Abstract
We have previously reported that premenopausal women with idiopathic osteoporosis (IOP) have profound microarchitectural deficiencies and heterogeneous bone remodeling. Those with the lowest bone formation rate have higher baseline serum IGF-1 levels and less robust response to teriparatide. Because IGF-1 stimulates bone formation and is critical for teriparatide action on osteoblasts, these findings suggest a state of IGF-1 resistance in some IOP women. To further investigate the hypothesis that osteoblast and IGF-1 related mechanisms mediate differential responsiveness to teriparatide in IOP, we studied circulating osteoblast progenitor (COP) cells and their IGF-1 receptor (IGF-1R) expression.
In premenopausal women with IOP, peripheral blood mononuclear cells (PBMCs) were obtained at baseline (n=25) and over 24 months of teriparatide treatment (n=11). Flow cytometry was used to identify and quantify COPs (non-hematopoetic lineage cells expressing osteocalcin and Runx2) and to quantify IGF-1R expression levels.
At baseline, both the percent of PBMCs that were COPs (%COP) and COP cell-surface IGF-1R expression correlated directly with several histomorphometric indices of bone formation in tetracycline-labeled transiliac biopsies. In treated subjects, both %COP and IGF-1R expression increased promptly after teriparatide, returning toward baseline by 18 months. While neither baseline %COP nor increase in %COP after three months predicted the BMD response to teriparatide, the percent increase in IGF-1R expression on COPs at 3 months correlated directly with the BMD response to teriparatide. Additionally, lower IGF-1R expression after teriparatide was associated with higher body fat, suggesting links between teriparatide resistance, body composition and the GH/IGF-1 axis.
In conclusion, these assays may be useful to characterize bone remodeling noninvasively, and may serve to predict early response to teriparatide, and possibly other bone formation stimulating medications. These new tools may also have utility in the mechanistic investigation of teriparatide resistance in premenopausal IOP, and perhaps in other populations.
Keywords: DXA, bone histomorphometry, osteoblasts, premenopausal osteoporosis, IGF-1
INTRODUCTION
Idiopathic osteoporosis (IOP) is an uncommon disorder that affects young, otherwise healthy individuals with intact gonadal function and no secondary cause of bone loss1. Using high resolution peripheral quantitative CT (HR-pQCT) of the distal radius and tibia2, central quantitative CT of the lumbar spine and proximal femur3 and microCT of iliac bone biopsies (obtained in 64 subjects and 40 controls)4, we reported that, compared to healthy premenopausal controls, premenopausal women with IOP have substantial microstructural deficits at both central and peripheral skeletal sites: thinner cortices; fewer, thinner, more widely separated and heterogeneously distributed trabeculae; and reduced bone stiffness by finite element analysis2–4. Bone remodeling, whether based upon serum bone turnover markers or tetracycline-based dynamic histomorphometry, was quite heterogeneous. However, compared to subjects in the highest tertile of bone remodeling as assessed by bone formation rate (BFR/BS), those in the lowest tertile had significantly higher serum insulin-like growth factor-1 (IGF-1) concentrations, lower mean wall thickness and trabecular bone volume fraction (BV/TV) and more disrupted trabecular microarchitecture. These observations suggest that women with IOP and low bone remodeling synthesize less bone matrix per remodeling site, and that osteoblast dysfunction, possibly due to resistance to IGF-1, may be involved in the pathogenesis4.
In 2008, we initiated an open-label, pilot study of teriparatide 20 μg daily in 21 premenopausal women with IOP. On average, we observed impressive 6–11% mean gains in areal BMD (aBMD) by dual energy X-ray absorptiometry (DXA)5. Paired transiliac bone biopsies performed before and after 18 months of treatment in 19 participants revealed marked improvements in trabecular structure and stiffness5. However, we also observed variability in response to teriparatide, with four of the 21 participants (19%) having no significant increase in aBMD at any site. BMD Non-Responders had 5-fold lower bone formation rate on pre-treatment biopsies and significantly (30%) higher baseline serum IGF-15. In addition, baseline serum IGF-1 was inversely associated with 12-month increase in spine aBMD in the entire group (r=−0.50; p = 0.03)5. Since IGF-1 stimulates bone formation and is critical for teriparatide action on osteoblasts, these data led us to hypothesize that in some women with IOP, osteoblasts are resistant to IGF-1 and hence teriparatide.
To investigate this hypothesis, we used a recently characterized population of circulating cells with osteogenic potential6. These circulating osteoblast progenitor (COP) cells express osteoblast specific markers (osteocalcin and Runx2) and can be identified, quantified, and isolated from peripheral blood mononuclear cells via flow cytometry7–9. Several groups have shown that these cells exhibit osteoblast-like behavior: COPs form mineralized nodules in vitro, correlate with other indices of bone remodeling assessed in serum and by quantitative histomorphometry in several populations, increase with puberty and increase in response to parathyroid hormone7–12. We measured COPs and the expression of the IGF-1 receptor (IGF-1R) on the COP cell surface within both the cross sectional (baseline) and teriparatide-treated IOP groups.
METHODS
Participants: Premenopausal women with idiopathic osteoporosis (IOP)
Premenopausal women, aged 18–48, were recruited at Columbia University Medical Center (CUMC), New York, NY and Creighton University, Omaha, NE by advertisement, self- or physician referral as previously described in detail13. Included were women with a documented low-trauma fracture after age 18 (regardless of whether aBMD was low) as well as women with low aBMD by dual energy x-ray absorptiometry (DXA; T score≤−2.5 or Z score≤−2.0) at the spine or hip without history of adult low trauma fracture. Inclusion and exclusion criteria were previously reported13. We defined premenopausal status as regular menses off hormonal contraception and early follicular phase follicle stimulating hormone (FSH) levels <20 mIU/mL. Secondary causes of osteoporosis were excluded by detailed history, physical and biochemical evaluation13. All subjects provided written informed consent. The Institutional Review Boards of both institutions approved these studies.
