Abstract
Osteolytic bone lesions are a hallmark of multiple myeloma bone disease. Bone destruction is associated with severely imbalanced bone remodeling, secondary to increased osteoclastogenesis and significant osteoblast suppression. Lytic lesions of the pelvis are relatively common in MM patients and are known to contribute to the increased morbidity due to the high risk of fracture that frequently demands extensive surgical intervention. After observing unexpected radiological improvement in serial large pelvic CT assessment in a patient treated in a total therapy protocol, the radiographic changes of pelvic osteolytic lesions by CT/PET scanning in patients who received total therapy 4 treatment for myeloma were retrospectively analyzed. Sixty-two (62) patients with lytic pelvic lesions > 1 cm in diameter were identified at baseline PET/CT scanning. Follow-up CT studies demonstrated that 27/62 patients (43%) with large baseline pelvic lesions achieved significant reaccumulation of radiodense mineralization at the lytic cortical site. The average size of lytic lesions in which remineralization occurred was 4 cm (range: 1.3 cm – 10 cm). This study clearly demonstrates that mineral deposition in large pelvic lesions occurs in a significant proportion of MM patients treated with Total Therapy 4, potentially affecting patient outcomes, quality-of-life and future treatment strategies.
INTRODUCTION
Multiple myeloma (MM) is characterized by the clonal proliferation of plasma cells in the bone marrow and is associated with extensive osteolytic bone lesions, which represent an independent factor for mortality and morbidity(1). Bone lesions are present in approximately 70% of MM patients at diagnosis(2); skull, spine, rib cage and pelvis are the most commonly involved disease sites(2). MM may present asymptomatically, but it is more frequently associated with painful fractures, hypercalcemia or spinal cord compression(3). Almost 50% of newly diagnosed patients will develop a fracture in the first year after diagnosis with or without treatment, and approximately 65% of newly diagnosed patients will develop a fracture during the course of their disease(4). Indeed, the development of pathological fracture confers a 20% increased risk of death, compared to patients without fracture(5). Myeloma bone disease places patients at significant risk of additional skeletal-related events (SREs), which impair quality of life as well as increase treatment costs and the need for surgery or/and palliative radiation(6). Indeed, the international myeloma working group includes the presence of one or more osteolytic lesions on skeletal radiography, CT or PET/CT as a myeloma defining event(7).
Pelvic and periacetabular lytic lesions are clinically challenging medical emergencies because these particular lesions offer fewer satisfactory surgical options(8), and the pelvic and periacetabular sites have an extremely high fracture risk, are weight bearing and can limit mobility for patients(9). The study described here was triggered by the clinical observation of a 49-year-old woman with kappa light-chain MM presented with a pathological fracture of the acetabulum and large sacroiliac lytic lesions. These lesions resulted in significant hip pain, severe discomfort and extremely limited mobility. The patient was evaluated by an orthopedic consultant; however, surgical intervention was not indicated or offered, and the patient proceeded to induction chemotherapy with VDTPACE (bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide, etoposide) followed by stem cell collection. Nine (9) weeks later after hematological recovery, the patient received VTD (velcade 1.0 mg/m2, thalidomide 200 mg and dexamethasone 40 mg) and melphalan 200 mg/m2 followed by autologous stem cell infusion. Maintenance therapy with velcade, revlimid and dexamethasone (VRD) was later initiated, and the patient achieved complete remission. The patient’s pelvic lesions never received radiation therapy, but serial CT imaging with reconstruction obtained throughout the course of treatment revealed progressive and significant remineralization of the large lytic lesion (Figure 1 A, B). From this unexpected finding, MM patients with large pelvic lytic lesions at presentation and with adequate radiological follow-up were identified to determine the effect of the total therapy approach on the remineralization of significant lytic defects of the pelvis.
Figure 1.
