Abstract
Background and Purpose
Approaches to prevent selective and progressive loss of insulin‐producing beta cells in Type 1 diabetes mellitus (T1DM) will help to manage this prevalent and devastating disease. Curcumin (CUR), a natural anti‐inflammatory substance, suppresses diabetes‐associated inflammation and cell death. However, very high doses need to be used because of poor oral bioavailability, making it difficult to translate the anti‐inflammatory actions to clinical situations.
Experimental Approach
We have prepared biodegradable nanosystems encapsulating curcumin (nCUR), resulting in at least nine‐fold improvement in oral bioavailability. Here, we tested the ability of nCUR to prevent streptozotocin (STZ)‐induced inflammation and apoptosis in pancreatic islets and beta cells, in rats.
Key Results
Non‐fasted rats pretreated with 10 or 50 mg·kg−1 nCUR 6 h prior to STZ challenge had up to 37% reduction in the glucose levels, while plain CUR (50 mg·kg−1) results in 12% reduction. This treatment with nCUR was accompanied by decreased islet or beta cell death, as shown by TUNEL assay and H&E staining. Both CUR and nCUR significantly decreased levels of inflammatory cytokines in pancreatic tissue homogenates that correlated well with minimal histiocytic infiltration. Pre‐treatment with nCUR, but not CUR, decreased 8‐oxo‐2′‐deoxyguanosine, a sensitive biomarker of ROS‐induced DNA damage, in pancreas. In normal rodents, daily dosing for 28 days, with nCUR (25‐100 mg·kg−1) did not cause any deleterious health issues by the carrier.
Conclusions and Implications
Together, these data indicate a potentially translatable dose of nCUR that is safe and efficacious in improving beta cell function, which could prevent T1DM.
Abbreviations
- 8‐oxo‐dG
8‐oxo‐2′‐deoxyguanosine
- BFM
bright field microscope
- CUR
curcumin
- HED
human equivalent doses
- Iba1
ionized calcium binding adaptor molecule 1
- nCUR
Nano‐curcumin
- RIN‐m5F
rat pancreatic beta‐insulinoma cells
- STZ
streptozotocin
- T1DM
Type 1 diabetes mellitus
- TdT
terminal deoxynucleotidyl transferase
Introduction
Type 1 diabetes mellitus (T1DM) is a heterogeneous disorder characterized by primary or secondary islet damage, making the beta cells antigenic, prompting T‐lymphocyte infiltration and production of pro‐inflammatory cytokines, such as GM‐CSF, G‐CSF, IL‐1β, TNF‐α and IFN‐γ (Eisenbarth, 1986; Hamilton, 2008; Skyler, 2013; Atkinson et al., 2014; Meah et al., 2016). The clinical manifestations of T1DM are only evident when nearly 80% of the beta cell mass has already been destroyed. To date, there is no record of success with preventative strategies (Reimann et al., 2009; Brooks‐Worrell and Palmer, 2013; Skyler and Pugliese, 2013; Wherrett, 2014; Skyler, 2015). Over the years, we have witnessed a plethora of developments in T1DM therapies that include insulin replacement, immunosuppression, antigen‐specific and cell‐based approaches. T1DM prevention in high‐risk individuals remains the highest priority where the goal is to maintain endogenous beta cell function (Creusot et al., 2016; Li et al., 2016). Therefore, protection of beta cells from cell death is considered as a new therapeutic target (Srimal and Dhawan, 1973; Ardestani and Maedler, 2016; Imai et al., 2016; Roy et al., 2016), where natural and safe anti‐inflammatory agents, such as curcumin (CUR)(Srimal and Dhawan, 1973; Castro et al., 2014), can perform better than some of the biological agents, such as canakinumab, a fully human anti‐IL‐1β monoclonal antibody (IgG‐1κ class), tested in trials with limited success (Cabrera et al., 2016). A critical barrier to the clinical translation of the anti‐inflammatory effects of CUR is its limited oral bioavailability (Shaikh et al., 2009). We have recently reported several studies using the large scale preparation of CUR encapsulated nanosystems (nCUR), and their therapeutic evaluation in models of diabetic complications (Ratnam et al., 2011; Grama et al., 2013a,b). We report here the ability of nCUR to prevent streptozotocin (STZ)‐induced inflammation and apoptosis in pancreatic islet or beta cells in rats, which was reflected in a reduction of glucose levels and of oxidative stress.
Methods
Preparation and characterization of nCUR
The nCUR were prepared as previously reported by our laboratory (Shaikh et al., 2009; Ratnam et al., 2011; Grama et al., 2013a,b). In brief, polylactide‐co‐glycolide (PLGA) (500 mg) was dissolved in 20 mL of ethyl acetate, to which 75 mg CUR pre dissolved in 5 mL of ethyl acetate was added then stirred for 1 h at 1000 r.p.m. on a magnetic stirrer. This organic phase was added dropwise to 50 mL of 1% (w/v) polyvinyl alcohol in water forming an oil‐in‐water emulsion followed by homogenization at 16 000 rpm for 30 min. The emulsion was added to excess water (250 mL) and stirred overnight to evaporate organic solvent. The suspension was centrifuged at 15 000 g for 30 min at 4°C. The pellet was re‐suspended in water (~15 mL). Sucrose (1.5 g) was dissolved separately in 5 mL of water then added to the re‐suspended pellet with the entire suspension volume made to 30 mL. Two milliliters of the suspension was added to a 5 mL vial and freeze dried. Three vials from the lot were used to measure particle size and CUR content using a particle size analyser (Brookhaven dynamic light scattering system), and a previously developed HPLC method respectively (Shaikh et al., 2009; Grama et al., 2013b).
In vitro testing of nCUR in RIN‐m5F cells
The RIN‐m5f cells were cultured according to the cell bank protocol. The cells (5×104 per well in 24‐well plates) were pretreated with various concentrations of CUR or nCUR (10 and 20 μM) for 1 h before challenging with 5 mM STZ (dissolved in citrate buffer, pH 4.5; containing trisodium citrate 0.1 M and citric acid 0.1 M) for 24 h. The cells were washed twice with 1% BSA in PBS, 0.5 μg·mL−1 propidium iodide (PI) was added before observation under an EVOS microscope. In another set of experiments, cells were fixed in 4% formaldehyde at room temperature for 30 min followed by washing with PBS. The effects of CUR/nCUR were evaluated by immunocytochemistry by staining fixed cells for caspase‐8.