We have previously conducted a cross sectional study to characterize bone microstructure and metabolism in premenopausal women with IOP (n=64)2–4. From that cross-sectional study, PBMC samples for COP studies were available in 25 of the original 64 participants; these 25 comprise the “cross-sectional” cohort for this study.
In 2008, we initiated an open-label, pilot study of teriparatide 20 μg daily in 21 premenopausal women with IOP5, who had previously participated in the cross-sectional study. From this longitudinal study of 21 women, PBMC samples for COP studies were available at baseline and follow up timepoints in 11 subjects. These 11 comprise the “longitudinal” teriparatide treated cohort for this study. Because all women in the longitudinal study had also participated in the cross sectional study, these 11 are also a subset of the 25 cross-sectional subjects.
Biochemical Assays
Fasting morning blood samples were collected during the early follicular phase of the menstrual cycle on the participant’s usual diet and supplements. Samples were stored at −80°C for batch analyses in a research laboratory. The following assays were used: 25-OHD, RIA (Diasorin, Stillwater, MN); intact PTH, RIA (Scantibodies, Santee, CA); N-terminal propeptides of procollagen type 1 (P1NP), (IDS, Scottsdale, AZ); C-telopeptide (CTx), ELISA (IDS, Scottsdale, AZ); Insulin-like growth factor 1 (IGF-1) was analyzed by radioimmunoassay (Alpco Diagnostics, Salem, NH) in the MECORE Laboratory, St. Josephs Hospital, Bangor, ME.
Areal BMD and body composition by DXA
Areal BMD at the lumbar spine, total hip, femoral neck and distal radius, and whole body (excluding head) and trunk fat were measured by DXA (Discovery, Hologic Inc., Walton, MA) at Columbia and Creighton University Medical Centers as previously described13.
Transiliac Bone Biopsy
After double-labeling with tetracycline, transiliac biopsy was performed using a Bordier-type trephine with an inner diameter of 7.5 mm14. The intact biopsy specimens were fixed and dehydrated in ethanol. Biopsy specimens were embedded in polymethylmethacrylate for quantitative histomorphometry, sectioned (7 μm and 20 μm) and stained (Goldner trichrome) according to established procedures15. Histomorphometry was performed with a digitizing image-analysis system (OsteoMeasure, Version 4.00C, OsteoMetrics, Inc, Atlanta, GA). All structural and remodeling variables were calculated according to American Society for Bone and Mineral Research recommendations16.
Quantification of peripheral blood osteogenic precursor (COP) cells
COP cells were analyzed by flow cytometry assay adapted from Khosla et al7,17. Briefly, frozen peripheral blood mononuclear cells (PBMCs) were thawed and immunostained for flow cytometry analysis.6–9 Cell number was assessed by Trypan blue exclusion method. PBMC (1×10^6 cells) were incubated with the FITC conjugated polyclonal anti-human osteocalcin (OCN) antibody (clone V19) (Santa Cruz Biotechnology, Santa Cruz, CA) and eFluor V450-conjugated anti–human lineage cocktail (LIN; from eBioscience, San Diego, CA) antibodies. For intracellular RUNX2 staining, cells were fixed and permeabilized using a kit (eBioscience, San Diego, CA). Next, cells were incubated with Alexa Fluor 647 conjugated anti-human Runx2 (Santa Cruz Biotechnology, Santa Cruz, CA). All antibody incubations were performed at 4°C for 1h. Acquisition and analysis were performed on the LSRII flow cytometer (Becton Dickinson, San Diego, CA) using FlowJo software (FloJo, LLC, Ashland, OR). Cells were gated for size, shape and granularity using forward and side scatter parameters. Hematopoietic lineage positive (LIN+) PBMCs were excluded prior to gating for specific osteogenic precursor populations: LIN−/OCN+/Runx2+. Isotype IgGs for each specific antibody were used as negative controls.
Surface IGF1R expression in COPs was measured by flow cytometry analysis using the PE-conjugated anti-IGF-1R antibody (CD221, BD Pharmingen, San Jose, CA) and calculating the Median Fluorescence Intensity (MFI) in COPs. An isotype IgG was used as a negative control to normalize the MFI and to ensure the specificity of antibody binding.
Our studies were performed on frozen and thawed PBMCs. In a separate small control cohort, we have shown that there is no significant difference in the measured parameters between fresh and frozen samples from the same subjects (see Supplemental Figure).
COP cell sample collection was initiated after the beginning of study enrollment for the longitudinal study of teriparatide. Only those subjects with available samples at baseline are included in these analyses.
Statistical Methods
Statistical analyses were performed using SAS software (SAS Institute, Cary NC, USA). Correlation analyses (Spearman) were used to describe relationships between variables in these populations. Multivariate linear regression analyses were used to control for covariates. All data are expressed as mean ± standard deviation (SD). Results were considered significant with p < 0.05.
RESULTS
COP cell samples and bone biopsies were available from 25 women from the cross-sectional study of IOP, of whom 11 participated in the longitudinal study of teriparatide for IOP. Characteristics of the study subjects are shown in Table 1.
TABLE 1.
