Abstract
Alteration in glutamate neurotransmission has been found to mediate the development of drug dependence, including nicotine. We and others, through using western blotting have reported that exposure to drugs of abuse reduced the expression of glutamate transporter-1 (GLT-1) as well as cystine/glutamate antiporter (xCT), which consequently increased extracellular glutamate concentrations in the mesocorticolimbic area. However, our previous studies did not reveal any changes in glutamate/aspartate transporter (GLAST) following exposure to drugs of abuse. In the present study, for the first time, we investigated the effect of chronic exposure to electronic (e)-cigarette vapor containing nicotine, for one hour daily for six months, on GLT-1, xCT, and GLAST expression in frontal cortex (FC), striatum (STR), and hippocampus in outbred female CD1 mice. In this study, we also investigated the expression of alpha-7 nicotinic acetylcholine receptor (α-7 nAChR), a major pre-synaptic nicotinic receptor in the glutamatergic neurons, which regulates glutamate release. We found that inhalation of e-cigarette vapor for six months increased α-7 nAChR expression in both FC and STR, but not in the HIP. In addition, chronic e-cigarette exposure reduced GLT-1 expression only in STR. Moreover, e-cigarette vapor inhalation induced downregulation of xCT in both the STR and HIP. We did not find any significant changes in GLAST expression in any brain region. Finally, using liquid chromatography-tandem mass spectrometry (LC-MS/MS) techniques, we detected high concentrations of nicotine and cotinine, a major metabolite of nicotine, in the FC tissues of e-cigarette exposed mice. These data provide novel evidence about the effects of chronic nicotine exposure on the expression of key glial glutamate transporters as well as α-7 nAChR. Our work may suggest that nicotine exposure via chronic inhalation of e-cigarette vapor may be mediated in part by alterations in the glutamatergic system.
Keywords: E-cigarettes, α-7 nAChR, GLT-1, xCT, Cotinine
1. Introduction
Electronic (e)-cigarettes are battery operated nicotine delivery devices that heat and aerosolize e-liquid, creating vapor [For review (Hahn et al., 2014)]. Most e-liquid on the market contains nicotine, thus users who are inhaling e-cigarette vapor are also inhaling nicotine (Crotty Alexander et al., 2015). Recently, e-cigarette use has increased globally as an alternative or supplement to conventional tobacco cigarette use (Yamin et al., 2010, Crotty Alexander et al., 2015, Schoenborn and Gindi, 2015). Emerging evidence demonstrates that exposure to e-cigarettes may induce several toxicological effects, including inflammation, decreased host defenses and DNA damage that is a precursor to neoplastic transformation (Vardavas et al., 2012, Hwang et al., 2016, Yu et al., 2016).
Although e-cigarettes were invented as a smoking cessation tool, they have been demonstrated to confer similar urges to smoke, as compared to conventional smoking (King et al., 2014). Withdrawal symptoms and high desire to smoke were associated with withholding of e-cigarettes (Dawkins et al., 2012). E-cigarettes deliver smaller amounts of nicotine per puff as compared to tobacco cigarettes, however, similar systemic nicotine and cotinine concentrations to combustible cigarettes have been achieved after exposure (Schroeder and Hoffman, 2014). It has been reported that some brands of e-cigarettes can deliver higher nicotine levels as compared to combustible cigarettes (Ramôa et al., 2015). It has been found that exposure to nicotine containing e-cigarette vapor during late prenatal and early postnatal life induced significant persistent behavioral alterations during adulthood compared to vehicle control (e-cigarettes without nicotine) and air-control mice (Smith et al., 2015).
In the present study, we investigated the effects of chronic exposure to e-cigarette vapor containing nicotine, using a well-established, clinically relevant mouse exposure system, on the glutamatergic system in female CD-1 mice. The rationale for using female, not male, mice in this study is that previous studies found that female animal models showed higher nicotine seeking behavior compared to male models (Donny et al., 2000, Torres et al., 2009). In addition, a prior study found that female rats exhibited higher motivation to consume nicotine as compared to male and female ovariectomized rats (Donny et al., 2000). This suggests that hormones, including estradiol, play a crucial role in nicotine seeking behavior. Moreover, nicotine rewarding effects have been enhanced in female rats as compared to male rats (Torres et al., 2009).
Nicotinic acetylcholine receptors (nAChRs), including alpha-7 nAChR (α-7 nAChR), have been found to be upregulated or stimulated following exposure to nicotine (Auta et al., 2000, Buisson and Bertrand, 2001, Konradsson-Geuken et al., 2009, Alsharari et al., 2015). Importantly, α-7 nAChR is localized mainly in pre-synaptic glutamatergic neurons in the mesocorticolimbic areas (Marchi et al., 2002, Jones and Wonnacott, 2004, Feduccia et al., 2012) and this receptor has been found to modulate the majority of glutamate release in prefrontal cortex (PFC) and other brain regions following nicotine exposure (Konradsson-Geuken et al., 2009, Feduccia et al., 2012, Bortz et al., 2013). However, there is little known about the effects of chronic nicotine exposure on α-7 nAChR expression. We here, for the first time, determined the expression of α-7 nAChR in frontal cortex (FC), striatum (STR) and hippocampus following six months of inhalation of e-cigarette vapor containing nicotine in female CD-1 mice.
Additionally, we and others have shown that chronic exposure to drugs of abuse reduced the expression of glutamate transporter-1 (GLT-1,) as well as cystine/glutamate antiporter (xCT) in the nucleus accumbens (NAc), HIP and amygdala (Knackstedt et al., 2009, Knackstedt et al., 2010, Alhaddad et al., 2014a, Alhaddad et al., 2014b, Aal-Aaboda et al., 2015). It is important to note that GLT-1 is responsible for clearance of a majority of the extracellular glutamate concentration into astrocytes (Tanaka et al., 1997, Danbolt, 2001). Decreased GLT-1expression was associated with significantly increased extracellular glutamate concentrations in the NAc in animals exposed to alcohol or heroin (Melendez et al., 2005, LaLumiere and Kalivas, 2008, Shen et al., 2014, Das et al., 2015). In addition, xCT plays a crucial role in glutamate homeostasis by exchanging extracellular cystine for intracellular glutamate (Baker et al., 2002, Shih et al., 2006). Moreover, the xCT system was found to be involved in attenuation of nicotine seeking (Knackstedt et al., 2009). Glutamate/aspartate transporter (GLAST) is another glutamate transporter, co-localized with GLT-1 in astrocytes, and is mainly expressed in the cerebellum and retina (Lehre and Danbolt, 1998, Danbolt, 2001). Although we did not observe any downregulation in GLAST expression following alcohol drinking for five weeks (Alhaddad et al., 2014b, Hakami et al., 2016), we aimed in this study to determine whether chronic nicotine exposure may affect the expression of this glia l glutamate transporter in the central reward brain regions. In the present study, the expression of GLT-1, xCT, and GLAST after six months of exposure to e-cigarette vapor containing nicotine was investigated in the FC, STR and HIP.
