Abstract
Objective
Physical activity benefits executive control, but the mechanism through which this benefit occurs is unclear. Sleep is a candidate mechanism given that it improves with exercise and has restorative effects on the prefrontal cortex. The present cross-sectional study examined the mediating role of sleep in the relationship between physical activity and executive control in young and older adults.
Participants
Young (n = 59) and older (n = 53) community-dwelling adults ages 21–30 and 55–80.
Methods
Participants wore an accelerometer for one week to assess sleep efficiency, total sleep time, and physical activity, operationalized as metabolic equivalent of task (METs) during time spent awake. Cognition was assessed in the laboratory across multiple measures of executive control, memory recall, and processing speed. Mediation analyses tested the role of sleep efficiency in the cross-sectional relationship between METs and cognitive performance accounting for age, sex, and education.
Results
METs were significantly associated with performance before, but not after accounting for covariates. METs were associated with sleep efficiency but not total sleep time. Sleep efficiency, but not total sleep time, mediated the relationship between METs and working memory, switching, verbal ability & fluency, and recall. Age group did not moderate the mediating role of sleep efficiency in the relationship between METs and performance.
Conclusions
Sleep efficiency is one pathway by which physical activity may be associated with executive control across young and older adults.
Keywords: Accelerometry, sleep continuity, total sleep time, executive control, mediation, aging
Introduction
Physical activity and sleep are positively associated with cognition, particularly memory consolidation and executive control. Executive control processes are responsible for the planning, initiating, and monitoring of complex goal-directed behavior (Royall et al., 2002), such as working memory, switching, and controlled memory retrieval processes (Bixby et al., 2007; Colcombe & Kramer, 2003; Colcombe et al., 2004; Erickson, Hillman, & Kramer, 2015; Hillman, Erickson, & Kramer, 2008; Pace-Schott & Spencer, 2011; Walker, 2009; Wilckens, Erickson, & Wheeler, 2012). The direct link between sleep and executive control is supported by the negative impact of sleep loss on multiple domains of cognition (Goel, Rao, Durmer, & Dinges, 2009). Further, deeper stages of sleep, such as slow-wave sleep involve neural synchrony generated by the prefrontal cortex (Steriade, McCormick, & Sejnowski, 1993) which may enhance efficiency of the executive control network to support executive processing (Wilckens, Hall, Nebes, Monk, & Buysse, 2016). It is unclear however, what mechanisms (i.e., mediators) link physical activity with executive control.
Physical activity, defined by any bodily movement that results in energy expenditure, and exercise, which is the planned, structured, and repetitive engagement in physical activity (Caspersen, Powell, & Christenson, 1985), are positively related to sleep, specifically sleep efficiency, sleep latency, and sleep depth (Kline et al., 2013; Montgomery & Dennis, 2002; Reid et al., 2010; Tworoger et al., 2003; Youngstedt, 2005). Recent studies have demonstrated that higher sleep efficiency (the amount of time in bed spent sleeping) is associated with greater executive control abilities in young and older adults (Blackwell et al., 2006; Nebes, Buysse, Halligan, Houck, & Monk, 2009; Wilckens et al., 2016). In contrast to sleep efficiency where higher values are associated with better executive control, longer total sleep time is rarely associated with better executive control in adults, particularly older adults (Blackwell et al., 2006; Cavuoto et al., 2016; Nebes et al., 2009). Rather, longer self-reported sleep duration which may include brief bouts of wakefulness has been associated with poorer performance (Lo, Groeger, Cheng, Dijk, & Chee, 2016). This dissociation between sleep efficiency and total sleep time may be due to the dampened sleep drive and greater sleep fragmentation that can occur with longer sleep durations (Harrison & Horne, 1996; Monk, Buysse, Begley, Billy, & Fletcher, 2009; Youngstedt & Kripke, 2004) and the greater likelihood of progressing through sleep stages that benefit the prefrontal cortex with higher sleep efficiency (Wilckens et al., 2012). Thus sleep efficiency is a candidate sleep feature that may mediate the relationship between physical activity and executive control across the adult lifespan (Wilckens et al., 2012).
Given the positive relationships between physical activity, sleep efficiency, and prefrontally-based processes (Blackwell et al., 2006; Nebes et al., 2009; Wilckens et al., 2016), the present study tested the hypothesis that sleep efficiency mediates the cross-sectional relationship between physical activity and multiple domains of executive control. These included working memory, switching, inhibition, verbal ability & fluency (Jurado & Rosselli, 2007), as well as delayed recall, a controlled memory process dependent on the prefrontal cortex (Buckner, 2003) due to critical search and post-retrieval monitoring processes (Tomita, Ohbayashi, Nakahara, Hasegawa, & Miyashita, 1999; Velanova et al., 2003). In contrast, we hypothesized that mediation models with processing speed would not be significant, based on evidence that exercise interventions, physical activity, and sleep efficiency are tenuously linked to processing speed (Colcombe & Kramer, 2003; Lim & Dinges, 2010; Nebes et al., 2009). Because total sleep time is less often associated with executive control in adults (Blackwell et al., 2006; Nebes et al., 2009; Wilckens et al., 2016), we hypothesized that total sleep time would not be a significant mediator in the relationship between physical activity and executive control domains.
There is equivocal evidence regarding age-related changes in the importance of physical activity and sleep to executive control (Duffy, Willson, Wang, & Czeisler, 2009; Young, Angevaren, Rusted, & Tabet, 2015). Thus, although the present study was designed to test the above hypotheses, we also conducted an exploratory moderated mediation analysis to test whether sleep efficiency similarly mediated the relationship between physical activity and executive control across young and older adulthood (Lopez, 2008). We hypothesized that despite anticipated negative effects of age on each of these factors, the mechanisms linking these factors would be unaffected by age. Therefore, mediating associations involving sleep efficiency would not be moderated by age.
Methods
Participants
One hundred and twenty-eight community-dwelling volunteers, ages 21 to 30 and 55 to 80 were recruited to participate in cognitive testing over two sessions separated by one week during which sleep was assessed. All participants provided written informed consent in line with the University of Pittsburgh’s Institutional Review Board. Participants were paid at a rate of $10 per hour of cognitive testing and were paid $50 for wearing an accelerometer device to measure sleep and physical activity for one week. Exclusion criteria included a self-reported diagnosis of depression, current psychiatric medication use, dependence on drugs or alcohol, or a diagnosis of a neurodegenerative disease reported by the participant at screening. Mood was assessed by self-report questionnaire for descriptive purposes, but was not used as exclusion criteria. Self-reported sleep apnea was assessed but was also not used as exclusion criteria partly justified by evidence that mild to moderate sleep apnea has little impact on cognitive function (Quan et al., 2014; Quan et al., 2006). All participants had normal or corrected-to-normal vision. Participants were excluded from the present analyses if they wore the accelerometer armband for less than 4 days (n = 3), if they did not return for the second cognitive testing session (n = 10), or in the case of technical malfunction during the computer-based portion of the experiment (n = 3). All participants had mini mental state exam (MMSE) scores ≥ 26. To maximize statistical power for mediation analyses, and to maximize individual variation in relation to sleep, physical activity, and cognition, participants were not excluded based on any other criteria for the present analyses including sleep criteria. Table 1 provides participant characteristics in the final sample of 59 young and 53 older participants.