SUBJECT CHARACTERISTICS
| Mean ± SD | Cross-sectional N=25 |
Longitudinal Subset (at Baseline) N=11/25 |
|---|---|---|
|
| ||
| Age (yrs) | 38 ± 7 | 38 ± 7 |
|
| ||
| BMI (kg/m2) | 21.8 ±3.2 | 20.9 ± 3.0 |
| DXA whole body fat (%) | 32 ± 6 | 32 ± 6 |
| DXA trunk fat (%) | 28 ± 8 | 29 ± 8 |
|
| ||
| BMD Z score Lumbar Spine | −1.8 ± 1.1 | −2.0 ± 0.6 |
| Total Hip | −1.3 ± 0.9 | −1.7 ± 0.5 |
| Femoral Neck | −1.7 ± 1.0 | −2.0 ± 0.5 |
|
| ||
| # of adult fractures | 1.5 ± 1.6 | 1.9 ± 1.9 |
|
| ||
| Bone Biopsy: Histomorphometry | ||
| BV/TV(%) | 20.3 ± 7.4 | 21.5 ± 7.9 |
| Wall Width (μm) | 34.2 ± 3.8 | 34.6 ± 4.8 |
| Mineralized Perimeter (%) | 4.0 ± 2.0 | 3.8 ± 2.2 |
| Mineral Apposition Rate (MAR: μm/day) | 0.62 ± 0.12 | 0.65 ± 0.14 |
| Bone Formation Rate (BFR: mm2/mm/yr) | 0.009 ± 0.006 | 0.009 ± 0.006 |
| Osteoblast Number (#/mm) | 1.1 ± 0.5 | 1.1 ± 0.5 |
|
| ||
| Biochemistries | ||
| Serum Calcium (albumin corrected; mg/dL) | 9.0 ± 0.3 | 9.0 ± 0.3 |
| PTH (pg/mL) | 30 ± 14 | 37 ± 13 |
| 25(OH) Vitamin D (ng/mL) | 41 ± 13 | 44 ± 14 |
| IGF-1 (ng/mL) | 175 ± 43 | 169 ± 47 |
| Serum CTX (ng/mL) | 0.346 ± 0.114 | 0.366 ± 0.128 |
| Serum P1NP (μg/L) | 48 ± 16 | 48 ± 12 |
|
| ||
| Peripheral Cell Studies/Flow Cytometry | ||
| Percentage COP cells (% LIN−/OCN+/Runx2+ of PBMCs) | 20.8 ± 11.3 | 22.1 ± 12.2 |
| IGF-1 Receptor Expression on COPs (MFI) | 1129 ± 594 | 1173 ± 730 |
Peripheral COP Cell Measures Correlate with Biopsy Measures in the Cross-Sectional Cohort
The percentage of PBMCs identified as COP cells (%COPs) correlated well with biopsy-based dynamic indices of bone remodeling (Table 2). %COP correlated most strongly with mineral apposition rate. IGF-1R expression on COPs correlated most strongly with mineralized perimeter and bone formation rate (Table 2). Trends were seen for correlations between serum CTX and biopsy parameters, but results were not significant (Table 2). No significant correlations were seen between %COP/IGF-1R expression and serum CTX, PINP or IGF-1 (p =0.2–0.9). Both %COP and IGF-1R expression were directly related to trabecular bone volume fraction, though the relationships were not significant (p=0.06–0.1).
Table 2.
Spearman Correlations; r,p With Body Composition and Bone Biopsy Measures In 25 IOP women
| %COP | IGF-1R MFI | CTX | PINP | Serum IGF-1 | |
|---|---|---|---|---|---|
|
| |||||
| Age | −0.11, 0.6 | 0.15, 0.5 | −0.30, 0.2 | −0.17, 0.4 | −0.60, 0.003 |
|
| |||||
| BMI | −0.23, 0.3 | −0.30, 0.1 | −0.03, 0.9 | 0.11, 0.6 | −0.22, 0.3 |
|
| |||||
| Whole body fat by DXA | −0.18, 0.4 | −0.44, 0.03 | −0.44, 0.04 | −0.11, 0.6 | −0.18, 0.4 |
|
| |||||
| Trunk fat by DXA | −0.26, 0.2 | −0.42, 0.04 | −0.39, 0.07 | −0.07, 0.8 | −0.18, 0.42 |
|
| |||||
| BMD by DXA (g/cm2) | |||||
| Lumbar Spine | −0.31, 0.14 | −0.40, 0.05 | −0.08, 0.7 | 0.17, 0.4 | −0.31, 0.2 |
| Total Hip | 0.07, 0.7 | −0.23, 0.3 | 0.16, 0.5 | 0.04, 0.9 | 0.15, 0.5 |
| Femoral Neck | −0.07, 0.7 | −0.22, 0.3 | 0.16, 0.5 | 0.14, 0.5 | 0.10, 0.65 |
|
| |||||
| Transiliac Crest Bone Biopsies: | |||||
|
| |||||
| BV/TV | 0.34, 0.10 | 0.38, 0.06 | 0.28, 0.2 | −0.16, 0.5 | −0.08, 0.7 |
|
| |||||
| Cancellous Wall Width (μm) | 0.51, 0.01 | 0.16, 0.4 | 0.10, 0.7 | −0.17, 0.4 | −0.05, 0.8 |
|
| |||||
| Mineralized Perimeter (%) | 0.21, 0.3 | 0.62, 0.0009 | 0.38, 0.08 | 0.13, 0.5 | −0.24, 0.3 |
|
| |||||
| Mineral Apposition Rate (MAR: μm/day) | 0.75, <0.0001 | 0.21, 0.3 | 0.27, 0.22 | 0.01, 0.9 | −0.05, 0.8 |
|
| |||||
| Bone Formation Rate (BFR: mm2/mm/yr) | 0.41, 0.04 | 0.57, 0.003 | 0.43, 0.05 | 0.08, 0.7 | −0.09, 0.7 |
|
| |||||
| Osteoblast Number (#/mm) | 0.01, 0.9 | 0.28, 0.2 | −0.02, 0.9 | 0.01, 0.9 | −0.37, 0.09 |
We have previously categorized bone turnover status (low, middle, high) in IOP women based on bone formation rate (BFR) on bone biopsy at baseline4. Among the 25 subjects included here, 6 had been categorized as low turnover (BFR: 0.002 ± 0.001 mm2/mm/yr), 10 as middle turnover (BFR: 0.008 ± 0.001 mm2/mm/yr), and 9 as high turnover (BFR: 0.015 ± 0.006 mm2/mm/yr). As we have previously reported4, serum IGF-1 levels were highest in the low turnover subjects (Figure 1). Conversely, both %COP and IGF-1R expression were significantly lower among those categorized as low turnover compared to the high turnover group (Figure 1).
