Abstract
Blood pressure (BP) can differ substantially when measured in the clinic versus outside of the clinic setting. Few population-based studies with ambulatory blood pressure monitoring (ABPM) include African Americans. We calculated the prevalence of clinic hypertension and ABPM phenotypes among 1,016 participants in the population-based Jackson Heart Study, an exclusively African-American cohort. Mean daytime systolic BP was higher than mean clinic systolic BP among participants not taking antihypertensive medication (127.1[standard deviation 12.8] versus 124.5[15.7] mmHg, respectively) and taking antihypertensive medication (131.2[13.6] versus 130.0[15.6] mmHg, respectively). Mean daytime diastolic BP was higher than clinic diastolic BP among participants not taking antihypertensive medication (78.2[standard deviation 8.9] versus 74.6[8.4] mmHg, respectively) and taking antihypertensive medication (77.6[9.4] versus 74.3[8.5] mmHg, respectively). The prevalence of daytime hypertension was higher than clinic hypertension for participants not taking antihypertensive medication (31.8% versus 14.3%) and taking antihypertensive medication (43.0% versus 23.1%). A high percentage of participants not taking and taking antihypertensive medication had nocturnal hypertension (49.4% and 61.7%, respectively), white coat hypertension (30.2% and 29.3%, respectively), masked hypertension (25.4% and 34.6%, respectively), and a non-dipping BP pattern (62.4% and 69.6%, respectively). In conclusion, these data suggest hypertension may be misdiagnosed among African Americans without using ABPM.
Keywords: Ambulatory blood pressure monitoring, nocturnal hypertension, masked hypertension, non-dipping, African American
Blood pressure (BP) often differs when measured in the clinic and outside of the clinic setting.1 Ambulatory blood pressure monitoring (ABPM) may provide a better estimate of an individual’s BP than measurements taken during a clinic visit by obtaining measurements every 15 to 30 minutes, typically over 24 hours, as well as measuring BP in a more natural environment.2,3 In addition to obtaining mean BP levels, several measures can be determined from ABPM including daytime, nocturnal and 24-hour hypertension and a non-dipping BP pattern. ABPM can also be used to identify mismatches between clinic and out-of-clinic BP levels including white-coat and masked hypertension.1-3 Several ABPM phenotypes, including masked hypertension, nocturnal hypertension, and a non-dipping BP pattern have been associated with an increased risk for target-organ damage and cardiovascular disease outcomes, independent of clinic BP levels.4-6
Previous population-based studies have reported a high prevalence of ABPM phenotypes in whites and Asians, but few data exist on the prevalence of ABPM phenotypes in the general population of African Americans.7 The prevalence of hypertension, based on clinic BP measurements, is high among African Americans.8,9 Additionally, a high prevalence of nocturnal hypertension and non-dipping BP has been reported in African Americans.10-13 However, many of these studies were performed in narrowly defined clinic populations (e.g., patients with kidney disease) with relatively small sample sizes. Population-based studies of African Americans are needed to obtain accurate prevalence estimates of ABPM phenotypes. If the prevalence of ABPM phenotypes is high in African Americans, it may support the use of ABPM in this population. Therefore, we determined mean BP levels based on clinic measurements and ABPM and the prevalence of clinic hypertension and ABPM phenotypes in the Jackson Heart Study (JHS), a population-based study comprised exclusively of African Americans.
Methods
Study population
The JHS is a population-based study designed to investigate cardiovascular disease in African Americans. Details regarding the design of the JHS have been published elsewhere.14,15 The JHS enrolled 5,306 African Americans, 20-95 years of age, between 2000 and 2004 from urban and rural areas of 3 counties (Hinds, Madison, and Rankin) that comprise the Jackson, Mississippi metropolitan area. We conducted a cross-sectional analysis using JHS data from the baseline examination among participants who underwent ABPM (n=1,146). We restricted these analyses to 1,016 participants with a complete ABPM recording (defined below), clinic BP measurements, and information on self-reported antihypertensive medication use. The protocol for the JHS was approved by the institutional review boards at the participating institutions, including Jackson State University, Tougaloo College, and the University of Mississippi Medical Center. All participants provided written informed consent prior to participation. The analyses of JHS data for the current manuscript were approved by the Institutional Review Board at the University of Alabama at Birmingham.