Animal study
All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee of the Texas A&M University, College Station (Study 1, efficacy) or the University of Strathclyde, Glasgow, under the Animals (Scientific Procedures) Act 1986 (UK) (Study 2, safety). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). Three‐month‐old male Sprague Dawley (SD) rats (250 ± 15 g, n = 6) were used in Study 1; 2‐month‐old male SD rats (200 ± 15 g, n = 4) were used in Study 2. All animals were fed normal diet ad libitum throughout the studies. All endpoint adjudication was carried out without knowledge of the treatments.
Experimental protocol
Study 1
After acclimatization for 2 weeks, the rats (male SD) were pretreated with a single dose of CUR or nCUR (10 or 50 mg·kg−1; p.o.), 6 h before STZ challenge. All rats received a single intraperitoneal injection of 60 mg·kg−1 STZ dissolved in 0.1 M sodium citrate buffer, pH 4.5, after 8 h fasting. The glucose levels in non‐fasted rats were monitored at 24, 48 and 72 h after STZ injection using precision Xtra glucometer (Abbott Diabetes Care Inc, Alameda, CA), and all rats were killed at 72 h post STZ. The control groups received neither CUR nor STZ. Blood and pancreas were collected and processed for analysis of various markers, histological evaluation and immunohistochemistry. The study had the following six groups: Group 1 (Control); Group 2 (STZ) diabetic control (STZ 60 mg·kg−1 intraperitoneal injection); Group 3 (STZ + CUR10) 10 mg·kg−1 curcumin; Group 4 (STZ + CUR50) 50 mg·kg−1 curcumin; Group 5 (STZ + nCUR10) 10 mg·kg−1 nano‐curcumin; Group 6 (STZ + nCUR50) 50 mg·kg−1 nano‐curcumin.
Study 2
After acclimatization for 2 weeks, the rats were treated with CUR 500 mg·kg−1 or nCUR 25, 50, or 100 mg·kg−1 daily by oral gavage for 28 days (Supporting Information Table S1). The control groups received equal volumes of vehicle (water in this case), while the nblank group received blank nanoparticles equivalent to the highest dose of CUR (100 mg·kg−1) for 28 days. Upon termination of the experiment at the end of 28 days, blood and other organs were collected and processed for haematological and histological analyses (detailed procedures and data are presented in Supporting Information).
Blood collection and pancreas separation
Rats in each group on Study 1 were killed as per IACUC protocol at 72 h after STZ injection. Blood was collected via cardiac puncture in heparinized tubes and centrifuged at 1620 g for 30 min. The plasma was immediately stored at −80°C for further use. The pancreas was surgically excised from the rats. These tissues were either stored at −80°C until further analysis or fixed in 10% buffered formalin for histological assessment.
Preparation of tissue homogenate
A 10% pancreatic tissue homogenate was prepared using a probe sonicator (qsonica) at 30% amplitude for 30 s, in ice cold homogenizing buffer (phosphate buffer/pH 7.4 supplemented with protease and phosphatase inhibitors). The homogenates were centrifuged for 30 min at 20 817 g at 4 °C. The supernatant was collected and stored at −80°C until further use.
Determination of plasma insulin
Plasma insulin levels were determined by using rat insulin elisa kits.
Multiplex immunoassay for cytokines
The cytokine panel of 14 markers was measured in plasma and pancreas homogenates using a Bio‐Plex® MAGPIX™ Multiplex Reader multiplex immunoassay. The markers analysed included IL‐1α, G‐CSF, IL‐10, IL‐17A, IL‐1 β, IL‐6, TNF‐α, IL‐4, GM‐CSF, IFN‐γ, IL‐2, IL‐5, IL‐13, IL‐12p70.
Histological studies
Pancreata from normal and experimental rats were fixed in 10% neutral buffered formalin (NBF) and processed for routine histology and paraffin embedding. Four micrometre tissue sections were stained with haematoxylin and eosin and evaluated histologically to determine the degree of islet destruction.
Immunohistochemistry to evaluate histiocytic infiltration in pancreatic islets
Sections were incubated on a hot plate for 45–60 min at 60°C, followed by deparaffinizing in xylene for 5 min, and rehydration in decreasing grades of ethanol (100, 95 and 70%). Antigen retrieval was performed by boiling the sections using a microwave, in 0.01 M sodium‐citrate buffer pH 6.0 for 10 min and blocked with blocking solution (3% goat or horse serum in PBS). Slides were washed three times with PBS and incubated in PBS containing goat serum with (2 μg·mL−1) anti‐Iba1 antibody specific to human, mouse and rat microglia and macrophages at 4°C overnight. Slides were washed three times with PBS and the binding of primary antibodies visualized by the corresponding anti‐Ig HRP detection kit according to the manufacturers' instructions. Slides were counterstained with Mayer's‐haematoxylin (Sigma‐Aldrich).
Immunofluorescence of insulin, TUNEL and 8‐oxo‐2′‐deoxyguanosine (8‐oxo‐dG)
Pancreata from normal and experimental rats were fixed in 10% NBF (24 h), incubated in 30% sucrose solution, and stored in OCT medium at −80°C, until being processed for cryosectioning. Four micrometre cryosections were used for the immunohistochemical staining for insulin, and 8‐oxo‐dG.
After antigen retrieval, cryosections were immunoreacted with respective primary antibodies (Abs) overnight at 4°C. All steps were preceded by rinsing with PBS (pH 7.4). Immunostaining was performed according to the standard protocol routinely used for immunohistopathology. Primary anti‐insulin Ab [clone INS05 (2D11‐H5)] was diluted to 1:200. Primary monoclonal Ab to 8‐hydroxyguanosine (8OHG), a marker of oxidative damage to nucleic acids was diluted to 1:200. Goat polyclonal anti‐rabbit IgG conjugated with Alexa Fluor® 488 (green), 594 (red) were used as the secondary antibody at a dilution of 1:1000. All samples were mounted with Vectashield mounting medium containing DAPI. Goat polyclonal anti‐mouse conjugated with Alexa Fluor® 594 IgG was used as the secondary antibody for insulin detection at a dilution of 1:1000 in PBS. TUNEL was performed on the same sections using Terminal deoxynucleotidyl transferase (TdT) to label blunt ends of double stranded DNA breaks. The end‐labelling method TUNEL enables the highly sensitive detection of apoptosis in tissue (In Situ Cell Death Detection Kit).
