Abstract
Background
Early theories for cervical dystonia, as promoted by Hassler, emphasized the role of midbrain interstitial nucleus of Cajal. Focus then shifted to basal ganglia, and it was further supported with the success of deep brain stimulation. Contemporary theories suggested the role of cerebellum. But even more recent hypotheses renewed interest in midbrain. Although pretectum was visited on several occasions, we still do not know about the physiology of midbrain neurons in cervical dystonia.
Methods
We analyzed the unique database of pretectal neurons collected in 1970s and 1980s during historic stereotactic surgeries aimed to treat cervical dystonia. This database is valuable because such recordings could otherwise never be obtained from humans.
Results
We found three types of eye or neck movement sensitivity, eye-only neurons responded to pure vertical eye movements; the neck-only neurons were sensitive to pure neck movements; and the combined eye-neck neurons. There were two neuronal subtypes – burst-tonic and tonic. The eye-neck or eye-only neurons sustained their activity during eccentric gaze holding. In contrast, the response of neck-only and eye-neck neurons exponentially decayed during neck movements.
Conclusions
Modern quantitative analysis of historic database of midbrain single-units from patients with cervical dystonia might support novel hypotheses for normal and abnormal head movements. This data, collected almost four decades ago, must be carefully viewed, especially because it was acquired using a less sophisticated technology available at that time, the aim was not to address specific hypothesis but to make an accurate lesion providing optimal relief from dystonia.
Keywords: head tremor, interstitial nucleus of Cajal, dystonic tremor
Introduction
The neural pathways responsible for cervical dystonia (CD) are not well understood. Historically, abnormalities in different neural structures have been described including the basal ganglia, cerebellum, midbrain, vestibular, and proprioceptive systems.1–14 In some of the studies conducted as early as in the 1950s, Hassler and colleagues called attention to the midbrain, because experimental manipulations of specific regions reproducibly resulted in abnormal head movements.15–18 Following a series of methodical studies in cats, Hassler and Hess proposed that the interstitial nucleus of Cajal (INC) was responsible for turning the head, while the prestitial and precommissural regions mediated vertical movements.16 More recently, these concepts were further refined in studies of non-human primates.19–24 These studies revealed that the INC and surrounding regions in non-human primates control the position of the head in all three planes.22, 23
While the empirical evidence for the involvement of specific regions of the midbrain in the control of head posture is strong, many questions remain. One important question is how results from animals with experimentally induced abnormal head postures might be related to the pathogenesis of CD in humans. In the current report, we describe a unique historical dataset taken during stereotactic surgeries of the midbrain in patients with CD. In the 1970s through 1980s, before the introduction of botulinum toxin and deep brain stimulation surgery for CD, a handful of centers around the world offered lesions of the INC or nearby regions as a treatment for CD. Most of the reports came from Japan, Europe, and Russia.
Sano and colleagues in Japan were among the first to report that manipulations of the INC could cause relief of dystonic posturing in CD.25, 26 The treatment of CD using the interventions involving the INC were further emphasized by Bertram and colleagues in Europe.27 Similar procedures were provided at the Burdenko Neurosurgical Institute in Moscow, where data regarding intraoperative neuronal recordings from stereotactic surgeries were collected and stored for 12 patients with CD29. These records provide a unique glimpse into the behavior of midbrain neurons in human patients with CD. When considering these data, it is essential to recognize the technical and methodological limitations associated with analyzing historical data by modern standards. It is also important to appreciate the challenges associated with interpreting results in light of modern theories of CD pathogenesis. Nevertheless, these data are uniquely valuable because they provide a glimpse into the behavior of neurons involved in the control of neck muscles in humans, the same neurons that have been proposed to be central to the pathogenesis of CD.
Methods
Patient population
We analyzed single-unit activity recorded from 55 neurons found in and around INC (here collectively called “pretectal neurons”) in 12 CD patients who underwent physiologic mapping for therapeutic stereotactic ablation of this area in 1970s and 1980s. All of the surgical procedures and physiological investigations were explained to the patients before the operations. All subjects provided written consent for the procedure, which was considered a viable treatment option for CD at the time.15, 18, 26, 28, 29 No special populations such as prisoners or children were included. The data (single-unit activity, electromyography (EMG) of the neck muscles, electrooculography (EOG), and audio signals) were collected as part of the targeting procedure and stored in the medical records. All data were de-identified and are presented here. The ethics committee at the Burdenko Neurosurgery Institute granted permission for these analyses. Although all patients consented to the procedures and collection of data as part of their medical treatments rather than for research, most were deceased and relatives were not available for re-consenting. Therefore this research could not have feasibly been conducted by re-contacting the patients or relatives. Supplementary Table 1 depicts demographic details and subjective clinical outcomes.
Stereotactic surgical procedures and data collection
The procedure was performed under local anesthesia. A Reichert-Mundinger stereotactic frame was used. Extracellular recordings of spike activity were made using tungsten microelectrodes with a tip diameter of 1 to 2 μm (resistance 1 to 2 MegaOhm). The trajectory of the microelectrode was directed from the premotor cortex toward the surgical target using preconfigured target coordinates of the pretectum. Mapping of the eye and/or neck movement sensitive neurons was performed between 65 and 85 mm depth. Fig 1 depicts stereotactic reconstructions of analyzed single-units in the sagittal plane from the human brain atlas of Schaltenbrand and Bailey (1959). Supplementary Table 1 depicts stereotactic coordinates of the pretectum for each patient. The averaged target coordinates were posterior: 13±1.8; inferior: 3.4±0.8; and lateral: 2.9±1 with respect to the middle of anterior and posterior commissure. Single-unit activity in response to isometric contractions of the neck muscles and eye movements were used for the identification of the pretectum.
Fig 1.
