Abstract
Aims
The purpose of this study was to determine prospectively whether p53 protein accumulation in biopsies of Barrett’s metaplasia (BM) is a predictor of malignant progression, without relying on dysplasia grading.
Methods and results
Sections of formalin fixed paraffin embedded tissue from the initial biopsies of 275 patients with BM, who had no high grade dysplasia (HGD) or esophageal adenocarcinoma (EAC), were stained for p53 by immunohistochemistry. Mean follow up was 41 months. p53-positive biopsies were divided into 4 groups: scattered positive cells, multifocal scattered positive cells, aggregates of positive cells, and multifocal aggregates of positive cells. Kaplan-Meier analysis with the log-rank test was used to determine the rate of progression to high grade dysplasia (HGD)/esophageal adenocarcinoma (EAC). Of the 275 patients, 227 had initial biopsies completely negative for p53 and of these one (0.4%) progressed to HGD/EAC; none of 24 (0%) patients with scattered positive cells and none of 4 (0%) of patients with multifocal scattered positive cells progressed. By contrast, 5 of 16 (31.25%) patients with aggregates of positive cells and 3 of 4 (75%) of those with multifocal aggregates of positive cells progressed to HGD/EAC. Kaplan-Meier analysis with log rank statistics showed the difference in progression rate between the five groups to be highly significant (p<0.0001).
Conclusions
We conclude that p53 protein accumulation, detected by IHC in aggregates of cells, is a significant predictor of malignant progression in patients with BM.
Keywords: Barrett’s oesophagus, immunohistochemistry, dysplasia, oesophageal cancer
INTRODUCTION
Barrett’s metaplasia (BM) is a condition in which the normal squamous lining of the esophagus is replaced by columnar epithelium containing intestinal-type goblet cells as a result of chronic gastroesophageal reflux disease (GERD) (1–3). Barrett’s metaplasia (BM) is considered a premalignant condition, predisposing patients to an increased risk of esophageal adenocarcinoma (EAC). Studies have shown that esophageal adenocarcinomas (EAC) detected in patients with Barrett’s metaplasia (BM) on surveillance tend to be of lower stage and better outcome than esophageal adenocarcinomas (EAC) detected in patients not enrolled in surveillance programs (4–8).
The frequency of endoscopic surveillance and clinical management of patients with BM depend on the presence and grade of dysplasia in the esophageal biopsy as determined by histopathologic examination(1–3,9). Unfortunately, there is significant interobserver and intraobserver variation in the grading of dysplasia in BM, even among expert gastrointestinal pathologists (10–13) which may have a negative impact on the effectiveness and cost effectiveness of endoscopic surveillance, clinical management, and design of prevention studies. We previously proposed that p53 protein accumulation may be an objective marker of malignant progression in BM (14–16). Our previous studies were retrospective, had limited sample size, and factored in dysplasia grading on initial biopsy. The purpose of this study was to determine prospectively whether p53 protein accumulation determined by immunohistochemical staining (IHC) of initial (index) esophageal biopsies from patients with BM is a predictor of malignant progression, independent of dysplasia diagnosis.
MATERIALS AND METHODS
Patients
The study protocol was first approved by the Institutional Review Board (IRB) for Baylor College of Medicine and Affiliated Hospitals in the year 2000. Patients were enrolled from academic as well as community gastroenterology practices. Biopsies were performed at the following endoscopy suites in Texas Medical Center in Houston, TX: Houston Methodist Hospital, Medical Center Endoscopy, and Diagnostic Clinic of Houston (now part of Houston Methodist).
