Abstract
In virtually all cells, store-operated Ca2+ entry signals are vital in controlling a spectrum of functions. The signals are mediated by STIM proteins in the ER and Orai channels in the PM which undergo a dynamic coupling process within discrete ER-PM junctional regions. This coupling is initiated by depletion of ER stored Ca2+ triggering STIM proteins to undergo an intricate activation process. Thereafter, STIM proteins become trapped in the ER-PM junctions where they tether and gate PM Orai Ca2+ channels. STIM1 exists as a dimer, with a single STIM-Orai activating region (SOAR) buried in the resting protein that becomes exposed upon activation. An exposed region on SOAR including the Phe-394 residue forms a critical Orai1 interacting site. Using dimeric SOAR concatemers, we reveal only one of the two sites in the SOAR dimer is needed for Orai1 activation. This unimolecular interaction of SOAR with Orai1 suggests STIM1 can cross-link Orai channels with important significance for Ca2+ signaling. A critical “nexus” region in Orai1 close to the STIM1-binding site can be mutated to constitutively activate the channel mimicking the gating action of STIM1. This indicates STIM1 remotely controls Orai1 channel gating through an allosteric switch triggered by STIM1 binding only to the exposed C-terminal tail of the Orai1 channel.
Keywords: calcium signals, calcium channels, STIM1, Orai1, store-operated
Graphical Abstract
Store-operated Ca2+ entry signals play a vital role in controlling a spectrum of cell functions in virtually all cell types (1). These signals are mediated by the combined actions of STIM proteins in the ER membrane and Orai channels in the PM. The two proteins undergo a dynamic coupling process within discrete ER-PM junctional regions (2–4). This coupling process is initiated in response to depletion of Ca2+ stored in the ER, which triggers the ER Ca2+-sensing STIM proteins to undergo an intricate activation process (2). Thereafter, the STIM proteins migrate and become trapped in the ER-PM junctions where they tether and gate Orai Ca2+ channels located in the PM (2–5). The opened Orai1 channel mediates Ca2+ signals critical in the control of gene expression, cell growth, secretion, and cell motility. The entering Ca2+ also plays an important role in the homeostatic control of cell Ca2+ and assuring that Ca2+ within the ER is maintained to protect the crucial protein processing functions of the ER. Although the STIM-Orai interaction is well established as being necessary for Orai channel activation, considerable uncertainty surrounds the molecular characteristics of the coupling process and how such coupling results in channel gating (3,5,6). Here we describe some recent advances in understanding the STIM-Orai coupling interface
The STIM-Orai Coupling Interface
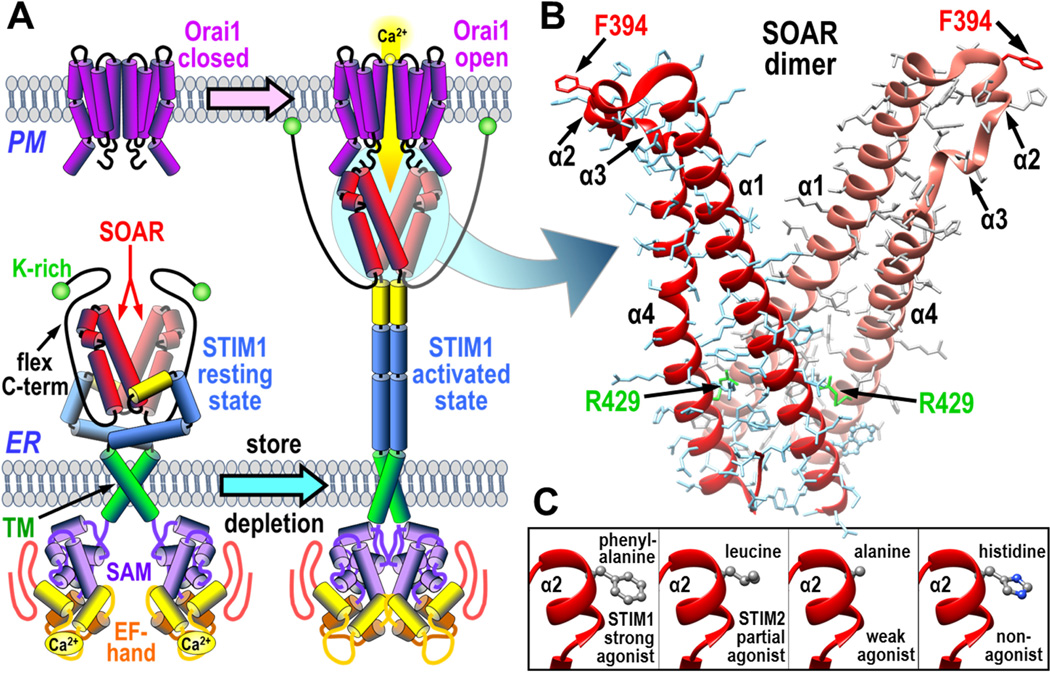
The two STIM proteins, STIM1 and STIM2, are single transmembrane-spanning proteins resident in the ER membrane (2). Their activation and coupling with Orai channels in the PM are shown in Fig. 1A. A decrease in Ca2+ stored in the ER lumen is sensed by a pair of Ca2+-binding EF hand domains on the luminal N-termini of the STIM proteins. STIM proteins exist as dimers (7,8), undergoing a scissor-like conformational rearrangement in response to Ca2+ store-depletion in which the N-terminal domains associate to induce an unfolding and extension of the C-terminal cytoplasmic domains (9). The C-termini of the STIM dimer includes a highly conserved compact region known generally now as the STIM-Orai activating region (SOAR; 344–442) (10). Slightly longer regions were named the channel-activating domain (CAD; 342–448) (11) or the Orai1-activating small fragment (OASF; 233–450) (7). Remarkably, when expressed alone as a cytoplasmic protein, the SOAR domain from STIM1 is sufficient to completely activate the Orai1 channel (10). SOAR is the smallest functional unit of STIM1. The crystal structure of SOAR reveals it to be a dimer, each of the two peptides comprising four α-helices (12) (Fig. 1B). This dimeric SOAR structure seems to be preserved within the C-terminal STIM1 dimer, the SOAR dimer itself being an important core helping to hold the two STIM1 monomers together (7,8,12,13). In the resting STIM1 dimer, the dimeric SOAR unit remains occluded within the large folded cytoplasmic STIM1 C-terminus (13). After STIM1 activation and unfolding of its cytoplasmic domain, the extended STIM1 molecule becomes trapped in ER-PM junctions as a result of interactions between the lysine-rich far C-terminus of STIM1 and acidic phospholipids in the PM (11,14). Simultaneously, the now-exposed SOAR domain is able to directly bind to Orai channels in the PM causing them to be tethered in the ER-PM junctions and activated to allow entry of Ca2+ (12,13,15). The Ca2+ entry signals mediated by Orai channels trigger numerous Ca2+-dependent responses including calcineurin-induced NFAT dephosphorylation and transcriptional activation (16,17)
Figure 1.