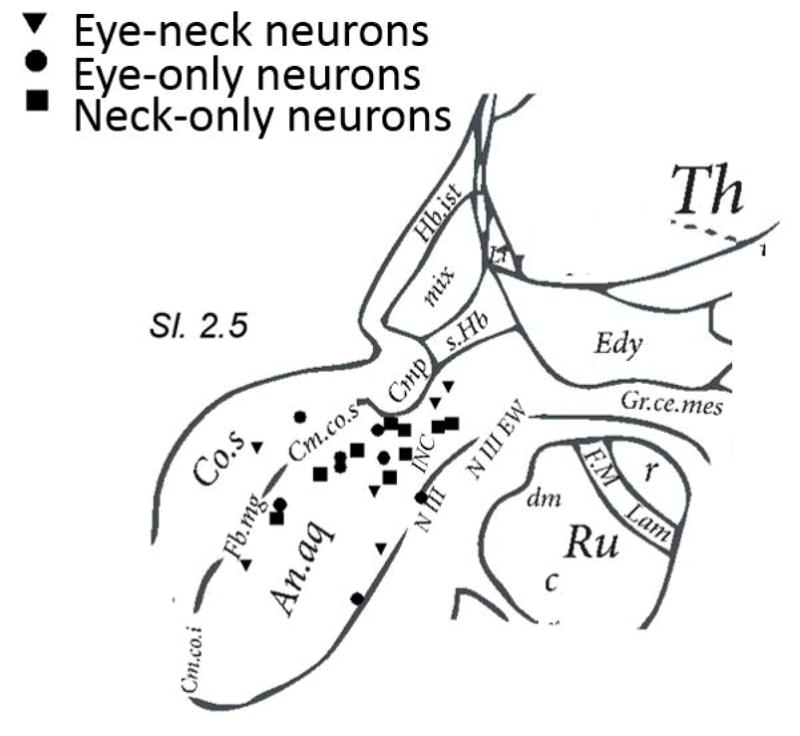
Reconstruction of recorded single units overlaid on the schematic from Schaltenbrand and Bailey (1959) atlas. The schematic is in the sagittal plane. Co.S: superior colliculus; Th: Thalamus; Ru: Red nucleus (dm:dorsomedial, r:rostral, c:caudal); NIII: oculomotor nucleus; NIII W: edinger westephal nucleus; An.aq: Annulus aquaeductus Edy: N. Endymalis; Cm.co.s.: Commissure of the superior colliculus; Fb.mg: basal forebrain magnocellular complex; Cm.co.i: Commissure of the inferior colliculus; Hb.ist: habenula interstitial; Cmp: Posterior commissure; INC: Interstitial nucleus of Cajal.
Neck EMG from sternocleidomastoid, trapezius, and splenius; EOG; and audio channel of the instructions to the patient were recorded simultaneously with single unit activity using a 14-channel tape recorder (EAM-500, TESLA, Czech Republic) and an 8-channel electroencephalograph (Medikor, Hungary). Data from the tape recorder were passed through an analog-to-digital converter L-1250 (L-CARD, Moscow, Russia) with a sampling rate of 20 kHz for the microelectrode signal, 10 kHz for the phonogram, and 1 kHz for all the other signals.
Once a neuron was identified, the subjects were asked to turn their eyes to the right, left, up, and down without activation of the neck muscles. In the second paradigm the subjects were asked to shrug their shoulder (activation of trapezius) or rotate head to the side against resistance (exert isometric contraction of the sternocleidomastoid and splenius capitus) while keeping the eyes in straight ahead position. The subjects’ head was immobilized in a stereotactic frame for the purpose of surgical procedure, so no actual movement of the head occurred. Neck EMG, EOG, and single unit activity were simultaneously recorded. The cells were isolated at approximately every 300–500 μm along each trajectory. Spontaneous neuronal activity of well-isolated units was collected for up to 20 seconds.
Data analysis
EOG, EMG, and audio signals were digitized, band-pass filtered (50 Hz to 3 kHz band-pass with a 6-dB/octave roll off), aligned, and imported into Spike 2 software (CED, Cambridge, UK). Further offline analysis in Spike 2 included single-unit isolation using characteristic amplitude and spike shape. The methods involving single-unit recordings, microstimulation, and neck muscle EMG recordings to study the neural control of head movements are not new, they have been extensively used in past in animal experiments19–21, 30–32. Because of the variability of spike amplitude during the bursts that can hinder accurate spike sorting, we manually discriminated them and ensured that the spikes in a burst were correctly defined in one spike train. Only cells having a good signal-to-noise ratio that could be clearly discriminated were included in subsequent analyses of spike characteristics, such as burst detection, firing rates, and neuron-types classification, which was carried out using custom-prepared software in MATLABTM (The MathWorks, Natick, Massachusetts) and R (R Development Core Team, https://www.r-project.org/).
Neuronal responses to eye and neck movements were identified from peri-event histograms (bin 50–200 ms) and raster plots. Increases or decreases in activity were considered significant when the mean firing rate in at least 3 consecutive 50 ms bins was more than 2 standard deviations from that measured during the hold period. In order to compare the amount of neck muscle reactivity to increases in the discharge of pretectal neck movement sensitive neurons we normalized the neural response rate with the corresponding peak. Similar normalization routine was used for EMG activity. In order to measure persistence of neural firing rate or the EMG activity we found the best goodness-of-fit to the exponential equation y=Ae^Tau*x to corresponding data. In the fitted equation value of A is constant while Tau is the time constant, x is time and y is firing rate (or EMG response to be analyzed).
Results
We analyzed single-unit activity of 55 pretectal neurons recorded during historic stereotactic ablation surgery in 12 patients with CD. Fig 1 depicts stereotactic reconstruction of the single-units analyzed in the human brain atlas of Schaltenbrand and Bailey (1959). The neurons were classified according to their burst-tonic or tonic discharge characteristics, and responsiveness to eye (change in EOG activity) or neck (change in EMG activity) movements. 10 neurons were only sensitive to eye movements (eye-only neurons); 9 eye-only neurons were tonic, while 1 was burst-tonic. Eight eye-only neurons were excited during upward eye positions, and inhibited during downward eye positions; while 2 eye-only neurons had the opposite response. Ten cells selectively activated during neck muscle activity (neck-only neurons), 4 neck-only neurons were burst-tonic and 6 were tonic. Four neck-only neurons were inhibited during contralateral neck muscle contraction, all inhibitory neck-only cells had burst-tonic characteristic. Seven cells were reactive to both eye and neck movements (eye-neck combined neurons), 3 were tonic and 4 were burst-tonic. 24 neurons responded neither to eye nor to neck movement. Below we provide the qualitative physiological characteristics of one neuron from each category. Subsequent a quantitative summary of results from all of the neurons is presented.
