Abstract
Vitiligo is the most frequent human pigmentary disorder, characterized by progressive autoimmune destruction of mature epidermal melanocytes. Of the current treatments offering partial and temporary relief, ultraviolet (UV) light is the most effective, coordinating an intricate network of keratinocyte and melanocyte factors that control numerous cellular and molecular signaling pathways. This UV-activated process is a classic example of regenerative medicine, inducing functional melanocyte stem cell populations in the hair follicle to divide, migrate, and differentiate into mature melanocytes that regenerate the epidermis through a complex process involving melanocytes and other cell lineages in the skin. Using an in-depth correlative analysis of multiple experimental and clinical data sets, we generated a modern molecular research platform that can be used as a working model for further research of vitiligo repigmentation. Our analysis emphasizes the active participation of defined molecular pathways that regulate the balance between stemness and differentiation states of melanocytes and keratinocytes: p53 and its downstream effectors controlling melanogenesis; Wnt/β-catenin with proliferative, migratory, and differentiation roles in different pigmentation systems; integrins, cadherins, tetraspanins, and metalloproteinases, with promigratory effects on melanocytes; TGF-β and its effector PAX3, which control differentiation. Our long-term goal is to design pharmacological compounds that can specifically activate melanocyte precursors in the hair follicle in order to obtain faster, better, and durable repigmentation.
Keywords: vitiligo, repigmentation, melanocyte stem cell, melanoblast, regeneration
1. REPIGMENTATION IN VITILIGO: CLINICAL PROFILE
A. General Aspects
Vitiligo is an acquired autoimmune disorder of polygenic multifactorial inheritance, affecting 0.3–0.5% of the population worldwide.1 The typical presentation is a progressive depigmentation of the skin and mucous membranes due to melanocyte disappearance. The striking visual contrast between the normal pigmented and lesional skin causes psychological and social stigma among all patients, which can be devastating on darker skin phototypes. While the vitiligo lesional epidermis is eventually completely devoid of melanocytes,2 the pigmentation of terminal hairs is usually preserved. This observation suggests the presence of an intact hair bulb, and an intact bulge melanocyte reservoir in depigmented vitiligo skin, both of which are spared from the effects of the immune attack. The presence of these melanocyte precursors in the hair follicles of vitiligo depigmented lesions3 was recently confirmed by our immunostaining studies.4 Proposed explanations for the presence of an intact hair follicle bulge and bulb are as follows: (i) sequestration of the melanocyte-associated antigens from the recognition by autoreactive CD8+ T cells,5 due to the deep location of melanocytes within the hair follicle, and (ii) the effect of immune privilege in the hair follicle5, 6 that protects melanocytes in these locations from cytotoxic T cells.
The most accepted hypothesis for vitiligo pathogenesis is that both genetic and nongenetic factors affect melanocyte function and survival, eventually leading to their immune-mediated destruction.7 Circulating skin-homing cytotoxic T cells8 and infiltrates of activated cytotoxic CD8+ T cells and macrophages were described at the margins of active vitiligo lesions,9–12 suggesting that the attack of CD8+ T cells on epidermal melanocytes is a key event for depigmentation.13 Recent reports show that CD8+ T cells are major producers of interferon (IFN)-γ,14 which is also found to be required for depigmentation in mouse models of vitiligo; IFN-γ induces the expression of the chemokine CXCL10, which promotes the migration of autoreactive T cells into the epidermis.15 The genetic factors in vitiligo are meaningful, since 15–20% of patients report one or more affected first-degree relatives, and the studies on monozygotic twins showa concordance rate of ~23%.16 Genome-wide association and sequencing analyses have produced a rich yield of vitiligo susceptibility genes, encoding proteins with immune- or melanocyte-specific functions.17, 18 Studies using melanocyte cultures from non-lesional vitiligo skin showed that long-term exposure to sub-cytotoxic oxidative stress induced a pro-senescent hypermitotic phenotype, which might explain the disappearance of functional melanocytes in the lesional epidermis.19 It was proposed that the loss of normal pigment regeneration may be caused by depletion of melanocyte stem or progenitor cells;19 however, this is contradicted by our immunostaining study4 that showed that the melanocyte stem cell populations are preserved in the hair follicle bulge of the untreated vitiligo skin, in similar numbers as in narrow band ultraviolet B (NBUVB) treated vitiligo or to skin collected from healthy controls. It is not yet proven whether or not these hair follicle bulge melanocyte stem cells from vitiligo skin are impaired in their ability to proliferate, migrate, and differentiate. Other mechanisms proposed for melanocyte destruction in vitiligo include involvement of cytotoxicity by antibodies,20 defects of melanocyte adhesion, neurogenic and biochemical damage, and autocytotoxicity.1
The goal of classic vitiligo therapies is to replenish the white spots with new active melanocyte precursors recruited in response to UV light and/or topical and oral drugs,21, 22 either from the hair follicle infundibulum (INF) or bulge through the process called repigmentation. In vitiligo, the generic term “repigmentation” refers to the replenishment of pigment cells only, since the basal keratinocytes appear in normal number in the depigmented skin. Standard vitiligo treatment has not had any conceptual advance since the introduction of psoralen plus ultraviolet A (PUVA) in the late 1940s.23 It is recognized that the main weaknesses of vitiligo treatment are its long duration and failure to provide definitive and complete repigmentation, producing an unsatisfactory clinical outcome. The lack of progress in vitiligo repigmentation is largely due to an incomplete understanding of the regulatory pathways involved in melanocyte stem cell activation in the hair follicle, and of their migration, proliferation, and differentiation to finally repopulate the basal epidermis. In this context, there is a compelling need for a better understanding of repigmentation biology. Exploring the full potential of melanocyte stem cells for the development of vitiligo therapies is a promising approach to achieve complete and stable repigmentation of the affected skin.
B. Clinical Patterns of Repigmentation
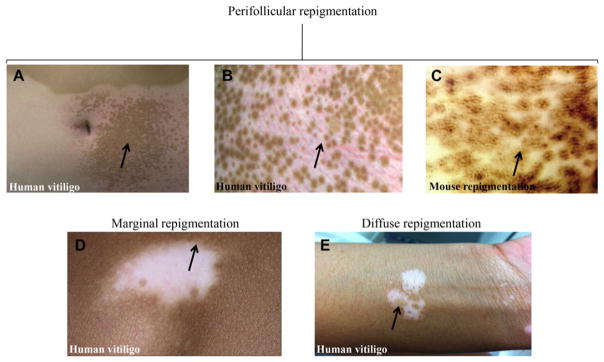
Repigmentation in human vitiligo occurs in different clinical patterns, of which the most prevalent has a perifollicular distribution24, 25 (Fig. 1A, B), indicating that the hair follicle is the main source of repigmentation. Using the double transgenic K14-Steel factor (SLF)/Dct-lacZ mice,26, 27 investigators depleted the amplifying populations of melanoblasts with c-KIT-blocking antibodies and then observed a perifollicular mottled repigmentation pattern similar to that seen in repigmenting human vitiligo (Fig. 1C).28 In this humanized model, the expression of SLF induced the intraepidermal migration of melanocytes; the use of Krt14 promoter forced constitutive expression of SLF in mouse epidermis, similar to that seen in human skin.
Figure 1.
Repigmentation patterns in human vitiligo and mouse model. (A, B, C) Perifollicular repigmentation pattern: (A, B) in human vitiligo after narrow band ultraviolet B (NBUVB) therapy; (C) in K14-Steel factor (SLF)/Dct-lacZ double transgenic mouse treated with c-Kit-blocking antibodies. (D) Marginal repigmentation pattern in human vitiligo. (E) Diffuse repigmentation pattern in human vitiligo. Panels A–C: Reproduced from [28]. Panel D: Reproduced from [3]. Panel E: Authors’ original. Arrows point toward the specific repigmentation pattern described in each panel.
The marginal repigmentation pattern presents as a hyperpigmented rim at the border of the white vitiligo macules (Fig. 1D),3, 25 occurring independently of or in combination with the perifollicular pattern. This pattern suggests activation of functional epidermal melanocytes at the lesional borders.29
Two other patterns have been observed in the minority of patients:25 diffuse (generalized darkening occurred across the patches supposedly from dermal or epidermal melanocyte precursors that persist in the center of the lesions)29 (Fig. 1E) and combined (including more than one pattern, or when the repigmentation does not fit into any single type).29
Recently, a fifth repigmentation pattern called “medium spotted” was described in a cohort of European pediatric population.30 This pattern is located in areas with no hairs or with low density of hairs (palms, soles, lips, ankles, and anterior wrists), and appears as irregular brown macules without a central hair follicle,30 and corresponds to a pattern previously described in a case report.31
Vitiligo repigmentation can be induced by stimulation with different types of UV light1 and only modestly with topical products - corticosteroids, calcineurin inhibitors, or vitamin D analogs. Repigmentation is unpredictable, not proportional to the magnitude of the lesions, and often cosmetically insufficient.20 In the same patient, new vitiligo depigmentation in association with repigmentation of other regions is common.1 Depigmented areas where hair follicles are absent (palms, soles, mucosal, or semimucosal surfaces) are in general refractory to treatment due to the absence of main melanocyte reservoir.3
Clinical studies on Indian24 and Korean25 vitiligo populations analyzed the evolution of various repigmentation patterns under therapy. Accordingly, the perifollicular and marginal patterns showed the greatest stability post-therapy.24 The speed of repigmentation was significantly higher in the diffuse type compared to any of the other three types.24 The ability to retain areas of repigmentation was dependent on the type of stimulus and site of activation: NBUVB with emission peak at 311 nm predominantly induced a perifollicular pattern;25 PUVA-treated lesions exhibited a similar pattern, which in addition was described as being long-lasting and stable,24 while topical or systemic steroids induced a diffuse type of repigmentation.24
C. The Role of Melanocyte Stem Cell Reservoirs in Repigmentation
The clinical observations of perifollicular and marginal patterns of repigmentation, suggesting the implication of hair follicle bulge and of epidermal melanocyte reservoirs, prompted the need for confirmatory studies at the cellular and molecular level. Early studies identified an amelanotic cell population (3,4-dihydroxyphenylalanine [DOPA] [-]) in the outer root sheath (ORS) of hair follicles that could not be distinguished morphologically from the surrounding keratinocytes.32–34 This population could be activated after excision of epidermis by UV therapy,33, 34 or after ionizing radiation.32 UV exposure of depigmented vitiligo skin induced the appearance of pigmented islands with follicular cores containing melanocytes with large cell bodies.35 This was confirmed in vitiligo patients treated with a Chinese herbal treatment36 that stimulated inactive melanocytes in the middle and/or lower parts of the hair follicles; the inactive melanocytes proliferated and migrated along the ORS to the nearby epidermis and expanded radially, exhibiting perifollicular repigmentation.36 The follicular reservoir of melanocyte precursors was also suggested by detection of DOPA (+) melanocytes in the scalp hair follicles after autologous transplantation of the lower portion of the hair follicles into depigmented vitiligo skin.37 Furthermore, in vitro experiments using cultures of human hair follicles revealed the existence of melanocyte lineage cells with significant potential for proliferation and further melanization.38 In the double transgenic K14-Steel factor (SLF)/Dct-lacZ mouse model,27 the observed upward migration of bulge Dct-lacZ(+) melanocyte precursors to the interfollicular epidermis (IE) provided confirmatory evidence that these precursors represent a reservoir for epidermal melanocytes.
Our group has recently designed an experimental platform that enabled the study of cellular and molecular mechanisms of perifollicular repigmentation in human vitiligo.4 The populations of melanocytes located in the bulge, infundibulum, and interfollicular epidermis of depigmented skin augmented by NBUVB exposure were identified based on the expression of dopachrome tautomerase (DCT) and C-KIT (markers of melanocyte development), and tyrosinase (TYR; marker of melanocyte differentiation). Mature melanocytes (TYR(+)) were not found in the bulge, infundibulum, or interfollicular epidermis of depigmented vitiligo skin, although these areas were enriched in melanocyte stem cells (DCT(+)/KIT(−)) and melanoblasts (DCT(+)/C-KIT(+)), ready for activation. Furthermore, these three populations had some proliferative [KI-67(+)] and migratory [(MCAM(+)] abilities. In addition, a fourth population [DCT(−)/C-KIT(+)/TYR(−)], found in the hair follicle and interfollicular epidermis of the treated skin, may represent a secondary melanocyte germ.
The existence of a reservoir of precursors in the epidermis has been supported earlier by the identification in the rete pegs of normal human skin of a subpopulation of dendritic and immature c-KIT(+)/TYR(−) pigment cells.39 An independent study described an epidermal population of PAX3 (+) melanocytes with suspected regenerative capacity, distributed in the deepest part of the rete ridges.40 In a more recently reported case of palmar vitiligo repigmentation after PUVA,31 which is most consistent with the newly described “medium spotted” pattern,30 the repigmented lesions contained TYR(+) melanocytes in the epidermis, while adjacent vitiliginous skin lacked these cells. The active pigment production was hypothesized to originate from a non-follicular, intra-epidermal precursor source within the glabrous skin31 that can implicate immature melanocyte c-KIT(+)/TYR(−), or PAX3(+), as described above.