Several studies have detected nicotine and cotinine (the major metabolite and biomarker of nicotine) in plasma and urine (Hengen and Hengen, 1978, Curvall et al., 1982, Horstmann, 1985, Degen and Schneider, 1991, Mercelina-Roumans et al., 1996, Acosta et al., 2004, Chang et al., 2005, Levine et al., 2013). Few studies have assessed the concentration of nicotine and cotinine in specific brain regions, such as NAc and STR (Chang et al., 2005, Katner et al., 2015). However, there is little known about the bioavailability of nicotine in the brain following six months of inhalation. As compared to other routes of administration, nicotine inhalation has a fast and high rate of absorption as well as high rate of brain distribution [For review see (Le Houezec, 2003)]. Detection of high concentrations of nicotine and cotinine in the brain during chronic nicotine inhalation might suggest the degree of nicotine exposure associated with any changes in the glutamatergic system. In this study, Western blotting was used to quantify changes in the amount of proteins that are expressed in neurons and glia in different brain regions for air control and e-cigarette vapor containing nicotine groups. In addition, we determined nicotine and cotinine concentrations in the FC following six months of exposure to e-cigarette vapor.
2. Materials and Methods
2.1 E-cigarettes
All e-cigarette atomizers (tanks; 2.4 ohm, plastic, refillable) and e-liquids (propylene glycol, vegetable glycerin, nicotine, with no additives) used in the study were bought online from Xtreme Vaping. E-liquids were prepared in the lab by mixing 50% propylene glycol, 50% vegetable glycerin, and 24 mg/mL nicotine. This composition is commonly used in multiple brands of e-cigarettes as well as with users who make their own e-cigarettes. E-cigarette batteries (280 mAh fixed, automatic, rechargeable, stainless steel) were purchased online from FastTech. Fresh e-cigarette vapor was used for all murine exposures, as described previously (Hwang et al., 2016). Briefly, voltage was applied to the heating coil in the e-liquid for 4 seconds every 20 seconds, and at the same time 2L/min negative pressure was applied to draw the e-liquid through the atomizer, creating the e-cigarette vapor.
2.2 Mouse inhalation of e-cigarette vapor
Six week old, female CD-1 mice were obtained from Jackson Labs. CD-1 mice are outbred, and thus have higher genetic diversity than most mouse lines. Significant findings in this mouse line are thought to have more clinical relevance, as their genetic diversity leads to more heterogeneity in the results, requiring a more powerful signal to reach significance. The inExpose system (SciReq) was used as previously described (Hwang et al., 2016) to provide nose-only e-cigarette vapor for inhalation, thus limiting the exposure to the respiratory system. Both e-cigarette and control mice are placed into the soft-mesh inExpose restraints for 60 minutes per day, five days per week, but the control mice breath room air only. At the age of 7–8 weeks, e-cigarette mice were exposed to e-cigarette vapor containing 24 mg/mL nicotine for 12 seconds per minute, for 60 minutes per day, five days per week, for six months.(Smith et al., 2015, Drummond et al., 2016, Hwang et al., 2016)Control mice were placed in the same restraints, but breathed room air only (Air control group). To create appropriate experimental controls, air control and e-cigarette exposed mice were placed into identical, individual mesh restraints. Although the air control group was intended to only breathe air, they were placed into the same hood as the e-cigarette mice attached to the InExpose device, to ensure similar temperature, light and sound exposures. Mice were allowed to recover for 30 minutes in pre-warmed cages following restraint. Mice were euthanized 1-2 hours after the last treatment with terminal intracardiac bleeding, under general anesthesia [100mg/kg ketamine (Zoetis) and 10mg/kg xylazine (Vedco)]. Note all animals were used in accord with the NIH guidelines for animal use under protocols approved by the IACUC committee at the University of California, San Diego and San Diego VA health system.
2.3 Brain Tissue Harvesting
Brains were gently removed from the skull. Specific brain regions, including FC, STR and HIP, were dissected according to stereotaxic coordinates from the Mouse Brain Atlas (Franklin and Paxinos). Brain sections were immediately snap frozen in liquid nitrogen and stored at -80°C for immunoblot testing.
2.4 Western blot protocol for detection of a-7 nAChR, GLT-1, xCT and GLAST
Western blotting was used to quantify changes in the expression of α-7 nAChR, GLT-1, xCT, GLAST and GAPDH in FC, STR and HIP tissues between e-cigarette and air control groups, as described previously (Alasmari et al., 2015, Alasmari et al., 2016a, Hakami et al., 2016). Brain tissues (FC, STR and HIP) were homogenized in lysis buffer-containing protease inhibitors. Total protein in each homogenized brain sample was quantified using Bio-Rad quantification reagents, DC™ (detergent compatible) protein assay (Bio-Rad, Hercules, CA, USA). Equal amounts of protein from FC, STR, and HIP tissue samples were loaded on 10-20% polyacrylamide gels for electrophoretic separation. Proteins were transferred to PVDF membranes, which were blocked using 5% (α -7 nAChR) or 3% (GLT-1, xCT, GLAST and GAPDH) milk in Tris-buffered saline with Tween-20 (TBST) for 30 minutes at room temperature. Membranes were incubated at 4°C overnight with one of the following primary antibodies: rat anti- α-7 nAChR (1:500 Abcam), guinea pig anti-GLT-1 (1:5000 Millipore),rabbit anti-xCT (1:1000 Abcam), and rabbit anti-GLAST (1:5000 Abcam). Mouse anti-GAPDH(1:5000 Millipore) was used as the loading control. On the following day, the membranes werewashed with TBST and blocked with 3% milk in TBST for 30 minutes at room temperature,followed by incubation with horseradish peroxidase-labeled (HRP) secondary antibodies (anti-ratIgG, anti-guinea pig IgG, anti-rabbit IgG or anti-mouse IgG) at 1:3000 for 90 minutes. Themembranes were washed five times with TBST and dried. Chemiluminescence was detectedusing a commercially available kit (Super Signal West Pico, Pierce Inc.) applied to the driedmembranes for one minute prior to exposure. An SRX-101A processor was used to develop theradiographic films (HyBlot CL Film Thermo Fisher Scientific) that were exposed to membranesincubated with chemiluminescence reagent. The digitized images of the α-7 nAChR, GLT-1, xCT, GLAST and GAPDH bands were quantified and assessed using MCID software. The data obtained from air control animals were used as 100% to focus on changes in the expression of studied proteins in brain regions of animals exposed to e-cigarette vapor containing nicotine.
