Abstract
Extreme phosphate levels (P) have been associated with mineralization defects and increased fracture risk. Whether P within normal range is related to bone health in the general population is not well understood. To investigate the association of P with bone mineral density (BMD) and fracture risk, we assessed two population-based cohorts: the Dutch Rotterdam Study (RS-I, RS-II, RS-III; n=6791) and the US Osteoporotic Fractures in Men (MrOS; n=5425) study. The relationship of P with lumbar spine (LS) and femoral neck (FN) BMD was tested in all cohorts via linear models; fracture risk was tested in RS-I, RS-II and MrOS through Cox models, after follow-up of 8.6, 6.6 and 10.9 years, respectively. Adjustments were made for age, body mass index, smoking, serum levels of calcium, potassium, 25-hydroxyvitamin D, and estimated glomerular filtration rate (eGFR), FN-BMD, prevalent diabetes and cardiovascular disease. Additional adjustments were made for phosphate intake, parathyroid hormone, and fibroblast growth factor 23 levels in MrOS. We further stratified by eGFR. Results were pooled through study-level meta-analyses. Hazard ratios (HR) and betas (β) (from meta-analyses) are expressed per 1 mg/dL P increase. P was positively associated with fracture risk in men and women from RS and findings were replicated in MrOS (pooled HR all (95% CI): 1.47 (1.31–1.65)). P was associated with fracture risk in subjects without chronic kidney disease (CKD): all (1.44 (1.26–1.63)) and in men with CKD (1.93 (1.42–2.62)). P was inversely related to LS-BMD in men (β: −0.06 (−0.11 to −0.02)) and not to FN-BMD in either sex. In summary, serum P was positively related to fracture risk independently from BMD and phosphate intake after adjustments for potential confounders. P and LS-BMD were negatively related in men. Our findings suggest that increased P levels even within normal range might be deleterious for bone health in the normal population.
Keywords: phosphate levels, fractures, BMD, calcium, 25-hydroxyvitamin D
Introduction
Phosphorus is the main mineral in the bone, where it is deposited together with calcium.(1) The intracellular compartment contains approximately 14% of phosphorus, and only 1% circulates freely in plasma as phosphate (P).(2) Within bone, phosphorus accumulates in the form of hydroxyapatite.(3) Phosphorus bioavailability is crucial for appropriate mineralization;(4) conditions of low phosphate are characterized by defective mineralization and excessive amount of unmineralized bone, or osteoid, typical of rickets/osteomalacia.(5, 6) On the other hand, extreme hyperphosphatemia induces tumoral calcinosis, characterized by ectopic calcifications but also mineralization defects. (7–9)
The recent finding that P regulation is exerted also by the phosphatonins α-Klotho and the osteocyte-derived fibroblast growth factor 23 (FGF23)(7,10) has established the concept that bone is not only a P reservoir but also acts as an endocrine organ (3) regulating P levels and mineralization. Therefore, a potential bidirectional relationship between P levels and bone can be postulated, in which adequate P availability allows bone mineralization (1) while osteocytes regulate P levels through FGF23 synthesis and through master control of bone remodeling. (11, 12)
Despite this important role of P in bone it is not known whether serum P is associated with bone mineral density (BMD) or fracture risk at the population level. This research has been scarce and assessed mostly in chronic kidney disease (CKD) patients. (13, 14)
The aims of this research were to study the relation between P and BMD and fractures in two population-based cohorts, the influence of potential confounders and to assess the existence of sex-specific effects, which have been previously described for some clinical outcomes mainly in the field of cardiovascular disease. (15, 16)
Materials and methods
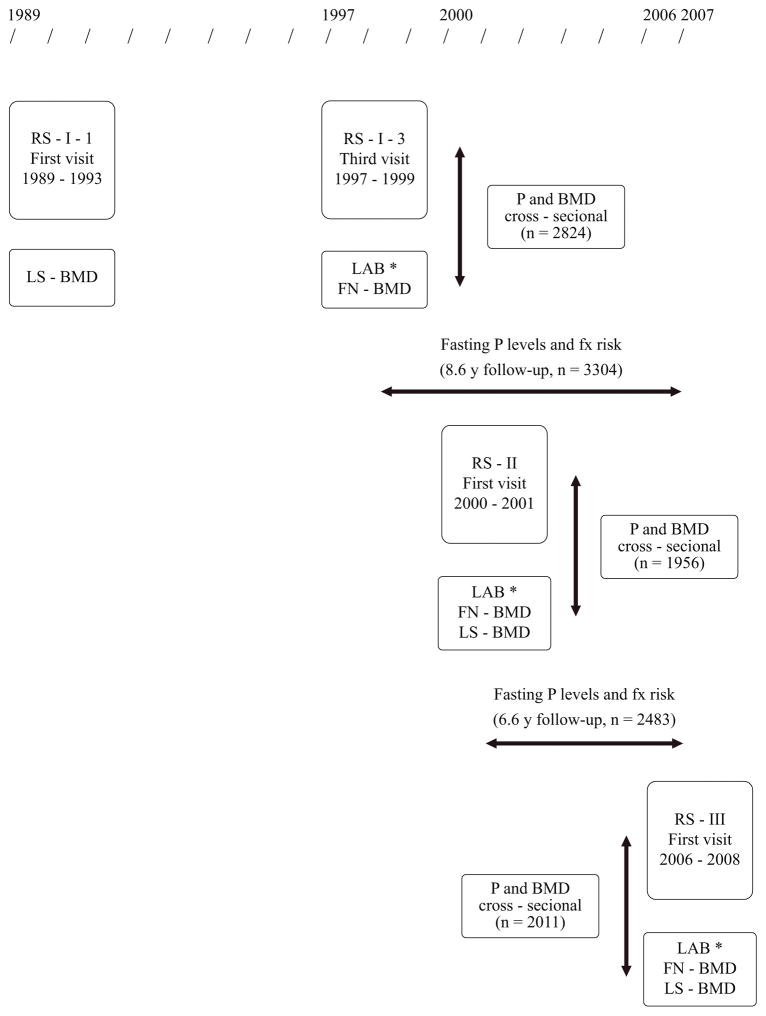
This research was performed in three cohorts from the Dutch Rotterdam Study (17) (RS-I, recruitment period 1989–1993, original n=7983; RS-II, recruitment period 2000–2001, original n=3011; RS-III, recruitment period 2006–2008, original n=3932; all subjects aged 45 or more) and in the US Osteoporotic Fractures in Men (MrOS) study (18, 19) (recruitment period 2000–2002, original n=5994; all male subjects aged 65 or more). Fasting serum P levels were measured in the third follow-up visit of RS-I, and in baseline visits of RS-II, RS-III and MrOS (Figure 1). Fasting P levels were chosen because the fasting state might modify the association of P with clinical outcomes. (20) Fracture incidence was collected prospectively until January 1, 2007, in RS-I and RS-II; and until January 8, 2015, in MrOS. Fracture incidence was not assessed in RS-III. A total of 12.216 and 11.196 participants were included for the BMD and fracture analyses, respectively, all of them with signed informed consent. The Rotterdam Study was approved by the Medical Ethics Committee of Erasmus Medical Center; MrOS was approved by the Institutional Review Board of each of the six clinical centers that enrolled participants.
Figure 1.
Flowchart for time line, design and sample size for the analyses, the Rotterdam Study cohorts
LAB*: includes fasting phosphate levels
FN – BMD: femoral neck BMD
LS – BMD: lumbar spine BMD
P: fasting phosphate levels
Fx risk: fracture risk
Laboratory measurements
The Rotterdam Study
The concentration of phosphorus in serum corresponds to the inorganic fraction, or phosphate (P), based on the formation of ammonium phosphomolybdate.(1) Total Calcium (Ca) determination was done through a colorimetric o-cresolphthalein complexone method. Levels of 25-hydroxyvitamin D (25OHD) were determined through an electrochemiluminescence-based immunoassay (Elecsys Vitamin D Total, Roche Diagnostics, Mannheim, Germany); the test sensitivity was 10 nmol/L, the test range was 7.5 nmol/L to 175 nmol/L, the within-run precision <6.5% and the total precision <11.5%. We applied cosinor regressions to adjust 25OHD levels for season and year. (21) Creatinine was determined through a sarcosine-based colorimetric assay and standardized against isotope dilution mass spectrometry (ID-MS).
