Abstract
Background
Lorazepam is one of the preferred agents used for intravenous treatment of status epilepticus (SE). We combined data from two pediatric clinical trials to characterize the population pharmacokinetics of intravenous lorazepam in infants and children 3 months to 17 years of age with active SE or a history of SE.
Methods
We developed a population pharmacokinetic model for lorazepam using the software NONMEM. We then assessed exploratory exposure-response relationships using the overall efficacy and safety study endpoints and performed dosing simulations.
Results
A total of 145 patients contributed 439 pharmacokinetic samples. The median (range) age and dose were 5.4 years (0.3–17.8) and 0.10 mg/kg (0.02–0.18), respectively. A two-compartment pharmacokinetic model with allometric scaling described the data well. In addition to total body weight (WT), younger age was associated with slightly higher weight-normalized clearance (CL). The following relationships characterized the typical values for the central compartment volume (V1), CL, peripheral compartment volume (V2), and intercompartmental clearance (Q), using individual subject WT (kg) and age (years): V1 (L) = 0.879*WT; CL (L/h) = 0.115*(Age/4.7) 0.133*WT0.75; V2 (L) = 0.542*V1; Q (L/h) = 1.45*WT0.75. No pharmacokinetic parameters were associated with clinical outcomes. Simulations suggest uniform pediatric dosing (0.1 mg/kg, up to a maximum of 4 mg) can be used to achieve concentrations of 50–100 ng/mL in children with SE, which have been previously associated with effective seizure control.
Conclusions
The population pharmacokinetics of lorazepam were successfully described using a sparse sampling approach and a two-compartment model in pediatric patients with active SE.
Keywords: lorazepam, population pharmacokinetics, status epilepticus, seizures, pediatrics
1 Introduction
Status epilepticus (SE) is a life-threatening condition of prolonged and repeated seizures. In the United States, SE is estimated to affect 12.5/100,000 individuals, with a higher incidence in children <10 years of age (14.3/100,000) [1]. In-hospital mortality in adults and children is approximately 9% and 3%, respectively [1,2]. Etiologies of pediatric SE include epilepsy, acute neurological conditions (e.g., stroke, central nervous system infection), and atypical febrile seizures [3,4].
Benzodiazepines are standard of care for emergent initial treatment of SE [5]. Lorazepam is a preferred agent for intravenous (i.v.) treatment (diazepam is the other), but it is not approved by the Food and Drug Administration (FDA) for children <18 years of age. The Neurocritical Care Society treatment guidelines for SE recommend a lorazepam i.v. dose of 0.1 mg/kg up to a 4 mg maximum [6]. This lorazepam dose was evaluated in children 3 months to 18 years of age with SE in a double-blind, randomized clinical trial supported under the Best Pharmaceuticals for Children Act [7]. Lorazepam was found to be effective in 72.9% of participants, and 17.6% experienced respiratory depression.
The pharmacokinetics of lorazepam in children are not well delineated. Lorazepam undergoes hepatic metabolism mediated by uridine 5′-diphospho-glucuronosyltransferases (UGTs) to an inactive glucuronide metabolite, which is then excreted in urine [8,9]. Specifically, UGT2B4, 2B7, and 2B15 have been shown in vitro to be important for the glucuronidation of the R- and S-lorazepam enantiomers, whereas R-lorazepam is also metabolized by the extrahepatic enzymes UGT1A7 and UGT1A10 [10]. Available data suggest that there are age-dependent changes in lorazepam disposition, particularly shortly after birth. One study in 10 critically ill term neonates found mean weight-normalized clearance (CL) was 80% lower compared to adults [11]. A separate investigation in 37 children (age 2 to ≤12 years) and 13 adolescents (>12 to 18 years of age) noted that the mean weight-normalized volume of distribution was 50% higher than adults for both groups whereas weight-normalized lorazepam CL was similar to adults [12]. We leveraged pharmacokinetic data collected through two clinical trials of pediatric SE [7,13] to characterize the population pharmacokinetics of lorazepam in children 3 months to 17 years of age. We then explored the relationship between lorazepam parameters and concentrations with safety and efficacy endpoints in one of the studies.
2 Methods
2.1 Patient Population
Data from two studies were used to characterize lorazepam disposition in pediatric patients with SE. The inclusion criteria were the same for both studies: 1) age 3 months to less than 18 years and 2) generalized tonic-clonic SE. The first study (“Status 1”) was a multicenter, prospective trial of lorazepam pharmacokinetics in participants with SE or a history of SE (ClinicalTrials.gov Identifier: NCT00114569). The pharmacokinetic results of this study have been previously published [13]. Briefly, there were two subject cohorts: one cohort received lorazepam (0.05–0.1 mg/kg by slow i.v. push [4 mg maximum]) with repeated dosing if necessary as per standard of care in the emergency department (n=48). This cohort included sparse pharmacokinetic sample collection. The second cohort received elective lorazepam (0.05 mg/kg i.v., single-dose) with intensive pharmacokinetic sampling as part of a pharmacokinetic study in a clinical research center (n=15).
The second study contributing data (“Status 2”) was a multicenter, prospective, randomized efficacy and safety study comparing i.v. lorazepam and diazepam in infants (>3 months of age), children, and adolescents for the treatment of acute SE (ClinicalTrials.gov Identifier: NCT00621478). Patients randomized to lorazepam therapy received 0.1 mg/kg (4 mg maximum) administered by a slow i.v. push. Patients were able to receive a second 0.05 mg/kg dose at 5 min if they were still experiencing active seizures. Pharmacokinetic data were available in 83 lorazepam-treated patients. The primary efficacy findings have been previously published, and the conclusion was that there was no statistically significant difference between lorazepam and diazepam in terms of cessation of SE by 10 min and no recurrence within 30 min [7]. The pharmacokinetic results of the Status 2 study are first reported in this publication.
