Abstract
Background and Purpose
Fatty acid amide hydrolase (FAAH) inhibitors are postulated to possess anti‐hypertensive potential, because their acute injection decreases BP in spontaneously hypertensive rats (SHR), partly through normalization of cardiac contractile function. Here, we examined whether the potential hypotensive effect of chronic FAAH inhibition by URB597 in hypertensive rats correlated with changes in cardiac performance.
Experimental Approach
Experiments were performed using perfused hearts and left atria isolated from 8‐ to 10–week‐old SHR, age‐matched deoxycorticosterone acetate (DOCA)‐salt rats and normotensive controls chronically treated with URB597 (1 mg·kg−1) or vehicle.
Key Results
URB597 decreased BP only in the DOCA‐salt rats, along with a reduction of ventricular hypertrophy and diastolic stiffness, determined in hypertension. We also observed normalization of the negative inotropic atrial response to the cannabinoid receptor agonist CP55940. In the SHR model, URB597 normalized (atria) and enhanced (hearts) the positive ino‐ and chronotropic effects of the β‐adrenoceptor agonist isoprenaline respectively. Ventricular CB1 and CB2 receptor expression was decreased only in the DOCA‐salt model, whereas FAAH expression was reduced in both models. URB597 caused translocation of CB1 receptor immunoreactivity to the intercalated discs in the hearts of SHR. URB597 increased cardiac diastolic stiffness and modified the ino‐ and lusitropic effects of isoprenaline in normotensive rats.
Conclusion and Implications
Hypotensive effect of chronic FAAH inhibition depend on the model of hypertension and partly correlate with improved cardiac performance. In normotensive rats, chronic FAAH inhibition produced several side‐effects. Thus, the therapeutic potential of these agents should be interpreted cautiously.
Abbreviations
- AEA
anandamide
- AM3506
5‐(4‐hydroxyphenyl)pentanesulfonyl fluoride
- CP55940
2‐[(1R,2R,5R)‐5‐hydroxy‐2‐(3‐hydroxypropyl)cyclohexyl]‐5‐(2‐methyloctan‐2‐yl)phenol
- CPP
coronary perfusion pressure
- DOCA
deoxycorticosterone acetate
- FAAH
fatty acid amide hydrolase
- HR
heart rate
- LVP
left ventricular pressure
- RPP
rate‐pressure product
- SBP
systolic BP
- SHR
spontaneously hypertensive rats
- URB694
6‐hydroxy‐[1,1′‐biphenyl]‐3‐yl‐cyclohexyl‐[11C–carbonyl]carbamate
- URB597
3‐(3‐carbamoylphenyl)phenyl N‐cyclohexylcarbamate
- WKY
Wistar–Kyoto rats
Introduction
Hypertensive heart disease, which includes ventricular hypertrophy, contractile dysfunction and their clinical manifestations (arrhythmias and heart failure), is a leading cause of death associated with high BP (Drazner, 2011). The endocannabinoid system is suggested to buffer increases in BP in hypertension. In support of this, the plasma level of the best known endocannabinoid, anandamide (AEA), was higher in hypertensive patients (Engeli et al., 2012) and in spontaneously hypertensive rats (SHR; Li et al., 2009). Moreover, in acute experiments, (1) the CB1 receptor‐mediated hypotension elicited by AEA was stronger in anaesthetized SHR than in normotensive controls (Lake et al., 1997; Bátkai et al., 2004); (2) in conscious SHR, AEA decreased BP, but increased it in normotensive controls (Lake et al., 1997); and (3) the systemic acute blockade of fatty acid amide hydrolase (FAAH; the key enzyme responsible for AEA degradation) by URB597 (Bátkai et al., 2004) and AM3506 (Godlewski et al., 2010) has been shown to normalize BP in SHR. Consequently, FAAH inhibitors are postulated to be potential antihypertensive agents (Bátkai et al., 2004; Godlewski et al., 2010). The chronic administration of URB597 to deoxycorticosterone acetate (DOCA)‐salt hypertensive rats reduced BP in older rats (in a manner partly dependent on vascular changes) and diminished cardiac hypertrophy in younger animals (Baranowska‐Kuczko et al., 2016; Toczek et al., 2016).
Cannabinoids have a negative inotropic effect in human (Bonz et al., 2003) and rat atrial muscle (Sterin‐Borda et al., 2005) via CB1 receptors and a positive inotropic effect via CB2 receptors (Sterin‐Borda et al., 2005). Coronary vasodilation is mediated by CB1 (Wagner et al., 2005) or via non‐CB1/CB2 receptors (Ford et al., 2002). The reduction of increased cardiac contractility in hypertension is suggested to be one of the major components of the antihypertensive action of FAAH inhibitors after their acute injection (Bátkai et al., 2004; Godlewski et al., 2010). Chronic treatment with the FAAH inhibitor URB694 normalized resting heart rate (HR) and prevented the occurrence of arrhythmia in stressed animals (Carnevali et al., 2015a). In FAAH knockout mice, age‐related cardiac dysfunction (Bátkai et al., 2007) was decreased, but doxorubicin‐induced cardiotoxicity was enhanced (Mukhopadhyay et al., 2011). Thus, the first aim of our study was to examine whether the potential hypotensive effect of chronic FAAH inhibition by URB597 in hypertensive rats correlated with changes in cardiac performance.
In hypertension, an elevated sympathetic tone alters β‐adrenoceptor‐induced effects (Guyenet, 2006), which might additionally be modified by cannabinoids. There is evidence that cannabinoid agonists HU210 (Maslov et al., 2004), AEA (Gaskari et al., 2005) and WIN55212‐2 (Liao et al., 2013) can diminish the following responses to the non‐selective β‐adrenoceptor agonist isoprenaline: its positive ino‐ and/or chronotropic effects and/or stimulation of cAMP production in isolated hearts, ventricular papillary muscle and neonatal cardiomyocytes respectively. Moreover, acute administration of URB694 reduced the occurrence of isoprenaline‐induced ventricular tachyarrhythmias (Carnevali et al., 2015b). Therefore, the second aim of our study was to examine the influence of chronic URB597 administration on β‐adrenoceptor‐mediated effects in cardiac tissues isolated from normotensive and hypertensive rats.
Methods
Animals
All animal care, surgical procedures and experimental protocols were performed in accordance with the European Directive (2010/63/EU) and Polish legislation and were approved by the local Animal Ethics Committee in Białystok (Poland). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). The study was carried out in compliance with the replacement, refinement or reduction (the 3Rs). Male rats were obtained from the Centre for Experimental Medicine of the Medical University of Białystok (Poland). They were housed in collective plastic cages (two rats per cage) with sawdust on the bottom in a temperature‐controlled room at 22 ± 1°C under a 12:12 h light–dark cycle with free access to water and standard laboratory food unless otherwise noted. We used age‐matched rats with comparable primary (SHR; the most frequently studied genetic hypertensive model) and secondary (DOCA‐salt) hypertension in order to distinguish changes induced by hypertension from those related to any one particular hypertension model. We used the DOCA‐salt model because a salt‐rich diet is one of the main lifestyle factors leading to hypertension.
DOCA‐salt hypertension was induced in Wistar rats (6–7 weeks old; initially weighing 260–300 g) as described previously (Toczek et al., 2016). Animals were anaesthetized by i.p. injection of pentobarbital sodium (70 mg·kg−1, i.e. ~300 μmol·kg−1). The right kidney was removed in all rats via a right lateral abdominal incision. After 1 week of recovery, hypertension was induced by subcutaneous injections of 11‐DOCA (25 mg·kg−1, i.e. ~67 μmol·kg−1; 0.4 mL·kg−1) twice weekly for 6 weeks and replacement of drinking water with 1% NaCl solution. Control sham‐operated rats (SHAM) received the vehicle for DOCA (N,N‐dimethylformamide) twice weekly and drank tap water.
Chronic treatment with URB597
We applied the same protocol and dose of URB597 as reported previously (Toczek et al., 2016) and age‐matched DOCA‐salt hypertensive rats, in which 2 weeks of URB597 administration was previously reported to decrease BP (Toczek et al., 2016). URB597 (1 mg·kg−1, i.e. ~3 μmol·kg−1; 1 mL·kg−1) or its vehicle – a mixture of DMSO, Tween 80 and saline (1:2:7) – was injected i.p. for 14 days, twice daily for the following four groups of rats: hypertensive (1) DOCA‐salt (4 weeks after the onset of DOCA‐salt administration); (2) 8‐ to 10–week‐old male SHR (270–320 g) and their respective normotensive controls: (3) age‐matched SHAM (4 weeks after the onset of vehicle for DOCA‐salt administration); and (4) Wistar–Kyoto rats (WKY; 290–390 g). Rats were assigned randomly to the different experimental groups. Before the first dose of URB597 or its vehicle and 12 h after the final dose, systolic BP (SBP) was recorded in conscious rats by the non‐invasive tail‐cuff method using the Rat Tail Blood Pressure Monitor from Hugo Sachs Elektronik‐Harvard Apparatus (March–Hugstetten, Germany).