Image analysis
Images shown are representative of at least three rats that gave similar results. At least 10 images were taken at the same magnification from all immunostained probes. Immunostained sections were examined on a Zeiss LSM780. Microscopy images were processed using ZEN2 image processing software.
Data and statistical analysis
The data and statistical analysis in this study comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). Results are expressed as means ± SEM (n = 6). Statistical analysis was performed using PRISM GRAPHPAD version 5.01. One‐way ANOVA was used to compare multiple data sets and when the P value obtained from ANOVA was significant (P<0.05), Tukey's test was applied to test for differences among groups. P < 0.05 was taken to indicate a significant difference between group means.
Materials
STZ was purchased from Sigma‐Aldrich (St. Louis, MO, USA), and all other chemicals were obtained from Fisher Scientific (USA) unless otherwise mentioned. CUR was purchased from Acros organics (New Jersey, USA). Antibodies were purchased from Thermo Fisher Scientific (Rockford, IL, USA), Santa Cruz (Dallas, Texas), Life technologies, USA and Cell Signaling, USA. DAPI was purchased from Vector Laboratories, USA, TUNEL was purchased from Roche Diagnostics (Mannhem, Germany). Rat insulin elisa kits were purchased from Mercodia [10–1250‐01] (Uppsala, Sweden), ProcartaPlex Rat Th complete panel was purchased from Affymetrix, eBiosciences (14plex EPX140–30120‐901, USA) and, Histomount was purchased from Ted Pella Inc. Rat pancreatic beta‐insulinoma cells (RIN‐m5f) were purchased from ATCC and maintained in RPMI‐1640 medium.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015).
Results
Preparation and characterization of nCUR
The nCUR preparation process has been thoroughly optimized over the years in our laboratory (Shaikh et al., 2009; Grama et al., 2013a). The preparation used in this study has a particle size of ~300 nm with ~100 μg CUR per mg polymer (Supporting Information Figure S1). These freeze‐dried preparations are rendered easy to reconstitute with comparable characteristics to the fresh preparation in terms of size and CUR content in the particles. We have established shelf‐storage stability of nCUR in our earlier studies (Grama et al., 2013a).
In vitro testing of nCUR in rat pancreatic β‐insulinoma (RIN‐m5F) cells
Rat (RIN‐m5F) insulinoma cells are widely used in vitro, as models for pancreatic beta cells (Skelin et al., 2010), to study effects of STZ‐induced cellular stress and apoptosis. Here, we have used this model to test the ability of CUR and nCUR to protect against STZ‐induced apoptosis. RIN‐m5F cells, untreated or pretreated with CUR or nCUR for 1 h, were exposed to STZ for 24 h, and viability was assessed using the PI assay, which stains dead cells. Treatment with STZ significantly reduced cell viability, whereas CUR and nCUR increased the viability of STZ‐treated RIN‐m5F cells (Figure 1A). Similar effects of CUR and nCUR were observed on preventing activation of caspase 8, thereby preventing cell death and improving cell survival (Figure 1B).
Figure 1.
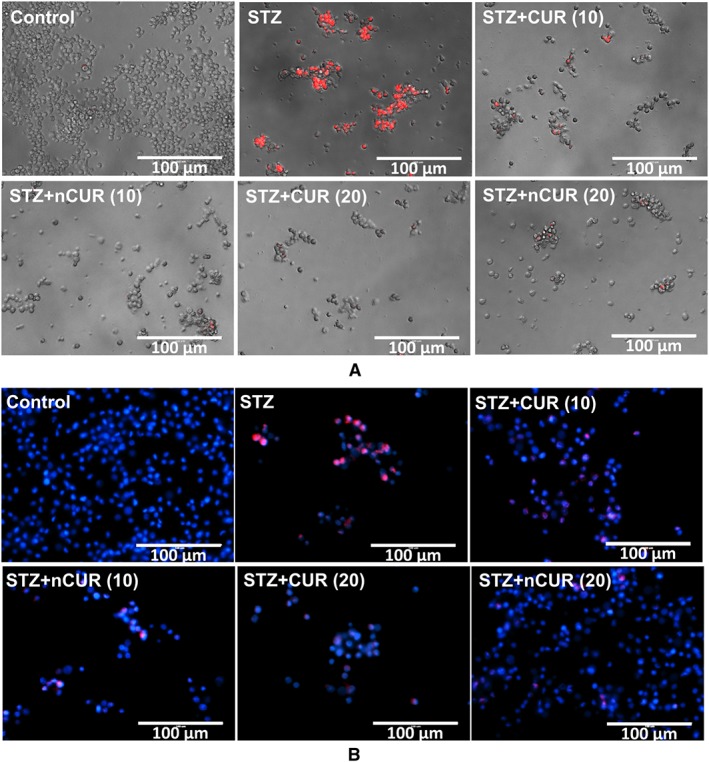
Pretreatment with CUR or nCUR prevents STZ induced cell death in RIN‐m5f cells. (A) Cell death was identified by PI staining (20× magnification, scale bar represents 200 μm). (B) The presence of active caspase‐8 was examined by immunofluorescence using antibodies specific for cleaved caspase‐8 (40× magnification, scale bar represents 100 μm).
Multiplex immunoassay for cytokines
Activated macrophages produce proinflammatory cytokines that are involved in up‐regulation of inflammatory reactions, promoting apoptosis. Therefore, we measured a panel of 14 cytokines including IL‐1 α, G‐CSF, IL‐10, IL‐17A, IL‐1 β, IL‐6, TNF‐α, IL‐4, GM‐CSF, IFN γ, IL‐2, IL‐5, IL‐13, IL‐12p70, in plasma and pancreas. Treatment with STZ insult significantly increased all the 14 markers studied. Pre‐treatment with nCUR prevented the STZ‐induced rise in G‐CSF/GM‐CSF (P < 0.05), but pre‐treatment with CUR failed to block the rise of G‐CSF. Both CUR and nCUR were successful in normalizing the pro‐inflammatory cytokines in pancreas tissue homogenates (Figure 2). However, analysis of plasma revealed no significant differences between controls, STZ or CUR treatment groups for the duration of the study (72 h) (Supporting Information Table S2).
Figure 2.