Table 1.
Age differences in mean (sd) or frequency (%) for demographic and health characteristics, physical activity, sleep, and cognitive performance. METs were limited to minutes awake. MMSE reflects score using the backwards spelling of the word “world”).
| Young | Older | Difference t(110) | p | |
|---|---|---|---|---|
| N | 59 | 53 | ||
| Age | 23.05 (2.42) | 62.68 (6.08) | 46.17 | <0.001 |
| Education | 16.09 (1.65) | 15.22 (3.05) | 1.90 | 0.06 |
| N Female | 38 (64.4%) | 36 (67.9%) | 0.38 | 0.689 |
| N African American/Black | 7 (11.9%) | 14 (26.4%) | ||
| N Asian | 3 (5.1%) | 0 | ||
| N Caucasian/White | 48 (81.4%) | 39 (73.6%) | ||
| N Pacific Islander | 1 (1.7%) | 0 | ||
| MMSE | 29.58 (0.70) | 28.89 (1.03) | 4.18 | <0.001 |
| Geriatric Mood Scale | 2.04 (2.29) | 1.50 (2.25) | 1.19 | 0.237 |
| BMI | 24.91 (6.07) | 29.07 (5.85) | 3.68 | <0.001 |
| N Smoker | 4 (6.8%) | 14 (26.4%) | 2.91 | 0.004 |
| Self-report sleep apnea | 0 | 8 (15%) | 3.44 | 0.001 |
| METs | 1.87 (0.34) | 1.51 (0.32) | 5.65 | <0.001 |
| Sleep Efficiency | 0.83 (0.07) | 0.82 (0.09) | 0.47 | 0.643 |
| Total sleep time (mins) | 382.51 (59.80) | 355.83 (67.76) | 2.21 | 0.029 |
| Working Memory | 0.12 (0.45) | −0.13 (0.57) | 2.60 | 0.010 |
| Switching | 0.29 (0.60) | −0.32 (0.628) | 5.32 | <0.001 |
| Inhibition | 0.12 (0.49) | −0.12 (0.47) | 2.65 | 0.009 |
| Verbal Ability & Fluency | 0.20 (0.63) | −0.22 (0.77) | 3.13 | 0.002 |
| Memory Recall | 0.36 (0.86) | −0.40 (1.00) | 4.26 | <0.001 |
| Processing Speed | 0.50 (0.55) | −0.56 (0.74) | 8.71 | <0.001 |
Protocol Overview
Participants completed a battery of assessments over two sessions, each taking place between 9:00 am and 3:00 pm depending on the participant’s preference. Participants were given an accelerometer armband to wear for one week between the two visits. The first session included computer-based tasks, self-report questionnaires, and instructions for wearing the accelerometer and completing a sleep diary. The second session included paper-and-pencil neuropsychological tasks. The computer-based tasks and self-report questionnaires at the first session lasted approximately 2 hours. Computer-based tasks were performed consecutively with approximately 2 minutes intervening between tasks. Following the computer-based tasks, participants could take a break before completing self-report questionnaires. At the second session, the paper-and-pencil tasks lasted approximately 1 hour. Paper-and-pencil based tasks were performed consecutively with no breaks.
Cognitive tasks
Cognitive abilities were assessed using the computer-based and paper-and-pencil-based tasks. In selecting cognitive tasks, our goal was to measure a wide array of tasks that would tap multiple domains within the umbrella of executive control based on evidence that tasks dependent on the prefrontal cortex are more relevant to sleep and physical activity (Kramer et al., 1999; Wilckens et al., 2012). Within that umbrella were working memory, task switching, inhibition, and verbal fluency. Additionally, given the dependence of controlled memory processes on the prefrontal cortex (Buckner, 2003) and their proposed dependence on sleep (Wilckens et al., 2012), we extended our assessment of executive tasks to memory recall. Finally, we aimed to contrast executive control domains with traditional tests of processing speed, which have been associated with temporo-parietal white matter integrity (Turken et al., 2008).
Computer-based tasks were two working memory tasks (Sternberg task (Sternberg, 1966) and the N-back task (Jaeggi et al., 2003; Lee et al., 2012)), a cued task-switching paradigm (Wilckens, Woo, Erickson, & Wheeler, 2014), and two inhibitory control tasks (color-word Stroop task (Stroop, 1935) and a Flanker task (Gothe et al., 2014)). Several of these tasks have been used previously in studies testing a wide range of executive tasks (Lee et al., 2012). The paper-and-pencil-based tasks were trail-making tasks A and B (Reitan, 1958; Wechsler, 1997), the digit-symbol substitution task (Wechsler, 1997), the National Adult Reading Test (Nelson & Willison, 1991), categorical and lexical fluency tasks (Borkowski, Benton, & Spreen, 1967), and the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) (Morris et al., 1989). These tasks are further described in the supplementary materials section.
Across all response time measures in computerized tasks, we accounted for processing speed and general age-related slowing by calculating costs in response time. Costs reflected the difference between response times associated with correct trials on the easier task condition and those of the more difficult task condition. To reduce the number of statistical tests performed, relevant conditions for each task were transformed to z-scores and averaged to arrive at each of six cognitive domains (Wilckens, Woo, Kirk, Erickson, & Wheeler, 2014). Working memory was assessed with accuracy and response time costs on the Sternberg and N-back tasks. Switching was assessed with Trails B switch costs (Trails B minus Trails A) and accuracy and response time switch costs on the cued task-switching paradigm. Inhibition was assessed with percent interference (incongruent/congruent)/congruent*100) from the Stroop task and Flanker task. Verbal Ability & Fluency were assessed with words read correctly on the National Adult Reading Test and number of words verbally generated within 60 seconds in the categorical and lexical fluency tasks. Each of these are a test of verbal ability and are highly correlated with one another (Crawford, Moore, & Cameron, 1992). Memory recall was assessed with number of words correctly recalled on the CERAD delayed recall task, with recall taking place after a delay of approximately 10 minutes. Delayed word recognition from the CERAD was collected as an “automatic” (i.e., not requiring executive control) form of memory, but performance on this task was at ceiling across groups and was therefore excluded from the present analyses. Processing speed was assessed with Trails A (time to complete) and the number of correct symbols written on the digit-symbol substitution test within 60 seconds. One young participant did not complete the Stroop task, and was therefore excluded from analyses with Inhibition. Because performance in all cognitive domains is represented in terms of z-scores, performance ranged from −2.31 to 1.82 across young and older adults. In each domain, higher scores indicate better performance (higher accuracy and lower RT costs).