Isolated Langendorff heart preparation
Twelve hours after the final dose of URB597 (or its vehicle), rats were anaesthetized with 300 μmol·kg−1 pentobarbital sodium i.p. and then injected with 500 IU heparin i.p. (Polfa, Warsaw, Poland). Hearts were rapidly excised, weighed and immediately mounted in a Langendorff system (Hugo Sachs Elektronik‐Harvard Apparatus GmbH, March‐Hugstetten, Germany). Hearts were perfused at a constant flow rate (12 mL·min−1) with oxygenated (95% O2 and 5% CO2) Krebs–Henseleit solution of the following composition (mM): NaCl 118.0, KCl 4.8, CaCl2 1.8, NaHCO3 24.0, KH2PO4 1.2, MgSO4 1.2, glucose 11.0, Na‐pyruvate 5.0, EDTA 0.03 (pH 7.4; 37°C). A distilled water filled latex balloon connected to a pressure transducer and inflated to increase the left ventricular end‐diastolic pressure to approximately 8–10 mmHg was inserted into the left ventricle through the mitral valve to measure the left ventricular pressure (LVP). Another pressure transducer located just above the aorta recorded coronary perfusion pressure (CPP). Hearts were allowed to beat spontaneously. After a 5 min stabilization period, increments in balloon volume (using a 50 mL Hamilton syringe connected to the fluid‐filled pressure transduction circuit) were applied to the heart at 0, 5, 10, 15, 20 and 30 mmHg, and left ventricular end‐diastolic pressure was recorded. Myocardial diastolic stiffness was calculated as the diastolic stiffness constant (κ, dimensionless), the slope of the linear relation between tangent elastic modulus (E, dyne·cm−2) and stress (σ, dyne/cm2) as previously described (Loch et al., 2007).
Heart parameters were continuously monitored using the ISOHEARTR software. Preparations were allowed to stabilize for 25 min. Two hearts (one from WKY URB597 and one from SHAM URB597 group) with persistent arrhythmias and poor contractility (LVP < 75 mmHg) were excluded from the study. After the stabilization period, increasing concentrations of isoprenaline (0.01 nM – 1 μM) were infused by a peristaltic pump (Ascor, Warsaw, Poland) at one hundredth of the current flow rate into the coronary arteries until a plateau was reached; concentrations referred to the final concentrations in the heart. Measurements recorded after the initial equilibration period were considered as the baseline values. The cardiac performance of the Langendorff‐perfused heart was evaluated from HR, LVP (which is an index of contractile activity) and the rate‐pressure product (RPP: HR × LVP, mmHg × beats·min−1), which is an index of cardiac work (Angelone et al., 2008), the maximum rate of positive +(LVdP/dt)max (inotropism) or negative −(LVdP/dt)max (lusitropism) changes in LVP and CPP as an index of coronary dilation. All parameters are expressed as Δ changes from baseline before addition of the first concentration of isoprenaline. After experiments, the left atrium and the right and left ventricle with septum were weighed, and their weights were expressed as a ratio of tissue weight (mg) to total body weight (g).
Isolated left atria
The left atria were dissected before the isolated heart perfusions (see above). They were weighed and mounted in 10 mL organ baths containing Krebs solution of the following composition (mM): NaCl 118, KCl 4.8, MgSO4 1, NaHCO3 29, NaHPO4x12H20 1, CaCl2 2.25, glucose 10, Na‐pyruvate 5, EDTA 0.04 (pH 7.4; 37°C). Atria were continuously stimulated electrically with square wave pulses (just over threshold, 5 ms duration, 2 Hz) using a pair of platinum electrodes. Contractions were recorded using an isometric force transducer (PIM 100RE, Bio‐Sys‐Tech, Białystok, Poland). Tissue was allowed to equilibrate for 60 min. When a stable amplitude of contractions had been reached, the cannabinoid receptor agonist CP55940 (1 nM – 30 μM) was cumulatively added to the atria. Atria were then washed several times and sensitized by a brief exposure to 5 × 10−6 M isoprenaline (Dincer et al., 2000). When steady basal values were obtained again, concentration–response curves for isoprenaline (0.01 nM – 3 μM) were constructed. The initial contractile forces (in mN) of atria in normotensive SHAM and WKY measured before the first concentration of CP55940 were as follows: 2.4 ± 0.1 (n = 11) and 2.5 ± 0.1 (n = 10), respectively, and before the first concentration of isoprenaline were the same 2.4 ± 0.1 for SHAM (n = 10) and WKY (n = 9). Hypertension and URB597 did not affect the basal values of this parameter (data not shown). Agonist responses were measured as the decrease or increase in the basal force (expressed as % of basal values) of the left atrium.
Western blots
Routine Western blotting procedure was used to examine protein expression, as has been described previously (Baranowska‐Kuczko et al., 2016). Briefly, samples from the left ventricles were homogenized in RIPA buffer containing a cocktail of protease and phosphatase inhibitors (Roche Diagnostics GmbH, Mannheim, Germany). In addition, protein concentration was measured using the bicinchoninic acid method with BSA as a standard. Following this, homogenates were reconstituted in Laemmli buffer, separated by 10% SDS‐PAGE and transferred onto nitrocellulose membranes. The membranes were incubated overnight at 4°C with the corresponding primary antibodies in appropriate dilutions: CB1 (1:200, cat no. Ab23703; Abcam, UK), CB2 (1:500, cat no. Ab3561; Abcam, UK), FAAH1 (1:500; cat no. Ab54615; Abcam, UK) and GAPDH (1:500, cat no. sc‐32 233; Santa Cruz Biotechnology, USA). Thereafter, nitrocellulose membranes were incubated with the appropriate secondary antibody conjugated to horseradish peroxidase (Santa Cruz Biotechnology, USA). After adding a suitable substrate for horseradish peroxidase (Thermo Scientific, Rockford, IL, USA), protein bands were quantified densitometrically using a ChemiDoc visualization system EQ (Bio‐Rad, Warsaw, Poland). Equal protein loading in each line was confirmed by Ponceau S staining. The level of protein expression was standardized to GAPDH.
Immunohistochemistry
For immunohistochemistry, the EnVision method was used (Baranowska‐Kuczko et al., 2016) using antibodies against: CB1 (1:2000 rabbit; Anti‐CB1 cat no. Ab23703; Abcam, UK) and CB2 (1:200 rabbit Anti‐CB2 cat no. Ab3561; Abcam, UK). To test the specificity of the antibodies, a negative control was included, in which the antibodies were replaced by normal rabbit serum (Vector Laboratories, CA, USA) at the respective dilution (no staining), and a positive control, which was prepared from rat hippocampus for CB1 and human tonsil for CB2 (data not shown). The obtained results of immunohistochemical staining were viewed on an Olympus BX41 microscope with an Olympus DP12 camera under a magnification of 200× (20× lens and 10× eyepiece).
Data and statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). The results are given as the mean ± SEM (n = number of animals). The exact group size (n) for each experimental group/condition is provided in Tables 1, 2, 3, 4 and ‘n’ refers to independent values, not replicates. Researchers were not blinded to the experimental conditions, but efforts were made to be close to the conditions of blinded assays, and our analysis did not include any subjective evaluation. Thus, (1) all the cardiac tissues were obtained via the same procedures; (2) they were treated in the same way; and (3) all data were obtained via direct recording of physiological parameters.
Table 1.