Data collection
Baseline data were collected during an in-home interview and a clinic examination. Intervieweradministered questionnaires were used to collect information on age, sex, highest level of education obtained, current smoking, self-reported medication use, and history of cardiovascular disease (CVD). Antihypertensive medication use was determined by self-report and statin use was defined based on a pill bottle review. During the examination, trained staff measured height, weight, and clinic BP. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Current smoking was defined by affirmative responses to the questions “Have you smoked >400 cigarettes in your lifetime?” and “Do you now smoke cigarettes?” Fasting total cholesterol, serum glucose, and hemoglobin A1c (HbA1c) were measured from blood samples obtained during the clinic examination. Diabetes was defined as a fasting glucose ≥ 126 mg/dL, HbA1c ≥ 6.5% (48 mmol/mol), or use of insulin or other glucose lower medications within 2 weeks prior to the examination. Urinary albumin and creatinine were quantified from a 24-hour urine collection or from a spot urine sample using the nephelometric immunoassay and enzymatic methods, respectively.15 Albuminuria was defined as a urinary albumin/creatinine ratio ≥ 30 mg/g. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.16 Reduced eGFR was defined as <60 mL/min/1.73 m2. Chronic kidney disease (CKD) was defined by the presence of albuminuria or reduced eGFR. History of CVD was defined as a history of myocardial infarction per self-report or electrocardiogram, self-reported history of carotid angioplasty, or self-reported history of stroke. Following the clinic examination, a subset of participants completed ABPM.
Clinic BP measurements
Clinic BP was measured by trained staff using a Hawksley random zero sphygmomanometer and Littman stethoscope following a standardized protocol. Each participant’s right arm circumference was measured at the midpoint of the upper arm to determine the appropriate cuff size. Participants rested for 5 minutes prior to their BP measurement. Two BP measurements were taken 1 minute apart while the participant was seated in an upright position with their feet flat on the floor and back supported. The average of these two measurements was used for the current analyses. As described previously, the random zero BP measurements were calibrated to a semi-automated oscillometric device (Omron HEM-907XL, Omron Healthcare Inc., Lake Forest, IL).17
ABPM measurements
ABPM was conducted using a SpaceLabs 90207 oscillometric device. The appropriate cuff size was determined by measuring the participant’s non-dominant arm circumference at the midpoint of the upper arm.15 Measurements were recorded every 20 minutes over a 24-hour monitoring period. Using the International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes (IDACO) criteria, daytime was defined as 10am to 8pm and nighttime was 12am to 6am.18 Participants with at least 10 daytime and 5 nighttime systolic blood pressure (SBP) and diastolic blood pressure (DBP) measurements were considered to have a complete ABPM recording.
Outcome definitions
Clinic hypertension was defined as mean clinic SBP ≥ 140 mmHg or mean clinic DBP ≥ 90 mmHg. The average of all ABPM measurements between 10am and 8pm, midnight and 6am, and over the full monitoring period were used to define mean daytime, nighttime, and 24-hour BP levels, respectively. ABPM phenotypes evaluated in the current study include daytime hypertension, nocturnal hypertension, 24-hour hypertension, sustained hypertension, white coat hypertension, and masked hypertension. These phenotypes are defined in Supplemental Table 1. While masked hypertension usually describes individuals not taking antihypertensive medication and masked uncontrolled hypertension describes individuals taking antihypertensive medication, we simplified the current definition for masked hypertension to refer to all participants regardless of antihypertensive medication use. Also, SBP dipping ratio was defined as the percent decline from daytime to nighttime and was calculated as [(mean daytime SBP − mean nighttime SBP)/mean daytime SBP]. A non-dipping BP pattern was defined as a dipping ratio >0.90.19
Statistical analyses
All analyses were stratified by antihypertensive medication use, as we hypothesized that the prevalence of ABPM phenotypes would be substantially higher for participants taking versus not taking antihypertensive medication. Additionally, analyses were conducted for the overall population and in subgroups defined by age (<45, 45-64 and ≥65 years), sex, and clinic BP level (SBP/DBP < 120/80 mm Hg, 120-139/80-89 mm Hg, and ≥ 140/90 mmHg). We calculated mean clinic, daytime, nighttime, and 24-hour SBP and DBP. Among participants with clinic hypertension, we calculated the white coat effect as clinic BP minus daytime BP. Among participants without clinic hypertension, we calculated the masked effect as daytime BP minus clinic BP. The statistical significance of differences in the white coat effect and the masked effect across sub-groups was calculated by analyses of variance. The prevalence and 95% confidence interval of clinic, daytime, and sustained hypertension was calculated. The prevalence and 95% confidence interval of white coat hypertension was calculated among participants with clinic hypertension and the prevalence and 95% confidence interval of masked hypertension was calculated among participants without clinic hypertension. We calculated the prevalence and 95% confidence interval of nocturnal hypertension, 24-hour hypertension, a nondipping BP pattern, and dipping ratio categories (≤0.8, >0.8 to 0.9, >0.9 to 1.0, >1.0). We used chi-square tests to determine the statistical significance of differences in the prevalence of ABPM phenotypes among participants taking versus not taking antihypertensive medication. P-values <0.05 were considered statistically significant. All analyses were conducted using SAS Version 9.4 (SAS Institute, Cary NC).