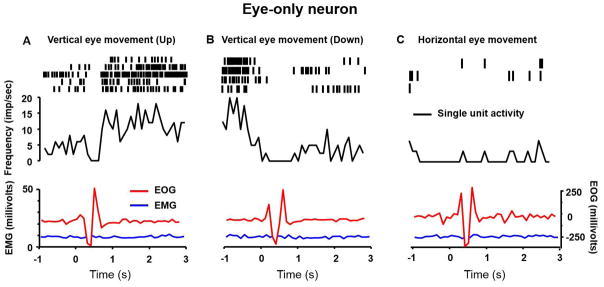
Eye-only neuron
Fig 2 depicts an example of an eye-only neuron that selectively responded to vertical eye position. The discharge rate of this neuron increased with an upward eye position (Fig 2A), but the downward eye position correlated with a reduction in the neural response (Fig 2B). There was no response to horizontal eye movement (Fig 2C). Isolated eye movement was objectively confirmed with EOG (red trace in Fig 2A,B) without any change in the neck EMG (blue trace in Fig 2A,B). The neural activity paused when the eyes were in transition (Fig 2A,B), but it resumed at a new firing rate that correlated with the vertical eye eccentricity. The persistence of neural activity was quantified by measuring a time constant of the exponential function fitted to the neural firing rate. Higher values of time constants depict persistence of neural activity. The neural discharge corresponding to the vertical eye position persisted with a time constant of 7.5 s. The neuronal discharge preceded the EOG response suggesting that an eye movement was driven by recorded premotor neuron and it was not a reaction to altered orbital proprioception in response to orbital muscle stretch (Fig 2A,B).
Fig 2.
An example of eye only neuron depicting increase in firing with upward eye movement (A), but reduction in firing with downward gaze (B). The neuron lacks consistent changes with horizontal (C) eye movements. In each panel the neural discharge rate (black trace), EOG activity (red trace), and EMG activity (blue trace) are plotted on the y-axis, while x-axis depicts corresponding time. Raster plot in inset of each panel graphically depicts a marker at the time a spike was presented in the recorded voltage trace. An increase in firing rate correlates with higher raster density.
Neck-only neuron
Fig 3 illustrates an example of a neck-only pretectal neuron that selectively responded to neck muscle contractions. The neural discharge preceded EMG activity by 100 ms (blue trace, Fig 3) but there were no corresponding eye movements as confirmed by EOG (red trace, Fig 3). The neck EMG and the discharge of the neck-only pretectal neuron exponentially declined. The decay time constant of exponentially decreasing activity of the illustrated neuron was 0.9 s, while the time constant corresponding to decaying EMG activity was 1.4 s.
Fig 3.
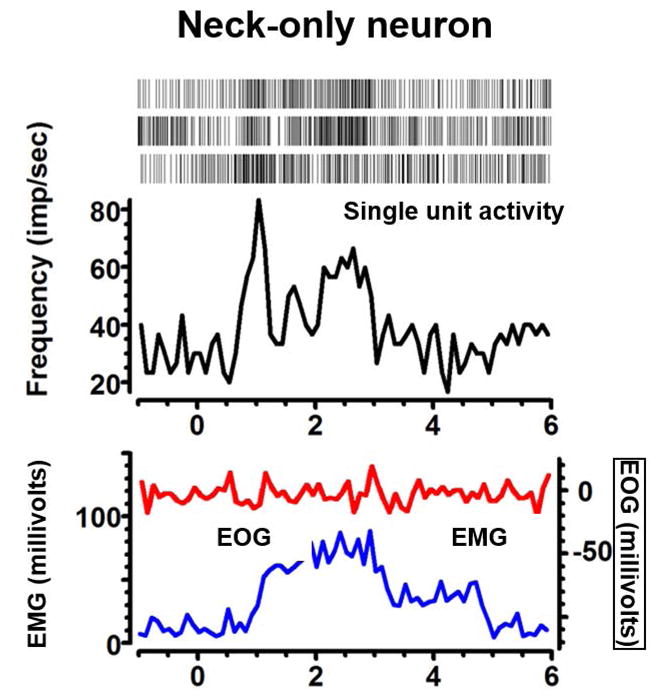
An example of neck only neuron depicting increase in the firing rate (black trace) with isometric neck muscle (sternocleidomastoid) contraction (blue traces) without coactivation of eye movements (red trace). Inset of the panel depicts raster plot, each marker of the raster depicts the presence of spike at a given time.
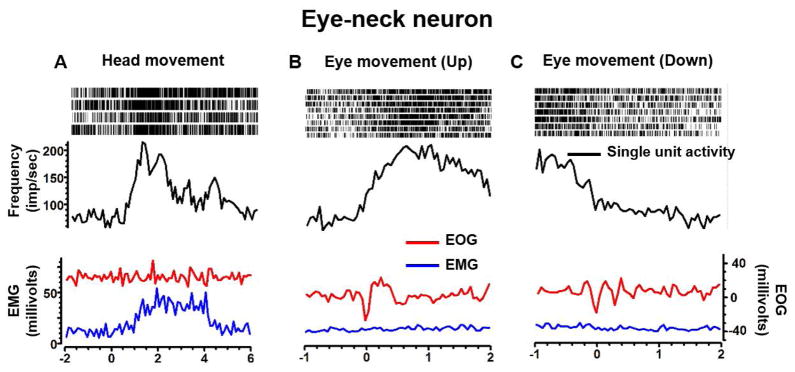
Eye-neck combined neuron
Fig 4A illustrates the activity of an eye-neck combined neuron in response to the isolated neck movements as confirmed by selective deflection in the neck EMG signal (blue trace, Fig 4A), while EOG remained non-reactive (red trace, Fig 4A). After isolated neck muscle exertion, the activity of the eye-neck neuron exponentially declined with a short time constant of 1.1 s (Fig 4A). Fig 4B,C depict an example of the same neuron and corresponding isolated eye movements as confirmed by selective deflection in the EOG signal (red traces, Fig 4B,C), but non-reactive EMG (blue trace, Fig 4B,C). The firing rate of the eye-neck neuron increased in response to isolated upward eye movement and the response persisted with a relatively longer time constant of 4.6 s (Fig 4B). In response to isolated downward eye movements, the neural response decreased and persisted with an exponential time constant of 5.3 s (Fig 4C).
Fig 4.