Influence of URB597 on physiological parameters of DOCA‐salt and SHR and their respective normotensive SHAM and WKY
| SHAM | SHAM URB597 | DOCA‐salt | DOCA‐salt URB597 | WKY | WKY URB597 | SHR | SHR URB597 | ||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | n | 10 | 10 | 10 | 11 | 10 | 9 | 10 | 10 |
| SBP (mmHg) | |||||||||
| day 0 | 131 ± 7 | 131 ± 8 | 217 ± 16* | 201 ± 13 | 104 ± 8 | 105 ± 6 | 183 ± 7* | 199 ± 12 | |
| day 14 | 137 ± 5 | 118 ± 10 | 217 ± 17* | 159 ± 10†, # | 105 ± 6 | 92 ± 4 | 196 ± 10* | 182 ± 7 | |
| HR (beats·min−1) | |||||||||
| day 0 | 362 ± 8 | 363 ± 10 | 360 ± 17 | 374 ± 11 | 343 ± 23 | 333 ± 9 | 383 ± 12 | 376 ± 10 | |
| day 14 | 357 ± 6 | 350 ± 14 | 370 ± 15 | 355 ± 11 | 280 ± 8# | 317 ± 12† | 357 ± 8* | 349 ± 7# | |
| Body weight (g) | |||||||||
| day 0 | 302 ± 7 | 291 ± 10 | 264 ± 2* | 267 ± 4 | 333 ± 10 | 340 ± 11 | 288 ± 6* | 295 ± 6 | |
| day 14 | 310 ± 8 | 304 ± 10 | 271 ± 9* | 277 ± 6 | 334 ± 10 | 343 ± 10 | 287 ± 5* | 291 ± 5 | |
| Heart weight/body weight (mg·g−1) | 4.0 ± 0.2 | 4.7 ± 0.3 | 5.9 ± 0.3* | 5.5 ± 0.2 | 4.7 ± 0.2 | 4.8 ± 0.2 | 5.6 ± 0.1* | 5.8 ± 0.1 | |
| LV + septum weight/body weight (mg·g−1) | 2.26 ± 0.07 | 2.32 ± 0.11 | 3.60 ± 0.14* | 3.15 ± 0.14† | 2.53 ± 0.06 | 2.47 ± 0.04 | 3.33 ± 0.04* | 3.54 ± 0.10 | |
| RV weight/body weight (mg·g−1) | 0.61 ± 0.05 | 0.66 ± 0.04 | 0.87 ± 0.04* | 0.74 ± 0.05 | 0.71 ± 0.03 | 0.67 ± 0.02 | 0.76 ± 0.02 | 0.78 ± 0.02 | |
| LA weight/body weight (mg·g−1) | 0.073 ± 0.010 | 0.082 ± 0.004 | 0.155 ± 0.014* | 0.129 ± 0.009 | 0.091 ± 0.005 | 0.088 ± 0.002 | 0.079 ± 0.002 | 0.076 ± 0.004 | |
| Diastolic stiffness constant (κ) | 22.9 ± 0.7 | 26.3 ± 0.8† | 32.4 ± 1.0* | 26.2 ± 0.9† | 21.6 ± 1.5 | 35.1 ± 1.1† | 30.8 ± 0.9* | 29.6 ± 2.8 |
Units are given in brackets. URB597 (1 mg·kg−1) or its vehicle was injected i.p. every 12 h for 14 days. SBP and HR were recorded before (day 0) the first dose of URB597 or its vehicle and 14 days later. Heart and body weights were determined 12 h after the last dose of URB597 or its vehicle. Diastolic stiffness was measured at the beginning of Langendorff experiment. Weights of right and left ventricle (RV and LV), septum and left atrium (LA) were determined after the end of in vitro experiments. Data are given as the means ± SEM.
P < 0.05 DOCA‐salt and SHR versus SHAM and WKY respectively;
P < 0.05, significant effect of URB597; ANOVA with Bonferroni post hoc test
P < 0.05, significantly different from values before URB597 treatment (day 0 vs. day 14); Student's t‐test for paired data.
Table 2.
Influence of URB597 on basal parameters of hearts isolated from DOCA‐salt and SHR and their respective normotensive SHAM and WKY
| SHAM | SHAM URB597 | DOCA‐salt | DOCA‐salt URB597 | WKY | WKY URB597 | SHR | SHR URB597 | ||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | n | 10 | 10 | 10 | 11 | 10 | 9 | 10 | 10 |
| HR (beats·min−1) | 273 ± 7 | 293 ± 9 | 273 ± 9 | 269 ± 8 | 274 ± 14 | 254 ± 7 | 282 ± 10 | 260 ± 8 | |
| LVP (mmHg) | 120 ± 8 | 131 ± 10 | 132 ± 10 | 131 ± 9 | 90 ± 5 | 90 ± 3 | 134 ± 10* | 137 ± 8 | |
| +(LVdP/dt)max (mmHg·s−1) | 3353 ± 217 | 3472 ± 274 | 3482 ± 240 | 3492 ± 268 | 2431 ± 222 | 2161 ± 631 | 3610 ± 204* | 3307 ± 122 | |
| −(LVdP/dt)max (mmHg·s−1) | −2422 ± 174 | −2846 ± 250 | −2560 ± 240 | −2798 ± 222 | −1918 ± 125 | −1924 ± 58 | −2898 ± 234* | −3031 ± 165 | |
| RPP (mmHg·beats·min−1) | 30 210 ± 2309 | 36 106 ± 3190 | 33 676 ± 2488 | 33 698 ± 2527 | 21 773 ± 1143 | 20 162 ± 620 | 34 243 ± 1587* | 32 286 ± 1666 | |
| CPP (mmHg) | 94 ± 8 | 92 ± 9 | 86 ± 8 | 85 ± 5 | 64 ± 4 | 66 ± 3 | 75 ± 3 | 76 ± 3 |
Units are given in brackets. URB597 (1 mg·kg−1) or its vehicle was injected i.p. every 12 h for 14 days. Hearts were isolated 12 h after the final dose of URB597 or its vehicle. Data are given as the means ± SEM.
P < 0.05, SHR significantly different from WKY; ANOVA with Bonferroni post hoc test; LVP, the maximum rate of positive +(LVdP/dt)max and negative −(LVdP/dt)max changes in LVP.
Table 3.
Influence of URB597 on the isoprenaline (0.01 nM – 1 μM)‐induced changes in parameters of hearts isolated from DOCA‐salt and SHR and their respective normotensive SHAM and WKY
| SHAM | SHAM URB597 | DOCA‐salt | DOCA‐salt URB597 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | 10 | 10 | 10 | 11 | |||||
| Parameters | pEC50 | Emax | pEC50 | Emax | pEC50 | Emax | pEC50 | Emax | |
| HR (beats·min−1) | 8.9 ± 0.2 | 107 ± 6 | 8.3 ± 0.2 | 98 ± 10 | 8.5 ± 0.2 | 89 ± 12 | 8.8 ± 0.3 | 94 ± 12 | |
| LVP (mmHg) | 9.8 ± 0.7 | 55 ± 7 | 8.8 ± 0.4 | 30 ± 6* | 8.7 ± 0.5 | 29 ± 6† | 8.8 ± 0.3 | 39 ± 5 | |
| +(LVdP/dt)max (mmHg·s−1) | 8.7 ± 0.4 | 4925 ± 522 | 8.5 ± 0.2 | 4124 ± 420 | 8.4 ± 0.2 | 2885 ± 397† | 8.6 ± 0.2 | 3933 ± 512 | |
| −(LVdP/dt)max (mmHg·s−1) | 9.3 ± 0.4 | −2697 ± 303 | 8.8 ± 0.3 | −2543 ± 335 | 8.5 ± 0.3 | −2148 ± 325 | 8.7 ± 0.3 | −2225 ± 350 | |
| RPP (mmHg·beats·min−1) | 9.5 ± 0.5 | 30 487 ± 3894 | 8.7 ± 0.3 | 22 267 ± 2928 | 8.7 ± 0.4 | 20 097 ± 4442 | 8.7 ± 0.3 | 24 931 ± 4466 | |
| CPP (mmHg) | 9.4 ± 0.6 | −28 ± 6 | 9.3 ± 0.3 | −32 ± 6 | – | – | – | – | |
| WKY | WKY URB597 | SHR | SHR URB597 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | 10 | 9 | 10 | 10 | |||||
| pEC50 | Emax | pEC50 | Emax | pEC50 | Emax | pEC50 | Emax | ||
| HR (beats·min−1) | 8.5 ± 0.3 | 95 ± 7 | 9.0 ± 0.1 | 105 ± 7 | 8.4 ± 0.3 | 83 ± 11 | 8.8 ± 0.2 | 116 ± 11* | |
| LVP (mmHg) | 9.7 ± 0.3 | 81 ± 9 | 8.7 ± 0.1 | 85 ± 8 | 9.0 ± 0.6 | 32 ± 10† | 8.6 ± 0.4 | 40 ± 8 | |
| +(LVdP/dt)max (mmHg·s−1) | 9.4 ± 0.3 | 5526 ± 684 | 8.4 ± 0.2* | 5279 ± 615 | 8.4 ± 0.2† | 4413 ± 585 | 8.3 ± 0.2 | 4410 ± 454 | |
| −(LVdP/dt)max (mmHg·s−1) | 9.2 ± 0.4 | −2970 ± 290 | 8.6 ± 0.2 | −3166 ± 451 | 8.3 ± 0.2 | −1847 ± 177† | 8.2 ± 0.2 | −1878 ± 222 | |
| RPP (mmHg·beats·min−1) | 9.5 ± 0.3 | 29 409 ± 3123 | 8.6 ± 0.1* | 37 038 ± 1066 | 8.7 ± 0.2† | 22 539 ± 2833 | 8.7 ± 0.2 | 24 680 ± 680 | |
| CPP (mmHg) | 9.7 ± 0.5 | −26 ± 4 | 9.1 ± 0.2 | −28 ± 3 | – | – | – | – | |
Values are based on the concentration–response curves shown in Figures 1 and 2. Maximal effects (Emax) determined for isoprenaline (0.01 nM – 0.1 μM) represent maximum changes from baseline. Units for Emax are given in brackets. Data are given as the means ± SEM.
P < 0.05, significant effect of URB597.
P < 0.05 DOCA‐salt and SHR significantly different from SHAM and WKY respectively; ANOVA with Bonferroni post hoc test; LVP, the maximum rate of positive +(LVdP/dt)max and negative −(LVdP/dt)max changes in LVP.
Table 4.