Figure 1.
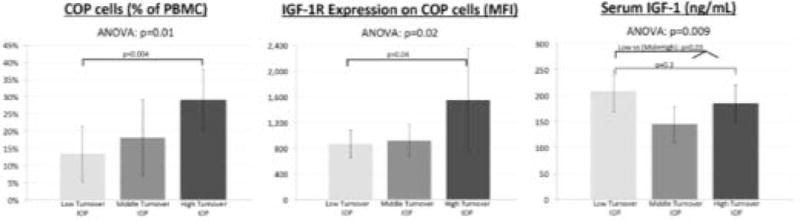
%COP, IGF-1R expression, and serum IGF-1 differ among IOP bone turnover groups defined by BFR on bone biopsy
Correlation with body fat
At baseline, %COP did not correlate with BMI or body fat. In contrast, higher body fat and trunk fat were significantly associated with lower IGF-1R expression (Table 2).
Correlation with aBMD response to teriparatide
Of the 25 subjects included in the cross-sectional cohort, 11 also participated in a longitudinal study of teriparatide. In this group, BMD increased substantially in response to teriparatide - by 12.9 ± 5.7% at the spine and 6.9 ± 4.6% at the femoral neck. As we had observed in the entire treatment group of 21 subjects, response was quite variable and one of the 11 was previously categorized as a non-responder (lumbar spine % change < 3%)5.
COP cell samples were obtained at baseline, 1 month, 3 months and 18 months after treatment. %COP increased with teriparatide (Figure 2), and was 221% above baseline by 3 months (p=0.02). Similarly, IGF-1R expression (MFI) also increased at 3 months by 128% (p<0.009). Changes in bone turnover markers occurred as expected and have been previously described5: serum PINP doubled by 1 month, peaked at 6 months (150% above baseline), and returned to baseline by 18–24 months. Serum IGF-1 did not change in response to teriparatide.
Figure 2.
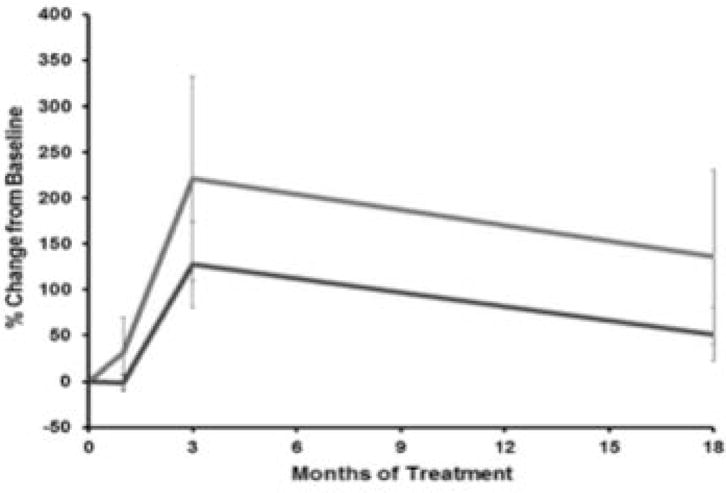
%COP cells (grey line) increased with teriparatide, peaking at 3 months by 221% (p=0.02). IGF-1R expression (black line, lower line) also increased, peaking at 3 months by 128% (p=0.009)
Higher baseline BMI, body fat and trunk fat strongly and significantly predicted a lower increase in IGF-1R expression in response to teriparatide (Table 3).
TABLE 3.
Spearman Correlations; r,p
| %COP: 3M %change from baseline | IGF-1R MFI: 3M %change from baseline | |
|---|---|---|
| Age | 0.04, 0.9 | −0.04, 0.9 |
| BMI | −0.15, 0.7 | −0.80, 0.003 |
| Whole body fat by DXA | −0.44, 0.2 | −0.90, 0.0002 |
| Trunk fat by DXA | −0.55, 0.08 | −0.87, 0.0005 |
| DXA aBMD %change vs baseline in IOP women on TPTD: | ||
| LS BMD: 6M | 0.52, 0.1 | 0.70, 0.02 |
| LS BMD: 12M | 0.40, 0.2 | 0.55, 0.08 |
| LS BMD: 24M | 0.21, 0.6 | 0.48, 0.2 |
| FN BMD: 6M | 0.21, 0.5 | 0.67, 0.02 |
| FN BMD: 12M | 0.10, 0.8 | 0.49, 0.1 |
| FN BMD: 24M | −0.29, 0.5 | −0.50, 0.2 |
All % change variables are corrected for baseline values.
Baseline %COP and the increase in %COP at 3 months did not predict spine or hip DXA BMD response to teriparatide (Table 3). In contrast, the percent increase in the IGF-1R expression (MFI) on COP cells at 3 months correlated directly with early aBMD response to teriparatide (Table 3). Changes in IGF-1R expression (MFI) in response to teriparatide in 2 subjects, a responder and a non-responder, are shown in Figure 3. This Non-Responder also had higher body fat, lower baseline BFR, and higher baseline serum IGF-1 than the Responder.