Results
Mean clinic and ABPM blood pressure levels
Participants taking antihypertensive medication were older and more likely to be female, have less than a high school education, be taking a statin, and have diabetes, CKD, and a history of CVD compared with their counterparts not taking antihypertensive medication (Table 1). Participants taking antihypertensive medication had a higher BMI and lower total cholesterol levels.
Table 1.
Characteristics of the Jackson Heart Study participants included in the current analysisby antihypertensive medication use.
| Taking antihypertensive medication | ||
|---|---|---|
| No (n=441) | Yes (n=575) | |
| Age in years, % | ||
| <45 | 21.1 | 5.7 |
| 45-64 | 57.4 | 53.9 |
| ≥ 65 | 21.5 | 40.4 |
| Female, % | 63.3 | 72.0 |
| Less than high school education, % | 14.6 | 22.3 |
| Current smoking, % | 11.9 | 8.9 |
| Body mass index, kg/m2 | 30.0 ± 6.4 | 32.0 ± 6.3 |
| Fasting total cholesterol, mg/dL | 203.5 ± 40.6 | 199.8 ± 39.1 |
| Statin medication use, % | 4.5 | 20.7 |
| Diabetes mellitus, % | 12.1 | 34.6 |
| Chronic kidney disease, % | 7.6 | 21.1 |
| History of cardiovascular disease, % | 6.4 | 13.7 |
| Clinic BP, mmHg, % | ||
| SBP/DBP < 120/80 | 37.2 | 25.9 |
| SBP/DBP 120-139/80-89 | 48.5 | 51.0 |
| SBP/DBP ≥ 140/90 | 14.3 | 23.1 |
Numbers in the table are percentage, except for body mass index and total cholesterol, which are presented as mean ± standard deviation.
BP: Blood pressure.
SBP: Systolic blood pressure.
DBP: Diastolic blood pressure.
Mean daytime SBP and DBP were higher than mean clinic SBP and DBP among participants not taking and taking antihypertensive medication, overall, and in all subgroups except for those with clinic SBP/DBP ≥ 140/90 mmHg (Supplemental Table 2). Among participants with clinic SBP/DBP ≥ 140/90 mmHg, mean daytime SBP was lower than mean clinic SBP and mean daytime DBP was within one mmHg of mean clinic DBP. Mean nighttime SBP and DBP were lower than mean clinic, daytime, or 24-hour SBP and DBP, respectively.
White coat effect
Among participants with clinic SBP/DBP ≥ 140/90, the mean white coat effect for SBP was 11.4 mmHg and 11.2 mmHg for those not taking and taking antihypertensive medication, respectively (Supplemental Table 3). The white coat effect for SBP did not differ by age or sex. The white coat effect for DBP was not statistically significant among participants not taking and taking antihypertensive medication.
Masked effect
Among participants without clinic hypertension (clinic SBP/DBP < 140/90), the mean masked effect was 4.9 mmHg and 5.0 mmHg for those not taking and taking antihypertensive medication, respectively (Supplemental Table 4). Among participants taking antihypertensive medication, the masked effect for SBP was larger in males compared with females. The mean masked effect for DBP was 4.2 mmHg and 4.3 mmHg among participants not taking and taking antihypertensive medication, respectively. Among participants not taking and taking antihypertensive medication, the masked effect for DBP was larger in males compared with females.
Prevalence of ABPM phenotypes
Among participants not taking and taking antihypertensive medication, the prevalence of daytime, nocturnal, and 24-hour hypertension were each higher than the prevalence of clinic hypertension, overall, (Figure 1) and in all subgroups (Table 2). Among participants not taking and taking antihypertensive medication, the prevalence of sustained hypertension was 10.0% and 16.4%, respectively. Among participants with SBP/DBP ≥ 140/90 mmHg, the prevalence of white-coat hypertension was 30.2% and 29.3% for those not taking and taking antihypertensive medication, respectively. Among participants with SBP/DBP < 140/90 mmHg, the prevalence of masked hypertension was 25.4% and 34.6% for those not taking and taking antihypertensive medication, respectively. A non-dipping BP pattern was present in 62.4% of participants and 69.6% of participants not taking and taking antihypertensive medication, respectively (Supplemental Table 5). Reverse dipping (dipping ratio >1.00) was present in 10.4% of participants not taking antihypertensive medication and 23.8% of participants taking antihypertensive medication (Figure 2).
Figure 1. Prevalence of clinic hypertension and ambulatory blood pressure monitoring phenotypes among participants taking and not taking antihypertensive medication.