Both pediatric studies were reviewed and approved by local institutional review boards. For the Status 1 study, patients either pre-consented to participate in the study prior to presentation of SE or consented to participate in the study after they had received lorazepam for the clinical treatment of SE in the emergency department. For the Status 2 study, participating hospitals submitted a site-specific plan to their institutional review board regarding the specific activities to address the requirements for the Exception from Informed Consent for Emergency Research (21 Code of Federal Regulations [CFR] 50.24). Thus, patients were treated prior to consent, and then once their condition had stabilized, the family was approached to discuss the study and obtain written consent for continued study participation.
2.2 Pharmacokinetic Sampling
In the Status 1 study, participants receiving lorazepam per standard of care had up to 5 pharmacokinetic samples collected between 0 and 48 hours following lorazepam administration. In the second Status 1 cohort who received elective lorazepam, participants had up to 13 pharmacokinetic samples collected from 0 to 48 hours. The Status 2 study used a pharmacokinetic sampling scheme that collected a pre-dose sample and up to 3 post-study drug samples up to 24 hours post-dose. The pre-dose sample could be collected through the line used to administer the study drug. Post-dose samples were drawn through a separate i.v. line. Post-dose sample collection times were determined based on the time when the second i.v. line was established.
2.3 Bioanalytical Assay
Plasma lorazepam concentrations were quantified using a validated liquid chromatography-tandem mass spectrometry assay developed by the Pediatric Trials Network (PTN) central laboratory (OpAns, Durham, NC). Many of the pharmacokinetic samples were also previously assayed by another laboratory, but this laboratory failed to meet FDA quality control documentation requirements, and thus the samples were reanalyzed. A comparability analysis evaluating the lorazepam concentrations from both laboratories showed that they were highly concordant, and thus the lorazepam concentration reanalysis results were used in the analyses described herein.
The following high-performance liquid chromatography (HPLC) instrumentation and conditions were used by the PTN central laboratory: Agilent 1200 Series HPLC system (Agilent Technologies, Inc., CA, USA); Poroshell 120 EC-C18, 50 × 3 mm i.d., 2.7 μm Agilent analytical column; flow rate, 0.8 mL/min; column temperature, 30°C; run time, 4.7 min; and typical injection volume, 7 μL. Two mobile phase components were used: mobile phase A, water containing 0.1% (v/v) formic acid; and mobile phase B, methanol containing 0.1% (v/v) formic acid. The following gradient conditions were used (%A/%B, time): 80%/20%, 0–3 min; 100% B, 3–4.2 min; 80%/20%, 4.2–4.7 min.
An Agilent 6400 Series Triple Quadrupole was used with the following conditions: ionization interface, positive mode electrospray; gas temperature, 350°C; gas flow, 10 L/min; nebulizer pressure, 50 psi; polarity, positive; capillary voltage, 4000 V; sheath gas temperature, 250°C; and sheath gas flow, 11 L/min. [2H4]-lorazepam was used as the internal standard.
Calibration standards varying from 1 to 500 ng/mL in human plasma were used for method validation. Using 1/×2 weighted power regression, the correlation coefficients obtained were better than 0.99 for all runs. Accuracy assessed using quality control samples (3, 40, and 400 ng/mL) were within 15% of the theoretical value. Also, precision values for all runs did not exceed a difference of 15%.
2.4 Population Pharmacokinetic Analysis
The lorazepam concentration versus time data from both studies were combined and modeled using the software NONMEM version 7.2 (ICON; Ellicott City, MD, USA). Diagnostic plots were executed in PLT Tools 5.1 (PLTSoft; San Francisco, CA, USA), the R Project 3.0.1 (downloaded from University of California, Los Angeles, CA, USA), and SAS 9.2 (Cary, NC, USA). The bootstrap analysis was performed using Wings for NONMEM version 7.2 (University of Auckland, Auckland, New Zealand), and 1000 bootstrap sample datasets were generated. The visual predictive check was performed using the modeling toolkit Perl-speaks-NONMEM (PsN, version 3.6.2).
One- and two-compartment structural pharmacokinetic models were tested. The base model included allometric scaling using total body weight (WT) to account for size differences before consideration of other covariates. The two-compartment pharmacokinetic model equations are shown below (Equations 1–4).
| (1) |
| (2) |
| (3) |
| (4) |
where CL, Q, V1, and V2 are the individual values for CL from the central compartment, intercompartmental CL, central compartment volume, and peripheral compartment volume, respectively; θCL, θQ, and θV1 represent the population estimates for each respective parameter; V2 was estimated as a function of V1, and thus θV2 is unit less; and WTi is the individual subject weight. The steady-state volume of distribution (Vss) was calculated as the sum of V1 and V2. ηi,CL, ηi,V1, and ηi,V2 are random variables with mean equal to zero and variance ω2CL, ω2V1, and ω2V2 that denote the deviation from the group value (patients with the same weight) for CL, V1, and V2, respectively, in the ith individual. No between-subject variability was estimated for Q. The off-diagonal covariance for between-subject variability parameters was assessed. For a two-compartment model, standard pharmacokinetic models and equations incorporated into NONMEM ADVAN3 and TRANS4 subroutines were used [14].
Diagnostic plots were used to assess the goodness of fit and appropriateness of the base model structure. Additive, combined additive plus proportional, and power residual errors were explored. Separate residual errors based on study (Status 1 versus 2) and intensive sampling (Status 1, Cohort 2) was evaluated given the differences in study design and the sampling scheme (Status 1 had a cohort of patients with intensive pharmacokinetic sampling). For the power residual error model, the relationship between observations and individual predictions was described according to Equation 5:
| (5) |
where Yij is the jth observed concentration in ith subject; Fij represents the corresponding prediction; ERRSTUDY1 and ERRSTUDY2 are the residual error terms for the Status 1 and Status 2 studies (samples from their respective study utilize their study specific ERR term and take a value of 0 for samples from the other study); and θPOWER represents the power exponent for weighting of the individual prediction when applied to the residual error.