2.5 Quantitation of Nicotine and Cotinine via ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS)
As described below, a portion of the FC was spiked with extraction buffer containing LC-MS internal standards, homogenized, extracted using a solid phase resin, and analyzed using LC-MS/MS via multiple reaction monitoring (MRM) to quantitate nicotine and cotinine. Treatment duration, route, conditions, and identity of samples were blinded to the LC-MS experimenter. Briefly, FC tissue (10-30 mg [n = 12]) was weighed and transferred to a 1.5 mL RINO tube (Next Advance Inc.) followed by addition of tissue extraction reagent (100 μL [Invitrogen] containing a protease inhibitor cocktail [Sigma]) and a solution of ammonium acetate (200 μLat 50 mM) containing two internal standards at a fixed concentration, Cotinine-d3 (5 μM) andNicotine-d4 (5 μM). The solution was homogenized with The Bullet Blender Storm (Next Advance Inc.) for 1 min (speed 8) followed by centrifugation at 13,500 rpm for 2 min. The supernatant was collected, the remaining pellet washed with ammonium acetate (200 μL at 50 mM), centrifuged at 13,500 rpm for 2 min, and the combined supernatants applied to a solid phase extraction resin (Evolute Express WCX cartridge; size = 30 mg/1 mL [Biotage]) using a Vacmaster manifold (Biotage). Cartridges were washed with ammonium acetate (1 mL at 50 mM), analytes eluted with methanol (1 mL) and evaporated to dryness via high flow purge with argon. Samples were reconstituted in 100 μL of 95:5 (ammonium acetate/methanol) for LC-MS analysis via a Shimadzu Nexera XR UPLC coupled with a Shimadzu 8050 triple quadrupole mass spectrometer. The instrument was optimized for MRM transitions using analytical grade standards of Cotinine, Nicotine, Cotinine-d3, and Nicotine-d4 (Sigma Aldrich and Cambridge Isotopes). UPLC utilized a Kinetex-core column (Phenomenex): 2.6 um C18 100 Å, 100 × 4.6 mm. UPLC Method: aqueous ammonium acetate (10mM, solvent A); methanolic Ammonium Acetate (10mM, solvent B); flow rate 1.0 mL/min (utilized post-column T-split, MS effective flow-rate = 0.3 mL/min). Gradient: t = 0 min, 0% B; t = 4 min, 95% B; t = 5.50 min, 95% B; t = 5.51, 0% B. Stop time 10 min. The resulting total ion chromatograms (TIC) for each MRM event demonstrated parent ion transition to three qualified fragment ions and were integrated to determine the area under the curve (AUC) of each analyte in each sample. Quantitation was carried out using a calibration curve and the ratio of the AUC of each analyte peak with the corresponding internal standard (AUCNic/AUCNicd4 and AUCCot/ AUCCotd3).
Forebrain tissue was spiked with varying concentrations of each analyte (Nicotine and Cotinine at 0.05, 0.1, 1, 5, and 10 μM) and processed as described for experimental samples above. A calibration curve was generated and extraction efficiency calculated based on the ratio of the AUC of the analyte peak to the AUC of the corresponding internal standard peak. The calibration curve was used to calculate concentration of analytes after normalization according to the weight of FC tissue used in each instance. Cotinine levels in serum were assessed via cotinine ELISA (Calbiochem), following the standard protocol and using a standard curve.
2.6 Statistical analyses
Western blot data obtained for proteins of interest in FC, STR and HIP tissues between e-cigarette and air control groups were analyzed using an independent unpaired t-test. The densities of immunoblot bands from the air control group were defined as 100%. LC-MS used one-way ANOVA with Tukey's post-test. All statistical analyses were represented as a p<0.05 level of significance.
3. Results
3.1 Effects of e-cigarettes on a-7 nAChR expression in the FC, STR and HIP
We determined the expression of α-7 nAChR following six months of e-cigarette vapor inhalation in the FC, STR and HIP. Unpaired t-test analyses showed an increase in the expression of α-7 nAChR in both FC (p<0.01; Fig. 1) and STR (p< 0.05; Fig. 1) in the e- cigarette group as compared to air controls. However, unpaired t-test analyses did not show any changes in α-7 nAChR in HIP (p> 0.05; Fig. 1).
Fig. 1.
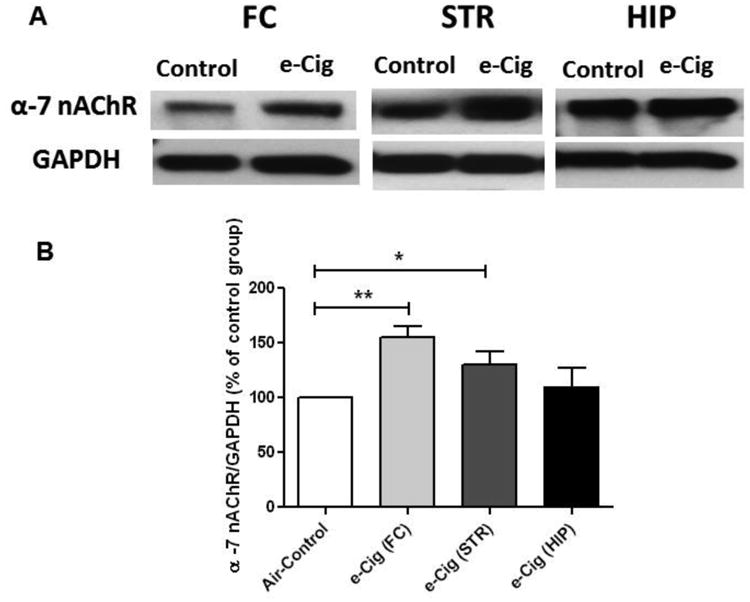
Effects of six months exposure to e-cigarette vapor (e-Cig) containing nicotine on α-7 nAChR expression in the FC, STR and HIP in female CD-1 mice. A) Immunoblot bands for α-7 nAChR and GAPDH (loading control) expression in the FC, STR and HIP. B) Unpaired t-test analysis of immunoblots showed a significant increase in the ratio of α-7 nAChR / GAPDH in FC and STR, but not in HIP, in e-cigarette exposed mice as compared with air controls (control value set to 100%). Data are shown as mean ± SEM (**p<0.01; *p<0.05), (n=4-5 for each group).
3.2 Effects of e-cigarettes on GLT-1 expression in the FC, STR and HIP
We investigated the effects of chronic e-cigarette use on the expression of GLT-1 in FC, STR and HIP brain regions. E-cigarette vapor inhalation reduced GLT-1 expression in the STR significantly, as compared to air controls (p< 0.05; Fig. 2). However, six months of exposure to e-cigarettes did not induce significant alterations in GLT-1 expression in either the FC or HIP brain regions (p> 0.05; Fig. 2).
Fig. 2.
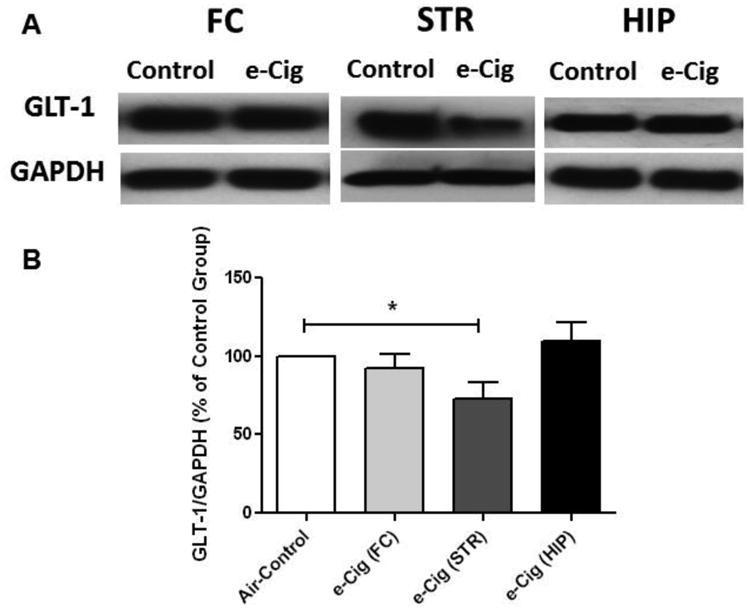
Effects of six months of inhalation of e-cigarette vapor containing nicotine (e-Cig) on GLT-1 expression in the FC, STR and HIP in female CD-1 mice. A) Immunoblot bands for GLT-1 and GAPDH (loading control) expression in the FC, STR and HIP. B) Unpaired t-test analysis of immunoblots showed a significant decrease in the ratio of GLT-1 / GAPDH in STR, and not significant in FC and HIP, in e-cigarette exposed mice compared with air controls (control value set to 100%). Data are shown as mean ± SEM (*p<0.05), (n=4-5 for each group).