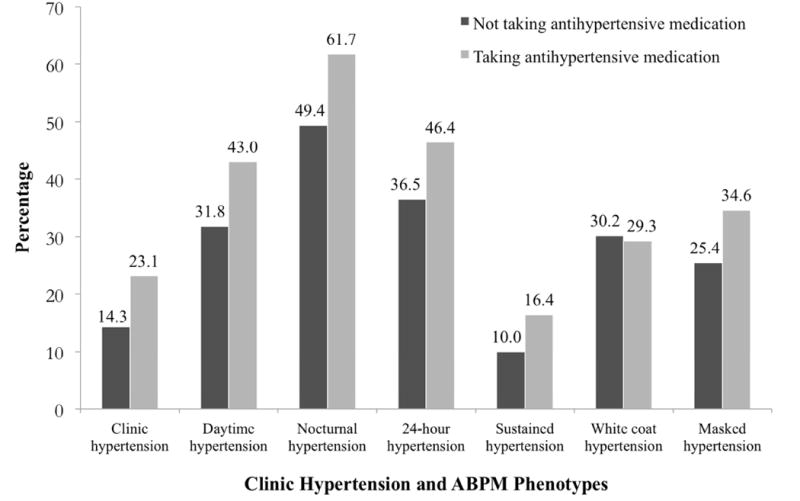
ABPM: Ambulatory blood pressure monitoring.
Definitions of clinic hypertension and ABPM phenotypes are provided in Table 1.
The prevalence of white coat hypertension was calculated among participants with systolic/diastolic blood pressure ≥ 140/90 mm Hg and the prevalence of masked hypertension was calculated among participants with systolic/diastolic blood pressure < 140/90 mm Hg.
Each p-value was < 0.01 comparing the prevalence of clinic hypertension, daytime hypertension, nocturnal hypertension, 24-hour hypertension, sustained hypertension, and masked hypertension for participants taking and not taking antihypertensive medication. The p-value was 0.905 comparing the prevalence of white coat hypertension for participants taking and not taking antihypertensive medication.
Table 2.
Prevalence of clinic hypertension and ambulatory blood pressure monitoring phenotypes for participants not taking antihypertensive medication (top panel) and taking antihypertensive medication (bottom panel).
| Clinic | Hypertension phenotype on ambulatory blood pressure monitoring | ||||||
|---|---|---|---|---|---|---|---|
| Daytime | Nocturnal | 24-Hour | Sustained | White Coat | Masked | ||
| Not taking antihypertensive medication | |||||||
| Overall | 14.3 (11.2-17.9) | 31.8 (27.4-36.3) | 49.4 (44.7-54.2) | 36.5 (32.0-41.2) | 10.0 (7.3-13.2) | 30.2 (19.2-43.0) | 25.4 (21.1-30.1) |
| Age groups, years | |||||||
| <45 | 7.5 (3.1-14.9) | 20.4 (12.8-30.1) | 32.3 (22.9-42.8) | 21.5 (13.7-31.2) | 5.4 (1.8-12.1) | 28.6 (3.7-71.0) | 16.3 (9.2-25.8) |
| 45-64 | 13.8 (9.8-18.7) | 30.0 (24.5-36.1) | 49.8 (43.5-56.1) | 36.8 (30.8-43.0) | 9.9 (6.5-14.2) | 28.6 (14.6-46.3) | 23.4 (17.9-29.6) |
| ≥ 65 | 22.1 (14.2-31.8) | 47.4 (37.0-57.9) | 65.3 (54.8-74.7) | 50.5 (40.1-61.0) | 14.7 (8.3-23.5) | 33.3 (14.6-57.0) | 41.9 (30.5-53.9) |
| Male | 18.5 (12.9-25.4) | 39.5 (31.9-47.5) | 58.0 (50.0-65.7) | 43.2 (35.5-51.2) | 14.8 (9.7-21.2) | 20.0 (7.7-38.6) | 30.3 (22.6-38.9) |
| Female | 11.8 (8.3-16.2) | 27.2 (22.1-32.9) | 44.4 (38.5-50.5) | 32.6 (27.2-38.5) | 7.2 (4.4-10.9) | 39.4 (22.9-57.9) | 22.8 (17.7-28.5) |
| Clinic BP, mmHg | |||||||
| SBP/DBP < 120/80 | 7.9 (4.3-13.2) | 25.6 (19.1-33.0) | 11.0 (6.6-16.8) | 7.9 (4.3-13.2) | |||
