Abstract
Background
Drugs activating the mu opioid receptor are routinely used to treat severe acute and chronic pain. Unfortunately, side effects including nausea, constipation, respiratory depression, addiction and tolerance can limit clinical utility. In contrast, kappa opioid receptor (KOPr) agonists, such as Salvinorin A (SalA), have analgesic properties with little potential for abuse.
Methods
We evaluated SalA and the novel analogue β-tetrahydropyran Salvinorin B (β-THP SalB) for the ability to modulate pain and inflammation in vivo. The hot water tail-withdrawal assay, intradermal formalin-induced inflammatory pain and paclitaxel-induced neuropathic pain models were used to evaluate analgesic properties in mice. Tissue infiltration of inflammatory cells was measured by histology and flow cytometry.
Results
β-THP SalB produced a longer duration of action in the tail-withdrawal assay compared to the parent compound SalA, and, like SalA and U50,488, β-THP SalB is a full agonist at the KOPr. In the formalin-induced inflammatory pain model, β-THP SalB and SalA significantly reduced pain score, paw oedema and limited the infiltration of neutrophils into the inflamed tissue. β-THP SalB and SalA supressed both mechanical and cold allodynia in the paclitaxel-induced neuropathic pain model, in a dose-dependent manner.
Conclusions
Structural modification of SalA at the C-2 position alters its analgesic potency and efficacy in vivo. Substitution with a tetrahydropyran group at C-2 produced potent analgesic and anti-inflammatory effects, including a reduction in paclitaxel-induced neuropathic pain. This study highlights the potential for KOPr agonists as analgesics with anti-inflammatory action and little risk of abuse.
Introduction
Pain is the most common reason to seek healthcare (St Sauver et al., 2013) and it is estimated that 19% of Europeans live with chronic pain (Breivik et al., 2006) creating a large socio-economic burden (Breivik et al., 2013). In the United States, pain costs $635 billion annually (Gaskin and Richard, 2012) whilst 40% of chronic pain suffers report insufficient treatment (Breivik et al., 2006), highlighting the need to develop more effective pharmacotherapies.
Drugs targeting mu-opioid receptors (MOPr), such as morphine, are the standard treatment for severe acute and chronic pain. While effective, long-term use of these therapies results in tolerance (Chu et al., 2006) and side effects including nausea, respiratory depression and constipation (Glare et al., 2006). Globally, rates of unintentional opioid overdose have been steadily rising (Compton and Volkow, 2006; Kuehn, 2007), with evidence showing misuse of prescription opioids to be a ‘gateway’ to other drugs of abuse (Siegal et al., 2003).
Activation of kappa-opioid receptors (KOPr) also have analgesic (Gallantine and Meert, 2008), anti-inflammatory (Bileviciute-Ljungar et al., 2006; Binder et al., 2001), and anti-pruritic effects (Akiyama et al., 2015; Kumagai et al., 2010). The endogenous KOPr ligand, dynorphin, is upregulated following pain (Iadarola et al., 1988) and pain responses are increased in KOPr knock-out mice (Simonin et al., 1998). In contrast to MOPr agonists, KOPr agonists have limited abuse potential (Di Chiara and Imperato, 1988), do not inhibit gastrointestinal transit (Porreca et al., 1984) or cause respiratory depression (Freye et al., 1983). However, side effects such as sedation, aversion and depression (Braida et al., 2009; Mague et al., 2003; Shippenberg and Herz, 1987) limit their clinical use.
Development of KOPr agonists with fewer side-effects (Law et al., 2013; Zhang et al., 2015) and reduced abuse potential (Morani et al., 2009) have recently received increased attention (Kivell and Prisinzano, 2010; Tsukahara-Ohsumi et al., 2011; Váradi et al., 2015; White et al., 2015). The recent clinical success of the potent KOPr agonist nalfurafine (Seki et al., 1999; Kumagai et al., 2010) illustrates the clinical utility of KOPr agonists for the management of pain and pruritus. Salvinorin A (SalA) is a potent, selective KOPr agonist isolated from the plant Salvia divinorum (Chavkin et al., 2004; Roth et al., 2002). In mice, SalA has anti-nociceptive effects in the tail-withdrawal, hot-plate (McCurdy et al., 2006), abdominal constriction and formalin footpad models (Guida et al., 2012; McCurdy et al., 2006). Whilst SalA has reduced side-effects compared to traditional KOPr agonists, the short duration of action limits its usefulness (Butelman et al., 2009; Prisinzano, 2005; Ranganathan et al., 2012). However, longer acting SalA analogues have potential for development of non-addictive analgesics if centrally mediated side-effects can be reduced.
The novel SalA analogue β-THP SalB is a potent, selective KOPr agonist that attenuates drug-seeking behaviours in preclinical models without sedative effects (Prevatt-Smith et al., 2011). In this study, we evaluated the analgesic and anti-inflammatory effects of SalA and β-THP SalB, including the potential to attenuate paclitaxel-induced mechanical and cold allodynia.
Materials and Methods
Animals
Adult male mice (B6-SJL, 23–30 g) were bred and housed at the Victoria University of Wellington (VUW) vivarium, New Zealand. All experiments were approved by the VUW Animal Ethics Committee and carried out in accordance with their guidelines for animal care. All animals were maintained on a 12-h light/dark cycle with lights on at 7:00. Access to food and water was provided ad libitum except during experimental sessions. All procedures were conducted during the light cycle. Animals were habituated to the experimental room and experimenter for 60 min prior to testing.
Drug preparation
The KOPr agonist SalA was isolated and purified from Salvia divinorum leaves (>98% pure by HPLC). β-THP SalB was synthesised from SalA as previously described (Prevatt-Smith et al., 2011). ICI 204,448 hydrochloride and paclitaxel were purchased from Tocris Bioscience. U50,488 was purchased from Sigma-Aldrich (NZ). All KOPr agonists were dissolved in 80% propylene glycol, 20% DMSO diluted with the same volume phosphate buffered saline (PBS) and delivered at 1–2 mg/kg via intraperitoneal (i.p.) injection. Paclitaxel was dissolved in 1:1:18 ratio of cremophor EL (Sigma-Aldrich)/ethanol/saline.
Hot water tail-withdrawal assay
The tail-withdrawal assay was carried out as previously described (Simonson et al., 2014). Briefly, mice were allowed to habituate in Plexiglas restrainers (internal diameter 24 mm) for 15 min prior to testing. Latencies were measured by immersing one-third of the tail in a hot water bath (50 ± 0.5 °C) and the time taken to show the withdrawal response recorded. A maximum cut-off latency of 10 s was used to avoid tissue damage. Following i.p. administration of the KOPr agonist or vehicle, latencies were repeatedly measured at 5, 10, 15, 30, 45, 60, 90 and 120 min. The maximum possible effect (MPE) was calculated using the following formula: % MPE = 100 × (test latency–control latency)/(10−control latency). Cumulative dose-response effects were evaluated in the tail-withdrawal assay at 30 min following each subcutaneous (s.c.) injection of KOPr agonist as previously described using a within animal design (Bohn et al., 2000). Non-linear regression analysis was used to determine potency (EC50) and efficacy (Emax) and data normalised to the known full KOPr agonist U50,488.