An example of eye-neck combined neuron depicting response to head (A) and eye (B,C) movements. Neural discharge rate (black trace), EMG activity (blue trace), and EOG (red trace) are plotted on the y-axis, while x-axis depicts corresponding time. Raster plot in the inset of each panel graphically depicts a marker at the time a spike was presented in the recorded voltage trace. Increase in firing rate correlates with higher raster density.
Summary of neural responses
Supplementary Table 2 summarizes the spontaneous firing rate, coefficient of variance depicting the regularity, and the latency (the time difference between the onset of neural discharge and evoked motor activity) of the eye-only, neck-only, eye-neck combined, and non-reactive neurons. The spontaneous firing rate of the eye-neck combined neurons was higher than remaining three groups. The coefficient of variability of spontaneous activity was lowest in the eye-neck combined neurons (Supplementary Table 2). The latency of evoked motor response was comparable in all three groups (Supplementary Table 2, Mann Whitney U Test, p>0.1).
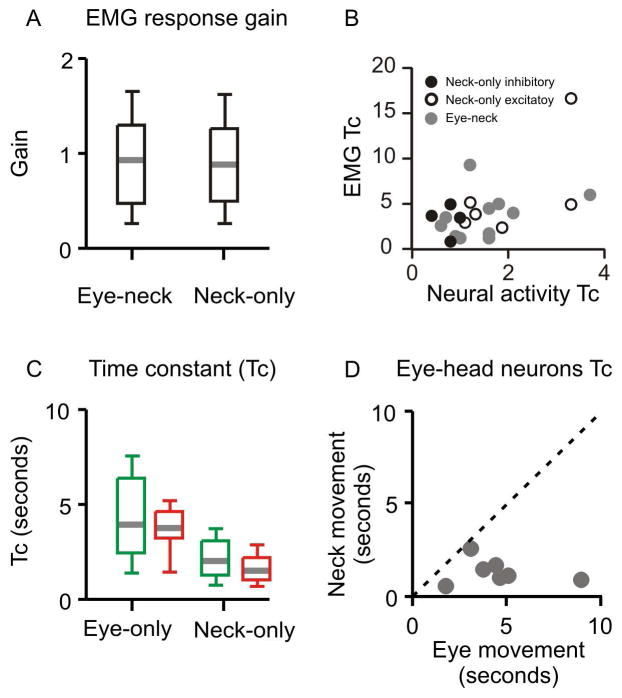
We measured the normalized change in the firing activity of the neck movement sensitive neurons (eye-neck combined and neck-only neurons) and corresponding normalized change in the EMG activity. The ratio of normalized change in firing activity and corresponding EMG quantitatively measured the reactivity of neck muscles to the change in pretectal neck movement sensitive neurons (EMG response gain). Box plots in Fig 5A depict the summary of EMG response gains for neck-only and eye-neck combined neurons. Mean and standard deviation of neuron response gain was 1.1 ± 0.3 and 1.3 ± 0.4 for neck-only and eye-neck neurons, respectively. Fig 5B compares the time constants of pretectal neck movement sensitive neuron (neck-only and eye-neck combined) with the time constants in corresponding EMG activity. Such a relationship for neck-only neurons had a correlation coefficient of 0.13. The relationship between the time constant of eye-neck combined neurons and the EMG activity had a correlation coefficient of 0.4.
Fig 5.
Summary results from all recorded neurons. (A) Summary of EMG response gain (ratio of normalized neural activity and normalized EMG activity) of eye-neck and neck-only neurons. The values of gain are plotted on the y-axis. Grey horizontal line depicts median value, the length of box is interquartile interval while whiskers depict the range (B) Comparison of decay time constant of the neural activity (x-axis) and the muscle EMG (y-axis). The dashed line is an equality line. Each symbol depicts one neuron, while symbol type classifies neurons in eye-neck or excitatory and inhibitory neck-only neurons. (C) Summary of decay time constant of neural discharge rate measured from eye only during excitatory or inhibitory responses (green and red colors, respectively; n=10 for each responses) and excitatory neck-only (n=10, green color) or inhibitory neck-only (n=4, red color) neurons is depicted. Time constant for the decay in neural discharge is plotted on y-axis, while each box plot depicts one group. There is a significant reduction in the neural discharge time constant for neck-only neuron as compared to eye-only neuron. (D) Comparison of decay time constant of neural discharge of the eye-neck combined neuron in response to isolated neck movement (y-axis) and isolated eye movement (x-axis). Each symbol depicts one neuron. Dashed grey line is an equality line. All data points fall below equality line suggesting consistently shorted time constant for the head movement as compared to the eye movement.
Fig 5C compares the time constants measured from the eye-only and neck-only neurons. Inhibitory neural response was quantified by measuring the time-constant of growth of re-emerging neural activity; while excitatory neurons were quantified by measuring the decay time constant. The mean time constant for eye-only neurons during excitation was 4.24 ± 2.2 s, and it was 3.48 ± 1.8 s during inhibition. The response were significantly larger compared to neck-only neurons (excitatory neck only neurons: 2.55 ± 2.1 s; inhibitory neck only neurons: 0.75± 0.3 s); the difference was statistically significant (Mann Whitney U Test, p = 0.002). Then we compared the time constant of the same eye-neck combined neurons during isolated neck movement and isolated eye movement (Fig 5D). Since the eye movements are normal in CD, the neural response to isolated eye movement was considered as a control, and was used for the comparison. In Fig 5D, the data points, each depicting individual eye-neck neuron, fell below the equality line. The fitted linear function had the slope of 0.25, which was not significantly different from 0 (p>0.05). The shallow slope suggests a consistent reduction in the response time constant of eye-neck neurons during neck movements but not during eye movements. The mean time constant of eye-neck neurons during eye movements was 4.5 ± 2.2 s, while it was 1.4 ± 0.6 s during head movements; the difference was statistically significant (Mann Whitney U Test, p=0.001).
Discussion
Here we report results from a unique historical dataset comprising single neuron activity captured from pretectum in patients with CD who had stereotactic ablation surgery. To our knowledge this is the first study of single unit electrophysiology of head and/or eye movement sensitive neurons from the human midbrain. There were three distinct categories of eye and neck movement sensitive neurons in the pretectum. One group of neurons responded only to eye movements. The second group of neurons responded only to neck movements. Due to intraoperative restrictions that limited actual neck movements, the neck movement sensitive neurons could only be assessed by isometric neck muscle contractions in the stereotactic frame or by shoulder shrugs with activation of the trapezius, which is an accessory neck muscle. We could not identify directional tuning of the neck movement sensitive neurons. The third category of neurons responded to both eyes and neck. These three distinct neuronal populations are consistent with the reports of cells found in non-human primate and feline pretectum, where more methodical explorations have revealed neurons responsive to specific directions of eye or head movements 33–37.