The presence of a dermal, extrafollicular melanocyte stem cell reservoir with capacity to replace the damaged melanocytes in the vitiligo epidermiswas previously hypothesized based on clinical and experimental findings,41 and was eventually confirmed. An extrafollicular dermal source of melanoblasts DCT(+) was identified in the secretory portion of the eccrine sweat glands after skin exposure to ionizing radiation.42 It seems that the precursors of these cells colonize sweat glands during development and can renew themselves in response to genomic stress (e.g., ionizing radiation); they are also capable of providing their differentiating progeny to the epidermis.42
In vitro experiments show that multipotent dermal stem cells isolated from human foreskin lacking hair follicles displayed a capacity for self-renewal and expressed neural crest stem cell markers (NGFRp75 and nestin), as well as an embryonic stem cell marker (OCT4), but not melanocyte markers, when grown as 3D spheres. In addition, cells derived from single-cell clones were able to differentiate into multiple lineages including melanocytes. In a 3D skin equivalent model, sphere-forming cells differentiated into HMB45 (+) melanocytes, which migrated from dermis to epidermis and aligned singly among the basal layer keratinocytes in a similar fashion to pigmented melanocytes isolated from epidermis.43
The clinical and experimental observations discussed above confirm the role of the hair follicle melanocyte stem cell reservoir in vitiligo repopulation, and suggest the epidermal immature melanocytes as possible source for the non-perifollicular patterns. It is not yet experimentally proven whether the dermal extrafollicular melanocyte reservoir identified in sweat glands,42 or the source of melanocytes originated from multipotent dermal stem cells43 can or cannot regenerate the depigmented basal layer of vitiligo skin.
2. BUILDING A WORKING MODEL OF MELANOCYTE REPOPULATION IN VITILIGO REPIGMENTATION
In the “epidermal melanin unit”,44, 45 the melanocytes and keratinocytes are in a tight anatomical and functional relationship that continues downwards in the hair follicle bulge.27, 46 Changes of these intimate interactions characterize numerous physiological and pathological states of the pigmentary system, including vitiligo depigmentation and repigmentation. Keratinocytes provide a catalog of paracrine factors and adhesion molecules that play a dominant role in regulating melanocyte survival, proliferation, and differentiation.46 Several reports have claimed that the ability of UV light to induce melanocyte regeneration requires a deep anatomic penetration, targeting the bulge4, 47, 48 (where UV light augments migration, proliferation, and differentiation of melanocyte stem cells). Alternatively, UV light may activate the secretion by keratinocyte of a network of cytokines and paracrine growth factors in the interfollicular epidermis of the hair follicle, diffusing downwards in the bulge to stimulate the migration, proliferation, and differentiation of melanocyte stem cells.
Besides keratinocytes, multiple other skin cells have been shown to support optimal melanocyte function: for example, fibroblasts, adipocytes, endothelial cells, inflammatory cells,49 and dermal mast cells.50, 51 All of these cell types potentially have a role in vitiligo repigmentation.51 In the following paragraphs, we will discuss repigmentation induced by UV light, which appears to be more complex and have superior stimulatory potential on melanocyte stem cells than other treatment alternatives.
The UV-induced repigmentation process consists of discrete components (Figs. 2–5):52–99 keratinocyte stimulation and melanocyte activation by UV light (Fig. 2), melanocyte migration (including decoupling from the basement membrane and from keratinocytes, cell movement, and recoupling to the basement membrane and to keratinocytes; Fig. 3), melanocyte proliferation, and melanocyte differentiation (Fig. 4). These components are strictly regulated by complex signaling pathways, and will be further described in the next sections.
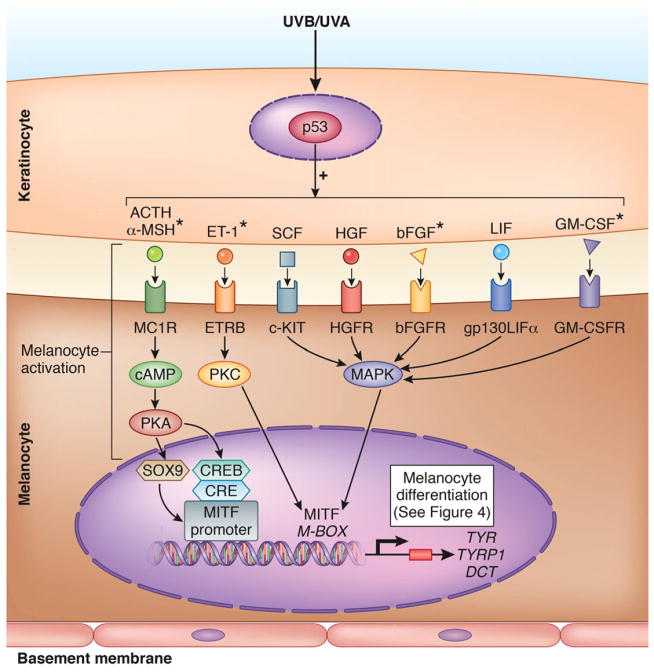
Figure 2.
Melanocyte activation integrated in the schematic view of the UV-induced pigmentation pathway in the normal skin. Once stimulated by UV light, p53 initiates the release by keratinocyte of melanogenic paracrine growth factors and cytokines: adrenocorticotropic hormone (ACTH),52 α-melanocyte-stimulating hormone (α-MSH),52,53 endothelin-1 (ET-1),54–56 stem cell factor (SCF),57 hepatocyte growth factor (HGF),58 basic fibroblast growth factor (bFGF),54,56 leukemia inhibitory factor (LIF),59 and granulocyte macrophage colony-stimulating factor (GM-CSF).60,61 The keratinocyte factors further interact with their corresponding receptors on melanocytes, and induce melanocyte activation, with subsequent stimulation of microphtalmia-associated transcription factor (MITF)62,63 and its downstream targets, the melanogenic enzymes Tyrosinase (TYR), Tyrosinase-related protein 1 (TYRP1), and Dopachrome tautomerase (DCT); this cascade leads to synthesis of melanin and melanocyte differentiation (see Fig. 4). MITF, the master regulator of melanogenic pathway, is activated through three signaling pathways regulated by cAMP,64,65 protein kinase C (PKC),63,66,67 and mitogen-activated protein kinase (MAPK)63,67 to subsequently induce proliferation and differentiation of human melanocytes.68 Figure based and modified from [62, 63]. UVB was shown to induce all keratinocyte factors included in the figure. *Factors activated by both UVB and UVA. Figure 2 was done by the medical illustrator Debbie Maizels.
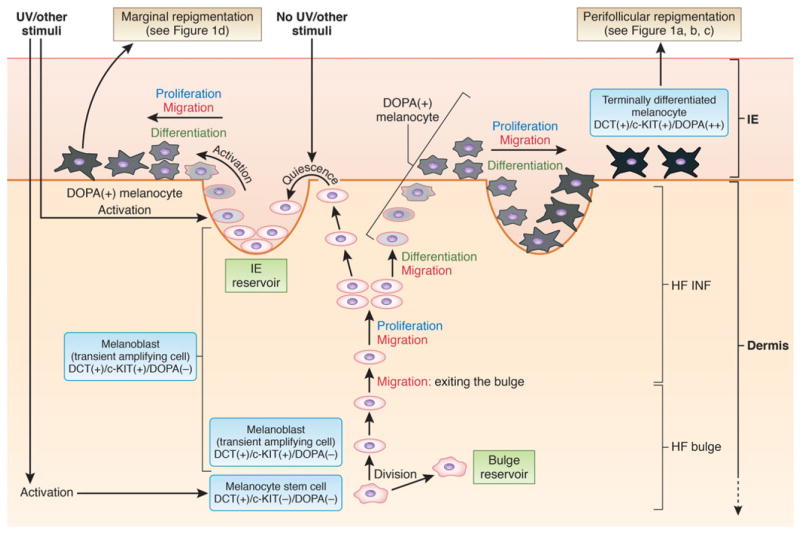
Figure 5.
Working model of the repigmentation pathway. This scheme was reproduced and modified from [48] and is based on data produced in a K14-Steel factor (SLF); Dct-lacZ double transgenic mouse,27 in a non-vitiligo Dct-LacZ+ mouse after wounding or UVB exposure,98 or in C57 black male mice after PUVA,99 and in human vitiligo after NBUVB4 or PUVA treatment,35 or after application of topical Chinese herbal medication.36 DCT, dopachrome tautomerase; DOPA, 3,4-dihydroxyphenylalanine; HF, hair follicle; IE, interfollicular epidermis; INF, infundibulum. Figure 5 was done by the medical illustrator Debbie Maizels.
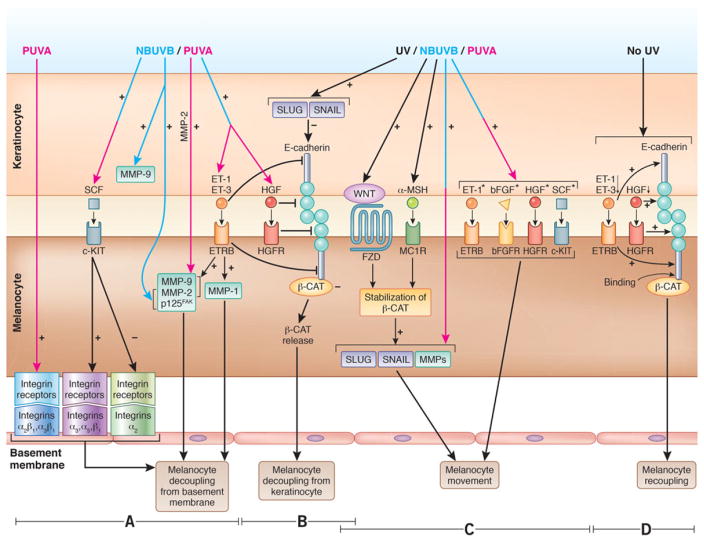
Figure 3.
Component processes of melanocyte migration (A–C).
(A) Melanocyte decoupling from basement membrane. Left-hand side section: PUVA induces the expression of α2 βl and α3 βl receptors in human melanocyte, which mediates melanocyte detachment from basement membrane.69 Middle section: SCF downregulates the expression of α2 receptor, and upregulates the expression of α3, α5, and β1 integrin receptors in human neonatal melanocytes, which enhance melanocyte decoupling.70 Right-hand side section: NBUVB increases the expression of endothelin receptor B (ETRB)-induced metalloproteinases (MMPs) and p125FAK in melanocyte, melanoma cells, and keratinocyte,54,71 which enhances melanocytic cells decoupling from the basement membrane. PUVA also induces significant upregulation of MMP-2.72
(B) Decoupling of melanocyte from keratinocyte. Melanocyte decoupling from keratinocytes is orchestrated by ET-173 and hepatocyte growth factor (HGF),58 both inhibitors of E-cadherin; decreased expression of E-cadherin in UVB-irradiated melanocytic cells73–75 and keratinocytes76 produces loss of cell–cell adhesions. E-cadherin is also repressed in human keratinocytes by transcription factors SLUG and SNAIL77,78 whose expression is elevated by UV,79 possibly through WNT/β-catenin activation.80
(C) Melanocyte movement. UV-induced loss of E-cadherin releases β-catenin,76 which forms a complex with a Frizzled (FZD) receptor, and further binds a WNT ligand expressed by keratinocyte; these events lead to β-catenin translocation from cytoplasm to the nucleus where β-catenin induces the expression of WNT target genes (SNAIL/SLUG, MMP-2, MMP-9) which under UV, stimulate melanocyte migration.72, 74,81–84 Melanocyte migration is also stimulated by α-MSH-MC1R85,86 and SCF/c-KIT signaling pathways,87 ET-1,88 bFGF,88 and HGF.88,89
(D) Melanocyte recoupling. Melanocyte recoupling to keratinocyte to reform the epidermal melanin unit can be supposedly initiated by increased expression of E-cadherin90 in absence of UV-induced stimulation of ET-1, ET-3, and HGF. Subsequently, E-cadherin can bind β-catenin, sequestering it in the cytoplasm, thus reestablishing the melanocyte–keratinocyte attachment. Experimental evidence used for this figure was generated in human, and mouse models, or using data from in vitro experiments performed on melanocyte/melanoma/epidermoid carcinoma cells. Pink, blue, and pink–blue arrows denote the action of either UVA or UVB, or of both, respectively. *Factors that stimulatemelanocyte proliferation.91–93 Figure 3 was done by the medical illustrator Debbie Maizels.