Formalin test and oedema
Mice were allowed to habituate to the test environment for 15 min before testing. The height of the hind paw was measured with digital callipers. Animals were given KOPr or vehicle treatment via i.p. injection 5 min prior to administration of 20 μL of 2% formaldehyde or PBS injected under the plantar surface of the right hind paw. Pain behaviours were recorded using a digital video camera for 60 min with a 45° angle mirror under the box facilitating observations of the injected hind paw as previously described (Lamb et al., 2012). The methods of Dubuisson and Dennis (1977) were used to assess pain behaviour using a weight bearing score. Briefly, the pain was scored as 0 if the mouse showed normal behaviour; 1 for partial weight bearing with only the digits touching the floor; 2 with no weight bearing with the paw raised; and 3 where the paw was bitten, licked or shaken. An average pain score was calculated for each 5 min time period. Pain behaviour scores were assigned at 5 s intervals for 60 min by an observer blinded to the treatment groups. Following the 60 min test period, the paw height was measured again to calculate the change in paw oedema. The mice were sacrificed and the footpad tissue retained for flow cytometry or histological evaluation.
Cell isolation and Flow cytometry
Cell isolation from footpad tissue was carried out using methods modified from Leong et al. (2012). Immediately following the formalin test (60 min following formalin injection), the footpad tissue was removed and digested for 2 h in 125 μg/mL DNAse I (Sigma-Aldrich) and 5 mg/mL collagenase I (Sigma-Aldrich) at 37°C in complete Iscove’s modified Dulbecco’s medium (cIMDM with 10% heat-inactivated FBS, 1% penicillin-streptomycin). The digest was pressed through a 40 nm filter and cells were washed and resuspended in cIMDM. Cells were washed in fluorescence activated cell sorter (FACS) buffer, resuspended in an antibody solution containing 1:800 peridinin-chlorophyll-protein (PerCP) CD45, 1:800 FITC CD11b, 1:400 PacBlueGr-1, 1:200 PE F4/80, 1:200 AF700 Ly6C, 1:200 APC CD11c and 1:500 Live-Dead (BD Bioscience, San Jose, CA, USA) in FACS buffer and incubated in the dark (15 min, 4°C). Cells were resuspended in FACS buffer containing 1:200 PerCP streptavidin (BD Bioscience) and incubated in the dark (15 min, 4°C). Cells were fixed in 10% formalin at room temperature for 10 min and resuspended in FACS buffer. Flow cytometry was analysed on a LSRII Special Order Research Product (BD Bioscience). Single-stained samples of negative control cells were included for each fluorophore. Leukocytes were distinguished from non-haematological cells by their CD45+ expression. Neutrophils were identified as CD45+/Ly6C-/CD11b+/Gr-1high. Monocytes were identified as CD45+/Ly6C+/CD11b+/Gr-1low. Macrophages were identified as CD45+/Ly6C+/CD11b+/F4-80+. All gating strategies removed the dead cells using Live-Dead Fixable blue.
Quantification of neutrophils in inflamed footpad tissue
The footpad tissue was removed 60 min following formalin or PBS injection and assessed for neutrophil infiltration in Haematoxylin and Eosin Y (H&E) stained sections. Footpad tissue was fixed in 4% formaldehyde for 24 h and cryo-protected in 30% sucrose and stored at −80 °C. Sagittal 5 μm sections were stained with H&E and neutrophil numbers counted at 100x magnification in 30 fields of view per biological replicate. The researcher performing the cell counts was blind to the treatment group.
Paclitaxel-induced neuropathic pain
Based on previously published methods (Deng et al., 2015), male C57BL/6 mice were given paclitaxel 4 mg/kg i.p. injections on four alternate days to give a cumulative dose of 16 mg/kg. Mechanical and cold allodynia were assessed every second day to measure the progression of paclitaxel-induced effects. On days with a measurement and a paclitaxel dose, measurements were always taken prior to the administration of paclitaxel.
Mechanical allodynia was measured using a 20-piece set of von Frey filaments (Stoelting, IL, USA). Filaments numbered from 2 to 9 were used, with testing always beginning with filament number 5. A simplified up-down method was used (Bonin et al., 2014), where a positive response resulted in the use of next lower filament in the subsequent test; if no response was observed, the next higher filament was used. This continued until 5 filaments had been administered and the final threshold was calculated as previously described (Bonin et al., 2014). A positive response was defined as a sharp withdrawal of the paw upon application or removal of the filament.
Cold allodynia was carried out as previously described (Deng et al., 2015). Briefly, a bubble of acetone was administered to the hind paw and the time taken to react was recorded. A positive reaction was defined as elevating, licking, biting or shaking of the paw.
On day 15 following the initial paclitaxel injection, the cumulative dose-response effects of the KOPr agonists were assessed in the paclitaxel-treated animals. The KOPr agonists or equivalent volumes of the vehicle were administered via s.c. injection every 30 minutes and the mechanical and cold allodynia measured in a within animals design.
Data Analysis
GraphPad Prism software v6.07 (San Diego, CA, USA) was used to determine statistical significance. Comparison of multiple treatment data was analysed using one-way ANOVA followed by Bonferroni post-tests. Comparisons of multiple effects were analysed using two-way ANOVA followed by Bonferroni post-tests. Dose-response effects were evaluated by non-linear regression analysis. Values represented as mean ± standard error of the mean (SEM) and were considered significant when p<0.05.
Results
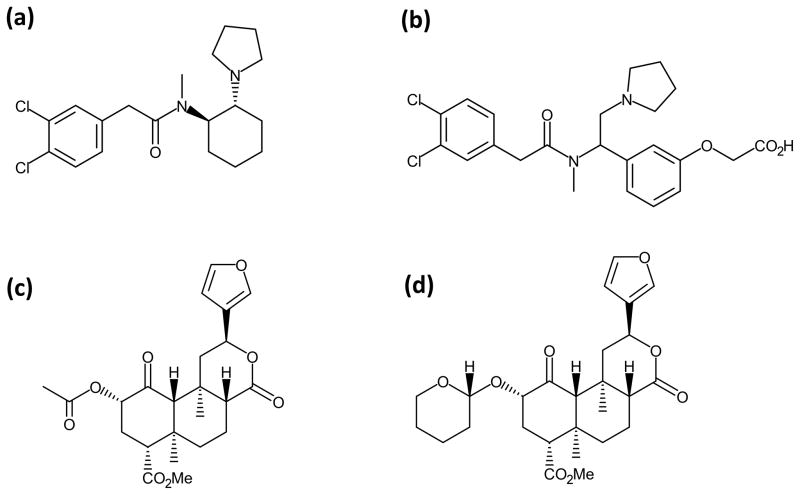
SalA, a neoclerodane diterpene isolated from Salvia divinorum is a full agonist at the KOPr (Roth et al., 2002). β-THP SalB is a semi-synthetic analogue of SalA with a tetrahydropyran substitution at the C-2 position (Fig. 1). β-THP SalB has been previously reported as a selective and potent full KOPr agonist with similar binding affinity and potency at the KOPr compared to SalA (Prevatt-Smith et al., 2011).
Figure 1.
Chemical structures for (a) U50,488, (b) ICI 204,448, (c) SalA and (d) β-THP SalB.