All patients were consented before they were enrolled in the study. After excluding patients with high-grade dysplasia (HGD) or EAC on initial biopsy, the study population consisted of 275 patients with endoscopically and histopathologically confirmed diagnosis of BM. Patient ages ranged from 21–91 years (mean 62, median 63); and the male to female ratio was 3.7:1. Follow up range was 3–112 months (mean 41 months, median 43 months). All patients were prescribed high dose proton pump inhibitors to be taken throughout the duration of the study; for those who could not afford this drug, or their insurance did not cover it, free proton pump inhibitors were provided. None of the patients received ablation therapy while on the study. During the initial and follow up endoscopies, four quadrant biopsies were taken from the Barrett’s segment every 1–2 cm, and from any visible lesion, using regular biopsy forceps. Biopsies were sent to pathology for routine histology processing (formalin fixed and paraffin embedded). Index/initial biopsies are those obtained the first time a patient consented to participate in this study. Histopathologic evaluation was carried out by experienced gastrointestinal pathologists (M.Y, G.Y.L.). Each biopsy was independently reviewed by two pathologists and all disagreements were resolved by joint review.
Immunohistochemical staining for p53
Immunohistochemical staining (IHC) was performed using a Dako automated autostainer (Dako, Carpenteria, CA). The positive and negative controls were sections of formalin-fixed and paraffin-embedded cell lines HT29 and MCF7, respectively. Following steam heat antigen retrieval in 10 mM citrate buffer at pH 6.0 for 20 minutes followed by 10 minutes cooling off at room temperature, sections were incubated with 1:2000 dilution of the anti-p53 monoclonal antibody BP-53-12 (BioGenex, Fremont, CA) for 30 minutes at room temperature. The sections were washed in wash buffer and the bound antibody was detected using Dako’s Envision Plus mouse peroxidase detection system with DAB as chromogen according to the manufacturer’s instructions. The sections were finally counterstained in hematoxylin, mounted, and coverslipped.
The initial batches of p53 immunostaining were jointly scored by two pathologists (M.Y. and K.B.), however because of the excellent agreement p53 immunostaining on the remaining slides was evaluated and scored by one author (K.B.), who was blinded to patient information, clinical and endoscopic data, and grade of dysplasia. Positive staining (protein accumulation) was defined as nuclear staining with the same staining intensity (3+) as the positive control cell line HT29. p53-positive biopsies were further subdivided into 4 groups:
P: scattered positive cells, when there is a minimum of one cell with nuclear p53 accumulation (3+) but no aggregates of positive cells.
PM: multifocal scattered positive cells, where there are scattered p53 positive cells as defined in “P” above but in biopsies from more than one location in the esophagus obtained in the same endoscopy session (such as in biopsies from 32 cm and in biopsies from 30 cm).
PA: aggregates of positive cells, when there is at least one gland with at least 50% of the nuclei positive for p53 protein accumulation (3+).
PAM: multifocal aggregates of positive cells, where there is at least one gland with at least 50% of the nuclei positive for p53 protein accumulation (3+) in biopsies from more than one location in the esophagus obtained in the same endoscopy session.
Statistical analysis
Kaplan-Meier analysis with the log-rank test was used to determine the rate of progression in the different groups according to the p53 status in the initial biopsies with progression to either high-grade dysplasia (HGD) or EAC considered an end result (failure). Fisher’s exact test was used for all other analysis. Statistical analysis was performed utilizing Prism version 6.0 for Macintosh (GraphPad Software, San Diego, CA) with two-tailed p test and 95% confidence interval.
RESULTS
275 patients with at least one follow up biopsy participated in the study. The median age of the patients was 63 years (range 21–91), and the male to female ratio was 3.6:1.
Examples of p53 immunostaining are shown in Figure 1. Of the 275 patients enrolled in this large prospective study, 227 had initial (index) biopsies completely negative for p53 and of these only one (0.4%) progressed to high-grade dysplasia (HGD)/EAC. None of 24 (0%) patients with P and none of 4 (0%) patients with PM progressed. By contrast, five of 16 (31.25%) patients with PA and 3 of 4 (75%) patients with PAM on initial biopsy progressed to HGD/EAC. Kaplan-Meier analysis show significant rate of progression to HGD/EAC in patients with BM whose index biopsies showed either PA or PAM pattern of p53 accumulation compared with patients with the other patient groups (p<0.0001, Figure 2A).
FIGURE 1.