3.3 Effects of e-cigarettes on xCT expression in the FC, STR and HIP
We further investigated the expression of xCT after six months of exposure to e-cigarettes. As compared to the air control group, unpaired t-test analyses showed significantly decreased the expression of xCT in the STR (p<0.05; Fig. 3) and HIP (p< 0.01; Fig. 3) in e-cigarette vapor exposed mice. However, e-cigarette vapor inhalation for six months did not cause any changes in xCT expression in the FC (p> 0.05; Fig. 3).
Fig. 3.
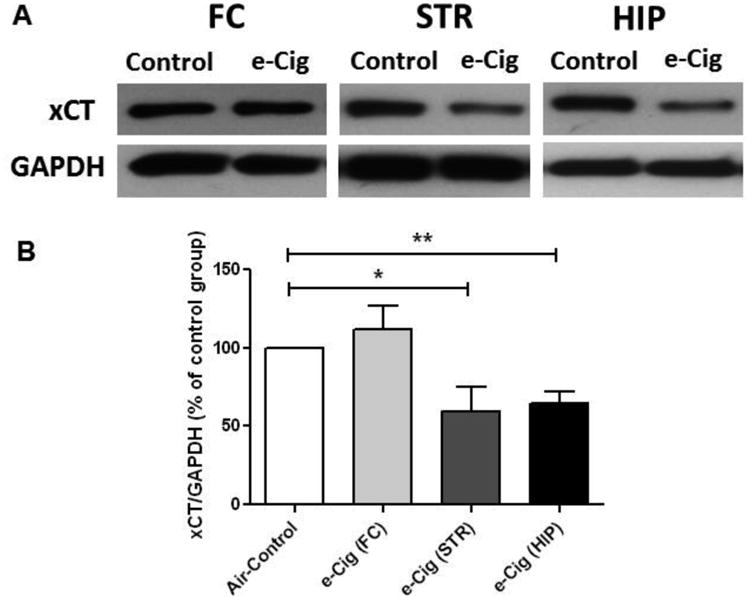
Effects of six months e-cigarette vapor containing nicotine inhalation (e-Cig) on xCT expression in the FC, STR and HIP of female CD-1 mice. A) Immunoblot bands for xCT and GAPDH (loading control) expression in FC, STR and HIP tissues. B) Unpaired t-test analysis of immunoblots showed a significant decrease in the ratio of xCT / GAPDH in STR and HIP, but not significant in FC, in e-cigarette mice as compared with air controls (control value set to 100%). Data are shown as mean ± SEM (**p<0.01; *p<0.05), (n=4-5 for each group).
3.4 Effects of e-cigarettes on GLAST expression in the FC, STR and HIP
The effects of nicotine containing e-cigarette vapor inhalation for six months on GLAST expression in the FC, STR and HIP were determined. Independent t-test analyses did not reveal any changes in GLAST expression in any of the three brain regions (p> 0.05; Fig. 4).
Fig. 4.
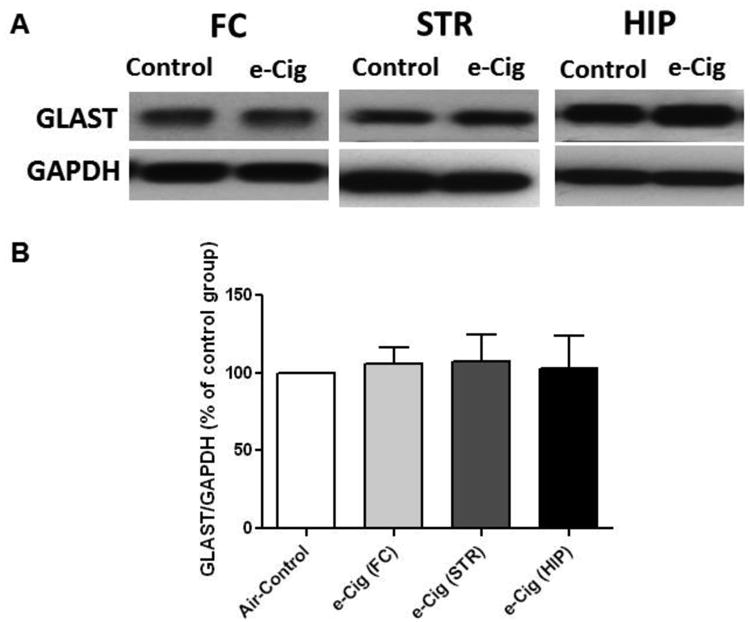
Effects of six months e-cigarette vapor containing nicotine inhalation (e-Cig) on GLAST expression in the FC, STR and HIP. A) Immunoblot bands for GLAST and GAPDH (loading control) expression in FC, STR and HIP tissues. B) Unpaired t-test analysis of immunoblots showed non-significant changes in the ratio of GLAST / GAPDH in FC, STR and HIP in e-cigarette exposed mice as compared with air controls (control value set to 100%). Data are shown as mean ± SEM, (n=4-5 for each group).
3.5 Determination of nicotine and cotinine concentrations in the FC, and cotinine in the plasma
Nicotine and cotinine concentrations were determined quantitatively using LC-MS based on a calibration curve correlating the area under the curve relationship of isotope labeled analytical standards (Nicotine-d4 and Cotinne-d3) at fixed concentrations with varying concentrations of nicotine and cotinine. Nicotine and cotinine were both found in the FC at significant levels in e-cigarette exposed mice, 18.82 μM (± 3.7) and 16.65 μM (± 9.2), respectively. The relatively short biological half-life of nicotine (reported at ∼10-30 min), makes our finding of significant interest. It suggests that chronic e-cigarette use could result in accumulation of nicotine and its chief metabolite, cotinine, in the FC. Cotinine has a 16 hour half-life in vivo, therefore it has more stable levels over time as compared to nicotine, making it an ideal biomarker for daily nicotine intake via smoking and vaping (Hukkanen et al., 2005). Cotinine in mouse plasma immediately after e-cigarette vapor exposure was 243 +/- 14 ng/mL. Human cotinine levels are typically between 250-300 ng/mL in cigarette smokers (Benowitz et al., 1983, Gori and Lynch, 1985). Nonsmokers exposed to secondhand smoke have serum cotinine levels of less than 1 ng/mL, but heavy exposure can lead to levels of 1–10 ng/mL. Active smokers consistently have serum levels higher than 10 ng/mL, averaging in the 250-300 ng/mL range, and sometimes higher than 500-800 ng/mL in heavy tobacco users (Hukkanen et al., 2005). Thus, our mouse e-cigarette exposure protocol leads to blood cotinine levels similar to that of smokers.