MrOS
Serum P, creatinine, and Ca were measured using a Roche COBAS Integra 800 automated analyzer. P detectable range was 0.3–20.0 mg/dl, creatinine was 0.2–15.0 mg/dl, and Ca was 0.1–20.0 mg/dl. Concentrations of 25OHD2 and 25OHD3 were analyzed by liquid chromatography/tandem mass spectrometry (MS) in a subgroup (n=2351) and added together to obtain total 25OHD levels using multiple reaction monitoring as previously described. (22) Additionally, free concentrations of 25OHD were measured in a subgroup (n=541) by ELISA (DIASource ImmunoAssays) at Future Diagnostics Solutions. This measurement was validated by comparison with equilibrium dialysis at 37 C in 15 normal samples yielding a correlation of 0.83. The lower limit of detection was 1.9 pg/ml and its precision was less than 6%. (23) Serum 25OHD levels were adjusted by season. Measurements were performed at the Mayo Medical Laboratories in Rochester, Minnesota.
Parathyroid hormone (PTH) levels were completed using fasting morning blood samples, and samples were frozen until measurement. Immunoradiometric Assay from Scantibodies (3KG600) at Columbia University was used to measure total intact PTH (pg/ml). Fibroblast growth factor 23 (FGF23) levels were completed at the UC Davis Medical Center by two-site monoclonal antibody ELISA using the millipore method. The lower limit of detection was 3.3 pg/ml. Bone turnover markers were measured in a specialized laboratory (CCBR, Synarc, Lyon, France); type I collagen N-propeptide (PINP, Roche Diagnostics, Mannhein, Germany) was measured as marker of bone formation, with intra and interassay coefficient of variation (CV) of <4.4%. For bone resorption, βC-terminal cross-linked telopeptide of type I collagen (βCTX, Roche Diagnostics) was measured, with intra and interassays CVs <4.2%. (24)
DXA scanning
Trained radiographic technicians performed BMD measurements using dual-energy X-ray absorptiometry (DXA). RS-I participants were assessed at baseline (lumbar spine-LS-BMD, RS-I-1, 1989–1991) and at the third visit (femoral neck FN-BMD, RS-I, 1997–1999), while RS-II and RS-III participants were assessed at both skeletal sites at baseline visits (2000–2001; 2006–2008; respectively); as depicted in Figure 1. A GE Lunar DPX-L densitometer was used in the assessments of RS-I and RS-II; and a ProdigyTM total body fan-beam densitometer (25) in RS-III (GE Lunar Corp, Madison, WI, USA). MrOS participants were assessed at both skeletal sites at the baseline visit; each US center used a DXA machine of the same model and manufacturer (QDR 4500 Hologic Inc, Waltham, MA, USA).(18) Machines across all 6 sites were cross-calibrated.
Fracture assessment
In the Rotterdam Study, information on incident clinical fracture events (of all skeletal sites) was obtained from computerized records of general practitioners (GPs) and hospital registries in the research area (covering 80% of the cohort) which are regularly checked by research physicians who review and code the fracture information according to ICD-10; (26) additionally research physicians regularly followed participant information in the GP’s records outside the research area and made an independent review and encoding of all reported events. (27) All fractures are described by a radiologist and in case of doubt the actual radiographs were reviewed. Finally, an expert in osteoporosis reviewed all coded events for final classification.(28, 29)
Because access to medical specialists in The Netherlands is possible only through the GP, we do not anticipate that a considerable number of fractures could have been treated by orthopaedic or traumatology surgeons without previous notification by GP. In the Netherlands there is a 24 h general practitioner evening and night center available after regular working hours and the GP is automatically informed after discharge with a report about the diagnosis. Additionally, insurance companies do not cover expenses from the emergency room when patients have not been referred by the GP. Therefore a significant underestimation of fractures is not anticipated in RS cohorts.
Incident fracture events were reported by participants in MrOS at 4-month intervals on brief mailed questionnaires. (30) The response rates exceeded 99%. Subsequently, study physicians centrally adjudicated reported fractures from medical records. Incident fractures were confirmed by radiology reports or radiographic images when reports were not available. (31) Only fractures that are confirmed by the adjudication process are included in MrOS dataset. Health care service providers sent a film copy or digital image of the X-ray to the Coordinating Center for review and confirmation by a radiologist.
Fracture outcomes
Initially we tested the association between P and all-fracture incidence; subsequently we analyzed fractures located at the hip, vertebrae, wrist, humerus and rib. Additionally we included osteoporotic fractures, defined as fractures at any skeletal site except fingers, toes, skull and facial fractures. (32)
Covariates
Due to previously reported differences in P levels for men and women,(33) we compared its distribution across sexes in the Rotterdam Study applying t-tests. We assessed the distribution of potential confounders in subjects with FN-BMD information available across P quintiles, applying age-adjusted tests for trend. We included age, body mass index (BMI), smoking status, FN-BMD, prevalent diabetes mellitus and levels of total Ca, 25OHD, potassium, creatinine and estimated glomerular filtration rate (eGFR). Prevalent diabetes mellitus and cardiovascular disease were determined as previously described. (34) Alcohol intake was estimated at baseline through a validated food frequency questionnaire. The Chronic Kidney Disease Epidemiology Collaboration equations based on creatinine levels (35) and the Modification of Diet in Renal Disease (MDRD) study equation (36) were applied to estimate eGFR (mL/min) in the Rotterdam Study and MrOS, respectively. Phosphate intake information collected at the same visit as fasting P was available in a subgroup from MrOS. This dietary information is from the Block Dietary Systems Food Frequency Questionnaire (FFQ), which was specially designed for the MrOS study as a brief FFQ for older adults, based on the NHANES III dietary recall data and including 69 items.
Statistical analyses
A potential association between P levels and BMD was tested through generalized linear models, allowing Gaussian but also non-normal distributions. BMD in sex-specific standard deviations (SD) was set as the dependent variable and P levels in mg/dL (1mg/dL=0.32 mmol/L) was set as the independent variable, adjusted for age, BMI and smoking; site and race adjustments were included in MrOS. Betas (β) are expressed per 1 mg/dL increase in P levels. Fitness of different models was compared through the Akaike Information Criteria – AIC (37); linear models with normal distributions displayed lower AIC values, corresponding to a better fit. The results from these analyses were meta-analyzed. LS-BMD was not measured simultaneously to P assessment in RS-I (Fig. 1).
We explored associations of P levels with fracture risk applying Cox models, testing the proportionality of the hazards through Schoenfeld residuals tests. (38) Results from RS-I, RS-II and MrOS were pooled through study-level meta-analysis, applying a fixed effects model due to the small number of studies involved. (39)The analysis time was set at the date of blood draw for fasting P levels. Subjects were followed until the first of the following events happened: first fracture, death, loss to follow-up, or censoring. Hazard ratios (HRs) are expressed per 1 mg/dL increase of P levels or in study-specific quintiles.
Adjustments were done first for a basic model including age, BMI and smoking; (40–42) site and race were also included in MrOS (Model I). Analyses in RS cohorts were also adjusted for a dummy variable to account for different DXA machines. We further adjusted the analyses for additional covariates included in a full model (Model II), composed of FN-BMD, calcium, potassium, eGFR, alcohol intake, and prevalent cardiovascular and diabetes mellitus; additionally this Model included season-corrected 25OHD adjustment in the full RS cohorts. We have adjusted for total 25OHD levels in MrOS in a subgroup with this information available.
Due to sex differences in P levels (33, 43) and in the association of P with several outcomes,(15,16) we explored relations of P with bone traits in sex-combined and in sex-stratified models in RS cohorts.
Sensitivity analyses
To account for the potential confounding effect of renal impairment in the association between P levels and bone traits we stratified the fracture analyses at an eGFR threshold of 58 mL/min, the estimated cut-off for P counterregulatory hormones triggering in early kidney disease. (44) In MrOS, subgroup analyses were performed in subjects with laboratory results of total and free 25OHD, PTH and FGF23. Also in MrOS, we adjusted the fracture analyses for phosphate intake (available in 99.3% of the study population).
Additionally, we repeated analyses including only subjects from both cohorts with P levels within normal range (0.81–1.45 mmol/L; 2.5–4.5 mg/dL).
Primary analyses were done with subjects with complete information on covariates. The completeness of information on covariates for those participants with available P samples was more than 99% in MrOS (with the exception of subgroup analyses) and approximately 75% in the Rotterdam Study cohorts. Subsequently, missing values in the Rotterdam Study cohorts were imputed via multiple imputation with chained equations, following guidelines for imputation for the Cox model.
Analyses were performed with SPSS (version 21.0, Armonk, NY: IBM Corp), Stata (version 13, College Station TX: Stata Corp LP) and Comprehensive Meta-Analysis (version 2.0).
Results
The distribution of relevant covariates across quintiles of P is depicted in Tables 1a and 1b. P and Ca levels were higher in women than men in the three RS cohorts (p<0.001).
Table 1a.