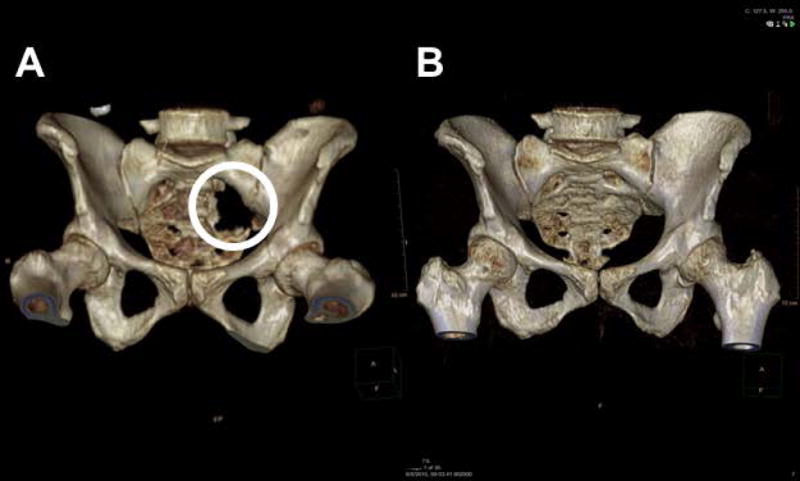
A- Three dimensional CT reconstruction renderings at the time of diagnosis (pretreatment)
B- Three dimensional CT reconstruction renderings after completion of Melphalan 200mg/m2 based autologous stem cell transplant
MATERIAL AND METHODS
The University of Arkansas for Medical Science (UAMS) myeloma patient database was interrogated to identify patients enrolled in the total therapy 4 (TT4) trial and who had pelvic lesions at least one cm in diameter at the time of presentation. TT4 is a protocol designed for newly diagnosed, low-risk MM patients, defined by a baseline plasma cell gene expression profile (GEP) score < than 0.66(10). All patients in this trial were either untreated or had not received more than one cycle of systemic MM therapy, excluding bisphosphonates and localized radiation. Enrolled patients also had a Zubrod score ≤ 2, unless symptoms and mobility were solely due to MM-related bone disease(6). All patients had preserved renal function, defined as serum creatinine level of < 3 mg/dL. Patients were randomized upfront to a standard or lite arm.
The standard treatment arm included two cycles of induction therapy with M (melphalan)-VTD-PACE and peripheral blood stem cell (PBSC) collection after the first cycle (melphalan 10 mg/m2, bortezomib 1 mg/m2 days 1, 4, 8, 11, dexamethasone 40 mg days 1–4, thalidomide 200 mg days 1–4, cisplatin 10 mg/m2 days 1–4, doxorubicin 10 mg/m2 days 1–4, cyclophosphamide 400 mg/m2 days 1–4 and etoposide 40 mg/m2× days 1–4). M-based tandem transplants were administered six weeks to three months apart, applying a single dose of M (200 mg/m2) with specific adjustments for age and renal function. Consolidation consisted of two additional cycles of dose-reduced VTD-PACE (bortezomib 1 mg/m2 days 1, 4, 8, 11, dexamethasone 40 mg days 1–4, thalidomide 200 mg days 1–4, cisplatin 7.5 mg/m2 days 1–4, doxorubicin 7.5 mg/m2 days 1–4, cyclophosphamide 300 mg/m2 days 1–4 and etoposide 30 mg/m2× days 1–4). Maintenance treatment consisted of VRD (bortezomib 1.0 mg/m2 day 1, 8, 15, 22, Revlimid 15 mg day 1–21, dexamethasone 20 mg day 1, 8, 15, 22 and weekly) given for three years. Only one patient received radiation therapy for extramedullary disease involving the adenoid glands. During the treatment protocol, patients were allowed to receive bisphosphonate therapy. The lite arm differed from the standard arm because it included a single induction and consolidation as well as a fractionated melphalan VTD high-dose chemotherapy (melphalan 50 mg/m2 day -4, -3, -2 -1, Velcade 1 mg/m2 day -4, -11, dexamethasone 40 mg day -4, -3, -2, -1 and thalidomide 200 mg day -4, -3, -2, -1).