2.5 Covariate Analysis
The investigation of the relationship between potential covariates and pharmacokinetic parameters proceeded by estimating the individual empirical Bayesian estimates using the base model. Once the base model was identified, covariates were tested for their influence on pharmacokinetic parameters CL and V2. The covariates evaluated included postnatal age, race, sex, hematocrit, aspartate aminotransferase, serum creatinine, and concomitant medications (carbamazepine, phenytoin, phenobarbital, and valproate). Race, sex, and concomitant medications were tested as categorical covariates, whereas the remaining covariates were tested as continuous variables. We did not look at dose or combination of concomitant medications, but did evaluation grouping of subjects taking at least one of the enzyme-inducing antiepileptic drugs as a categorical variable. With the individual parameter estimates, their deviation from the typical population parameter values (i.e., etas) was also generated. Next, graphical assessment of the relationships between pharmacokinetic parameters and potential covariates was performed by plotting the etas versus potential clinically relevant covariates. When trends were identified between covariates and etas, these variables were tested for model inclusion. We employed a forward inclusion and backward elimination approach to covariate selection using p-value cutoffs of 0.05 and 0.005 (change in the objective function value of 3.84 and 7.88), respectively.
2.6 Model Simulations
Monte Carlo simulations were performed using the final population pharmacokinetic model to determine the distribution of lorazepam concentrations following a lorazepam 0.1 mg/kg i.v. push. The effect of a second dose at 0.05 mg/kg was also evaluated. Two hundred virtual patients with similar demographics as those in the original studies were simulated for each age group, and these simulations were compared to a target concentration range of 50–100 ng/mL based on published lorazepam pharmacodynamics and an average maximal concentration (Cmax) of 70 ng/mL seen in adults following 4 mg administered intravenously [9,15,16].
2.7 Associations between Exposure and Efficacy and Safety Endpoints
Only subjects in the Status 2 study had efficacy and safety measurements collected in a manner that allowed the exploratory assessment. They represent more than two thirds of the overall subjects in this analysis, and characteristics were similar to the entire dataset. More detailed description of the Status 2 subject characteristics can be found in the primary publication [7]. For these patients, exploratory exposure-response relationships were assessed using the overall efficacy and safety endpoints. The overall efficacy endpoint was defined as SE cessation within 10 min and sustained absence of seizures for 30 min. The overall safety endpoint was defined as absence of life-threatening respiratory depression (requiring assisted ventilation). Potential associations between predicted lorazepam drug concentrations at 10, 30, 60 and 240 min, pharmacokinetic parameters, and key clinical characteristics were initially compared in patients who met pharmacodynamic endpoints with those who did not using the Wilcoxon test. This was followed by a stepwise logistic regression analysis for characterization of potential independent effects of drug exposure that included age of the patient and other anti-epileptic drugs (carbamazepine, phenytoin, phenobarbital, and valproate) individually and collectively as concomitant enzyme inducers (carbamazepine, phenytoin, and phenobarbital). The potential association between pharmacokinetic parameters and pharmacodynamic endpoints was evaluated because the former are expected to alter drug exposure.
3 Results
3.1 Patient Data
A total of 145 patients (62 in Status 1 and 83 in Status 2) contributed 439 (283 from Status 1 and 156 from Status 2) measured lorazepam concentrations in the final dataset. One subject from the original Status 1 dataset was excluded from analysis as all samples were of inadequate volume for re-analysis. There were 125 (28%), 184 (42%), and 130 (30%) total samples collected from patients 3 months to <3 years, 3-<13 years, and ≥13 years of age, respectively, across both studies. Out of the 15 patients enrolled in the intensive pharmacokinetic sampling cohort of the Status 1 study, the median age was 13.9 years (range 0.8–17.5); only 1 subject was <1 year of age. All specimens had detectable lorazepam above the limit of quantification except pre-dose samples. Demographic characteristics are shown in Table 1. The median (range) number of doses administered per patient in the Status 1 and 2 studies were 1 (1–18) and 1 (1–2), respectively. The median (range) observed lorazepam concentration was 34.9 (1.1–342.3) ng/mL with only isolated concentrations greater than 150 ng/mL. Seventy (16%) and 96 samples (22%) were collected within 0.5 and 1 hours after last dose, respectively.
Table 1.
Clinical data used for population pharmacokinetic model development.
| Variable | 3 months – < 3 years (N=54) | 3 – <13 years (N=69) | ≥13 years (N=22) | Total (N=145) |
|---|---|---|---|---|
| Dose (mg/kg) | 0.10 (0.04–0.18) | 0.09 (0.02–0.12) | 0.04 (0.02–0.10) | 0.10 (0.02–0.18) |
| Age (years) | 1.3 (0.3–2.9) | 6.5 (3.0–12.0) | 15.9 (13.8–17.8) | 5.4 (0.3–17.8) |
| Weight (kg) | 10 (5–20) | 23 (10–83) | 68 (36–105) | 18 (5–105) |
| Serum creatinine (mg/dL) | 0.3 (0.1–0.6) | 0.5 (0.3–1.4) | 0.9 (0.6–1.7) | 0.4 (0.1–1.7) |
| Albumin (g/dL) | 3.8 (3.3–4.4) | 4.1 (2.8–5.0) | 4.3 (3.3–4.9) | 4.1 (2.8–5.0) |
| AST (U/L) | 38 (7–103) | 29 (11–128) | 28 (14–100) | 33 (7–128) |
| Hematocrit (%) | 35 (27–42) | 37 (30–46) | 40 (29–45) | 36.3 (27–46) |
| Male | 43% | 52% | 59% | 50% |
| Race (N, White/Black/Other) | 22/25/7 | 23/40/6 | 3/16/3 | 48/81/16 |
| Concomitant medications | ||||
| Carbamazepine | 4% | 13% | 32% | 12% |
| Phenobarbital | 31% | 14% | 0 | 19% |
| Phenytoin | 17% | 23% | 18% | 20% |
| Valproate | 2% | 22% | 18% | 14% |
For continuous variables, data are presented as median (range). AST: aspartate aminotransferase.
3.2 Population Pharmacokinetic Analysis
A two-compartment structural model described the data well. Age and serum creatinine were the most impactful covariates for CL and V2, respectively, in the univariate screen; both resulted in a reduction in the objective function value of >8 points individually. However, the graphical associations between these covariates and pharmacokinetic parameters were modest. The multivariable analysis did not support inclusion of both age on CL and serum creatinine on V in a combined model, nor did any other potential covariates improve either of these models. The age-CL and serum creatinine-V2 models were similar in terms of goodness of fit. While the model incorporating serum creatinine on V2 had a slightly lower objective function value (2793.4 for serum creatinine-V2 vs. 2794.4 for age-CL), this model’s parameter estimates were less precise in a bootstrap analysis than the model with age as a covariate on CL. Therefore, we selected the model with age on CL as the final model.