General characteristics of subjects with femoral neck BMD information available in RS-I, RS-II and RS-III according to quintiles of fasting phosphate levels
| Men | Women | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphate in quintiles | Phosphate in quintiles | |||||||||||
| 1 | 2 | 3 | 4 | 5 | p* | 1 | 2 | 3 | 4 | 5 | p* | |
| I) RS-I | ||||||||||||
| N Phosphate Mean (mg/dL) |
242 (2.56) | 243 (2.92) | 243 (3.14) | 243 (3.37) | 243 (3.76) | 322 (3.02) | 322 (3.40) | 322 (3.62) | 322 (3.84) | 322 (4.22) | ||
| Range (mg/dL) | 1.9–2.8 | 2.8–3.0 | 3.0–3.3 | 3.3–3.5 | 3.5–4.9 | 2.3–3.3 | 3.3–3.5 | 3.5–3.7 | 3.7–3.9 | 3.9–5.1 | ||
| Age (y) | 71.9 | 72.3 | 71.7 | 72.3 | 71.9 | 0.982 | 72.8 | 72.2 | 72.9 | 72.1 | 72.3 | 0.297 |
| BMI (kg/m2) | 26.6 | 26.5 | 26.4 | 26.1 | 26.1 | 0.020 | 28.7 | 27.7 | 27.2 | 26.6 | 25.8 | <0.001 |
| Smoke (%) | 87% | 87% | 93% | 92% | 94% | 0.002 | 47% | 49% | 52% | 52% | 48% | 0.615 |
| Calcium (mg/dL) | 9.58 | 9.66 | 9.62 | 9.64 | 9.72 | 0.001 | 9.77 | 9.79 | 9.77 | 9.80 | 9.86 | 0.006 |
| 25OHD (nmol/L) | 63.4 | 61.7 | 60.5 | 58.3 | 59.1 | 0.013 | 47.2 | 47.7 | 45.9 | 49.7 | 50.5 | 0.057 |
| FN-BMD (g/cm2) | 0.90 | 0.90 | 0.91 | 0.90 | 0.88 | 0.124 | 0.82 | 0.80 | 0.79 | 0.79 | 0.78 | <0.001 |
| Glucose (mmol/L) | 6.07 | 6.01 | 5.99 | 5.99 | 6.16 | 0.593 | 6.13 | 5.83 | 5.93 | 5.72 | 5.76 | 0.001 |
| Prevalent DM (%) | 12% | 14% | 13% | 9% | 15% | 0.623 | 16% | 10% | 12% | 8% | 9% | 0.003 |
| Creatinine (mg/dL) | 1.04 | 1.05 | 1.02 | 1.03 | 1.06 | 0.548 | 0.82 | 0.82 | 0.82 | 0.81 | 0.82 | 0.977 |
| eGFR (mL/min) | 73.5 | 72.3 | 74.7 | 73.9 | 73.3 | 0.676 | 73.2 | 73.5 | 73.5 | 74.2 | 73.9 | 0.632 |
| Na+ (mmol/L) | 142.3 | 142.1 | 142.4 | 141.8 | 142.1 | 0.218 | 142.3 | 142.5 | 142.7 | 142.3 | 142.5 | 0.957 |
| K+ (mmol/L) | 4.32 | 4.41 | 4.45 | 4.43 | 4.53 | <0.001 | 4.30 | 4.37 | 4.44 | 4.43 | 4.49 | <0.001 |
| II) RS-II | ||||||||||||
| N Phosphate Mean (mg/dL) |
181 (2.48) | 182 (2.84) | 182 (3.06) | 182 (3.29) | 182 (3.70) | 209 (2.91) | 209 (3.31) | 210 (3.52) | 209 (3.76) | 210 (4.14) | ||
| Range (mg/dL) | 1.4–2.7 | 2.7–2.9 | 2.9–3.2 | 3.2–3.4 | 3.4–4.7 | 1.8–3.2 | 3.2–3.4 | 3.4–3.6 | 3.6–3.9 | 3.9–5.1 | ||
| Age (y) | 63.4 | 64.1 | 64.5 | 63.5 | 63.2 | 0.555 | 64.2 | 64.5 | 63.4 | 63.8 | 62.2 | 0.002 |
| BMI (kg/m2) | 27.2 | 26.7 | 26.7 | 26.8 | 27.1 | 0.791 | 28.8 | 27.7 | 27.4 | 26.5 | 26.1 | <0.001 |
| Smoke (%) | 87% | 78% | 82% | 88% | 89% | 0.052 | 57% | 63% | 60% | 57% | 63% | 0.690 |
| Calcium (mg/dL) | 9.52 | 9.58 | 9.54 | 9.57 | 9.62 | 0.014 | 9.64 | 9.65 | 9.69 | 9.68 | 9.74 | 0.005 |
| 25OHD (nmol/L) | 65.5 | 68.5 | 66.3 | 65.7 | 63.8 | 0.355 | 59.0 | 56.8 | 59.6 | 58.2 | 63.4 | 0.294 |
| FN-BMD (g/cm2) | 0.98 | 0.98 | 0.95 | 0.98 | 0.97 | 0.478 | 0.89 | 0.88 | 0.91 | 0.88 | 0.87 | <0.001 |
| Glucose (mmol/L) | 6.06 | 5.98 | 6.17 | 5.89 | 6.49 | 0.041 | 6.13 | 5.81 | 5.77 | 5.83 | 5.87 | 0.194 |
| Prevalent DM (%) | 12% | 9% | 15% | 11% | 20% | 0.024 | 13% | 8% | 10% | 10% | 9% | 0.450 |
| Creatinine (mg/dL) | 0.98 | 0.99 | 0.99 | 0.98 | 0.99 | 0.571 | 0.78 | 0.77 | 0.79 | 0.77 | 0.78 | 0.640 |
| eGFR (mL/min) | 81.8 | 81.3 | 80.2 | 81.9 | 82.4 | 0.714 | 80.8 | 81.7 | 80.4 | 82.3 | 82.4 | 0.843 |
| Na+ (mmol/L) | 140.9 | 141.1 | 141.2 | 141.1 | 141.1 | 0.318 | 141.2 | 141.4 | 141.5 | 141.6 | 141.7 | 0.032 |
| K+ (mmol/L) | 4.16 | 4.21 | 4.21 | 4.27 | 4.26 | <0.001 | 4.17 | 4.23 | 4.24 | 4.25 | 4.28 | <0.001 |
| III) RS-III | ||||||||||||
| N Phosphate Mean (mg/dL) |
174 (2.56) | 174 (2.94) | 174 (3.20) | 174 (3.45) | 174 (3.87) | 228 (2.97) | 228 (3.39) | 228 (3.63) | 228 (3.85) | 229 (4.26) | ||
| Range (mg/dL) | 1.6–2.8 | 2.8–3.0 | 3.0–3.3 | 3.3–3.6 | 3.6–5.4 | 2.1–3.2 | 3.2–3.5 | 3.5–3.7 | 3.7–3.9 | 3.9–5.1 | ||
| Age (y) | 57.4 | 57.6 | 57.4 | 56.6 | 55.8 | 0.008 | 56.2 | 57.5 | 57.2 | 57.6 | 56.8 | 0.304 |
| BMI (kg/m2) | 28.2 | 28.2 | 27.6 | 27.4 | 27.4 | 0.017 | 29.2 | 27.9 | 27.1 | 27.1 | 26.9 | <0.001 |
| Smoke (%) | 77% | 74% | 83% | 76% | 72% | 0.640 | 64% | 67% | 69% | 60% | 69% | 0.720 |
| Calcium (mg/dL) | 9.68 | 9.79 | 9.82 | 9.87 | 9.88 | <0.001 | 9.74 | 9.78 | 9.85 | 9.90 | 10.0 | <0.001 |
| 25OHD (nmol/L) | 57.5 | 60.0 | 59.1 | 63.1 | 63.4 | 0.011 | 56.3 | 58.1 | 62.3 | 59.9 | 62.3 | 0.014 |
| FN-BMD (g/cm2) | 0.98 | 0.99 | 0.99 | 1.00 | 0.98 | 0.902 | 0.93 | 0.92 | 0.92 | 0.91 | 0.92 | 0.701 |
| Glucose (mmol/L) | 5.92 | 5.71 | 5.74 | 5.78 | 5.92 | 0.661 | 5.40 | 5.50 | 5.38 | 5.41 | 5.72 | 0.346 |
| Prevalent DM (%) | 12% | 8% | 10% | 12% | 14% | 0.364 | 5% | 7% | 4% | 6% | 5% | 0.764 |
| Creatinine (mg/dL) | 0.94 | 0.97 | 0.99 | 0.97 | 0.97 | 0.140 | 0.78 | 0.77 | 0.77 | 0.77 | 0.78 | 0.671 |
| eGFR (mL/min) | 88.0 | 85.7 | 85.9 | 86.5 | 88.2 | 0.391 | 85.4 | 86.0 | 86.2 | 85.6 | 85.5 | 0.664 |
| Na+ (mmol/L) | 141.6 | 141.9 | 142.1 | 142.3 | 142.3 | 0.017 | 141.8 | 142.0 | 142.2 | 142.0 | 142.9 | <0.001 |
| K+ (mmol/L) | 4.29 | 4.39 | 4.42 | 4.41 | 4.45 | <0.001 | 4.30 | 4.33 | 4.38 | 4.38 | 4.44 | <0.001 |
P values corresponds to age-adjusted significance of trend across quintiles. BMI: body mass index. Smoke: ever smoke. 25OHD: 25-hydroxyvitamin D levels; FN-BMD: femoral neck bone mineral density; prevalent DM: prevalent diabetes mellitus; creatin: creatinine; eGFR: estimated glomerular filtration rate according to Chronic Kidney Disease Epidemiology Collaboration equations based on creatinine levels. Conversion to SI Units: to convert 25-hydroxyvitamin D levels to ng/mL multiply by 0.4; to convert glucose to mg/dL multiply by 18.02
Table 1b.