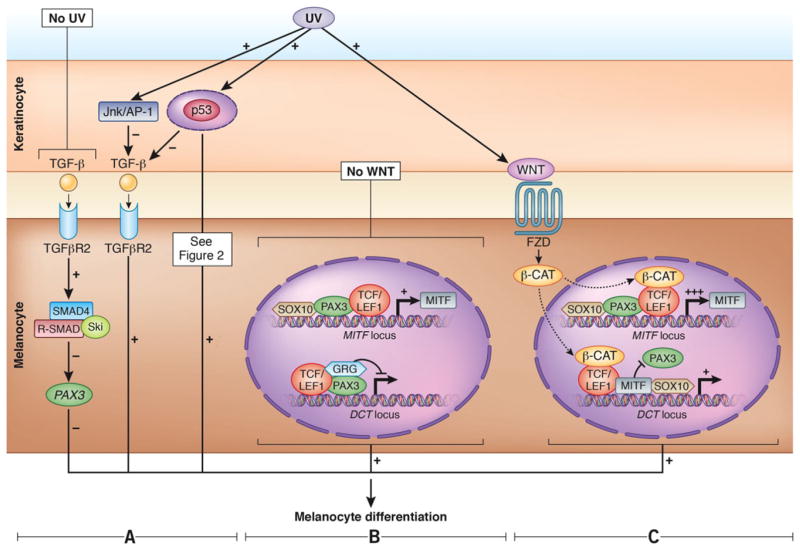
Figure 4.
Melanocyte differentiation. Transforming growth factor β (TGF-β) signaling pathway.
(A) In the absence of UV radiation (left-hand side section), keratinocytes express TGF-β that blocks melanocyte differentiation via SMAD signaling94,95 by repressing PAX3. In the presence of UV, the JNK/AP-1 pathway downregulates TGF-β (middle section). TGF-β is itself repressed in epidermal keratinocytes by p53 that promotes melanocyte differentiation.95,96 (B, C) Canonical WNT signaling pathway.
(B) In the absence of WNT: Upper side in the melanocyte nucleus-the complex of PAX3-SOX10 can activate the expression of MITF. Lower side in the melanocytes nucleus-the transcriptional repressor complex PAX3-LEF1-groucho-related proteins (GRGs) represses DCT transcription.97
(C) In the presence of WNT: Upper side in the melanocyte nucleus-β-catenin is activated by WNT and, together with LEF1, upregulates expression of MITF. Lower side in the melanocyte nucleus-activated β-catenin also forms an activator complex on the DCT promoter with MITF and LEF1 and displaces the repressor complex containing PAX3. SOX10 can also synergistically activate DCT with MITF. Experimental evidence used for this figure was generated in human and mouse. Parts B and C of Figure 4 are based on and modified from [97]. Figure 4 was done by the medical illustrator Debbie Maizels.
A. Melanocyte Activation Following UV Stimulation
Studies of human scalp hair indicated that the DOPA (−) ORS melanocytes contain some of the early melanocyte structural proteins, but none of the enzymatic components necessary for melanogenesis.100 It is conceivable that activation of ORS melanocytes in the hair follicle bulge initiates the synthesis of the structural and enzymatic proteins needed for melanin production. Several studies showed that the activation process is closely followed by migration and proliferation, which are paralleled by progressive differentiation,4, 33, 35 as the melanocytes move up along the hair follicle into the nearby epidermis.
An intimate interaction between melanocytes and keratinocytes is essential for the activation process. In hypermelanosis developed after repeated UV exposure, melanocyte activation is mediated by p53, as an immediate response to cellular and DNA damage.101, 102 Activation of p53 coordinates the release of keratinocyte paracrine/growth factors with melanogenic activity, and subsequently results in the induction of microphtalmia-associated transcription factor (MITF) and of downstream enzymes required for melanin biosynthesis62, 103 (Fig. 2).
B. Melanocyte Migration (Decoupling—Cell Movement—Recoupling)
Melanocyte migration, following the activation process, is initiated by the decoupling of melanocytes from the basement membrane44 and from keratinocytes.69 The release of paracrine, adhesion, and growth factors by keratinocytes which affect cell–cell adhesion is essential for the melanocyte decoupling–migration–recoupling cyclic process, and these factors will be described in the next paragraphs.
1. Melanocyte Decoupling from the Basement Membrane: Integrins
Integrins are the essential mediators of interactions between melanocytes and the basement membrane104 through attachment to vitronectin, fibronectin, and type I and IV collagen.105 Conditions resulting in increased cell migration (embryonic development, wound healing, angiogenesis and metastasis) are accompanied by active changes in integrin expression106 that initiate a shift from the stationary to motile cellular status. Similarly, during the repigmentation process, changes in expression of integrins’ subunits in melanocyte precursors can decrease cell adhesion to the basement membrane and orchestrate cell decoupling. In the absence of a repigmentation stimulus, no difference was found in the expression of integrin subunits α2, α3, α5, αv, α6, β1 and β3 in vitiligo lesional skin, compared to nonlesional skin and to normal human skin.107 Variation in the expression of integrins was observed in cultured melanocytes, following stimulation with UV or induction by stem cell factor (SCF), as outlined in detail in Fig. 3A.
2. Melanocyte Decoupling from the Basement Membrane: Metalloproteinases and Basement Membrane Abnormalities
Metalloproteinases (MMPs) are highly expressed during tissue remodeling, due to their capacity to cleave components of the extracellular matrix,108 preparing an optimal setting for cell migration. MMPs induce the decoupling of melanocytes from keratinocytes and coordinate the attachment of melanocytes to the next epidermal unit. Significantly lower expression of MMP-2 and MMP-9 has been reported in the vitiligo lesional border, as compared to the normal skin.108 This finding suggested that the defective function of MMPs in depigmented skin might impact the migratory ability of melanocyte precursors along the ORS to the lesional epidermis, impairing epidermal repopulation. Additional interesting data supporting the involvement of MMPs in the movement of melanocytic cells are summarized in Fig. 3A.
Ultrastructural abnormalities of the basement membrane have been observed frequently in vitiligo (e.g., focal gaps and multiple replication or layering directly beneath melanocytes).109, 110 The basement membrane has also been shown to contain increased amounts of the anti-adhesive molecule tenascin at the contact sites in melanocytes located near the lesional skin.107, 111 Thus, a defective basement membrane may contribute to melanocyte detachment in active vitiligo and can have a negative impact on melanocyte attachment to keratinocyte during epidermal repopulation with melanocyte precursors. Alternatively, increased tenascin and melanocyte detachment near the border of vitiligo patches may indicate an attempt by the surrounding epidermis to repigment the patch.
3. Melanocyte Decoupling from Keratinocytes: E-Cadherin
The main melanocyte-keratinocyte adhesion mediator is E-cadherin112 based on its role in formation of adherens junctions. Decreased expression of E-cadherin, producing loss of cell-cell adhesion and increased migratory capacity of melanocytic cells, has been observed during neural crest cell migration,113 nevi formation,114 and melanoma development and metastasis.112, 114 There are multiple factors that contribute to the reduced E-cadherin expression, and thus melanocytic cell migration, as presented in Fig. 3B. Immunostaining studies have shown that E-cadherin is discontinuously distributed across melanocyte membranes in the non-lesional skin of human vitiligo patients, several months before clinical vitiligo lesions become apparent. This abnormality was associated with the detachment of melanocytes from the basal and suprabasal keratinocytes.115
The E-cadherin cytoplasmic domain binds to β-catenin and forms a complex80 (Fig. 3B) whose aberrant skin expression was associated with a variety of human malignancies and disorders resulting from epithelial–mesenchymal transition.116 Modulation of this signaling pathway may hold the potential for facilitating melanocyte migration in vitiligo repigmentation and should be further investigated.
4. Melanocyte Movement
Numerous studies have shown significant stimulatory effects of keratinocyte-derived cytokines and paracrine growth factors released by keratinocytes on melanocyte movement: α-MSH-MC1R85, 86 and SCF/c-KIT signaling pathways,87, 117 ET-1,88, 117 bFGF,88, 117 and HGF88, 89 (Fig. 3C). Several experimental models that allow the manipulation of these signaling pathways4, 26, 27, 48, 98, 118, 119 (summarized in Table I) have provided valuable insights into the migration of melanocyte precursors.
Table I.
Models for Study of Skin Repigmentation
| Model specie | Model type | Findings | Ref. | |
|---|---|---|---|---|
| Mouse | ||||
| SLFTg1-1 injected with anti-c-Kit monoclonal antibody ACK2 | Repigmentation non-vitiligo humanized | This transgenic mouse expresses in the basal layer steel factor (SLF), forced using the Krt14 promoter. As a result, these mice presented hyperpigmented areas (gums, paws, etc.) that are unpigmented, or hypopigmented in the wild-type mice; treatment with the anti-c-KIT (ACK2) antibody eliminated c-Kit-dependent melanoblasts in the hair follicle and induced complete skin and hair depigmentation maintained for at least 1 week after birth; there was a population of residual melanocyte stem cells c-Kit((−))/Dct(+) maintained in the skin of the SLF transgenic mice; this population was able to migrate and cover most of the epidermis after several months | 26 | |
| K14-SLF/+; Dct-lacZ/+ double transgenic (Tg/+) | Repigmentation non-vitiligo humanized | The K14-SLF; Dct-LacZ/+ double transgenic (Tg/+) expresses SLF mutation and the lacZ reporter gene under the control of the Dct promoter; these mice were treated with ACK2 antibody at neonatal stage; melanocyte stem cells exited the bulge and migrated along the ORS to the inferfollicular epidermis in the presence of SLF expressed by epidermal keratinocytes; close to the epidermal surface some melanocytes became pigmented, indicative of active differentiation; the findings provided evidence that the bulge stem cells represent a reservoir for epidermal melanocytes | 27 | |
| F1 hairless HR-1 × HR/De | Repigmentation non-vitiligo | These mice are homozygous or heterozygous dominant for the main coat color genes; starting at 2 weeks of age, they lose their hair coat rapidly due to an abnormal second hair cycle, becoming completely hairless at week 3. At week 4 they are depigmented. They represent a unique model in which delayed pigmented spots are induced long after UV irradiation; the melanocyte precursors with migratory ability were observed to exit the hair follicle bulge and move along the infundibulum to the epidermis following UVB exposure; melanocyte stem cells proliferated in the bulge and differentiated into melanoblasts that migrated to the epidermis and became melanotic cells | 48 | |
| Dct-LacZ+ | Repigmentation non-vitiligo | Following the exposure to UVB or upon induction of a wound on the back of the mouse, melanocyte stem cells were shown to exit the bulge and migrate along the ORS infundibulum without proliferation; they proliferated and differentiated in the epidermis | 98 | |
| Mc1re/e | Repigmentation non-vitiligo | The wounded Mc1re/e mice (expressing a non-functional Melanocortin 1 receptor - Mc1r) presented a lower number of melanocyte stem cells that directly migrated from bulge to the epidermis, as compared to its control littermate Mc1r+/+ mice, suggesting impaired migration in the mutant mice Mc1re/e; this finding suggested that the defective melanocyte migration of Mc1re/e mice is attributed to the lack of Mc1r function | 98 | |
| Tyr-CreERT2; β-cateninfl(ex3)/+ | Pigmentation non-vitiligo | Wnt-stimulated melanocyte stem cell differentiation into pigment-producing melanocyte, and that the subsequent induction of aberrant β-catenin activation was followed by ectopic pigmentation in the bulge, a region devoid of pigment | 118 | |
| Human | ||||
| Punch grafts | Repigmentation vitiligo | Punch grafts were done on depigmented vitiligo lesions, and then they were exposed to Khellin + UV light. Immunostaining experiments revealed the migratory capacity of melanocytes (horizontal migration to depigmented areas) | 119 | |
| Skin biopsies | Repigmentation vitiligo | Skin biopsies taken from vitiligo untreated patients and from patients treated with NBUVB for 3 and 6 months were triple immunostained using melanocyte antibodies (anti-DCT, anti-C-KIT, anti-TYR, or anti-PAX3), anti-KI-67 antibody (labels proliferative cells) and/or anti-MCAM antibody (MCAM being associated with motile phenotypes), and a keratinocyte antibody (anti-K14); NBUVB was associated with a significant increase in the number of melanocytes in the infundibulum and with restoration of the normal melanocyte population in the epidermis, which was lacking in the untreated vitiligo biopsies; several precursor populations (melanocyte stem cells, melanoblasts, and other immature phenotypes), and progressively differentiating melanocytes, some with putative migratory and/or proliferative abilities, were identified | 4 | |
DCT, dopachrome tautomerase; MC1R, melanocortin 1 receptor; NBUVB, narrow band ultraviolet B; ORS, outer root sheath; Tyr, tyrosinase; UV, ultraviolet.
Tetraspanins CD9 and CD151 have been implicated in affecting the motility of primary human melanocytes and intermelanocyte and melanocyte–keratinocyte adhesion based on their localization in areas of homotypic intercellular contact (including the tips of melanocyte dendrites), and on the ability of tetraspanin monoclonal antibodies to abrogate melanocyte migration.120 Although minimally explored so far, tetraspanins can be potential players in vitiligo repigmentation.