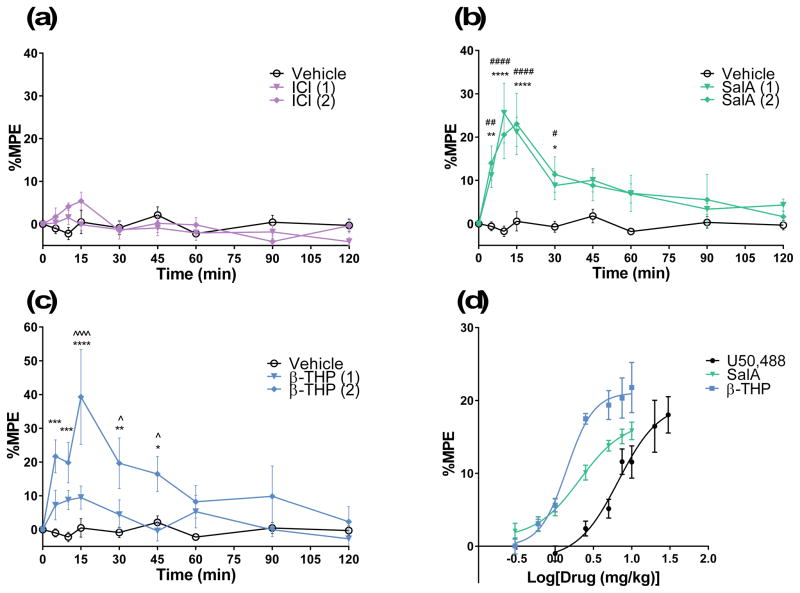
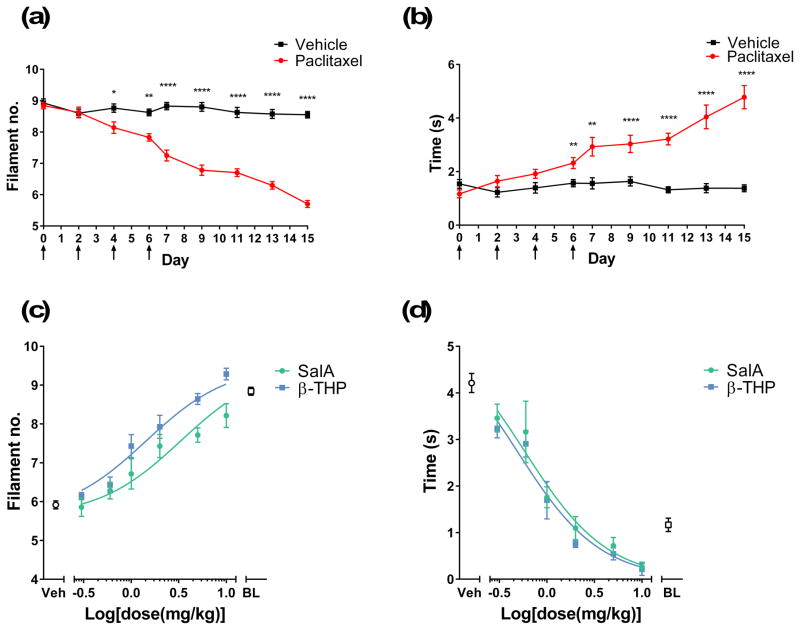
The hot-water tail-withdrawal assay was used to assess the duration of action of analgesic effects and the central penetrance of the KOPr agonists in vivo. For comparison, the KOPr agonist, ICI 204,448 was also evaluated, which showed no significant effect of treatment (F(16,144)=1.702, p>0.05)(Fig. 2a). SalA showed a significant effect compared to vehicle (F(16,216)=3.516, p<0.0001) and significantly increased tail-withdrawal latencies at 5–30 min (Fig. 2b). β-THP SalB showed a significant treatment effect (F(16,144)=4.833, p<0.0001) with the 2 mg/kg dose showing significant effects between 5–45 min and a significant difference between the 1 mg/kg and 2 mg/kg doses at 15–45 min (Fig. 2c). The cumulative dose-response effects of SalA and β-THP SalB were compared to the known full KOPr agonist U50,488 (Fig. 2d). Non-linear regression analysis revealed that SalA and β-THP SalB, like U50,488, are full agonists at KOPr with β-THP SalB being the most potent (EC50 1.4 mg/kg) followed by SalA (EC50 2.1 mg/kg) and U50,488 (EC50 6.7 mg/kg)(Table 1).
Figure 2.
The analgesic effects of KOPr agonists in the hot water tail-withdrawal assay in mice. The maximal possible effect (%MPE ± SEM) at each time point was calculated as a percent based on pre-treatment control latencies. Values shown in brackets represent dose in mg/kg. Mice were treated with the KOPr agonists (a) ICI 204,448, (b) SalA and (c) β-THP SalB. Analysed using two-way repeated measures ANOVA followed by Bonferroni post-test. (d) Non-linear regression analysis showing dose-response effect of KOPr agonists. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 for 2 mg/kg doses compared to vehicle. #p<0.05, ##p<0.01, ###p<0.001, ####p<0.0001 for 1 mg/kg doses compared to vehicle. ^p<0.05 and ^^^^p<0.001 for comparison between the two dosages. (n=6–12 per group).
Table 1.
Dose-response effects in the 50 °C tail-withdrawal assay in mice
| KOPr agonist | ED50 ± SEM (mg/kg) | Emax ± SEM (%) |
|---|---|---|
| U50,488 | 6.7 ± 0.1 | 100 ± 23 |
| SalA | 2.1 ± 0.1 | 88 ± 12 |
| β-THP SalB | 1.4 ± 0.5 | 107 ± 7 |
Cumulative dose-response effects in tail-withdrawal latency in vivo following s.c. administration in the mouse.
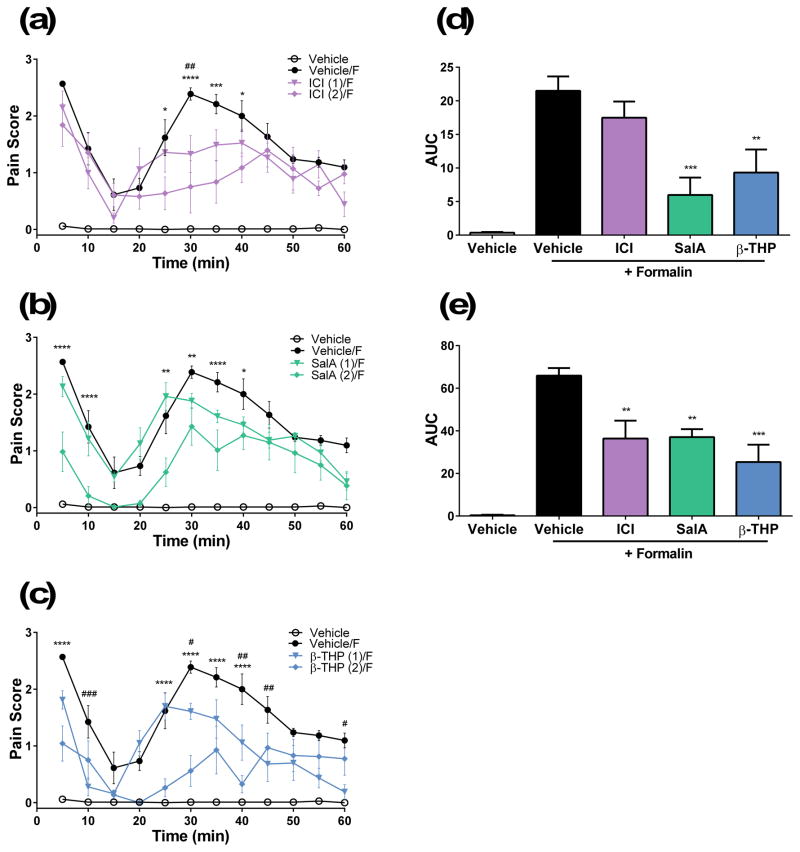
Administration of 2% formalin to the mouse hind paw is known to induce two phases of pain. The first phase involves rapid activation of nociceptors and lasts up to 15 min. The second phase, called the inflammatory phase, lasts between 20–60 min. We used this preclinical model to assess the anti-nociceptive and anti-inflammatory effects of the novel KOPr agonists. ICI 204,448, showed no effect in phase 1 nociceptive pain but did show a significant decrease in the pain score between 25–40 min at a dose of 2 mg/kg (Fig. 3a). SalA showed a significant interaction compared to vehicle (F(33,220)=3.169, p<0.0001) and a reduction phase 1 nociceptive pain at 5–10 min, and in phase 2 inflammatory pain at 25–40 min (Fig. 3b). β-THP SalB showed significant interaction effects (F(33,220)=6.839, p<0.0001) and showed dose-dependent effects in phase 1 and 2 behavioural pain scores. β-THP SalB (2 mg/kg) attenuated the first phase of pain at 5 min and the second phase between 25–40 min (Fig. 3c). β-THP SalB (1 mg/kg) also showed reduced pain scores at 10 min and at 30, 40–45 and 60 min (Fig. 3c).