General characteristics of subjects with femoral neck BMD information available in men from MrOS according to quintiles of fasting phosphate levels
| Men | ||||||
|---|---|---|---|---|---|---|
| Phosphate in quintiles | ||||||
| 1 | 2 | 3 | 4 | 5 | p* | |
| MrOS | ||||||
| N Phosphate Mean (mg/dL) |
1086 (2.6) |
1085 (2.9) |
1085 (3.2) |
1085 (3.4) |
1084 (3.8) |
|
| Range | 1.8–2.8 | 2.8–3.0 | 3.1–3.3 | 3.3–3.5 | 3.5–6.8 | |
| Age (y) | 73.2 | 73.2 | 73.7 | 73.9 | 73.7 | 0.002 |
| BMI (kg/m2) | 27.3 | 27.3 | 27.4 | 27.5 | 27.6 | 0.006 |
| Smoke (%) | 61% | 60% | 63% | 63% | 67% | <0.001 |
| Calcium (mg/dL) | 9.28 | 9.30 | 9.31 | 9.32 | 9.37 | <0.001 |
| 25OHD (nmol/L) | 63.3 | 65.5 | 65.4 | 63.6 | 62.8 | 0.534 |
| FN-BMD (g/cm2) | 0.79 | 0.79 | 0.79 | 0.78 | 0.79 | 0.723 |
| Glucose (mmol/L) | 5.79 | 5.89 | 5.80 | 5.85 | 6.00 | 0.003 |
| Prevalent DM (%) | 7% | 10% | 10% | 11% | 18% | <0.001 |
| Creatinine (mg/dL) | 0.99 | 0.99 | 1.02 | 1.02 | 1.07 | <0.001 |
| eGFR (mL/min) | 88 | 89 | 86 | 85 | 82 | <0.001 |
| Na+ (mmol/L) | 141.4 | 141.3 | 141.4 | 141.5 | 141.4 | 0.296 |
| K+ (mmol/L) | 4.19 | 4.21 | 4.25 | 4.30 | 4.36 | <0.001 |
P values corresponds to age-adjusted significance of trend across quintiles
eGFR: estimated glomerular filtration rate according to Modification of Diet in Renal Disease (MDRD) study equation
P levels lie within normal range (0.81–1.45 mmol/L; 2.5–4.5 mg/dL) in the vast majority (~95%) of each study population.
Phosphate is not associated with FN-BMD; it is negatively correlated with LS-BMD in men from Rotterdam Study but not MrOS
Tables 2a and 2b show the relationship between P levels and BMD. We found no association between P and FN-BMD (Table 2a) in men (pooled β (95% CI)) (β: −0.04 (−0.08 to 0.01), p=0.096). In women, a negative association was found in the age-only adjusted model (β: −0.15 (−0.22 to −0.08), p <0.001) but it became non-significant after adjustment for BMI.
Table 2a.
Phosphate levels and femoral neck BMD in RS-I, RS-II, RS-III and MrOS
| Model I | Model II | |||||
|---|---|---|---|---|---|---|
| n | β (95% CI)1 | p | N | β (95% CI)1 | p | |
| RS-I | ||||||
| Men | 1214 | −0.11 (−0.24 to 0.01) | 0.084 | 1204 | −0.06 (−0.18 to 0.06) | 0.328 |
| Women | 1610 | −0.24 (−0.35 to −0.13) | <0.001 | 1596 | −0.05 (−0.16 to 0.05) | 0.314 |
| RS-II | ||||||
| Men | 909 | −0.09 (−0.23 to 0.06) | 0.232 | 905 | −0.07 (−0.21 to 0.07) | 0.311 |
| Women | 1047 | −0.19 (−0.32 to −0.05) | 0.005 | 1040 | −0.01 (−0.13 to 0.12) | 0.916 |
| RS-III | ||||||
| Men | 870 | −0.03 (−0.17 to 0.11) | 0.692 | 870 | 0.01 (−0.12 to 0.15) | 0.849 |
| Women | 1141 | −0.02 (−0.13 to 0.10) | 0.762 | 1140 | 0.07 (−0.04 to 0.19) | 0.196 |
| RS combined2 | ||||||
| Men | 2993 | −0.08 (−0.16 to 0.00) | 0.050 | 2979 | −0.04 (−0.12 to 0.03) | 0.287 |
| Women | 3798 | −0.15 (−0.22 to −0.08) | <0.001 | 3776 | 0.01 (−0.06 to 0.07) | 0.988 |
| MrOS | ||||||
| Men | 5425 | −0.02 (−0.08 to 0.04) | 0.458 | 5422 | −0.03 (−0.09 to 0.02) | 0.215 |
| Studies combined2 | ||||||
| Men | 8418 | −0.04 (−0.09 to 0.01) | 0.079 | 8401 | −0.04 (−0.08 to 0.01) | 0.096 |
| Women | 3798 | −0.15 (−0.22 to −0.08) | <0.001 | 3776 | 0.01 (−0.06 to 0.07) | 0.988 |
| Sex-combined | 12216 | −0.08 (−0.11 to −0.04) | <0.001 | 12177 | −0.03 (−0.06 to 0.01) | 0.171 |
Model I: age adjusted
Model II: age, BMI and smoking adjusted; additional race and site adjustments in MrOS
Betas are expressed per 1 mg/dL increase in P levels; BMD is expressed in SD
Studies were pooled applying a fixed effects model
Table 2b.
Phosphate levels and lumbar spine BMD in RS-I, RS-II, RS-III and MrOS
| Model I | Model II | |||||
|---|---|---|---|---|---|---|
| n | β (95% CI)1 | p | n | β (95% CI)1 | p | |
| RS-I | ||||||
| Men | 1458 | −0.13 (−0.24 to −0.02) | 0.021 | 1437 | −0.10 (−0.21 to 0.01) | 0.084 |
| Women | 2003 | −0.21 (−0.31 to −0.11) | <0.001 | 1943 | −0.09 (−0.19 to 0.01) | 0.084 |
| RS-II | ||||||
| Men | 910 | −0.19 (−0.34 to −0.04) | 0.012 | 906 | −0.18 (−0.32 to −0.03) | 0.017 |
| Women | 1059 | −0.15 (−0.28 to −0.02) | 0.027 | 1051 | −0.02 (−0.15 to 0.11) | 0.730 |
| RS-III | ||||||
| Men | 766 | −0.12 (−0.27 to 0.03) | 0.126 | 766 | −0.09 (−0.24 to 0.06) | 0.238 |
| Women | 1039 | −0.06 (−0.18 to 0.07) | 0.374 | 1038 | 0.02 (−0.10 to 0.14) | 0.772 |
| RS combined2 | ||||||
| Men | 3134 | −0.14 (−0.22 to −0.07) | <0.001 | 3109 | −0.12 (−0.19 to −0.04) | 0.002 |
| Women | 4101 | −0.15 (−0.22 to −0.08) | <0.001 | 4032 | −0.04 (−0.10 to 0.03) | 0.247 |
| MrOS | ||||||
| Men | 5390 | −0.02 (−0.08 to 0.04) | 0.495 | 5387 | −0.03 (−0.09 to 0.03) | 0.360 |
| Studies combined2 | ||||||
| Men | 8524 | −0.07 (−0.11 to −0.02) | 0.005 | 8496 | −0.06 (−0.11 to −0.02) | 0.007 |
| Women | 4101 | −0.15 (−0.22 to −0.08) | <0.001 | 4032 | −0.04 (−0.10 to 0.03) | 0.247 |
| Sex-combined | 12625 | −0.10 (−0.13 to −0.06) | <0.001 | 12528 | −0.06 (−0.09 to −0.02) | 0.004 |
Model I: age adjusted
Model II: age, BMI and smoking adjusted; additional race and site adjustments in MrOS
Betas are expressed per 1 mg/dL increase in P levels; BMD is expressed in SD
Studies were pooled applying a fixed effects model
We found a negative relationship between P levels and LS-BMD (Table 2b) in the pooled results from men (β: −0.06 (−0.11 to −0.02), p=0.007) which was driven by men from RS cohorts (β: −0.12 (−0.19 to −0.04), p=0.002) and not significant in men from MrOS (β: −0.03 (−0.09 to 0.03), p=0.360). In women, a negative association was found in the age-adjusted model (pooled β: −0.15 (−0.22 to −0.08), p<0.001) but this became non-significant after adjustment for BMI. Therefore, the significant association between P levels and LS-BMD in sex-combined analysis (β: −0.06 (−0.09 to −0.02), p=0.004) was driven by a significant negative association in men.