Physiological data collection
A SenseWear® armband was used to estimate participants’ physical activity and sleep. Participants were asked to wear the armband for one week between the two experiment sessions. Sleep efficiency was chosen as the measure of sleep continuity based on existing data showing that sleep efficiency and sleep latency are associated with physical activity, and sleep efficiency with executive control, but additionally given established limitations with estimating sleep latency with actigraphy (Slater et al., 2015; Tryon, 2004).
Pertinent to physical activity, the SenseWear device estimated the average metabolic equivalent of task (METs) every 60 seconds. The METs measure was calculated by the device using a proprietary algorithm that takes into account the participant’s age, height, weight, and energy expenditure based on sensor data including activity, skin conductance, and heat flux. From the minute to minute MET estimates produced by the SenseWear device, METs during wakefulness were averaged to obtain a single measure of daily physical activity outside of time spent sleeping. This measure of METs while awake was chosen to assess the overall level of physical activity throughout the week. The device has been shown to correlate well with energy expenditure measured with indirect calorimetry during exercise (Andre et al., 2006).
Pertinent to sleep, the SenseWear device estimated on a minute-by-minute basis whether participants were lying down or asleep based on body axis, heat flux, activity, galvanic skin response, body temperature, and near body temperature. These additional measures avoid issues with overestimation of sleep when the armband is off the body (Sunseri et al., 2009). This device has been shown have 90% concordance with polysomnography during sleep (Sunseri et al., 2009). Participants also recorded when they went to bed for the final time and got out of bed each day they wore the accelerometer. These records were used with the SenseWear actigraphy data to define the nighttime sleep bout. From the sleep and lying down estimates within the nighttime sleep bout, average sleep efficiency (time spent asleep/time lying down) and average total sleep time were calculated. By calculating the average across the week of accelerometer time with a minimum of four days per participant, we made the assumption that the MET, sleep efficiency, and total sleep time averages reflected participants’ habitual behavior.
Statistical analyses
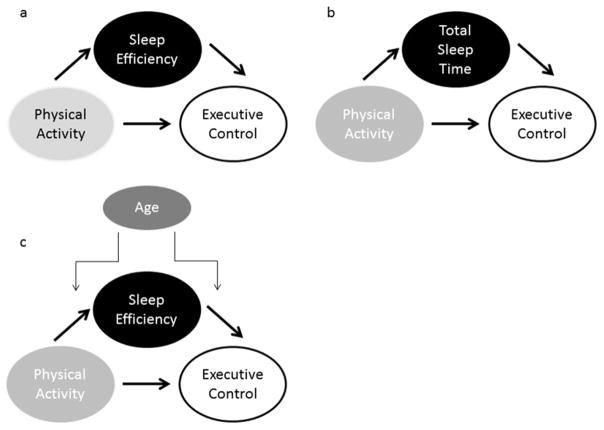
Relationships among physical activity, sleep, and cognitive variables were tested with Pearson correlations and hierarchical regression analyses controlling for age, sex, and education. Bootstrapping mediation and moderated mediation analyses were tested with conditional process modeling using the Process macro in Statistical Package for the Social Sciences (SPSS) (Hayes, 2013). Five thousand bootstrap samples were run with a 95% confidence interval. For all primary mediation analyses, awake METs was the independent variable, sleep efficiency (Figure 1a) or total sleep time (Figure 1b) were the proposed mediators, and a given cognitive domain was the dependent variable.
Figure 1.
Set of hypothesized (a) and alternative (b & c) mediation models: a) hypothesized model whereby sleep efficiency is mediator in the relationship between physical activity and executive control; b) total sleep time as a mediator in the relationship between physical activity and executive control; c) age a moderator of the mediating role of sleep efficiency in the relationship between physical activity and executive control.
Additionally, given that higher sleep efficiency may lead to more physical activity (Lambiase, Gabriel, Kuller, & Matthews, 2013), we statistically tested whether physical activity mediated the relationship between sleep efficiency and executive control (presented in the supplementary materials section).
The present study was designed to have adequate power to test the mediation models described above based on recommended sample sizes of Fritz & MacKinnon (2007). As an exploratory analysis, we further tested whether age group moderated the mediating role of sleep efficiency in the relationship between physical activity and cognition. For moderated mediation analyses, age group was the proposed moderator. Moderated mediation was tested in two ways: at the level of the association between physical activity and sleep efficiency and at the level of the association between sleep efficiency and cognition (Figure 1c). Although there is evidence that sleep time has both linear and non-linear relationships with cognition, this is particularly the case for self-reported sleep which often includes brief bouts of wakefulness (Lo et al., 2016). In addition, the turning point at which longer sleep times become detrimental may differ between young and older adults (Steptoe, Peacey, & Wardle, 2006). Such a non-linear model would therefore require a separate analysis between the young and older group, which would be underpowered here. Thus, the present report focuses on linear relationships. Age, sex and education were included as covariates in all mediation and moderated mediation analyses. In all cases, separate analyses were run for each sleep variable and each cognitive domain.
To address potential confounds of day-to-day sleep variability, sleep apnea, and vigorous bouts of exercise, three sets of sensitivity analyses were run: To rule out day-to-day variability in total sleep time as a potential confound in the relationship between sleep efficiency and cognition, sensitivity analyses included the standard deviation in total sleep time across days as a covariate for significant mediating associations of sleep efficiency. To address the potential role of sleep apnea in the links between sleep efficiency, physical activity, and cognition, we used participants’ self-reported diagnoses of sleep apnea as a covariate in significant mediation analyses. Finally, to assess whether significant mediating associations were driven by bouts of exercise, as opposed to overall physical activity, we controlled for average minutes spent in moderate to vigorous physical activity (six or more METs).
The main requirement for mediation is that the indirect effect of the independent variable (physical activity) through the mediator (sleep) on the dependent variable (cognition) be significant. Using the bootstrapping approach, there is no requirement that relationships between the independent variable and dependent variable be significant due to potential unmeasured suppressor variables (Gelfand, Mensinger, & Tenhave, 2009; Preacher & Hayes, 2008; Zhao, Lynch, & Chen, 2010). Thus, we tested mediation for all relationships between METs and performance regardless of whether the bivariate relationship was statistically significant.