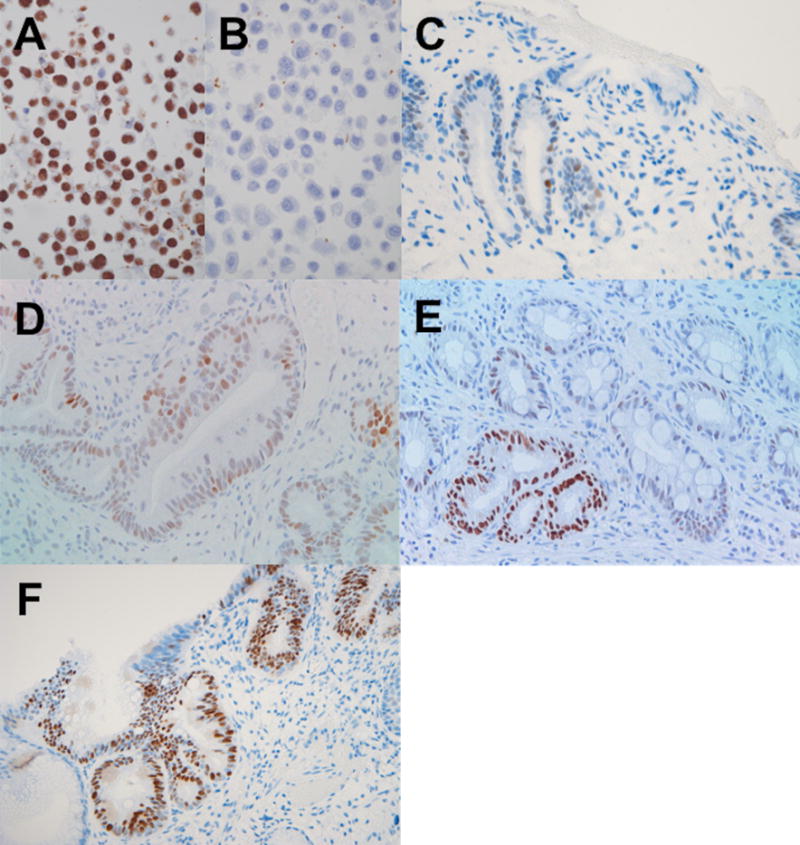
Examples of positive p53 immunohistochemical staining. A) HT-29 colon cancer cell line used as positive control in this study, showing strong (3+) nuclear staining. B) Breast cancer cell line MCF7 used as negative control, here stained in the same batch as HT29 with the same p53 antibody. C-F) sections of formalin fixed paraffin embedded biopsies from patients with Barrett’s metaplasia (BM) showing a biopsy with one 2+ positive nucleus and several 1+ (weak) positive nuclei, considered negative (C), a biopsy rare 3+, occasional 2+, and several weakly positive nuclei scored as “scattered positive” or “P” (D), and two biopsies (E and F) with at least one gland showing more than 50% of the cells with 3+ nuclei (similar staining intensity to HT29 in A) both scored as “positive aggregate” or “PA”. IHC with hematoxylin counterstain.
FIGURE 2.
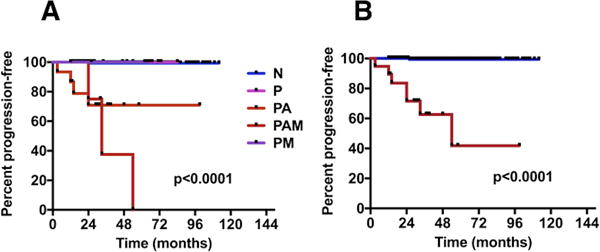
A) Progression of Barrett’s metaplasia to HGD/CA according to p53 status on initial biopsy determined by immunohistochemistry (IHC). N: Negative; P: Positive with scattered p53-positive cells in biopsies from one level in the esophagus; PM: Positive with scattered p53-positive cells in biopsies from two or more level in the esophagus; PA: Positive with one or more aggregates of p53-positive cells in biopsies from one level in the esophagus; PAM: Positive with one or more aggregates of p53-positive cells in biopsies from two or more level in the esophagus. B) Progression of Barrett’s metaplasia to HGD/CA according to p53 status on initial biopsy determined by immunohistochemistry (IHC). Blue line represents cases negative for p53 protein overexpression, red line represents cases positive for p53 protein overexpression. This figure represent the same findings in Figure 2 after combining the results of biopsies with N, P, and PM into negative and groups PA and PAM into positive.