Phosphate is associated with all-type fracture risk in men and women
Table 3 shows results from analyses of P levels and fracture risk in RS-I, RS-II and MrOS after follow-up of 8.6, 6.6 and 10.9 years, respectively. During the follow-up period, a total of 1825 cases of incident fractures were recorded. In the basic model, each 1 mg/dL increase in P levels was significantly associated with an increase in all-type fracture risk in male subjects from the Rotterdam Study and in MrOS and borderline significantly in women. In the full model the associations were statistically significant in all groups: results for men were hazard ratio (HR): 1.52 (1.34–1.74), p<0.001; results for women were 1.32 (1.04–1.67), p=0.023; results for sex and study-combined analyses were HR: 1.47 (1.31–1.65), p<0.001. In MrOS, further adjustments for season-corrected total 25OHD in the full model yielded similar results: HR:1.49 (1.17–1.90), p=0.001; n=2345). In both cohorts adjustments for vitamin D (using different methods) did not influence results; furthermore season adjustment in MrOS did not change results. In the full model, there was no statistical evidence for sex interaction in the association between P and fracture risk in RS cohorts (p heterogeneity=0.258).
Table 3.
Risk of incidence of all types of fractures as a function of phosphate levels in RS-I, RS-II and MrOS
| Model I | Model II | |||||
|---|---|---|---|---|---|---|
| nofxs/total n | HR1,2 (95% CI) | p | nofxs/total n | HR1,2 (95% CI) | p | |
| RS-I | ||||||
| Men | 152/1476 | 1.95 (1.37–2.77) | <0.001 | 116/1094 | 1.74 (1.12–2.69) | 0.013 |
| Women | 390/1828 | 1.33 (1.05–1.69) | 0.017 | 279/1325 | 1.48 (1.11–1.97) | 0.007 |
| Sex-combined | 542/3304 | 1.50 (1.23–1.83) | <0.001 | 395/2419 | 1.54 (1.21–1.95) | <0.001 |
| RS-II | ||||||
| Men | 75/1127 | 1.33 (0.78–2.25) | 0.292 | 51/876 | 1.58 (0.84–2.95) | 0.153 |
| Women | 162/1356 | 0.90 (0.62–1.31) | 0.583 | 116/1012 | 1.02 (0.66–1.56) | 0.937 |
| Sex-combined | 237/2483 | 1.03 (0.76–1.40) | 0.829 | 167/1888 | 1.18 (0.83–1.69) | 0.351 |
| RScombined2 | ||||||
| Men | 227/2603 | 1.73 (1.29–2.32) | <0.001 | 167/1970 | 1.69 (1.18–2.41) | 0.004 |
| Women | 552/3184 | 1.19 (0.97–1.45) | 0.092 | 395/2337 | 1.32 (1.04–1.67) | 0.023 |
| MrOS | ||||||
| Men | 1046/5409 | 1.54 (1.34–1.77) | <0.001 | 1046/5409 | 1.50 (1.30–1.72) | <0.001 |
| Studies combined3 | ||||||
| Men | 1273/8012 | 1.57 (1.39–1.78) | <0.001 | 1213/7379 | 1.52 (1.34–1.74) | <0.001 |
| Women | 552/3184 | 1.19 (0.97–1.45) | 0.092 | 395/2337 | 1.32 (1.04–1.67) | 0.023 |
| Sex-combined | 1825/11196 | 1.45 (1.31–1.62) | <0.001 | 1608/9716 | 1.47 (1.31–1.65) | <0.001 |
no fxs: number of fractures
Model I: age, BMI and smoking adjusted; additional race and site adjustments in MrOS
Model II: adjusted for age, BMI, smoking, FN-BMD, alcohol intake, prevalent diabetes mellitus, prevalent cardiovascular disease, eGFR and serum levels of potassium, and calcium; additional adjustments for season-adjusted 25(OH)D in RS cohorts and race and site adjustments in MrOS
.Hazard ratios are expressed per increase in 1 mg/dL of P levels.
HRs from Cox models
.Studies combined applying a fixed effects model
Phosphate in quintiles and fracture risk
Analyses of P in quintiles and fracture risk suggested a dose-effect relation in both RS-I (the RS cohort with more fracture events) and MrOS (Tables 4a and 4b). After adjustments in Model I, data from RS-I showed a significant trend for increasing P and fracture risk in both men (HRs for the fourth: 2.07 (1.21–3.57), p=0.008 and fifth quintile: 2.27 (1.33–3.90), p= 0.003 against the first quintile; p trend <0.001) and women (HRs for the fourth: 1.50 (1.08–2.09), p=0.016 and fifth quintile: 1.47 (1.05–2.05), p=0.026 against the first quintile; p trend = 0.022). A similar trend was observed in MrOS (HRs for the fourth: 1.23 (1.01–1.49), p=0.040 and fifth quintile: 1.59 (1.32–1.93), p<0.001 against the first quintile; p trend <0.001).
Table 4a.
Risk of incidence of all types of fractures as a function of phosphate levels categorized in quintiles in men and women from RS-I
| Men | Women | ||||||
|---|---|---|---|---|---|---|---|
| P levels1 mean (range) | events/ no risk | HR2,3(95% CI) | p | P levels1 mean (range) | events/ no risk | HR2,3 (95% CI) | P |
| 2.6 (1.9–2.8) | 20/295 | 1.00 (reference) | 3.0 (2.3–3.3) | 59/365 | 1.00 (reference) | ||
| 2.9 (2.8–3.0) | 22/295 | 1.12 (0.61–2.06) | 0.708 | 3.4 (3.3–3.5) | 78/366 | 1.33 (0.95–1.87) | 0.099 |
| 3.1 (3.1–3.2) | 32/295 | 1.66 (0.95–2.91) | 0.075 | 3.6 (3.5–3.7) | 76/365 | 1.25 (0.89–1.76) | 0.197 |
| 3.4 (3.3–3.5) | 38/295 | 2.07 (1.21–3.57) | 0.008 | 3.8 (3.7–3.9) | 90/366 | 1.50 (1.08–2.09) | 0.016 |
| 3.8 (3.5–7.6) | 40/296 | 2.27 (1.33–3.90) | 0.003 | 4.2 (4.0–5.2) | 87/366 | 1.47 (1.05–2.05) | 0.026 |
| p trend <0.001 | p trend = 0.022 | ||||||
P levels expressed in mg/dL
Hazard ratios are age, BMI and smoking adjusted; first quintile was set as reference
Hazard ratios are derived from Cox models
Table 4b.
Risk of incidence of all types of fractures as a function of phosphate levels categorized in quintiles in men from MrOS
| Men | |||
|---|---|---|---|
| P levels1 mean (range) | events/no risk | HR2,3(95% CI) | p |
| 2.6 (1.8–2.8) | 188/1085 | 1.00 (reference) | |
| 2.9 (2.8–3.0) | 206/1081 | 1.14 (0.94–1.39) | 0.194 |
| 3.2 (3.1–3.3) | 190/1081 | 1.06 (0.87–1.30) | 0.558 |
| 3.4 (3.3–3.5) | 213/1083 | 1.23 (1.01–1.49) | 0.040 |
| 3.8 (3.5–6.8) | 249/1079 | 1.59 (1.32–1.93) | <0.001 |
| p trend <0.001 | |||
P levels expressed in mg/dL
Hazard ratios are age, BMI, smoking, site and race adjusted; first quintile was set as reference
Hazard ratios are derived from Cox models
Subtypes of fractures
Results of different subtypes of fractures can be found in Supplementary Table 1. In studies and sexes combined we found that P levels were related to all types of fractures. Although effects sizes could not be compared due to the difference in numbers of fractures, it appeared that the strongest associations were found for (clinical) vertebral fractures in men while women displayed a stronger association for humerus fractures.
Sensitivity analyses
The stratified fracture analyses according to eGFR (Supplementary Table 2) showed that the association between P and fractures was not abolished after restricting the analyses to subjects with eGFR>58 mL/min (pooled results for sex and studies combined: Model I HR: 1.43 (1.27–1.61), p<0.001; Model II HR: 1.44 (1.26–1.63), p<0.001).