All patients underwent a detailed clinical staging at initial registration, including full blood work, CBC, analysis of blood chemistry, and standard MM-related serological and urinary measurements, including FLc, SPEP/UPEP. Immunofixation analyses of serum and urine were performed to define the nature of the monoclonal proteins present in serum and/or urine. Bone marrow aspirates and biopsies were obtained for cytological and histopathological evaluation of the degree of plasma cell infiltration, including immunohistochemical clonality assessment, metaphase cytogenetics FISH and GEP studies from purified plasma cells, which are routine at our institution. Imaging studies included baseline standard metastatic bone surveys, MRI T1 and short T1 inversion recovery (STIR) sequences of the spine, pelvis, proximal humeri and sternum as well as PET-CT. The MRI and PET-CT imaging were repeated before each individual treatment cycle, during maintenance at regular intervals and as needed if clinically indicated. Osteolytic pelvic lesions observed on PET/CT scans or CT scans of the pelvis, obtained for CT guided biopsies of focal lesions at baseline, were compared to the most recent CT imaging available. All identified cases were reviewed by a board-certified skeletal radiologist to confirm radiological findings. Remineralization was considered positive when cortical bone with a minimum calculated thickness of 1 mm, not present at the baseline exam, was observed during the follow-up examination.
All patients enrolled in this study signed an IRB approved consent form.
STUDY DESIGN
This study consisted of a retrospective review of changes in mineralization at the site of a baseline pelvic lytic lesion. All patients signed an IRB-approved consent indicating the proposed treatment and potential examination of their medical records, in accordance with the institutional approved IRB.
STATISTICAL ANALYSIS
All statistical analysis were performed using R 3.2.2(11). The Wilcoxon rank sum was used to determine significant differences in median ALP variation between the two groups at each time point measured. Descriptive statistics were compared with boxplots and significance determined by the Student’s t-test. A probability value < 0.05 was considered statistically significant and is reported as such.
RESULTS
A total of 374 patients enrolled on the TT4 study were screened to identify those patients with baseline lytic pelvic lesions. Sixty-two (62) patients were identified as eligible and were included in this analysis. Patients were followed for a median of 41 months between the baseline and follow-up radiological study. All patients completed induction, HDCT, consolidation and maintenance; 33 patients were randomized to the lite and 29 to standard arm. The average size of lytic lesions at enrollment was 4.0 cm in maximum diameter (minimum 1.3 cm – maximum 10 cm). Baseline patient characteristics of the whole group and each subgroup are shown in Table 1. Comparing the baseline to follow-up PET CT studies, 43% (27/62) of large baseline pelvic lesions achieved the accumulation of radiodense bone mineral at the specific periosteal site with a minimal calculated thickness of 1 mm (Figure 2 A, B, C). Such radiological evidence of remineralization appeared progressively evident throughout the treatment phases and was already visible in the post-transplant phase in 80% of subjects. Axial and coronal images of the remineralized bony sites demonstrated the presence of woven-like bone. Within the group of patients with evidence of mineral deposition at the time of follow-up exam, complete remission status (CR) was present in 5 cases (19%), stringent complete response (sCR) in 8 (30%), very good partial response (VGPR) in 4 (15 %), partial remission (PR) 3 (11%) and progressive disease was evident in 6 subjects (22 %). One patient was in relapse at the time of follow up CT scanning (4%). PR or better responses in the group of patients without evidence of bone remineralization were not different from the compared group (Figure 3). Serum levels of alkaline phosphatase (ALP) were followed throughout the course of treatment. The median serum ALP level variation during the protocol showed an early peak similar to that observed in previous trials (Figure 4). Mean ALP in the non-mineralized and mineralized groups was 71.5 IU/L and 65.4 IU/L respectively (Figure 4). Genetic and laboratorial prognostic factors were also analyzed. Baseline GEP 70 defined risk assessment, molecular subgroups and cytogenetic distribution was not different between patients with evidence of bone remineralization compared to negative controls.