Diagnostic plots and the visual predictive check for the final model are shown in Figures 1–3. The relationship between CL and half-life versus age is shown in Online Resource 1. The estimated values for the population pharmacokinetic parameters, covariates, and variances, along with the percent relative standard error of these estimates, bootstrap medians, and the 95% confidence intervals for these values, are listed in Table 2. Eta shrinkage estimates for CL, V1, and V2 were 9%, 20%, and 61%, respectively. The removal of the between-subject variability on V2 worsened the data fit. Also, because the exploratory pharmacodynamic analysis was focused on defining early lorazepam individual predicted concentrations, which are heavily dependent on V1 and V2, the eta on V2 was included in the final model. The power residual error model and separate proportional errors for each study enhanced the model goodness of fit. Inclusion of an additive residual error did not improve the model. The median bootstrap V1 and CL estimates were within 10% of population estimates from the original data set. While the observed V2 and Q were within the 95% confidence interval bootstrap estimates, the observed values are lower than the median bootstrap value, indicating less precision in these parameter estimates. However, the calculated Vss was within 5% of the value derived from the bootstrap estimates for V1 and V2. Overall there was significant unexplained between-subject variability (>40%) for CL, V1, and V2. The visual predictive check indicated that the model adequately described the data; less than 10% of the observed data fell outside the 90% prediction interval. Individual empirical Bayesian estimates are shown in Table 3. When scaled by linear body weight, individual subject post-hoc CL estimates decreased slightly and half-life increased with age. There was no apparent effect of age on Vss.
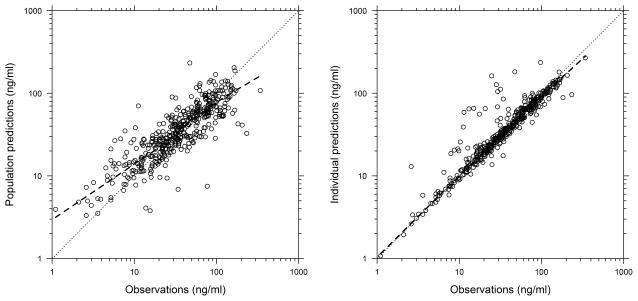
Figure 1.
Observations versus population and individual predictions from the final population pharmacokinetic model. The dashed lines represent the line of unity and the loess curve.
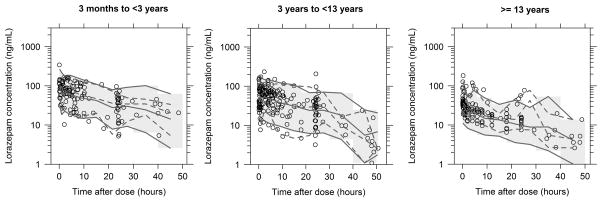
Figure 3.
Visual predictive check for final population pharmacokinetic model stratified by postnatal age. The open circles represent the observed data. The dashed and solid lines represent the 5th, 50th, and 95th percentiles for the observed and simulated data, respectively. The shaded grey region represents the 90% prediction interval. One thousand simulations were performed.
Table 2.
Pharmacokinetic parameter estimates for the final population model.
| Final Model | Bootstrap (n=1,000) | |||||
|---|---|---|---|---|---|---|
| Model parametera | Symbol | Point Estimate | RSE (%) | 2.5% | Median | 97.5% |
| V1 (L/kg) | θV1 | 0.879 | 11.2 | 0.607 | 0.786 | 0.969 |
| CL (L/h/kg0.75) | θCL | 0.115 | 5.5 | 0.108 | 0.119 | 0.131 |
| V2 (xV1) | θV2 | 0.542 | 32.7 | 0.452 | 0.737 | 1.250 |
| Q (L/h/kg0.75) | θQ | 1.450 | 50.8 | 1.100 | 2.520 | 4.981 |
| Exponent for (Age/4.7 yr) on CL | θCL-AGEY | 0.133 | 33.2 | 0.024 | 0.094 | 0.188 |
| Between-Subject Variability (CV%)b | ||||||
| ηV1 | ω2V1 | 47% | 37% | 50% | 60% | 75% |
| ηCL | ω2CL | 40% | 18% | 75% | 81% | 86% |
| ηV2 | ω2V2 | 57% | 91% | 17% | 48% | 85% |
| Residual variance (WSV) (CV%)c | ||||||
| ERR, STUDY=1 | σ2 STUDY1 | 33% | 66% | 31% | 42% | 61% |
| ERR, STUDY=2 | σ2 STUDY2 | 22% | 82% | 18% | 29% | 51% |
| Weighting Exponent | θPOWER | 1.290 | 13.9 | 0.956 | 1.170 | 1.340 |
V1: central compartment volume; CL: clearance; V2: peripheral compartment volume; Q: intercompartmental clearance; RSE: relative standard error of estimate.
V1 (L)=0.879*WT; CL (L/h)=0.115*WT0.75*(AGE/4.7)0.133; V2 (L)=0.542*V1; Q (L/h) = 1.45*WT0.75.
The covariance (cov) between etas for V1 and CL (covV1,CL), V1 and V2 (covV1,V2), and CL and V2 (covCL,V2) were 0.163, −0.159, and 0.0348, respectively.
The relationship between observations and individual predictions was described according to the following relationship: Y=IPRED+ERR(1)*(θSTUDY1*(2-STUDY)+θSTUDY2*(STUDY-1))*(IPRED**θPOWER), where IPRED represents the individual prediction; θSTUDY1 and θSTUDY2 are the residual error terms for the Status 1 and Status 2 trials, respectively; ERR(1) was fixed to unity; and the individual predictions were raised to a power exponent denoted as θPOWER.
Table 3.