Results
Bivariate relationships among physical activity, sleep efficiency, and cognition
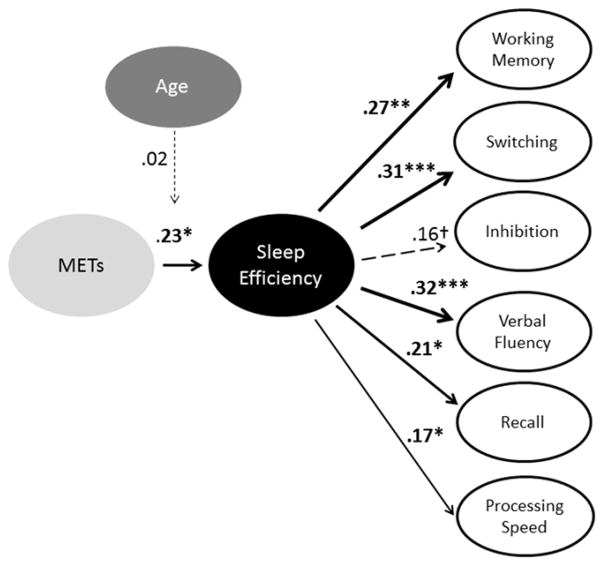
Bivariate relationships involving METs and each sleep and cognitive domain are presented in Table 2. METs and sleep efficiency were significantly associated with one another after controlling for covariates. METs and total sleep time were not significantly related before or after accounting for covariates. There were significant zero-order correlations between METs and cognition for working memory, inhibition, recall, and processing speed. However, these relationships were no longer significant after accounting for age, sex, and education (Table 2). Sleep efficiency was significantly associated with each cognitive domain, except for inhibition, which was marginally significant (Figure 2; Supplementary material Table 6).
Table 2.
Associations among METs, sleep variables, and cognitive variables. Fully adjusted regression model includes age, sex, and education as covariates. Significant associations are highlighted in bold font.
| Zero-order Correlations with METs (r) | p | Fully Adjusted Regression (β) | p | |
|---|---|---|---|---|
| Sleep Efficiency | 0.15 | 0.114 | 0.23 | 0.037 |
| Total Sleep Time | 0.13 | 0.170 | 0.08 | 0.452 |
| Working Memory | 0.20 | 0.038 | 0.13 | 0.251 |
| Switching | 0.15 | 0.108 | −0.08 | 0.449 |
| Inhibition | 0.22 | 0.021 | 0.08 | 0.436 |
| Verbal Ability & Fluency | 0.15 | 0.112 | −0.01 | 0.908 |
| Recall | 0.19 | 0.042 | 0.04 | 0.670 |
| Processing Speed | 0.43 | <0.001 | 0.13 | 0.131 |
Figure 2.
Beta values for associations between METs, sleep efficiency, and each cognitive domain accounting for age, sex, and education. †p < 0.1, *p < 0.05, **p < 0.01, ***p < 0.001. Line thickness represents the strength of the association. Non-significant relationships are represented by dotted lines. Exact p values for regressions between sleep efficiency and performance are presented in the supplementary materials section. Other p values are presented where results are reported.
Indirect (mediating) role of sleep in the relationship between physical activity and cognition
Consistent with our primary hypothesis, sleep efficiency significantly mediated the relationship between METs and working memory, switching, verbal ability & fluency, and recall (Table 3). Sleep efficiency was a marginally significant mediator for inhibition (significant with a 90% confidence interval). These mediating associations with sleep efficiency remained significant after controlling for variability in total sleep time, self-reported sleep apnea, and moderate to vigorous activity (all confidence intervals excluded zero). Total sleep time was not a significant mediator in the relationship between METs and any cognitive domain (Table 4).
Table 3.
Indirect mediating role of sleep efficiency on the relationship between METs and cognition. Significant mediation is highlighted in bold font.
| Domain | Effect (Boot SE) | Lower : Upper 95% Confidence Interval |
|---|---|---|
| Working Memory | 0.08 (0.06) | 0.001 : 0.28 |
| Switching | 0.14 (0.08) | 0.023: 0.36 |
| Inhibition | 0.05 (0.04) | −0.002: 0.15 |
| Verbal Ability & Fluency | 0.15 (0.09) | 0.02: 0.41 |
| Recall | 0.13 (0.08) | 0.01: 0.35 |
| Processing Speed | 0.08 (0.06) | −0.0004: 0.26 |
Table 4.
Indirect mediating role of total sleep time in the relationship between METs and cognition.
| Domain | Effect (Boot SE) | Lower : Upper 95% Confidence Interval |
|---|---|---|
| Working Memory | 0.02 (0.04) | −0.03 : 0.17 |
| Switching | 0.03 (0.05) | −0.03 : 0.18 |
| Inhibition | 0.01 (0.02) | −0.01 : 0.10 |
| Verbal Ability & Fluency | 0.02 (0.04) | −0.02 : 0.20 |
| Recall | 0.02 (0.04) | −0.03 : 0.17 |
| Processing Speed | 0.02 (0.03) | −0.02 : 0.14 |
Moderated mediation with age group
Age group did not moderate the mediating role of sleep efficiency at the level of the association between METs and sleep efficiency as illustrated in Figure 2 (coefficient = 0.02, se = 0.05, t = 0.38, p = 0.70; p = 0.74 for inhibition in which n = 111). Nor was moderated mediation significant for age group at the level of the association between sleep efficiency and any cognitive domain (Supplementary Material Table 7).
Summary of Results
Figure 2 illustrates the results in the context of the mediation and moderated mediation models with sleep efficiency. METs were significantly associated with performance before, but not after accounting for covariates. METs were associated with sleep efficiency but not total sleep time. Sleep efficiency, but not total sleep time, mediated the relationship between METs and working memory, switching, verbal ability & fluency, and recall. Age group did not moderate the mediating role of sleep efficiency in the relationship between METs and cognition.
Discussion
Physical activity and sleep efficiency are often associated with executive control (Colcombe & Kramer, 2003; Erickson et al., 2015; Nebes et al., 2009; Wilckens et al., 2016; Wilckens, Woo, Erickson, et al., 2014). Here we report for the first time, that sleep efficiency significantly mediates cross-sectional associations between physical activity and cognition; in this case, executive control. This novel finding fits in line with the broad view that uninterrupted sleep may promote brain health and that this process may be facilitated through physical activity.
Sleep efficiency was a significant, or in the case of inhibition, a marginally significant mediator in the relationship between METs and each cognitive domain, except for processing speed. These findings are consistent with the view that physical activity specifically benefits executive control, as opposed to having domain general links with cognition, including domains that decline with aging, such as processing speed (Salthouse, 1996). Accordingly, these findings suggest that physical activity is linked to executive control through efficient sleep, and that this link is partially independent of age. Aging has a robust effect on processing speed, and processing speed has been posited to underlie many age-related cognitive deficits (Salthouse, 1996). The current findings are not necessarily at odds with this view, nor are they at odds with the view that age-related decrements are disproportionately sensitive to executive control (West, 1996). Rather, these findings demonstrate that there is a link between physical activity, sleep efficiency, and executive control that is independent of age and may be most relevant for prefrontal-based cognitive processes. Nonetheless, future studies using latent constructs across a range of cognitive tasks to disentangle processing speed and executive control will be necessary to identify the role of processing speed in associations examined here.