Table 1.
Baseline Patient Characteristics
| Baseline Patient Characteristics | Remineralized n/N (%) | Non-mineralized n/N (%) |
|---|---|---|
| Age: Mean (Minimum to Maximum) | 61 (34 – 75) | 57 (35 – 76) |
| Male | 15/27 (56%) | 28/35 (80%) |
| LDH >= 190 U/L | 3/27 (11%) | 5/35 (14%) |
| Albumin < 3.5 g/dL | 19/27 (70%) | 23/35 (66%) |
| B2M >= 3.5 mg/L | 17/27 (63%) | 17/35 (49%) |
| Creatinine >= 2.0 mg/dL | 1/27 (4%) | 1/35 (3%) |
| Platelet Count < 150 × 109/L | 4/27 (15%) | 3/35 (9%) |
| GEP70 CD-1 Subgroup | 3/27 (11%) | 1/35 (3%) |
| GEP70 CD-2 Subgroup | 5/27 (19%) | 9/35 (26%) |
| GEP70 HY Subgroup | 12/27 (44%) | 17/35 (49%) |
| GEP70 LB Subgroup | 3/27 (11%) | 4/35 (11%) |
| GEP70 PR Subgroup | 3/27 (11%) | 2/35 (6%) |
| GEP70 MF Subgroup | 1/27 (4%) | 0/35 (0%) |
| GEP70 MS subgroup | 0/27 (0%) | 2/35 (6%) |
| del13q | 10 (36%) | 12 (32%) |
| del17p | 5 (18%) | 7 (18%) |
| t(4;14) | 0 | 2 (5%) |
| t(14;20) | 0 | 0 |
| t(14;16) | 0 | 0 |
| IgA Isotype | 6/27 (22%) | 5/35 (14%) |
| IgG Isotype | 16/27 (59%) | 20/35 (57%) |
| IgD Isotype | 0/27 (0%) | 1/35 (3%) |
| Nonsecretory Isotype | 0/27 (0%) | 1/35 (3%) |
| Light Chain Isotype | 5/27 (19%) | 8/35 (23%) |
| Response PR or better | 25/27 (93%) | 33/35 (94%) |
Figure 2.
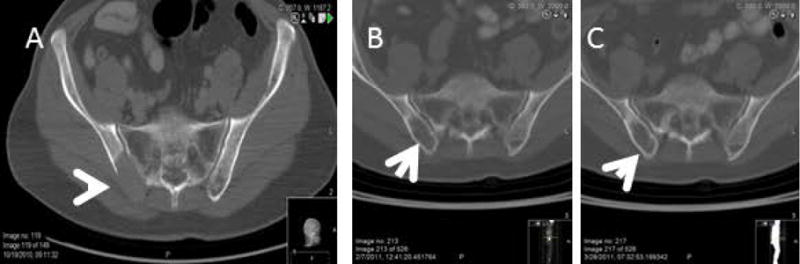
A -CT of the Pelvis showing the lytic lesion at the time of presentation (Pretreatment)
B- CT after fractionated Melphalan-VTD ASCT # 1
C –CT after fractionated Melphalan-VTD ASCT #2
Figure 3.
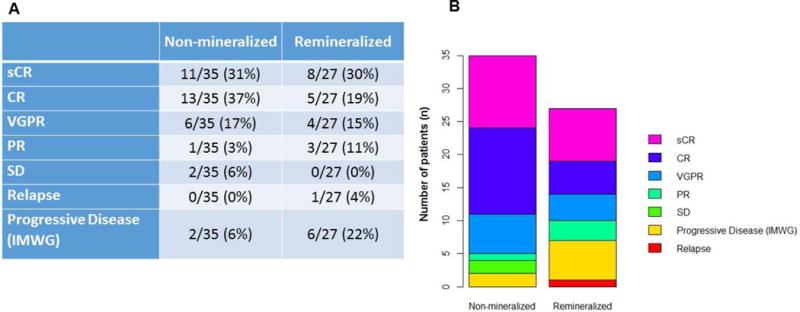
Myeloma response in the remineralized and non-mineralized group.
sCR- Stringent complete response, CR- Complete response, VGPR-Very good partial response, PR– partial response, SD-stable disease
Figure 4.