In our cohort from humans we found two subtypes of neurons in each category; 90 percent of eye-only neurons were tonic while 10 percent were burst tonic. Among neck-only neurons 40 percent were burst tonic while 60 percent were tonic. Similar tonic or burst-tonic pretectal neurons were also found in recordings from non-human primates.37 The location of the patient neurons cannot be determined precisely, because modern imaging techniques for localization were not available when this study was done 40 years ago. We therefore inferred the location of these neurons based on coordinate-based reconstructions according to the atlas of Schaltenbrand and Bailey (1959) as outlined in figure 1. All fall in an analogous region described in detail in prior animal studies.33, 35–41
The primary goal of the original surgery in these patients was to stereotactically and electrophysiologically locate the pretectum in order to create appropriate lesion to effectively treat dystonia. The neurophysiology signal was only used to characterize the neuronal population. Therefore confirmatory neurophysiology signals such as EMG and EOG were not calibrated to shoulder, neck or eye position. Due to the lack of calibrated EMG signal we used normalized values of EMG and neural response to compute the response gain during the neck movements. The audio data was simultaneously captured, where we could confirm that in order to test for eye movement sensitivity the subjects were asked to keep eyes sustained in eccentric position while neck muscle activity was tested by asking subjects to exert sustained muscle contractions.
In a separate analysis we measured how persistently the neurons could maintain the firing rate after the movements were initiated. Persistence in the neural firing rate was quantified by fitting the exponential function and corresponding time constant. The decay time constant was used for excitatory neurons while the inhibitory neuron response was measured through growth time constant. The time constant was larger in neurons with eye movement sensitivity compared to the neck movement sensitive neurons. The eye-neck neurons had longer time constant when activated with eye movements as compared to their activation with isolated neck movements. There are at least two explanations for the differences in the decay time constant of the eye-only and neck-only neurons. One explanation is simply that eye movement and neck movement cells have different subtypes and they have different membrane properties. However, comparable spontaneous firing rates, and the presence of disparity in time constant in combined eye-neck neurons makes such a possibility less likely. The other explanation is that the subjects were able to sustain eccentric eye positions, but they were not able to do so for the neck due to dystonia or restraint in the stereotactic frame. As a result the neurons could not maintain their firing rates and showed an effort-dependent reduction in the response. While this technical issue is important in interpreting our results, we invariably found an exponential decay in neural activity questioning the possibility of effort-dependence as the sole explanation to our results. The pretectum receives proprioceptive projections from the neck muscles42. The proprioceptive driving of pretectal neuron was however unlikely, because the neural discharge consistently preceded the neck EMG activity.
Although comparisons of time constants of neck EMG and single-unit activity had a positive slope, they had a weak correlation coefficient, the data points were not on the equality line, and decaying neck EMG activity typically had a higher time constant compared to the driving neurons. Such disparity in the time constants of the pretectal neck movement sensitive neurons and neck muscle activity can be described by additional muscle plant dynamics, such as mechanoelastic forces, influencing neck muscle activity. There was a broad variability in the scatter depicting the relationship between evoked EMG activity and the pretectal neural reactivity. Such dispersion in the neck muscle reactivity could be attributed to the fact that all recorded neurons are not optimally tuned to the measured neck muscles. The neck muscle may receive inputs from more than one pretectal areas, or the given pretectal area may have variable tuning properties for different neck muscles.
It is interesting to note that some of the results are consistent with modern theories of CD pathogenesis.43–45 A series of experiments from non-human primates have shown that the INC is responsible for converting a head velocity signal (the pulse) into steady state neural firing encoding steady head position (the step).22, 23 Such pulse-to-step conversion is a function of a neural network that acts (in mathematical sense) as an integrator for head movement. While the existence of a head neural integrator was established in studies of non-human primates, we do not know whether a similar integrator exists in humans. Our prior quantitative investigations of head movements in CD were compatible with the concept of a defect in a head neural integrator, but the results are indirect.11, 22, 23, 44, 45 Results from the current studies provide more direct evidence. The eye-only pretectal neurons sustained their neural activity during vertical gaze holding, consistent with normal function of an ocular motor neural integrator. In contrast the neck-only pretectal neurons did not sustain activity, their responses decreased exponentially and corresponded to drifts in the neck EMG. This observation is compatible with the hypothesis of impaired head neural integration in CD.11, 44, 45 We also found that the combined eye-neck neurons had a shorter time constant for head holding as compared to eye holding. Such differences in the reactivity of the same neuron during two independent tasks implies that abnormal activity during neck movements is not due to an intrinsic abnormality in the integrator, but it could be related to some other influence, such as abnormal head movement feedback. Indeed the cerebellum and basal ganglia, two main suspects for the pathophysiology of CD, are important input sources projecting to the head neural integrator 7–10, 42, 46–54.
While these data are compatible with the neural integrator hypothesis, any interpretations must be made with caution. First, these data were not collected specifically to test the neural integrator hypothesis, so some important information was not collected, for example, we only had verbal verification that subjects complied with all tasks. Second, pretectal neurons are known to respond to vestibular signals 33–36, 38–40, 55–57. Several studies assessed vestibular function in subjects with CD, and a few of these even attributed the role of INC in influence of vestibular system in pathogenesis of dystonia58–64. While it would be desirable to directly measure the effects of vestibular system on the function of pretectal neurons, testing vestibular sensitivity by means of passive or active head movements is not possible during stereotactic neurosurgery. The scientific literature investigating the eye-neck neurons has utilized complex behavioral protocols teasing apart coordinated eye and neck movements in experimental animals. Such complex protocols were not used during historic stereotactic surgeries. Nevertheless, protocol to examine eye-neck combined neurons selectively induced eye or neck movements. We confirmed such selective eye or neck muscle activation by simultaneously recorded EOG and EMG.