The coupling interface between STIM1 and Orai1. (A) Overall scheme for activation and coupling between the STIM1 dimer in the endoplasmic reticulum (ER) and Orai1 channel in the plasma membrane (PM). STIM1 is shown in its resting state (left) and activated state after store depletion and dissociation of luminal Ca2+ (right). The Orai1 channel is shown in its resting closed state (left) and activated open state coupled to the STIM1 protein (right). The STIM1 domains shown include the STIM-Orai activating region (SOAR), the lysine-rich C-terminus (K-rich), the flexible C-terminal domain (flex C-term), the transmembrane region (TM), the sterile-α motif (SAM), and the Ca2+-binding EF-hand segment. (B) Structure of dimeric SOAR domain from STIM1 revealing the four α-helical domains (α1, α2, α3, and α4), the active STIM1-binding site residue, Phe-394, and the structurally important Arg-429 residue. (C) Detail of the α2 domain of SOAR to show the wildtype Phe-394 residue, and substitutions of this residue with leucine (found in STIM2) or with alanine or histidine which block the interaction with Orai1.
The plasma membrane Orai1 channel is revealed to be a hexameric multimer of Orai1 subunits (18,19). Each Orai1 subunit of the hexamer is a four transmembrane-spanning protein. The N-terminal transmembrane helix (TM1) forms the Orai1 channel pore in the center of the hexamer (18). The second and third transmembrane helices (TM2 and TM3) in each subunit are closely packed around the pore, and the fourth (C-terminal) transmembrane helix (TM4) has a helical cytoplasmic extension known to be the major site of interaction with STIM proteins (11,18). The exact nature of the coupling between the unfolded STIM1 and Orai1 channels is a critical question that is not yet understood (3,5,6). Curiously, the stoichiometry of STIM1-Orai1 coupling is variable (20,21). The greatest Orai1 channel activation occurs with a STIM1:Orai1 ratio of 2:1 (21,22), suggesting six STIM1 dimers interact with a single Orai1 hexameric channel. In contrast, recent structural studies with partial fragments of SOAR and the C-terminal STIM1-binding helices from Orai1, suggest a bimolecular interaction such that a STIM1 dimer binds to two adjacent Orai1 subunits within the Orai1 hexameric channel (23–25). This model would be consistent with a stoichiometry of 1:1, but would not explain how a variable stoichiometry might occur. Our studies, described here, suggest a different configuration of the STIM-Orai coupling interface.
Use of SOAR-dimer Constructs to Assess STIM-Orai Coupling
In order to better understand how STIM1-Orai1 coupling occurs, we utilized constructs that included mutation of an important residue (Phe-394) we recently identified in STIM1 as defining a crucial Orai1-binding site (26). We determined that although the SOAR region in STIM2 is almost identical to STIM1, the STIM2 molecule differs in having a leucine at the equivalent position (Leu-485) which results in STIM2 having reduced affinity for Orai1 and functioning as only a partial agonist of Orai1 channel activation (Fig. 1C). When we mutated this residue to alanine, STIM1 worked only as a weak agonist of Orai1. Mutating this same STIM1 residue to a histidine (F394H) provided a powerful and complete block of the Orai1-binding site and completely prevented STIM1 interacting with or activating the Orai1 channel (26). Yet, despite blocking STIM1-Orai1 coupling, the F394 mutation does not change the STIM1 resting state or the ability of STIM1 to become activated by store-depletion and move into ER-PM junctions.
We made a series of concatenated dimers of SOAR in which either one or both of the F394 residues in each SOAR peptide were mutated to histidine. Based on the previously postulated bimolecular interaction with Orai1 subunits, we predicted that heterodimers of SOAR which included one normal monomer and one with the F394H mutation, would be nonfunctional. To our surprise, the heterodimers were fully functional in activating Orai1 channels (27). This unexpected result reveals that full activation of the Orai1 channel requires only one of the two active sites on the SOAR dimer to activate the Orai1 channel.