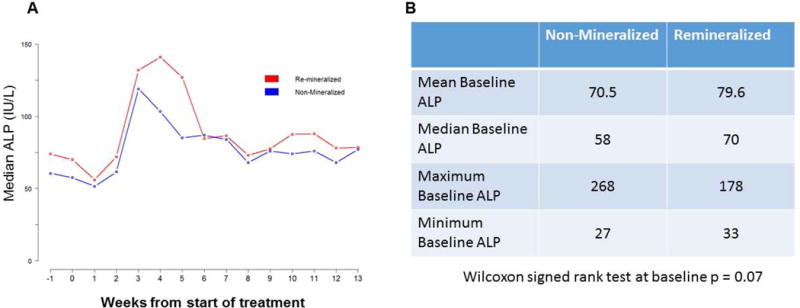
ALP variations in both groups
DISCUSSION
Myeloma bone disease is characterized by significant imbalances between bone formation and bone resorption(12). Plasma cells adhere and interact with bone marrow stromal cells as well as with normal resident bone cells to stimulate the production of receptor activator of Nf kappa β ligand (RANKL) and other bone resorption mediators such as interleukin-6 (IL-6), IL-11, tumor necrosis factor β (TNF-β), macrophage inflammatory protein 1α and 1β, resulting in enhanced osteoclast activation and significant bone destruction(13). An increased RANKL/osteoprotegerin (OPG) ratio in the bone marrow microenvironment is responsible for the significantly increased osteoclast activity(14). At the same time, osteoblast formation is suppressed largely via the inhibition of osteoblast transcription factors Runx-2/Cbfa-1 and osterix, as well as the Wnt/b-catenin signaling pathway(15–17). Dickkopf-1(DKK-1) is a Wnt pathway antagonist up-regulated in MM patients with osteolytic lesions(17, 18). Sclerostin, the product of the Sost gene and secreted by osteocytes, is another potent Wnt inhibitor that also appears to be increased in myeloma patients(19).
Little is known regarding lytic bone lesion changes in patients undergoing anti-myeloma therapy and it is generally held that lytic lesions rarely (if ever) heal, even when the disease is in apparent remission(20). Therefore, the goal in myeloma bone lesion therapy has been primarily palliative and focused on pain control and the prevention of skeletal fractures and SREs. This is achieved largely by the use of bisphosphonate therapy, palliative radiation and surgical intervention(21) (22). Indeed, palliative radiation therapy is useful to control pain in impending fractures and can significantly improve the quality-of-life in a significant percentage of patients(23). Bisphosphonates are potent antiresorptive agents currently recommended for patients with MM with or without detectable osteolytic bone lesions by conventional radiography and who are receiving anti-myeloma therapy as well as for patients with diffuse osteopenia or osteoporosis resulting from MM(6).