Total sleep time did not mediate the relationship between physical activity and executive control. This may be partially explained by the lack of a relationship between physical activity and total sleep time. Further, in contrast to total sleep time, measures of sleep continuity such as sleep efficiency are most consistently associated with executive control among adults (Blackwell et al., 2006; Nebes et al., 2009; Wilckens et al., 2016). Thus it is not merely any aspect of sleep that mediates the relationship between physical activity and cognition, but rather ease of falling asleep and staying asleep (Buysse, 2014) that may be a critical link between physical activity with executive control.
Awakenings during sleep are less likely to occur during the deepest stage of sleep, slow-wave sleep (Neckelmann & Ursin, 1993). Slow-wave sleep involves neural synchrony predominantly over the prefrontal cortex, reflecting synchronized depolarizing of neurons (Steriade et al., 1993). Such neural synchrony over the prefrontal cortex may potentiate synapses within networks important for executive control. Slow-wave sleep has been shown to increase with exercise (Kline et al., 2013), and is linked to executive control and memory consolidation (Anderson & Horne, 2003; Mander et al., 2013; Wilckens et al., 2016). One proposed mechanism supporting a link between physical activity and sleep is the restoration hypothesis which proposes that energy expenditure stimulates a restoration process whereby sleep allows the body and brain to recuperate (Buman & King, 2010; Driver & Taylor, 2000; Lopez, 2008). Accordingly, slow-wave sleep has been proposed to preferentially “restore” prefrontal cortex function (Anderson & Horne, 2003; Maquet et al., 1997; Muzur, Pace-Schott, & Hobson, 2002; Picchioni, Duyn, & Horovitz, 2013; Wilckens et al., 2016). Additionally, low sleep efficiency may reflect the disruption of multiple sleep features involved in cognition, including stage N2 spindles and rapid eye movement sleep. Future research will determine whether the sleep mechanism linking physical activity with executive control is synchronized neural firing, a restoration processes, or a combination of sleep features working together to enhance executive control.
Age group did not moderate the mediating role of sleep efficiency in any cognitive domain. This finding suggests that sleep efficiency’s mediating role applies across age groups. It should be noted however, that while our sample size across age groups was sufficiently large to test mediation, it may have been under powered to test hypotheses of moderated mediation (Fritz & MacKinnon, 2007). Future studies should further examine the effect of age on relationships involving physical activity and sleep efficiency with executive control. Further, this study did not recruit participants between the ages of 30–55. Thus, these results may be missing information about changes in the mediating role of sleep efficiency on the relationship between physical activity and executive control across the adult lifespan.
Despite an established literature demonstrating a relationship between physical activity and executive control (Erickson et al., 2015; Hillman et al., 2008), associations between METs and executive domains were no longer significant after accounting for age, sex, and education in the present sample. However, the intuitive rationale that a direct relationship between physical activity and cognition must first be established before mediation can be tested, is inaccurate (Zhao et al., 2010). The direct relationship between physical activity and cognition reflects the total relationship with cognition including the proposed mediator of sleep efficiency as well as other mediators not measured here, some of which may suppress the association between physical activity and cognition (Preacher & Hayes, 2008; Zhao et al., 2010). Thus, only the indirect association is required to establish mediation (Preacher & Hayes, 2008).
The lack of an association between physical activity and performance, after accounting for covariates, could be interpreted as consistent with published meta-analyses reporting little effect of exercise on executive control (Kelly et al., 2014; Young et al., 2015). However, such “negative” meta-analysis findings are likely due to a number of factors, including heterogeneity in scientific rigor among randomized control trials and absence of critical factors such as adherence (Kelly et al., 2014). Indeed as described above, heterogeneity of the current sample likely contributed to the suppression of the relationship between physical activity and performance. The current findings suggest that objective sleep should also be measured in exercise trials to determine whether exercise benefits are more successful in improving executive control when sleep efficiency is also enhanced.
Despite growing acknowledgement that physical activity, sleep, and executive control are linked (Garcia & Gunstad, 2015; Lopez, 2008; Vitiello, 2008; Wilckens et al., 2012), there are very few studies that have examined all of these factors together in one study. In a pilot study of 12 participants, Benloucif et al. (2004) found that both subjective sleep quality and neuropsychological performance improved with two weeks of physical activity. However, this study was not sufficiently large to test a mediation model as reported here. Lambiase et al. (2014) found that the relationship between sleep efficiency and neuropsychological performance was diminished at higher levels of physical activity, suggesting that higher physical activity may counteract negative effects of poor sleep efficiency. Notably as a contrast, sensitivity analyses reported here revealed that higher levels of physical activity did not explain the significant mediation results. This finding suggests that a broader measure of physical activity as tested here can account for sleep efficiency-related differences in cognition. A number of reviews have posited sleep as a mediator in the relationship between physical activity and cognition (Lopez, 2008; Vitiello, 2008; Wilckens et al., 2012), but this is the first published study to our knowledge to investigate and demonstrate sleep as a mechanism linking physical activity and cognition, in this case, executive control.
Limitations and future directions
One major limitation of the present study was the cross-sectional design. As a result, causal mediation cannot be established. Rather, statistical mediation used here may be used to generate hypotheses for future exercise intervention studies (Kraemer, Kiernan, Essex, & Kupfer, 2008).
There are some pros and cons in the use of the accelerometer armband for measuring sleep and physical activity. The device measures sleep similarly to more commonly used actigraphic devices, but it additionally takes into account additional physiological measures such as skin conductance and heart rate, which arguably makes the device more sensitive to sleep and wakefulness. Though polysomnographic sleep is considered the gold standard for sleep measurement, there have been some validation studies for SenseWear against established sleep and physical activity metrics. Based on the findings of these validation studies, there remains a possibility that the current results may have been influenced by an underestimation or overestimation in sleep or METs. For instance, despite overall 90% concordance with polysomnography, the SenseWear device shows 50% concordance with polysomnography during short periods of wakefulness, suggesting that sleep efficiency may have been underestimated in the current study. Nonetheless, in line with the goal of measuring habitual sleep in the participant’s natural sleep environment, there are many advantages with using actigraphy over traditional polysomnography. For instance, this approach allowed for sampling over multiple nights in the participants normal sleep environment with minimal interference from equipment and wires. Measures of habitual sleep may be more effective in revealing the chronic effects of sleep on cognition. Further, although objective actigraphic and polysomnographic measures are typically favored over self-report measures, there is evidence for stronger relationships between sleep and cognition for self-report measures, specifically in poor sleepers (Bastien et al., 2003). This suggests that there are aspects of nighttime wakefulness important for cognition, for which objective measures may be either overly sensitive or unable to measure with traditional methods. Thus, one goal for future work will be to disentangle the critical underlying sleep features supporting the link between physical activity and executive control as captured by the variety of sleep measurement techniques.