Although once considered a viable treatment option, midbrain lesions are no longer routinely offered to patients with CD. While the procedure appeared to benefit some individuals, outcomes were variable. Some of this variability could be due to imprecise understanding of how individual midbrain regions contributed to different abnormal head postures, the heterogeneous nature of CD and its causes, the lack of precise surgical targeting methods (MRI was not widely available until the late 1980s), and unpredictable side effects resulting from lesions of nearby midbrain structures. Nevertheless, the current data are uniquely valuable for focusing attention on a region where further studies may lead to alternative treatment strategies for CD.
Supplementary Material
Acknowledgments
Funding support: Aasef Shaikh was supported by Dystonia Medical Research Foundation Clinical Fellowship Award, and Dystonia Coalition/Dystonia Medical Research Foundation Career Development Award. Buz Jinnah is the recipient of NIH grant U54NS065701.
This manuscript presents historic data collected in 1970s and 1980s during microelectrode recording guided stereotactic neurosurgeries performed by Dr. Vladimir Shabalov. Dr. Svetlana Raeva (deceased: 2013) performed microelectrode recordings during these ablation procedures. Aasef Shaikh was supported by Dystonia Medical Research Foundation Clinical Fellowship Award, and Dystonia Coallition/Dystonia Medical Research Foundation Career Development Award. H.A. Jinnah is the recipient of NIH grants U54NS065701 and TR001456.
Footnotes
Financial disclosure: The authors have no financial disclosure pertinent to this study.
Contributions:
VS and SR collected data. AS (Sedov), VP, and AS (Shaikh) conceptualized the manuscript and analyzed data. AS (Sedov), VP, AS (Shaikh), and HJ wrote and edited the manuscript.
Reference List
- 1.Putnam TJ, Herz E, Glaser GH. Spasmodic torticollis; surgical treatment. Arch Neurol Psychiatry. 1949;61(3):240–247. doi: 10.1001/archneurpsyc.1949.02310090015002. [DOI] [PubMed] [Google Scholar]
- 2.Herz E, Glaser GH. Spasmodic torticollis; clinical evaluation. Arch Neurol Psychiatry. 1949;61(3):227–239. doi: 10.1001/archneurpsyc.1949.02310090002001. [DOI] [PubMed] [Google Scholar]
- 3.Avanzino L, Fiorio M. Proprioceptive dysfunction in focal dystonia: from experimental evidence to rehabilitation strategies. Front Hum Neurosci. 2014;8:1000. doi: 10.3389/fnhum.2014.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13(7):281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 5.Hallett M. Dystonia: abnormal movements result from loss of inhibition. Adv Neurol. 2004;94:1–9. [PubMed] [Google Scholar]
- 6.Ma K, Babij R, Cortes E, Vonsattel JP, Louis ED. Cerebellar pathology of a dual clinical diagnosis: patients with essential tremor and dystonia. Tremor Other Hyperkinet Mov (N Y) 2012:2. doi: 10.7916/D8JD4VJ5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neychev VK, Fan X, Mitev VI, Hess EJ, Jinnah HA. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain. 2008;131(Pt 9):2499–2509. doi: 10.1093/brain/awn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neychev VK, Gross RE, Lehericy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiol Dis. 2011;42(2):185–201. doi: 10.1016/j.nbd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pizoli CE, Jinnah HA, Billingsley ML, Hess EJ. Abnormal cerebellar signaling induces dystonia in mice. J Neurosci. 2002;22(17):7825–7833. doi: 10.1523/JNEUROSCI.22-17-07825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prudente CN, Hess EJ, Jinnah HA. Dystonia as a network disorder: what is the role of the cerebellum? Neuroscience. 2014;260:23–35. doi: 10.1016/j.neuroscience.2013.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaikh AG, Zee DS, Jinnah HA. Oscillatory head movements in cervical dystonia: Dystonia, tremor, or both? Mov Disord. 2015;30(6):834–842. doi: 10.1002/mds.26231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starr PA, Rau GM, Davis V, et al. Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson’s disease and normal macaque. J Neurophysiol. 2005;93(6):3165–3176. doi: 10.1152/jn.00971.2004. [DOI] [PubMed] [Google Scholar]
- 13.Vitek JL. Pathophysiology of dystonia: a neuronal model. Mov Disord. 2002;17(Suppl 3):S49–62. doi: 10.1002/mds.10142. [DOI] [PubMed] [Google Scholar]
- 14.Vitek JL, Chockkan V, Zhang JY, et al. Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Ann Neurol. 1999;46(1):22–35. doi: 10.1002/1531-8249(199907)46:1<22::aid-ana6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Hassler R, Dieckmann G. Stereotactic treatment of different kinds of spasmodic torticollis. Confin Neurol. 1970;32(2):135–143. doi: 10.1159/000103408. [DOI] [PubMed] [Google Scholar]
- 16.Hassler R, Hess WR. Experimental and anatomic studies of rotatory movements and their control mechanisms (in German) Archives of Psychiatry Nervenkr. 1954;192:488–526. doi: 10.1007/BF00342860. [DOI] [PubMed] [Google Scholar]
- 17.Hassler R, Riechert T. Indications and targetting methods of stereotactic surgery (in German) Nervenrzt. 1954;25:441–447. [PubMed] [Google Scholar]
- 18.Hassler R, Vasilescu C, Dieckmann G. Electromyographic activity of neck muscles in patients affected by retrocollis under the influence of stimulation and coagulation of the prestitial nucleus of the midbrain. Appl Neurophysiol. 1981;44(5–6):291–301. doi: 10.1159/000102211. [DOI] [PubMed] [Google Scholar]