Figure 3.
Antinocicpetive effects of KOPr agonists in the 2% formalin assay in mice. Time course of pain behaviours in mice treated with (a) ICI 204,448, (b) SalA and (c) β-THP SalB. Data shown as mean ± SEM. Values shown in brackets represent dose in mg/kg. Two-way repeated measures ANOVA followed by Bonferroni post-test. Area under the curve (AUC) calculated for the (d) phase 1 (5–15 min) and (e) phase 2 (15–60 min) pain for 2 mg/kg doses. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 for 2 mg/kg doses compared to vehicle. #p<0.05, ##p<0.01, ###p<0.001, ####p<0.0001 for 1 mg/kg doses compared to formalin control. F indicates intradermal administration of formalin as opposed to PBS. (n=6 for each group).
The area under the curve (AUC) was calculated for both phase 1 (0–15 min) and phase 2 pain (20–60 min), at the 2 mg/kg dosages, with a significant effect of treatment found in both phase 1 (F(4,25)=12.67, p<0.0001) and phase 2 (F(4,25)=17.16, p<0.0001). Results show ICI 204,448 had no effect in phase 1 pain, whilst SalA and β-THP SalB showed a significant reduction compared to formalin controls (formalin vs. SalA at 2 mg/kg: p=0.0005; formalin vs. β-THP SalB at 2 mg/kg: p=0.0058) (Fig. 3d). In the inflammatory phase of pain, ICI 204,448, SalA and β-THP SalB all showed a significant reduction in the AUC analysis (formalin vs. ICI 204,448 at 2 mg/kg: p=0.0047; formalin vs. SalA at 2 mg/kg: p=0.0059; formalin vs. β-THP SalB at 2 mg/kg: p=0.0001) (Fig. 3e).
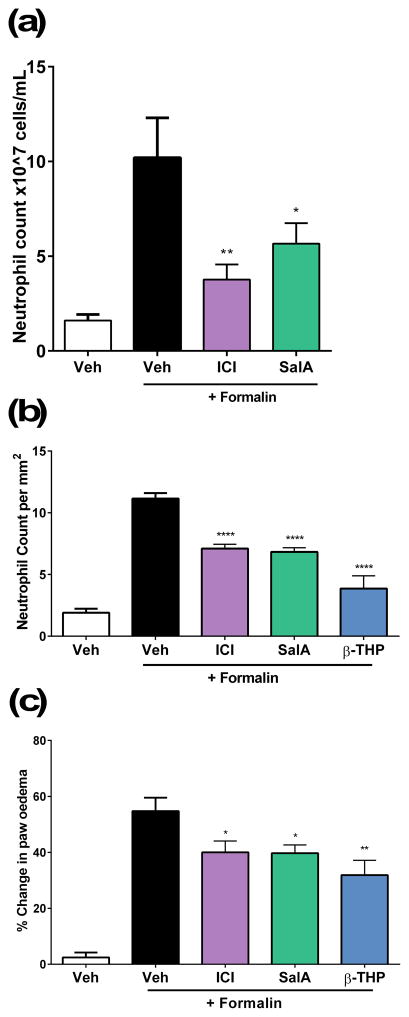
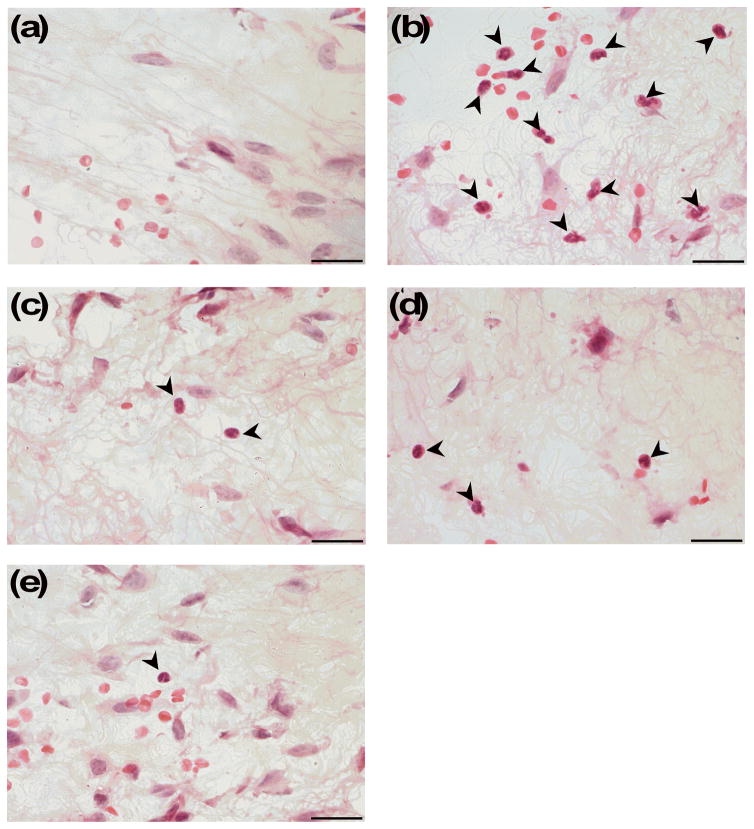
To further assess the anti-inflammatory effects of the KOPr agonists, we used flow cytometry as a preliminary screen to understand the effect of the KOPr agonists on inflammatory cell populations using an array of antibodies to identify inflammatory cell populations. One-way ANOVA analysis showed a significant effect of treatment (F(3,55)=5.387, p=0.0016) for the neutrophil population. SalA (p=0.0387) and ICI 204,448 (p=0.0045) both show a significant reduction in the neutrophil cell counts (Fig. 4a). No changes were seen in monocyte and macrophage cell numbers (data not shown). Following the flow cytometry data, we further quantified neutrophil numbers in H&E stained tissue sections prepared from the mice that underwent the formalin assay. A significant effect of treatment was observed (F(4,31)=43.37, p<0.0001). ICI 204,448 (p<0.0001), SalA (p<0.0001) and β-THP SalB (p<0.0001) all showed a significant reduction in neutrophil numbers (Fig. 4b, Fig. 5).
Figure 4.
Anti-inflammatory effects of KOPr agonists. (a) Neutrophil counts in footpad tissue evaluated using antibody markers and flow cytometry following administration of ICI 204,448 (2 mg/kg i.p.) and SalA (2 mg/kg i.p.) with no significant changes in any other inflammatory cells (data not shown) (n=13–23). (b) Neutrophil counts in H&E stained footpad tissue (per mm2 of sectioned footpad tissue)(n=6–12). (c) Paw oedema following 2% formalin. Data shown as mean ± SEM. One-way ANOVA with Bonferroni post-test *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Figure 5.