5. Melanocyte Recoupling
Molecular models of melanoma formation and metastasis showed that melanoma cells escaped from keratinocytes by downregulation of E-cadherin and upregulation of N-cadherin. Re-expression of E-cadherin in human melanoma cells restored keratinocyte coupling and inhibited the invasive potential,90 underlining the importance of the N- to E-cadherin switch in cell recoupling. E-, N-, and P-cadherin expressions (Fig. 3D) have been reported in normal human melanocytes,121 but their implication in melanocyte recoupling in vitiligo repopulation is unexplored, and awaits further investigation.
C. Melanocyte Proliferation
Studies of melanocyte proliferation after UV exposure have produced divergent results (Tables II4, 35, 48, 91, 99, 122–128 and III84, 98, 125, 129–133). Studies supporting melanocyte proliferation (Table II) found an increased number of melanocytes, after between 4 days and 6 months of UVA or UVB exposure. The vast majority of studies observed an increased number of melanocytes in the epidermis4, 48, 91, 99, 122, 123, 125–128, while a few observed melanocyte proliferation in the hair follicle ORS.4, 35, 48 The hypothetical mechanisms by which UV light stimulates melanocyte proliferation include direct effects on melanocytes or their stimulation by keratinocyte-derived factors: ET-1,68, 92, 93 SCF,68 bFGF,68 and α-MSH68, 93 (Fig. 3). Consistent with the latter mechanism, increased serum levels of bFGF, SCF, and HGF were found in vitiligo patients undergoing active repigmentation with PUVA, as compared to untreated patients or to healthy controls.132
Table II.
Overview of the In Vitro, In Vivo, and Clinical Studies Supporting Melanocyte Proliferation Following UV Exposure
| Test system | UV exposure | Results | Ref. | |
|---|---|---|---|---|
| In vitro data | ||||
| Melanocytes from black and white donors | UVA | Melanocyte proliferation 7 and 14 days after UV exposure vs non-irradiated cells | 122 | |
| F1 mice of HR-1 × HR/De | UVB | Increased melanocyte number starting on day 7 and markedly increased on day 14 | 48 | |
| In vivo data | ||||
| C57B1 mice | UVA | Melanocyte proliferation in epidermis after UV exposure | 123 | |
| C57B1 mice | UVB | Recruitment of melanocytes or their precursors from outside of epidermis contributed to proliferation of melanocyte after UV exposure | 124 | |
| C57 black mice | UVA | Melanocyte proliferation 6–7 days after UV exposure | 99 | |
| C57 black mice | UVB | Increased melanocyte number 2 weeks after UV exposure | 91 | |
| SKH-2 mice | UVB | Melanocyte proliferation 4 days after UV exposure | 125 | |
| Humanized F1 hairless HR-1 × HR/De humanized mouse | UVB | Increased melanocyte number in epidermis on days 7 and 14 of UV exposure | 48 | |
| Clinical data | ||||
| Vitiligo human skin, Fitzpatrick II, IV | PUVA | Melanocyte proliferation in the hair follicle ORS after 2 months of PUVA | 35 | |
| Normal human skin, Fitzpatrick III, IV | UVA and UVB | Increased melanocyte number on days 7 and 14 of UV exposure | 126 | |
| Human nevi | UVA and UVB | Increased melanocyte proliferation in nevi excised 7 days after UV exposure | 127 | |
| Normal human skin, Fitzpatrick IV, V | UVa | Increased melanocyte proliferation on day 14 after UV exposure | 128 | |
| Vitiligo depigmented and repigmented skin | NBUVB | Increased melanocyte number in HF and IE after 3 and 6 months of phototherapy | 4 | |
UV, ultraviolet; IE, interfollicular epidermis; HF, hair follicle; NB, narrow band; vs, versus; ORS, outer root sheath; PUVA, psoralen + UVA.
Type of UV used not specified.
UV effects on melanocyte proliferation appear to be highly dependent on the type of UV used during clinical treatment or in the in vivo or in vitro studies. A single high dose of radiation seemed to be superior to repeated suberythemal exposures,125, 128 likely because it induced more DNA damage, more inflammation, and consequently a more prominent increase in melanocyte number. These findings suggest that UV-induced erythema is necessary to further stimulate melanocyte proliferation. This may explain the higher efficacy of NBUVB in inducing repigmentation versus PUVA (UVA requires 1000-fold higher dose than UVB to induce erythema)134 or topical compounds (e.g., calcineurin inhibitors135, 136 and vitamin D derivatives,21 which induce modest inflammation and have been shown to suppress melanocyte growth).21, 135, 137
In contrast with the findings discussed, other studies (Table III) have reported that melanocyte migrate and/or differentiate rather than proliferate during repigmentation,98, 133 and even that melanocyte proliferation was actually inhibited by UV exposure,130–132 thus leaving this question wide open for further investigation. Moving forward, standardization of the testing methods for UV-induced melanocyte proliferation, investigated endpoints, and data evaluation is imperative to explain the conflicting results. Toward this goal, we provide an outline for possible future research directions in Table IV.
Table III.
Overview of the In Vitro, In Vivo, and Clinical Studies Reporting Absent or Negative UV Effect on Melanocyte Proliferation
| Test system | UV exposure | Results | Ref. | |
|---|---|---|---|---|
| In vitro data | ||||
| Human melanocytes | UVa | Decreased melanocyte proliferation upon direct UV exposure and activated melanin synthesis | 129 | |
| Human melanocytes | UVB | Inhibition of melanocyte proliferation upon direct UV exposure | 130 | |
| Human melanocytes | PUVA | PUVA inhibited melanocyte proliferation | 131 | |
| Human melanocytes | UVA and UVB | Decreased number of melanocytes after UVB exposure, whereas no change in their number after UVA exposure | 132 | |
| Human melanocytes | PUVA, NBUVB | PUVA did not induce immediate effects on melanocyte proliferation vs NBUVB | 84 | |
| In vivo data | ||||
| Pigmented hairless Hr (SKH-2) mouse | UVA | UVA did not increase melanocyte proliferation | 125 | |
| Transgenic Dct-LacZ mice (wounded) | UVB | Presence of wound or UVB exposure did not increase melanocyte proliferation | 98 | |
| Clinical data | ||||
| Vitiligo human skin | PUVA | PUVA did not stimulate melanocyte proliferation but rather their migration | 133 | |
75% UVB and 25% UVA.
NBUVB, narrow band UVB; PUVA, psoralen and UVA; UV, ultraviolet; vs, versus.
Table IV.
Future Research Directions for Development of Research Platforms
| Repigmentation process component | Future directions |
|---|---|
| Melanocyte activation |
|
| Melanocyte migration |
|
| Melanocyte proliferation |
|
| Melanocyte differentiation |
|
MMP, metalloproteinase; NBUVB, narrow band ultraviolet B; PUVA, psoralen and ultraviolet A; TGF, transforming growth factor; UV, ultraviolet.
D. Melanocyte Differentiation/Maturation/Melanization
During this process, the precursors from the bulge and hair follicles acquire melanocyte specialized features, specifically the formation and maturation of melanosomes and the ability to synthesize melanin. Pathways governed by p5396, 101–103 (Fig. 2), TGF-β94, 95 (Fig. 4), and WNT/β-catenin48, 80, 97, 119 (Fig. 4), which involve both melanocytes and keratinocytes, are essential in melanocyte differentiation, all of them converging to activation of MITF and its downstream melanogenic enzymes. It seems that fibroblast-derived paracrine factors, dickkopf-related protein 1 (DKK1), and neuregulin-1 (NRG1) contribute to regulation of melanogenesis. DKK1, which is secreted at high levels in the dermis of the palms/soles by fibroblasts, suppresses melanocyte growth and function by inhibiting the Wnt/β-catenin signaling pathway.49, 138, 139 NRG1, which is highly expressed by fibroblasts derived from darker skin, was shown to have significantly increased pigmentation in a reconstructed skin model and in cultured human melanocytes. It has been suggested that NRG1, acting through the ErbB3 or ErbB4 receptors, leads to the activation of intracellular signaling that include the PI3K and the MAPK pathways to regulate melanogenesis.49, 140
The specific implication of these pathways in vitiligo repopulation awaits exploration. Melanocyte differentiation has been shown to accompany the proliferation and/or migration processes in different animal or human models of pigmentation, as outlined in Table I.4, 27, 48, 98, 118
This review reveals a rather limited knowledge of the interactions between different factors involved in NBUVB activation, migration, proliferation, and differentiation of melanocytes during the process of repigmentaion of human vitiligo lesions. Based on clinical observations and the research efforts of our group and others, we propose the following working model for melanocyte repigmentation in vitiligo (illustrated schematically in Fig. 5). Melanocyte stem cells residing in the bulge reservoir (identified as DCT(+)/c-KIT(−)/DOPA(−)),48 divide27, 48 and generate transient amplifying cells27 that are melanoblasts141 identified as DCT(+)/c-KIT(+)/DOPA(−).48 In response to different stimuli (like ultraviolet [UV] irradiation, or wounds created in the skin), melanoblasts can exit the bulge.27, 48, 98 Once in the infundibulum, the melanoblasts migrate along the ORS to the interfollicular epidermis;27, 48, 98 in parallel they proliferate,27, 48 while some differentiate, synthesizing melanin27 and becoming DOPA(+).4 Our current understanding is that some of these melanocyte precursors can reach the interfollicular epidermis in an immature state27 and enter into a quiescent state (maintaining an epidermal reservoir of precursors, as presented in the upper left-hand side of the figure), until activation by UV or other stimuli. These precursors may represent the source of marginal repigmentation observed clinically.31, 39 Another population of melanocytes derived from the hair follicle reaches the interfollicular epidermis and continues toward terminal differentiation (as presented in the upper right-hand side of the figure).27, 48 These cells appear in concentric layers in the epidermis around the hair follicle ostia (perifollicular repigmentation),27 and continue to migrate colonizing the vacant areas27 in a radial pattern36 and gradually becoming strongly pigmented melanocytes [DCT(+)/c-KIT(+)/DOPA(++)].27, 36, 48, 98 We believe that the model presented here becomes functional in situations of crisis, such as vitiligo and skin wounds, when epidermal melanocyte and keratinocytes are depleted, or significantly dysregulated.
3. HARNESSING THE POWER OF REGENERATIVE MEDICINE IN VITILIGO USING NEW TECHNOLOGIES
Besides activation of melanocyte precursors in the hair follicles with UV light or drugs, regeneration of vitiligo epidermiswas achieved with different transplantation techniques using tissue and cellular grafting,142 which do not involve the hair follicle melanocyte source. Although autologous transplantation is a therapy used currently, it is based on adult melanocytes which are difficult to culture and amplify in large numbers in vitro.143
Of the numerous pluripotent stem cell sources used for successful melanocytes induction (Table V),143–152 the multilineage differentiating stress-enduring (Muse) cells isolated from human dermal fibroblasts are considered the most promising for epidermal regeneration, due to their location in accessible mesenchymal tissue, and their ease of isolation by simple labeling with SSEA-3 marker.150 They can be readily reprogramed into functional melanocytes (Muse melanocytes) by using certain combinations of factors and cytokines. Muse melanocytes express several melanocyte-specific markers, were DOPA (+), produce melanin, and can integrate themselves into the basal layer of epidermis when they are incorporated into 3D-cultured skin models. Thus, functional Muse melanocytes generated from adult human fibroblasts can be applied to autologous transplantation for pigment disorders such as vitiligo.150
Table V.
Harnessing the Power of Regenerative Medicine in Vitiligo: Multiple Candidate Cell Populations for Initiating Vitiligo Repigmentation
| Cell lineage | Characteristics and technology | Ref. |
|---|---|---|
| Mesenchymal stem cells (MSCs) |
|
143 |
| Embryonic stem (ES) cells and induced pluripotent stem (iPS) cells |
|
144–149 |
|
51 | |
|
148, 149 | |
| Multilineage differentiating stress-enduring (Muse) cells |
|
150 |
|
151 | |
| Skin-derived precursor cells (SKPs) |
|
152 |
Recognizing the diverse differentiation ability of human pluripotent stem cells (hPSCs), and the power of modern techniques of isolation, purification, and reprogramming, we anticipate that future studies will explore the therapeutic potential of different candidate cell populations (presented in Table V)147–149 in initiating vitiligo repigmentation.