Thus, although SOAR always exists as a dimer (expressed either by itself or inside the full-length STIM1 molecule), it need only undergo monomeric interaction with Orai1 to fully open the channel. Indeed, the SOAR unit alone or within STIM1 must be a dimer to activate Orai1. Mutating the residues that hold the SOAR dimer together, prevents SOAR from binding or activating Orai1 channels (12,23,25). Thus, the R429 residue is known from crystallization studies (12) to mediate dimer-dimer interactions (see Fig. 1B) and the spontaneously occurring R429C mutation in humans causes loss of STIM1 function (25). But, complete dissociation of the SOAR dimer may not result from the R429C mutation – thus, it appears to only modify the SOAR dimer secondary structure, without causing monomer dissociation. In whole STIM1, the R429C mutation not only prevents coupling to Orai1, but also causes the entire STIM1 C-terminus to unfold exposing the K-rich terminal region causing constitutive movement into ERPM junctions but no channel activation (25). Our results with the F394H mutation in SOAR reveal that its effect on coupling is very different from the actions of the R429C mutation (27). Homodimers of STIM1 with the F394H substitution can still become activated by store-depletion and move into junctions as a result of exposure of the K-rich C-terminus being exposed and its binding to acidic phospholipids in the PM. The only deficiency it has is the functional coupling to Orai1 channels which is required for Orai1 gating. Indeed, the F394 residue resides at a critical locus in the SOAR structure, at the prominently exposed apex of each of the SOAR monomers (Fig. 1B). This clearly contrasts with the R429 residue which is deeply buried within the residues involved in monomer-monomer interactions in the SOAR dimer (Fig. 1B).
A Unimolecular Coupling Model for STIM1-Orai1 Interactions
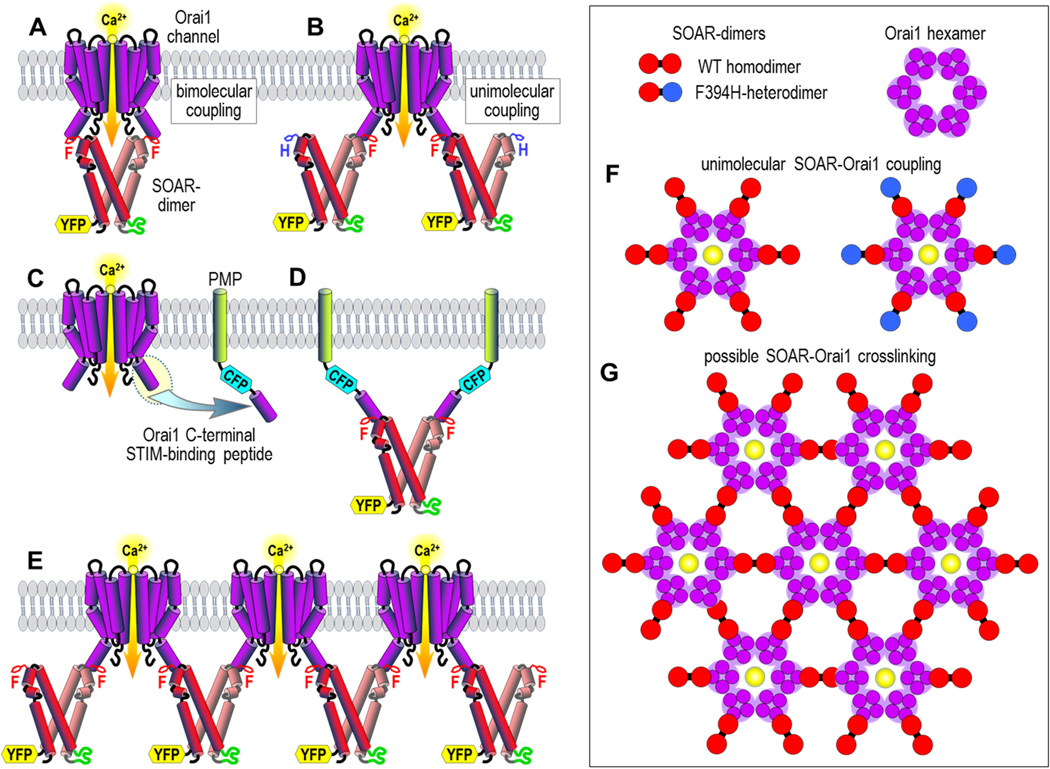
Our results suggest a STIM-Orai coupling mechanism that is quite different to that previously proposed. Thus, the predominant model for coupling has been that SOAR dimers bind across two juxtaposed Orai1 subunits to activate the channel, as shown in Fig. 2A. Recent NMR studies were considered to support this bimolecular model (23,24). However, those studies were based on short SOAR-derived peptides that had no Orai1-activating function. Moreover, the proposed Orai1-interacting sequences were exactly those same residues involved in SOAR-SOAR interactions that are buried deeply in the SOAR dimer. Another factor is that the site proposed earlier (23,24) did not include the exposed and highly active SOAR region surrounding the F394 residue that we suggest is a crucial locus for Orai1 interactions. Our surprising results with heteromeric SOAR concatemers suggested that a “unimolecular” interaction of SOAR with Orai1 is sufficient to activate the channel. This revealed for the first time that it is not necessary for both the symmetrical active sites in the SOAR dimer to be able to bind to adjacent Orai1 channel subunits in the bimolecular configuration shown in Fig. 2A. Instead, each monomer in the SOAR dimer can undergo a unimolecular interaction with a single Orai1 subunit as shown in Fig. 2B.
Figure 2.