Additionally, men with eGFR ≤ 58mL/min from both populations displayed a significant relation between P and fracture risk in both the basic and full models (RS men, Model I HR: 2.24 (1.01–4.98), p=0.048, Model II HR: 4.05 (1.38–11.9), p=0.011; men from MrOS, Model I HR: 1.90 (1.40–2.58), p<0.001; Model II HR: 1.81 (1.32–2.49), p<0.001). The pooled result for men yielded: Model I HR: 1.94 (1.46–2.58), p<0.001 and Model II HR: 1.93 (1.42–2.62), p<0.001.
Women with eGFR≤58 mL/min displayed no significant association between P and fracture risk.
Results for P and fracture risk after excluding subjects with abnormal values of P were significant in men (RS men HR: 1.79 (1.26–2.56), p=0.001; MrOS men: 1.55 (1.33–1.81), p=0.001) (data not shown). The pooled results yielded HR: 1.59 (1.38–1.83), p<0.001). In women, the relation between normal P and fracture risk was not statistically significant (HR: 1.12 (0. 89–1.40), p=0.330). In study and sex-combined analyses the results were HR: 1.44 (1.28–1.62), p<0.001.
Analyses after applying multiple imputation did not substantially modify the results obtained in the analyses with the complete cases (data not shown).
Additional adjustments in MrOS
The additional adjustments for total and free 25OHD, FGF23 and PTH levels in a subgroup of men from MrOS (Supplementary Table 3a) did not substantially modify the significant association between serum P and fracture risk (PTH-adjusted HR: 1.50 (1.18–1.90), p=0.001; FGF23-adjusted HR: 1.69 (1.25–2.29), p=0.001; total 25OHD-adjusted HR: 1.49 (1.18–1.89), p=0.001; free 25OHD-adjusted HR: 1.73 (1.16–2.59), p=0.008). The multivariate analyses showed no modification in the results either.
The same pattern was observed after stratification by kidney function (GFR 58 mL/min; Supplementary Table 3b) in both strata.
Further adjustments for dietary phosphate intake in men from MrOS did not change results (Model I HR: 1.53 (1.33–1.76) n=5394, Model I adjusted for dietary phosphate, calcium and energy intake HR: 1.53 (1.33–1.76), n=5394; Model II HR: 1.48 (1.16–1.89) n=2333, Model II adjusted for dietary phosphate, calcium and energy intake HR: 1.48 (1.16–1.89), n=2333).
Additional analyses performed in MrOS in a subset (n= 988) with bone turnover markers available did not change results: (Model I HR: 1.34 (0.94–1.90) n=937, Model I adjusted for PINP and βCTX HR: 1.34 (0.94–1.90), n=933; Model II HR: 1.35 (0.94–1.95) n=933, Model II adjusted for PINP and βCTX HR: 1.36 (0.94–1.97), n=933).
Discussion
In these population-based cohorts, serum P levels were positively and significantly associated with fracture risk in both sexes. These associations were independent of BMD and not explained by multiple potential confounders. Although associations appeared stronger in men than women in the Rotterdam Study, there was no statistical evidence for a sex-difference. P was inversely associated with LS-BMD only in men from the Rotterdam Study although in combined analyses of sexes and cohorts this association remained significant. No associations were found with FN-BMD. In women, a relation between P and BMD at both skeletal sites was completely explained by a previously described association of P with BMI.(43)
The results from fracture analyses with P in categories suggested a potential threshold of P (3.3 mg/dL (1.1 mmol/L) in men –consistent in both cohorts- and 3.7 mg/dL (1.2 mmol/L) in women) above which fracture risk was increased. However, trend analysis suggests that risk may start to increase even at lower levels. Analyses restricted to subjects with P levels within normal range still showed a significant relation between P and fracture risk although results were statistically significant in men only.
Previous cross-sectional studies reported P levels to be higher in elderly subjects and in CKD patients on hemodialysis with previous fragility fractures (13, 14, 45) compared to subjects without fractures, but to the best of our knowlegde no prospective studies have been reported at the population level.
Regarding the mechanisms underlying the relation between P levels and bone traits several potential pathways can be hypothesized, namely: a) effects through P regulatory hormones, b) direct effects of P on BMD and/or bone quality (and vice versa) and/or fracture risk and c) P as a reflection of bone turnover. Regarding the first possibility, P levels are regulated by a complex set of hormones that play an important role in bone metabolism, such as FGF23, PTH and 1,25-hydroxyvitamin D. Abnormal FGF23 levels have been associated with impaired mineralization,(6,7,46,47) through P-dependent and independent effects. (48) However, consistent with previous research (49) adjustments for FGF23 levels did not influence the association of P with fracture risk in men from MrOS.
High PTH levels in hypovitaminosis D may also increase fracture risk. (50) Nevertheless, we found that adjustments for 25OHD in RS cohorts and additionally for total and free 25OHD and PTH levels in a subgroup of men from MrOS did not basically modify the association between P and fracture risk. The exclusion of subjects with CKD yielded similar results both in men and women; therefore we conclude that our findings are not likely explained by secondary elevations of FGF23 or PTH in CKD or by vitamin D deficiency.
On the other hand, we also observed a strong relation between P and fracture risk in men from RS cohorts with CKD, which was replicated in men from MrOS. These results are consistent with an increased gradient of risk for fracture stemming from the increased P load that patients with CKD display, (51) even without overt hyperphosphatemia. This finding was not abolished or even attenuated after adjustment for FGF23 and PTH levels in MrOS suggesting that high P itself and not underlying hormonal disturbances may explain the increased fracture risk in this group. As a potential therapeutic possibility, the feasibility of a multicenter randomized trial testing whether P lowering is able to decrease several clinical outcomes in patients with CKD, including bone pain and fracture risk is currently being evaluated. (52)
In contrast, the association between P and fracture risk in women with CKD was not statistically significant.
Regarding our findings of a negative association between P levels and LS-BMD in the pooled results from men, which was driven by men from RS cohorts, we can only speculate whether this is a chance finding or related to the fact that LS-BMD contains more trabecular than cortical bone. It has been previously described that FGF23 expression differs across human bone tissue (53) and that it tends to cluster in osteocytes near the trabecular periphery and the lacuna-canalicular systems, (54–58) in contrast to the expression pattern of other osteocytes (DMP1 osteocytes), which are diffusely located throughout bone. This observation needs to be tested in other cohorts,(59) and if confirmed, it deserves further research. As LS-BMD measurements can be affected by degenerative changes, (60) more accurate techniques for trabecular volumetric bone assessment might be desirable as well as novel methods to assess more accurately bone microarchitecture which might be influenced by serum phosphate. (7,8)
It is also possible that P may have direct effects on bone metabolism. P itself exerts key roles in growth plate maturation, secondary ossification center formation and osteoblast differentiation.(2, 4) Moreover, high P diets have been shown to increase bone resorption and development of osteoporosis in senescent mice. (61–63) Studies on rats fed high P showed disturbances in P homeostasis and reduced bone mineralization over short- and long term periods.(64) Therefore, a direct negative effect of increased P intake on bone is quite well possible. In MrOS, adjustment for dietary phosphate intake did not influence the associations between P and fracture risk but it is currently difficult to accurately estimate phosphorus intake e.g., because phosphorus additives from processed food are often not labeled on food products.(65) But if a relation can be shown between dietary phosphate intake and (bone) health this may have implications in light of the increasing use of P additives in our diet. (65)
It is important to emphasize that fracture risk was found to be increased within normal values of serum phosphate suggesting that for bone health the current upper limit may be too high. It has been shown that high dietary intake of P is related to postprandial elevation of serum P,( 66) which may not be reflected as fasting P. Indeed there was no association between dietary P intake and fasting serum P levels in MrOS. Interestingly, the same threshold above which fracture risk was increased in men from both populations (3.3 mg/dL), was previously related to increased cardiovascular risk. (67) To the best of our knowledge, this is the first report to describe this association in a prospective fashion,(13, 14) and it may have important clinical consequences for subjects with and without CKD.
Thirdly, we cannot exclude the possibility that high serum P is merely a reflection of high bone turnover although we think this is not very likely as under normal circumstances, P exchange with the skeleton yields a neutral balance and bone turnover abnormalities rarely give rise to clinically relevant disturbances in P homeostasis.(11,68,69) Additionally, the results from adjustments for bone turnover markers in MrOS suggest that bone turnover does not explain the association between P and fracture risk.
Lastly, we cannot rule out that P levels were associated with fracture risk through non-skeletal effects, but an effect through falling appears to be unlikely as P levels were also strongly related to vertebral fractures and these fractures are often not preceded by a fall. Nevertheless, we cannot discard that other potential mechanisms through muscle mass or function might play a role in our findings.