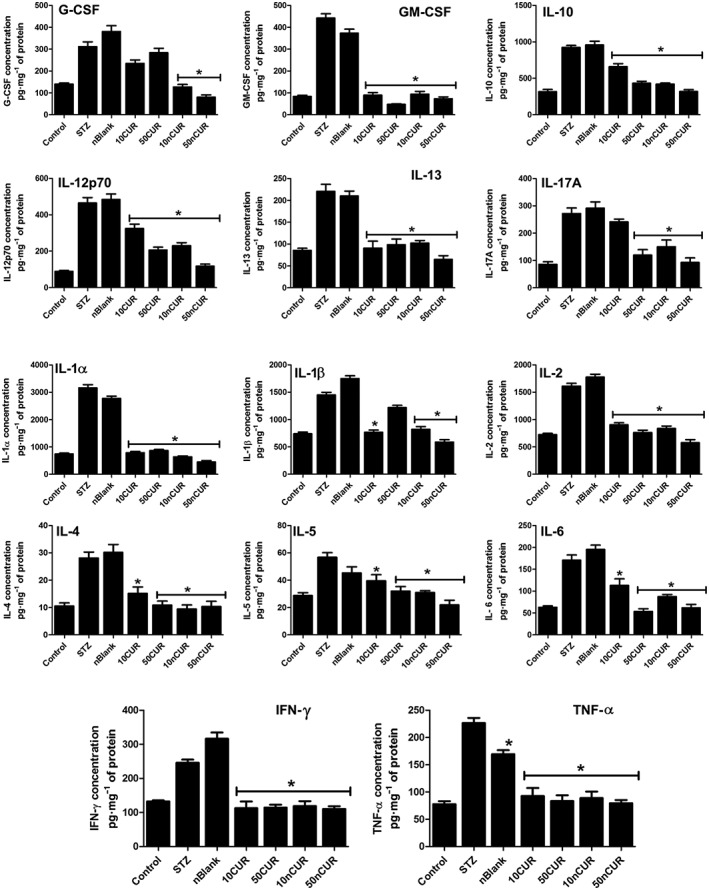
Pretreatment with CUR or nCUR (10 or 50 mg·kg−1) orally prior to STZ challenge regulates inflammatory cytokines. Pancreatic tissue homogenates were analysed 72 h after injection of STZ, using a multiplex assay. CUR/nCUR regulated the expression of pro‐ and anti‐inflammatory cytokines induced by STZ. Data are presented as mean ± SEM, (n = 6). *P < 0.05, treatment groups (STZ + 10CUR, 50CUR, 10nCUR, 50nCUR), significantly different from untreated diabetic controls (STZ); one‐way ANOVA, with Tukey's test.
Effect of CUR on blood glucose and plasma insulin level
The selective pancreatic beta cell apoptosis caused by STZ challenge is expected to increase blood glucose levels, and protection from beta cell death should, in principle, provide better glucose control. Treatment with nCUR, but not CUR, significantly decreased blood glucose in non‐fasted rats, by 32% and 37% for nCUR10 and nCUR50, respectively, at 72 h after STZ injection. This reduction in glucose levels also reflects in a slight increase in plasma insulin levels, suggesting improved beta cell function (Figure 3A). Interestingly, statistically significant differences were found in the blood glucose levels at 48 and 72 h, suggesting possible beta cell regeneration. However, more detailed experiments on repeated administration are needed for both pre‐and post‐STZ challenges.
Figure 3.
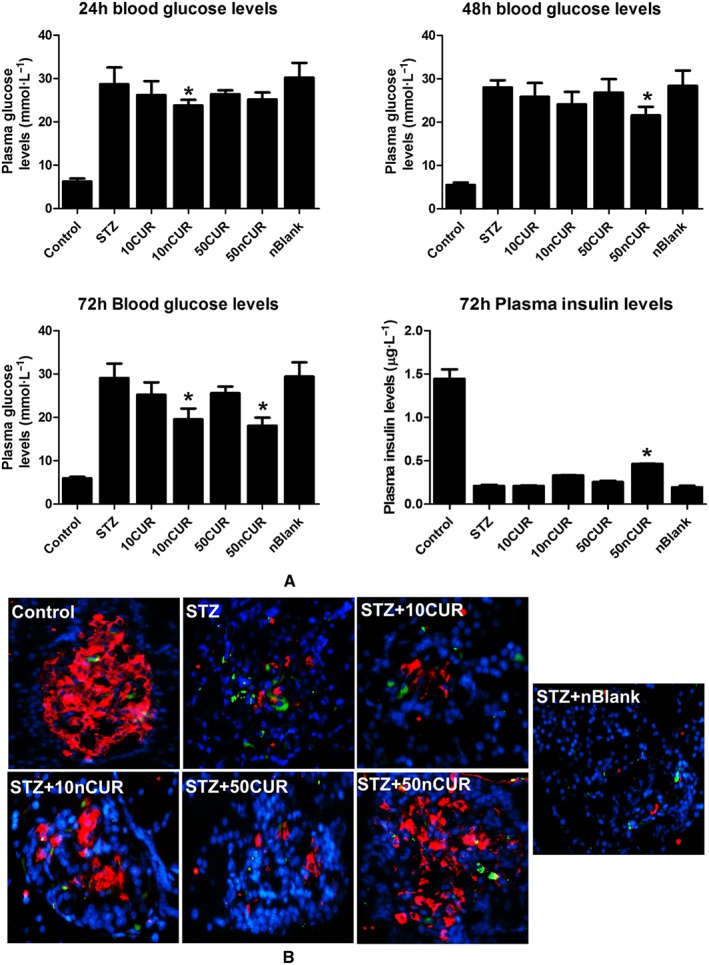
Pretreatment with nCUR prior to STZ challenge protects against elevated blood glucose or insulin and loss of pancreatic islet cell numbers through apoptosis. (A) The blood glucose levels (24, 48 and 72 h) and 72 h plasma insulin level, after injection of STZ, were monitored by glucose strips and elisa kits (n = 6). (B) Pancreatic islets (72 h) were identified by insulin immunofluorescence (red), and apoptotic cells were determined by TUNEL (green); nuclei were visualized by DAPI staining and shown in blue. Images were acquired using EVOIS‐FL microscope at 40x magnification. Data are presented as the mean ± SEM (n = 6). *P < 0.05, treatment groups (STZ + 10CUR, 50CUR, 10nCUR, 50nCUR), significantly different from untreated diabetic controls (STZ); one‐way ANOVA, with Tukey's test.