Individual post-hoc pharmacokinetic parameter estimates derived using the final model.
| Parameter | 3 months – <3 years (N=54) | 3 – <13 years (N=69) | ≥13 years (N=22) | Total (N=145) |
|---|---|---|---|---|
| CL (L/h) | 0.60 (0.24–1.96) | 1.23 (0.31–8.44) | 2.56 (0.48–6.67) | 1.02 (0.24–8.44) |
| CL_WT (L/h/kg) | 0.058 (0.022–0.169) | 0.058 (0.012–0.173) | 0.049 (0.006–0.095) | 0.056 (0.006–0.173) |
| Vss (L) | 10.37 (4.88–19.33) | 19.84 (6.37–156.74) | 55.64 (20.41–111.0) | 17.61 (4.88–156.74) |
| Vss_WT (L/kg) | 1.40 (0.62–2.30) | 1.40 (0.43–4.98) | 1.25 (0.35–1.79) | 1.40 (0.35–4.98) |
| Alpha half-life (h) | 0.26 (0.13–0.39) | 0.29 (0.11–0.65) | 0.34 (0.11–0.50) | 0.28 (0.11–0.65) |
| Beta half-life (h) | 17.56 (8.57–25.41) | 16.51 (8.19–39.19) | 20.08 (12.02–53.57) | 17.36 (8.19–53.57) |
All data are presented as median (range). CL: clearance; CL_WT: weight-adjusted clearance; Vss: steady-state volume of distribution; Vss_WT: weight-adjusted steady-state volume of distribution.
3.3 Lorazepam Predicted Concentrations Based on Individual Pharmacokinetic Estimates
Individual empirical Bayesian estimates for pharmacokinetic parameters were used to predict Cmax for study patients. The median predicted Cmax based on a dosage of 0.1 mg/kg (maximum dose of 4 mg) was 104 ng/mL; more than 92% of patients had predicted Cmax values greater than 50 ng/mL. While 54% had Cmax concentrations >100 ng/mL, with the typical alpha half-life of less than 20 min, typical lorazepam concentrations fell to 75–80 ng/mL after 10–15 min.
3.4 Dosing Simulations
Using the final pharmacokinetic model and Monte Carlo simulations, lorazepam exposure following a 0.1 mg/kg dose was assessed. The initial lorazepam concentrations 5 min after administration were >50 ng/mL in approximately 90% of simulated pediatric patients, and half of simulated patients had concentrations >100 ng/mL. By 4 hours, concentrations in 7% of simulated patients were >100 ng/mL, yet concentrations were maintained above 50 ng/mL in 56% of simulated patients. As expected, administration of a second dose of lorazepam (0.05 mg/kg) resulted in higher concentrations than those following single-dose administration. However, less than one-third of simulated patients with two doses had protracted concentrations >100 ng/mL for 4 hours.
3.5 Associations between Exposure and Efficacy and Safety Endpoints
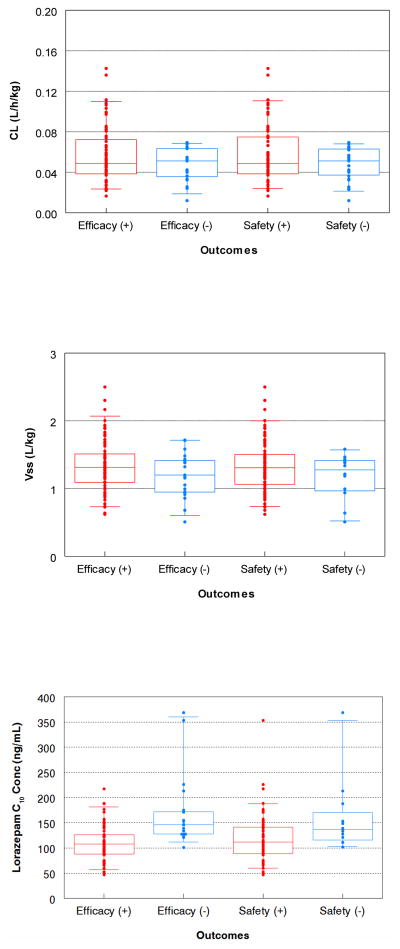
For the Status 2 study data, there were no associations among dose-independent pharmacokinetic parameters, CL (L/h/kg), Vss (L/kg), or V1 (L/kg) and efficacy (p=0.41, p=0.21, and p=0.35, per Wilcoxon test, respectively) or safety defined as the need for mechanical ventilation (p=0.515, p=0.32, and p=0.32, Wilcoxon test, respectively) (Figure 4). Mean (±SD) predicted lorazepam concentrations at 10 min (C10min), 30 min (C30min), and 4 hours (C240min) after initiating therapy were higher in patients who failed the safety criteria (C10min: 160±73 versus 119±47 ng/mL, p=0.017; C30min: 131±60 versus 100±37 ng/mL, p=0.034; C240min: 103±57 versus 76±31 ng/mL, p=0.058). Higher lorazepam concentrations were also seen in patients who failed the efficacy criteria (C10min: 168±70 versus 109±32 ng/mL; C30min: 144±52 versus 91±28 ng/mL; C240min: 112±46 versus 58±26 ng/mL, all p<0.0001).
Figure 4.
Clearance (top), steady-state volume of distribution (middle), and predicted concentration at 10 min (C10 lower) versus outcomes. The box is the interquartile range, and the whiskers represent the 5th and 95th percentiles.
Patients with continued or recurrent seizures received a larger total lorazepam dosage than those who met the efficacy criteria: total median dose 0.15 mg/kg versus 0.10 mg/kg, respectively (p<0.0001, Wilcoxon test). In addition, more phenobarbital use was seen in patients with continued or recurrent seizures (43% versus 5%, p<0.001) and more phenytoin/fos-phenytoin use in patients who failed the safety criteria (58% versus 8.5%, p=<0.001), likely due to additional use of these anti-epileptic drugs for prolonged seizures.
Stepwise logistic regressions of pharmacokinetic parameters, predicted lorazepam concentrations during the first 4 hours post-dose, cumulative lorazepam dose, concomitant anti-epileptic drugs, and age did not identify any pharmacokinetic parameter or lorazepam concentration time point as an independent predictor of efficacy or safety outcomes. Limiting the safety analysis to include only the patients who met the efficacy criteria did not change the analysis results.