Influence of URB597 on the isoprenaline (0.01 nM – 3 μM)‐induced positive inotropic and the CP55940 (1 nM – 30 μM)‐induced negative inotropic effects in left atria isolated from DOCA‐salt and SHR and their respective normotensive SHAM and WKY
| Group | SHAM | SHAM URB597 | DOCA‐salt | DOCA‐salt URB597 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | n | n | n | |||||||||
| pEC50 | Emax | pEC50 | Emax | pEC50 | Emax | pEC50 | Emax | |||||
| Isoprenaline | 8.1 ± 0.2 | 74 ± 9 | 9 | 7.9 ± 0.2 | 81 ± 12 | 9 | 8.3 ± 0.2 | 65 ± 13 | 10 | 8.2 ± 0.2 | 81 ± 15 | 11 |
| CP55940 | 6.1 ± 0.3 | −38 ± 4 | 10 | 6.8 ± 0.4 | −36 ± 4 | 10 | 5.0 ± 0.2# | −25 ± 2 | 8 | 6.1 ± 0.2† | −48 ± 6† | 10 |
| WKY | n | WKY URB597 | n | SHR | n | SHR URB597 | n | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pEC50 | Emax | pEC50 | Emax | pEC50 | Emax | pEC50 | Emax | |||||
| Isoprenaline | 8.3 ± 0.1 | 156 ± 12 | 9 | 8.3 ± 0.1 | 230 ± 15† | 10 | 8.3 ± 0.1 | 67 ± 6# | 10 | 8.6 ± 0.1 | 125 ± 11† | 8 |
| CP55940a | 7.3 ± 0.2 | −54 ± 4 | 9 | 7.3 ± 0.1 | −48 ± 4 | 10 | 6.6 ± 0.2 | −34 ± 6# | 9 | 7.3 ± 0.2† | −36 ± 5 | 10 |
Values are based on the concentration–response curves shown in Figure 3. Maximal effects (Emax) are expressed in % of basal values.
In the case of CP55940, Emax was determined for the concentration of 30 μM. Data are given as the means ± SEM.
P < 0.05, significant effect of URB597;
P < 0.05 DOCA‐salt and SHR significantly different from SHAM and WKY respectively. ANOVA with Bonferroni post hoc test.
Maximal effects of agonists (Emax) and their potencies (as pEC50 values) were determined from the individual concentration–response curves. Values of Emax for isolated hearts and atria represent respective maximal changes from baseline and % of basal values, as their basal values in control normotensive SHAM and WKY were different in hearts (Table 2) and comparable in atria (see above). Due to dual cardiac effect of isoprenaline and the lack of the pronounced Emax for CP55940 in atria, Emax were determined for the following agonist concentrations: isoprenaline in the heart: 0.01 nM – 0.1 μM; and CP55940 in atria – 30 μM. Statistical analysis was performed using Graph Pad Prism 5 (GraphPad Software, La Jolla, CA, USA). Intergroup statistical comparisons were made by ANOVA followed by Bonferroni's multiple comparison test of the entire data set. Post hoc tests were only performed where the F‐ratio of the ANOVA highlighted a significant difference (P < 0.05). The Student's t‐test for paired and unpaired data was used as appropriate. Differences were considered significant at P < 0.05.
Materials
CP55940 (Tocris, Bristol, UK); heparin sodium (Polfa, Warsaw, Poland); 11‐DOCA, N,N‐dimethylformamide, (−)‐isoprenaline (±)‐bitartrate salt, Tween 80 (Sigma‐Aldrich, Munich, Germany); URB597; Cayman Chemical Company, Ann Arbor, MI, USA); pentobarbital sodium (Biowet, Puławy, Poland). Stock solutions of isoprenaline were prepared in distilled water and further diluted with Krebs solution. CP55940 was dissolved in DMSO; further dilutions were made with Krebs solution such that the final concentration of the DMSO in the organ bath was less than 0.01%.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015a,b).
Results
General
Before application of the first dose of URB597, or its vehicle, SBP was about 70% higher in both hypertensive models. compared with normotensive controls (Table 1). URB597 reduced SBP in DOCA‐salt by about 20% but did not change SBP in SHR. HR or body weight were also unchanged in DOCA‐salt and SHR. Basal HR was reduced (by about 10%) in WKY and SHR both in the absence and the presence of URB597, 2 weeks after the experiment began.
Heart weight to body weight ratios were higher by about 50 and 20% in DOCA‐salt and SHR compared with SHAM and WKY respectively (Table 1). The cardiac hypertrophy in DOCA‐salt was connected with an increase in hypertrophy indices in all cardiac compartments. In contrast, in SHR, cardiac hypertrophy was associated with an increase in the ratio of left ventricular and septum weight to body weight only. URB597 reduced the elevated index of left ventricular and septum weight to body weight observed in DOCA‐salt.
Diastolic stiffness was increased by about 40% in hearts of both hypertensive models (Table 1). URB597 diminished the enhanced diastolic stiffness observed in hypertension by about 20% in DOCA‐salt, but not in SHR. Surprisingly, in normotensive animals, URB597 increased diastolic stiffness by about 15 and 50% in SHAM and WKY respectively.
Influence of hypertension and URB597 on basal parameters of isolated hearts
Values of all basal parameters of hearts isolated from DOCA‐salt and SHAM were comparable (Table 2). In SHR, indices of cardiac contractility (LVP), work (RPP), maximal rate of LV contraction and relaxation were higher by about 50% than in WKY. These values were comparable with the respective values in DOCA‐salt. URB597 did not modify any basal parameters of isolated hearts in normo‐ or hypertension.
Effects of hypertension and chronic URB597 administration on isoprenaline‐induced cardiostimulatory effects in isolated hearts
Isoprenaline (0.01 nM – 1 μM) caused concentration‐dependent increases of all cardiac parameters, which were comparable in hearts isolated from normotensive rats of both groups (for the respective pEC50 and Emax values determined for isoprenaline 0.01 nM – 0.1 μM, see Table 3; Figures 1 and 2). One exception was a lower increase in LVP (for Emax P < 0.05; but not its pEC50 value) in SHAM compared with WKY. Hypertension attenuated all cardiostimulatory effects of isoprenaline (mainly at the lower concentrations; Table 3; Figures 1 and 2). Maximal responses were reduced by about 50–60% for LVP (DOCA‐salt and SHR) and by about 40% for +(LVdP/dt)max (DOCA‐salt) and for −(LVdP/dt)max (SHR). Vasodilatory effects of isoprenaline on coronary arteries were completely prevented by hypertension. A weaker potency of isoprenaline was only observed for two parameters in SHR: +(LVdP/dt)max and RPP.
Figure 1.

Influence of URB597 on the isoprenaline‐induced changes in LVP (A, A′), the maximum rate of positive +(LVdP/dt)max (B, B′) and negative −(LVdP/dt)max (C, C′) changes in LVP (ΔLVP) of hearts isolated from DOCA‐salt and SHR and their respective normotensive SHAM and WKY. URB597 (1 mg·kg−1) or its vehicle was injected i.p. every 12 h for 14 days. Values shown are changes from baseline (Table 2). Data are given as the means ± SEM of 9–11 rats. *P < 0.05, significant effect of URB597, in normotensive rats only: # P < 0.05, DOCA‐salt and SHR significantly different from SHAM and WKY respectively; ANOVA with Bonferroni post hoc test. In few cases, SEM is smaller than or equal to the size of symbols.
Figure 2.
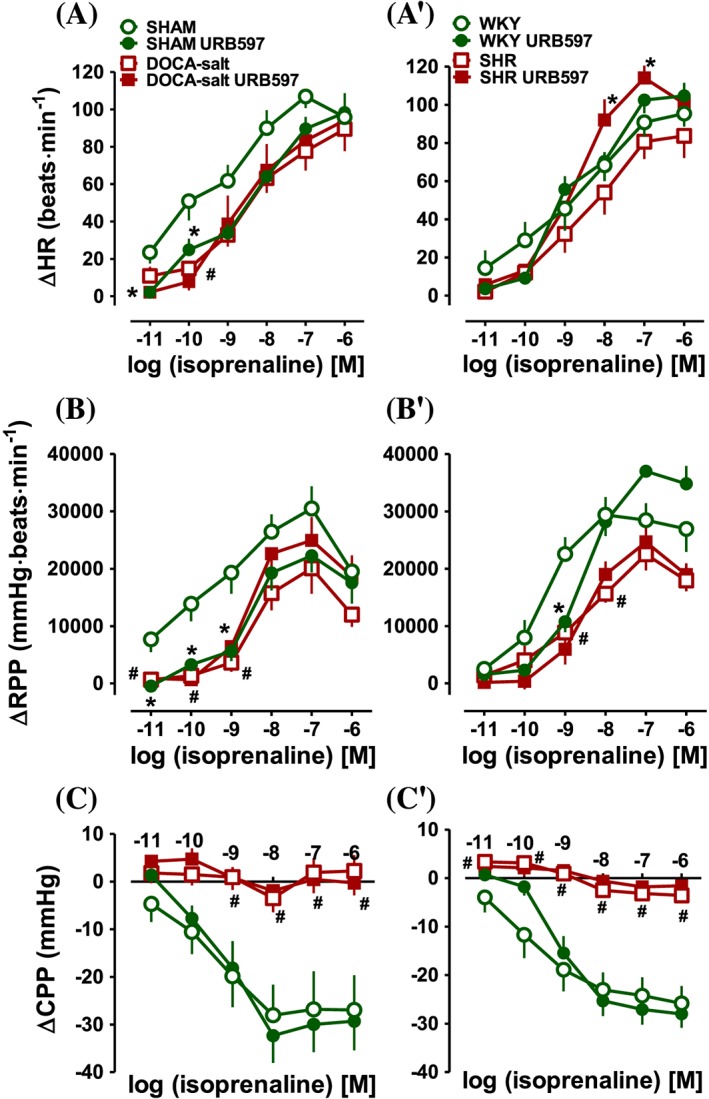
Influence of URB597 on the isoprenaline‐induced changes in HR (A, A′), RPP (B, B′) and CPP (C, C′) of hearts isolated from DOCA‐salt and SHR and their respective normotensive SHAM and WKY. URB597 (1 mg·kg−1) or its vehicle was injected i.p. every 12 h for 14 days. Values shown (ΔHR etc) are changes from baseline (Table 2). Data are given as the means ± SEM of 9–11 rats. *P < 0.05, significant effect of URB597, in normotensive rats only with the exception of HR in SHR; # P < 0.05, DOCA‐salt and SHR significantly different from SHAM and WKY respectively; ANOVA with Bonferroni post hoc test. In few cases, SEM is smaller than or equal to the size of symbols.