Effect of CUR on pancreatic islets/beta cells
Inflammation is associated with cell death and the ability to effectively target inflammatory pathways will prevent cell death (Carrington et al., 2011; Santin and Eizirik, 2013). The ability of CUR/nCUR to prevent STZ induced beta cell death was examined by TUNEL assay. The qualitative data clearly suggest that treatment with nCUR offered better protection than treatment with plain CUR (Figure 3B), and this was corroborated with the corresponding blood glucose levels (Figure 3A). Immunostaining for insulin is shown in Supporting Information Fig. S2.
The results of the histological analysis of pancreas showed well defined islets of Langerhans, along with intact exocrine pancreatic tissues in normal control rats, which were lost in the sections from rats treated with STZ only. Although there was an improvement in the histology of pancreatic tissue from both CUR and nCUR treated groups, these differences were not statistically significant (Figure 4A). Histiocytes were identified by staining for ionized calcium binding adaptor molecule 1 (Iba1), which is also called allograft inflammatory factor 1. The immunohistochemical staining for Iba1 showed that the number of infiltrating histiocytes was significantly reduced with the CUR and nCUR treatments (Figure 4B). Under hyperglycaemic condition, generation of reactive oxygen intermediates and apoptosis is expected to increase. Therefore, oxidative damage to DNA was assessed by immunostaining for 8‐oxo‐dG. A higher intensity staining for 8‐oxodG was observed in diabetic pancreas, compared to control, and pre‐treatment with nCUR prevented this damage, whereas CUR had little effect (Figure 4C).
Figure 4.
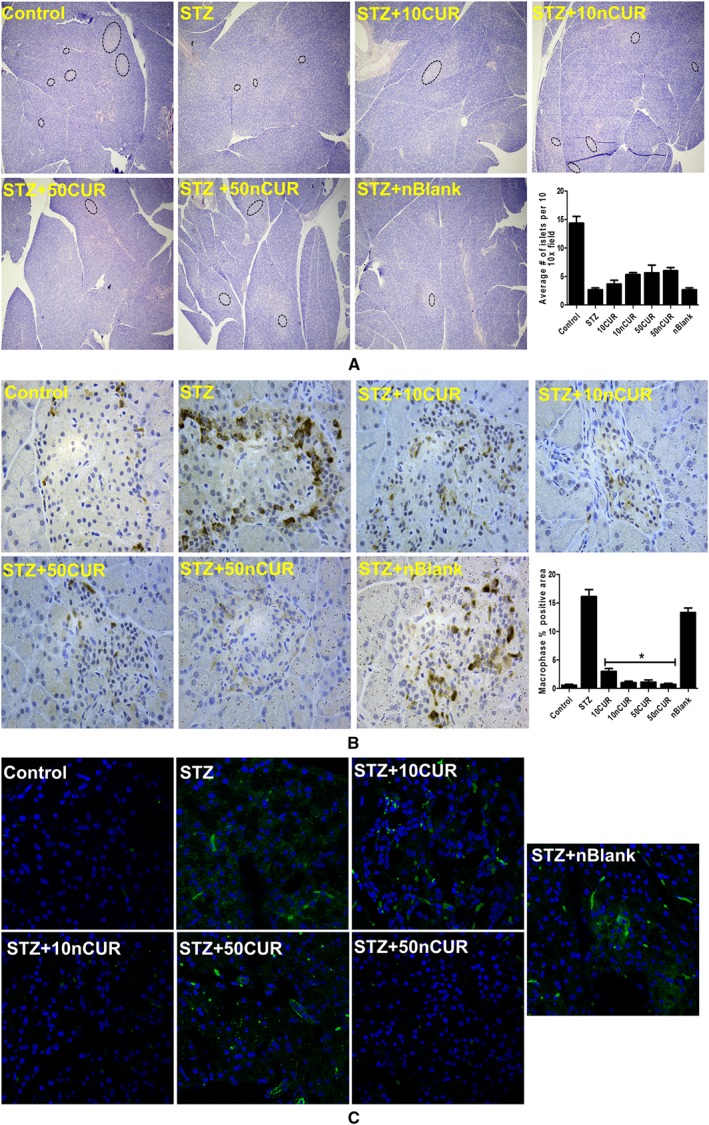
Pretreatment with CUR/nCUR prior to STZ challenge supports pancreatic islet cell numbers while reducing histiocytic infiltration and oxidative stress. (A) Histological changes in pancreatic tissue were observed by haematoxylin and eosin staining. Images were captured using bright field microscope (BFM) at 10× magnification and islets were marked in black dotted ellipses. The average number of islets per field at 10× magnification was plotted, (n = 6). (B) Histiocytes were identified by Iba1 immunohistochemistry using DAB (brown) and counterstained with haematoxylin. Images were acquired at 40× magnification using BFM. Histiocytic infiltration as a percentage of field of view area was calculated by NIH‐imageJ software. Data are presented as mean ± SEM, (n = 6–12 images from each group). *P < 0.05, treatment groups (STZ + 10CUR, 50CUR, 10nCUR, 50nCUR), significantly different from untreated diabetic controls (STZ); one‐way ANOVA, with Tukey's test. (C) Oxidative stress was identified by 8‐oxo‐dG immunofluorescent staining with FITC (green) and nuclei are counterstained with DAPI. Immunostained sections were examined on a Zeiss microscope. Microscopy images were captured using CLSM at 40× original magnification for entire layout. Confocal images were processed using ZEN software.
Safety of CUR and nCUR on sub‐acute dosing
From a regulatory standpoint it is important to establish that the carrier systems for pharmacological agents do not cause any deleterious effects on the biological system to which they are administered. Repeated dosing (once daily for 28 days) of CUR at 500 mg·kg−1 or nCUR at 25, 50 and 100 mg·kg−1 or the blank (CUR‐void) nanosystems, equivalent to 100 mg·kg−1 CUR had no noticeable effects on inflammatory/oxidative stress, antioxidant enzyme levels or haematological parameters (Supporting Information Tables S3–S7). At 50 mg·kg−1 (the dose used in the efficacy study), the plasma levels of curcumin were significantly higher in the nCUR group, compared to those in rats receiving 500 mg·kg−1 plain CUR. This difference presumably reflected the improved absorption and the avoidance of enzymic and non‐enzymic degradation of encapsulated CUR, provided by the nCUR formulation (Supporting Information Table S8). Further, no noticeable changes were observed in histological evaluation of a range of rat tissues, including small intestine, spleen, lung, kidney, heart and testis (Supporting Information Fig. S3).