4 Discussion
Pediatric SE represents a particularly difficult study population in which to perform pharmacokinetic/pharmacodynamic evaluations due to the life-threatening nature of the condition, the need to dose medications until an adequate response is observed, use of concomitant medications that can alter drug elimination or serve as a confounder, and challenges associated with obtaining informed consent and collecting blood samples during an emergent situation. However, clinical pharmacology studies in pediatric SE are important because drug disposition may be altered as a result of pathophysiological changes (e.g., metabolic or respiratory acidosis, hemodynamic instability), and optimal dosing regimens need to be used during these emergent events.
The present study evaluated the population pharmacokinetics of lorazepam in 145 pediatric patients as young as 3 months of age with acute SE. A significant number of pharmacokinetic samples were collected shortly after drug administration in order to characterize the early lorazepam concentrations and the initial volume of distribution, both of which are important factors for SE patients. The resulting population pharmacokinetic model characterized lorazepam’s disposition parameters in these infants and children with reasonable certainty. As previously described, lorazepam’s population pharmacokinetics in children were well described using a two-compartment structural pharmacokinetic model [13]. Relative to our previous publication [13], combining the data from two pediatric studies allowed us to more than double the number of children age 3 months to <3 years in our analysis. CL and volume of distribution estimates were in the range seen in non-seizing pediatric and adult populations, strongly suggesting that acute SE does not have pronounced, persistent influence on lorazepam pharmacokinetics [17,18]. The rapid anti-seizure effects of lorazepam and logistic difficulties of collecting very early pharmacokinetic samples limited the pharmacokinetic data during ongoing seizures, and thus the current study may have missed transient acute pharmacokinetic effects of SE. Although the number of patients receiving other anticonvulsants was limited, the presence of treatment with concomitant anti-epileptic drugs was not identified as significant covariates for pharmacokinetics.
While a maturation-age effect was seen in our model when size was allometrically scaled (L/h/kg0.75), weight-normalized CL was 0.058 L/h/kg in younger patients (<13 years) compared to 0.048 L/h/kg in adolescents. This is consistent with another study where a modest (yet non-significant) age effect on weight-normalized CL was also reported: mean (SD) 0.076 (0.034) L/h/kg in children age ≤12 years versus 0.062 (0.024) L/hg/kg for children >12 years of age [12]. In contrast, in 10 critically ill term neonates with seizures (mean [SD] gestational age 39.6 [1.4] weeks, postnatal age not reported), lorazepam weight-normalized CL was lower (mean [SD] 0.014 [0.0066] L/h/kg) and was found to correlate with gestational age [11]. Thus, it is likely that only a modest age effect was observed in our study as all children were >3 months of age. It is also consistent with available in vitro and in vivo data that suggests significant developmental changes in UGT activity during the first 6 months following birth, and less profound age-dependent changes thereafter [13,19–21].
Since lorazepam dosing is weight-based, one would expect greater exposure in older patients. However, the therapeutic approach of lorazepam use in SE is as a single- or two-dose regimen. This loading approach translates into persistent but not higher concentrations. Also, the truncation of dosing at 40 kg body weight (maximum single dose of 4 mg) has the net effect of giving lower mg/kg doses in adolescents in accordance with their lower weight-normalized CL. The CL difference between ages <3 years and 3-<13 years was small when compared to ages ≥13 years. Since the oldest age group represents adolescents, most with body weights above 40 kg, the dose limit of 4 mg will help prevent prolonged high concentrations in this age group.
In adults, a 4 mg i.v. dose is recommended for the treatment of SE [9]. If seizures continue after a 10–15–min observation period, then an additional 4 mg dose may be warranted [9]. In children, dosing recommendations are lacking in the product labeling. The primary analysis of the Status 2 study showed that a 0.1 mg/kg i.v. dose (4 mg maximum) achieved cessation of SE for 10 min without recurrence within 30 min in 72.9% (97/133) of children [7]. Approximately 18% of children who received lorazepam required assisted ventilation in this study. Another study in children (median age 32 months [range 8–91]) with severe malaria and convulsions reported complete cessation of seizures with an i.v. lorazepam dose of 0.1 mg/kg, but 4/15 [27%] later had seizures recur [22].
The ideal target range for lorazepam concentrations in children for the treatment of SE is unknown; however, in adults, one study noted seizure improvement (intractable complex seizures) with concentrations of 20–30 ng/mL [16]. In another study that evaluated the sedative effects of lorazepam in adult intensive care unit patients, a target concentration of 50 ng/mL was used [15]. In adults, a 4 mg dose results in an initial concentration of approximately 70 ng/mL [9]. Based on simulations performed using our final model, a 0.1 mg/kg dose (4 mg maximum) achieved concentrations >50 ng/mL in the majority of virtual patients 5 min after administration, and half had simulated concentrations >100 ng/mL. At 4 hours following administration, over half of virtual patients continued to have a lorazepam concentration above 50 ng/mL. This may help explain the clinical findings in the Status 2 study that approximately 60% of patients had not returned to baseline mental status by 6 hours. We found no association between pharmacokinetic parameters and efficacy or safety endpoints in children with SE. There were higher lorazepam concentrations in children who failed the safety or efficacy criteria, which is due to the additional lorazepam doses allowed in patients with continued or recurrent seizures and possibly greater use of other anti-epileptic drugs. Of note, these patients may have had more severe epilepsy (more refractory to baseline treatments and lorazepam therapy) and thus needed higher doses to control their disease.