URB597 tended to enhance, the reduced in hypertension increases in LVP, +(LVdP/dt)max and RPP (but not other parameters), which were induced by higher (0.01 nM – 0.1 μM) isoprenaline concentrations in DOCA‐salt hypertensive rats; in the case of maximal responses by about 35, 40 and 25% respectively (for the respective pEC50 and Emax values, see Table 3, Figures 1 and 2). In contrast, the only effect of URB597 in SHR was enhancement of the positive chronotropic effect of isoprenaline at higher (0.01 nM – 0.1 μM) concentrations (by approximately 40% for Emax). However, the potency of isoprenaline was not affected (Figure 2A′, Table 3).
In both normotensive groups, URB597 reduced the following cardiostimulatory effects of isoprenaline at lower concentrations: LVP, +(LVdP/dt)max, −(LVdP/dt)max and RPP. Moreover, in SHAM, URB597 decreased the increases in LVP and HR induced by higher and lower concentrations of isoprenaline respectively (Figures 1 and 2). In WKY, URB597 diminished the potency of isoprenaline for +(LVdP/dt)max and RPP (Table 3). However, isoprenaline‐induced coronary vasodilatation in hypertensive and normotensive rats (Figure 2C, C′) was not altered by FAAH inhibition.
Effects of hypertension and chronic URB597 administration on isoprenaline‐ and CP55940‐induced changes in the contractile force of isolated left atria
Isoprenaline (0.01 nM – 3 μM) induced a concentration‐dependent increase in the contractile force of atria isolated from normo‐ and hypertensive rats. Hypertension and URB597 did not alter the positive inotropic effect of isoprenaline in DOCA‐salt and in SHAM (for the respective pEC50 and Emax values, see Table 4, Figure 3A).
Figure 3.
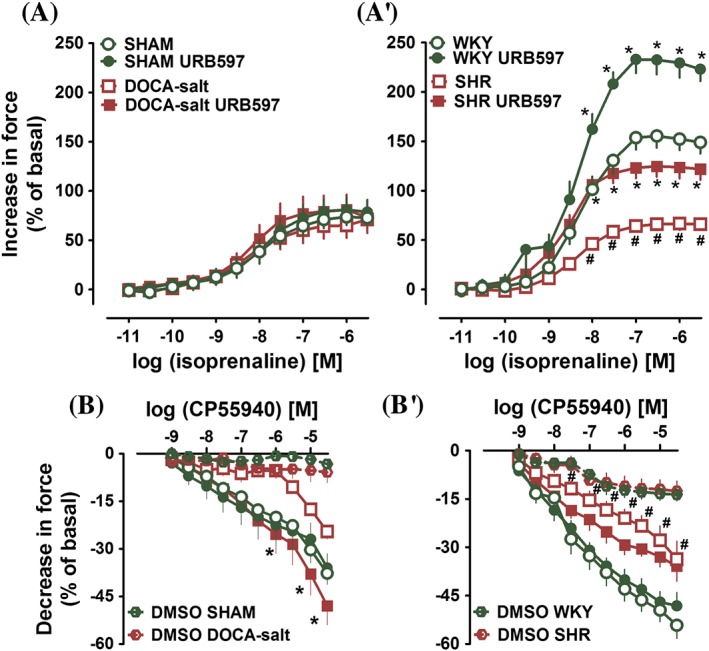
Influence of URB597 on the isoprenaline‐induced (A, A′) increases and on the CP55940‐induced (B, B′) decreases in contractile force of left atria isolated from DOCA‐salt and SHR and their respective normotensive SHAM and WKY. URB597 (1 mg·kg−1) or its vehicle was injected i.p. every 12 h for 14 days. Data are expressed in % of basal values (see Results). Data are given as the means ± SEM of 8–11 rats; *P < 0.05, significant effect of URB597; # P < 0.05, DOCA‐salt and SHR significantly different from SHAM and WKY respectively; ANOVA with Bonferroni post hoc test. In few cases, SEM is smaller than or equal to the size of symbols.
The inotropic effect of the higher concentration of isoprenaline in atria isolated from WKY was higher than in SHAM (in the case of the Emax value by approximately 110%; P < 0.05). The Emax value in SHR was lower by about 60% than in WKY. URB597 almost completely restored the positive inotropic effect of isoprenaline in SHR. Interestingly, it also strongly enhanced isoprenaline‐induced increase in contractile force observed in WKY. The maximal response was increased by about 50% (for the respective pEC50 and Emax values, see Table 4, Figure 3A′). There was no alteration in the potency of isoprenaline (Table 4) in either model of hypertension or after administration of URB597.
The vehicle alone for CP55940 reduced atrial contractile force by about 5–10% in all experimental groups (Figure 3), in hypertension and in the presence of URB597 (data not shown). CP55940 (1 nM – 30 μM) caused concentration‐dependent decreases in the contractile force of left atria (a direct negative inotropic effect); stronger in WKY than in SHAM (P < 0.05 for pEC50 and Emax values; Figure 3B, B′; for the respective pEC50 and Emax values induced by CP55940 at 30 μM, see Table 4). The concentration–response curves for the negative inotropic effect of CP55940 in normotensive rats were shifted to the right, in atria isolated from DOCA‐salt and SHR. URB597 completely restored the negative inotropic effect of CP55940 in the DOCA‐salt and the potency of CP55940 in SHR. URB597 did not modify the negative inotropic effect of CP55940 observed in atria isolated from normotensive animals (Figure 3B, B′, Table 4).
Influence of hypertension and chronic administration of URB597 on cardiac expression of CB1, CB2 receptors and FAAH
Analysis of CB receptor and FAAH expression in left cardiac ventricles by Western blotting showed a single immunoreactive band of the molecular size expected for CB1 receptors (60 kDa), CB2 (40 kDa) and FAAH (67 kDa). The expression of CB1, CB2 receptors and FAAH decreased in tissues from hypertensive DOCA‐salt rats, whereas in SHR, only FAAH was down‐regulated. URB597 did not modify expression levels in normotensive animals. It just prevented the previously observed decrease in FAAH expression and tended to do so with respect to CB1 and CB2 receptor expression, in DOCA‐salt but not in SHR (Figure 4).
Figure 4.
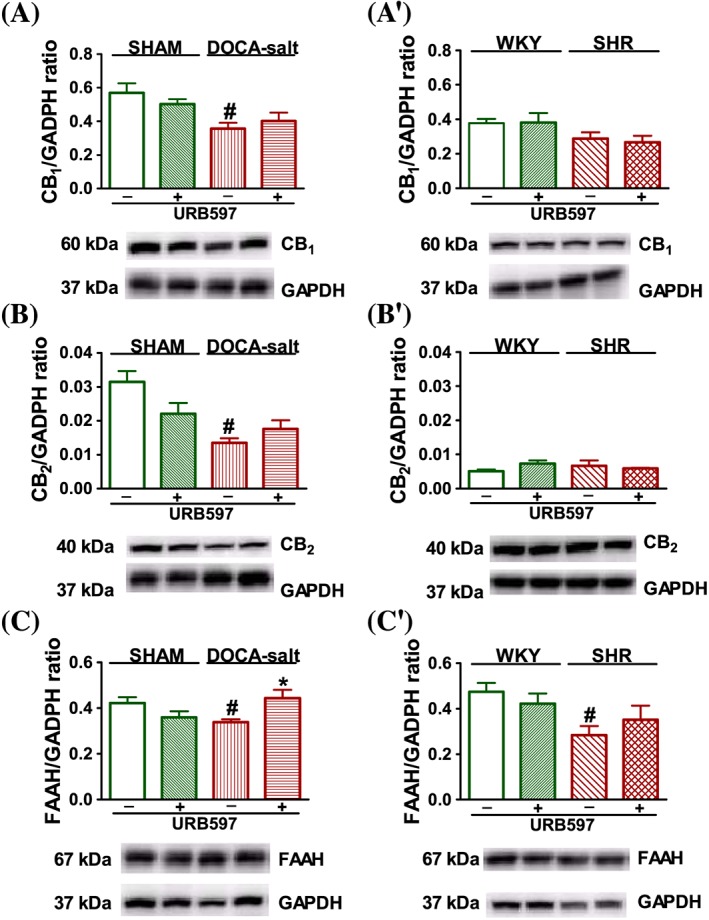
Western blots of CB1 (A, A′), CB2 (B, B′) and FAAH (C, C′) protein in left ventricle isolated from DOCA‐salt and SHR and their respective normotensive SHAM and WKY. URB597 (1 mg·kg−1) or its vehicle was injected i.p. every 12 h for 14 days. On the bottom of the panel, representative Western blots for CB1, CB2, FAAH and GADPH (which served as loading control) are given. Mean ± SEM of six rats; *P < 0.05, significant effect of URB597; # P < 0.05, DOCA‐salt and SHR significantly different from SHAM and WKY respectively; ANOVA with Bonferroni post hoc test.