Discussion
To the best of our knowledge, ours is the first study to demonstrate the ability of nCUR to prevent inflammation and beta cell apoptosis. In doing so, we have also established the biological safety of the nanosystems, which is critical for clinical translation.
Reformulating drugs or drug‐like compounds to address stability, pharmacokinetic and pharmacodynamic issues is a common strategy and biodegradable nanosystems have been hailed as a breakthrough technology for this purpose (Bhardwaj et al., 2005). Despite the huge, largely untapped therapeutic benefit of CUR, a natural anti‐inflammatory agent, numerous clinical trials have failed owing to poor oral bioavailability, leading to reduced enthusiasm for CUR therapy (Ratnam et al., 2006, 2011; Kumar, 2012). Our laboratory has conducted a series of investigations on improving CUR stability and oral bioavailability by encapsulating CUR into biodegradable nanosystems that were shown to have improved efficacy in diabetes models (Ratnam et al., 2011; Grama et al., 2013a,b).
The current study describes the use of nCUR for preventing beta cell apoptosis as a possible intervention for diabetes prevention and management. The nCUR used in this study have a particle size of ~300 nm with ~100 μg CUR per mg polymer. Although there is no clear cut‐off size for oral delivery, it is generally believed the smaller particle sizes are better absorbed (Ratnam et al., 2013). Drug loading is critical for developing translatable dosage forms as initial loading and entrapment will influence the bio‐distribution, safety and efficacy (Bhardwaj et al., 2005). This becomes really important for chronic conditions such as diabetes, in that better drug loads will minimize excess use of excipients. According to the USFDA, for conventional formulations, conversion of animal doses to Human Equivalent Doses (HED) based on body surface area requires the animal dose, in mg·kg−1, to be divided by 6.2 or multiplied by 0.16 to obtain the HED, also in mg·kg−1. Based on these assumptions, the HED of the dose used in this study would be 480 mg for a 60 kg human, with ~4.8 g of associated polymer. Similar doses (360 mg·day−1, Trial #NCT02104752) of CUR nanosystems made of gum ghatti are currently in use clinically. Based on the preclinical studies in rodents, the polymer nanosystems developed in the current study may not require daily administration. This point, however, needs to be tested in higher animals or Phase 1 studies in humans to better understand the HED conversion of unconventional delivery systems.
Effective prevention or management of diabetes requires the preservation of the insulin‐secreting beta cells. A large body of data suggests that oxidative stress plays a key role in the development of diabetes and its complications (Acharya and Ghaskadbi, 2010). The weak defence status of islets combined with inefficiency in repairing oxidative damage to DNA, compared to other tissues, renders the pancreas more vulnerable to oxidative stress, leading to pancreatic beta cell death. STZ is a toxin that leads to oxidative stress and inflammation and apoptosis of beta cells in the pancreas mimicking autoimmune diabetes. In the present study, we used the RINm5F cell line as a model of pancreatic beta cells, to investigate the ability of CUR and nCUR to prevent STZ‐induced apoptosis. In general, nCUR offered better cytoprotective properties, compared to unformulated CUR, probably due to intracellular accumulation of nCUR and the subsequent sustained release of the encapsulated CUR (Prokop and Davidson, 2008). There is good evidence for the role of caspase‐8 in beta cell apoptosis, leading to the development of diabetes (Choi et al., 2011). In our work, the immunofluorescence data of caspase‐8 corroborated with the PI assay. Pre‐incubation of RINm5F cells with CUR or nCUR prevented STZ‐induced cell death caused by activation of DNA endonuclease, resulting in apoptotic DNA fragmentation (Kruidering and Evan, 2000).
To determine the protective effects of pretreating rats with CUR or nCUR followed by injection of STZ in vivo, we examined changes in blood glucose and cytokine levels and correlated them with changes in pancreatic tissue function. We have measured G‐CSF and GM‐CSF in plasma and pancreatic tissue homogenates. The cell sources for G‐CSF include stroma, endothelium, fibroblasts, monocytes and macrophages, and for GM‐CSF, T lymphocytes, endothelium, fibroblasts, monocytes, macrophages and smooth muscle (Root and Dale, 1999). While the plasma levels of these cytokines were not significantly different between control and STZ groups, there was a significant increase in pancreatic tissue homogenates from STZ‐treated rats. The systemic manifestations of inflammation could be duration‐dependent and such differences were also noticed by other workers (Maier et al., 2008; Kirwin et al., 2009). In pancreatic tissue homogenates, both CUR and nCUR were effective in reducing GM‐CSF, while only nCUR reduced G‐CSF. Pro‐inflammatory cytokines play a critical role in the series of events ultimately leading to tissue destruction in diabetes. Among others, pro‐inflammatory cytokines are produced in macrophages and monocytes that trigger the differentiation of T‐cells (IL‐6) followed by a sequence of events, such as the proliferation of cytotoxic T‐cells (IL‐2), activation of T‐cells and macrophages (IL‐1 and IFN‐γ), infiltration of cells into tissues, local inflammation (TNF‐α) and local tissue destruction (IL‐1) (Wang et al., 2010). Pre‐treatment with CUR or nCUR significantly decreased pro‐inflammatory cytokine production which had been increased by STZ. These findings are in agreement with an increase in histiocytic infiltration in the STZ groups, shown by the immunohistochemical staining for Iba1.
The blood glucose levels at 72 h were significantly lower in nCUR groups probably due to the enhanced oral bioavailability of nCUR at the dose of 50 mg·kg−1. This decrease in glucose was accompanied by a corresponding increase in insulin levels. These findings are in agreement with insulin immunofluorescence, islet numbers and the apoptotic cells determined by TUNEL. TUNEL positive cells in pancreatic islets increased notably in the STZ group and these numbers were markedly decreased in rats pretreated with nCUR.
It is widely recognized that macrophages are involved in the final stage of autoimmune‐mediated beta cell destruction, where activated macrophages can directly kill beta cells (Yang, 2008). Oxidative damage is known to correlate well with beta cell apoptosis and glucose levels in diabetes. Further, oxidative stress in diabetes is responsible for a cascade of events such as, stimulation of the polyol pathway, activation of protein kinase C, formation of advanced glycation end products, and subsequent formation of reactive oxygen radicals. Hyperglycaemia, not only generates more ROS but also attenuates anti‐oxidative mechanisms by scavenging enzymes and substances. We examined oxidative damage to DNA by measuring 8‐oxo‐dG immunofluorescence, where pre‐treatment with nCUR, but not CUR, offered protection,. The results from the sub‐acute toxicity study suggested nCUR at 100 mg·kg−1 did not cause any deleterious general health effects attributable to the carrier. No significant haematological, histological or biochemical changes were recorded.