4. Discussion
Several studies have investigated the effects of nicotine on the glutamatergic system in different brain regions in the mesocorticolimbic areas (Wang et al., 2007, Kenny et al., 2009, Knackstedt et al., 2009, Konradsson-Geuken et al., 2009). Nicotine increases glutamate neurotransmission as well as upregulates post-synaptic glutamate receptor in these areas (Neff et al., 1998, Wang et al., 2007, Kenny et al., 2009, Konradsson-Geuken et al., 2009). It has been reported that the prefrontal cortex (PFC) sends glutamatergic inputs into NAc, a major brain region in the ventral STR (Kalivas and Volkow, 2005). In addition, the PFC sends as well as receives glutamatergic projections into and from the HIP (Hyman et al., 1987, Gigg et al., 1994, Parent et al., 2010). Moreover, the HIP has been shown to send glutamatergic inputs into the NAc (Britt et al., 2012). This glutamatergic interaction between different brain regions indicates the important role of the glutamatergic system following exposure to nicotine. In these studies, we investigated changes in the glutamatergic system, including nicotinic receptors, glutamate transporters and glutamate antiporters in FC, STR (location of NAc) and HIP brain regions following six months of nicotine-containing e-cigarette vapor inhalation by female CD-1 mice. We designed our mouse e-cigarette vapor exposure system based on the most common conventional cigarette smoke exposure, which entails 1 second of cigarette smoke every 60 seconds for 1 hour daily (1 minute total of cigarette smoke). Users take longer drags from e-cigarettes (3-4 seconds) as compared to conventional cigarettes (1 second), and have been found to consume more e-cigarette vapor daily when directly compared to cigarettes, thus our system activates the e-cigarette for 4 seconds, three times per minute. The total e-cigarette vapor exposure of one hour per day, 5 days per week, is modest compared to what heavy e-cigarette users are reporting. Alternatively, vapor inhalation for drug delivery has been well used in several drugs of abuse anima l models.
However, it is important to consider that vapor inhalation delivery may or not induce hormonal changes in animals as compared to animals exposed to drugs of abuse using different delivery routes. Studies are warranted to determine whether cigarette vapor inhalation can lead to hormonal changes in female and male animal models.
The effects of nicotine exposure on α-7 nAChR have been studied extensively (McGehee et al., 1995, Auta et al., 2000, Alkondon and Albuquerque, 2005, Konradsson-Geuken et al., 2009). However, there is little known about the effects of chronic nicotine exposure on α-7 nAChR expression. Gutamatergic synaptic transmission has been enhanced at least in part by stimulatory effects of nicotine on pre-synaptic α-7 nAChR (Cheng and Yakel, 2014). Stimulating α-7 nAChR by nicotine has been suggested to be one of the main mechanisms for nicotine-induced high extracellular glutamate concentrations in the mesocorticolimbic areas (Konradsson-Geuken et al., 2009, Cheng and Yakel, 2014). Chronic exposure to nicotine was found to be associated with upregulation of post-synaptic glutamate receptors (Wang et al., 2007, Kenny et al., 2009, Alasmari et al., 2016a) and that the effect has been suggested to be associated with altered glutamate neurotransmission in the mesocorticolimbic brain regions (Wang et al., 2007). Importantly, α-7 nAChR is expressed in glutamatergic projections from PFC to central brain reward regions such as NAc and ventral tegmental area (VTA) [For review see (Feduccia et al., 2012)]. This indicates that α-7 nAChR, expressed in the PFC, mediates glutamatergic projections into NAc and VTA. In this study, we determined a significant increase in α-7 nAChR expression in the FC in e-cigarette exposed mice as compared to air controls (Fig. 1). Moreover, we found that six months exposure to e-cigarettes upregulated α-7 nAChR in the STR as compared to air controls. It is important to note that, in a previous study, one minute local perfusion of nicotine (10 nmol/minute) into the STR was able to upregulate α-7 nAChR significantly in Flinders Sensitive rats as compared to Flinders Resistant rats, which was associated with significant increases in dopamine concentrations (Auta et al., 2000). Stimulation of α-7 nAChR in STR by a receptor agonist, through a microdialysis probe, induced glutamate release and, consequently, dopamine release (Campos et al., 2010). These and our findings suggest that α-7 nAChR in STR may be critical in the development of nicotine dependence through stimulation of glutamate and dopamine release.
A prior study reported that two subcutaneous nicotine injections in male rat pups at postnatal days 14 or 15 did not induce any upregulatory effects on α-7 nAChR expression in HIP slices (Alkondon and Albuquerque, 2005). Our results also showed that α-7 nAChR expression in the HIP region is not changed following chronic (six month) inhalation of nicotine within e-cigarette vapor. It is important to note that α-7 nAChR is highly expressed in HIP (Clarke et al., 1985, Seguela et al., 1993, Marks et al., 1996), which may suggest that neuroadaptation mediating α-7 nAChR expression in the HIP is a key factor that may reduce the effects of chronic nicotine exposure on the changes of the expression of this receptor.
The effects of drugs of abuse on GLT-1 and xCT have been investigated extensively (Knackstedt et al., 2009, Knackstedt et al., 2010, Alhaddad et al., 2014a, Alhaddad et al., 2014b, Hakami et al., 2016). Our lab showed that chronic exposure to ethanol for five weeks reduced the expression of GLT-1 and xCT in NAc, HIP, and amygdala in male rats (Alhaddad et al., 2014b, Aal-Aaboda et al., 2015, Hakami et al., 2016). In addition, β-lactam antibiotics and (R)-(-)-5-methyl-1-nicotinoyl-2-pyrazoline (MS-153) upregulated GLT-1 and xCT in these brain regions and consequently attenuated ethanol drinking behavior (Alhaddad et al., 2014b, Aal-Aaboda et al., 2015, Alasmari et al., 2015, Alasmari et al., 2016b). Moreover, ceftriaxone, a β-lactam antibiotic, and N-acetylcysteine restored the expression of both GLT-1 and xCT in rats exposed to cocaine and consequently reduced relapse-like cocaine seeking behavior (Knackstedt et al., 2010). GLT-1 and xCT have been suggested to play a crucial role in nicotine-seeking behavior such as nicotine-self administration and reinstatement of nicotine, as well as nicotine tolerance (Knackstedt et al., 2009, Schroeder et al., 2011, Alajaji et al., 2013, Gipson et al., 2013, Ramirez-Niño et al., 2013). A recent study from our lab showed that ceftriaxone attenuated ethanol-, nicotine-, and a mixed solution of ethanol and nicotine-drinking behavior at least in part by upregulating GLT-1 in both NAc and PFC (Sari et al., 2016). Importantly, self-administration of nicotine (0.03 mg/kg) base/infusion for 21 days induced down-regulatory effects on GLT-1 in NAc but not in other brain regions, including VTA, amygdala and PFC (Knackstedt et al., 2009). The same study found that nicotine self-administration for 21 days reduced the expression of xCT in both NAc and VTA (Knackstedt et al., 2009). In the present study, we used a model of nicotine intake with high physiological relevance to human e-cigarette users. We found that inhalation of e-cigarette vapor-containing nicotine for six months reduced GLT-1 expression in the STR but not in the FC and HIP. This suggests that GLT-1 protein in the STR is reduced following chronic nicotine exposure, and that this reduction may be due to the motivational effects of drugs of abuse, including nicotine (Knackstedt et al., 2009, Reissner and Kalivas, 2010).
We report here a significant reduction in xCT expression in the STR and HIP but not in FC tissues in e-cigarette exposed mice as compared to air controls. It is important to note that in contrast to continuous exposure to nicotine (such as via in-dwelling pumps), phasic exposure to nicotine, which was applied in our study, has been shown to decrease xCT expression (Knackstedt et al., 2009). Alternatively, we did not find any significant changes in GLAST, which is primarily located in the cerebellum and retina, expression level in any brain region studied between e-cigarette and air control groups. This is consistent with prior findings which showed that ethanol ingestion for five weeks did not induce any changes in GLAST expression in NAc and PFC in male rats (Alhaddad et al., 2014b, Hakami et al., 2016).