Interactions between the SOAR dimer from STIM1 and the Orai1 channel. (A) The bimolecular SOAR-Orai1 coupling model, involving the interaction of the active sites of both of the two SOAR monomers in the SOAR dimer with two adjacent subunits of the Orai1 channel to effect pore opening. Only two Orai1 subunits from the Orai1 channel hexamer are shown. SOAR is shown as the concatemeric dimer construct with two wildtype F394 residues, and tagged with YFP. (B) The unimolecular SOAR-Orai1 coupling model, in which only one of the two SOAR monomers in the SOAR dimer is required to interact with a single Orai1 channel subunit to induce gating. The concatemeric SOAR heterodimer dimer construct has one wildtype SOAR unit and one F394H mutated SOAR unit. (C) Scheme showing attachment of the Orai1 C-terminal STIM-binding peptide (the M4 extension of Orai1) to the plasma membrane-directed peptide (PMP) linked to CFP (PMP-CFP-Orai1CT). (D) Result of FRET experiments revealing that two PMP-CFP-Orai1CT units can bind to a single SOAR dimer. (E) Hypothetical cross-linking of Orai1 channels through unimolecular interactions with dimeric SOAR. (F) Scheme showing theoretical arrangement of SOAR dimers around a hexameric Orai1 channel. The unimolecular coupling model predicts that both the wildtype SOAR homodimer (left) and the heterodimer (right) of SOAR containing one SOAR-F394H mutant unit (blue), would still both bind to and fully activate Orai1. (G) Hypothetical clustering of Orai1 channel hexamers through cross-linking by dimeric SOAR molecules.
We sought to examine the validity of a unimolecular interaction model by investigating whether each monomer within the SOAR dimer molecule could independently interact with the STIM1-binding site on the Orai1 channel. It has been well established that that the cytosolic C-terminal peptide of Orai1 is the strong binding for STIM1 or SOAR as described in earlier studies (11,28). Therefore, we made a new construct by attaching this C-terminal 35 amino acid Orai1 peptide (Orai1CT; residues 267–301) to the C-terminus of CFP, then linking the N-terminus of CFP to a PM-directed single transmembrane-spanning helical peptide (PMP) to give the construct shown in Fig. 2C (27). We expressed this construct (PMP-CFP-Orai1CT) in HEK cells together with our four YFP-tagged SOAR dimers containing either, two F394 (WT) residues, one F394H mutated residue (either the first of second unit in the SOAR dimer), or both SOAR dimers mutated to F394H. We quantitatively analyzed the FRET between PMP-CFP-Orai1CT and each of the YFP-SOAR dimers revealing that the homomeric WT-SOAR dimer could bind twice the amount of PMP-CFP-Orai1CT compared to either of the two heteromeric SOAR-dimer units containing a single F394H monomer. From this result we were able to deduce that the SOAR dimer can indeed simultaneously bind to two separate STIM1-binding sites on Orai1 (Fig. 2D).
Implications of the Unimolecular Coupling Model
The finding that just one active site in the SOAR dimer is sufficient to activate Orai1 prompted us to consider the utility of the other site. The observation that the dimeric SOAR unit in STIM1 can bind to two Orai1 C-termini indicates that STIM1 may be able to bridge between separate Orai1 channels. Thus, we hypothesize that cross-linking of Orai1 channels by STIM1 can give rise to clustering (Fig. 2E). A suggestion that the slightly larger CAD fragment from STIM could cross-link Orai1 channels had earlier been made from electron microscopy approaches (11). Interestingly, a more recent study has revealed uniform spacing between Orai1 channels clustered in ER-PM junctions by STIM1 with a distance of approximately 15 nm (29). The crystal structure of the hexameric Drosophila Orai channel gives a dimension across the cytoplasmic surface of the hexamer of approximately 7 nm, and from the SOAR dimer crystal structure the distance between F394 residues in the SOAR dimer is approximately 5 nm. The distance between centers would theoretically be 12 nm based on a model similar to Fig. 2E, however, some unfolding of the C-terminal STIM1 binding site on Orai1 (18) might account for a larger distance bridged between channels by STIM1 dimers.
A most interesting further correlative feature of the cross-linking model is that it provides an explanation for the variable stoichiometry of interaction reported for STIM1 and Orai1 (20–22). The unimolecular coupling model we propose envisages six STIM1 dimers interacting with six single Orai1 channel subunits within the hexameric structure (Fig. 2F, left), giving a STIM:Orai1 stoichiometry of 2:1. Full activation still occurs with the SOAR-WT/F394H heterodimer since only one monomer of the dimer is required for channel activation (Fig. 2F, right). The earlier bimolecular model for STIM1-Orai1 interaction (23,24) would not be compatible with this observation nor with the 2:1 stoichiometry. A hypothetical lattice between STIM1 dimers and Orai1 channels (Fig. 2G) would have a variable STIM1-Orai1 stoichiometry of between 1:1 and 2:1 dependent on the size of clusters formed, the larger the cluster, the closer the stoichiometry would approach 1:1. Thus, the model could explain the variable STIM1-Orai1 stoichiometry observed in functional studies. The operation of a STIM1-Orai1 lattice model may have great significance in enhancing the kinetics of activation and deactivation of channels and also in spatially restricting or “concentrating” Ca2+ entry signals which may be important for the differential activation of downstream effectors (16,17).
How Does STIM1 Cause Gating of the Orai1 Channel?