Immunolocalization of CB1 and CB2 receptors (Figure 5) revealed their appearance in cardiomyocytes of the left cardiac ventricle, although the immunoreactivity for CB1 receptors appeared more intense. Both CB1 and CB2 immunoreactivities were less intense in both models of hypertension (especially in DOCA‐salt) compared with their respective normotensive controls. URB597 increased labelling for CB1 receptors in both hypertensive models and for CB2 receptors in DOCA‐salt only. Interestingly, in both models of hypertension treated with URB597, a clearly visible signal for CB1 receptors was noticed (stronger in SHR rather than DOCA‐salt rats), in the region of the intercalated discs of the heart.
Figure 5.

Representative micrographs of immunohistochemical staining of CB1 and CB2 receptors in cross sections of left ventricle isolated from DOCA‐salt and SHR and their respective normotensive SHAM and WKY treated i.p. every 12 h for 14 days with URB597 (1 mg·kg−1) or its vehicle. Scale bar: 50 μm.
Discussion
Chronic URB597 administration produces age‐dependent hypotension in DOCA‐salt hypertensive rats (Toczek et al., 2016), which is partly dependent on vascular changes (Baranowska‐Kuczko et al., 2016). Here, we examined whether the potential hypotensive effect of chronic FAAH inhibition in hypertension was dependent on cardiac performance. Cardiac function was assessed in two in vitro models using tissue taken from the same animal: Langendorff hearts and paced left atria in which contractility is dependent or independent of contraction frequency respectively.
Changes related to hypertension
Hypertensive animals had higher SBP, cardiac hypertrophy and diastolic stiffness. Isoprenaline‐induced vasodilatation and its ino‐ (with the exception of atria from DOCA‐salt animals), chrono‐ and lusitropic effects in hearts and/or atria were attenuated in hypertension. This may result from a reduction of myocardial β‐adrenoceptor density in SHR (Böhm et al., 1988). In DOCA‐salt hypertension, no change or a decrease in the number of β‐adrenoceptors has been reported (Böhm et al., 1992).
The cannabinoid receptor agonist CP55940 induced a concentration‐dependent decrease in atrial contractile force, with a stronger effect in WKY compared with SHAM. The maximum negative inotropic effect of CP55940 was comparable with that induced by AEA in atrial muscle of humans (Bonz et al., 2003) and rats (Sterin‐Borda et al., 2005). This is the first study to demonstrate that the negative inotropic effects of CP55940 are diminished in hypertension. This was accompanied by decreased ventricular immunoreactivity and/or expression of FAAH and CB receptors (especially in DOCA‐salt), which can induce negative (CB1) and positive (CB2) inotropic responses in rat left atria (Sterin‐Borda et al., 2005). By contrast, Bátkai et al. (2004) reported an up‐regulation of cardiac CB1 receptors and FAAH, associated with an AEA‐induced sustained decrease in cardiac contractility, in anaesthetized SHR. However, Bátkai et al. (2004) used older rats for their in vivo experiments (8–10 months vs. 8–10 weeks in our study), in which the negative inotropic effect of AEA was modified by a decreased HR. We examined atrial but not ventricular contractility. However, hypertension‐induced ventricular changes are accompanied by modifications of atrial function (Pluteanu et al., 2015). In contrast to the reduced effects and expression of cardiac CB1 receptors in DOCA‐salt reported here, we have found in the same model up‐regulation of vascular CB1 receptors and CB1 receptor‐mediated vasodilatation (Baranowska‐Kuczko et al., 2016) and enhanced inhibition of sympathetic noradrenaline release (Toczek et al., 2015) in resistance vessels. This suggests that these receptors may play a protective role in hypertension.
Influence of chronic URB597 administration on hearts of hypertensive rats
FAAH inhibitors are postulated to possess anti‐hypertensive potential, because their acute systemic injection decreased BP in SHR partially through normalization of cardiac contractile performance, associated with up‐regulation of cardiac CB1 receptors and FAAH (Bátkai et al., 2004; Pacher et al., 2008; Godlewski et al., 2010; O'Sullivan, 2015). We found that the effect of chronic FAAH inhibition was dependent on the hypertension model. More marked alterations were observed in secondary hypertension. This was probably due to the more dynamic progress of hypertension in DOCA‐salt compared with SHR (the comparable hypertension of about 200 mmHg before the first dose of URB597 was obtained after 4 weeks of DOCA‐salt procedure vs. 8–10 weeks of hypertension development in SHR) and the potential development of adaptive changes in SHR (Zicha and Kunes, 1999).
Chronic URB597 administration decreased BP only in DOCA‐salt (consistent with our previous studies; Baranowska‐Kuczko et al., 2016; Toczek et al., 2016) but not in SHR. We measured BP 12 h after the final dose of URB597 (1 mg·kg−1) in conscious rats. Higher doses were not used due to potential side effects and neurotoxicity (1.5 and 3 mg·kg−1; Su et al., 2015). In contrast, the strong hypotensive effects of acute administration of the FAAH inhibitors URB597 (used at the maximally effective dose of 10 mg·kg−1; Bátkai et al., 2004) and AM3506 (Godlewski et al., 2010) were determined immediately after i.v. injection in older anaesthetized SHR.
The present results demonstrate that URB597 induced a fall in BP in DOCA‐salt hypertension, which was partly mediated by vascular (Baranowska‐Kuczko et al., 2016) and cardiac mechanisms. URB597 reduced hypertrophy of the left ventricle and septum and cardiac diastolic stiffness (observed in hypertension) and completely restored the negative inotropic effect of CP55940 in isolated atria. These changes were accompanied by enhanced ventricular expression of CB1 and CB2 receptors (immunohistochemistry) and FAAH (Western blot) that was reduced in hypertension. It is the first report demonstrating the up‐regulation of FAAH expression following chronic FAAH inhibition. So far, a decrease (Baranowska‐Kuczko et al., 2016; Su et al., 2016) or no change (Okine et al., 2012; Baranowska‐Kuczko et al., 2016) in FAAH expression has been demonstrated in other tissues, after chronic URB597 administration. We can only speculate that in our hands, inhibition of enzyme activity might cause a compensatory increase in enzyme expression (King et al., 1991). The possible involvement of cardiac mechanisms in the hypotensive effect of URB597 is partly confirmed by the observation that, in SHR, the same procedure did not decrease BP or normalize cardiac hypertrophy, diastolic stiffness, the negative inotropic effect of CP55940 or expression of CB receptors and FAAH. Our results on isolated atria from DOCA‐salt validated the findings from in vivo experiments (Bátkai et al., 2004) that changes in cannabinoid‐induced inotropic effects are related to a direct influence on the myocardium but not through inhibition of sympathetic tone. Moreover, the significance of CB1 receptor mediated cardiac alternations underlined observations that chronic administration of the CB1 receptor antagonist rimonabant improved cardiac function and remodelling after myocardial infarction, in obese SHR (Slavic et al., 2013).
The most visible effects of URB597 in SHR were the normalization (atria) and enhancement (hearts) of the positive ino‐ and chronotropic effects of isoprenaline respectively. It remains to be established whether these changes are related to redistribution of CB1 receptors to the region of intercalated discs, clearly observed in SHR. We are the first to demonstrate this intriguing relationship between cardiac intercalated discs and CB1 receptors. Intercalated discs are responsible for mechanical (desmosomes and adheren junctions) and electrical (gap junctions) communication between adjacent cardiomyocytes (Campbell et al., 2014). AEA can inhibit gap junctions in certain cell types, such as endothelial cells, but not myocytes (Oz, 2006). Improving cardiac gap junction communication, which is also modified by β‐adrenoceptor stimulation (Campbell et al., 2014), has been proposed as a new antiarrhythmic mechanism (Dhein et al., 2010). Thus, normalization of basal HR and prevention of arrhythmia by chronic URB694 administration to stressed animals (Carnevali et al., 2015b) could be associated with gap junction modification.