The present study measured sleep efficiency which includes both sleep latency and nighttime awakenings, which have both been associated with physical activity in prior studies (Kline et al., 2013; Reid et al., 2010). However, issues with defining sleep onset undermine the validity of actigtaphically-measured sleep latency (Slater et al., 2015; Tryon, 2004). Thus, it is unclear based on the current findings what aspect of sleep efficiency is the critical mediator in the relationship between physical activity and executive control. Although the neural processes underlying slow-wave sleep remain a plausible mechanism linking sleep efficiency to both exercise and executive control, we cannot draw conclusions about sleep stages using actigraphy.
There are established age differences in the time of day preferable for cognitive performance between young and older adults (Hasher, Goldstein, & May, 2005). The present study did not control for time of day, but it did accommodate individual participants’ preferences. This study would have benefitted from a time of day control or manipulation. Nonetheless, such a control would have likely enhanced the strength of associations by increasing the signal to noise ratio.
Finally, although the present study benefitted from a wide range of sleep behaviors across participants, the inclusion of participants regardless of sleep disorders could have influenced the current findings. For instance, sleep apnea is related to both cognition (Emamian et al., 2016; Fulda & Schulz, 2001) and physical activity (Iftikhar, Kline, & Youngstedt, 2014). Sensitivity analyses provide preliminary evidence that the present mediation findings are independent of sleep apnea. However, sleep apnea may have been underestimated in the current sample. Future work using well-validated and objective measures of sleep apnea are critical to determine if the benefits of physical activity to executive control through sleep are driven by sleep efficiency broadly or partly through symptoms of sleep apnea.
Conclusions
Sleep efficiency, but not total sleep time, was found to statistically mediate the non-significant relationship between physical activity and a variety of measures of executive control. This mediating role of sleep efficiency applies across young and older adults suggesting that the sleep mechanisms linking physical activity with executive control in prior studies may be similar across the adult lifespan.
Supplementary Material
Acknowledgments
The authors thank Sarah Woo, Afton Kirk, Krupa Patel, Marina Lukac, and Leslie Denlinger for assistance with data collection. This work was performed at the University of Pittsburgh Learning Research and Development Center. This project was supported by the National Institute of Mental Health (MH086492, PI: M.E.W.). K.A.W. was supported by training grants T32 GM081760 and T32 MH019986, and a career development award K01 AG049879 from the National Institutes of Health.
Contributor Information
Kristine A. Wilckens, University of Pittsburgh School of Medicine, Department of Psychiatry, 3811 O’Hara Street E1124, Pittsburgh, PA 15213.
Kirk I. Erickson, University of Pittsburgh, Department of Psychology, 3601 Sennott Square, Pittsburgh, PA 15260.
Mark E. Wheeler, Georgia Institute of Technology, School of Psychology, 654 Cherry Street, Atlanta GA, 30332.
References
- Anderson C, Horne JA. Prefrontal cortex: links between low frequency delta EEG in sleep and neuropsychological performance in healthy, older people. Psychophysiology. 2003;40(3):349–357. doi: 10.1111/1469-8986.00038. [DOI] [PubMed] [Google Scholar]
- Andre D, Pelletier R, Farringdon J, Safier S, Talbott W, Stone R, … Vishnubhatla S. The development of the SenseWear® armband, a revolutionary energy assessment device to assess physical activity and lifestyle. BodyMedia Inc 2006 [Google Scholar]
- Bastien CH, Fortier-Brochu É, Rioux I, LeBlanc M, Daley M, Morin CM. Cognitive performance and sleep quality in the elderly suffering from chronic insomnia: relationship between objective and subjective measures. Journal of Psychosomatic Research. 2003;54(1):39–49. doi: 10.1016/s0022-3999(02)00544-5. [DOI] [PubMed] [Google Scholar]
- Benloucif S, Orbeta L, Ortiz R, Janssen I, Finkel SI, Bleiberg J, Zee PC. Morning or evening activity improves neuropsychological performance and subjective sleep quality in older adults. Sleep. 2004;27(8):1542–1551. doi: 10.1093/sleep/27.8.1542. [DOI] [PubMed] [Google Scholar]
- Bixby WR, Spalding TW, Haufler AJ, Deeny SP, Mahlow PT, Zimmerman JB, Hatfield BD. The unique relation of physical activity to executive function in older men and women. Medicine & Science in Sports & Exercise. 2007;39(8):1408–1416. doi: 10.1249/mss.0b013e31806ad708. [DOI] [PubMed] [Google Scholar]
- Blackwell T, Yaffe K, Ancoli-Israel S, Schneider JL, Cauley JA, Hillier TA, … Stone KL. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. The Journals of Gerontology: Series A. 2006;61(4):405–410. doi: 10.1093/gerona/61.4.405. 61/4/405. [DOI] [PubMed] [Google Scholar]
- Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5(2):135–140. [Google Scholar]
- Buckner RL. Functional–anatomic correlates of control processes in memory. The Journal of Neuroscience. 2003;23(10):3999–4004. doi: 10.1523/JNEUROSCI.23-10-03999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buman MP, King AC. Exercise as a Treatment to Enhance Sleep. American Journal of Lifestyle Medicine. 2010;4(6):500–514. doi: 10.1177/1559827610375532. [DOI] [Google Scholar]
- Buysse DJ. Sleep health: can we define it? Does it matter. Sleep. 2014;37(1):9–17. doi: 10.5665/sleep.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Reports. 1985;100(2):126. [PMC free article] [PubMed] [Google Scholar]
- Cavuoto MG, Ong B, Pike KE, Nicholas CL, Bei B, Kinsella GJ. Objective but not subjective sleep predicts memory in community-dwelling older adults. Journal of Sleep Research. 2016 doi: 10.1111/jsr.12391. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF. Fitness Effects on the Cognitive Function of Older Adults: A Meta-Analytic Study. Psychological Science. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, … Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences. 2004;101(9):3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford J, Moore J, Cameron I. Verbal fluency: a NART-based equation for the estimation of premorbid performance. British Journal of Clinical Psychology. 1992;31(3):327–329. doi: 10.1111/j.2044-8260.1992.tb00999.x. [DOI] [PubMed] [Google Scholar]