In conclusion, this study demonstrates that nCUR is safe and prevents STZ‐induced diabetes in rats, at least partly, by the suppression of oxidative stress, inflammation and pancreatic beta cell apoptosis. Further studies on repeated long‐term administration and application to the prevention of Type‐2 diabetes are clearly needed. It would also be interesting to assess the effects of insulin combined with nCUR, on the management of both Type 1 and 2 diabetes.
Author contributions
M.N.V.R.K., R.G., M.A., S.G., A.R.H. and H.G.J participated in research design. R.G., M.A and V.P.V. conducted the experiments. M.N.V.R.K, R.G., M.A., S.G., A.R.H., H.G.J., R.B., P.J. and B.Z. performed data analysis. M.N.V.R.K. and R.G. wrote the manuscript. All authors read, revised and approved the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1 Representative dynamic light scattering particle size distribution of nCUR and nBlank used in the study.
Figure S2 Pre‐treatment with CUR/nCUR 10 or 50 mg/kg orally, prior to STZ challenge. Pancreatic tissue sections were immunostained for insulin.
Figure S3 Histological images of organs after 28 days subacute toxicity study.
Table S1 Randomization of rats for subacute toxicity study.
Table S2 Pre‐treatment with CUR/nCUR (10 or 50 mg/kg) orally prior to STZ challenge does not affect inflammatory cytokines in the plasma. Plasma was analysed 72 h post STZ challenge using multiplex assay. In contrast to the results with pancreas tissue homogenates (c.f. Figure 2), levels in the plasma showed no significant differences between groups.
Table S3 Absolute white cell counts at the end of the study.
Table S4 Erythrocyte numbers at the end of the study.
Table S5 Differential white cell counts at the end of the study.
Table S6 Platelet (PLT) count and mean platelet volume (MPV) at the end of the study.
Table S7 Plasma markers indicative of oxidative stress and inflammation, and antioxidant enzyme levels at the end of the study.
Table S8 Curcumin concentration in plasma at the end of the study, 24 h post last dose.
Acknowledgements
Thanks are due to Dr. Ficht, Health Science Center, for providing access to particle size analyser. Seed grant from College of Pharmacy, Texas A&M University; partial support from The Cunningham Trust, Scotland, UK and The Royal Golden Jubilee PhD Program, Thailand.
Ganugula, R. , Arora, M. , Jaisamut, P. , Wiwattanapatapee, R. , Jørgensen, H. G. , Venkatpurwar, V. P. , Zhou, B. , Rodrigues Hoffmann, A. , Basu, R. , Guo, S. , and Majeti, N. V. R. K. (2017) Nano‐curcumin safely prevents streptozotocin‐induced inflammation and apoptosis in pancreatic beta cells for effective management of Type 1 diabetes mellitus. British Journal of Pharmacology, 174: 2074–2084. doi: 10.1111/bph.13816.
References
- Acharya JD, Ghaskadbi SS (2010). Islets and their antioxidant defense. Islets : 225–235. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015). The concise guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardestani A, Maedler K (2016). MST1: a promising therapeutic target to restore functional beta cell mass in diabetes. Diabetologia 59: 1843–1849. [DOI] [PubMed] [Google Scholar]
- Atkinson MA, Eisenbarth GS, Michels AW (2014). Type 1 diabetes. Lancet 383: 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj V, Hariharan S, Bala I, Lamprecht A, Kumar N, Panchagnula R et al. (2005). Pharmaceutical aspects of polymeric nanoparticles for oral drug delivery. J Biomed Nanotechnol 1: 235–258. [Google Scholar]
- Brooks‐Worrell B, Palmer JP (2013). Prevention versus intervention of type 1 diabetes. Clin Immunol 149: 332–338. [DOI] [PubMed] [Google Scholar]
- Cabrera SM, Wang X, Chen YG, Jia S, Kaldunski ML, Greenbaum CJ et al. (2016). Interleukin‐1 antagonism moderates the inflammatory state associated with type 1 diabetes during clinical trials conducted at disease onset. Eur J Immunol 46: 1030–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington EM, Kos C, Zhan Y, Krishnamurthy B, Allison J (2011). Reducing or increasing beta‐cell apoptosis without inflammation does not affect diabetes initiation in neonatal NOD mice. Eur J Immunol 41: 2238–2247. [DOI] [PubMed] [Google Scholar]
- Castro CN, Barcala Tabarrozzi AE, Winnewisser J, Gimeno ML, Antunica Noguerol M, Liberman AC et al. (2014). Curcumin ameliorates autoimmune diabetes. Evidence in accelerated murine models of type 1 diabetes. Clin Exp Immunol 177: 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D, Schroer SA, Lu SY, Cai EP, Hao Z, Woo M (2011). Redundant role of the cytochrome c‐mediated intrinsic apoptotic pathway in pancreatic beta‐cells. J Endocrinol 210: 285–292. [DOI] [PubMed] [Google Scholar]
- Creusot RJ, Battaglia M, Roncarolo MG, Fathman CG (2016). Concise review: cell‐based therapies and other non‐traditional approaches for type 1 diabetes. Stem Cells 34: 809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SPA, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth GS (1986). Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med 314: 1360–1368. [DOI] [PubMed] [Google Scholar]
- Grama CN, Suryanarayana P, Patil MA, Raghu G, Balakrishna N, Kumar MNVR et al. (2013a). Efficacy of biodegradable curcumin nanoparticles in delaying cataract in diabetic rat model. PLoS One 8: e78217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grama CN, Venkatpurwar VP, Lamprou DA, Kumar MNVR (2013b). Towards scale‐up and regulatory shelf‐stability testing of curcumin encapsulated polyester nanoparticles. Drug Deliv Transl Res 3: 286–293. [DOI] [PubMed] [Google Scholar]
- Hamilton JA (2008). Colony‐stimulating factors in inflammation and autoimmunity. Nat Rev Immunol 8: 533–544. [DOI] [PubMed] [Google Scholar]
- Imai Y, Dobrian AD, Morris MA, Taylor‐Fishwick DA, Nadler JL (2016). Lipids and immunoinflammatory pathways of beta cell destruction. Diabetologia 59: 673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwin SJ, Kanaly ST, Linke NA, Edelman JL (2009). Strain‐dependent increases in retinal inflammatory proteins and photoreceptor FGF‐2 expression in streptozotocin‐induced diabetic rats. Invest Ophthalmol Vis Sci 50: 5396–5404. [DOI] [PubMed] [Google Scholar]