Previous studies found that inhalation of e-cigarettes as well as combustible cigarettes induced significantly increased cotinine concentrations in the plasma and urine (Brazell et al., 1984, Xu et al., 2014, Ha et al., 2015, Ponzoni et al., 2015, Smith et al., 2015, Drummond et al., 2016). In this study, we further detected nicotine and cotinine concentrations in FC tissues, which provide novel evidence about the distribution of nicotine in e-cigarette vapor into the brain. We found significant nicotine and cotinine concentrations in the brain of anima ls exposed to nicotine containing e-cigarette vapor, as compared to air control groups. This indicates the ability of inhaled nicotine to cross the blood brain barrier. Previous and our findings may indicate that inhaled nicotine of cigarettes is absorbed into the circulatory system and distributed into central nervous system. Since e-cigarettes and air-control groups were placed into the same mesh restraints, the environmental levels of e-cigarette vapor within the fume hood led to very low concentrations of cotinine in the FC of the air control mice.
5. Conclusion
We conclude here that nicotine delivery via e-cigarette vapor inhalation, using a physiological and clinically relevant exposure method, induced changes in the expression of glial glutamate transporters and nicotinic receptors. Chronic exposure to nicotine through inhalation of e-cigarette vapor containing nicotine increased α-7 nAChR expression in the mesocorticolimbic area. Moreover, e-cigarette exposure also reduced the expression of GLT-1 and xCT, which may lead to high extracellular glutamate concentrations in central reward brain regions. These data demonstrated that nicotine exposure alters glial glutamate transporters as well as nicotinic receptors, which might be key proteins in the development of nicotine dependence.
Fig. 5.
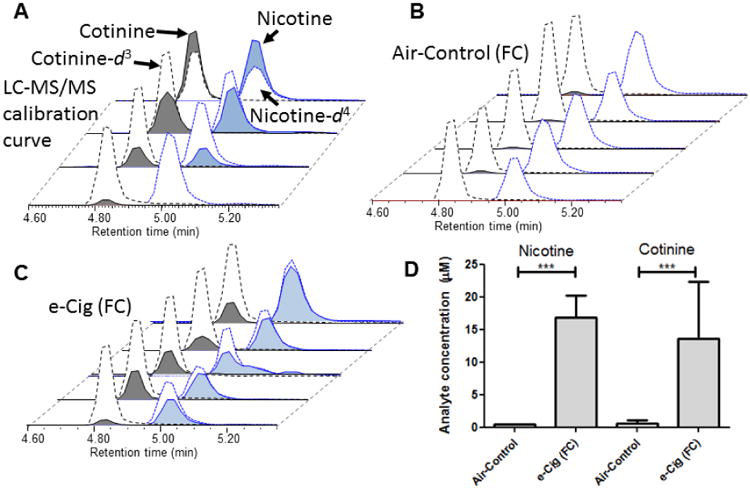
LC-MS/MS was used to quantify levels of nicotine and cotinine based on the ratio of the area under the curve of the MRM transitions for each analyte and a corresponding isotope labeled internal standard (cotinine-d3 and nicotine-d4). A) calibration curve for extracted blank brain samples spiked with varying concentrations of nicotine and cotinine with fixed concentration of nicotine-d4 and cotinine-d3. LC-MS/MS total ion chromatograms (TIC) for each analyte within each sample of B) Air-control group and C) e-cigarettes (e-Cig) group. D) calculated concentrations of each analyte based on TIC shown in A-C. Data analyzed using oneway ANOVA and shown as mean ± SEM (***= p<0.001), (n=5 for each group).
Highlights.
Inhalation of e-Cig vapor containing nicotine upregulates α-7 nAChR in FC and STR.
Inhalation of e-Cig vapor containing nicotine downregulates GLT-1 in STR.
Inhalation of e-Cig vapor containing nicotine downregulates xCT in STR and HIP.
GLAST is not changed following chronic exposure to e-Cig vapor containing nicotine.
Abbreviations
- α-7 nAChR
alpha-7 nicotinic acetylcholine receptor
- e-cigarettes
electronic cigarette
- FC
frontal cortex
- GLAST
glutamate/aspartate transporter
- GLT-1
Glutamate transporter-1
- HIP
hippocampus
- NAc
nucleus accumbens
- PFC
prefrontal cortex
- STR
striatum
- VTA
ventral tegmental area
- xCT
cystine/glutamate antiporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aal-Aaboda M, Alhaddad H, Osowik F, Nauli SM, Sari Y. Effects of (R)-(−)-5-methyl-1- nicotinoyl-2-pyrazoline on glutamate transporter 1 and cysteine/glutamate exchanger as well as ethanol drinking behavior in male, alcohol-preferring rats. Journal of neuroscience research. 2015;93:930–937. doi: 10.1002/jnr.23554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta MC, Buchhalter AR, Breland AB, Hamilton DC, Eissenberg T. Urine cotinine as an index of smoking status in smokers during 96-hr abstinence: comparison between gas chromatography/mass spectrometry and immunoassay test strips. Nicotine & tobacco research. 2004;6:615–620. doi: 10.1080/14622200410001727867. [DOI] [PubMed] [Google Scholar]
- Alajaji M, Bowers MS, Knackstedt L, Damaj MI. Effects of the beta-lactam antibiotic ceftriaxone on nicotine withdrawal and nicotine-induced reinstatement of preference in mice. Psychopharmacology (Berl) 2013;228:419–426. doi: 10.1007/s00213-013-3047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasmari F, Abuhamdah S, Sari Y. Effects of ampicillin on cystine/glutamate antiporter and glutamate transporter 1 isoforms as well as ethanol drinking in male P rats. Neuroscience letters. 2015;600:148–152. doi: 10.1016/j.neulet.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasmari F, Al-Rejaie SS, AlSharari SD, Sari Y. Targeting glutamate homeostasis for potential treatment of nicotine dependence. Brain research bulletin. 2016a;121:1–8. doi: 10.1016/j.brainresbull.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasmari F, Rao PS, Sari Y. Effects of cefazolin and cefoperazone on glutamate transporter 1 isoforms and cystine/glutamate exchanger as well as alcohol drinking behavior in male alcohol-preferring rats. Brain research. 2016b;1634:150–157. doi: 10.1016/j.brainres.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad H, Das SC, Sari Y. Effects of ceftriaxone on ethanol intake: a possible role for xCT and GLT-1 isoforms modulation of glutamate levels in P rats. Psychopharmacology. 2014a;231:4049–4057. doi: 10.1007/s00213-014-3545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad H, Kim NT, Aal-Aaboda M, Althobaiti YS, Leighton J, Boddu SH, et al. Effects of MS-153 on chronic ethanol consumption and GLT1 modulation of glutamate levels in male alcohol-preferring rats. Frontiers in behavioral neuroscience. 2014b;8:366. doi: 10.3389/fnbeh.2014.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Nicotinic receptor subtypes in rat hippocampal slices are differentially sensitive to desensitization and early in vivo functional up-regulation by nicotine and to block by bupropion. Journal of Pharmacology and Experimental Therapeutics. 2005;313:740–750. doi: 10.1124/jpet.104.081232. [DOI] [PubMed] [Google Scholar]
- Alsharari SD, King JR, Nordman JC, Muldoon PP, Jackson A, Zhu AZ, et al. Effects of menthol on nicotine pharmacokinetic, pharmacology and dependence in mice. PloS one. 2015;10:e0137070. doi: 10.1371/journal.pone.0137070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auta J, Lecca D, Nelson M, Guidotti A, Overstreet DH, Costa E, et al. Expression and function of striatal nAChRs differ in the flinders sensitive (FSL) and resistant (FRL) rat lines. Neuropharmacology. 2000;39:2624–2631. doi: 10.1016/s0028-3908(00)00082-4. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. The Journal of neuroscience. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Kuyt F, Jacob P, 3rd, Jones RT, Osman AL. Cotinine disposition and effects. Clin Pharmacol Ther. 1983;34:604–611. doi: 10.1038/clpt.1983.222. [DOI] [PubMed] [Google Scholar]
- Bortz D, Mikkelsen J, Bruno J. Localized infusions of the partial alpha 7 nicotinic receptor agonist SSR180711 evoke rapid and transient increases in prefrontal glutamate release. Neuroscience. 2013;255:55–67. doi: 10.1016/j.neuroscience.2013.09.047. [DOI] [PubMed] [Google Scholar]