| SBP/DBP 120-139/80-89 | 38.8 (32.2-45.7) | 57.9 (51.0-64.6) | 44.9 (38.1-51.8) | 38.8 (32.2-45.7) | |||
| SBP/DBP ≥ 140/90 | 100.0 | 69.8 (57.0-80.8) | 82.5 (70.9-91.0) | 74.6 (62.1-84.7) | 69.8 (57.0-80.8) | 30.2 (19.2-43.0) | |
| Taking antihypertensive medication | |||||||
| Overall | 23.1 (19.7-26.8) | 43.0 (38.9-47.1) | 61.7 (57.6-65.7) | 46.4 (42.3-50.6) | 16.4 (13.4-19.6) | 29.3 (21.8-37.8) | 34.6 (30.2-39.3) |
| Age groups, years | |||||||
| <45 | 3.0 (0.1-15.8) | 36.4 (20.4-54.9) | 60.6 (42.1-77.1) | 33.3 (18.0-51.8) | 3.0 (0.1-15.8) | 34.4 (18.6-53.2) | |
| 45-64 | 21.0 (16.6-25.9) | 41.0 (35.4-46.7) | 57.1 (51.4-62.7) | 43.2 (37.6-48.9) | 13.6 (9.9-17.9) | 35.4 (23.9-48.2) | 34.7 (28.8-41.0) |
| ≥ 65 | 28.9 (23.1-35.2) | 46.6 (40.0-53.2) | 68.1 (61.7-74.1) | 52.6 (46.0-59.2) | 22.0 (16.8-27.9) | 23.9 (14.3-35.9) | 34.6 (27.3-42.3) |
| Male | 27.3 (20.6-34.9) | 54.7 (46.6-62.5) | 77.0 (69.7-83.3) | 62.1 (54.1-69.6) | 21.1 (15.1-28.2) | 22.7 (11.5-37.8) | 46.2 (36.9-55.6) |
| Female | 21.5 (17.6-25.8) | 38.4 (33.7-43.3) | 55.8 (50.9-60.7) | 40.3 (35.6-45.2) | 14.5 (11.3-18.3) | 32.6 (23.0-43.3) | 30.5 (25.5-35.8) |
| Clinic BP, mmHg | |||||||
| SBP/DBP < 120/80 | 22.2 (15.8-29.7) | 43.0 (34.9-51.3) | 23.5 (16.9-31.1) | 22.2 (15.8-29.7) | |||
| SBP/DBP 120-139/80-89 | 41.0 (35.3-46.8) | 60.4 (54.6-66.1) | 44.0 (38.3-49.9) | 41.0 (5.3-46.8) | |||
| SBP/DBP ≥ 140/90 | 100.0 | 70.7 (62.2-78.3) | 85.7 (78.6-91.2) | 77.4 (69.4-84.2) | 70.7 (62.2-78.3) | 29.3 (21.8-37.8) | |
Numbers in table are percentage (95% confidence interval).
Cells are greyed out for values that are 0%.
95% confidence intervals are not provided because, by definition, participants with SBP/DBP ≥ 140/90 mm Hg have clinic hypertension.
BP: blood pressure; SBP: systolic blood pressure; DBP: diastolic blood pressure.
Figure 2. Distribution of participants taking and not taking antihypertensive medication by dipping ratio categories.
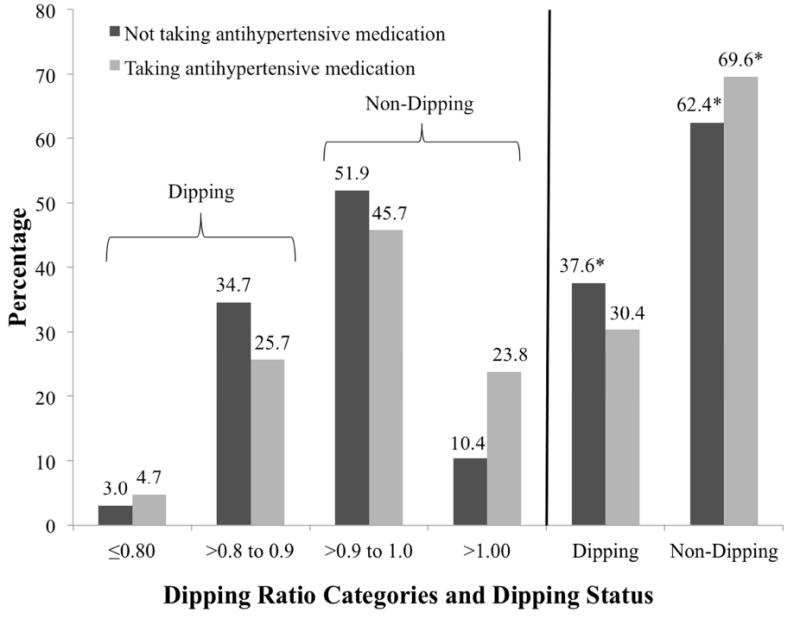
* The prevalence of dipping ratio categories ≤0.80 and >0.80 to 0.90 may not sum to the dipping category and the prevalence of dipping ratio categories >0.90 to 1.00 and >1.00 may not sum to the no dipping category due to rounding.
The p-value was <0.05 comparing the prevalence of non-dipping status for participants taking and not taking antihypertensive medication.