Although there was no statistical evidence for an interaction by sex in the main analysis, the relations between P and bone traits seemed stronger in men than women with larger effect sizes. Such an observation has been reported before for other clinical outcomes, such as all-cause mortality, subclinical atherosclerosis, CKD progression and incident coronary disease. (15,16, 70) More research is needed to fully elucidate if the relation of P and fractures (and other outcomes) is indeed stronger in men.
Our study has some limitations. LS-BMD and fasting P levels were not assessed simultaneously at RS-I and only 75% of individuals from RS cohorts had complete data for all covariates of interest. But results were similar after applying multiple imputation. There are several strengths, though, namely the availability of several well-characterized and large cohorts with BMD and prospective fracture information and the ability to replicate the association between P and fracture risk in another large population-based cohort. Although no DXA cross-calibration between MrOS and RS cohorts was performed, statistical adjustments to account for use of different machines did not materially change results.
Additionally, the association between P and fracture risk was significant despite multiple adjustments, including levels of FGF23 and PTH in men from MrOS. Therefore, we consider it unlikely that our results are explained by residual confounding.
In conclusion, we found that in two population-based studies, increasing P levels were positively associated with fracture risk in men and women. These results were independent from BMD although there was an inverse association between P and LS-BMD in men. The association between P and fractures was also independent of dietary phosphate intake. Results were also not explained by serum levels of calcium, 25OHD, PTH, FGF23 or by comorbidity, including CKD, but associations between P and fractures were also found in men with CKD. The association between P and fractures calls for future research testing whether lowering of serum P or reducing P intake may reduce the risk of fracture. Additionally, further well-powered studies are needed to clarify if there is a sex-difference in the relation between P and bone traits.
The findings of our study also suggest that the current upper limit of serum P may be too high and they call for more research into the effects of high P diets and the use of P as food additives on bone health.
Supplementary Material
Acknowledgments
The authors thank the participants and staff of the research center of the Rotterdam Study and the MrOS study. The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam, Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam. The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Institute on Aging, the National Center for Research Resources, and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01 AG027810, and UL1 TR000128. CMN is supported by NIAMS K01 AR062655. The funding sources had no influence in the study design, collection, analysis, interpretation of data, writing of the report and in the decision to submit.
Footnotes
Disclosures
The authors state that they have no conflicts of interest.
None of the authors have any conflicts of interest
Author contribution: Study design: NCO, NK and MCZ. Data collection: AH, AU, MCZ, CMN. Data analysis: NCO, ERH, CMN and MCZ. Drafting of the manuscript: NCO, NK, CMN, ERH and MCZ. Critical review of the manuscript: all authors. Statistical analyses: NCO and ERH. Approving final version of manuscript: NCO, NK, CMN, ERH and MCZ. Obtained funding: AH and AU. Study supervision: MCZ. CMN and ERH had full access to all of the data in MrOS study; NCO and MCZ had full access to all of the data in Rotterdam Study cohorts. NCO and MCZ take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Berner YN, Shike M. Consequences of phosphate imbalance. Annu Rev Nutr. 1988;8:121–48. doi: 10.1146/annurev.nu.08.070188.001005. [DOI] [PubMed] [Google Scholar]
- 2.Koeppen BM, Stanton BA. Berne & Levy Physiology. 6. Philadelphia, PA: Koeppen, BM and Stanton BA; 2010. The Renal System; pp. 557–636. [Google Scholar]
- 3.Hu MC, Shiizaki K, Kuro-o M, Moe OW. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol. 2013;75:503–33. doi: 10.1146/annurev-physiol-030212-183727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang R, Lu Y, Ye L, et al. Unique roles of phosphorus in endochondral bone formation and osteocyte maturation. J Bone Miner Res. 2011;26(5):1047–56. doi: 10.1002/jbmr.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albright F, Butler AM, Bloomberg E. Rickets resistant to vitamin D therapy. Am J Dis Child. 1937;54(3):529–47. [Google Scholar]
- 6.Pettifor JM, Thandrayen K. Hypophosphatemic rickets: unraveling the role of FGF23. Calcif Tissue Int. 2012;91(5):297–306. doi: 10.1007/s00223-012-9651-0. [DOI] [PubMed] [Google Scholar]
- 7.Shimada T, Kakitani M, Yamazaki Y, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113(4):561–8. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ichikawa S, Imel EA, Kreiter ML, et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest. 2007;117(9):2684–91. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masi L, Gozzini A, Franchi A, et al. A novel recessive mutation of fibroblast growth factor-23 in tumoral calcinosis. J Bone Joint Surg Am. 2009;91(5):1190–8. doi: 10.2106/JBJS.H.00783. [DOI] [PubMed] [Google Scholar]
- 10.Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444(7120):770–4. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 11.Martin A, David V, Quarles LD. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev. 2012;92(1):131–55. doi: 10.1152/physrev.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–38. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jadoul M, Albert JM, Akiba T, et al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2006;70(7):1358–66. doi: 10.1038/sj.ki.5001754. [DOI] [PubMed] [Google Scholar]
- 14.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–18. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 15.Onufrak SJ, Bellasi A, Cardarelli F, et al. Investigation of gender heterogeneity in the associations of serum phosphorus with incident coronary artery disease and all-cause mortality. Am J Epidemiol. 2009;169(1):67–77. doi: 10.1093/aje/kwn285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onufrak SJ, Bellasi A, Shaw LJ, et al. Phosphorus levels are associated with subclinical atherosclerosis in the general population. Atherosclerosis. 2008;199(2):424–31. doi: 10.1016/j.atherosclerosis.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Hofman A, Brusselle GG, Darwish Murad S, et al. The Rotterdam Study: 2016 objectives and design update. Eur J Epidemiol. 2015;30(8):661–708. doi: 10.1007/s10654-015-0082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the Osteoporotic Fractures in men Study (MrOS) Contemp Clin Trials. 2005;26(5):557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Chang AR, Grams ME. Serum phosphorus and mortality in the Third National Health and Nutrition Examination Survey (NHANES III): effect modification by fasting. Am J Kidney Dis. 2014;64(4):567–73. doi: 10.1053/j.ajkd.2014.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson-Cohen C, Hoofnagle AN, Ix JH, et al. Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. JAMA. 2013;310(2):179–88. doi: 10.1001/jama.2013.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh RJ, Taylor RL, Reddy GS, Grebe SK. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab. 2006;91:3055–61. doi: 10.1210/jc.2006-0710. [DOI] [PubMed] [Google Scholar]
- 23.Heureux N, Anciaux M, Poncelet M, et al. Development of an ELISA for the measurement of free 25OHD vitamin D. Endocrine Abstracts. 2015:37. [Google Scholar]
- 24.Bauer DC, Garnero P, Harrison SL, et al. Biochemical Markers of Bone Turnover, Hip Bone Loss, and Fracture in Older Men: The MrOS Study. J Bone Miner Res. 2009;24:2032–38. doi: 10.1359/JBMR.090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofman A, Breteler MM, van Duijn CM, et al. The Rotterdam Study: 2010 objectives and design update. Eur J Epidemiol. 2009;24(9):553–72. doi: 10.1007/s10654-009-9386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. International Statistical Classification of Diseases and Related Problems 10th. 1992. Revision (ICD-10) [Google Scholar]
- 27.de Laet C, van Hout BA, Burger H, et al. Hip fracture prediction in elderly men and women: validation in the Rotterdam Study. J Bone Miner Res. 1998;13:1587–93. doi: 10.1359/jbmr.1998.13.10.1587. [DOI] [PubMed] [Google Scholar]