- Driver HS, Taylor SR. Exercise and sleep. Sleep Medicine Reviews. 2000;4(4):387–402. doi: 10.1053/smrv.2000.0110. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Willson HJ, Wang W, Czeisler CA. Healthy older adults better tolerate sleep deprivation than young adults. Journal of the American Geriatrics Society. 2009;57(7):1245–1251. doi: 10.1111/j.1532-5415.2009.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emamian F, Khazaie H, Tahmasian M, Leschziner GD, Morrell MJ, Hsiung G-YR, … Sepehry AA. The association between obstructive sleep apnea and Alzheimer’s disease: a meta-analysis perspective. Frontiers in Aging Neuroscience. 2016;8 doi: 10.3389/fnagi.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Hillman CH, Kramer AF. Physical activity, brain, and cognition. Current Opinion in Behavioral Sciences. 2015;4:27–32. doi: 10.1016/j.cobeha.2015.01.005. [DOI] [Google Scholar]
- Fritz MS, MacKinnon DP. Required Sample Size to Detect the Mediated Effect. Psychological Science. 2007;18(3):233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S, Schulz H. Cognitive dysfunction in sleep disorders. Sleep Medicine Reviews. 2001;5(6):423–445. doi: 10.1053/smrv.2001.0157. [DOI] [PubMed] [Google Scholar]
- Garcia S, Gunstad J. Sleep and physical activity as modifiable risk factors in age-associated cognitive decline. Sleep & Biological Rhythms. 2015:1–9. [Google Scholar]
- Gelfand LA, Mensinger JL, Tenhave T. Mediation analysis: a retrospective snapshot of practice and more recent directions. Journal of General Psychology. 2009;136(2):153–176. doi: 10.3200/GENP.136.2.153-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Seminars in Neurology. 2009;29(4):320. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothe NP, Fanning J, Awick E, Chung D, Wójcicki TR, Olson EA, … Kramer AF. Executive function processes predict mobility outcomes in older adults. Journal of the American Geriatrics Society. 2014;62(2):285–290. doi: 10.1111/jgs.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison Y, Horne J. Long-term extension to sleep-Are we really chronically sleep deprived? Psychophysiology. 1996;33(1):22–30. doi: 10.1111/j.1469-8986.1996.tb02105.x. [DOI] [PubMed] [Google Scholar]
- Hasher L, Goldstein D, May CP. It’s About Time: Circadian Rhythms, Memory, and Aging. 2005. [Google Scholar]
- Hayes AF, editor. Introduction to mediation, moderation, and condition process analysis: A regression-based approach. New York: Guilford Press; 2013. [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nature Reviews Neuroscience. 2008;9(1):58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Iftikhar IH, Kline CE, Youngstedt SD. Effects of exercise training on sleep apnea: a meta-analysis. Lung. 2014;192(1):175–184. doi: 10.3389/fnagi.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Seewer R, Nirkko AC, Eckstein D, Schroth G, Groner R, Gutbrod K. Does excessive memory load attenuate activation in the prefrontal cortex? Load-dependent processing in single and dual tasks: functional magnetic resonance imaging study. NeuroImage. 2003;19(2):210–225. doi: 10.1016/S1053-8119(03)00098-3. [DOI] [PubMed] [Google Scholar]
- Jurado MB, Rosselli M. The elusive nature of executive functions: a review of our current understanding. Neuropsychology review. 2007;17(3):213–233. doi: 10.1007/s11065-007-9040-z. [DOI] [PubMed] [Google Scholar]
- Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, Brennan S. The impact of exercise on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Ageing Research Reviews. 2014;16:12–31. doi: 10.1016/j.arr.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Kline CE, Irish LA, Krafty RT, Sternfeld B, Kravitz HM, Buysse DJ, … Hall MH. Consistently high sports/exercise activity is associated with better sleep quality, continuity and depth in midlife women: the SWAN sleep study. Sleep. 2013;36(9):1279. doi: 10.5665/sleep.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Kiernan M, Essex M, Kupfer DJ. How and Why Criteria Defining Moderators and Mediators Differ Between the Baron & Kenny and MacArthur Approaches. Health Psychology. 2008;27(2 Suppl):S101–S108. doi: 10.1037/0278-6133.27.2(Suppl.).S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, … Boileau RA. Ageing, fitness and neurocognitive function. Nature. 1999;400(6743):418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Lambiase MJ, Gabriel KP, Kuller LH, Matthews KA. Temporal relationships between physical activity and sleep in older women. Medicine & Science in Sports & Exercise. 2013;45(12) doi: 10.1249/MSS.0b013e31829e4cea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambiase MJ, Gabriel KP, Kuller LH, Matthews KA. Sleep and Executive Function in Older Women: The Moderating Effect of Physical Activity. The Journals of Gerontology: Series A. 2014;69(9):1170–1176. doi: 10.1093/gerona/glu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Boot WR, Basak C, Voss MW, Prakash RS, Neider M, … Gratton G. Performance gains from directed training do not transfer to untrained tasks. Acta Psychologica. 2012;139(1):146–158. doi: 10.1016/j.actpsy.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychological bulletin. 2010;136(3):375. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo JC, Groeger JA, Cheng GH, Dijk DJ, Chee MW. Self-reported sleep duration and cognitive performance in older adults: a systematic review and meta-analysis. Sleep Medicine. 2016;17:87–98. doi: 10.1016/j.sleep.2015.08.021. [DOI] [PubMed] [Google Scholar]
- Lopez M. Exercise and Sleep Quality. In: Spirduso WW, Poon LW, Chodzko-Zajko W, editors. Exercise and Its Mediating Effects on Cognition. Vol. 2. Champaigne, IL: Human Kinetics; 2008. [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, … Walker MP. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nature Neuroscience. 2013 doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquet P, Degueldre C, Delfiore G, Aerts J, Peters JM, Luxen A, Franck G. Functional neuroanatomy of human slow wave sleep. Journal of Neuroscience. 1997;17(8):2807–2812. doi: 10.1523/JNEUROSCI.17-08-02807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ, Begley AE, Billy BD, Fletcher ME. Effects of a Two-Hour Change in Bedtime on the Sleep of Healthy Seniors. Chronobiology International. 2009;26(3):526–543. doi: 10.1080/07420520902821119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery P, Dennis JA. Physical exercise for sleep problems in adults aged 60+ The Cochrane Database of Systematic Reviews. 2002;(4) doi: 10.1002/14651858.CD003404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, … Clark C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/WNL.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Muzur A, Pace-Schott EF, Hobson JA. The prefrontal cortex in sleep. Trends in Cognitive Science. 2002;6(11):475–481. doi: 10.1016/S1364-6613(02)01992-7. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Buysse DJ, Halligan EM, Houck PR, Monk TH. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. The Journals of Gerontology: Series B. 2009;64(2):180–187. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckelmann D, Ursin R. Sleep stages and EEG power spectrum in relation to acoustical stimulus arousal threshold in the rat. Sleep. 1993;16(5):467–477. [PubMed] [Google Scholar]