- Brazell R, Stiff A, Henderson G, Jenkins R, Romig P, Auerbach O. Plasma nicotine and cotinine in tobacco smoke exposed beagle dogs. Toxicology and applied pharmacology. 1984;73:152–158. doi: 10.1016/0041-008x(84)90063-2. [DOI] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Chronic exposure to nicotine upregulates the human α4β2 nicotinic acetylcholine receptor function. The Journal of Neuroscience. 2001;21:1819–1829. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos F, Alfonso M, Duran R. In vivo modulation of alpha7 nicotinic receptors on striatal glutamate release induced by anatoxin-A. Neurochemistry international. 2010;56:850–855. doi: 10.1016/j.neuint.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Chang YL, Tsai PL, Chou YC, Tien JH, Tsai TH. Simultaneous determination of nicotine and its metabolite, cotinine, in rat blood and brain tissue using microdialysis coupled with liquid chromatography: pharmacokinetic application. Journal of Chromatography A. 2005;1088:152–157. doi: 10.1016/j.chroma.2005.01.042. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Yakel JL. Presynaptic alpha7 nicotinic acetylcholine receptors enhance hippocampal mossy fiber glutamatergic transmission via PKA activation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:124–133. doi: 10.1523/JNEUROSCI.2973-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P, Schwartz RD, Paul SM, Pert CB, Pert A. Nicotinic binding in rat brain: autoradiographic comparison of [3H] acetylcholine,[3 H] nicotine, and [125I]-alpha- bungarotoxin. The Journal of Neuroscience. 1985;5:1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty Alexander LE, Vyas A, Schraufnagel DE, Malhotra A. Electronic cigarettes: the new face of nicotine delivery and addiction. Journal of thoracic disease. 2015;7:E248–251. doi: 10.3978/j.issn.2072-1439.2015.07.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curvall M, Kazemi-Vala E, Enzell CR. Simultaneous determination of nicotine and cotinine in plasma using capillary column gas chromatography with nitrogen-sensitive detection. Journal of Chromatography B: Biomedical Sciences and Applications. 1982;232:283–293. doi: 10.1016/s0378-4347(00)84168-7. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Progress in neurobiology. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Das SC, Yamamoto BK, Hristov AM, Sari Y. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. 2015;97:67–74. doi: 10.1016/j.neuropharm.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins L, Turner J, Hasna S, Soar K. The electronic-cigarette: effects on desire to smoke, withdrawal symptoms and cognition. Addictive behaviors. 2012;37:970–973. doi: 10.1016/j.addbeh.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Degen PH, Schneider W. Rapid and sensitive determination of low concentrations of nicotine in plasma by gas chromatography with nitrogen-specific detection. Journal of chromatography. 1991;563:193–198. doi: 10.1016/0378-4347(91)80295-n. [DOI] [PubMed] [Google Scholar]
- Donny E, Caggiula A, Rowell P, Gharib M, Maldovan V, Booth S, et al. Nicotine self- administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology. 2000;151:392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- Drummond CA, Alexander LEC, Haller ST, Fan X, Xie JX, Kennedy DJ, et al. Cigarette Smoking Causes Epigenetic Changes Associated With Cardiorenal Fibrosis. Physiological Genomics physiolgenomics. 2016 doi: 10.1152/physiolgenomics.00070.2016. 00070.02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feduccia AA, Chatterjee S, Bartlett SE. Neuronal nicotinic acetylcholine receptors: neuroplastic changes underlying alcohol and nicotine addictions. Frontiers in molecular neuroscience. 2012;5:83. doi: 10.3389/fnmol.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. Paxinos and Franklin's The mouse brain in stereotaxic coordinates [Google Scholar]
- Gigg J, Tan AM, Finch DM. Glutamatergic hippocampal formation projections to prefrontal cortex in the rat are regulated by GABAergic inhibition and show convergence with glutamatergic projections from the limb ic thalamus. Hippocampus. 1994;4:189–198. doi: 10.1002/hipo.450040209. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, et al. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proceedings of the National Academy of Sciences. 2013;110:9124–9129. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori GB, Lynch CJ. Analytical cigarette yields as predictors of smoke bioavailability. Regul Toxicol Pharmacol. 1985;5:314–326. doi: 10.1016/0273-2300(85)90045-5. [DOI] [PubMed] [Google Scholar]
- Ha MA, Smith GJ, Cichocki JA, Fan L, Liu YS, Caceres AI, et al. Menthol attenuates respiratory irritation and elevates blood cotinine in cigarette smoke exposed mice. PloS one. 2015;10:e0117128. doi: 10.1371/journal.pone.0117128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J, Monakhova YB, Hengen J, Kohl-Himmelseher M, Schussler J, Hahn H, et al. Electronic cigarettes: overview of chemical composition and exposure estimation. Tobacco induced diseases. 2014;12:23. doi: 10.1186/s12971-014-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakami AY, Hammad AM, Sari Y. Effects of Amoxicillin and Augmentin on Cystine-Glutamate Exchanger and Glutamate Transporter 1 Isoforms as well as Ethanol Intake in Alcohol-Preferring Rats. Frontiers in neuroscience. 2016;10:171. doi: 10.3389/fnins.2016.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengen N, Hengen M. Gas-liquid chromatographic determination of nicotine and cotinine in plasma. Clinical chemistry. 1978;24:50–53. [PubMed] [Google Scholar]
- Horstmann M. Simple high-performance liquid chromatographic method for rapid determination of nicotine and cotinine in urine. Journal of chromatography. 1985;344:391–396. doi: 10.1016/s0378-4347(00)82046-0. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Lyes M, Sladewski K, Enany S, McEachern E, Mathew DP, et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. Journal of Molecular Medicine. 2016:1–13. doi: 10.1007/s00109-016-1378-3. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR. Alzheimer's disease: glutamate depletion in the hippocampal perforant pathway zone. Annals of neurology. 1987;22:37–40. doi: 10.1002/ana.410220110. [DOI] [PubMed] [Google Scholar]