The p-value was < 0.001 comparing the prevalence of dipping ratio categories for participants taking and not taking antihypertensive medication.
Discussion
In the current analysis of a population-based study of African Americans, mean daytime SBP was higher than clinic SBP in participants not taking and taking antihypertensive medication. Mean DBP was also higher than clinic DBP. The prevalence of daytime, nocturnal, and 24-hour hypertension were each higher than the prevalence of clinic hypertension. There was a substantial mismatch in the diagnosis of hypertension using clinic BP and ABPM. Approximately 30% of participants with clinic SBP/DBP ≥ 140/90 mmHg had white coat hypertension, while over 25% of participants with clinic SBP/DBP < 140/90 mmHg had masked hypertension. Additionally, a majority of participants had a non-dipping BP pattern. These data indicate that hypertension may be misdiagnosed in a substantial percentage of African Americans without using ABPM.
BP measured outside of the clinic setting is known to differ from measurements in the clinic setting.20 Elevated ambulatory BP is associated with target-organ damage and cardiovascular disease events, independent of clinic BP.4,21,22 Previous studies have reported that African Americans have both higher clinic and out-of-clinic BP compared with whites.8,12,23 There are few population-based studies of ABPM conducted in African Americans and, as a result, previous results may have limited generalizability. In a previous population-based study, African Americans had higher daytime and nighttime SBP when compared with whites, even after multivariable adjustment that included clinic BP.23 However, the sample size of African Americans was relatively small (n=178) and the population was comprised entirely of young adults (mean age: 29.8 years; range 23 to 35 years). In the current study, mean daytime SBP was higher than clinic SBP in all subgroups except for participants with clinic SBP/DBP ≥ 140/90 mmHg. This finding suggests African Americans may experience higher BP levels than what is measured in the clinic setting. This mismatch should be considered in the management of hypertension in African Americans.
The white-coat effect, higher clinic BP compared with out-of-clinic BP, and masked effect, higher out-of-clinic BP compared with clinic BP, are terms used to describe the mismatch between clinic and out-of-clinic BP. It is estimated that 15% to 30% of adults diagnosed with hypertension based on clinic BP may have white-coat hypertension.21 White-coat hypertension is not associated with increased risk for cardiovascular outcomes or mortality when compared with sustained normotensive BP (clinic SBP/DBP < 140/90 mm Hg and daytime SBP/DBP ≥ 135/85 mm Hg).22,24 In the current study, 30.2% of African Americans not taking antihypertensive medication and 29.3% of African Americans taking antihypertensive medication had white-coat hypertension. These data suggest a substantial percentage of African Americans may be overdiagnosed with hypertension and over-treated with antihypertensive medication. Also, it is estimated that 15% to 30% of individuals with non-elevated clinic BP have masked hypertension, a phenotype that is associated with increased risk of target-organ damage and cardiovascular events.25 The current analyses suggest that a substantial percentage of African Americans taking and not taking antihypertensive medication have masked hypertension. Therefore, a large number of African Americans may be under-diagnosed and under-treated. It has been suggested that the higher prevalence of masked hypertension in individuals taking versus not taking antihypertensive medication, as found in the current study, is the result of the over-reliance on clinic BP, converting patients with sustained hypertension to masked hypertension.26 Additionally, given the high prevalence of participants with clinic SBP 120-139/DBP 80-89 mmHg in the current study and the high risk for hypertension-related cardiovascular disease in African Americans, the use of ABPM may be particularly important for the detection of masked hypertension in this population. These findings support recommendations that ABPM be used for the diagnosis of hypertension and guiding antihypertensive treatment.21,22,27,28
In the current analysis, the prevalence of daytime, nocturnal, 24-hour and masked hypertension were high and a majority of participants had a non-dipping BP pattern. These findings are consistent with other studies reporting a high prevalence of ABPM phenotypes, as well as a nondipping BP pattern, among African Americans.10-13 Furthermore, the prevalence of ABPM phenotypes were higher among participants taking versus not taking antihypertensive medication, suggesting that ABPM may be particularly useful in the management of hypertension in African Americans. The current study extends previous results from clinic-based samples to a population-based cohort that provides more generalizability to the larger population of African Americans. Previous studies have also demonstrated that people with nocturnal hypertension and/or a non-dipping BP pattern are at increased risk for target-organ damage and cardiovascular events.4-6 Home blood pressure monitoring (HBPM) has been suggested as a possible alternative to ABPM.29 Although the United States Preventive Services Task Force (USPSTF) found good evidence supporting the use of HBPM to identify white coat hypertension, current devices to conduct HBPM that are available in the US are unable to measure BP at night and, therefore, cannot identify nocturnal hypertension or a non-dipping BP pattern. Given the high prevalence of nocturnal hypertension and non-dipping BP in African Americans, HBPM may not be as beneficial as ABPM in this population.