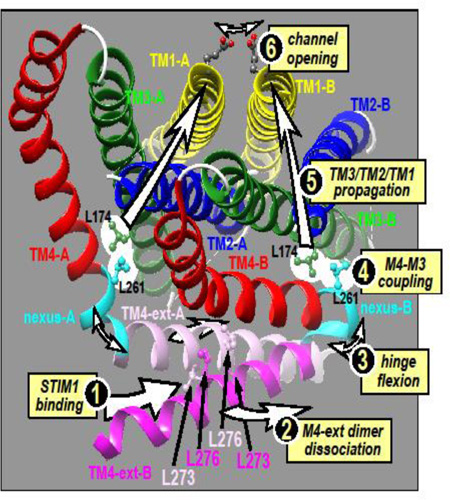
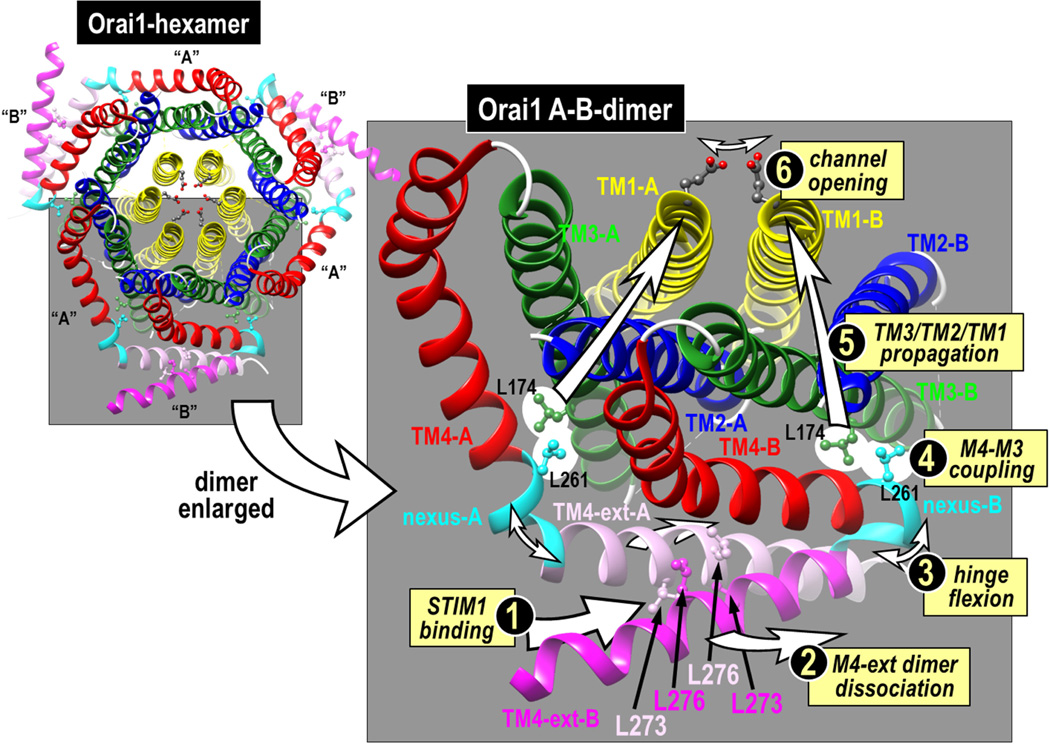
In a recent series of studies on Orai1 we identified a crucial segment that links the C-terminal STIM-binding helical extension of TM4 (TM4-ext) with the closely apposed TM3 helix in Orai1 (30). We termed this five-amino acid segment (residues 261–265 in human Orai1; LVSHK) the “nexus” through which information from the external STIM-binding site is transmitted to the core of the channel to mediate channel gating (Fig. 3). When the LVSHK nexus sequence in Orai1 is mutated to ANSGA, the channel is constitutively active and all the properties of the opened channel appear identical to those of the Orai1 channel activated by STIM1. Thus the mutation seems to exactly mimic the conformational change of the TM4-ext caused by STIM1-binding. The nexus has two regions – a “hinge” domain (SHK; 263–265)(18,31,32) and two residues that form a “hinge plate” (LV; 261–262) through which the nexus appears to be hydrophobically attached to TM3. The hinge region was studied earlier, and substitution with a proline or cross-linking with cysteine, locks the hinge and prevents binding of STIM1 and channel opening (31,32). But, the more crucial LV “hinge-plate” was not examined. It seems L261 can hydrophobically couple to L174 within the TM3 helix and this coupling mediates Orai1 channel gating. If we substitute L174 or L261 with charged residues we block STIM1-induced activation of the wildtype Orai1 channel. If we replace both residues with cysteines and cross link them, we enhance channel activation. Thus, the nexus functions as a vital conduit between STIM1 binding and activation of the channel. We believe that the nexus mediates a coupling trigger between the TM4 and TM3 helices, and that this conformational change propagates through the tightly associated TM3, TM2 and TM1 helices to cause channel activation resulting from rearrangement of critical residues in the pore. This model is supported by experiments examining Tb3+ luminescence of purified Orai1 which show that STIM1 association with the cytoplasmic side of Orai1 causes a conformational alteration of the external entrance of the pore, in particular at the E106 residue that constitutes the Ca2+-selectivity, as well as adjacent residues in the pore, including V102 (33).
Figure 3.