5. CONCLUSIONS
The limited success of current topical treatments and the failure of NBUVB to induce satisfactory repigmentation in most vitiligo patients highlight the pressing need for a better understanding of repigmentation biology. In this review, we have summarized data (generated using in vitro testing platforms, human and mouse models of vitiligo, studies on normal melanocytes, neural crest cells, melanoma cells, the wound healing process, and finally, clinical observations) suggesting that repigmentation in vitiligo requires the coordinated participation of essential molecular players in the key pathways that coordinate the balance between stemness and differentiation states of melanocytes including the following: p53 and its downstream effectors with melanogenic effects; Wnt/β-catenin with pro-proliferative, migratory, and differentiation roles in different pigmentation systems; integrins, cadherins, tetraspanins, MMPs, all with promigratory roles on melanocytes; TGF-β, its downstream effector PAX3 and their interaction with SOX10, all with essential roles in regulating melanocyte maturation. We have emphasized the exciting potential for designing future studies, based on standardized in vitro, in vivo, and clinical methods that can provide reproducible, reliable, and correlative results that define the mechanism(s) of repigmentation in vitiligo. An improved understanding of the molecular mechanisms that control melanocyte stem cells has the potential to lead to the identification of new drugs that can activate melanocyte stem cells and regenerate normal pigmented epidermis in vitiligo. While still relatively unexplored, the use of hPSCs represents a promising alternative that holds great potential for the future design of regenerative medicine treatments for vitiligo and other pigmentary disorders.
Acknowledgments
Contract grant sponsor: University of Colorado Anschutz Medical Campus Skin Disease Research Center; Contract grant number: P30AR057212.
We thank Dr. Jiang Chen, University of Stony Brook, for his valuable advice on this manuscript. This work was supported by the University of Colorado Anschutz Medical Campus Skin Disease Research Center (P30AR057212), and by an American Skin Association Research Scholar Award in Vitiligo to SAB.
Biographies
Stanca A. Birlea, M.D. Ph.D., is Associate Professor of Dermatology at the School of Medicine, University of Colorado. She has a background in clinical and investigative dermatology. She received her M.D. and her Ph.D. with a thesis on vitiligo, at the University of Medicine and Pharmacy Iuliu-Hatieganu, Cluj-Napoca Romania. She also completed a Dermatology Residency at the same institution. She worked in Romania as dermatologist for 5 years, specializing in general dermatology and developing numerous clinical trials for patients with skin autoimmune diseases. She completed her 6-year fellowship in genetics and immunology of vitiligo and complex traits at the School of Medicine, University of Colorado, having as mentors Professor David Norris, the Chair of the Department of Dermatology, and Professor Richard Spritz, the Director of Human Medical Genetics and Genomics program. Working in the Spritz Laboratory, she played an active role in the identification of several vitiligo susceptibility genes in founder and several unrelated vitiligo populations. In collaboration with Dr. Norris, she developed a translational research program named “Vitiligo: Regenerative Medicine/Stem Cell Approach to Repigmentation-VREMSAR” that she has been directing since 2012. This program is a collaboration between the Department of Dermatology and the Gates Stem Cell Center, directed by Dr. Dennis Roop, and seeks to develop innovative treatments for vitiligo, by combining clinical observation with the study of cellular and molecular pathways that govern repigmentation. Using the laser capture microdissection technique, her team has been characterizing the molecular pathways and triggers that control vitiligo repigmentation.
Gertrude-E. Costin, Ph.D., M.B.A. is a biochemist with a long history studying melanocyte function in the skin. Her research on the pigment cell field began during the Ph.D. training at the Institute of Biochemistry of the Romanian Academy in Bucharest, Romania. Her Ph.D. thesis investigated the effect of glycosylation inhibitors on tyrosinase maturation and intracellular trafficking in melanoma cells. She completed her postdoctoral training in Dr. Vincent Hearing’s laboratory at the National Cancer Institute, The National Institutes of Health. Her projects were focused on the intracellular trafficking and maturation of melanosomal proteins, using melanocytes isolated from mouse models for oculocutaneous albinism (OCA). She also derived and immortalized new melanocyte lines from mice carrying different pigmentation mutations, now available to the scientific world for research. In 2005, she started as a Senior Research Scientist in the New Technology Department of Avon Products, Inc., Global R&D, Suffern, NY, where she focused on antiageing and skin whitening platforms. Since 2008, she has been working as a Toxicologist and Study Director at the Institute for In Vitro Sciences, Inc. Gaithersburg, MD. At IIVS, Dr. Costin optimized an in vitro method for the rapid screening of melanogenesis modulators based on reconstructed pigmented tissues models. Dr. Costin has developed and applied in vitro toxicology methods for screening personal care and cosmetic products for dermal safety assessment. She is a member of several scientific societies including the PanAmerican Society for Pigment Cell Research (PASPCR) where she currently serves as the Newsletter Editor.
Dennis R. Roop, Ph.D., is Professor of Dermatology and the Director of the Charles C. Gates Center for Regenerative Medicine at the School of Medicine, University of Colorado. He received his B.A. from Berea College, Berea Kentucky and a Masters and Ph.D. in Microbiology from the University of Tennessee, Knoxville, Tennessee. His laboratory has a longstanding interest in identifying genes required for normal skin development and understanding how they function. His team has discovered the genetic basis for multiple blistering and hyperkeratotic skin diseases. His team is currently generating induced pluripotent stem cells (iPSCs) from patients with inherited skin fragility syndromes using methods which do not require viral vectors, and determining whether genome editing techniques can be used to correct the genetic defect in these patient-specific iPSCs. Their ultimate goal is to return keratinocytes derived from genetically corrected iPSCs to the same patient as an autograft. His laboratory is also isolating and characterizing skin cancer stem cells, of which an improved understanding could result in the development of novel therapeutic strategies that specifically target these precursors for destruction and prevention of tumor recurrence. Dr. Roop’s laboratory has been continuously funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and the National Cancer Institute since 1989. He has served on the Advisory Council of NIAMS and he is a former President of the Society for Investigative Dermatology. He is the author of more than 250 scientific manuscripts, books, reviews, and scientific proceedings.
David A. Norris, M.D., is Professor of Dermatology and the Chairman of the Department of Dermatology at the School of Medicine, University of Colorado. He received his B.A. from the Johns Hopkins University, and an M.D. from Duke University, and completed Dermatology residency training at the University of Colorado, where he has been a member of the faculty since 1977. Between 1987 and 1992, he was the Editor in Chief of the Journal of Investigative Dermatology. He has served in many capacities for the Society for Investigative Dermatology, including a term as President. For 13 years he was the Director for the Annual Montagna Symposium on the Biology of Skin. He is a member of the American Society for Clinical Investigation and is the Chair of the Medical Advisory Committee for the American Skin Association. He has held similar positions with the Psoriasis Foundation, and the National Alopecia Areata Foundation. He was the co-PI of the National Alopecia Areata Registry, now the Alopecia Areata Registry, Biobank, and Clinical Trials Network. Dr. Norris’ research has focused on cell death in normal skin, in autoimmune/inflammatory skin diseases, and in skin cancer including basal cell carcinoma and malignant melanoma. His collaborative research has been exploring cytotoxic mechanism in cutaneous disease, melanoma resistance to apoptosis, control of migration in melanoma, resistance to activation-induced cell death in cutaneous T cell lymphoma, and role of bacterial toxins in human skin diseases. He has made seminal mechanistic observations in vitiligo, photosensitive lupus, alopecia areata, and malignant melanoma. He is the author of >150 scientific manuscripts, books, reviews, and scientific proceedings. Dr. Norris is the organizing co-chair of the XXIII International Pigment Cell Conference that will be held in Denver in 2017.
References
- 1.Birlea SA, Spritz RA, Norris DA. Vitiligo. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick’s Dermatology in General Medicine. 8. New York, NY: McGraw-Hill; 2012. pp. 792–803. [Google Scholar]
- 2.Le Poole IC, van den Wijngaard RM, Westerhof W, Dutrieux RP, Das PK. Presence or absence of melanocytes in vitiligo lesions: An immunohistochemical investigation. J Invest Dermatol. 1993;100:816–822. doi: 10.1111/1523-1747.ep12476645. [DOI] [PubMed] [Google Scholar]
- 3.Falabella R. Vitiligo and the melanocyte reservoir. Indian J Dermatol. 2009;54:313–318. doi: 10.4103/0019-5154.57604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein NB, Koster MI, Hoaglin LG, Spoelstra NS, Kechris KJ, Robinson SE, Robinson WA, Roop DR, Norris DA, Birlea SA. Narrow band ultraviolet B treatment for human vitiligo is associated with proliferation, migration, and differentiation of melanocyte precursors. J Invest Dermatol. 2015;135:2068–2076. doi: 10.1038/jid.2015.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito T, Ito N, Saatoff M, Hashizume H, Fukamizu H, Nickoloff BJ, Takigawa M, Paus R. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. J Invest Dermatol. 2008;128:1196–1206. doi: 10.1038/sj.jid.5701183. [DOI] [PubMed] [Google Scholar]
- 6.Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 7.Spritz RA. The genetics of generalized vitiligo. Curr Dir Autoimmun. 2008;10:244–257. doi: 10.1159/000131501. [DOI] [PubMed] [Google Scholar]
- 8.Ogg GS, Rod Dunbar P, Romero P, Chen JL, Cerundolo V. High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. J Exp Med. 1998;188:1203–1208. doi: 10.1084/jem.188.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gross A, Tapia FJ, Mosca W, Perez RM, Briceño L, Henriquez JJ, Convit J. Mononuclear cell subpopulations and infiltrating lymphocytes in erythema dyschromicum perstans and vitiligo. Histol Histopathol. 1987;2:277–283. [PubMed] [Google Scholar]
- 10.Badri AM, Todd PM, Garioch JJ, Gudgeon JE, Stewart DG, Goudie RB. An immunohistological study of cutaneous lymphocytes in vitiligo. J Pathol. 1993;170:149–155. doi: 10.1002/path.1711700209. [DOI] [PubMed] [Google Scholar]
- 11.Le Poole IC, Das PK, van den Wijngaard RM, Bos JD, Westerhof W. Review of the etiopathomechanism of vitiligo: A convergence theory. Exp Dermatol. 1993;2:145–153. doi: 10.1111/j.1600-0625.1993.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 12.Le Poole IC, van den Wijngaard RM, Westerhof W, Das PK. Presence of T cells and macrophages in inflammatory vitiligo skin parallels melanocyte disappearance. Am J Pathol. 1996;148:1219–1228. [PMC free article] [PubMed] [Google Scholar]