The analyses described herein leveraged data from two pediatric clinical trials to characterize lorazepam’s population pharmacokinetics in children age 3 months to 18 years with acute SE. Combining the data from both these studies allowed us to maximize the amount of pediatric data used during population pharmacokinetic model development and explore the relationship between lorazepam parameters and concentrations with safety and efficacy endpoints in one of the studies. Although the pharmacokinetic results are consistent with previous studies, a few notable limitations should be acknowledged. First, due to the sparse pharmacokinetic sampling scheme used, a robust exposure-response analysis could not be performed. However, the sparse pharmacokinetic sampling approach is still the most practical approach for performing a clinical trial in this difficult-to-study patient population. Second, although the overwhelming majority of patients achieved lorazepam concentrations believed to be therapeutic, the ideal concentration range for children with acute SE is unknown. Last, we only had pharmacokinetic data for infants >3 months of age, which may have limited our ability to detect a more pronounced age effect previously described in neonates. Despite these limitations, we believe that our study provides important information regarding the disposition and dosing of lorazepam in children >3 months of age with acute SE. Future studies should be designed to help provide a better understanding of SE and treatment considerations in patients with SE.
5 Conclusion
The population pharmacokinetics of lorazepam were successfully described using a two-compartment model in pediatric patients with acute SE. After accounting for body size, age was a statistically significant covariate with a modest impact on CL across the age continuum, likely reflecting developmental differences in UGT activity. No pharmacokinetic parameters were associated with efficacy or safety in one of the studies, but the study design limited determining the relationship between lorazepam concentrations and outcomes. These analyses suggest that uniform pediatric dosing (0.1 mg/kg, up to a maximum of 4 mg) can be used to achieve concentrations in children with SE that have been previously associated with effective seizure control.
Supplementary Material
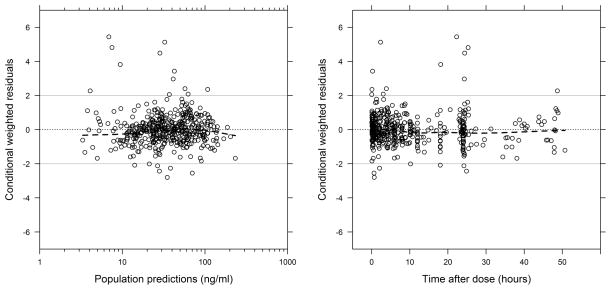
Figure 2.
Conditional weighted residuals (CWRES) versus time after dose and population predictions for the final population pharmacokinetic model. The dashed black lines represent the line at which the CWRES is equal to zero and the loess curve. The solid grey lines represent the CWRES values of 2 and −2.
Key Points.
Lorazepam is one of the preferred agents for treatment of status epilepticus (SE), but few studies have characterized its disposition in pediatric patients.
Pediatric SE represents a particularly difficult study population in which to perform clinical pharmacology studies due to the life-threatening nature of the condition, the need to dose medications until an adequate response is observed, and challenges associated with obtaining informed consent and collecting blood samples during an emergent situation, among other factors.
Monte Carlo simulations performed using a developed population pharmacokinetic model suggest uniform pediatric dosing (0.1 mg/kg, up to a maximum of 4 mg) in the setting of SE can be used to achieve lorazepam concentrations of 50–100 ng/mL in children >3 months of age. This simple uniformed mg/kg dose can be used in the broad age range encompassing pediatrics, which will facilitate clinical implementation.
Acknowledgments
The assay measuring lorazepam concentrations was performed at OpAns Laboratory (Durham, NC, USA) by Christine Grosse, Kenneth Lewis, and Robert Wurm.
The Best Pharmaceuticals for Children Act – Pediatric Trials Network Steering Committee
Daniel K. Benjamin Jr., MD, PhD, Katherine Y. Berezny, Duke Clinical Research Institute, Durham, NC; Gregory L. Kearns, Arkansas Children’s Hospital, Little Rock, AR; Matthew M. Laughon, MD, MPH, University of North Carolina, Chapel Hill, NC; Ian M. Paul, Penn State College of Medicine, Hershey, PA; Michael J. Smith, University of Louisville, Louisville, KY; P. Brian Smith, MD, MPH, MHS, Duke Clinical Research Institute, Durham, NC; John van den Anker, George Washington University School of Medicine and Health, Washington, DC; Kelly Wade, Children’s Hospital of Philadelphia, Philadelphia, PA.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development: David Siegel, Perdita Taylor-Zapata, Anne Zajicek, Zhaoxia Ren, Ekaterini Tsilou, Alice Pagan.
The EMMES Corporation (Data Coordinating Center): Ravinder Anand, Traci Clemons, Gina Simone.
Pediatric Trials Network Lorazepam Study Team, Principal Investigators, and Study Coordinators
Duke Clinical Research Institute: Michael Cohen-Wolkowiez, Kevin Watt, Jeffrey T. Guptill, and Barrie Harper
The University of North Carolina at Chapel Hill: Daniel Gonzalez
University of California, San Diego: Edmund V. Capparelli
Footnotes
Compliance with Ethical Standards
Funding and Conflicts of Interest: This work was funded under National Institute of Child Health and Human Development (NICHD) contract HHSN275201000003I (PI: Benjamin) for the Pediatric Trials Network. Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award number UL1TR001117. D.G. receives support for research from NICHD (K23HD083465), the nonprofit organization Thrasher Research Fund (www.thrasherresearch.org), and from industry (Cempra, Inc. and Jacobus Pharmaceutical Company, Inc.) for drug development in adults and children. M.C.-W. receives support for research from the NIH (1R01-HD076676-01A1), the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the National Institute of Allergy and Infectious Disease (NIAID) (HHSN272201500006I and HHSN272201300017I), NICHD (HHSN275201000003I), the Food and Drug Administration (1U01FD004858-01), the Biomedical Advanced Research and Development Authority (BARDA) (HHSO100201300009C), and the nonprofit organization Thrasher Research Fund (www.thrasherresearch.org) for drug development in adults and children (www.dcri.duke.edu/research/coi.jsp). J.T.G. receives research support through K23NS085049 from the National Institute of Neurological Disorders and Stroke (NINDS). The remaining authors have no funding to disclose. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Ethical approval: Both pediatric studies were reviewed and approved by local institutional review boards.
Informed consent: For the Status 1 study, patients either pre-consented to participate in the study prior to presentation of SE or consented to participate in the study after they received lorazepam if they presented to the emergency department in SE. For Status 2 study, participating hospitals submitted a site-specific plan to their institutional review board regarding the specific activities to address the requirements for the Exception from Informed Consent for Emergency Research (21 CFR 50.24). Thus, patients were treated prior to consent and then once their condition had stabilized the family was approached to discuss the study and obtain written consent for continued study participation.