Next, we combined groups PA and PAM as positive for p53 protein accumulation defined as any biopsy with at least one crypt showing 50% or more of the nuclei with strong p53 staining, and all other groups (N, P, and PM) as negative for p53 protein accumulation. There were 20 cases with p53 protein overexpression on initial biopsies of which 8 (40%) progressed to HGD/EAC. This is in sharp contrast to the 255 cases with initial biopsies negative for p53 protein accumulation of which only one (0.3%) progressed to HGD/EAC (p <0.0001, Figure 2B). Of the combined p53 positive initial (index) biopsies (PA and PAM) 5 were ND, 5 were IND and 10 were LGD by histopathologic examination. Of the combined p53 negative initial biopsies 187 were ND, 49 were IND and 19 were LGD.
DISCUSSION
The incidence of EAC in the United States has been rising since the early 1970s and while the most dramatic increase has been in white males, an increase in the incidence in Hispanics and in white females appear to be at a similar rate but at much smaller extent (17). EAC is associated with very poor survival(17). The precursor lesion for EAC is BM. It has been estimated that 5.6% of the US population have BM(18). In order to decrease the mortality from EAC, patients with BM are enrolled in surveillance programs in which esophageal endoscopy and biopsy are performed at regular intervals depending on several factors the most important of which is the grade of dysplasia found on biopsy(3,9). Unfortunately, the cornerstone of surveillance programs, dysplasia grading, suffers from a significant variation in reproducibility even among experts in gastrointestinal pathology. Pathologists in general practice were found to have even more problems with dysplasia grading in BM(19,20), and it has been recommended that the grade of dysplasia be confirmed by an expert pathologist(3).
We previously proposed that p53 protein accumulation in biopsies from patients with BM, detected by IHC, is a predictor of malignant progression(14–16). Although initial studies of p53 expression in BM focused largely on correlation with the grade of dysplasia(21–28), subsequent studies confirmed that p53 protein accumulation in BM correlates with malignant progression (29–33). However, these studies, including our own, were retrospective in nature and were all, in some way, dependent on dysplasia grading.
In this prospective study, our results clearly show that p53 accumulation is a significant predictor of cancer risk in biopsies with BM negative for HGD or EAC. Importantly, malignant progression is predicted without having to first stratify the biopsies based on dysplasia. The initial biopsies entered in this study had 54 cases of IND and 29 LGD for a combined LGD/IND of 83. Traditionally, this is considered the high risk group that is most likely to benefit from surveillance and/or prevention measures. By contrast, p53 accumulation, according to our criteria, narrows down this group of at risk patients to just 20 patients. Scattered p53 positive cells likely represent physiologic accumulation of p53, whereas aggregates of p53 positive cells likely represent clonal expansion of cells with abnormal p53. As with dysplasia, it is not surprising that p53 accumulation (in aggregate of cells) in multiple levels within the esophagus (multifocal) further increases the risk of malignant progression.
Two recent studies, including a large prospective study, confirmed the predictive value of p53 IHC in biopsies of patients with BM. The authors of these studies defined a pattern of p53 overexpression that is generally similar to ours as strong nuclear staining except in single nuclei (34, 35). The scoring of p53 overexpression was also found to be more reproducible and better predictor of progression to HGD/ECA than dysplasia grading on H&E (34, 35). Murray et al included “diffuse staining” together with strong staining in one category as positive p53 staining and concluded that p53 IHC has low sensitivity as a biomarker of malignant progression in BM (36). Therefore, it is important to restrict the definition of positive staining to strong nuclear staining that is more than in just scattered nuclei.