- Nelson HE, Willison J. National Adult Reading Test (NART) Nfer-Nelson; 1991. [Google Scholar]
- Pace-Schott EF, Spencer RM. Age-related changes in the cognitive function of sleep. In: Green AM, Chapman CE, Kalaska JF, Lepore F, editors. Progress in Brain Research. Vol. 191. New York, NY: Elsevier; 2011. [DOI] [PubMed] [Google Scholar]
- Picchioni D, Duyn JH, Horovitz SG. Sleep and the functional connectome. NeuroImage. 2013;80:387–396. doi: 10.1016/j.neuroimage.2013.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavioral Research Methods. 2008;40(3):879–891. doi: 10.3758/BRM.40.3.879. [DOI] [PubMed] [Google Scholar]
- Quan SF, Budhiraja R, Batool-Anwar S, Gottlieb DJ, Eichling P, Patel S, … Kushida CA. Lack of impact of mild obstructive sleep apnea on sleepiness, mood and quality of life. Southwest Journal of Pulmonary & Critical Care. 2014;9(1):44. doi: 10.13175/swjpcc082-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan SF, Wright R, Baldwin CM, Kaemingk KL, Goodwin JL, Kuo TF, … Bootzin RR. Obstructive sleep apnea–hypopnea and neurocognitive functioning in the Sleep Heart Health Study. Sleep Medicine. 2006;7(6):498–507. doi: 10.1016/j.sleep.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Reid KJ, Baron KG, Lu B, Naylor E, Wolfe L, Zee PC. Aerobic exercise improves self-reported sleep and quality of life in older adults with insomnia. Sleep Medicine. 2010;11(9):934–940. doi: 10.1016/j.sleep.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan Validity of the trail making test as an indicator of organic brain disease. Perceptual and Motor Skills. 1958;8:271–276. doi: 10.2466/pms.1958.8.3.271. [DOI] [Google Scholar]
- Royall DR, Lauterbach EC, Cummings JL, Reeve A, Rummans TA, Kaufer DI, … Coffey CE. Executive control function: a review of its promise and challenges for clinical research. A report from the Committee on Research of the American Neuropsychiatric Association. The Journal of Neuropsychiatry and Clinical Neurosciences. 2002;14(4):377–405. doi: 10.1176/jnp.14.4.377. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103(3):403. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Slater JA, Botsis T, Walsh J, King S, Straker LM, Eastwood PR. Assessing sleep using hip and wrist actigraphy. Sleep & Biological Rhythms. 2015;13(2):172–180. doi: 10.1111/sbr.12103. [DOI] [Google Scholar]
- Steptoe A, Peacey V, Wardle J. Sleep duration and health in young adults. Archives of internal medicine. 2006;166(16):1689–1692. doi: 10.1001/archinte.166.16.1689. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262(5134):679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153(3736):652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18(6):643. doi: 10.1037/h0054651. [DOI] [Google Scholar]
- Sunseri M, Liden C, Farringdon J, Pelletier R, Safier S, Stivoric J, … Vishnubhatla S. The SenseWear armband as a Sleep Detection Device. 2009:1–9. [Google Scholar]
- Tomita H, Ohbayashi M, Nakahara K, Hasegawa I, Miyashita Y. Top-down signal from prefrontal cortex in executive control of memory retrieval. Nature. 1999;401(6754):699–703. doi: 10.1038/44372. [DOI] [PubMed] [Google Scholar]
- Tryon WW. Issues of validity in actigraphic sleep assessment. Sleep. 2004;27(1):158–165. doi: 10.1093/sleep/27.1.158. [DOI] [PubMed] [Google Scholar]
- Turken Whitfield-Gabrieli S, Bammer R, Baldo J, Dronkers NF, Gabrieli JDE. Cognitive processing speed and the structure of white matter pathways: Convergent evidence from normal variation and lesion studies. NeuroImage. 2008;42(2):1032–1044. doi: 10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tworoger SS, Yasui Y, Vitiello MV, Schwartz RS, Ulrich CM, Aiello EJ, … McTiernan A. Effects of a yearlong moderate-intensity exercise and a stretching intervention on sleep quality in postmenopausal women. Sleep. 2003;26(7):830–838. doi: 10.1093/sleep/26.7.830. [DOI] [PubMed] [Google Scholar]
- Velanova K, Jacoby LL, Wheeler ME, McAvoy MP, Petersen SE, Buckner RL. Functional–anatomic correlates of sustained and transient processing components engaged during controlled retrieval. The Journal of Neuroscience. 2003;23(24):8460–8470. doi: 10.1523/JNEUROSCI.23-24-08460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello MV. Exercise, Sleep, and Cognition: Interactions in Aging. In: Spirduso WW, Poon LW, Chodzko-Zajko W, editors. Exercise and Its Mediating Effects on Cognition. Vol. 2. Champaigne, IL: Human Kinetics; 2008. [Google Scholar]
- Walker MP. The role of sleep in cognition and emotion. Annals of the New York Academy of Sciences. 2009;1156(1):168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. WAIS-III. Administration and scoring manual. 3. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychological bulletin. 1996;120(2):272. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Wilckens KA, Erickson KI, Wheeler ME. Age-related decline in controlled retrieval: The role of the PFC and sleep. Neural Plasticity, 2012. 2012 doi: 10.1155/2012/624795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilckens KA, Hall MH, Nebes RD, Monk TH, Buysse DJ. Changes in Cognitive Performance Are Associated with Changes in Sleep in Older Adults With Insomnia. Behavioral Sleep Medicine. 2016;14:1–16. doi: 10.1080/15402002.2014.1002034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilckens KA, Woo SG, Erickson KI, Wheeler ME. Sleep continuity and total sleep time are associated with task-switching and preparation in young and older adults. Journal of Sleep Research. 2014 doi: 10.1111/jsr.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilckens KA, Woo SG, Kirk AR, Erickson KI, Wheeler ME. The role of sleep continuity and total sleep time in executive function across the adult lifespan. Psychology & Aging. 2014;29(3) doi: 10.1037/a0037234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J, Angevaren M, Rusted J, Tabet N. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. The Cochrane Library. 2015 doi: 10.1002/14651858.CD005381.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstedt SD. Effects of exercise on sleep. Clinics in Sports Medicine. 2005;24(2):355–365. doi: 10.1016/j.csm.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Youngstedt SD, Kripke DF. Long sleep and mortality: rationale for sleep restriction. Sleep Medicine Reviews. 2004;8(3):159–174. doi: 10.1016/j.smrv.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Zhao X, Lynch JGJ, Chen Q. Reconsidering Baron and Kenny: Myths and Truths about Mediation Analysis. Journal of Consumer Research. 2010;37 doi: 10.1086/651257. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.