References
- 1.Dham BS, Hunter K, Rincon F. The epidemiology of status epilepticus in the United States. Neurocrit Care. 2014;20:476–83. doi: 10.1007/s12028-013-9935-x. [DOI] [PubMed] [Google Scholar]
- 2.Chin RFM, Neville BGR, Peckham C, Bedford H, Wade A, Scott RC. Incidence, cause, and short-term outcome of convulsive status epilepticus in childhood: prospective population-based study. Lancet. 2006;368:222–9. doi: 10.1016/S0140-6736(06)69043-0. [DOI] [PubMed] [Google Scholar]
- 3.Betjemann JP, Lowenstein DH. Status epilepticus in adults. Lancet Neurol. 2015;14:615–24. doi: 10.1016/S1474-4422(15)00042-3. [DOI] [PubMed] [Google Scholar]
- 4.Singh R, Gaillard W. Status epilepticus in children. Curr Neurol Neurosci Rep. 2009;9:137–44. doi: 10.1007/s11910-009-0022-9. [DOI] [PubMed] [Google Scholar]
- 5.Glauser T, Shinnar S, Gloss D, Alldredge B, Arya R, Bainbridge J, et al. Evidence-Based Guideline: Treatment of Convulsive Status Epilepticus in Children and Adults: Report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr. 2016;16:48–61. doi: 10.5698/1535-7597-16.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, et al. Guidelines for the Evaluation and Management of Status Epilepticus. Neurocrit Care. 2012;17:3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain JM, Okada P, Holsti M, Mahajan P, Brown KM, Vance C, et al. Lorazepam vs diazepam for pediatric status epilepticus: a randomized clinical trial. JAMA. 2014;311:1652–60. doi: 10.1001/jama.2014.2625. [DOI] [PubMed] [Google Scholar]
- 8.Greenblatt DJ, Shader RI, Franke K, MacLaughlin DS, Harmatz JS, Allen MD, et al. Pharmacokinetics and bioavailability of intravenous, intramuscular, and oral lorazepam in humans. J Pharm Sci. 1979;68:57–63. doi: 10.1002/jps.2600680119. [DOI] [PubMed] [Google Scholar]
- 9.Watson Laboratories, Inc. [Accessed February 12, 2016];Lorazepam injection package insert. Available from: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ae274b1f-27c3-483b-99f1-9a9249dc2459.
- 10.Uchaipichat V, Suthisisang C, Miners JO. The glucuronidation of R- and S-lorazepam: Human liver microsomal kinetics, UDP-glucuronosyltransferase enzyme selectivity, and inhibition by drugs. Drug Metab Dispos. 2013;41:1273–84. doi: 10.1124/dmd.113.051656. [DOI] [PubMed] [Google Scholar]
- 11.McDermott C, Kowalczyk A, Schnitzler E, Mangurten H, Rodvold K, Metrick S. Pharmacokinetics of lorazepam in critically ill neonates with seizures. J Pediatr. 1992;120:479–83. doi: 10.1016/s0022-3476(05)80925-4. [DOI] [PubMed] [Google Scholar]
- 12.Crom WR, Relling MV, Christensen ML, Rivera GK, Evans WE. Age-related differences in hepatic drug clearance in children: studies with lorazepam and antipyrine. Clin Pharmacol Ther. 1991;50:132–40. doi: 10.1038/clpt.1991.117. [DOI] [PubMed] [Google Scholar]
- 13.Chamberlain JM, Capparelli EV, Brown KM, Vance CW, Lillis K, Mahajan P, et al. Pharmacokinetics of intravenous lorazepam in pediatric patients with and without status epilepticus. J Pediatr. 2012;160:667–72. e2. doi: 10.1016/j.jpeds.2011.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibaldi M, Perrier D. Pharmacokinetics. 2. New York, NY: Marcel Dekker; 1982. Multicompartment models. [Google Scholar]
- 15.Barr J, Zomorodi K, Bertaccini EJ, Shafer SL, Geller E. A double-blind, randomized comparison of i.v. lorazepam versus midazolam for sedation of ICU patients via a pharmacologic model. Anesthesiology. 2001;95:286–98. doi: 10.1097/00000542-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Walker JE, Homan RW, Crawford IL. Lorazepam: a controlled trial in patients with intractable partial complex seizures. Epilepsia. 1984;25:464–6. doi: 10.1111/j.1528-1157.1984.tb03444.x. [DOI] [PubMed] [Google Scholar]
- 17.Relling MV, Mulhern RK, Dodge RK, Johnson D, Pieper Ja, Rivera GK, et al. Lorazepam pharmacodynamics and pharmacokinetics in children. J Pediatr. 1989;114:641–6. doi: 10.1016/s0022-3476(89)80713-9. [DOI] [PubMed] [Google Scholar]
- 18.Swart EL, Zuideveld KP, De Jongh J, Danhof M, Thijs LG, Strack van Schijndel RMJ. Comparative population pharmacokinetics of lorazepam and midazolam during long-term continuous infusion in critically ill patients. Br J Clin Pharmacol. 2003;57:135–45. doi: 10.1046/j.1365-2125.2003.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strassburg CP, Strassburg a, Kneip S, Barut a, Tukey RH, Rodeck B, et al. Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut. 2002;50:259–65. doi: 10.1136/gut.50.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Divakaran K, Hines RN, McCarver DG. Human hepatic UGT2B15 developmental expression. Toxicol Sci. 2014;141:292–9. doi: 10.1093/toxsci/kfu126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouwmeester NJ. Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Br J Anaesth. 2004;92:208–17. doi: 10.1093/bja/aeh042. [DOI] [PubMed] [Google Scholar]
- 22.Muchohi SN, Kokwaro GO, Ogutu BR, Edwards G, Ward SA, Newton CRJC. Pharmacokinetics and clinical efficacy of lorazepam in children with severe malaria and convulsions. Br J Clin Pharmacol. 2008;65:12–21. doi: 10.1111/j.1365-2125.2007.02966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.