Bortezomib is a first in class proteasome inhibitor that is a key agent in the treatment of MM, both in relapsed refractory and treatment naïve patients(24, 25), that stimulates osteoblast activation irrespective of the response to treatment(26). A Phase II prospective study in patients with relapsed/refractory MM demonstrated increased bone volume/total volume of random pelvic biopsies via comparative histomorphometric microCT analysis after three 3-week cycles of treatment in six out of seven patients(26). A previous report of 14 patients with relapsed/refractory MM receiving bortezomib demonstrated that two patients experienced substantial radiographic recovery of bone lesions by CT scan of the skull, clavicle and thoracic vertebrae(27). The analysis of the phase III VISTA trial of bortezomib plus melphalan, prednisone (VMP) vs. Melphalan –prednisone (MP) showed that worsening bone disease, skeletal adverse effects and the requirement for radiotherapy were lower among the patients in the VMP arm vs. MP arm (3% vs 11%, and 3% vs 8%, respectively)(28). Hinge and Dale have recently reported remineralization in 35 MM patients treated with combination of chemotherapy proteasome inhibitor and IMiD drugs. The authors reported sclerotic changes in 68% of target lesions(29). A similar bone anabolic effect was also described with salvage lenalidomide dexamethosne combination therapy in cases of myeloma patients refractory to high dose chemotherapy with bortezomib and autologous blood peripheral stem cell transplantation(30). All these studies support the bone anabolic activity of bortezomib and IMiD drugs, which we have incorporated into the total therapy 4 treatment protocol.
To the best of our knowledge, this is the first study to demonstrate a significant percentage (43%) of re-mineralization of such large pelvic lytic lesions obtained with combination therapy for MM. Even if we did not observe significant differences in serum ALP levels by mineralization status we still observed an ALP elevation during the first months of therapy as previously described. Bone mineralization in this small cohort of patients appeared not to be affected by response to treatment suggesting the possibility of an independent phenomenon as previously reported by other investigators(31–32).
Re-mineralization predominantly occurred at cortical bone sites but was also seen in cancellous bone. Since pelvic bones are flat bones that predominantly develop by membranous ossification(29), further investigation of the mechanism responsible for this profound new mineralization/bone formation is warranted in other flat bone sites such as scapula, sternum and ribs which share a similar ossification pattern. However, the observations are significantly robust to provide the basis for an ongoing study in long bones which regenerate by an endochondral mechanism(29). These data also demonstrate that, contrary to much current dogma, lytic MM bone lesions, at least in the pelvis, retain mineralization capacity which can restore the apparent bone mineral to near normal levels over time. In addition, these data also suggest the need to consider a more conservative approach in the treatment of lytic pelvic lesions in patients with MM.
The impact on functional capacity of the patients was not specifically addressed in this study. But clinical impressions suggest that re-mineralization also restores patient’s activity to levels more comparable with pre-diagnosis status. Further studies are ongoing to confirm if a similar phenomenon is achievable at different sites with different drug regimens.
Acknowledgments
MM, RSS, LJS, MZ designed and performed the research, analyzed the data and wrote the paper. DY, AFB, AB analyzed data and edited the paper. COM, RN, RM, SY contributed to research design and analyzed data. ST, CS, SY, FvR, FED, GJM wrote the paper.
Source(s) of support: Total Therapy 4 was supported by the National Cancer Institute, National Institutes of Health Program Project Grant CA55819 (to Dr. Gareth Morgan).
Footnotes
AUTHORSHIP
All authors declare no conflicts.