- Jones IW, Wonnacott S. Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:11244–11252. doi: 10.1523/JNEUROSCI.3009-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. The American journal of psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Katner SN, Toalston JE, Smoker MP, Rodd ZA, McBride WJ, Engleman EA. Time-course of extracellular nicotine and cotinine levels in rat brain following administration of nicotine: effects of route and ethanol coadministration. Psychopharmacology (Berl) 2015;232:551–560. doi: 10.1007/s00213-014-3681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Chartoff E, Roberto M, Carlezon WA, Jr, Markou A. NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2009;34:266–281. doi: 10.1038/npp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Smith LJ, McNamara PJ, Matthews AK, Fridberg DJ. Passive exposure to electronic cigarette (e-cigarette) use increases desire for combustible and e-cigarettes in young adult smokers. Tobacco control. 2014 doi: 10.1136/tobaccocontrol-2014-051563. tobaccocontrol-2014-051563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biological psychiatry. 2009;65:841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biological psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradsson-Geuken Å, Gash CR, Alexander K, Pomerleau F, Huettl P, Gerhardt GA, et al. Second-by-second analysis of alpha 7 nicotine receptor regulation of glutamate release in the prefrontal cortex of awake rats. Synapse. 2009;63:1069–1082. doi: 10.1002/syn.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. The journal of neuroscience. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Houezec J. Role of nicotine pharmacokinetics in nicotine addiction and nicotine replacement therapy: a review. The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2003;7:811–819. [PubMed] [Google Scholar]
- Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. The Journal of neuroscience. 1998;18:8751–8757. doi: 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine H, Berman T, Goldsmith R, Göen T, Spungen J, Novack L, et al. Exposure to tobacco smoke based on urinary cotinine levels among Israeli smoking and nonsmoking adults: a cross-sectional analysis of the first Israeli human biomonitoring study. BMC public health. 2013;13:1. doi: 10.1186/1471-2458-13-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi M, Risso F, Viola C, Cavazzani P, Raiteri M. Direct evidence that release- stimulating α7* nicotinic cholinergic receptors are localized on human and rat brain glutamatergic axon terminals. Journal of neurochemistry. 2002;80:1071–1078. doi: 10.1046/j.0022-3042.2002.00805.x. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Grun EU, Collins AC. ST/b and DBA/2 mice differ in brain α- bungarotoxin binding and α7 nicotinic receptor subunit mRNA levels: a quantitative autoradiographic analysis. Molecular brain research. 1996;39:207–222. doi: 10.1016/0169-328x(96)00027-7. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcoholism: Clinical and Experimental Research. 2005;29:326–333. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Mercelina-Roumans PE, Schouten H, Ubachs JM, Van Wersch JW. Cotinine concentrations in plasma of smoking pregnant women and their infants. Clinical Chemistry and Laboratory Medicine. 1996;34:525–528. doi: 10.1515/cclm.1996.34.7.525. [DOI] [PubMed] [Google Scholar]
- Neff RA, Humphrey J, Mihalevich M, Mendelowitz D. Nicotine enhances presynaptic and postsynaptic glutamatergic neurotransmission to activate cardiac parasympathetic neurons. Circulation research. 1998;83:1241–1247. doi: 10.1161/01.res.83.12.1241. [DOI] [PubMed] [Google Scholar]
- Parent MA, Wang L, Su J, Netoff T, Yuan LL. Identification of the hippocampal input to medial prefrontal cortex in vitro. Cerebral Cortex. 2010;20:393–403. doi: 10.1093/cercor/bhp108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzoni L, Moretti M, Sala M, Fasoli F, Mucchietto V, Lucini V, et al. Different physiological and behavioural effects of e-cigarette vapour and cigarette smoke in mice. European Neuropsychopharmacology. 2015;25:1775–1786. doi: 10.1016/j.euroneuro.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Ramirez-Niño AM, D'Souza MS, Markou A. N-acetylcysteine decreased nicotine self- administration and cue-induced reinstatement of nicotine seeking in rats: comparison with the effects of N-acetylcysteine on food responding and food seeking. Psychopharmacology. 2013;225:473–482. doi: 10.1007/s00213-012-2837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramôa CP, Hiler MM, Spindle TR, Lopez AA, Karaoghlanian N, Lipato T, et al. Electronic cigarette nicotine delivery can exceed that of combustible cigarettes: a preliminary report. Tobacco control. 2015 doi: 10.1136/tobaccocontrol-2015-052447. obaccocontrol-2015-052447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Kalivas PW. Using glutamate homeostasis as a target for treating addictive disorders. Behavioural pharmacology. 2010;21:514. doi: 10.1097/FBP.0b013e32833d41b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Toalston JE, Rao PS, Bell RL. Effects of ceftriaxone on ethanol, nicotine or sucrose intake by alcohol-preferring (P) rats and its association with GLT-1 expression. Neuroscience. 2016;326:117–125. doi: 10.1016/j.neuroscience.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenborn CA, Gindi RM. Electronic cigarette use among adults: United States, 2014. NCHS data brief. 2015;217:1–8. [PubMed] [Google Scholar]
- Schroeder JA, Quick KF, Landry PM, Rawls SM. Glutamate transporter (GLT-1) activation enhances nicotine antinociception and attenuates nicotine analgesic tolerance. Neuroreport. 2011;22:970. doi: 10.1097/WNR.0b013e32834d87eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder MJ, Hoffman AC. Electronic cigarettes and nicotine clinical pharmacology. Tob Control. 2014;23 Suppl 2:ii30–35. doi: 10.1136/tobaccocontrol-2013-051469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Hw, Scofield MD, Boger H, Hensley M, Kalivas PW. Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. The Journal of Neuroscience. 2014;34:5649–5657. doi: 10.1523/JNEUROSCI.4564-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, Erb H, Sun X, Toda S, Kalivas PW, Murphy TH. Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. The Journal of neuroscience. 2006;26:10514–10523. doi: 10.1523/JNEUROSCI.3178-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Aherrera A, Lopez A, Neptune E, Winickoff JP, Klein JD, et al. Adult behavior in male mice exposed to E-cigarette nicotine vapors during late prenatal and early postnatal life. PloS one. 2015;10:e0137953. doi: 10.1371/journal.pone.0137953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O'Dell LE. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology. 2009;206:303–312. doi: 10.1007/s00213-009-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest Journal. 2012;141:1400–1406. doi: 10.1378/chest.11-2443. [DOI] [PubMed] [Google Scholar]
- Wang F, Chen H, Steketee JD, Sharp BM. Upregulation of ionotropic glutamate receptor subunits within specific mesocorticolimbic regions during chronic nicotine self- administration. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2007;32:103–109. doi: 10.1038/sj.npp.1301033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Xu Y, Fan ZH. Cotinine concentration in serum correlates with tobacco smoke- induced emphysema in mice. Scientific reports. 2014;4 doi: 10.1038/srep03864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamin CK, Bitton A, Bates DW. E-cigarettes: a rapidly growing Internet phenomenon. Annals of internal medicine. 2010;153:607–609. doi: 10.7326/0003-4819-153-9-201011020-00011. [DOI] [PubMed] [Google Scholar]
- Yu V, Rahimy M, Korrapati A, Xuan Y, Zou AE, Krishnan AR, et al. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral oncology. 2016;52:58–65. doi: 10.1016/j.oraloncology.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