Figure 3.
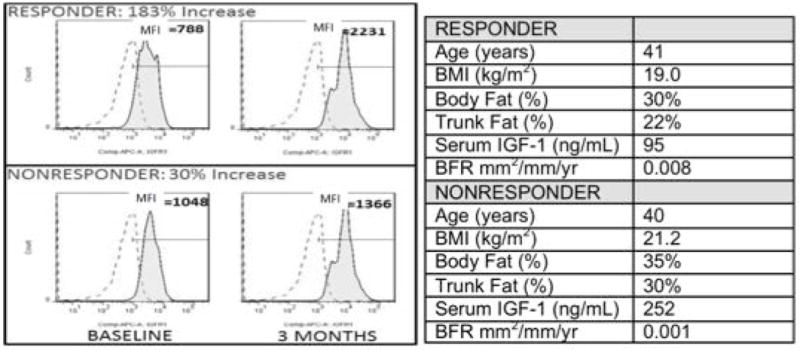
Figure shows reference standard and IGF-1R expression: 3 month increase in IGF-1R expression in a teriparatide responder (183% increase) and a nonresponder (30% increase).
DISCUSSION
In this study, we used flow cytometry to identify and quantify circulating osteoblast progenitor (COP) cells among peripheral blood mononuclear cells (PBMCs) and to quantify expression of the IGF-1 receptor (IGF-1R) on the COP cell surface in premenopausal women with IOP. We report that both %COP cells and expression of IGF-1R on the COP cell-surface correlated with tissue-level bone formation assessed on transiliac bone biopsies. Moreover, both %COP and IGF-1R expression increased promptly after teriparatide, returning toward baseline by 18 months. While neither baseline %COP nor increase in %COP at three months predicted the BMD response to teriparatide, the percent increase in IGF-1R expression on COPs at 3 months correlated directly with the BMD response to teriparatide at both the lumbar spine and the femoral neck.
Our results in premenopausal women with IOP are consistent with our earlier findings that suggest that COP cells reflect bone formation at the tissue level. Specifically, we have shown that COP cells correlate directly with histomorphometric parameters of bone formation in two other diseases, both characterized by low bone remodeling activity: hypoparathyroidism9 and Type 2 diabetes18. Moreover, we have also shown that COP cells change in response to PTH therapy. Specifically, COP cells increased markedly in patients with hypoparathyroidism who were treated with PTH(1-84) and correlated with increases in bone formation on biopsies in response to this osteo-anabolic drug9. We have also shown that COP cells sorted from PBMCs form mineralized nodules in culture and express osteoblast gene markers such as Runx2, a key transcriptional factor that initiates osteogenesis and promotes bone mineralization8,9. Our results in premenopausal women with IOP provide further confirmation that COP cells reflect tissue-level bone formation status in several populations.
Our data contrast somewhat with a prior study of osteoblast precursor cell response to teriparatide in 15 post-menopausal women with osteoporosis19. In these postmenopausal subjects, osteoblast precursor cells did not increase in response to teriparatide, but did increase their expression of both osteocalcin and alkaline phosphatase (AP), suggesting increased maturation in response to the medication. The difference from our results may be due to baseline differences in precursor quantity in a postmenopausal population, or due to the different definition of precursor cells – defined as CD15-/OC+/AP+ in these postmenopausal studies.
We examined the IGF-1R on the COP cells because several previous observations suggested to us that IGF-1 may be involved in the pathogenesis of premenopausal IOP. Although tissue-level bone remodeling activity varied substantially in our biopsy study of 64 premenopausal women with IOP2,4,13, a subset had profoundly low cancellous BFR and significantly lower wall width of completed bone modeling units, suggesting decreased osteoblast productivity4. In this and the prior analysis4, women with premenopausal IOP and low BFR had significantly higher serum IGF-1 than women with normal or high BFR. A U-shaped relationship was observed; both low and high turnover subjects had higher IGF-1. We hypothesize that the high IGF-1 in the low turnover group represents a pathologic deviation from the normal direct relationship between IGF-1 and bone turnover status. This is in contrast to men with IOP, who typically have low bone turnover and lower IGF-120,21. In addition, the four women who had no increase in BMD in response to teriparatide in our pilot study (n=21) had markedly lower bone formation rate on pre-treatment biopsies compared to the teriparatide responders (0.002 ± 0.001 vs 0.011 ± 0.006 mm2/mm/y; p < 0.001) and significantly higher serum IGF-1 (208 ± 54 vs 157 ± 44 ng/mL; p=0.03)5. Higher serum IGF-1 (and BMI) also predicted lower BMD response to teriparatide5. Interestingly, we found that expression of IGF-1R on COP cells exhibited relationships opposite to serum IGF-1. Lower expression of IGF-1R on COP cells and higher serum IGF-1 were associated with higher body fat and lower biopsy-based bone formation. Additionally, lower IGF-1R expression in response to teriparatide was associated with higher body fat and also predicted lower BMD response. The relationship between teriparatide response and IGF-1 action may also be supported by studies showing effects of teriparatide treatment on body composition and glucose homeostasis22–24. Our findings suggest that the amount of IGF-1R on COP cells and, presumably, osteoblasts (and potentially in other tissues) may reflect IGF-1 resistance downstream from the IGF-1R. To investigate further the possibility that IGF-1 resistance contributes to the pathogenesis of low turnover IOP, we are conducting studies to determine whether there may be evidence of IGF-1 resistance in tissues other than bone in the context of a randomized clinical trial of teriparatide in premenopausal IOP (NCT 01440803).