Supplemental Data: Total Therapy 4 Protocol
References
- 1.Croucher PI, Apperley JF. Bone disease in multiple myeloma. British journal of haematology. 1998;103(4):902–10. doi: 10.1046/j.1365-2141.1998.01082.x. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clinic proceedings. 2003;78(1):21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 3.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1588–94. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Roodman GD. Treatment strategies for bone disease. Bone marrow transplantation. 2007;40(12):1139–46. doi: 10.1038/sj.bmt.1705802. [DOI] [PubMed] [Google Scholar]
- 5.Roodman GD. Pathogenesis of myeloma bone disease. Journal of cellular biochemistry. 2010;109(2):283–91. doi: 10.1002/jcb.22403. [DOI] [PubMed] [Google Scholar]
- 6.Terpos E, Morgan G, Dimopoulos MA, Drake MT, Lentzsch S, Raje N, et al. International Myeloma Working Group recommendations for the treatment of multiple myeloma-related bone disease. J Clin Oncol. 2013;31(18):2347–57. doi: 10.1200/JCO.2012.47.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajkumar SV. Myeloma Today: Disease Definitions and Treatment Advances. American journal of hematology. 2015 doi: 10.1002/ajh.24236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakellariou VI, Mavrogenis AF, Savvidou O, Sim FH, Papagelopoulos PJ. Reconstruction of multiple myeloma lesions around the pelvis and acetabulum. European journal of orthopaedic surgery & traumatology : orthopedie traumatologie. 2015;25(4):643–53. doi: 10.1007/s00590-014-1555-4. [DOI] [PubMed] [Google Scholar]
- 9.Rock MG, Harrington K. Pathologic fractures of the acetabulum and the pelvis. Orthopedics. 1992;15(5):569–76. doi: 10.3928/0147-7447-19920501-08. [DOI] [PubMed] [Google Scholar]
- 10.Shaughnessy JD, Jr, Haessler J, van Rhee F, Anaissie E, Pineda-Roman M, Cottler-Fox M, et al. Testing standard and genetic parameters in 220 patients with multiple myeloma with complete data sets: superiority of molecular genetics. British journal of haematology. 2007;137(6):530–6. doi: 10.1111/j.1365-2141.2007.06586.x. [DOI] [PubMed] [Google Scholar]
- 11.Nrf R, R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing V; Austria: 2015. https://www.R-project.org/ [Google Scholar]
- 12.Sezer O. Myeloma bone disease: recent advances in biology, diagnosis, and treatment. The oncologist. 2009;14(3):276–83. doi: 10.1634/theoncologist.2009-0003. [DOI] [PubMed] [Google Scholar]
- 13.Abe M, Hiura K, Wilde J, Shioyasono A, Moriyama K, Hashimoto T, et al. Osteoclasts enhance myeloma cell growth and survival via cell-cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood. 2004;104(8):2484–91. doi: 10.1182/blood-2003-11-3839. [DOI] [PubMed] [Google Scholar]
- 14.Terpos E, Szydlo R, Apperley JF, Hatjiharissi E, Politou M, Meletis J, et al. Soluble receptor activator of nuclear factor kappaB ligand-osteoprotegerin ratio predicts survival in multiple myeloma: proposal for a novel prognostic index. Blood. 2003;102(3):1064–9. doi: 10.1182/blood-2003-02-0380. [DOI] [PubMed] [Google Scholar]
- 15.Giuliani N, Colla S, Morandi F, Lazzaretti M, Sala R, Bonomini S, et al. Myeloma cells block RUNX2/CBFA1 activity in human bone marrow osteoblast progenitors and inhibit osteoblast formation and differentiation. Blood. 2005;106(7):2472–83. doi: 10.1182/blood-2004-12-4986. [DOI] [PubMed] [Google Scholar]
- 16.Qiang YW, Barlogie B, Rudikoff S, Shaughnessy JD., Jr Dkk1-induced inhibition of Wnt signaling in osteoblast differentiation is an underlying mechanism of bone loss in multiple myeloma. Bone. 2008;42(4):669–80. doi: 10.1016/j.bone.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349(26):2483–94. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 18.Kaiser M, Mieth M, Liebisch P, Oberlander R, Rademacher J, Jakob C, et al. Serum concentrations of DKK-1 correlate with the extent of bone disease in patients with multiple myeloma. European journal of haematology. 2008;80(6):490–4. doi: 10.1111/j.1600-0609.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 19.Terpos E, Christoulas D, Katodritou E, Bratengeier C, Gkotzamanidou M, Michalis E, et al. Elevated circulating sclerostin correlates with advanced disease features and abnormal bone remodeling in symptomatic myeloma: reduction post-bortezomib monotherapy. International journal of cancer Journal international du cancer. 2012;131(6):1466–71. doi: 10.1002/ijc.27342. [DOI] [PubMed] [Google Scholar]