- 13.Mosenson JA, Zloza A, Nieland JD, Garrett-Mayer E, Eby JM, Huelsmann EJ, Kumar P, Denman CJ, Lacek AT, Kohlhapp FJ, Alamiri A, Hughes T, Bines SD, Kaufman HL, Overbeck A, Mehrotra S, Hernandez C, Nishimura MI, Guevara-Patino JA, Le Poole IC. Mutant HSP70 reverses autoimmune depigmentation in vitiligo. Sci Transl Med. 2013;5(174):1–25. doi: 10.1126/scitranslmed.3005127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang L, Wei Y, Sun Y, Shi W, Yang J, Zhu L, Li M. Interferon-gamma inhibits melanogenesis and induces apoptosis in melanocytes: A pivotal role of CD8+ cytotoxic T lymphocytes in vitiligo. Acta Derm Venereol. 2015;95:664–670. doi: 10.2340/00015555-2080. [DOI] [PubMed] [Google Scholar]
- 15.Harris JE. IFN-γ in vitiligo, is it the fuel or the fire? Acta Derm Venereol. 2015;95:643–644. doi: 10.2340/00015555-2137. [DOI] [PubMed] [Google Scholar]
- 16.Alkhateeb A, Fain PR, Thody A, Bennett DC, Spritz RA. Epidemiology of vitiligo and associated autoimmune diseases in Caucasian probands and their families. Pigment Cell Res. 2003;16:208–214. doi: 10.1034/j.1600-0749.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 17.Spritz RA. The genetics of vitiligo. J Invest Dermatol. 2011;131:E18–E20. doi: 10.1038/skinbio.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spritz RA. Modern vitiligo genetics sheds new light on an ancient disease. J Dermatol. 2013;40:310–318. doi: 10.1111/1346-8138.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellei B, Pitisci A, Ottaviani M, Ludovici M, Cota C, Luzi F, Dell’Anna ML, Picardo M. Vitiligo: A possible model of degenerative diseases. PLoS One. 2013;8:e59782. doi: 10.1371/journal.pone.0059782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norris DA, Kissinger RM, Naughton GM, Bystryn JC. Evidence for immunologic mechanisms in human vitiligo: Patients’ sera induce damage to human melanocytes in vitro by complement-mediated damage and antibody-dependent cellular cytotoxicity. J Invest Dermatol. 1988;90:783–789. doi: 10.1111/1523-1747.ep12461505. [DOI] [PubMed] [Google Scholar]
- 21.Birlea SA, Costin GE, Norris DA. New insights on therapy with vitamin D analogs targeting the intracellular pathways that control repigmentation in human vitiligo. Med Res Rev. 2009;29:514–546. doi: 10.1002/med.20146. [DOI] [PubMed] [Google Scholar]
- 22.Birlea SA, Serota M, Norris DA. Non-bullous skin diseases: Alopecia areata, vitiligo, psoriasis and urticaria. In: Mackay R, editor. Autoimmune Diseases. 5. Oxford and London, UK: Elsevier Academic Press; 2013. pp. 971–89. [Google Scholar]
- 23.Monem El Mofty A. A preliminary clinical report on the treatment of leucodermia with Ammi majus Linn. J Egypt Med Assoc. 1948;31:651–665. [PubMed] [Google Scholar]
- 24.Parsad D, Pandhi R, Dogra S, Kumar B. Clinical study of repigmentation patterns with different treatment modalities and their correlation with speed and stability of repigmentation in 352 vitiliginous patches. J Am Acad Dermatol. 2004;50:63–67. doi: 10.1016/s0190-9622(03)00786-2. [DOI] [PubMed] [Google Scholar]
- 25.Yang YS, Cho HR, Ryou JH, Lee MH. Clinical study of repigmentation patterns with either narrow-band ultraviolet B (NBUVB) or 308 nm excimer laser treatment in Korean vitiligo patients. Int J Dermatol. 2010;49:317–323. doi: 10.1111/j.1365-4632.2009.04332.x. [DOI] [PubMed] [Google Scholar]
- 26.Kunisada T, Yoshida H, Yamazaki H, Miyamoto A, Hemmi H, Nishimura E, Shultz LD, Nishikawa S, Hayashi S. Transgene expression of steel factor in the basal layer of epidermis promotes survival, proliferation, differentiation and migration of melanocyte precursors. Development. 1998;125:2915–2923. doi: 10.1242/dev.125.15.2915. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M, Jackson IJ, Barrandon Y, Miyachi Y, Nishikawa S. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–860. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- 28.Nishimura EK. Melanocyte stem cells: A melanocyte reservoir in hair follicles for hair and skin pigmentation. Pigment Cell Melanoma Res. 2011;24:401–410. doi: 10.1111/j.1755-148X.2011.00855.x. [DOI] [PubMed] [Google Scholar]
- 29.Kanwar AJ, Parsad D. Understanding the mechanism of repigmentation in vitiligo. In: Gupta S, Olsson MJ, Kanwar AJ, Ortonne JP, editors. Surgical Management of Vitiligo. 1. MA: JohnWiley & Sons; 2007. [Google Scholar]
- 30.Gan EY, Gahat T, Cario-André M, Seneschal J, Ezzedine K, Taïeb A. Clinical repigmentation patterns in paediatric vitiligo. Br J Dermatol. 2016;175(3):555–60. doi: 10.1111/bjd.14635. [DOI] [PubMed] [Google Scholar]
- 31.Davids LM, du Toit E, Kidson SH, Todd G. A rare repigmentation pattern in a vitiligo patient: A clue to an epidermal stem-cell reservoir of melanocytes? Clin Exp Dermatol. 2009;34:246–248. doi: 10.1111/j.1365-2230.2008.02793.x. [DOI] [PubMed] [Google Scholar]
- 32.Montagna W, Chase HB. Histology and cytochemistry of human skin. X. X-irradiation of the scalp. Am J Anat. 1956;99:415–445. doi: 10.1002/aja.1000990304. [DOI] [PubMed] [Google Scholar]
- 33.Staricco RG, Milinska Miller. Activation of the amelanotic melanocytes in the outer root sheath of the hair follicle following ultra violet rays exposure. J Invest Dermatol. 1962;39:163–164. doi: 10.1038/jid.1962.97. [DOI] [PubMed] [Google Scholar]
- 34.Staricco RG. Amelanotic melanocytes in the outer sheath of the human hair follicle and their role in the repigmentation of regenerated epidermis. Ann N Y Acad Sci. 1963;100:239–255. doi: 10.1111/j.1749-6632.1963.tb57123.x. [DOI] [PubMed] [Google Scholar]
- 35.Ortonne JP, Schmitt D, Thivolet J. PUVA-induced repigmentation of vitiligo: Scanning electron microscopy of hair follicles. J Invest Dermatol. 1980;74:40–42. doi: 10.1111/1523-1747.ep12514597. [DOI] [PubMed] [Google Scholar]
- 36.Cui J, Shen LY, Wang GC. Role of hair follicles in the repigmentation of vitiligo. J Invest Dermatol. 1991;97:410–416. doi: 10.1111/1523-1747.ep12480997. [DOI] [PubMed] [Google Scholar]
- 37.Arrunátegui A, Arroyo C, Garcia L, Covelli C, Escobar C, Carrascal E, Falabella R. Melanocyte reservoir in vitiligo. Int J Dermatol. 1994;33:484–487. doi: 10.1111/j.1365-4362.1994.tb02860.x. [DOI] [PubMed] [Google Scholar]
- 38.Tobin DJ, Colen SR, Bystryn JC. Isolation and long-term culture of human hair-follicle melanocytes. J Invest Dermatol. 1995;104:86–89. doi: 10.1111/1523-1747.ep12613573. [DOI] [PubMed] [Google Scholar]
- 39.Grichnik JM, Ali WN, Burch JA, Byers JD, Garcia CA, Clark RE, Shea CR. KIT expression reveals a population of precursor melanocytes in human skin. J Invest Dermatol. 106:967–971. doi: 10.1111/1523-1747.ep12338471. 199. [DOI] [PubMed] [Google Scholar]
- 40.Medic S, Ziman M. PAX3 expression in normal skin melanocytes and melanocytic lesions (naevi and melanomas) PLoS One. 2010;5:e9977. doi: 10.1371/journal.pone.0009977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoerter JD, Bradley P, Casillas A, Chambers D, Denholm C, Johnson K, Weiswasser B. Extrafollicular dermal melanocyte stem cells and melanoma. Stem Cells Int. 2012;2012:1–10. doi: 10.1155/2012/407079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okamoto N1, Aoto T, Uhara H, Yamazaki S, Akutsu H, Umezawa A, Nakauchi H, Miyachi Y, Saida T, Nishimura EK. A melanocyte-melanoma precursor niche in sweat glands of volar skin. Pigm Cell Melanoma Res. 2014;27:1039–1050. doi: 10.1111/pcmr.12297. [DOI] [PubMed] [Google Scholar]
- 43.Li L, Fukunaga-Kalabis M, Yu H, Xu X, Kong J, Lee JT, Herlyn M. Human dermal stem cells differentiated into functional epidermal melanocytes. J Cell Res. 2010;123:853–860. doi: 10.1242/jcs.061598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haass NK, Herlyn M. Normal human melanocyte homeostasis as a paradigm for understanding melanoma. J Investig Dermatol Symp Proc. 2005;10:153–163. doi: 10.1111/j.1087-0024.2005.200407.x. [DOI] [PubMed] [Google Scholar]
- 45.Hoath SB, Leahy DG. The organization of human epidermis: Functional epidermal units and phi proportionality. J Invest Dermatol. 2003;121:1440–1446. doi: 10.1046/j.1523-1747.2003.12606.x. [DOI] [PubMed] [Google Scholar]
- 46.Osawa M. Melanocyte stem cells. In: Gage F, Watt Fiona, editors. Stem Book. 1. Cambridge, MA: 2009. pp. 11–12. [Google Scholar]
- 47.White RA, Neiman JM, Reddi A, Han G, Birlea S, Mitra D, Dionne L, Fernandez P, Murao K, Bian L, Keysar SB, Goldstein NB, Song N, Bornstein S, Han Z, Lu X, Wisell J, Li F, Song J, Lu SL, Jimeno A, Roop DR, Wang XJ. Epithelial stem cell mutations that promote squamous cell carcinoma metastasis. J Clin Invest. 2013;123:4390–404. doi: 10.1172/JCI65856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamada T, Hasegawa S, Inoue Y, Date Y, Yamamoto N, Mizutani H, Nakata S, Matsunaga K, Akamatsu H. Wnt/β-catenin and kit signaling sequentially regulate melanocyte stem cell differentiation in UVB-induced epidermal pigmentation. J Invest Dermatol. 2013;133:2753–2762. doi: 10.1038/jid.2013.235. [DOI] [PubMed] [Google Scholar]
- 49.Kondo T, Hearing VJ. Updated on the regulation of mammalian melanocyte function and skin pigmentation. Expert Rev Dermatol. 2011;6:97–108. doi: 10.1586/edm.10.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aroni K, Voudouris S, Ioannidis E, Grapsa A, Kavantzas N, Patsouris E. Increased angiogenesis and mast cells in the centre compared to the periphery of vitiligo lesions. Arch Dermatol Res. 302:601–607. doi: 10.1007/s00403-010-1040-9. 201. [DOI] [PubMed] [Google Scholar]
- 51.Mull AN, Zolekar A, Wang YC. Melanocyte stem cells for disease modeling and regenerative medicine applications. Int J Mol Sci. 2015;16:30458–30469. doi: 10.3390/ijms161226207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Phsyiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- 53.Holzmann H, Altmeyer P, Stöhr L, Chilf GN. Modification of alpha-MSH by UVA irradiation of the skin. Hautarzt. 1983;34:294–297. [PubMed] [Google Scholar]
- 54.Wu CS, Yu CL, Wu CS, Lan CC, Yu HS. Narrow-band ultraviolet-B stimulates proliferation and migration of cultured melanocytes. Exp Dermatol. 2004;13:755–763. doi: 10.1111/j.0906-6705.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- 55.Imokawa G, Kobayashi T, Miyagishi M, Higashi K, Yada Y. The role of endothelin-1 in epidermal hyperpigmentation and signaling mechanisms of mitogenesis and melanogenesis. Pigment Cell Res. 1997;10:218–228. doi: 10.1111/j.1600-0749.1997.tb00488.x. [DOI] [PubMed] [Google Scholar]
- 56.Brenner M, Degitz K, Besch R, Berking C. Differential expression of melanoma-associated growth factors in keratinocytes and fibroblasts by ultraviolet A and ultraviolet B radiation. Br J Dermatol. 2005;153:733–739. doi: 10.1111/j.1365-2133.2005.06780.x. [DOI] [PubMed] [Google Scholar]
- 57.Hachiya A, Kobayashi A, Yoshida Y, Kitahara T, Takema Y, Imokawa G. Biphasic expression of two paracrine melanogenic cytokines, stem cell factor and endothelin-1, in ultraviolet B-induced human melanogenesis. Am J Pathol. 2004;165:2099–2109. doi: 10.1016/S0002-9440(10)63260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mildner M, Mlitz V, Gruber F, Wojta J, Tschachler E. Hepatocyte growth factor establishes autocrine and paracrine feedback loops for the protection of skin cells after UV irradiation. J Invest Dermatol. 2007;127:2637–2644. doi: 10.1038/sj.jid.5700938. [DOI] [PubMed] [Google Scholar]
- 59.McKenzie RC. Ultraviolet radiation B (UVB)-induction of leukaemia inhibitory factor (LIF) in human keratinocytes. Photodermatol Photoimmunol Photomed. 2001;17:284–285. doi: 10.1034/j.1600-0781.2001.170607.x. [DOI] [PubMed] [Google Scholar]
- 60.Imokawa G, Yada Y, Kimura M, Morisaki N. Granulocyte/macrophage colony-stimulating factor is an intrinsic keratinocyte-derived growth factor for humanmelanocytes inUVA-induced melanosis. Biochem J. 1996;313:625–631. doi: 10.1042/bj3130625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim DS, Kim HJ, Choi KH, Chung JH, Kim KH, Par KC. UVB-induced GM-CSF production is suppressed by dexamethasone in HaCaT cells. Photodermatol Photoimmunol Photomed. 2001;17:121–125. doi: 10.1034/j.1600-0781.2001.170303.x. [DOI] [PubMed] [Google Scholar]