- 28.Oei L, Zillikens MC, Dehghan A, et al. High bone mineral density and fracture risk in type 2 diabetes as skeletal complication of inadequate glucose control. Diabetes Care. 2013;36(6):1619–28. doi: 10.2337/dc12-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stolk L, van Meurs JB, Arp PP, et al. The RIZ Pro 704 insertion-deletion polymorphism, bone mineral density and fracture risk: the Rotterdam Study. Bone. 2008;42:286–93. doi: 10.1016/j.bone.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Barrett-Connor E, Nielson CM, Orwoll E, Bauer DC, Cauley JA Osteoporotic Fractures in Men Study G. Epidemiology of rib fractures in older men: Osteoporotic Fractures in Men (MrOS) prospective cohort study. BMJ. 2010;340:c1069. doi: 10.1136/bmj.c1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ettinger B, Liu H, Blackwell T, et al. Validation of FRC, a fracture risk assessment tool, in a cohort of older men: the Osteoporotic Fractures in Men (MrOS) Study. J Clin Densitom. 2012;15(3):334–42. doi: 10.1016/j.jocd.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oei L, Hsu YH, Styrkarsdottir U, et al. A genome-wide copy number association study of osteoporotic fractures points to the 6p25.1 locus. J Med Genet. 2014;51(2):122–31. doi: 10.1136/jmedgenet-2013-102064. [DOI] [PubMed] [Google Scholar]
- 33.Meng J, Ohlsson C, Laughlin GA, et al. Associations of estradiol and testosterone with serum phosphorus in older men: the Osteoporotic Fractures in Men study. Kidney Int. 2010;78(4):415–22. doi: 10.1038/ki.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Popele NM, Elizabeth Hak A, Mattace-Raso FU, et al. Impaired fasting glucose is associated with increased arterial stiffness in elderly people without diabetes mellitus: the Rotterdam Study. J Am Geriatr Soc. 2006;54(3):397–404. doi: 10.1111/j.1532-5415.2005.00614.x. [DOI] [PubMed] [Google Scholar]
- 35.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 37.Akaike H. An information criterion (AIC) Math Sci. 1976;14:5–9. [Google Scholar]
- 38.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–26. [Google Scholar]
- 39.Higgins JPT, Greenland S, editors. Cochrane Handbook for Systematic Reviews of intervention. Version 5.1.0. The Cochrane collaboration; 2011. (updated March 2011) [Google Scholar]
- 40.McCloskey E, Johansson H, Oden A, Kanis JA. Fracture risk assessment. Clin Biochem. 2012;45(12):887–93. doi: 10.1016/j.clinbiochem.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Johansson H, Kanis JA, Oden A, et al. A meta-analysis of the association of fracture risk and body mass index in women. J Bone Miner Res. 2014;29(1):223–33. doi: 10.1002/jbmr.2017. [DOI] [PubMed] [Google Scholar]
- 42.Bleicher K, Cumming RG, Naganathan V, et al. U-shaped association between serum 25-hydroxyvitamin D and fracture risk in older men: results from the prospective population-based CHAMP study. J Bone Miner Res. 2014;29(9):2024–31. doi: 10.1002/jbmr.2230. [DOI] [PubMed] [Google Scholar]
- 43.Håglin L, Lindblad A, Bygren LO. Original communication. Hypophosphatemia in the metabolic syndrome. Gender differences in body weight and blood glucose. Eur J Clin Nutr. 2001;55:493–8. doi: 10.1038/sj.ejcn.1601209. [DOI] [PubMed] [Google Scholar]
- 44.Isakova T, Wahl P, Vargas GS, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370–8. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Figueiredo CP, Rajamannan NM, Lopes JB, et al. Serum phosphate and hip bone mineral density as additional factors for high vascular calcification scores in a community-dwelling: the Sao Paulo Ageing & Health Study (SPAH) Bone. 2013;52(1):354–9. doi: 10.1016/j.bone.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 46.ADHR Consortium. Autosomal dominant hypophosphatemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26(3):345–8. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Yoshiko Y, Yamamoto R, et al. Overexpression of fibroblast growth factor 23 suppresses osteoblast differentiation and matrix mineralization in vitro. J Bone Miner Res. 2008;23(6):939–48. doi: 10.1359/jbmr.080220. [DOI] [PubMed] [Google Scholar]
- 48.Sitara D, Kim S, Razzaque MS, et al. Genetic evidence of serum phosphate-independent functions of FGF-23 on bone. PLoS Genet. 2008;4(8):e1000154. doi: 10.1371/journal.pgen.1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Isakova T, Cai X, Lee J, et al. Associations of FGF23 with change in bone mineral density and fracture risk in older individuals. J Bone Miner Res. 2016;31(4):742–8. doi: 10.1002/jbmr.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bruce DG, St John A, Nicklason F, Goldswain PR. Secondary hyperparathyroidism in patients from Western Australia with hip fracture: relationship to type of hip fracture, renal function, and vitamin D deficiency. J Am Geriatr Soc. 1999;47(3):354–9. doi: 10.1111/j.1532-5415.1999.tb03001.x. [DOI] [PubMed] [Google Scholar]
- 51.Schlieper G, Schurgers L, Brandenburg V, et al. Vascular calcification in chronic kidney disease: an update. Nephrol Dial Transplant. 2016;31:31–9. doi: 10.1093/ndt/gfv111. [DOI] [PubMed] [Google Scholar]
- 52.Bhargava R, Kalra PA, Brenchley P, Hurst H, Hutchison A. A Study to Inform the Design of a National Multicentre Randomised Controlled Trial to Evaluate If Reducing Serum Phosphate to Normal Levels Improves Clinical Outcomes including Mortality, Cardiovascular Events, Bone Pain, or Fracture in Patients on Dialysis. Int J Nephrol. 2015;2015:579434. doi: 10.1155/2015/579434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mirams M, Robinson BG, Mason RS, Nelson AE. Bone as a source of FGF23: regulation by phosphate? Bone. 2004;35(5):1192–9. doi: 10.1016/j.bone.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 54.Pereira RC, Juppner H, Azucena-Serrano CE, Yadin O, Salusky IB, Wesseling-Perry K. Patterns of FGF-23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone. 2009;45(6):1161–8. doi: 10.1016/j.bone.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stubbs JR, Liu S, Tang W, et al. Role of hyperphosphatemia and 1,25-dihydroxyvitamin D in vascular calcification and mortality in fibroblastic growth factor 23 null mice. J Am Soc Nephrol. 2007;18(7):2116–24. doi: 10.1681/ASN.2006121385. [DOI] [PubMed] [Google Scholar]
- 56.Divieti Pajevic P. Recent progress in osteocyte research. Endocrinol Metab (Seoul) 2013;28(4):255–61. doi: 10.3803/EnM.2013.28.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ubaidus S, Li M, Sultana S, et al. FGF23 is mainly synthesized by osteocytes in the regularly distributed osteocytic lacunar canalicular system established after physiological bone remodeling. J Electron Microsc (Tokyo) 2009;58(6):381–92. doi: 10.1093/jmicro/dfp032. [DOI] [PubMed] [Google Scholar]
- 58.Wesseling-Perry J, Pereira RC, Wang H, et al. Relationship between plasma fibroblast growth factor-23 concentration and bone mineralization in children with renal failure on peritoneal dialysis. J Clin Endocrinol Metab. 2009;94(2):511–7. doi: 10.1210/jc.2008-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jovanovich A, Buzkova P, Chonchol M, et al. Fibroblast growth factor 23, bone mineral density, and risk of hip fracture among older adults: the cardiovascular health study. J Clin Endocrinol Metab. 2013;98(8):3323–31. doi: 10.1210/jc.2013-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ, 3rd, Khaltaev N. A reference standard for the description of osteoporosis. Bone. 2008;42(3):467–75. doi: 10.1016/j.bone.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 61.Koyama Y, Rittling SR, Tsuji K, et al. Osteopontin deficiency suppresses high phosphate load-induced bone loss via specific modulation of osteoclasts. Endocrinology. 2006;147(6):3040–9. doi: 10.1210/en.2005-0671. [DOI] [PubMed] [Google Scholar]
- 62.Shah BG, Krishnarao GV, Draper HH. The relationship of Ca and P nutrition during adult life and osteoporosis in aged mice. J Nutr. 1967;92(1):30–42. doi: 10.1093/jn/92.1.30. [DOI] [PubMed] [Google Scholar]
- 63.Garcia-Contreras F, Paniagua R, Avila-Diaz M, et al. Cola beverage consumption induces bone mineralization reduction in ovariectomized rats. Arch Med Res. 2000;31(4):360–5. doi: 10.1016/s0188-4409(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 64.Amato D, Maravilla A, Montoya C, et al. Acute effects of soft drink intake on calcium and phosphate metabolism in immature and adult rats. Rev Invest Clin. 1998;50(3):185–9. [PubMed] [Google Scholar]
- 65.European Food Safety Authority. Assessment of one published review on health risks associated with phosphate additives in food. EFSA Journal. 2013;11(11):27. [Google Scholar]
- 66.Nishida Y, Taketani Y, Yamanaka-Okumura H, et al. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int. 2006;70(12):2141–7. doi: 10.1038/sj.ki.5002000. [DOI] [PubMed] [Google Scholar]
- 67.Dhingra R, Sullivan LM, Fox CS, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167(9):879–85. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 68.Peacock M. Calcium metabolism in health and disease. Clin J Am Soc Nephrol. 2010;5(Suppl 1):S23–30. doi: 10.2215/CJN.05910809. [DOI] [PubMed] [Google Scholar]
- 69.Coen G, Ballanti P, Mantella D, et al. Bone turnover, osteopenia and vascular calcifications in hemodialysis patients. A histomorphometric and multislice CT study. Am J Nephrol. 2009;29(3):145–52. doi: 10.1159/000151769. [DOI] [PubMed] [Google Scholar]
- 70.Bellasi A, Madreoli M, Baldrat L, et al. Chronic kidney disease progression and outcome according to seum phosphorus in mild-to-moderate kidney dysfunction. Clin J Am Soc Nephrol. 2011;6(4):883–9. doi: 10.2215/CJN.07810910. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.