Representative images showing neutrophil infiltration into inflamed footpad tissue following (a) vehicle/PBS, (b) vehicle/2% formalin, (c) ICI/2% formalin, (d) SalA/2% formalin and (e) β-THP SalB/2% formalin. KOPr agonists (2 mg/kg i.p.) and vehicle treatments given 5 min prior to 2% formalin or PBS intradermal injection. Tissue samples taken one hour following formalin or PBS administration. Markers indicate neutrophils. Images were taken at 100 x magnification. Scale bar = 20 μm.
Paw oedema was calculated by measuring the paw size before and after administration of formalin. The results showed a significant effect of treatment (F(4,84)=20.10, p<0.0001) and a significant reduction in paw oedema with all compounds (ICI 204,448 vs. formalin p=0.0289, SalA vs. formalin p=0.0113, β-THP SalB vs. formalin p=0.0040) (Fig. 4c).
To induce neuropathic pain, mice were given a cumulative dose of 16 mg/kg of paclitaxel. Hypersensitivity was measured on alternate days, showing a significant decrease in the mechanical threshold (F(8,192)=28.27, p<0.0001)(Fig. 6a) and a significant increase in the response time to cold stimulation (F(8,192)=11.45, p<0.0001)(Fig. 6b) in paclitaxel-treated animals. On day 15, paclitaxel-treated animals underwent a cumulative dosing experiment to assess the dose-response effects of SalA and β-THP SalB. Non-linear regression analysis revealed that SalA and β-THP SalB were able to reduce mechanical allodynia, thereby showing an increase in the mechanical threshold. β-THP SalB was the most potent (EC50 1.4 mg/kg) followed by SalA (EC50 3.3 mg/kg)(Fig. 6c). Cold allodynia response time was also reduced by β-THP SalB (EC50 0.49 mg/kg) and SalA (EC50 0.59 mg/kg)(Fig. 6d) to a level lower than the pre-paclitaxel baseline.
Figure 6.
The effect KOPr agonists on paclitaxel-induced neuropathic pain. (a) Paclitaxel treatment resulted in withdrawal responses to a lower filament number. (b) Paclitaxel treatment increased the time responding to acetone stimulation. Two-way repeated measures ANOVA followed by Bonferroni post-test. Arrows indicate the days that animals received either paclitaxel or vehicle injections. Dose-response effects of SalA and β-THP SalB on (c) mechanical and (d) cold allodynia (n=7 per group). Veh refers to paclitaxel-treated animals administered with vehicle as opposed to the KOPr agonists. BL refers to pre-paclitaxel baseline values. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 for paclitaxel-treated animals compared to vehicle-treated.
Discussion
SalA is the active ingredient of Salvia divinorum, a plant from the sage family native to Mexico (Valdés, 1994; Wasson, 1962) and is the first naturally occurring non-nitrogenous KOPr agonist to be discovered (Roth et al., 2002). While its hallucinogenic properties (Valdés et al., 1983) and short duration of action (Butelman et al., 2009; Prisinzano, 2005; Ranganathan et al., 2012; Teksin et al., 2009) limit its clinical usefulness, SalA has proven preclinical analgesic and anti-inflammatory properties (Aviello et al., 2011; Fichna et al., 2012; Guida et al., 2012; John et al., 2006; McCurdy et al., 2006; Rossi et al., 2016). As a KOPr agonist, SalA is structurally unique and structure–activity studies have shown that the C-2 position of SalA is important for binding to KOPr and metabolic stability (Chavkin et al., 2004; Prisinzano, 2005).
In this study, we tested the analgesic and anti-inflammatory properties of a SalA analogue with a C-2 modification, β-THP SalB (Figure 1). The analogue has a large protecting group at the C-2 position which we postulated would increase the duration of action. β-THP SalB has similar binding affinity and efficacy at the KOPr compared to SalA (Prevatt-Smith et al., 2011), and both have previously been shown to attenuate cocaine-induced drug-seeking behaviour without sedation in spontaneous locomotion tests in rats (Morani et al., 2009; Morani et al., 2012; Prevatt-Smith et al., 2011).
In the hot water tail-withdrawal assay, we assessed the nociceptive processes at the spinal cord level. Utilising this test we showed that SalA significantly attenuates pain between 5 and 30 min, whilst the novel KOPr agonist β-THP SalB has a longer duration of action. β-THP SalB is as fast acting as SalA, showing anti-nociceptive effects at 5 min and lasting until 45 min. This suggests that β-THP SalB may have an improved metabolic profile and bioactivity in vivo compared to SalA. Previous studies on SalA have shown analgesic effects at around 20 min following subcutaneous administration (McCurdy et al., 2006) and a half-life of approximately 8 min in non-human primate brain (Hooker et al., 2008). The cumulative dose-response effects of β-THP SalB, SalA and U50,488 reveal differences in both potency (ED50) with β-THP SalB> SalA> U50,488 and efficacy (Emax). It is interesting to note that in vitro, all are full agonists at KOPr (Prevatt-Smith et al., 2011). Further exploration of in vivo effects and potential identification of biased agonism effects warrants further investigation.
To further investigate the analgesic properties of β-THP SalB, we utilised the formalin model which measures both phase 1 nociceptive pain and phase 2 inflammatory pain. Previous studies using SalA have shown a dose-dependent reduction in both phase 1 and 2 pain in mice, effects that were prevented by the administration of the KOPr antagonist nor-binaltorphimine (Aviello et al., 2011). The current study supports this, with SalA showing a reduction in both phases of pain (Fig. 3b). The peripherally restricted compound ICI 204,448 did not reduce the centrally-mediated phase 1 pain, but significantly reduced phase 2 inflammatory pain, supporting its known reduced CNS-mediated effects (Fig. 3a). We evaluated the effects of novel β-THP SalB in the formalin model, which showed significant attenuation of both phase 1 and 2 pain (Fig 3c).
Previous studies have shown that KOPr agonists have anti-inflammatory properties. The KOPr agonist U69,593 significantly reduced paw volume and histological score in the Freud’s adjuvant model of arthritic pain in Wistar rats (Binder et al., 2001). Administration of U50,488 also reduced the spread of inflammation following injection of Mycobacterium butyricum into the ankle joint (Bileviciute-Ljungar et al., 2006) and in the adjuvant arthritis model in Lewis rats (Wilson et al., 1996). Previously, SalA has also been shown to reduce carrageenan-induced paw oedema in mice (Aviello et al., 2011). The current study measured the level of oedema resulting from intradermal 2% formalin administration. Results show SalA and β-THP SalB both reduce formalin-induced oedema when compared to the vehicle-treated controls (Fig. 4c). SalA and β-THP SalB reduced the level of inflammatory pain, in parallel with a reduction in inflammation, indicating that the KOPr agonists may be having peripheral anti-inflammatory effects.
To better understand the effect of KOPr agonists on formalin-induced inflammation, we utilised two methods to quantify the infiltration of immune cells. As a preliminary screen to understand the immune cell populations in digested footpad tissue, cells were labelled with an array of antibodies to known immune cell surface markers and flow cytometry used to profile immune cell infiltration. Both SalA and ICI 204,448 significantly reduced neutrophil infiltration into the paw. In the present study, however, we did not see a reduction in the macrophage, monocytes or dendritic cell numbers (data not shown). This is likely due to the short one-hour duration of the formalin model of acute inflammation. We then measured the neutrophils via histological counts in H&E stained footpad tissue (Figs. 4c and 5) matched from the animals in the formalin assay. The results confirmed that SalA and ICI 204,448 significantly reduced neutrophil numbers. β-THP SalB also showed a significant reduction in the neutrophil numbers compared to the formalin control, indicating an anti-inflammatory effect.