Up to only a few years ago, all studies examining p53 expression in BM using IHC focused on p53 protein overexpression/accumulation as the only p53 abnormality, and that was largely considered to represent p53 gene mutation that led to decreased degradation/stabilization and accumulation of the p53 protein in the nucleus. Recently, a second pattern of abnormal p53 expression has been described (34) and called the “absent pattern”. It was found in a smaller number of cases, and was defined as absence of p53 staining in a focus in an area of interest compared to the low-level staining in non-dysplastic glandular epithelium which is referred as normal expression (34). We have not detected “normal expression” in our cases, and therefore detection of the “absent” pattern in our series was not possible. We titrate down our p53 antibody using two formalin-fixed and paraffin-embedded cell lines one know to harbor p53 mutation (and overexpress p53) while the other has wild-type p53. We optimized our staining conditions to produce strong nuclear staining in the cell line with p53 mutation and no staining in the cell line with wild type p53, and this may have resulted in our inability to detect wild type p53 in our cases. However, the absent pattern seems to be largely detected in HGD and EAC (34), and since we excluded such cases from our cohort we would not be expected to see the absent pattern in our initial biopsies even if we used higher antibody concentration. Furthermore, because of its detection largely in cases with HGD/EAC it is likely that absent p53 stating pattern is a late event in the neoplastic progression in BM, and may not be valuable as a predictive marker.
The number of patients in our study who progressed to HGD/EAC was lower than expected. This is probably due to the fact that all participating patients were on high dose proton pump inhibitors (37–42). Because our findings largely confirm those of another large prospective study (34), and p53 IHC scoring was found to be largely reproducible (34,35) future research should focus on whether p53 protein accumulation could replace dysplasia grading which may lead to increased cost-effectiveness of surveillance and prevention programs for patients with BM.
Acknowledgments
The authors wish to acknowledge the valuable contributions of the late Juan Lechago, MD, PhD, to the planning of the study, and the evaluation of the H&E slides for dysplasia.
This study was supported by National Institutes of Health grant R01 CA81570 (M.Y.), and in parts by Eisai Inc. and Ortho-McNeil Janssen Scientific Affairs, LLC and Ertan Educational/Research Foundation.
Footnotes
Conflict of interest statement:
The authors have nothing to disclose (no conflict of interest)
Author contributions:
Mamoun Younes, MD: Study concept and design, planning, data collection, data interpretation and analysis, obtained funding, study supervision, and drafting of manuscript.
Atilla Ertan, MD: planning, data collection, obtained funding, critical revision of the manuscript.
Keith Brown, MD: data interpretation, data collection, and critical revision of the manuscript.
Gregory Y. Lauwers, MD: data interpretation, data collection, and critical revision of the manuscript.
Gulchin Ergun, MD: data collection, and critical revision of the manuscript.
Frank Meriano, MD: data collection, and critical revision of the manuscript.
A. Carl Schmulen, MD: data collection, and critical revision of the manuscript.
Alberto Barroso, MD: data collection, and critical revision of the manuscript.