In the majority of patients, teriparatide is associated with a rapid rise in bone formation markers25 followed by a later rise in bone resorption markers26 and marked increases in BMD. Teriparatide is also very effective in reducing fractures, particularly vertebral fractures. However, the response to teriparatide is quite variable: from 5% to 20% of patients have minimal increases in BMD5,27–29. To date, few clinical factors have been found to predict response to teriparatide. Since teriparatide is an expensive, injectable medication, there is a need for validated predictors of response and deeper understanding of the mechanisms of nonresponse. PTH/teriparatide treatment is known to be less effective in states of low bone turnover30,31. In postmenopausal women, baseline serum resorption and formation markers26,27,32, and early changes in formation markers correlate directly with BMD response to teriparatide26,27,29,32,33. Using data from the Fracture Prevention Trial that included over 1500 postmenopausal women, Chen et al. reported that increases in bone formation markers (PICP [C-terminal propeptides of procollagen type I] at 1 month and PINP at 3 months) correlated best with increases in lumbar spine BMD at 18 months (r=0.65 and 0.61, respectively; p < 0.05) and that the relationships with spine BMD were stronger than the relationship with femoral neck BMD response26. Similarly, in our prior study of 21 women with IOP, 1 month change in both osteocalcin and PINP predicted both 12 and 24 month increases in spine (r=0.50–0.58; p< 0.05), but not hip, BMD5. In the longitudinal component of this study (n=11), the relationships between 1 month change in osteocalcin or PINP and 12 month change in spine BMD were comparable (r=0.48–0.66; p=0.05–0.2; data not shown). Based on these new data, it appears that the early change in IGF-1R expression on COP cells is predictive of BMD response, similar to early changes in markers of bone formation, and could be studied further as a novel noninvasive and mechanistic predictor of response with potential clinical utility both in other osteoporotic populations and in studies of new medications that stimulate bone formation.
This study has several limitations. The data, while obtained prospectively, were from a small cohort of premenopausal Caucasian women with a rare form of unexplained osteoporosis that presented before menopause and the teriparatide data were from an open-label, non-randomized clinical trial, limiting the generalizability of the results. PMBCs were obtained at few time-points and their trajectory over the entire course of teriparatide treatment is unknown. There remains debate concerning the true identity of COP cells, and whether the LIN−/OCN+/Runx2+ subset truly represents cells that can travel to bone and contribute to bone remodeling6,8,12. This study does not prove that these cells themselves travel to the bone and form osteoblasts on bone tissue, but it does suggest that these cells reflect tissue-level osteoblast activity and osteoblast response to a potent osteo-anabolic drug.
In conclusion, we found that %COP cells and IGF-1R expression on COP cells reflect tissue level bone formation in premenopausal women with idiopathic osteoporosis, as they do in other conditions such as hypoparathyroidism and Type 2 diabetes. We also found that the early increase in IGF-1R expression on COPs may predict the BMD response to teriparatide. These assays may be useful to characterize bone remodeling noninvasively. Future studies are needed to assess whether measurement of IGF-1R expression on COPs could serve as an early predictor of response to teriparatide and a new tool in the investigation of teriparatide resistance in premenopausal idiopathic osteoporosis, and perhaps other populations such as patients with postmenopausal or glucocorticoid-induced osteoporosis.
Supplementary Material
Supplemental Figure: Assessments of COP cells and COP cell expression of IGF-1R, osteocalcin and RUNX2 demonstrated no significant differences between fresh and frozen/thawed samples from the same control subjects (n=3).
Acknowledgments
The cross sectional study was funded by the NIH: R01 AR049896 (E.S.), K24 AR 05266 (E.S.) and K23 AR054127 (A.C.).
The treatment study was investigator-initiated. The principal investigators designed and conducted these studies. Eli Lilly, USA, supplied teriparatide and provided financial support for the treatment study, which was registered with clinical trial registration number NCT01440803. Additional funding was provided by the NIH for ancillary studies to explore IGF-1 related mechanisms, R03 AR064016 (A.C.).
These studies were also supported by the Thomas L. Kempner, Jr. and Katheryn C. Patterson Foundation.
Statisticians and investigators at Columbia University Medical Center performed all analyses.
The content is solely the responsibility of the authors.
These studies were supported by the following NIH funding sources: R01 AR049896 (E.S.), K24 AR 05266 (E.S.) and K23 AR054127 (A.C.)
Footnotes
DISCLOSURE STATEMENT: AC, SK, BB, JGS, JL, JSM, DJM, MB, MK, and JS have nothing to declare. ES is the Principal Investigator of a grant from Eli Lilly to Columbia University that provided partial funding for this study. RRR and DWD have consulted for Eli Lilly and their total compensation exceeds $5000. DWD has received grant support from Eli Lilly.