Pre‐incubation of ventricular papillary muscle (isolated from rats with cirrhotic cardiomyopathy) with the CB1 receptor antagonist 1‐(2,4‐dichlorophenyl)‐5‐(4‐iodophenyl)‐4‐methyl‐N‐piperidin‐1‐ylpyrazole‐3‐carboxamide restored its blunted contractile response to isoprenaline (Gaskari et al., 2005). In our hands, chronic URB597 administration restored the positive inotropic effect of isoprenaline in atria, which was reduced in SHR and tended towards the same in isolated hearts from DOCA‐salt.
Influence of chronic URB597 administration on hearts of normotensive rats
To date, it is generally accepted that endocannabinoids are not involved in the regulation of cardiovascular parameters under physiological conditions, as genetic ablation or acute pharmacological blockade of CB receptors or FAAH does not modify basal haemodynamic parameters including left ventricle stiffness (Pacher et al., 2005; Malinowska et al., 2012; O'Sullivan, 2015) and parameters of isolated rat hearts (Wagner et al., 2005). Surprisingly, we found that chronic URB597 administration produced two detrimental effects in normotensive rats, namely, increased cardiac diastolic stiffness and modified cardiostimulatory effects of isoprenaline. Thus, it diminished the following effects of lower concentrations of isoprenaline on the heart: ino‐ and lusitropic actions, increases in RPP and the chronotropic effect (the latter in SHAM only). Moreover, the positive inotropic effect of higher concentrations of isoprenaline was enhanced in atria from WKY. These data indicate that the modification of β‐adrenoceptor function by cannabinoids, found in acute experiments (Maslov et al., 2004; Gaskari et al., 2005; Liao et al., 2013; Carnevali et al., 2015b; see Introduction), may also extend to chronic studies.
There is no clear explanation of the negative effect of URB597 in normotensive rats. In the light of pro‐fibrotic effects of CB1 receptor activation in diabetic mice (Rajesh et al., 2012) or anti‐fibrotic influence of the CB1 receptor antagonist rimonabant in experimental metabolic syndrome (Slavic et al., 2013), it could be not so surprising to see increased cardiac stiffness in response to URB597 increasing level of endocannabinoids acting via CB receptors. URB597 is generally regarded as a selective FAAH inhibitor (Piomelli et al., 2006). However, proteome‐wide studies identified the off‐target effects of URB597 (Ahn et al., 2007). Thus, side‐effects of URB597 might be connected with its off‐target response and with direct or indirect activation of alternative pathways of AEA metabolism. Importantly, other unexpected effects of chronic URB597 administration in SHAM animals have been observed previously in small resistance arteries: impaired acetylcholine‐induced vasodilatation, potentiated phenylephrine‐induced vasoconstriction (Baranowska‐Kuczko et al., 2016) and enhanced oxidative stress in liver (Biernacki et al., 2016). Similarly, cardiac injury and enhanced oxidative/nitrative stress were greater in FAAH−/− compared with FAAH+/+ mice, and AEA was reported to enhance cell death in human cardiomyocytes pretreated with an FAAH inhibitor (Mukhopadhyay et al., 2011).
In summary, the present findings show that hypotensive effect of chronic FAAH inhibition depends on the model of hypertension used and partly correlates with improved cardiac performance. The fall in BP in response to chronic URB597 administration was observed only in DOCA‐salt rats. This was associated with decreased left ventricular hypertrophy and cardiac diastolic stiffness and an enhanced negative inotropic atrial response to CP55940. The most visible influence of URB597 in SHR was an enhanced positive inotropic effect to isoprenaline in isolated hearts, accompanied by a redistribution of CB1 receptors to the intercalated disc regions. However, chronic URB597 administration induced side‐effects in normotensive rats such as enhanced cardiac diastolic stiffness and modified cardiostimulatory effects to isoprenaline, stressing the importance of examination of bidirectional interactions of endocannabinoids with the cardiac effects of β‐adrenoceptors. Thus, the use of FAAH inhibitors as potential pharmacotherapy in normotensive patients, for example, for control of pain and inflammation (Fowler, 2015) or psychological‐cardiac comorbidity (Carnevali et al., 2016), should be approached cautiously, especially considering the side effects of FAAH inhibitors that have been reported in clinical studies (Mallet et al., 2016).
Author contributions
A.P.‐B. designed and conducted isolated heart experiments, analysed data and co‐wrote the manuscript; J.W. performed isolated atria experiments and analysed the data; M.T. performed the in vivo studies and helped in interpretation of data; M.B.K. contributed to the revised manuscript; I.K. designed and analysed immunohistochemical experiments; E.H.‐S. designed and analysed Western blot experiments; B.M. conceived, designed and supervised experiments and wrote the manuscript. All authors approved the final manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
The work was supported by the National Science Centre (Poland), research grant NCN 2012/05/B/NZ7/03102 and by the Medical University of Białystok (Poland) grant no. 143‐13793F. We wish to thank Mrs I. Malinowska and Mrs T. Makar for their excellent technical assistance.
Pędzińska‐Betiuk, A. , Weresa, J. , Toczek, M. , Baranowska‐Kuczko, M. , Kasacka, I. , Harasim‐Symbor, E. , and Malinowska, B. (2017) Chronic inhibition of fatty acid amide hydrolase by URB597 produces differential effects on cardiac performance in normotensive and hypertensive rats. British Journal of Pharmacology, 174: 2114–2129. doi: 10.1111/bph.13830.
References
- Ahn K, Johnson DS, Fitzgerald LR, Liimatta M, Arendse A, Stevenson T et al. (2007). Novel mechanistic class of fatty acid amide hydrolase inhibitors with remarkable selectivity. Biochemistry 46: 13019–13030. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The concise guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The concise guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelone T, Quintieri AM, Brar BK, Limchaiyawat PT, Tota B, Mahata SK et al. (2008). The antihypertensive chromogranin a peptide catestatin acts as a novel endocrine/paracrine modulator of cardiac inotropism and lusitropism. Endocrinology 149: 4780–4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranowska‐Kuczko M, Kozłowska H, Kloza M, Karpińska O, Toczek M, Harasim E et al. (2016). Protective role of cannabinoid CB1 receptors and vascular effects of chronic administration of FAAH inhibitor URB597 in DOCA‐salt hypertensive rats. Life Sci 151: 288–299. [DOI] [PubMed] [Google Scholar]
- Bátkai S, Pacher P, Osei‐Hyiaman D, Radaeva S, Liu J, Harvey‐White J et al. (2004). Endocannabinoids acting at cannabinoid‐1 receptors regulate cardiovascular function in hypertension. Circulation 110: 1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bátkai S, Rajesh M, Mukhopadhyay P, Haskó G, Liaudet L, Cravatt BF et al. (2007). Decreased age‐related cardiac dysfunction, myocardial nitrative stress, inflammatory gene expression, and apoptosis in mice lacking fatty acid amide hydrolase. Am J Physiol Heart Circ Physiol 293: H909–H918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernacki M, Łuczaj W, Gęgotek A, Toczek M, Bielawska K, Skrzydlewska E (2016). Crosstalk between liver antioxidant and the endocannabinoid systems after chronic administration of the FAAH inhibitor, URB597, to hypertensive rats. Toxicol Appl Pharmacol 301: 31–41. [DOI] [PubMed] [Google Scholar]
- Bonz A, Laser M, Küllmer S, Kniesch S, Babin‐Ebell J, Popp V et al. (2003). Cannabinoids acting on CB1 receptors decrease contractile performance in human atrial muscle. J Cardiovasc Pharmacol 41: 657–664. [DOI] [PubMed] [Google Scholar]
- Böhm M, Beuckelmann D, Diet F, Feiler G, Lohse MJ, Erdmann E (1988). Properties of cardiac alpha‐ and beta‐adrenoceptors in spontaneously hypertensive rats. Naunyn Schmiedebergs Arch Pharmacol 338: 383–391. [DOI] [PubMed] [Google Scholar]
- Böhm M, Gierschik P, Knorr A, Larisch K, Weismann K, Erdmann E (1992). Desensitization of adenylate cyclase and increase of Gi alpha in cardiac hypertrophy due to acquired hypertension. Hypertension 20: 103–112. [DOI] [PubMed] [Google Scholar]
- Campbell AS, Johnstone SR, Baillie GS, Smith G (2014). β‐Adrenergic modulation of myocardial conduction velocity: connexins vs. sodium current. J Mol Cell Cardiol 77: 147–154. [DOI] [PubMed] [Google Scholar]