The strengths of this study include the use of a large population-based sample of African Americans, data collection following a standardized protocol, and conduct of clinic BP and ABPM following a standardized protocol. Despite these strengths, the results need to be interpreted in the context of several limitations. Clinic BP was measured using a random-zero sphygmomanometer, a device that is no longer used in the US. However, we were able to calibrate random-zero measurements to a semi-automated oscillometric device. Additionally, clinic BP data were based on the average of two BP measurements obtained at a single visit, whereas current recommendations suggest obtaining measurements at a minimum of two separate visits.30 ABPM phenotypes were based on a single 24-hour assessment. While the reproducibility of many ABPM phenotypes is high, some phenotypes may not be present on repeat testing.31-33 ABPM was a voluntary procedure in the Jackson Heart Study and only a subsample of participants completed it. Although the Jackson Heart Study enrolled a large number of African Americans, all participants resided in the Jackson, MS metropolitan area and these results may not generalize to African Americans in other regions of the US. Lastly, BP cut-points for ABPM phenotypes were derived using data collected on Europeans and Asians; normative data do not exist for African Americans.
In conclusion, daytime SBP and DBP were higher than clinic SBP and DBP, respectively, in this population-based sample of African Americans. Furthermore, daytime, nocturnal and 24-hour hypertension were each more prevalent than clinic hypertension, a substantial percentage of participants with clinic hypertension had white coat hypertension, a substantial percentage of participants without clinic hypertension had masked hypertension, and a majority of participants had a non-dipping BP pattern. These findings suggest that hypertension may be misdiagnosed in African Americans and the reliance on clinic BP may be sub-optimal for the detection and management of hypertension.
Supplementary Material
Highlights.
Ambulatory blood pressure monitoring was conducted in African Americans.
Daytime hypertension was more prevalent than clinic hypertension.
Nocturnal hypertension was more prevalent than clinic hypertension.
White coat hypertension and masked hypertension were highly prevalent.
Use of ambulatory blood pressure monitoring may be warranted in this population.
Acknowledgments
The Jackson Heart Study is supported by contracts HHSN268201300046C, HSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities.
The authors thank the Jackson Heart Study team (University of Mississippi Medical Center Jackson State University, and Tougaloo College) and participants for their long-term commitment and significant contributions to the study. This analysis was supported by grant R01 HL117323 and K24-HL125704 from the National Heart, Lung, and Blood Institute and grant 15SFRN2390002 from the American Heart Association.
Footnotes
Disclaimer
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med. 2006;354(22):2368–2374. doi: 10.1056/NEJMra060433. [DOI] [PubMed] [Google Scholar]
- Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5):697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- Parati G, Stergiou G, O’Brien E, et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32(7):1359–1366. doi: 10.1097/HJH.0000000000000221. [DOI] [PubMed] [Google Scholar]
- Conen D, Bamberg F. Noninvasive 24-h ambulatory blood pressure and cardiovascular disease: a systematic review and meta-analysis. J Hypertens. 2008;26(7):1290–1299. doi: 10.1097/HJH.0b013e3282f97854. [DOI] [PubMed] [Google Scholar]
- Diaz KM, Veerabhadrappa P, Brown MD, Whited MC, Dubbert PM, Hickson DA. Prevalence, Determinants, and Clinical Significance of Masked Hypertension in a Population-Based Sample of African Americans: The Jackson Heart Study. Am J Hypertens. 2015;28(7):900–908. doi: 10.1093/ajh/hpu241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HQ, Li Y, Thijs L, et al. Prognostic value of isolated nocturnal hypertension on ambulatory measurement in 8711 individuals from 10 populations. J Hypertens. 2010;28(10):2036–2045. doi: 10.1097/HJH.0b013e32833b49fe. [DOI] [PubMed] [Google Scholar]
- Li Y, Wei FF, Thijs L, et al. Ambulatory hypertension subtypes and 24-hour systolic and diastolic blood pressure as distinct outcome predictors in 8341 untreated people recruited from 12 populations. Circulation. 2014;130(6):466–474. doi: 10.1161/CIRCULATIONAHA.113.004876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303(20):2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10):1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- Pogue V, Rahman M, Lipkowitz M, et al. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension. 2009;53(1):20–27. doi: 10.1161/HYPERTENSIONAHA.108.115154. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Stepnowsky C, Dimsdale J, Marler M, Cohen-Zion M, Johnson S. The effect of race and sleep-disordered breathing on nocturnal BP “dipping”: analysis in an older population. Chest. 2002;122(4):1148–1155. doi: 10.1378/chest.122.4.1148. [DOI] [PubMed] [Google Scholar]