- 20.Dimopoulos M, Terpos E, Comenzo RL, Tosi P, Beksac M, Sezer O, et al. International myeloma working group consensus statement and guidelines regarding the current role of imaging techniques in the diagnosis and monitoring of multiple Myeloma. Leukemia. 2009;23(9):1545–56. doi: 10.1038/leu.2009.89. [DOI] [PubMed] [Google Scholar]
- 21.Berenson JR, Rosen LS, Howell A, Porter L, Coleman RE, Morley W, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer. 2001;91(7):1191–200. doi: 10.1002/1097-0142(20010401)91:7<1191::aid-cncr1119>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Morgan GJ, Davies FE, Gregory WM, Szubert AJ, Bell SE, Drayson MT, et al. Effects of induction and maintenance plus long-term bisphosphonates on bone disease in patients with multiple myeloma: the Medical Research Council Myeloma IX Trial. Blood. 2012;119(23):5374–83. doi: 10.1182/blood-2011-11-392522. [DOI] [PubMed] [Google Scholar]
- 23.Balducci M, Chiesa S, Manfrida S, Rossi E, Za T, Frascino V, et al. Impact of radiotherapy on pain relief and recalcification in plasma cell neoplasms: long-term experience. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft [et al] 2011;187(2):114–9. doi: 10.1007/s00066-010-2155-9. [DOI] [PubMed] [Google Scholar]
- 24.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609–17. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 25.Barlogie B, Anaissie E, van Rhee F, Haessler J, Hollmig K, Pineda-Roman M, et al. Incorporating bortezomib into upfront treatment for multiple myeloma: early results of total therapy 3. Br J Haematol. 2007;138(2):176–85. doi: 10.1111/j.1365-2141.2007.06639.x. [DOI] [PubMed] [Google Scholar]
- 26.Zangari M, Yaccoby S, Pappas L, Cavallo F, Kumar NS, Ranganathan S, et al. A prospective evaluation of the biochemical, metabolic, hormonal and structural bone changes associated with bortezomib response in multiple myeloma patients. Haematologica. 2011;96(2):333–6. doi: 10.3324/haematol.2010.031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozaki S, Tanaka O, Fujii S, Shigekiyo Y, Miki H, Choraku M, et al. Therapy with bortezomib plus dexamethasone induces osteoblast activation in responsive patients with multiple myeloma. International journal of hematology. 2007;86(2):180–5. doi: 10.1532/IJH97.07030. [DOI] [PubMed] [Google Scholar]
- 28.Delforge M, Terpos E, Richardson PG, Shpilberg O, Khuageva NK, Schlag R, et al. Fewer bone disease events, improvement in bone remodeling, and evidence of bone healing with bortezomib plus melphalan-prednisone vs. melphalan-prednisone in the phase III VISTA trial in multiple myeloma. Eur J Haematol. 2011;86(5):372–84. doi: 10.1111/j.1600-0609.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- 29.Hinge M, Andersen KT, Lund T, Jørgensen HB, Holdgaard PC, Ormstrup TE, et al. Bone healing in multiple myeloma: a prospective evaluation of the impact of first-line and anti-myeloma treatment. Haematologica. 2016;101(10):e419–e422. doi: 10.3324/haematol.2016.144477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sekiguchi Y, Ichikawa K, Wakabayashi M, Sugimoto K, Tomita S, Izumi H, et al. Bone formation of following lenalidomide-dexamethasone combination therapy in cases of multiple myeloma refractory to high-dose chemotherapy with bortezomib and autologous peripheral blood stem cell transplantation; report of a case and review of the literature. Int J Clin Exp Pathol. 2015;(8):9609–19. [PMC free article] [PubMed] [Google Scholar]
- 31.Heider W, Kaiser M, Müller C, Jakob C, Zavrski I, Schulz CO, et al. Bortezomib increases osteoblast activity in myeloma patients irrespective of response to treatment. Eur J Haematol. 2016;77(3):233–8. doi: 10.1111/j.1600-0609.2006.00692.x. [DOI] [PubMed] [Google Scholar]