References
- 1.Sampliner RE. Practice guidelines on the diagnosis, surveillance, and therapy of Barrett’s esophagus. The Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 1998;93:1028–1032. doi: 10.1111/j.1572-0241.1998.00362.x. [DOI] [PubMed] [Google Scholar]
- 2.Sampliner RE, Practice Parameters Committee of the American College of Gastroenterology Updated guidelines for the diagnosis, surveillance, and therapy of Barrett’s esophagus. Am J Gastroenterol. 2002;97:1888–1895. doi: 10.1111/j.1572-0241.2002.05910.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang KK, Sampliner RE, Practice Parameters Committee of the American College of Gastroenterology Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788–797. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 4.Robertson CS, Mayberry JF, Nicholson DA, James PD, Atkinson M. Value of endoscopic surveillance in the detection of neoplastic change in Barrett’s oesophagus. Br J Surg. 1988;75:760–763. doi: 10.1002/bjs.1800750813. [DOI] [PubMed] [Google Scholar]
- 5.Whiting JL, Sigurdsson A, Rowlands DC, Hallissey MT, Fielding JWL. The long term results of endoscopic surveillance of premalignant gastric lesions. Gut. 2002;50:378–81. doi: 10.1136/gut.50.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verbeek RE, Leenders M, Kate Ten FJW, et al. Surveillance of Barrett’s Esophagus and Mortality from Esophageal Adenocarcinoma: A Population-Based Cohort Study. Am J Gastroenterol. 2014;109:1215–1222. doi: 10.1038/ajg.2014.156. [DOI] [PubMed] [Google Scholar]
- 7.Kastelein F, van Olphen S, Steyerberg EW, et al. Surveillance in patients with long-segment Barrett’s oesophagus: a cost-effectiveness analysis. Gut. 2015;64:864–871. doi: 10.1136/gutjnl-2014-307197. [DOI] [PubMed] [Google Scholar]
- 8.Kastelein F, van Olphen SH, Steyerberg EW, Spaander MCW, Bruno MJ, ProBar-study group Impact of surveillance for Barrett’s oesophagus on tumour stage and survival of patients with neoplastic progression. Gut. 2016;65:548–554. doi: 10.1136/gutjnl-2014-308802. [DOI] [PubMed] [Google Scholar]
- 9.Whiteman DC, Appleyard M, Bahin FF, et al. Australian clinical practice guidelines for the diagnosis and management of Barrett’s esophagus and early esophageal adenocarcinoma. J Gastroenterol Hepatol. 2015;30:804–820. doi: 10.1111/jgh.12913. [DOI] [PubMed] [Google Scholar]
- 10.Reid BJ, Haggitt RC, Rubin CE, et al. Observer variation in the diagnosis of dysplasia in Barrett’s esophagus. Hum Pathol. 1988;19:166–178. doi: 10.1016/s0046-8177(88)80344-7. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery E, Bronner MP, Goldblum JR, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol. 2001;32:368–378. doi: 10.1053/hupa.2001.23510. [DOI] [PubMed] [Google Scholar]
- 12.Coco DP, Goldblum JR, Hornick JL, et al. Interobserver variability in the diagnosis of crypt dysplasia in Barrett esophagus. Am J Surg Pathol. 2011;35:45–54. doi: 10.1097/PAS.0b013e3181ffdd14. [DOI] [PubMed] [Google Scholar]
- 13.Sonwalkar SA, Rotimi O, Scott N, et al. A study of indefinite for dysplasia in Barrett’s oesophagus: reproducibility of diagnosis, clinical outcomes and predicting progression with AMACR (alpha-methylacyl-CoA-racemase) Histopathology. 2010;56:900–907. doi: 10.1111/j.1365-2559.2010.03571.x. [DOI] [PubMed] [Google Scholar]
- 14.Younes M, Lebovitz RM, Lechago LV, Lechago J. p53 protein accumulation in Barrett’s metaplasia, dysplasia, and carcinoma: a follow-up study. Gastroenterology. 1993;105:1637–1642. doi: 10.1016/0016-5085(93)91058-p. [DOI] [PubMed] [Google Scholar]