- Profant J, Dimsdale JE. Race and diurnal blood pressure patterns. A review and metaanalysis. Hypertension. 1999;33(5):1099–1104. doi: 10.1161/01.hyp.33.5.1099. [DOI] [PubMed] [Google Scholar]
- Sherwood A, Routledge FS, Wohlgemuth WK, Hinderliter AL, Kuhn CM, Blumenthal JA. Blood pressure dipping: ethnicity, sleep quality, and sympathetic nervous system activity. Am J Hypertens. 2011;24(9):982–988. doi: 10.1038/ajh.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HA, Jr, Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15(4 Suppl 6):S6–4. 17. [PubMed] [Google Scholar]
- Carpenter MA, Crow R, Steffes M, et al. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328(3):131–144. doi: 10.1097/00000441-200409000-00001. [DOI] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdalla M, Booth JN, 3rd, Seals SR, et al. Masked Hypertension and Incident Clinic Hypertension Among Blacks in the Jackson Heart Study. Hypertension. 2016;68(1):220–226. doi: 10.1161/HYPERTENSIONAHA.115.06904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijs L, Hansen TW, Kikuya M, et al. The International Database of Ambulatory Blood Pressure in relation to Cardiovascular Outcome (IDACO): protocol and research perspectives. Blood Press Monit. 2007;12(4):255–262. doi: 10.1097/mbp.0b013e3280f813bc. [DOI] [PubMed] [Google Scholar]
- Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38(4):852–857. doi: 10.1161/hy1001.092640. [DOI] [PubMed] [Google Scholar]
- Myers MG, Valdivieso M, Kiss A. Use of automated office blood pressure measurement to reduce the white coat response. J Hypertens. 2009;27(2):280–286. doi: 10.1097/HJH.0b013e32831b9e6b. [DOI] [PubMed] [Google Scholar]
- Piper MA, Evans CV, Burda BU, et al. Screening for High Blood Pressure in Adults: A Systematic Evidence Review for the US Preventive Services Task Force. Rockville MD: 2014. [PubMed] [Google Scholar]
- Siu AL Force USPST. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163(10):778–786. doi: 10.7326/M15-2223. [DOI] [PubMed] [Google Scholar]
- Muntner P, Lewis CE, Diaz KM, et al. Racial differences in abnormal ambulatory blood pressure monitoring measures: Results from the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Hypertens. 2015;28(5):640–648. doi: 10.1093/ajh/hpu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton CM, Muntner P, Booth JN, 3rd, Shimbo D, Schwartz JE. Is white-coat hypertension associated with increased cardiovascular and mortality risk? J Hypertens. 2016;34(8):1655–1658. doi: 10.1097/HJH.0000000000000983. [DOI] [PubMed] [Google Scholar]
- Peacock J, Diaz KM, Viera AJ, Schwartz JE, Shimbo D. Unmasking masked hypertension: prevalence, clinical implications, diagnosis, correlates and future directions. J Hum Hypertens. 2014;28(9):521–528. doi: 10.1038/jhh.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin SS, O’Brien E, Thijs L, Asayama K, Staessen JA. Masked hypertension: a phenomenon of measurement. Hypertension. 2015;65(1):16–20. doi: 10.1161/HYPERTENSIONAHA.114.04522. [DOI] [PubMed] [Google Scholar]
- Pickering TG, White WB, Giles TD, et al. When and how to use self (home) and ambulatory blood pressure monitoring. J Am Soc Hypertens. 2010;4(2):56–61. doi: 10.1016/j.jash.2010.03.003. [DOI] [PubMed] [Google Scholar]
- O’Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31(9):1731–1768. doi: 10.1097/HJH.0b013e328363e964. [DOI] [PubMed] [Google Scholar]
- Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary: a joint scientific statement from the American Heart Association, American Society Of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52(1):1–9. doi: 10.1161/HYPERTENSIONAHA.107.189011. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- Hinderliter AL, Routledge FS, Blumenthal JA, et al. Reproducibility of blood pressure dipping: relation to day-to-day variability in sleep quality. J Am Soc Hypertens. 2013;7(6):432–439. doi: 10.1016/j.jash.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuspidi C, Meani S, Salerno M, et al. Reproducibility of nocturnal blood pressure fall in early phases of untreated essential hypertension: a prospective observational study. J Hum Hypertens. 2004;18(7):503–509. doi: 10.1038/sj.jhh.1001681. [DOI] [PubMed] [Google Scholar]
- Booth JN, 3rd, Muntner P, Abdalla M, et al. Differences in night-time and daytime ambulatory blood pressure when diurnal periods are defined by self-report, fixed-times, and actigraphy: Improving the Detection of Hypertension study. J Hypertens. 2016;34(2):235–243. doi: 10.1097/HJH.0000000000000791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