- 62.Costin GE, Hearing VJ. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007;21:976–994. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- 63.Hirobe T. How are proliferation and differentiation of melanocytes regulated? Pigment Cell Melanoma Res. 2011;24:462–478. doi: 10.1111/j.1755-148X.2011.00845.x. [DOI] [PubMed] [Google Scholar]
- 64.Mizutani Y, Hayashi N, Kawashima M, Imokawa G. A single UVB exposure increases the expression of functional KIT in human melanocytes by up-regulating MITF expression through the phosphorylation of p38/CREB. Arch Dermatol Res. 2010;302:283–294. doi: 10.1007/s00403-009-1007-x. [DOI] [PubMed] [Google Scholar]
- 65.Passeron T, Valencia JC, Bertolotto C, Hoashi T, Le Pape E, Takahashi K, Ballotti R, Hearing VJ. SOX9 is a key player in ultraviolet B-induced melanocyte differentiation and pigmentation. Proc Natl Acad Sci USA. 2007;104:13984–13989. doi: 10.1073/pnas.0705117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park HY, Wu C, Yonemoto L, Murphy-Smith M, Wu H, Stachur CM, Gilcrest BA. MITF mediates cAMP-induced protein kinase C-beta expression in human melanocytes. Biochem J. 2006;395:571–578. doi: 10.1042/BJ20051388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu K, Yu D, Cho YY, Bode AM, Ma W, Yao K, Li S, Li J, Bowden GT, Dong Z, et al. Sunlight UV-induced skin cancer relies upon activation of the p38 signaling pathway. Cancer Res. 2013;73:2181–2188. doi: 10.1158/0008-5472.CAN-12-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Halaban R. The regulation of normal melanocyte proliferation. Pigment Cell Res. 2000;13:4–14. doi: 10.1034/j.1600-0749.2000.130103.x. [DOI] [PubMed] [Google Scholar]
- 69.Norris DA, Horikawa T, Morelli JG. Melanocyte destruction and repopulation in vitiligo. Pigment Cell Res. 1994;7:193–203. doi: 10.1111/j.1600-0749.1994.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 70.Scott G, Ewing J, Ryan D, Abboud C. Stem cell factor regulates human melanocyte-matrix interactions. Pigment Cell Res. 1994;7:44–51. doi: 10.1111/j.1600-0749.1994.tb00017.x. [DOI] [PubMed] [Google Scholar]
- 71.Bagnato A, Rosanò L, Spinella F, Di Castro V, Tecce R, Natali PG. Endothelin B receptor blockade inhibits dynamics of cell interactions and communications in melanoma cell progression. Cancer Res. 2004;64:1436–1443. doi: 10.1158/0008-5472.can-03-2344. [DOI] [PubMed] [Google Scholar]
- 72.Lei TC, Vieira WD, Hearing VJ. In vitro migration of melanoblasts requires matrix metalloproteinase-2: Implications to vitiligo therapy by photochemotherapy. Pigment Cell Res. 2002;15:426–432. doi: 10.1034/j.1600-0749.2002.02044.x. [DOI] [PubMed] [Google Scholar]
- 73.Jamal S, Schneider RJ. UV-induction of keratinocyte endothelin-1 downregulates E-cadherin in melanocytes and melanoma cells. J Clin Invest. 2002;110:443–452. doi: 10.1172/JCI13729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li G, Satyamoorthy K, Herlyn M. N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res. 2001;61:3819–3825. [PubMed] [Google Scholar]
- 75.Seline PC, Norris DA, Horikawa T, Fujita M, Middleton MH, Morelli JG. Expression of E and P-cadherin by melanoma cells decreases in progressive melanomas and following ultraviolet radiation. J Invest Dermatol. 1996;106:1320–1324. doi: 10.1111/1523-1747.ep12349048. [DOI] [PubMed] [Google Scholar]
- 76.Hung CF, Chiang HS, Lo HM, Jian JS, Wu WB. E-cadherin and its downstream catenins are proteolytically cleaved in human HaCaT keratinocytes exposed to UVB. Exp Dermatol. 2006;15:315–321. doi: 10.1111/j.0906-6705.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- 77.Peinado H, Ballestar E, Esteller M, Cano A. Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24:306–319. doi: 10.1128/MCB.24.1.306-319.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Herfs M, Hubert P, Kholod N, Caberg JH, Gilles C, Berx G, Savagner P, Boniver J, Delvenne P. Transforming growth factor-beta1-mediated Slug and Snail transcription factor up-regulation reduces the density of Langerhans cells in epithelial metaplasia by affecting E-cadherin expression. Am J Pathol. 2008;172:1391–1402. doi: 10.2353/ajpath.2008.071004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hudson LG, Choi C, Newkirk KM, Parkhani J, Cooper KL, Lu P, Kusewitt DF. Ultraviolet radiation stimulates expression of Snail family transcription factors in keratinocytes. Mol Carcinog. 2007;46:257–268. doi: 10.1002/mc.20257. [DOI] [PubMed] [Google Scholar]
- 80.Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol. 2010;2:1–24. doi: 10.1101/cshperspect.a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Poser I, Domínguez D, de Herreros AG, Varnai A, Buettner R, Bosserhoff AK. Loss of E-cadherin expression in melanoma cells involves up-regulation of the transcriptional repressor Snail. J Biol Chem. 2001;276:24661–24666. doi: 10.1074/jbc.M011224200. [DOI] [PubMed] [Google Scholar]
- 82.Palmer MB, Majumder P, Green MR, Wade PA, Boss JM. A 3′ enhancer controls snail expression in melanoma cells. Cancer Res. 2007;67:6113–6120. doi: 10.1158/0008-5472.CAN-06-4256. [DOI] [PubMed] [Google Scholar]
- 83.Shirley SH, Grimm EA, Kusewitt DF. Ultraviolet radiation and the slug transcription factor induce proinflammatory and immunomodulatory mediator expression in melanocytes. J Skin Cancer. 2012:1–8. doi: 10.1155/2012/410925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu CS1, Lan CC, Wang LF, Chen GS, Wu CS, Yu HS. Effects of psoralen plus ultraviolet A irradiation on cultured epidermal cells in vitro and patients with vitiligo in vivo. Br J Dermatol. 2007;156:122–129. doi: 10.1111/j.1365-2133.2006.07584.x. [DOI] [PubMed] [Google Scholar]
- 85.Zhang XQ, Feng J, Mou KH, Ma HQ, Niu XW, Liu C. Effects of bFGF and alpha-MSH on adhesion and migration of human melanocytes in vitro. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2006;5:161–164. doi: 10.3785/j.issn.1008-9292.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 86.Bellei B, Pitisci A, Catricalà C, Larue L, Picardo M. Wnt/β-catenin signaling is stimulated by α-melanocyte-stimulating hormone in melanoma and melanocyte cells: Implication in cell differentiation. Pigment Cell Melanoma Res. 2011;24:309–325. doi: 10.1111/j.1755-148X.2010.00800.x. [DOI] [PubMed] [Google Scholar]
- 87.Enomoto A, Yoshihisa Y, Yamakoshi T, Ur Rehman M, Norisugi O, Hara H, Matsunaga K, Makino T, Nishihira J, Shimizu T. UV-B radiation induces macrophage migration inhibitory factor-mediated melanogenesis through activation of protease-activated receptor-2 and stem cell factor in keratinocytes. Am J Pathol. 2011;178:679–687. doi: 10.1016/j.ajpath.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vancoillie G, Lambert J, Nayaert JM. Melanocyte biology and its implications for the clinician. Eur J Dermatol. 1999;9:241–251. [PubMed] [Google Scholar]
- 89.Matsumoto K, Hashimoto K, Yoshikawa K, Nakamura T. Marked stimulation of growth and motility of human keratinocytes by hepatocity growth factor. Exp Cell Res. 1991;196:114–120. doi: 10.1016/0014-4827(91)90462-4. [DOI] [PubMed] [Google Scholar]
- 90.Santiago-Walker A1, Li L, Haass NK, Herlyn M. Melanocytes: From morphology to application. Skin Pharmacol Physiol. 2009;22:114–121. doi: 10.1159/000178870. [DOI] [PubMed] [Google Scholar]
- 91.Kawaguchi Y, Mori N, Nakayama A. Kit(+) melanocytes seem to contribute to melanocyte proliferation after UV exposure as precursor cells. J Invest Dermatol. 2001;116:920–925. doi: 10.1046/j.0022-202x.2001.01370.x. [DOI] [PubMed] [Google Scholar]
- 92.Yada Y, Higuchi K, Imokawa G. Effects of endothelins on signal transduction and proliferation in human melanocytes. J Biol Chem. 1991;266:18352–18357. [PubMed] [Google Scholar]
- 93.Tada A1, Suzuki I, Im S, Davis MB, Cornelius J, Babcock G, Nordlund JJ, Abdel-Malek ZA. Endothelin-1 is a paracrine growth factor that modulates melanogenesis of human melanocytes and participates in their responses to ultraviolet radiation. Cell Growth Differ. 1998;9:575–584. [PubMed] [Google Scholar]
- 94.Yang G, Li Y, Nishimura EK, Xin H, Zhou A, Guo Y, Dong L, Denning MF, Nickoloff BJ, Cui R. Inhibition of PAX3 by TGF-beta modulates melanocyte viability. Mol Cell. 2008;32:554–563. doi: 10.1016/j.molcel.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 95.Moustakas A. TGF-beta targets PAX3 to controlmelanocyte differentiation. Dev Cell. 2008;15:797–799. doi: 10.1016/j.devcel.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 96.Box NF, Terzian T. The role of p53 in pigmentation: Tanning and melanoma. Pigment Cell Melanoma Res. 2008;21:523–533. doi: 10.1111/j.1755-148X.2008.00495.x. [DOI] [PubMed] [Google Scholar]
- 97.Kubic JD, Young KP, Plummer RS, Ludvik AE, Lang D. Pigmentation PAX-ways: The role of Pax3 in melanogenesis, melanocyte stem cell maintenance, and disease. Pigment Cell Melanoma Res. 2008;21:627–645. doi: 10.1111/j.1755-148X.2008.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chou WC, Takeo M, Rabbani P, Hu H, Lee W, Chung YR, Carucci J, Overbeek P, Ito M. Direct migration of follicular melanocyte stem cells to the epidermis after wounding or UVB irradiation is dependent on Mc1r signaling. Nat Med. 2013;19:924–929. doi: 10.1038/nm.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jimbow K, Uesugi T. New melanogenesis and photobiological processes in activation and proliferation of precursor melanocytes after UV-exposure: Ultrastructural differentiation of precursor melanocytes from Langerhans cells. J Invest Dermatol. 1982;78:108–115. doi: 10.1111/1523-1747.ep12505758. [DOI] [PubMed] [Google Scholar]
- 100.Horikawa T, Norris DA, Johnson TW, Zekman T, Dunscomb N, Bennion SD, Jackson RL, Morelli JG. DOPA-negative melanocytes in the outer root sheath of human hair follicles express premelanosomal antigens but not a melanosomal antigen or the melanosome-associated glycoproteins tyrosinase, TRP-1, and TRP-2. J Invest Dermatol. 1996;106:28–35. doi: 10.1111/1523-1747.ep12326989. [DOI] [PubMed] [Google Scholar]
- 101.Burren R, Scaletta C, Frenk E, Panizzon RG, Applegate LA. Sunlight and carcinogenesis: Expression of p53 and pyrimidine dimers in human skin following UVA I, UVA I + II and solar simulating radiations. Int J Cancer. 1998;76:201–026. doi: 10.1002/(sici)1097-0215(19980413)76:2<201::aid-ijc6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 102.Will K, Neben M, Schmidt-Rose T, Deppert W, Wittern KP, Bergemann J. P53-dependent UVB responsiveness of human keratinocytes can be altered by cultivation on cell cycle-arrested dermal fibroblasts. Photochem Photobiol. 2000;71:321–326. doi: 10.1562/0031-8655(2000)071<0321:pduroh>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 103.Murase D, Hachiya A, Amano Y, Ohuchi A, Kitahara T, Takema Y. The essential role of p53 in hyperpigmentation of the skin via regulation of paracrine melanogenic cytokine receptor signaling. J Biol Chem. 2009;284:4343–4353. doi: 10.1074/jbc.M805570200. [DOI] [PubMed] [Google Scholar]
- 104.Zambruno G, Marchisio PC, Melchiori A, Bondanza S, Cancedda R, De Luca M. Expression of integrin receptors and their role in adhesion, spreading and migration of normal humanmelanocytes. J Cell Sci. 1993;105:179–190. doi: 10.1242/jcs.105.1.179. [DOI] [PubMed] [Google Scholar]
- 105.Hara M, Yaar M, Tang A, Eller MS, Reenstra W, Gilchrest BA. Role of integrins in melanocyte attachment and dendricity. J Cell Sci. 1994;107:2739–2748. doi: 10.1242/jcs.107.10.2739. [DOI] [PubMed] [Google Scholar]
- 106.Truong H, Danen EH. Integrin switching modulates adhesion dynamics and cell migration. Cell Adh Migr. 2009;3:179–181. doi: 10.4161/cam.3.2.8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Le Poole IC, van den Wijngaard RM, Westerhof W, Das PK. Tenascin is overexpressed in vitiligo lesional skin and inhibits melanocyte adhesion. Br J Dermatol. 1997;137:171–178. doi: 10.1046/j.1365-2133.1997.18011894.x. [DOI] [PubMed] [Google Scholar]