Predicted structure of Orai1 based on the crystal structure of Drosophila Orai. The full hexamer (left) comprises six Oai1 subunits, arranged as three dimers, each dimer consisting of one “A” and one “B” Orai1 monomer. A magnified version of just one dimer (right) reveals that dimers are held together by interactions between the two TM4 extensions (TM4-ext) on the monomers which are either straight (TM4-A; pink) or bent (TM4-B; hot pink) at the hinge/nexus region (cyan). The antiparallel binding of between the two TM4 extensions in the dimer is mediated by cross-interactions between the Leu-273 and Leu-276 residues on each monomer, as shown. We suggest that STIM1 binds to the TM4 extensions (1) causing them to dissociate (2) and the hinge to flex (3). Flexion of the hinge causes the hinge plate reside (Leu-261) to be displaced thereby displacing the closely-coupled Leu-174 residue on the TM3 helix (4). Thereafter, the allosteric alteration is propagated through the TM3, TM2 and TM1 helices (5) causing the E106 selectivity filter in the Orai1 pore to be displaced to open the channel (6).
Knowing that STIM1 (or, more specifically, the SOAR dimer in STIM1) undergoes a powerful interaction with the short cytosolic C-terminal peptide of Orai1, we asked the question whether this interaction was sufficient to activate the channel or whether STIM1 needed to undergo an additional interaction with Orai1 for channel gating to occur. Thus, a considerable body of evidence is interpreted to conclude that, after tethering the Orai1 C-terminus, STIM1 must undergo a second interaction with the cytosolic extension of the N-terminal pore-forming transmembrane helix (TM1) which causes direct opening of the channel pore (6,11,28,31,33–39). Indeed, it has been suggested that certain external-facing residues of the TM1-extension are the sites at which STIM1 can bind to effectively “pull open” the channel pore to effect gating (33). Specifically, the three residues Leu-81, Ser-82 and Lys-85 were implicated in this STIM1-mediated gating mechanism, and mutations in these residues prevented channel gating by STIM1. Using the STIM1-indedependent constitutively open Orai1-ANSGA construct, we mutated each of the three external TM1 sites and found the channel function was blocked exactly the same as for STIM1-induced activation of wildtype Orai1. This strongly indicates that interaction of STIM1 with these three amino acids is not required for gating of the channel. Instead, these residues are likely necessary for the integrity of the channel and their alteration simply blocks channel function. We conclude that the strong interaction of STIM1 with the C-terminal TM4-extension of Orai1 appears to be both necessary and sufficient for channel gating. While channel gating by STIM1 does not require STIM1 binding to the Orai1 N-terminus, we do not rule out that STIM1 undergoes interactions with the N-terminal portion of Orai1. Indeed it is probable that such interactions are necessary for mediating the Ca2+-dependent inhibition that is a characteristic of Orai1 channel activation by STIM1 (30,40,41)
The ANSGA mutation of Orai1 is likely mediating the same conformational alteration of the Orai1 channel that STIM1 confers and hence is a powerful tool to study the open channel configuration. From its action we deduce that the STIM1 gating mechanism is restricted to a conformational change due to a discrete reconfiguration of just the C-terminal TM4 extension close to the Orai1 nexus segment. In the hexameric Orai crystal structure, two adjacent Orai monomers interact through their TM4-extension sequences (Fig. 3). This interaction is nonsymmetrical and the two adjacent TM4 extensions differ in whether the hinge is almost straight (TM4-A) or bent close to 180° (TM4-B). The two TM4-extensions interact in an anti-parallel fashion, held together by hydrophobic interactions between the two lysine pairs (Leu-273 and Leu-276) shown in Fig. 3. Indeed, the hydrophobic pairing of these two TM4 extensions is required for STIM1 binding (32). Our new model proposes that STIM1 “teases apart” the TM4 extensions perhaps through interaction with the crucially important Phe-394 residue in the SOAR active site, described above. This separation of the two M4-extensions would cause the two SHK-hinges to flex, putting strain upon the LV-hinge plates, and causing displacement of the closely attached TM4 Leu-261 and TM3 Leu-174 residues. Thereafter, we proposed that a conformational change is propagated through the tightly clustered TM3/TM2/TMI helices, eventually leading to a reconfiguration of the mouth of the pore (the Glu-106) to open the channel. Our hypothetical activation mechanism for the channel envisages that STIM1 remotely controls opening of the outer pore by interaction only with the Orai1 C-terminus, without any direct contact of STIM1 with the N-terminal TM1 Orai1 pore helix.
In conclusion, our model for STIM1-induced Orai1 channel opening requires interaction of STIM1 only with the Orai1 C-terminus. This model is simpler than the previously suggested models involving interactions of STIM1 with both the C- and the N-termini of Orai1 (6,11,28,31,33–39). Clearly, the Orai1 channel has closed and open configurations, and we speculate that the binding of STIM1 to the C-terminal TM4 extension of Orai1 is sufficient to induce an allosteric transition from the closed to the open state. As described above, the interaction of STIM1 with the Orai1 channel was also somewhat simpler than previously envisaged, requiring only one of the two active sites of the SOAR dimer for full activation of the channel (27). Therefore, we conclude that Orai1 channel activation is mediated by a rather simple interaction between a monomeric active SOAR unit within the STIM1 dimer and just the cytoplasmic C-terminal TM4 extension of the Orai1 channel. Clearly, further study is needed to determine exactly how the conformational change from this interaction is transmitted within the Orai1 channel hexamer to induce the pore helices to transition into the open state.
HIGHLIGHTS.