- 15.Younes M, Ertan A, Lechago LV, Somoano JR, Lechago J. p53 Protein accumulation is a specific marker of malignant potential in Barrett’s metaplasia. Dig Dis Sci. 1997;42:697–701. doi: 10.1023/a:1018828207371. [DOI] [PubMed] [Google Scholar]
- 16.Younes M, Lechago J, Chakraborty S, et al. Relationship between dysplasia, p53 protein accumulation, DNA ploidy, and Glut1 overexpression in Barrett metaplasia. Scand J Gastroenterol. 2000;35:131–137. doi: 10.1080/003655200750024281. [DOI] [PubMed] [Google Scholar]
- 17.Younes M, Henson DE, Ertan A, Miller CC. Incidence and survival trends of esophageal carcinoma in the United States: racial and gender differences by histological type. Scand J Gastroenterol. 2002;37:1359–1365. doi: 10.1080/003655202762671215. [DOI] [PubMed] [Google Scholar]
- 18.Hayeck TJ, Kong CY, Spechler SJ, Gazelle GS, Hur C. The prevalence of Barrett’s esophagus in the US: estimates from a simulation model confirmed by SEER data. Dis Esophagus. 2010;23:451–457. doi: 10.1111/j.1442-2050.2010.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sangle NA, Taylor SL, Emond MJ, Depot M, Overholt BF, Bronner MP. Overdiagnosis of high-grade dysplasia in Barrett’s esophagus: a multicenter, international study. Mod Pathol. 2015;28:758–765. doi: 10.1038/modpathol.2015.2. [DOI] [PubMed] [Google Scholar]
- 20.Curvers WL, Kate ten FJ, Krishnadath KK, et al. Low-grade dysplasia in Barrett’s esophagus: overdiagnosed and underestimated. Am J Gastroenterol. 2010;105:1523–1530. doi: 10.1038/ajg.2010.171. [DOI] [PubMed] [Google Scholar]
- 21.Polkowski W, van Lanschot JJ, Kate Ten FJ, et al. The value of p53 and Ki67 as markers for tumour progression in the Barrett’s dysplasia-carcinoma sequence. Surg Oncol. 1995;4:163–171. doi: 10.1016/s0960-7404(10)80021-0. [DOI] [PubMed] [Google Scholar]
- 22.Flejou JF, Potet F, Muzeau F, Le Pelletier F, Fekete F, Henin D. Overexpression of p53 protein in Barrett’s syndrome with malignant transformation. J Clin Pathol. 1993;46:330–333. doi: 10.1136/jcp.46.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trakal E, Guidi A, Butti AL, Trakal JJ, Sambuelli R, Zarate FE. Detection of the risk of adenocarcinoma in Barrett’s esophagus by means of tumor markers (p53 and Ki67) Acta Gastroenterol Latinoam. 2010;40:211–215. [PubMed] [Google Scholar]
- 24.Khan S, Do KA, Kuhnert P, et al. Diagnostic value of p53 immunohistochemistry in Barrett’s esophagus: an endoscopic study. Pathology. 1998;30:136–140. doi: 10.1080/00313029800169076. [DOI] [PubMed] [Google Scholar]
- 25.Krishnadath KK, Tilanus HW, van Blankenstein M, Bosman FT, Mulder AH. Accumulation of p53 protein in normal, dysplastic, and neoplastic Barrett’s oesophagus. J Pathol. 1995;175:175–180. doi: 10.1002/path.1711750204. [DOI] [PubMed] [Google Scholar]
- 26.Kim R, Clarke MR, Melhem MF, et al. Expression of p53, PCNA, and C-erbB-2 in Barrett’s metaplasia and adenocarcinoma. Dig Dis Sci. 1997;42:2453–2462. doi: 10.1023/a:1018891923998. [DOI] [PubMed] [Google Scholar]
- 27.Rice TW, Goldblum JR, Falk GW, Tubbs RR, Kirby TJ, Casey G. p53 immunoreactivity in Barrett’s metaplasia, dysplasia, and carcinoma. J Thorac Cardiovasc Surg. 1994;108:1132–1137. [PubMed] [Google Scholar]
- 28.Hamelin R, Flejou JF, Muzeau F, et al. TP53 gene mutations and p53 protein immunoreactivity in malignant and premalignant Barrett’s esophagus. Gastroenterology. 1994;107:1012–1018. doi: 10.1016/0016-5085(94)90225-9. [DOI] [PubMed] [Google Scholar]
- 29.Weston AP, Banerjee SK, Sharma P, Tran TM, Richards R, Cherian R. p53 protein overexpression in low grade dysplasia (LGD) in Barrett’s esophagus: immunohistochemical marker predictive of progression. Am J Gastroenterol. 2001;96:1355–1362. doi: 10.1111/j.1572-0241.2001.03851.x. [DOI] [PubMed] [Google Scholar]
