolates. Res Vet Sci. 2004;76:1–18. doi: 10.1016/s0034-5288(03)00078-x. [DOI] [PubMed] [Google Scholar]
- 81.Rahman MT, Uddin MS, Sultana R, Moue A, Setu M. Polymerase Chain Reaction (PCR): a short review. AKMMC J. 2013;4:30–36. [Google Scholar]
- 82.Ramos DF, Tavares L, da Silva PE, Dellagostin OA. Molecular typing of Mycobacterium bovis isolates: a review. Braz J Microbiol. 2014;45:365–372. doi: 10.1590/s1517-83822014005000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rahman MM, Noor M, Islam KM, Uddin MB, Hossain FM, Zinnah MA, et al. Molecular diagnosis of bovine tuberculosis in bovine and human samples: implications for zoonosis. Future Microbiol. 2015;10:527–535. doi: 10.2217/fmb.14.139. [DOI] [PubMed] [Google Scholar]
- 84.Majeed MA, Ahmed WA, Manki AA. Amplification of a 500 base-pair fragment from routinely identified isolates of M. bovis from cow’s milk in Baghdad. Int J Adv Biol Res. 2013;3:163–167. [Google Scholar]
- 85.Alwathnani HA, Ashgan MH, Ihab MM. Nested polymerase chain reaction as a molecular tool for detection of Mycobacterium tuberculosis complex recovered from milk samples. Afr J Microbiol Res. 2012;6:1338–1344. [Google Scholar]
- 86.Mishra A, Singhal A, Chauhan DS, Katoch VM, Srivastava K, Thakral SS, et al. Direct detection and identification of Mycobacterium tuberculosis and Mycobacterium bovis in bovine samples by a novel nested PCR assay: correlation with conventional techniques. J Clin Microbiol. 2005;43:5670–5678. doi: 10.1128/JCM.43.11.5670-5678.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leite CQ, Anno IS, Leite SR, Roxo E, Morlock GP, Cooksey RC. Isolation and identification of Mycobacteria from livestock specimens and milk obtained in Brazil. Mem Inst Oswaldo Cruz. 2003;98:319–323. doi: 10.1590/s0074-02762003000300005. [DOI] [PubMed] [Google Scholar]
- 88.Sgarioni SA, Hirata RD, Hirata MH, Leite CQ, de Prince KA, Leite SR, et al. Occurrence of Mycobacterium bovis and non-tuberculous mycobacteria (NTM) in raw and pasteurized milk in the northwestern region of Paraná, Brazil. Braz J Microbiol. 2014;45:707–711. doi: 10.1590/s1517-83822014000200046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vitale F, Capra G, Maxia L, Reale S, Vesco G, Caracappa S. Detection of Mycobacterium tuberculosis complex in cattle by PCR using milk, lymph node aspirates, and nasal swabs. J Clin Microbiol. 1998;36:1050–1055. doi: 10.1128/jcm.36.4.1050-1055.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Senthil NR, Ranjani MR, Vasumathi K. Comparative diagnosis of Mycobacterium bovis by polymerase chain reaction and Ziel- Neilson staining technique using milk and nasal washing. J Res Agric Anim Sci. 2014;2:1–3. [Google Scholar]
- 91.Zumárraga MJ, Soutullo A, García MI, Marini R, Abdala A, Tarabla H, et al. Detection of Mycobacterium bovis–infected dairy herds using PCR in bulk tank milk samples. Foodborne Pathog Dis. 2012;9:132–137. doi: 10.1089/fpd.2011.0963. [DOI] [PubMed] [Google Scholar]
- 92.Supply P, Magdalena J, Himpens S, Locht C. Identification of novel intergenic repetitive units in a mycobacterial two-components system operon. Mol Microbiol. 1997;26:991–1003. doi: 10.1046/j.1365-2958.1997.6361999.x. [DOI] [PubMed] [Google Scholar]
- 93.Barnes PF, Cave MD. Molecular epidemiology of tuberculosis. N Eng J Med. 2003;349:1149–1156. doi: 10.1056/NEJMra021964. [DOI] [PubMed] [Google Scholar]
- 94.Pettersson E, Lundeberg J, Ahmadian A. Generations of sequencing technologies. Genomics. 2009;93:105–111. doi: 10.1016/j.ygeno.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 95.Senna SG, Battilana J, Costa JC, Silva MG, Duarte RS, Fonseca LS, et al. Sequencing of hsp65 gene for identification of Mycobacterium species isolated from environmental and clinical sources in Rio de Janeiro, Brazil. J Clin Microbiol. 2008;46:3822–3825. doi: 10.1128/JCM.00451-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ringuet H, Akoua-Koffi C, Honore S, Varnerot A, Vincent V, Berche P, et al. hsp65 sequencing for identification of rapidly growing Mycobacteria. J Clin Microbiol. 1999;37:852–857. doi: 10.1128/jcm.37.3.852-857.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pourahmad F, Thompson KD, Adams A, Richards RH. Comparative evaluation of Polymerase Chain Reaction-Restriction Enzyme Analysis (PRA) and sequencing of heat shock protein 65 (hsp65) gene for identification of aquatic mycobacteria. J Microbiol Methods. 2009;6:128–135. doi: 10.1016/j.mimet.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 98.Ereqat S, Bar-Gal GK, Nasereddin A, Said S, Greenblatt CL, Azmi K, et al. Pulmonary tuberculosis in the West Bank, Palestinian Authority: molecular diagnostic approach. Trop Med Int Health. 2011;16:360–367. doi: 10.1111/j.1365-3156.2010.02697.x. [DOI] [PubMed] [Google Scholar]
- 99.Carvalho RC, Castro VS, Fernandes DV, Moura G, Soares ES, Figueiredo ES, et al. Use of PCR for detection of bovine tuberculosis bacillus in milk of positive skin test cows. Braz J Vet Res Anim Sci. 2014;51:42–48. [Google Scholar]
- 100.Figueiredo EE, Conte CA, Junior, Furlaneto LV, Silvestre FG, Duarte RS, Silva JT, et al. Molecular techniques for identification of species of the Mycobacterium tuberculosis complex: the use of multiplex PCR and an adapted HPLC method for identification of Mycobacterium bovis and diagnosis of bovine tuberculosis. In: Cardona PJ, editor, editor. Understanding tuberculosis : global experiences and innovative approaches to the diagnosis. Rijeka: InTech; 2012. pp. 411–432. [Google Scholar]
- 101.Gilardoni LR, Fernández B, Morsella C, Mendez L, Jar AM, Paolicchi FA, et al. Mycobacterium paratuberculosis detection in cow’s milk in Argentina by immunomagnetic separation-PCR. Braz J Microbiol. 2016;47:506–512. doi: 10.1016/j.bjm.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Paolicchi F, Cirone K, Morsella C, Gioffré A. First isolation of Mycobacterium avium subsp paratuberculosis from commercial pasteurized milk in Argentina. Braz J Microbiol. 2012;43:1034–1037. doi: 10.1590/S1517-838220120003000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Botsaris G, Slana I, Liapi M, Dodd C, Economides C, Rees C, et al. Rapid detection methods for viable Mycobacterium avium subspecies paratuberculosis in milk and cheese. Int J Food Microbiol. 2010;141:S87–S90. doi: 10.1016/j.ijfoodmicro.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 104.World Health Organization . Global tuberculosis report. Geneva: WHO; 2016. [Google Scholar]