Finally, we measured the effect of the KOPr agonists in a neuropathic pain model. Paclitaxel is a taxane chemotherapy drug commonly used to treat breast, ovarian, head, neck and non-small cell lung cancers. As a side-effect, paclitaxel induces neuropathic pain, which is commonly characterised by spontaneous tingling or burning pain, mechanical and cold allodynia, and numbness, often occurring in the hands and feet in a ‘stocking and glove’ type distribution (Dougherty et al., 2004; Forman, 1990). Chemotherapy-induced neuropathic pain is often the reason for limiting the dose and length of treatment in cancer patients (Holmes et al., 1991; Rowinsky et al., 1993). There are limited options of clinically available treatments and neuropathic pain may continue months past the last chemotherapy dose (van den Bent et al., 1997), making this neuropathy difficult to control. Previous animal studies have shown morphine reduced cold and mechanical allodynia in paclitaxel-treated rats (Pascual et al., 2010; Rahn et al., 2008). However, in the present study, we believe we have the first evidence of KOPr agonists as a potential treatment for paclitaxel-induced neuropathy. Both SalA and β-THP SalB dose-dependently reduced mechanical and cold allodynia to pre-paclitaxel levels, with the novel β-THP SalB demonstrating increased potency over the parent compound. This finding indicates that KOPr agonists have the potential to treat this difficult to manage condition.
The recent success of nalfurafine is a promising example of the clinical benefits of developing KOPr agonists. The potent KOPr agonist (Seki et al., 1999) (also called TRK-820 and the trade-name Remitch) exhibits strong analgesic effects (Endoh et al., 1999; Endoh et al., 2000) and is the first KOPr agonist to be made clinically available for the treatment of medication-resistant pruritus in haemodialysis patients (Kumagai et al., 2010). Co-administration of nalfurafine with spinal morphine reduces morphine-induced pruritus without altering the analgesic effects (Ko and Husbands, 2009). Furthermore, there is no evidence of abuse liability (Ueno et al., 2013) or reinforcement of self-administration (Nakao et al., 2016), demonstrating that KOPr agonists can have therapeutic utility without abuse potential.
In conclusion, KOPr agonists show attenuation of acute nociceptive and inflammatory pain. Substitution of the tetrahydropyran group at the C-2 position increased the duration of action in vivo beyond that of SalA. In addition, β-THP SalB showed reduced phase 1 and 2 pain in the formalin assay and reduced formalin-induced oedema and neutrophil numbers in the inflamed footpad tissue, similar to SalA and ICI 204,448. More importantly, this is the first report of a KOPr agonist attenuating mechanical and cold allodynia in the paclitaxel-induced neuropathic pain model. β-THP SalB displays greater potency than SalA, highlighting the potential for the development of KOPr agonists as analgesics with little potential for abuse.
What’s already known about this topic?
Compounds activating the kappa opioid receptor modulate pain and inflammation with low abuse potential. However, side-effects of traditional kappa-opioid receptor agonists limit potential therapeutic utility.
What does this study add?
Salvinorin A and the novel analogue β-THP Salvinorin B show analgesic effects in the tail-withdrawal and formalin assays.
Reduce oedema and decrease neutrophil infiltration into inflamed tissue.
Suppress mechanical and cold allodynia in paclitaxel-induced neuropathic pain.
Acknowledgments
Funding sources
This work was supported by funding from The Wellington Medical Research Foundation and Victoria University of Wellington (to B.M.K.) and the National Institutes of Health, DA018151 (to T.E.P.), GM008545 (to R.S.C.) and AFPE Pre-doctoral Fellowship in Pharmaceutical Sciences (to R.S.C.). K.F.P. received a studentship award from Victoria University of Wellington, New Zealand.
The authors would like to thank Odette Shaw, Kristel Kodar, Nayana Wijayawardhane and Karmella Naidoo for their technical assistance.
Footnotes
Conflict of interest
No conflict of interest to declare.
Author Contributions
B.M.K. designed the study, contributed to the analysis of data and wrote the manuscript. K.F.P. performed the experiments, analysed the data and wrote the manuscript. N.K. performed the experiments and analysed the data. T.E.P. and R.S.C. provided SalA and β-THP SalB. J.L.H. provided input into the design of the study. All authors discussed the results and critically reviewed the manuscript.
References
- Akiyama T, Carstens MI, Piecha D, Steppan S, Carstens E. Nalfurafine suppresses pruritogen- and touch-evoked scratching behavior in models of acute and chronic itch in mice. Acta Derm Venereol. 2015;95:147–150. doi: 10.2340/00015555-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviello G, Borrelli F, Guida F, Romano B, Lewellyn K, Chiaro MD, Luongo L, Zjawiony JK, Maione S, Izzo AA, Capasso R. Ultrapotent effects of salvinorin A, a hallucinogenic compound from Salvia divinorum, on LPS-stimulated murine macrophages and its anti-inflammatory action in vivo. J Mol Med. 2011;89:891–902. doi: 10.1007/s00109-011-0752-4. [DOI] [PubMed] [Google Scholar]
- Bileviciute-Ljungar I, Saxne T, Spetea M. Anti-inflammatory effects of contralateral administration of the κ-opioid agonist U-50,488H in rats with unilaterally induced adjuvant arthritis. Rheumatology. 2006;45:295–302. doi: 10.1093/rheumatology/kei156. [DOI] [PubMed] [Google Scholar]
- Binder W, Machelska H, Mousa S, Schmitt T, Riviere PJ, Junien JL, Stein C, Schafer M. Analgesic and antiinflammatory effects of two novel kappa-opioid peptides. Anesthesiology. 2001;94:1034–1044. doi: 10.1097/00000542-200106000-00018. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. [mu]-Opioid receptor desensitization by [beta]-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- Bonin RP, Bories C, De Koninck Y. A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Molecular Pain. 2014;10:1–11. doi: 10.1186/1744-8069-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braida D, Capurro V, Zani A, Rubino T, Viganò D, Parolaro D, Sala M. Potential anxiolytic- and antidepressant-like effects of salvinorin A, the main active ingredient of Salvia divinorum, in rodents. Br J Pharmacol. 2009;157:844–853. doi: 10.1111/j.1476-5381.2009.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Breivik H, Eisenberg E, O’Brien T. The individual and societal burden of chronic pain in Europe: the case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health. 2013;13:1229. doi: 10.1186/1471-2458-13-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelman ER, Prisinzano TE, Deng H, Rus S, Kreek MJ. Unconditioned behavioral effects of the powerful kappa-opioid hallucinogen salvinorin A in nonhuman primates: fast onset and entry into cerebrospinal fluid. J Pharmacol Exp Ther. 2009;328:588–597. doi: 10.1124/jpet.108.145342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, Sud S, Jin W, Stewart J, Zjawiony JK, Siebert DJ, Toth BA, Hufeisen SJ, Roth BL. Salvinorin A, an active component of the hallucinogenic sage salvia divinorum is a highly efficacious kappa-opioid receptor agonist: structural and functional considerations. J Pharmacol Exp Ther. 2004;308:1197–1203. doi: 10.1124/jpet.103.059394. [DOI] [PubMed] [Google Scholar]
