Abstract
Store-operated calcium entry (SOCE) is a ubiquitous Ca2+ entry pathway that is activated in response to depletion of ER-Ca2+ stores and critically controls the regulation of physiological functions in miscellaneous cell types. The transient receptor potential canonical 1 (TRPC1) is the first member of the TRPC channel subfamily to be identified as a molecular component of SOCE. While TRPC1 has been shown to contribute to SOCE and regulate various functions in many cells, none of the reported TRPC1-mediated currents resembled ICRAC, the highly Ca2+-selective store-dependent current first identified in lymphocytes and mast cells. Almost a decade after the cloning of TRPC1 two proteins were identified as the primary components of the CRAC channel. The first, STIM1, is an ER-Ca2+ sensor protein involved in activating SOCE. The second, Orai1 is the pore-forming component of the CRAC channel. Co-expression of STIM1 and Orai1 generated robust ICRAC. Importantly, STIM1 was shown to also activate TRPC1 via its C-terminal polybasic domain, which is distinct from its Orai1-activating domain, SOAR. In addition, TRPC1 function critically depends on Orai1-mediated Ca2+ entry which triggers recruitment of TRPC1 into the plasma membrane where it is then activated by STIM1. More importantly, TRPC1 and Orai1 form discrete STIM1-gated channels that generate distinct Ca2+ signals and regulate specific cellular functions. Surface expression of TRPC1 can be modulated by trafficking of the channel to and from the plasma membrane, resulting in changes to the phenotype of TRPC1-mediated current and [Ca2+]i signals. Thus, TRPC1 is activated downstream of Orai1 and modifies the initial [Ca2+]i signal generated by Orai1 following store depletion. This review will summarize the important findings that underlie the current concepts for activation and regulation of TRPC1, as well as its impact on cell function.
Keywords: TRPC, STIM1, Orai1, SOCE, ER-PM junctions, lipid rafts, caveolin
Graphical abstract
A Brief History – SOCE and TRPC1
Store-operated Calcium Entry (SOCE), first described almost thirty years ago by Jim Putney [1], is a ubiquitous plasma membrane Ca2+ entry mechanism that is activated when ER-[Ca2+] is decreased. SOCE provides Ca2+ signals to regulate critical cell functions in many tissues. The same basic mechanism regulates SOCE in every cell type, although as discussed in this chapter, other components can contribute to and modulate SOCE and these could vary between cell types. The physiological trigger for activating SOCE is the generation of inositol 1,4,5-triphosphate (IP3) that occurs in response to agonist stimulation of cells and induces Ca2+ release from the ER. The consequent decrease in ER-[Ca2+] is the primary determinant in the regulation of SOCE. The exact mechanism of SOCE, whether it is mediated by a channel or transporter, as well as the molecular components involved in the process, remained unclarified for several years. A major advancement in the field came with the identification of the Calcium Release Activated Calcium (CRAC) channel that mediates the highly Ca2+ selective current (ICRAC) in mast and T cells, with its characteristic inward rectification and a reversal potential (Erev) >+40mV [2–8]. However, ER-Ca2+ store depletion activates different types of calcium currents in other cell types [9–11], giving rise to the suggestion that diverse channel components or regulatory proteins might be involved in SOCE. Elucidating the molecular components of SOCE, i.e. the channels as well as regulatory proteins, proved to be a challenging endeavor in the field for almost two decades.
The Drosophila Transient Receptor Potential (TRP) channel was first proposed as a candidate component of SOCE channels [12–14], fueling extensive search for its mammalian homologues. The TRP canonical 1 (TRPC1) was the first mammalian member of the TRPC channel family to be cloned [15, 16], with subsequent discoveries of TRPC2-7. TRPCs exhibit diverse channel properties and physiological functions in a wide range of cells. The conclusion that TRPC1 mediates SOCE was based on the following hallmark characteristics: (i) activation by agonist, thapsigargin (Tg), and TPEN; and (ii) inhibition by 1-5μM Gd3+ and 10-20 μM 2APB. Furthermore, whole cell patch clamp measurements under conditions used for CRAC channel measurements (inclusion of IP3 in the pipette solution or stimulation with either Tg or agonist) resulted in generation of cation currents ranging from relatively Ca2+-selective to non-selective [9, 17, 18]. These currents were also completely blocked by low [Gd3+] and 2APB. In addition, overexpression or knockdown of TRPC1 increased and decreased the current, respectively. The electrophysiological characteristics of TRPC1-associated current vary in different cell types, likely due to the TRPC channel composition. For example, in human submandibular gland (HSG) cells, TRPC1 mediates a relatively Ca2+-selective current with a slight inward rectification and a Erev ≈ +15 mV with little contribution from other TRPC channels [9, 19]. In contrast, TRPC1 forms a heteromeric complex with TRPC3 in human parotid gland ductal cells, generating a non-selective, linear current [9, 18].