References
- 1.Heshmati HM, Khosla S. Idiopathic osteoporosis: a heterogeneous entity. Ann Med Interne (Paris) 1998;149(2):77–81. [PubMed] [Google Scholar]
- 2.Cohen A, Liu XS, Stein EM, McMahon DJ, Rogers HF, Lemaster J, Recker RR, Lappe JM, Guo XE, Shane E. Bone microarchitecture and stiffness in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2009;94(11):4351–60. doi: 10.1210/jc.2009-0996. doi: jc.2009-0996 [pii] 10.1210/jc.2009-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen A, Lang TF, McMahon DJ, Liu XS, Guo XE, Zhang C, Stein EM, Dempster DW, Young P, Saeed I, Lappe JM, Recker RR, Shane E. Central QCT reveals lower volumetric BMD and stiffness in premenopausal women with idiopathic osteoporosis, regardless of fracture history. J Clin Endocrinol Metab. 2012;97(11):4244–52. doi: 10.1210/jc.2012-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen A, Dempster DW, Recker RR, Stein EM, Lappe JM, Zhou H, Wirth AJ, van Lenthe GH, Kohler T, Zwahlen A, Muller R, Rosen CJ, Cremers S, Nickolas TL, McMahon DJ, Rogers H, Staron RB, Lemaster J, Shane E. Abnormal Bone Microarchitecture and Evidence of Osteoblast Dysfunction in Premenopausal Women with Idiopathic Osteoporosis. J Clin Endocrinol Metab. 2011;96(10):3095. doi: 10.1210/jc.2011-1387. Epub 2011/08/13. doi: jc.2011-1387 [pii] 10.1210/jc.2011-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen A, Stein EM, Recker RR, Lappe JM, Dempster DW, Zhou H, Cremers S, McMahon DJ, Nickolas TL, Muller R, Zwahlen A, Young P, Stubby J, Shane E. Teriparatide for idiopathic osteoporosis in premenopausal women: a pilot study. J Clin Endocrinol Metab. 2013;98(5):1971–81. doi: 10.1210/jc.2013-1172. Epub 2013/04/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khosla S, Eghbali-Fatourechi GZ. Circulating cells with osteogenic potential. Ann N Y Acad Sci. 2006;1068:489–97. doi: 10.1196/annals.1346.022. doi: 1068/1/489 [pii] 10.1196/annals.1346.022. [DOI] [PubMed] [Google Scholar]
- 7.Eghbali-Fatourechi GZ, Lamsam J, Fraser D, Nagel D, Riggs BL, Khosla S. Circulating osteoblast-lineage cells in humans. N Engl J Med. 2005;352(19):1959–66. doi: 10.1056/NEJMoa044264. Epub 2005/05/13. doi: 352/19/1959 [pii] 10.1056/NEJMoa044264. [DOI] [PubMed] [Google Scholar]
- 8.Manavalan JS, Arpadi S, Tharmarajah S, Shah J, Zhang CA, Foca M, Neu N, Bell DL, Nishiyama KK, Kousteni S, Yin MT. Abnormal Bone Acquisition With Early-Life HIV Infection: Role of Immune Activation and Senescent Osteogenic Precursors. J Bone Miner Res. 2016 doi: 10.1002/jbmr.2883. Epub 2016/06/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin MR, Manavalan JS, Dempster DW, Shah J, Cremers S, Kousteni S, Zhou H, McMahon DJ, Kode A, Sliney J, Shane E, Silverberg SJ, Bilezikian JP. Parathyroid hormone stimulates circulating osteogenic cells in hypoparathyroidism. J Clin Endocrinol Metab. 2011;96(1):176–86. doi: 10.1210/jc.2009-2682. Epub 2010/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otsuru S, Tamai K, Yamazaki T, Yoshikawa H, Kaneda Y. Bone marrow-derived osteoblast progenitor cells in circulating blood contribute to ectopic bone formation in mice. Biochem Biophys Res Commun. 2007;354(2):453–8. doi: 10.1016/j.bbrc.2006.12.226. Epub 2007/01/24. [DOI] [PubMed] [Google Scholar]
- 11.Pignolo RJ, Kassem M. Circulating osteogenic cells: implications for injury, repair, and regeneration. J Bone Miner Res. 2011;26(8):1685–93. doi: 10.1002/jbmr.370. Epub 2011/05/04. [DOI] [PubMed] [Google Scholar]
- 12.Undale A, Srinivasan B, Drake M, McCready L, Atkinson E, Peterson J, Riggs BL, Amin S, Modder UI, Khosla S. Circulating osteogenic cells: characterization and relationship to rates of bone loss in postmenopausal women. Bone. 2010;47(1):83–92. doi: 10.1016/j.bone.2010.03.018. Epub 2010/04/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen A, Recker RR, Lappe J, Dempster DW, Cremers S, McMahon DJ, Stein EM, Fleischer J, Rosen CJ, Rogers H, Staron RB, Lemaster J, Shane E. Premenopausal women with idiopathic low-trauma fractures and/or low bone mineral density. Osteoporos Int. 2012;23(1):171–82. doi: 10.1007/s00198-011-1560-y. Epub 2011/03/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dempster D, Shane E. Bone quantification and dynamics of bone turnover. In: K B, editor. Principles and Practice of Endocrinology and Metabolism. Philadelphia, PA2002: pp. 475–9. [Google Scholar]
- 15.Cohen A, Dempster DW, Muller R, Guo XE, Nickolas TL, Liu XS, Zhang XH, Wirth AJ, van Lenthe GH, Kohler T, McMahon DJ, Zhou H, Rubin MR, Bilezikian JP, Lappe JM, Recker RR, Shane E. Assessment of trabecular and cortical architecture and mechanical competence of bone by high-resolution peripheral computed tomography: comparison with transiliac bone biopsy. Osteoporos Int. 2010;21(2):263–73. doi: 10.1007/s00198-009-0945-7. Epub 2009/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR, Parfitt AM. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28(1):2–17. doi: 10.1002/jbmr.1805. Epub 2012/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eghbali-Fatourechi GZ, Modder UI, Charatcharoenwitthaya N, Sanyal A, Undale AH, Clowes JA, Tarara JE, Khosla S. Characterization of circulating osteoblast lineage cells in humans. Bone. 2007;40(5):1370–7. doi: 10.1016/j.bone.2006.12.064. doi: S8756-3282(06)00952-5 [pii] 10.1016/j.bone.2006.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manavalan JS, Cremers S, Dempster DW, Zhou H, Dworakowski E, Kode A, Kousteni S, Rubin MR. Circulating osteogenic precursor cells in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97(9):3240–50. doi: 10.1210/jc.2012-1546. Epub 2012/06/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Amelio P, Tamone C, Sassi F, D’Amico L, Roato I, Patane S, Ravazzoli M, Veneziano L, Ferracini R, Pescarmona GP, Isaia GC. Teriparatide increases the maturation of circulating osteoblast precursors. Osteoporos Int. 2012;23(4):1245–53. doi: 10.1007/s00198-011-1666-2. Epub 2011/05/28. [DOI] [PubMed] [Google Scholar]