- 108.Kumar R, Parsad D, Kanwar AJ, Kaul D. Altered levels of Ets-1 transcription factor and matrix metalloproteinases in melanocytes from patients with vitiligo. Br J Dermatol. 2011;165:285–291. doi: 10.1111/j.1365-2133.2011.10324.x. [DOI] [PubMed] [Google Scholar]
- 109.Breathnach AS, Bor S, Wyllie LM. Electron microscopy of peripheral nerve terminals and marginal melanocytes in vitiligo. J Invest Dermatol. 1996;47:125–140. doi: 10.1038/jid.1966.117. [DOI] [PubMed] [Google Scholar]
- 110.Bose SK, Ortonne JP. Focal gaps in the basement membrane of involved and uninvolved skin of vitiligo: Are they normal? J Dermatol. 1994;21:152–159. doi: 10.1111/j.1346-8138.1994.tb01713.x. [DOI] [PubMed] [Google Scholar]
- 111.O’Donnel DB, Saddler M, Aronson PJ, Sikha MVKN. Tenascin is abundant in the skin of vitiligo patients. J Invest Dermatol. 1992;98(4):620. [Google Scholar]
- 112.Tang A, Eller MS, Hara M, Yaar M, Hirohashi S, Gilchrest BA. E-cadherin is the major mediator of human melanocyte adhesion to keratinocytes in vitro. J Cell Sci. 1994;107:983–992. doi: 10.1242/jcs.107.4.983. [DOI] [PubMed] [Google Scholar]
- 113.Kuriyama S, Mayor R. Molecular analysis of neural crest migration. Philos Trans R Soc Lond B Biol Sci. 2008;363:1349–1362. doi: 10.1098/rstb.2007.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cowley GP, Smith ME. Cadherin expression in melanocytic naevi and malignant melanomas. J Pathol. 1996;179:183–187. doi: 10.1002/(SICI)1096-9896(199606)179:2<183::AID-PATH554>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 115.Wagner RY, Luciani F, Cario-André M, Rubod A, Petit V, Benzekri L, Ezzedine K, Lepreux S, Steingrimsson E, Taieb A, et al. Altered E-cadherin levels and distribution in melanocytes precede clinical manifestations of vitiligo. J Invest Dermatol. 2015;135:1810–1819. doi: 10.1038/jid.2015.25. [DOI] [PubMed] [Google Scholar]
- 116.Tian X, Liu Z, Niu B, Zhang J, Tan TK, Lee SR, Zhao Y, Harris DC, Zheng G. E-cadherin/β-catenin complex and the epithelial barrier. J Biomed Biotechnol. 2011:1–16. doi: 10.1155/2011/567305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Horikawa T, Norris DA, Yohn JJ, Zekman T, Travers JB, Morelli JG. Melanocyte mitogens induce both melanocyte chemokinesis and chemotaxis. J Invest Dermatol. 1995;104:256–259. doi: 10.1111/1523-1747.ep12612795. [DOI] [PubMed] [Google Scholar]
- 118.Kovacs D, Abdel-Raouf H, Al-Khayyat M, Abdel-Azeem E, Hanna MR, Cota C, Picardo M, Anbar TS. Vitiligo: Characterization of melanocytes in repigmented skin after punch grafting. J Eur Acad Dermatol Venereol. 2015;29:581–590. doi: 10.1111/jdv.12647. [DOI] [PubMed] [Google Scholar]
- 119.Rabbani P, Takeo M, Chou W, Myung P, Bosenberg M, Chin L, Taketo MM, Ito M. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell. 2011;145:941–955. doi: 10.1016/j.cell.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.García-López MA, Barreiro O, García-Díez A, Sánchez-Madrid F, Peñas PF. Role of tetraspanins CD9 and CD151 in primary melanocyte motility. J Invest Dermatol. 2005;125:1001–1009. doi: 10.1111/j.0022-202X.2005.23882.x. [DOI] [PubMed] [Google Scholar]
- 121.Hsu MY1, Wheelock MJ, Johnson KR, Herlyn M. Shifts in cadherin profiles between human normal melanocytes and melanomas. J Investig Dermatol Symp Proc. 1996;1:188–194. [PubMed] [Google Scholar]
- 122.Yohn JJ, Lyons MB, Norris DA. Cultured human melanocytes from black and white donors have different sunlight and ultraviolet A radiation sensitivities. J Invest Dermatol. 1992;99:454–459. doi: 10.1111/1523-1747.ep12616151. [DOI] [PubMed] [Google Scholar]
- 123.Uesugi T, Katoh M, Oikawa O, Sugiyama S, Jimbow K. Fine structure of mitotic melanocytes in the epidermis of C57 black mouse after UV irradiation (UV-A) (author’s transl) Nihon Hifuka Gakkai Zasshi. 1977;87:925–928. [PubMed] [Google Scholar]
- 124.Rosdahl IK. Melanocyte mitosis in UVB-irradiated mouse skin. Acta Derm Venereol. 1978;58:217–221. [PubMed] [Google Scholar]
- 125.van Schanke A, Jongsma MJ, Bisschop R, van Venrooij GM, Rebel H, de Gruijl FR. Single UVB overexposure stimulates melanocyte proliferation in murine skin, in contrast to fractionated or UVA-1 exposure. J Invest Dermatol. 2005;124:241–247. doi: 10.1111/j.0022-202X.2004.23551.x. [DOI] [PubMed] [Google Scholar]
- 126.Rosen CF, Seki Y, Farinelli W, Stern RS, Fitzpatrick TB, Pathak MA, Gange RW. A comparison of the melanocyte response to narrow band UVA and UVB exposure in vivo. J Invest Dermatol. 1987;88:774–779. doi: 10.1111/1523-1747.ep12470474. [DOI] [PubMed] [Google Scholar]
- 127.Tronnier M, Alexander M, Wolff HH. Adhesion molecule expression in normal skin and melanocytic lesions. Role of UV-irradiation and architectural characteristics in nevi. J Cutan Pathol. 1997;24:278–287. doi: 10.1111/j.1600-0560.1997.tb00792.x. [DOI] [PubMed] [Google Scholar]
- 128.An HT, Yoo JY, Lee MK, Shin MH, Rhie GE, Seo JY, Chung JH, Eun HC, Cho KH. Single dose radiation is more effective for the UV-induced activation and proliferation of melanocytes than fractionated dose radiation. Photodermatol Photoimmunol Photomed. 2001;17:266–271. doi: 10.1034/j.1600-0781.2001.170604.x. [DOI] [PubMed] [Google Scholar]
- 129.Abdel-Malek Z, Swope V, Smalara D, Babcock G, Dawes S, Nordlund J. Analysis of the UV-induced melanogenesis and growth arrest of human melanocytes. Pigment Cell Res. 1994;7:326–332. doi: 10.1111/j.1600-0749.1994.tb00635.x. [DOI] [PubMed] [Google Scholar]
- 130.Medrano EE1, Im S, Yang F, Abdel-Malek ZA. Ultraviolet B light induces G1 arrest in human melanocytes by prolonged inhibition of retinoblastoma protein phosphorylation associated with long-term expression of the p21Waf-1/SDI-1/Cip-1 protein. Cancer Res. 1995;55:4047–4052. [PubMed] [Google Scholar]
- 131.Kao CH, Yu HS. Comparison of the effect of 8-methoxypsoralen (8-MOP) plus UVA (PUVA) on human melanocytes in vitiligo vulgaris and in vitro. J Invest Dermatol. 1992;98:734–740. doi: 10.1111/1523-1747.ep12499936. [DOI] [PubMed] [Google Scholar]
- 132.Abdel-Naser MB, Krasagakis K, Garbe C, Eberle J. Direct effects on proliferation, antigen expression and melanin synthesis of cultured normal human melanocytes in response to UVB and UVA light. Photodermatol Photoimmunol Photomed. 2003;19:122–127. doi: 10.1034/j.1600-0781.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- 133.Ortonne JP, MacDonald DM, Micoud A, Thivolet J. PUVA-induced repigmentation of vitiligo: A histochemical (split-DOPA) and structural study. Br J Dermatol. 1979;101:1–12. doi: 10.1111/j.1365-2133.1979.tb15285.x. [DOI] [PubMed] [Google Scholar]
- 134.Rhodes LE, Lim HW. The acute effects of UV radiation on the skin. In: Lim HW, Honigsmann H, Hawk LM, editors. Photodermatology. CRC Press by Taylor and Francis Group: Informa Healthcare, Harvard; 2007. pp. 75–89. [Google Scholar]
- 135.Kang HY, Choi YM. FK506 increases pigmentation and migration of human melanocytes. Br J Dermatol. 2006;155:1037–1040. doi: 10.1111/j.1365-2133.2006.07467.x. [DOI] [PubMed] [Google Scholar]
- 136.Sisti A, Sisti G, Oranges CM. Effectiveness and safety of topical tacrolimus monotherapy for repigmentation in vitiligo: A comprehensive literature review. An Bras Dermatol. 2016;91:187–195. doi: 10.1590/abd1806-4841.20164012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hong DK, Yoon TJ, Kim NI, Park JK, Haw CR. Effects of calcipotriol (MC 903), a novel synthetic derivative of vitamin D3 on the growth of cultured human keratinocytes and melanocytes. Korean J Dermatol. 1992;30:811–823. [Google Scholar]
- 138.Yamaguchi Y, Itami S, Watabe H, Yasumoto J, Abdel-Malek ZA, Kubo T, Rouzaud F, Tanemura A, Yoshikawa K, Hearing VJ. Mesenchymal-epithelial interactions in the skin: Increased expression of dickkopf1 by palmoplantar fibroblasts inhibits melanocyte growth and differentiation. J Cell Biol. 2004;165:275–285. doi: 10.1083/jcb.200311122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yamaguchi Y, Passeron T, Hoashi T, Watabe H, Rouzaud F, Yasumoto K, Hara T, Tohyama C, Katayama I, Miki T, et al. Dickkopf 1 (DKK1) regulates skin pigmentation and thickness by affecting Wnt/beta-catenin signaling in keratinocytes. FASEB J. 2008;22:1009–1020. doi: 10.1096/fj.07-9475com. [DOI] [PubMed] [Google Scholar]
- 140.Choi W, Wolber R, Gerwat W, Mann T, Batzer J, Smuda C, Liu H, Kolbe L, Hearing VJ. The fibroblast-derived paracrine factor neuregulin-1 has a novel role in regulating the constitutive color and melanocyte function in human skin. J Cell Sci. 2010;123:3102–3111. doi: 10.1242/jcs.064774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Borojevic R. Resident stem cell in the skin. In: Goldenberg RSdS, Campos de Carvalho AC., editors. Resident Stem Cells and Regenerative Therapy. Waltham, MA: Academic Press; 2013. pp. 89–120. [Google Scholar]
- 142.Van Geel N, Goh BK, Wallaeys E, De Keyser S, Lambert J. A review of non-cultured epidermal cellular grafting in vitiligo. J Cutan Aesthet Surg. 2011;4:17–22. doi: 10.4103/0974-2077.79181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Paino F, Ricci G, De Rosa A, D’Aquino R, Laino L, Pirozzi G, Tirino V, Papaccio G. Ectomesenchymal stem cells from dental pulp are committed to differentiate into active melanocytes. Eur Cell Mater. 2010;20:295–305. doi: 10.22203/ecm.v020a24. [DOI] [PubMed] [Google Scholar]
- 144.Jones JC, Sabatini K, Liao X, Tran HT, Lynch CL, Morey RE, Glenn-Pratola V, Boscolo FS, Yang Q, Parast MM, et al. Melanocytes derived from transgene-free human induced pluripotent stem cells. J Invest Dermatol. 2013;133:2104–2108. doi: 10.1038/jid.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yang R, Jiang M, Kumar SM, Xu T, Wang F, Xiang L, Xu X. Generation of melanocytes from induced pluripotent stem cells. J Invest Dermatol. 2011;13:2458–2466. doi: 10.1038/jid.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yamane T, Hayashi S, Mizoguchi M, Yamazaki H, Kunisada T. Derivation of melanocytes from embryonic stem cells in culture. Dev Dyn. 1999;216:450–458. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<450::AID-DVDY13>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 147.Ohta S, Imaizumi Y, Okada Y, Akamatsu W, Kuwahara R, Ohyama M, Amagai M, Matsuzaki Y, Yamanaka S, Okano H, Kawakami Y. Generation of humanmelanocytes from induced pluripotent stem cells. PLoS One. 2011;6:e16182. doi: 10.1371/journal.pone.0016182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mica Y, Lee G, Chambers SM, Tomishima MJ, Studer L. Modeling neural crest induction, melanocyte specification, and disease-related pigmentation defects in hESCs and patient-sepcific iPSCs. Cell Rep. 2013;3:1140–1152. doi: 10.1016/j.celrep.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Larribere L, Wu H, Novak D, Galach M, Bernhardt M, Orouji E, Weina K, Knappe N, Sachpekidis C, Umansky L, et al. NF1 loss induces senescence during human melanocyte differentiation in an iPSC-based model. Pigment Cell Melanoma Res. 2015;28:407–416. doi: 10.1111/pcmr.12369. [DOI] [PubMed] [Google Scholar]
- 150.Tsuchiyama K, Wakao S, Kuroda Y, Ogura F, Nojima M, Sawaya N, Yamasaki K, Aiba S, Dezawa M. Functional melanocytes are readily reprogrammable from multilineage-differentiating stress-enduring (muse) cells, distinct stem cells in human fibroblasts. J Invest Dermatol. 2013;133:2425–2435. doi: 10.1038/jid.2013.172. [DOI] [PubMed] [Google Scholar]
- 151.Heneidi S, Simerman AA, Keller E, Singh P, Li X, Dumesic DA, Chazenbalk G. Awakened by cellular stress: Isolation and characterization of a novel population of pluripotent stem cells derived from human adipose tissue. PLoS One. 2013;8:e64752. doi: 10.1371/journal.pone.0064752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Toma JG, McKenzie IA, Bagli D, Miller FD. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727–737. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]