- Kruidering M, Evan GI (2000). Caspase‐8 in apoptosis: the beginning of "the end"? IUBMB Life 50: 85–90. [DOI] [PubMed] [Google Scholar]
- Kumar MNVR (2012). Can efficient delivery systems leverage benefits of antioxidants leading to potential medicines? Drug Discov Today 17: 407–408. [DOI] [PubMed] [Google Scholar]
- Li X, Cheng J, Zhou Z (2016). Revisiting multiple models of progression of beta‐cell loss of function in type 1 diabetes: Significance for prevention and cure. J Diabetes 8: 460–469. [DOI] [PubMed] [Google Scholar]
- Maier R, Weger M, Haller‐Schober EM, El‐Shabrawi Y, Wedrich A, Theisl A et al. (2008). Multiplex bead analysis of vitreous and serum concentrations of inflammatory and proangiogenic factors in diabetic patients. Mol Vis 14: 637–643. [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meah FA, DiMeglio LA, Greenbaum CJ, Blum JS, Sosenko JM, Pugliese A et al. (2016). The relationship between BMI and insulin resistance and progression from single to multiple autoantibody positivity and type 1 diabetes among TrialNet Pathway to Prevention participants. Diabetologia 59: 1186–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop A, Davidson JM (2008). Nanovehicular intracellular delivery systems. J Pharm Sci 97: 3518–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnam DV, Ankola DD, Bhardwaj V, Sahana DK, Kumar MNVR (2006). Role of antioxidants in prophylaxis and therapy: a pharmaceutical perspective. J Control Release 113: 189–207. [DOI] [PubMed] [Google Scholar]
- Ratnam DV, Bhardwaj V, Kumar MNVR (2013). Can controversial nanotechnology promise drug delivery? Chem Rev 113: 1686–1735. [DOI] [PubMed] [Google Scholar]
- Ratnam DV, Wadsworth RM, Kumar MNVR (2011). Protective effects of nanoparticulate coenzyme Q10 and curcumin on inflammatory markers and lipid metabolism in streptozotocin‐induced diabetic rats: a possible remedy to diabetic complications. Drug Deliv Transl Res 1: 448–455. [DOI] [PubMed] [Google Scholar]
- Reimann M, Bonifacio E, Solimena M, Schwarz PE, Ludwig B, Hanefeld M et al. (2009). An update on preventive and regenerative therapies in diabetes mellitus. Pharmacol Ther 121: 317–331. [DOI] [PubMed] [Google Scholar]
- Root RK, Dale DC (1999). Granulocyte colony‐stimulating factor and granulocyte‐macrophage colony‐stimulating factor: comparisons and potential for use in the treatment of infections in nonneutropenic patients. J Infect Dis 179 (Suppl 2): S342–S352. [DOI] [PubMed] [Google Scholar]
- Roy MS, Janal MN, Crosby J, Donnelly R (2016). Plasma markers of inflammation and prediction of cardiovascular disease and mortality in African Americans with type 1 diabetes. Diabetes Res Clin Pract 114: 117–125. [DOI] [PubMed] [Google Scholar]
- Santin I, Eizirik DL (2013). Candidate genes for type 1 diabetes modulate pancreatic islet inflammation and beta‐cell apoptosis. Diabetes Obes Metab 15 (Suppl 3): 71–81. [DOI] [PubMed] [Google Scholar]
- Shaikh J, Ankola DD, Beniwal V, Singh D, Kumar MNVR (2009). Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9‐fold when compared to curcumin administered with piperine as absorption enhancer. Eur J Pharm Sci 37: 223–230. [DOI] [PubMed] [Google Scholar]
- Skelin M, Rupnik M, Cencic A (2010). Pancreatic beta cell lines and their applications in diabetes mellitus research. ALTEX 27: 105–113. [DOI] [PubMed] [Google Scholar]
- Skyler JS (2013). Primary and secondary prevention of type 1 diabetes. Diabet Med 30: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skyler JS (2015). Prevention and reversal of type 1 diabetes – past challenges and future opportunities. Diabetes Care 38: 997–1007. [DOI] [PubMed] [Google Scholar]
- Skyler JS, Pugliese A (2013). Immunotherapy trials for type 1 diabetes: the contribution of George Eisenbarth. Diabetes Technol Ther 15 (Suppl 2) S2‐13‐S12‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srimal RC, Dhawan BN (1973). Pharmacology of diferuloyl methane (curcumin), a non‐steroidal anti‐inflammatory agent. J Pharm Pharmacol 25: 447–452. [DOI] [PubMed] [Google Scholar]
- Wang C, Guan Y, Yang J (2010). Cytokines in the Progression of Pancreatic beta‐Cell Dysfunction. Int J Endocrinol 2010: 515136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherrett DK (2014). Trials in the prevention of type 1 diabetes: current and future. Can J Diabetes 38: 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LJ (2008). Big mac attack: does it play a direct role for monocytes/macrophages in type 1 diabetes? Diabetes 57: 2922–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Representative dynamic light scattering particle size distribution of nCUR and nBlank used in the study.
Figure S2 Pre‐treatment with CUR/nCUR 10 or 50 mg/kg orally, prior to STZ challenge. Pancreatic tissue sections were immunostained for insulin.
Figure S3 Histological images of organs after 28 days subacute toxicity study.
Table S1 Randomization of rats for subacute toxicity study.
Table S2 Pre‐treatment with CUR/nCUR (10 or 50 mg/kg) orally prior to STZ challenge does not affect inflammatory cytokines in the plasma. Plasma was analysed 72 h post STZ challenge using multiplex assay. In contrast to the results with pancreas tissue homogenates (c.f. Figure 2), levels in the plasma showed no significant differences between groups.
Table S3 Absolute white cell counts at the end of the study.
Table S4 Erythrocyte numbers at the end of the study.
Table S5 Differential white cell counts at the end of the study.
Table S6 Platelet (PLT) count and mean platelet volume (MPV) at the end of the study.
Table S7 Plasma markers indicative of oxidative stress and inflammation, and antioxidant enzyme levels at the end of the study.
Table S8 Curcumin concentration in plasma at the end of the study, 24 h post last dose.