- 20.Kurland ES, Rosen CJ, Cosman F, McMahon D, Chan F, Shane E, Lindsay R, Dempster D, Bilezikian JP. Insulin-like growth factor-I in men with idiopathic osteoporosis. J Clin Endocrinol Metab. 1997;82(9):2799–805. doi: 10.1210/jcem.82.9.4253. [DOI] [PubMed] [Google Scholar]
- 21.Ljunghall S, Johansson AG, Burman P, Kampe O, Lindh E, Karlsson FA. Low plasma levels of insulin-like growth factor 1 (IGF-1) in male patients with idiopathic osteoporosis. J Intern Med. 1992;232(1):59–64. doi: 10.1111/j.1365-2796.1992.tb00550.x. [DOI] [PubMed] [Google Scholar]
- 22.Anastasilakis A, Goulis DG, Koukoulis G, Kita M, Slavakis A, Avramidis A. Acute and chronic effect of teriparatide on glucose metabolism in women with established osteoporosis. Experimental and clinical endocrinology & diabetes: official journal, German Society of Endocrinology [and] German Diabetes Association. 2007;115(2):108–11. doi: 10.1055/s-2007-967090. Epub 2007/02/24. [DOI] [PubMed] [Google Scholar]
- 23.Passeri E, Dozio E, Mendola M, Costa E, Bandera F, Corsi Romanelli MM, Corbetta S. Treatment with teriparatide might be associated with cardiometabolic changes in postmenopausal severe osteoporotic women. Journal of biological regulators and homeostatic agents. 2015;29(4):931–40. Epub 2016/01/13. [PubMed] [Google Scholar]
- 24.Yang Y, Luo X, Xie X, Yan F, Chen G, Zhao W, Jiang Z, Fang C, Shen J. Influences of teriparatide administration on marrow fat content in postmenopausal osteopenic women using MR spectroscopy. Climacteric: the journal of the International Menopause Society. 2016;19(3):285–91. doi: 10.3109/13697137.2015.1126576. Epub 2016/01/09. [DOI] [PubMed] [Google Scholar]
- 25.Glover SJ, Eastell R, McCloskey EV, Rogers A, Garnero P, Lowery J, Belleli R, Wright TM, John MR. Rapid and robust response of biochemical markers of bone formation to teriparatide therapy. Bone. 2009;45(6):1053–8. doi: 10.1016/j.bone.2009.07.091. Epub 2009/08/15. [DOI] [PubMed] [Google Scholar]
- 26.Chen P, Satterwhite JH, Licata AA, Lewiecki EM, Sipos AA, Misurski DM, Wagman RB. Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res. 2005;20(6):962–70. doi: 10.1359/JBMR.050105. Epub 2005/05/11. [DOI] [PubMed] [Google Scholar]
- 27.Heaney RP, Watson P. Variability in the measured response of bone to teriparatide. Osteoporos Int. 2011;22(6):1703–8. doi: 10.1007/s00198-010-1376-1. Epub 2010/09/10. [DOI] [PubMed] [Google Scholar]
- 28.Siddique N, Healy M, Chan CG, Fallon N, Walsh JB, Casey MC, editors. American Society for Bone and Mineral Research Annual Meeting. 2011. How to predict a suboptimal response to recombinant parathyroid hormone treatment in osteoporosis. [Google Scholar]
- 29.Gallagher JC, Rosen CJ, Chen P, Misurski DA, Marcus R. Response rate of bone mineral density to teriparatide in postmenopausal women with osteoporosis. Bone. 2006;39(6):1268–75. doi: 10.1016/j.bone.2006.06.007. Epub 2006/08/04. doi: S8756-3282(06)00568-0 [pii] 10.1016/j.bone.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Delmas PD, Licata AA, Reginster JY, Crans GG, Chen P, Misurski DA, Wagman RB, Mitlak BH. Fracture risk reduction during treatment with teriparatide is independent of pretreatment bone turnover. Bone. 2006;39(2):237–43. doi: 10.1016/j.bone.2006.02.003. doi: S8756-3282(06)00260-2 [pii] 10.1016/j.bone.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Khosla S. Parathyroid hormone plus alendronate–a combination that does not add up. N Engl J Med. 2003;349(13):1277–9. doi: 10.1056/NEJMe038143. Epub 2003/09/23. [DOI] [PubMed] [Google Scholar]
- 32.Miller PD, Delmas PD, Lindsay R, Watts NB, Luckey M, Adachi J, Saag K, Greenspan SL, Seeman E, Boonen S, Meeves S, Lang TF, Bilezikian JP. Open-label Study to Determine How Prior Therapy with Alendronate or Risedronate in Postmenopausal Women with Osteoporosis Influences the Clinical Effectiveness of Teriparatide I. Early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab. 2008;93(10):3785–93. doi: 10.1210/jc.2008-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lane NE, Sanchez S, Genant HK, Jenkins DK, Arnaud CD. Short-term increases in bone turnover markers predict parathyroid hormone-induced spinal bone mineral density gains in postmenopausal women with glucocorticoid-induced osteoporosis. Osteoporos Int. 2000;11(5):434–42. doi: 10.1007/s001980070111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure: Assessments of COP cells and COP cell expression of IGF-1R, osteocalcin and RUNX2 demonstrated no significant differences between fresh and frozen/thawed samples from the same control subjects (n=3).