- 19.Farshadmanesh F, Byrne P, Keith GP, Wang H, Corneil BD, Crawford JD. Cross-validated models of the relationships between neck muscle electromyography and three-dimensional head kinematics during gaze behavior. J Neurophysiol. 2012;107(2):573–590. doi: 10.1152/jn.00315.2011. [DOI] [PubMed] [Google Scholar]
- 20.Farshadmanesh F, Byrne P, Wang H, Corneil BD, Crawford JD. Relationships between neck muscle electromyography and three-dimensional head kinematics during centrally induced torsional head perturbations. J Neurophysiol. 2012;108(11):2867–2883. doi: 10.1152/jn.00312.2012. [DOI] [PubMed] [Google Scholar]
- 21.Farshadmanesh F, Chang P, Wang H, Yan X, Corneil BD, Crawford JD. Neck muscle synergies during stimulation and inactivation of the interstitial nucleus of Cajal (INC) J Neurophysiol. 2008;100(3):1677–1685. doi: 10.1152/jn.90363.2008. [DOI] [PubMed] [Google Scholar]
- 22.Farshadmanesh F, Klier EM, Chang P, Wang H, Crawford JD. Three-dimensional eye-head coordination after injection of muscimol into the interstitial nucleus of Cajal (INC) J Neurophysiol. 2007;97(3):2322–2338. doi: 10.1152/jn.00752.2006. [DOI] [PubMed] [Google Scholar]
- 23.Klier EM, Wang H, Constantin AG, Crawford JD. Midbrain control of three-dimensional head orientation. Science. 2002;295(5558):1314–1316. doi: 10.1126/science.1067300. [DOI] [PubMed] [Google Scholar]
- 24.Klier EM, Wang H, Crawford JD. Interstitial nucleus of cajal encodes three-dimensional head orientations in Fick-like coordinates. J Neurophysiol. 2007;97(1):604–617. doi: 10.1152/jn.00379.2006. [DOI] [PubMed] [Google Scholar]
- 25.Sano K, Sekino H, Tsukamoto Y, Yoshimasu N, Ishijima B. Stimulation and destruction of the region of the interstitial nucleus in cases of torticollis and see-saw nystagmus. Confin Neurol. 1972;34(5):331–338. doi: 10.1159/000103078. [DOI] [PubMed] [Google Scholar]
- 26.Sano K, Yoshioka M, Mayanagi Y, Sekino H, Yoshimasu N. Stimulation and destruction of and around the interstitial nucleus of Cajal in man. Confin Neurol. 1970;32(2):118–125. doi: 10.1159/000103405. [DOI] [PubMed] [Google Scholar]
- 27.Bertrand C, Molina-Negro P, Martinez SN. Combined stereotactic and peripheral surgical approach for spasmodic torticollis. Appl Neurophysiol. 1978;41(1–4):122–133. doi: 10.1159/000102408. [DOI] [PubMed] [Google Scholar]
- 28.Dieckmann G. Stereotaxic treatment of extrapyramidal torticollis. Neurochirurgie. 1976;22(6):568–571. [PubMed] [Google Scholar]
- 29.Raeva SN, Vasin N, Shabalov VA, Medzhidov MR, Shashkov VV. Identification of nuclei of the pretectal area during stereotaxic surgery for spastic torticollis. Zh Vopr Neirokhir Im N N Burdenko. 1986;(5):39–43. [PubMed] [Google Scholar]
- 30.Corneil BD, Olivier E, Richmond FJ, Loeb GE, Munoz DP. Neck muscles in the rhesus monkey. II. Electromyographic patterns of activation underlying postures and movements. J Neurophysiol. 2001;86(4):1729–1749. doi: 10.1152/jn.2001.86.4.1729. [DOI] [PubMed] [Google Scholar]
- 31.Corneil BD, Olivier E, Munoz DP. Neck muscle responses to stimulation of monkey superior colliculus. II. Gaze shift initiation and volitional head movements. J Neurophysiol. 2002;88(4):2000–2018. doi: 10.1152/jn.2002.88.4.2000. [DOI] [PubMed] [Google Scholar]
- 32.Corneil BD, Olivier E, Munoz DP. Neck muscle responses to stimulation of monkey superior colliculus. I. Topography and manipulation of stimulation parameters. J Neurophysiol. 2002;88(4):1980–1999. doi: 10.1152/jn.2002.88.4.1980. [DOI] [PubMed] [Google Scholar]
- 33.Fukushima K, Fukushima J, Kase M. The origin of high and regular discharge rates of eye-movement-related neurons in the region of the interstitial nucleus of Cajal. Neurosci Res. 1991;12(2):379–387. doi: 10.1016/0168-0102(91)90005-j. [DOI] [PubMed] [Google Scholar]
- 34.Fukushima K, Suzuki Y, Fukushima J, Kase M. Latencies of response of eye movement-related neurons in the region of the interstitial nucleus of Cajal to electrical stimulation of the vestibular nerve in alert cats. Exp Brain Res. 1991;87(2):254–258. doi: 10.1007/BF00231842. [DOI] [PubMed] [Google Scholar]
- 35.Fukushima K, Takahashi K, Fukushima J, Ohno M, Kimura T, Kato M. Effects of lesion of the interstitial nucleus of Cajal on vestibular nuclear neurons activated by vertical vestibular stimulation. Exp Brain Res. 1986;64(3):496–504. doi: 10.1007/BF00340487. [DOI] [PubMed] [Google Scholar]
- 36.Fukushima K, Takahashi K, Kato M. Responses of vestibular neurons to stimulation of the interstitial nucleus of Cajal in the cat. Exp Brain Res. 1983;51(1):1–15. doi: 10.1007/BF00236797. [DOI] [PubMed] [Google Scholar]
- 37.King WM, Fuchs AF, Magnin M. Vertical eye movement-related responses of neurons in midbrain near intestinal nucleus of Cajal. J Neurophysiol. 1981;46(3):549–562. doi: 10.1152/jn.1981.46.3.549. [DOI] [PubMed] [Google Scholar]
- 38.Fukushima K, Fukushima J. In: Involvement of Interstitial nucleus of Cajal in the midbrain reticular formation in the positin-related, tonic component of vertical eye movement and head posture. Berthoz A, Graf W, Vidal PP, editors. The Head-Neck Seonsory Motor Sysem; Oxford: 1992. [Google Scholar]
- 39.Fukushima K, Murakami S, Matsushima J, Kato M. Vestibular responses and branching of interstitiospinal neurons. Exp Brain Res. 1980;40(2):131–145. doi: 10.1007/BF00237531. [DOI] [PubMed] [Google Scholar]
- 40.Fukushima K, Ohno M, Takahashi K, Kato M. Location and vestibular responses of interstitial and midbrain reticular neurons that project to the vestibular nuclei in the cat. Exp Brain Res. 1982;45(1–2):303–312. doi: 10.1007/BF00235791. [DOI] [PubMed] [Google Scholar]