- Chu LF, Clark DJ, Angst MS. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: a preliminary prospective study. J Pain. 2006;7:43–48. doi: 10.1016/j.jpain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: Concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Deng L, Guindon J, Cornett BL, Makriyannis A, Mackie K, Hohmann AG. Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1-dependent withdrawal. Biol Psychiatry. 2015;77:475–487. doi: 10.1016/j.biopsych.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- Dougherty PM, Cata JP, Cordella JV, Burton A, Weng HR. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109:132–142. doi: 10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- Endoh T, Matsuura H, Tajima A, Izumimoto N, Tajima C, Suzuki T, Saitoh A, Suzuki T, Narita M, Tseng L, Nagase H. Potent antinociceptive effects of TRK-820, a novel kappa-opioid receptor agonist. Life Sci. 1999;65:1685–1694. doi: 10.1016/s0024-3205(99)00417-8. [DOI] [PubMed] [Google Scholar]
- Endoh T, Tajima A, Suzuki T, Kamei J, Suzuki T, Narita M, Tseng L, Nagase H. Characterization of the antinociceptive effects of TRK-820 in the rat. Eur J Pharmacol. 2000;387:133–140. doi: 10.1016/s0014-2999(99)00815-8. [DOI] [PubMed] [Google Scholar]
- Fichna J, Dicay M, Lewellyn K, Janecka A, Zjawiony JK, MacNaughton WK, Storr MA. Salvinorin A has antiinflammatory and antinociceptive effects in experimental models of colitis in mice mediated by KOR and CB1 receptors. Inflamm Bowel Dis. 2012;18:1137–1145. doi: 10.1002/ibd.21873. [DOI] [PubMed] [Google Scholar]
- Forman A. Peripheral neuropathy in cancer patients: clinical types, etiology, and presentation. Part 2. Oncology (Williston Park) 1990;4:85–89. [PubMed] [Google Scholar]
- Freye E, Hartung E, Schenk GK. Bremazocine - an Opiate That Induces Sedation and Analgesia without Respiratory Depression. Anesth Analg. 1983;62:483–488. [PubMed] [Google Scholar]
- Gallantine EL, Meert TF. Antinociceptive and adverse effects of mu- and kappa-opioid receptor agonists: a comparison of morphine and U50488-H. Basic Clin Pharmacol Toxicol. 2008;103:419–427. doi: 10.1111/j.1742-7843.2008.00306.x. [DOI] [PubMed] [Google Scholar]
- Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13:715–724. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Glare P, Walsh D, Sheehan D. The adverse effects of morphine: a prospective survey of common symptoms during repeated dosing for chronic cancer pain. Am J Hosp Palliat Care. 2006;23:229–235. doi: 10.1177/1049909106289068. [DOI] [PubMed] [Google Scholar]
- Guida F, Luongo L, Aviello G, Palazzo E, De Chiaro M, Gatta L, Boccella S, Marabese I, Zjawiony JK, Capasso R, Izzo AA, de Novellis V, Maione S. Salvinorin A reduces mechanical allodynia and spinal neuronal hyperexcitability induced by peripheral formalin injection. Mol Pain. 2012;8:60. doi: 10.1186/1744-8069-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes FA, Walters RS, Theriault RL, Buzdar AU, Frye DK, Hortobagyi GN, Forman AD, Newton LK, Raber MN. Phase II Trial of Taxol, an Active Drug in the Treatment of Metastatic Breast Cancer. J Natl Cancer Inst. 1991;83:1797–1805. doi: 10.1093/jnci/83.24.1797-a. [DOI] [PubMed] [Google Scholar]
- Hooker JM, Xu Y, Schiffer W, Shea C, Carter P, Fowler JS. Pharmacokinetics of the potent hallucinogen, salvinorin A in primates parallels the rapid onset and short duration of effects in humans. NeuroImage. 2008;41:1044–1050. doi: 10.1016/j.neuroimage.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadarola MJ, Brady LS, Draisci G, Dubner R. Enhancement of dynorphin gene expression in spinal cord following experimental inflammation: stimulus specificity, behavioral parameters and opioid receptor binding. Pain. 1988;35:313–326. doi: 10.1016/0304-3959(88)90141-8. [DOI] [PubMed] [Google Scholar]
- John TF, French LG, Erlichman JS. The antinociceptive effect of Salvinorin A in mice. Eur J Pharmacol. 2006;545:129–133. doi: 10.1016/j.ejphar.2006.06.077. [DOI] [PubMed] [Google Scholar]
- Kivell B, Prisinzano TE. Kappa opioids and the modulation of pain. Psychopharmacology. 2010;210:109–119. doi: 10.1007/s00213-010-1819-6. [DOI] [PubMed] [Google Scholar]
- Ko MC, Husbands SM. Effects of atypical kappa-opioid receptor agonists on intrathecal morphine-induced itch and analgesia in primates. J Pharmacol Exp Ther. 2009;328:193–200. doi: 10.1124/jpet.108.143925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn BM. Prescription drug abuse rises globally. JAMA. 2007;297:1306–1306. doi: 10.1001/jama.297.12.1306. [DOI] [PubMed] [Google Scholar]
- Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: a Phase III, randomized, double-blind, placebo-controlled study. Nephrol Dial Transplant. 2010;25:1251–1257. doi: 10.1093/ndt/gfp588. [DOI] [PubMed] [Google Scholar]
- Lamb K, Tidgewell K, Simpson DS, Bohn LM, Prisinzano TE. Antinociceptive effects of herkinorin, a MOP receptor agonist derived from salvinorin A in the formalin test in rats: New concepts in mu opioid receptor pharmacology: From a symposium on new concepts in mu-opioid pharmacology. Drug Alcohol Depend. 2012;121:181–188. doi: 10.1016/j.drugalcdep.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law PY, Reggio PH, Loh HH. Opioid receptors: toward separation of analgesic from undesirable effects. Trends Biochem Sci. 2013;38:275–282. doi: 10.1016/j.tibs.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong AG, Herst PM, Harper JL. Indigenous New Zealand honeys exhibit multiple anti-inflammatory activities. Innate Immun. 2012;18:459–466. doi: 10.1177/1753425911422263. [DOI] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- McCurdy CR, Sufka KJ, Smith GH, Warnick JE, Nieto MJ. Antinociceptive profile of salvinorin A, a structurally unique kappa opioid receptor agonist. Pharmacol Biochem Behav. 2006;83:109–113. doi: 10.1016/j.pbb.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Morani AS, Kivell B, Prisinzano TE, Schenk S. Effect of kappa-opioid receptor agonists U69593, U50488H, spiradoline and salvinorin A on cocaine-induced drug-seeking in rats. Pharmacol Biochem Behav. 2009;94:244–249. doi: 10.1016/j.pbb.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morani AS, Schenk S, Prisinzano TE, Kivell B. Single injection of novel kappa opioid receptor agonist salvinorin A attenuates expression of cocaine induced behavioral sensitization in rats. Behav Pharmacol. 2012;23:162. doi: 10.1097/FBP.0b013e3283512c1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao K, Hirakata M, Miyamoto Y, Kainoh M, Wakasa Y, Yanagita T. Nalfurafine hydrochloride, a selective κ opioid receptor agonist, has no reinforcing effect on intravenous self-administration in rhesus monkeys. J Pharmacol Sci. 2016;130:8–14. doi: 10.1016/j.jphs.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Pascual D, Goicoechea C, Burgos E, Martín MI. Antinociceptive effect of three common analgesic drugs on peripheral neuropathy induced by paclitaxel in rats. Pharmacol Biochem Behav. 2010;95:331–337. doi: 10.1016/j.pbb.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Porreca F, Mosberg HI, Hurst R, Hruby VJ, Burks TF. Roles of mu, delta and kappa opioid receptors in spinal and supraspinal mediation of gastrointestinal transit effects and hot-plate analgesia in the mouse. J Pharmacol Exp Ther. 1984;230:341–348. [PubMed] [Google Scholar]