- Carnevali L, Rivara S, Nalivaiko E, Thayer JF, Vacondio F, Mor M et al. (2016). Pharmacological inhibition of FAAH activity in rodents: a promising pharmacological approach for psychological‐cardiac comorbidity? Neurosci Biobehav . https://doi.org/10.1016/j.neubiorev.2016.04.013. [DOI] [PubMed] [Google Scholar]
- Carnevali L, Vacondio F, Rossi S, Callegari S, Macchi E, Spadoni G et al. (2015a). Antidepressant‐like activity and cardioprotective effects of fatty acid amide hydrolase inhibitor URB694 in socially stressed Wistar Kyoto rats. Eur Neuropsychopharmacol 25: 2157–2169. [DOI] [PubMed] [Google Scholar]
- Carnevali L, Vacondio F, Rossi S, Macchi E, Spadoni G, Bedini A et al. (2015b). Cardioprotective effects of fatty acid amide hydrolase inhibitor URB694, in a rodent model of trait anxiety. Sci Rep 5: 18218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhein S, Hagen A, Jozwiak J, Dietze A, Garbade J, Barten M et al. (2010). Improving cardiac gap junction communication as a new antiarrhythmic mechanism: the action of antiarrhythmic peptides. Naunyn Schmiedebergs Arch Pharmacol 381: 221–234. [DOI] [PubMed] [Google Scholar]
- Drazner MH (2011). The progression of hypertensive heart disease. Circulation 123: 327–334. [DOI] [PubMed] [Google Scholar]
- Dincer UD, Ozcelikay AT, Yilmaz ED (2000). The effects of chronic L‐NAME and L‐arginine administration on β‐adrenergic responsiveness of STZ‐diabetic rat atria. Pharmacol Res 41: 565–570. [DOI] [PubMed] [Google Scholar]
- Engeli S, Blüher M, Jumpertz R, Wiesner T, Wirtz H, Bosse‐Henck A et al. (2012). Circulating anandamide and blood pressure in patients with obstructive sleep apnea. J Hypertens 30: 2345–2351. [DOI] [PubMed] [Google Scholar]
- Ford WR, Honan SA, White R, Hiley CR (2002). Evidence of a novel site mediating anandamide‐induced negative inotropic and coronary vasodilatator responses in rat isolated hearts. Br J Pharmacol 135: 1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ (2015). The potential of inhibitors of endocannabinoid metabolism for drug development: a critical review. Handb Exp Pharmacol 231: 95–128. [DOI] [PubMed] [Google Scholar]
- Gaskari SA, Liu H, Moezi L, Li Y, Baik SK, Lee SS (2005). Role of endocannabinoids in the pathogenesis of cirrhotic cardiomyopathy in bile duct‐ligated rats. Br J Pharmacol 146: 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlewski G, Alapafuja SO, Bátkai S, Nikas SP, Cinar R, Offertáler L et al. (2010). Inhibitor of fatty acid amide hydrolase normalizes cardiovascular function in hypertension without adverse metabolic effects. Chem Biol 17: 1256–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG (2006). The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SJ, Oparil S, Berecek KH (1991). Neuronal angiotensin‐converting enzyme (ACE) gene expression is increased by converting enzyme inhibitors (CEI). Mol Cell Neurosci 2: 13–20. [DOI] [PubMed] [Google Scholar]
- Lake KD, Martin BR, Kunos G, Varga K (1997). Cardiovascular effects of anandamide in anesthetized and conscious normotensive and hypertensive rats. Hypertension 29: 1204–1210. [DOI] [PubMed] [Google Scholar]
- Li D, Chen BM, Peng J, Zhang YS, Li XH, Yuan Q et al. (2009). Role of anandamide transporter in regulating calcitonin gene‐related peptide production and blood pressure in hypertension. J Hypertens 27: 1224–1232. [DOI] [PubMed] [Google Scholar]
- Liao Y, Bin J, Luo T, Zhao H, Ledent C, Asakura M et al. (2013). CB1 cannabinoid receptor deficiency promotes cardiac remodeling induced by pressure overload in mice. Int J Cardiol 167: 1936–1944. [DOI] [PubMed] [Google Scholar]
- Loch D, Hoey A, Morisseau C, Hammock BO, Brown L (2007). Prevention of hypertension in DOCA‐salt rats by an inhibitor of soluble epoxide hydrolase. Cell Biochem Biophys 47: 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowska B, Baranowska‐Kuczko M, Schlicker E (2012). Triphasic blood pressure responses to cannabinoids: do we understand the mechanism? Br J Pharmacol 165: 2073–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov LN, Lasukova OV, Krylatov AV, Uzhachenko RV, Pertwee R (2004). Selective cannabinoid receptor agonist HU‐210 decreases pump function of isolated perfused heart: role of cAMP and cGMP. Bull Exp Biol Med 138: 550–553. [DOI] [PubMed] [Google Scholar]
- Mallet C, Dubray C, Dualé C (2016). FAAH inhibitors in the limelight, but regrettably. Int J Clin Pharmacol Ther 54: 498–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P, Horváth B, Rajesh M, Matsumoto S, Saito K, Bátkai S et al. (2011). Fatty acid amide hydrolase is a key regulator of endocannabinoid‐induced myocardial tissue injury. Free Radic Biol Med 50: 179–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okine BN, Norris LM, Woodhams S, Burston J, Patel A, Alexander SP et al. (2012). Lack of effect of chronic pre‐treatment with the FAAH inhibitor URB597 on inflammatory pain behaviour: evidence for plastic changes in the endocannabinoid system. Br J Pharmacol 167: 627–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan SE (2015). Endocannabinoids and the cardiovascular system in health and disease. Handb Exp Pharmacol 231: 393–422. [DOI] [PubMed] [Google Scholar]
- Oz M (2006). Receptor‐independent actions of cannabinoids on cell membranes: focus on endocannabinoids. Pharmacol Ther 111: 114–144. [DOI] [PubMed] [Google Scholar]
- Pacher P, Bátkai S, Osei‐Hyiaman D, Offertáler L, Liu J, Harvey‐White J et al. (2005). Hemodynamic profile, responsiveness to anandamide, and baroreflex sensitivity of mice lacking fatty acid amide hydrolase. Am J Physiol Heart Circ Physiol 289: H533–H541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Mukhopadhyay P, Mohanraj R, Godlewski G, Bátkai S, Kunos G (2008). Modulation of the endocannabinoid system in cardiovascular disease: therapeutic potential and limitations. Hypertension 52: 601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D, Tarzia G, Duranti A, Tontini A, Mor M, Compton TR et al. (2006). Pharmacological profile of the selective FAAH inhibitor KDS‐4103 (URB597). CNS Drug Rev 12: 21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluteanu F, Heß J, Plackic J, Nikonova Y, Preisenberger J, Bukowska A et al. (2015). Early subcellular Ca2+ remodelling and increased propensity for Ca2+ alternans in left atrial myocytes from hypertensive rats. Cardiovasc Res 106: 87–97. [DOI] [PubMed] [Google Scholar]
- Rajesh M, Bátkai S, Kechrid M, Mukhopadhyay P, Lee WS, Horváth B et al. (2012). Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes 61: 716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavic S, Lauer D, Sommerfeld M, Kemnitz UR, Grzesiak A, Trappiel M et al. (2013). Cannabinoid receptor 1 inhibition improves cardiac function and remodelling after myocardial infarction and in experimental metabolic syndrome. J Mol Med (Berl) 91: 811–823. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterin‐Borda L, Del Zar CF, Borda E (2005). Differential CB1 and CB2 cannabinoid receptor‐inotropic response of rat isolated atria: endogenous signal transduction pathways. Biochem Pharmacol 69: 1705–1713. [DOI] [PubMed] [Google Scholar]
- Su SH, Wang YQ, Wu YF, Wang DP, Lin Q, Hai J (2016). Cannabinoid receptor agonist WIN55,212‐2 and fatty acid amide hydrolase inhibitor URB597 may protect against cognitive impairment in rats of chronic cerebral hypoperfusion via PI3K/AKT signaling. Behav Brain Res 313: 334–344. [DOI] [PubMed] [Google Scholar]
- Su SH, Wu YF, Lin Q, Yu F, Hai J (2015). Cannabinoid receptor agonist WIN55,212‐2 and fatty acid amide hydrolase inhibitor URB597 suppress chronic cerebral hypoperfusion‐induced neuronal apoptosis by inhibiting c‐Jun N‐terminal kinase signaling. Neuroscience 301: 563–575. [DOI] [PubMed] [Google Scholar]
- Toczek M, Schlicker E, Grzęda E, Malinowska B (2015). Enhanced function of inhibitory presynaptic cannabinoid CB1 receptors on sympathetic nerves of DOCA‐salt hypertensive rats. Life Sci 138: 78–85. [DOI] [PubMed] [Google Scholar]
- Toczek M, Baranowska‐Kuczko M, Grzęda E, Pędzińska‐Betiuk A, Weresa J, Malinowska B (2016). Age‐specific influences of chronic administration of the fatty acid amide hydrolase inhibitor URB597 on cardiovascular parameters and organ hypertrophy in DOCA‐salt hypertensive rats. Pharmacol Rep 68: 363–369. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Abesser M, Karcher J, Laser M, Kunos G (2005). Coronary vasodilator effects of endogenous cannabinoids in vasopressin‐preconstricted unpaced rat isolated hearts. J Cardiovasc Pharmacol 46: 348–355. [DOI] [PubMed] [Google Scholar]
- Zicha J, Kunes J (1999). Ontogenetic aspects of hypertension development: analysis in the rat. Physiol Rev 79: 1227–1282. [DOI] [PubMed] [Google Scholar]