- 41.Kokkoroyannis T, Scudder CA, Balaban CD, Highstein SM, Moschovakis AK. Anatomy and physiology of the primate interstitial nucleus of Cajal I. efferent projections. J Neurophysiol. 1996;75(2):725–739. doi: 10.1152/jn.1996.75.2.725. [DOI] [PubMed] [Google Scholar]
- 42.Fukushima K, Ohno M, Kato M. Responses of cat mesencephalic reticulospinal neurons to stimulation of superior colliculus, pericruciate cortex, and neck muscle afferents. Exp Brain Res. 1981;44(4):441–444. doi: 10.1007/BF00238838. [DOI] [PubMed] [Google Scholar]
- 43.Shaikh AG, Wong A, Zee DS, Jinnah HA. Why are voluntary head movements in cervical dystonia slow? Parkinsonism Relat Disord. 2015;21(6):561–566. doi: 10.1016/j.parkreldis.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaikh AG, Wong AL, Zee DS, Jinnah HA. Keeping your head on target. J Neurosci. 2013;33(27):11281–11295. doi: 10.1523/JNEUROSCI.3415-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaikh AG, Zee DS, Crawford JD, Jinnah HA. Cervical dystonia: A disorder of neural integrator. Brain. 2016 doi: 10.1093/brain/aww141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pelisson D, Goffart L, Guillaume A. Contribution of the rostral fastigial nucleus to the control of orienting gaze shifts in the head-unrestrained cat. J Neurophysiol. 1998;80(3):1180–1196. doi: 10.1152/jn.1998.80.3.1180. [DOI] [PubMed] [Google Scholar]
- 47.Pelisson D, Goffart L, Guillaume A. Control of saccadic eye movements and combined eye/head gaze shifts by the medio-posterior cerebellum. Prog Brain Res. 2003;142:69–89. doi: 10.1016/S0079-6123(03)42007-4. [DOI] [PubMed] [Google Scholar]
- 48.Brettler SC, Fuchs AF, Ling L. Discharge patterns of cerebellar output neurons in the caudal fastigial nucleus during head-free gaze shifts in primates. Ann N Y Acad Sci. 2003;1004:61–68. [PubMed] [Google Scholar]
- 49.Raike RS, Pizoli CE, Weisz C, van den Maagdenberg AM, Jinnah HA, Hess EJ. Limited regional cerebellar dysfunction induces focal dystonia in mice. Neurobiol Dis. 2013;49:200–210. doi: 10.1016/j.nbd.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prudente CN, Pardo CA, Xiao J, et al. Neuropathology of cervical dystonia. Exp Neurol. 2013;241:95–104. doi: 10.1016/j.expneurol.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Onodera S, Hicks TP. Projections from substantia nigra and zona incerta to the cat’s nucleus of Darkschewitsch. J Comp Neurol. 1998;396(4):461–482. [PubMed] [Google Scholar]
- 52.Blood AJ, Kuster JK, Woodman SC, et al. Evidence for altered basal ganglia-brainstem connections in cervical dystonia. PLoS One. 2012;7(2):e31654. doi: 10.1371/journal.pone.0031654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bakker DA, Richmond FJ, Abrahams VC. Central projections from cat suboccipital muscles: a study using transganglionic transport of horseradish peroxidase. J Comp Neurol. 1984;228(3):409–421. doi: 10.1002/cne.902280308. [DOI] [PubMed] [Google Scholar]
- 54.Ishii Y. Central afferent projections from the rat sternocleidomastoid and trapezius muscles. A study using transganglionic transport of horseradish peroxidase. Osaka Daigaku Shigaku Zasshi. 1989;34(1):193–212. [PubMed] [Google Scholar]
- 55.Markham CH, Precht W, Shimazu H. Effect of stimulation of interstitial nucleus of Cajal on vestibular unit activity in the cat. J Neurophysiol. 1966;29(3):493–507. doi: 10.1152/jn.1966.29.3.493. [DOI] [PubMed] [Google Scholar]
- 56.King WM, Precht W, Dieringer N. Synaptic organization of frontal eye field and vestibular afferents to interstitial nucleus of Cajal in the cat. J Neurophysiol. 1980;43(4):912–928. doi: 10.1152/jn.1980.43.4.912. [DOI] [PubMed] [Google Scholar]
- 57.Fukushima K, Terashima T, Kudo J, Inoue Y, Kato M. Projections of the group y of the vestibular nuclei and the dentate and fastigial nuclei of the cerebellum to the interstitial nucleus of Cajal. Neurosci Res. 1986;3(4):285–299. doi: 10.1016/0168-0102(86)90021-0. [DOI] [PubMed] [Google Scholar]
- 58.Stell R, Gresty M, Metcalfe T, Bronstein AM. Cervico-ocular function in patients with spasmodic torticollis. J Neurol Neurosurg Psychiatry. 1991;54(1):39–41. doi: 10.1136/jnnp.54.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diamond SG, Markham CH, Baloh RW. Ocular counterrolling abnormalities in spasmodic torticollis. Arch Neurol. 1988;45(2):164–169. doi: 10.1001/archneur.1988.00520260050019. [DOI] [PubMed] [Google Scholar]
- 60.Bronstein AM, Rudge P. The vestibular system in abnormal head postures and in spasmodic torticollis. Adv Neurol. 1988;50:493–500. [PubMed] [Google Scholar]
- 61.Bisdorff AR, Bronstein AM, Wolsley C, Lees AJ. Torticollis due to disinhibition of the vestibulo-collic reflex in a patient with Steele-Richardson-Olszewski syndrome. Mov Disord. 1997;12(3):328–336. doi: 10.1002/mds.870120311. [DOI] [PubMed] [Google Scholar]
- 62.Anastasopoulos D, Nasios G, Psilas K, Mergner T, Maurer C, Lucking CH. What is straight ahead to a patient with torticollis? Brain. 1998;121(Pt 1):91–101. doi: 10.1093/brain/121.1.91. [DOI] [PubMed] [Google Scholar]
- 63.Anastasopoulos D, Bhatia K, Bronstein AM, Marsden CD, Gresty MA. Perception of spatial orientation in spasmodic torticollis. Part 2: The visual vertical. Mov Disord. 1997;12(5):709–714. doi: 10.1002/mds.870120514. [DOI] [PubMed] [Google Scholar]
- 64.Anastasopoulos D, Bhatia K, Bisdorff A, Bronstein AM, Gresty MA, Marsden CD. Perception of spatial orientation in spasmodic torticollis. Part I: The postural vertical. Mov Disord. 1997;12(4):561–569. doi: 10.1002/mds.870120413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.