A critical exposed region in STIM1 is the active site for Orai1 binding
Only one Orai1-activating site on the STIM1 dimer is required for channel gating
The Orai1 nexus region remotely controls channel gating by STIM1
Acknowledgments
This work was supported by NIH Grants GM120783 and GM109279 to D.L.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Putney JW, Steinckwich-Besancon N, Numaga-Tomita T, Davis FM, Desai PN, D'Agostin DM, Wu S, Bird GS. The functions of store-operated calcium channels. Biochim. Biophys. Acta. 2016 doi: 10.1016/j.bbamcr.2016.11.028. [Epub: Nov 30, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soboloff J, Rothberg BS, Madesh M, Gill DL. STIM proteins: dynamic calcium signal transducers. Nat. Rev. Mol. Cell Biol. 2012;13:549–565. doi: 10.1038/nrm3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amcheslavsky A, Wood ML, Yeromin AV, Parker I, Freites JA, Tobias DJ, Cahalan MD. Molecular Biophysics of Orai Store-Operated Ca Channels. Biophys. J. 2015;108:237–246. doi: 10.1016/j.bpj.2014.11.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prakriya M, Lewis RS. Store-Operated Calcium Channels. Physiol. Rev. 2015;95:1383–1436. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shim AH, Tirado-Lee L, Prakriya M. Structural and functional mechanisms of CRAC channel regulation. J. Mol. Biol. 2015;427:77–93. doi: 10.1016/j.jmb.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rothberg BS, Wang Y, Gill DL. Orai channel pore properties and gating by STIM: implications from the Orai crystal structure. Sci Signal. 2013;6:pe9. doi: 10.1126/scisignal.2003971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muik M, Fahrner M, Derler I, Schindl R, Bergsmann J, Frischauf I, Groschner K, Romanin C. A cytosolic homomerization and a modulatory domain within STIM1 C-terminus determine coupling to ORAI1 channels. J. Biol. Chem. 2009;284:8421–8426. doi: 10.1074/jbc.C800229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Covington ED, Wu MM, Lewis RS. Essential role for the CRAC activation domain in store-dependent oligomerization of STIM1. Mol. Biol. Cell. 2010;21:1897–1907. doi: 10.1091/mbc.E10-02-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma G, Wei M, He L, Liu C, Wu B, Zhang SL, Jing J, Liang X, Senes A, Tan P, Li S, Sun A, Bi Y, Zhong L, Si H, Shen Y, Li M, Lee MS, Zhou W, Wang J, Wang Y, Zhou Y. Inside-out Ca(2+) signalling prompted by STIM1 conformational switch. Nat Commun. 2015;6:7826. doi: 10.1038/ncomms8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat. Cell Biol. 2009;11:337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X, Jin H, Cai X, Li S, Shen Y. Structural and mechanistic insights into the activation of Stromal interaction molecule 1 (STIM1) Proc. Natl. Acad. Sci. U. S. A. 2012;109:5657–5662. doi: 10.1073/pnas.1118947109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y, Srinivasan P, Razavi S, Seymour S, Meraner P, Gudlur A, Stathopulos PB, Ikura M, Rao A, Hogan PG. Initial activation of STIM1, the regulator of store-operated calcium entry. Nat. Struct. Mol. Biol. 2013;20:973–981. doi: 10.1038/nsmb.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korzeniowski MK, Popovic MA, Szentpetery Z, Varnai P, Stojilkovic SS, Balla T. Dependence of STIM1/Orai1-mediated Calcium Entry on Plasma Membrane Phosphoinositides. J. Biol. Chem. 2009;284:21027–21035. doi: 10.1074/jbc.M109.012252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma G, Wei M, He L, Liu C, Wu B, Zhang SL, Jing J, Liang X, Senes A, Tan P, Li S, Sun A, Bi Y, Zhong L, Si H, Shen Y, Li M, Lee MS, Zhou W, Wang J, Wang Y, Zhou Y. Inside-out Ca2+ signalling prompted by STIM1 conformational switch. Nat Commun. 2015;6:7826. doi: 10.1038/ncomms8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kar P, Parekh AB. Distinct Spatial Ca2+ Signatures Selectively Activate Different NFAT Transcription Factor Isoforms. Mol. Cell. 2015;58:232–243. doi: 10.1016/j.molcel.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y, Trebak M, Gill DL. Calcium signals tune the fidelity of transcriptional responses. Mol. Cell. 2015;58:197–199. doi: 10.1016/j.molcel.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou X, Pedi L, Diver MM, Long SB. Crystal structure of the calcium release-activated calcium channel Orai. Science. 2012;338:1308–1313. doi: 10.1126/science.1228757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai X, Zhou Y, Nwokonko RM, Loktionova NA, Wang X, Xin P, Trebak M, Wang Y, Gill DL. The Orai1 Store-operated Calcium Channel Functions as a Hexamer. J. Biol. Chem. 2016;291:25764–25775. doi: 10.1074/jbc.M116.758813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scrimgeour N, Litjens T, Ma L, Barritt GJ, Rychkov GY. Properties of Orai1 mediated store-operated current depend on the expression levels of STIM1 and Orai1 proteins. J. Physiol. 2009;587:2903–2918. doi: 10.1113/jphysiol.2009.170662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoover PJ, Lewis RS. Stoichiometric requirements for trapping and gating of Ca2+ release-activated Ca2+ (CRAC) channels by stromal interaction molecule 1 (STIM1) Proc. Natl. Acad. Sci. U. S. A. 2011;108:13299–13304. doi: 10.1073/pnas.1101664108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z, Liu L, Deng Y, Ji W, Du W, Xu P, Chen L, Xu T. Graded activation of CRAC channel by binding of different numbers of STIM1 to Orai1 subunits. Cell Res. 2011;21:305–315. doi: 10.1038/cr.