- Prevatt-Smith KM, Lovell KM, Simpson DS, Day VW, Douglas JT, Bosch P, Dersch CM, Rothman RB, Kivell B, Prisinzano TE. Potential Drug Abuse Therapeutics Derived from the Hallucinogenic Natural Product Salvinorin A. Medchemcomm. 2011;2:1217–1222. doi: 10.1039/C1MD00192B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisinzano TE. Psychopharmacology of the hallucinogenic sage Salvia divinorum. Life Sci. 2005;78:527–531. doi: 10.1016/j.lfs.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Rahn EJ, Zvonok AM, Thakur GA, Khanolkar AD, Makriyannis A, Hohmann AG. Selective activation of cannabinoid CB2 receptors suppresses neuropathic nociception induced by treatment with the chemotherapeutic agent paclitaxel in rats. J Pharmacol Exp Ther. 2008;327:584–591. doi: 10.1124/jpet.108.141994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan M, Schnakenberg A, Skosnik PD, Cohen BM, Pittman B, Sewell RA, D’Souza DC. Dose-related behavioral, subjective, endocrine, and psychophysiological effects of the kappa opioid agonist Salvinorin A in humans. Biol Psychiatry. 2012;72:871–879. doi: 10.1016/j.biopsych.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Pace S, Tedesco F, Pagano E, Guerra G, Troisi F, Werner M, Roviezzo F, Zjawiony JK, Werz O, Izzo AA, Capasso R. The hallucinogenic diterpene salvinorin A inhibits leukotriene synthesis in experimental models of inflammation. Pharmacol Res. 2016;106:64–71. doi: 10.1016/j.phrs.2016.01.032. [DOI] [PubMed] [Google Scholar]
- Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. Salvinorin A: A potent naturally occurring nonnitrogenous κ opioid selective agonist. PNAS. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowinsky EK, Chaudhry V, Forastiere AA, Sartorius SE, Ettinger DS, Grochow LB, Lubejko BG, Cornblath DR, Donehower RC. Phase I and pharmacologic study of paclitaxel and cisplatin with granulocyte colony-stimulating factor: neuromuscular toxicity is dose-limiting. J Clin Oncol. 1993;11:2010–2020. doi: 10.1200/JCO.1993.11.10.2010. [DOI] [PubMed] [Google Scholar]
- Seki T, Awamura S, Kimura C, Ide S, Sakano K, Minami M, Nagase H, Satoh M. Pharmacological properties of TRK-820 on cloned μ-, δ- and κ-opioid receptors and nociceptin receptor. Eur J Pharmacol. 1999;376:159–167. doi: 10.1016/s0014-2999(99)00369-6. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A. Place preference conditioning reveals the involvement of D1-dopamine receptors in the motivational properties of mu- and kappa-opioid agonists. Brain Res. 1987;436:169–172. doi: 10.1016/0006-8993(87)91571-x. [DOI] [PubMed] [Google Scholar]
- Siegal HA, Carlson RG, Kenne DR, Swora MG. Probable relationship between opioid abuse and heroin use. Am Fam Physician. 2003;67:942, 945. [PubMed] [Google Scholar]
- Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, Le Meur M, Roques BP, Maldonado R, Kieffer BL. Disruption of the κ-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective κ-agonist U-50,488H and attenuates morphine withdrawal. The EMBO Journal. 1998;17:886–897. doi: 10.1093/emboj/17.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson B, Morani AS, Ewald AWM, Walker L, Kumar N, Simpson D, Miller JH, Prisinzano TE, Kivell BM. Pharmacology and anti-addiction effects of the novel κ opioid receptor agonist Mesyl Sal B, a potent and long-acting analogue of salvinorin A. Br J Pharmacol. 2014;172:515–531. doi: 10.1111/bph.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Sauver JL, Warner DO, Yawn BP, Jacobson DJ, McGree ME, Pankratz JJ, Melton LJ, 3rd, Roger VL, Ebbert JO, Rocca WA. Why patients visit their doctors: assessing the most prevalent conditions in a defined American population. Mayo Clin Proc. 2013;88:56–67. doi: 10.1016/j.mayocp.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teksin ZS, Lee IJ, Nemieboka NN, Othman AA, Upreti VV, Hassan HE, Syed SS, Prisinzano TE, Eddington ND. Evaluation of the transport, in vitro metabolism and pharmacokinetics of Salvinorin A, a potent hallucinogen. Eur J Pharm Biopharm. 2009;72:471–477. doi: 10.1016/j.ejpb.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara-Ohsumi Y, Tsuji F, Niwa M, Hata T, Narita M, Suzuki T, Sasano M, Aono H. The kappa opioid receptor agonist SA14867 has antinociceptive and weak sedative effects in models of acute and chronic pain. Eur J Pharmacol. 2011;671:53–60. doi: 10.1016/j.ejphar.2011.09.169. [DOI] [PubMed] [Google Scholar]
- Ueno Y, Mori A, Yanagita T. One year long-term study on abuse liability of nalfurafine in hemodialysis patients. Int J Clin Pharmacol Ther. 2013;51:823–831. doi: 10.5414/CP201852. [DOI] [PubMed] [Google Scholar]
- Valdés LJ. Salvia divinorum and the unique diterpene hallucinogen, Salvinorin (divinorin) A. J Psychoactive Drugs. 1994;26:277–283. doi: 10.1080/02791072.1994.10472441. [DOI] [PubMed] [Google Scholar]
- Valdés LJ, Díaz J, Paul AG. Ethnopharmacology of ska María Pastora (Salvia divinorum, Epling and Játiva-M.) J Ethnopharmacol. 1983;7:287–312. doi: 10.1016/0378-8741(83)90004-1. [DOI] [PubMed] [Google Scholar]
- van den Bent MJ, van Raaij-van den Aarssen VJ, Verweij J, Doorn PA, Sillevis Smitt PA. Progression of paclitaxel-induced neuropathy following discontinuation of treatment. Muscle Nerve. 1997;20:750–752. doi: 10.1002/(sici)1097-4598(199706)20:6<750::aid-mus15>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Váradi A, Marrone GF, Eans SO, Ganno ML, Subrath JJ, Le Rouzic V, Hunkele A, Pasternak GW, McLaughlin JP, Majumdar S. Synthesis and Characterization of a Dual Kappa-Delta Opioid Receptor Agonist Analgesic Blocking Cocaine Reward Behavior. ACS Chem Neurosci. 2015;6:1813–1824. doi: 10.1021/acschemneuro.5b00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasson RG. A new Mexican psychotropic drug from the mint family. Bot Mus Leafl. 1962;20:77–84. [Google Scholar]
- White KL, Robinson JE, Zhu H, DiBerto JF, Polepally PR, Zjawiony JK, Nichols DE, Malanga CJ, Roth BL. The G protein-biased kappa-opioid receptor agonist RB-64 is analgesic with a unique spectrum of activities in vivo. J Pharmacol Exp Ther. 2015;352:98–109. doi: 10.1124/jpet.114.216820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JL, Nayanar V, Walker JS. The site of anti-arthritic action of the kappa-opioid, U-50, 488H, in adjuvant arthritis: importance of local administration. Br J Pharmacol. 1996;118:1754–1760. doi: 10.1111/j.1476-5381.1996.tb15601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LS, Wang J, Chen JC, Tao YM, Wang YH, Xu XJ, Chen J, Xu YG, Xi T, Hu XW, Wang YJ, Liu JG. Novel kappa-opioid receptor agonist MB-1C-OH produces potent analgesia with less depression and sedation. Acta Pharmacol Sin. 2015;36:565–571. doi: 10.1038/aps.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]