- 30.Kaye PV, Haider SA, Ilyas M, et al. Barrett’s dysplasia and the Vienna classification: reproducibility, prediction of progression and impact of consensus reporting and p53 immunohistochemistry. Histopathology. 2009;54:699–712. doi: 10.1111/j.1365-2559.2009.03288.x. [DOI] [PubMed] [Google Scholar]
- 31.Sikkema M, Kerkhof M, Steyerberg EW, et al. Aneuploidy and overexpression of Ki67 and p53 as markers for neoplastic progression in Barrett’s esophagus: a case-control study. Am J Gastroenterol. 2009;104:2673–2680. doi: 10.1038/ajg.2009.437. [DOI] [PubMed] [Google Scholar]
- 32.Horvath B, Singh P, Xie H, Thota PN, Sun X, Liu X. Expression of p53 predicts risk of prevalent and incident advanced neoplasia in patients with Barrett’s esophagus and epithelial changes indefinite for dysplasia. Gastroenterol Rep. 2015 doi: 10.1093/gastro/gov045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skacel M, Petras RE, Rybicki LA, et al. p53 expression in low grade dysplasia in Barrett’s esophagus: correlation with interobserver agreement and disease progression. Am J Gastroenterol. 2002;97:2508–2513. doi: 10.1111/j.1572-0241.2002.06032.x. [DOI] [PubMed] [Google Scholar]
- 34.Kastelein F, Biermann K, Steyerberg E, et al. Aberrant p53 protein expression is associated with an increased risk of neoplastic progression in patients with Barrett’s oesophagus. Gut. 2013;62:1676–1683. doi: 10.1136/gutjnl-2012-303594. [DOI] [PubMed] [Google Scholar]
- 35.Kaye PV, Ilyas M, Soomro I, et al. Dysplasia in Barrett’s oesophagus: p53 immunostaining is more reproducible than haematoxylin and eosin diagnosis and improves overall reliability, while grading is poorly reproducible. Histopathology. 2016;69:431–440. doi: 10.1111/his.12956. [DOI] [PubMed] [Google Scholar]
- 36.Murray L, Sedo A, Scott M, et al. TP53 and progression from Barrett’s metaplasia to oesophageal adenocarcinoma in a UK population cohort. Gut. 2006;55:1390–1397. doi: 10.1136/gut.2005.083295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hillman LC, Chiragakis L, Shadbolt B, Kaye GL, Clarke AC. Proton-pump inhibitor therapy and the development of dysplasia in patients with Barrett’s oesophagus. Med J Aust. 2004;180:387–391. doi: 10.5694/j.1326-5377.2004.tb05991.x. [DOI] [PubMed] [Google Scholar]
- 38.El-Serag HB, Aguirre TV, Davis S, Kuebeler M, Bhattacharyya A, Sampliner RE. Proton pump inhibitors are associated with reduced incidence of dysplasia in Barrett’s esophagus. Am J Gastroenterol. 2004;99:1877–1883. doi: 10.1111/j.1572-0241.2004.30228.x. [DOI] [PubMed] [Google Scholar]
- 39.Hillman LC, Chiragakis L, Shadbolt B, Kaye GL, Clarke AC. Effect of proton pump inhibitors on markers of risk for high-grade dysplasia and oesophageal cancer in Barrett’s oesophagus. Aliment Pharmacol Ther. 2008;27:321–326. doi: 10.1111/j.1365-2036.2007.03579.x. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen DM, El-Serag HB, Henderson L, Stein D, Bhattacharyya A, Sampliner RE. Medication usage and the risk of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2009;7:1299–1304. doi: 10.1016/j.cgh.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kastelein F, Spaander MCW, Steyerberg EW, et al. Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2013;11:382–388. doi: 10.1016/j.cgh.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 42.Lada MJ, Nieman DR, Han M, et al. Gastroesophageal reflux disease, proton-pump inhibitor use and Barrett’s esophagus in esophageal adenocarcinoma: Trends revisited. Surgery. 2013;154:856–864. doi: 10.1016/j.surg.2013.07.020. [DOI] [PubMed] [Google Scholar]