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stathopulos PB, Schindl R, Fahrner M, Zheng L, Gasmi-Seabrook GM, Muik M, Romanin C, Ikura M. STIM1/Orai1 coiled-coil interplay in the regulation of store-operated calcium entry. Nat Commun. 2013;4:2963. doi: 10.1038/ncomms3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fahrner M, Muik M, Schindl R, Butorac C, Stathopulos P, Zheng L, Jardin I, Ikura M, Romanin C. A coiled-coil clamp controls both conformation and clustering of stromal interaction molecule 1 (STIM1) J. Biol. Chem. 2014;289:33231–33244. doi: 10.1074/jbc.M114.610022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maus M, Jairaman A, Stathopulos PB, Muik M, Fahrner M, Weidinger C, Benson M, Fuchs S, Ehl S, Romanin C, Ikura M, Prakriya M, Feske S. Missense mutation in immunodeficient patients shows the multifunctional roles of coiled-coil domain 3 (CC3) in STIM1 activation. Proc. Natl. Acad. Sci. U. S. A. 2015;112:6206–6211. doi: 10.1073/pnas.1418852112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Wang Y, Zhou Y, Hendron E, Mancarella S, Andrake MD, Rothberg BS, Soboloff J, Gill DL. Distinct Orai-coupling domains in STIM1 and STIM2 define the Orai-activating site. Nat Commun. 2014;5:3183. doi: 10.1038/ncomms4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Y, Wang X, Wang X, Loktionova NA, Cai X, Nwokonko RM, Vrana E, Wang Y, Rothberg BS, Gill DL. STIM1 dimers undergo unimolecular coupling to activate Orai1 channels. Nat Commun. 2015;6:8395. doi: 10.1038/ncomms9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNally BA, Somasundaram A, Jairaman A, Yamashita M, Prakriya M. The C- and N-terminal STIM1 binding sites on Orai1 are required for both trapping and gating CRAC channels. J. Physiol. 2013;591:2833–2850. doi: 10.1113/jphysiol.2012.250456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perni S, Dynes JL, Yeromin AV, Cahalan MD, Franzini-Armstrong C. Nanoscale patterning of STIM1 and Orai1 during store-operated Ca2+ entry. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E5533–E5542. doi: 10.1073/pnas.1515606112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y, Cai X, Loktionova NA, Wang X, Nwokonko RM, Wang X, Wang Y, Rothberg BS, Trebak M, Gill DL. The STIM1-binding site nexus remotely controls Orai1 channel gating. Nat Commun. 2016;7:13725. doi: 10.1038/ncomms13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palty R, Stanley C, Isacoff EY. Critical role for Orai1 C-terminal domain and TM4 in CRAC channel gating. Cell Res. 2015;25:963–980. doi: 10.1038/cr.2015.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tirado-Lee L, Yamashita M, Prakriya M. Conformational Changes in the Orai1 C-Terminus Evoked by STIM1 Binding. PLoS One. 2015;10:e0128622. doi: 10.1371/journal.pone.0128622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gudlur A, Quintana A, Zhou Y, Hirve N, Mahapatra S, Hogan PG. STIM1 triggers a gating rearrangement at the extracellular mouth of the ORAI1 channel. Nat Commun. 2014;5:5164. doi: 10.1038/ncomms6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z, Lu J, Xu P, Xie X, Chen L, Xu T. Mapping the interacting domains of STIM1 and Orai1 in CRAC channel activation. J. Biol. Chem. 2007;282:29448–20456. doi: 10.1074/jbc.M703573200. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi Y, Murakami M, Watanabe H, Hasegawa H, Ohba T, Munehisa Y, Nobori K, Ono K, Iijima T, Ito H. Essential role of the N-terminus of murine Orai1 in store-operated Ca2+ entry. Biochem. Biophys. Res. Commun. 2007 doi: 10.1016/j.bbrc.2007.02.107. [DOI] [PubMed] [Google Scholar]
- 36.Derler I, Plenk P, Fahrner M, Muik M, Jardin I, Schindl R, Gruber HJ, Groschner K, Romanin C. The extended transmembrane Orai1 N-terminal (ETON) region combines binding interface and gate for Orai1 activation by STIM1. J. Biol. Chem. 2013;288:29025–29034. doi: 10.1074/jbc.M113.501510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lis A, Zierler S, Peinelt C, Fleig A, Penner R. A single lysine in the Nterminal region of store-operated channels is critical for STIM1-mediated gating. J. Gen. Physiol. 2010;136:673–686. doi: 10.1085/jgp.201010484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y, Meraner P, Kwon HT, Machnes D, Oh-hora M, Zimmer J, Huang Y, Stura A, Rao A, Hogan PG. STIM1 gates the store-operated calcium channel ORAI1 in vitro. Nat. Struct. Mol. Biol. 2010;17:112–116. doi: 10.1038/nsmb.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng H, Zhou MH, Hu C, Kuo E, Peng X, Hu J, Kuo L, Zhang SL. Differential Roles of the C and N Termini of Orai1 Protein in Interacting with Stromal Interaction Molecule 1 (STIM1) for Ca2+ Release-activated Ca2+ (CRAC) Channel Activation. J. Biol. Chem. 2013;288:11263–11272. doi: 10.1074/jbc.M113.450254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mullins FM, Yen M, Lewis RS. Orai1 pore residues control CRAC channel inactivation independently of calmodulin. J. Gen. Physiol. 2016;147:137–152. doi: 10.1085/jgp.201511437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mullins FM, Lewis RS. The inactivation domain of STIM1 is functionally coupled with the Orai1 pore to enable Ca2+-dependent inactivation. J. Gen. Physiol. 2016;147:153–164. doi: 10.1085/jgp.201511438. [DOI] [PMC free article] [PubMed] [Google Scholar]