TRPC1 contributes to SOCE in a wide range of cells, with knockdown of endogenous TRPC1 producing the most consistent and significant effects, such as in HSG cells, smooth muscle cells, endothelial cells and platelets [19–25], and in preparations of salivary gland and pancreatic acinar cells as well as aortic endothelial cells from TRPC1 knockout mice [26–28]. Additionally, TRPC1-mediated Ca2+ entry is associated with the regulation of physiological functions in many tissues including migration of intestinal epithelial cells [29, 30] and human malignant gliomas [31]; proliferation of neural stem and hippocampal neural progenitor cells [32–34]; and synaptic plasticity of neuromuscular junctions [34]. TRPC1 contributes to maintenance of endothelial cell barrier, wound healing in the intestinal epithelial layer, attenuation of cytotoxicity, contraction of glomerular mesangial cells, and osteoclast formation and function [35–39]. Studies with TRPC1−/− mice show that it is a vital Ca2+ entry component in several tissues although the mice display normal viability, development and behavior. Acinar cells from salivary glands and pancreas, and aortic endothelial cells exhibit dramatically attenuated SOCE with corresponding reduction in Ca2+-dependent processes such as the activation of KCa and Cl− channels. Importantly, agonist-stimulated fluid and protein secretion from the salivary glands and pancreas, respectively, as well as vasorelaxation of the aorta, are adversely impacted [26–28, 40–42]. It is worth noting that the residual Orai1 cannot compensate for the loss of TRPC1 function in these cells, which clearly establishes the non-redundancy of TRPC1.
Despite the relatively strong data supporting that TRPC1 is involved in SOCE, the electrophysiological characteristics of the currents associated with TRPC1 do not resemble ICRAC. For that reason, the TRPC1-associated current was named store-operated calcium current (ISOC) to distinguish it from ICRAC [19]. Further, it was also established that TRPC1 was not a component of CRAC channels as knockdown of TRPC1 did not affect CRAC channel function. Thus, the search for CRAC channel components continued for almost two decades, resulting in the discovery of a four-transmembrane protein named Orai1, which was confirmed as the primary pore-forming component of CRAC channel and essential for T cell function [43–46]. Importantly, siRNA screening led to identification of the ER-resident Ca2+-sensing protein, STIM1, as the primary activator of Orai1 [47, 48]. STIM1 is an ER protein with an EF hand domain that is located within the lumen of the ER. Rapid advance in understanding SOCE came from studies which showed that disassociation of Ca2+ from the EF hand domain of STIM1 triggers extensive intramolecular and intermolecular rearrangement of STIM1. In resting cells, STIM1 tracks along microtubules while after store-depletion, it oligomerizes and translocates to the cell periphery, aggregating within ER-plasma membrane (ER-PM) junctions. These junctions are formed by close apposition of the ER and plasma membrane which is promoted when ER-residing STIM1 anchors to the plasma membrane PIP2, a process that brings ER closer to the PM. Orai1 is recruited by STIM1 into these junctions and where it is activated following store depletion. Several other proteins contribute to the assembly, architecture, and stability of Orai1-STIM1 interactions within this domain.
Activation of Orai1 is elicited by interaction of the channel with the STIM1 Orai1 Activating Region (SOAR) in the C-terminal region of STIM1 [43, 45, 47–51]. Importantly, TRPC1 also aggregates with Orai1 and STIM1 within the same ER-PM junctions. Further, TRPC1 interacts with and is activated by STIM1. The residues in STIM1 that are involved in gating TRPC1 are distinct from those that activate Orai1. However, activation of TRPC1 is a bit more complicated as it requires an additional crucial functional interaction with Orai1. This observation raised much debate and contention till it was finally resolved by data showing that Orai1-mediated Ca2+ entry triggers recruitment of TRPC1 into the plasma membrane where it is activated by STIM1 [52–54] (Figure 1). Thus Orai1/STIM1 and TRPC1/STIM1 assemble into two distinct channels which provide Ca2+ signals for regulation of distinct cellular functions. The characteristics of the current generated in the cell depend on whether one or both channels are functional. These previous and more recent studies will be further discussed below.
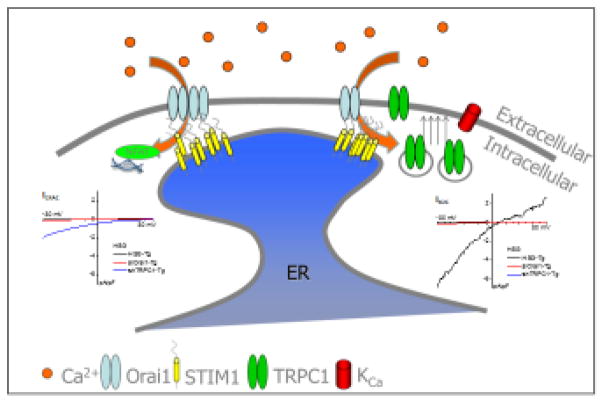
Figure 1. Proposed model for TRPC1 activation.
Following store depletion, STIM1 aggregates and translocates to the ER-PM junction. Orai1 is recruited to the STIM1 puncta resulting in CRAC channel activation. The resulting [Ca2+]i increase leads to NFAT activation and insertion of TRPC1-containing vesicles into the plasma membrane (Modified from [55]). Insets in the figure show I-V relationships of the currents recorded under the various conditions; ICRAC generated by Orai1+STIM1 (left) and ISOC generated by combination of TRPC1+STIM1 and Orai1+STIM1 channels.
Regulation of TRPC1 by STIM1 and Orai1
The ER-resident STIM proteins, STIM1 and STIM2, are the major regulatory components of SOCE channels. While there is relatively little information regarding role of STIM2 in TRPC1-mediated SOCE, knockdown of STIM1 dramatically reduces endogenous TRPC1-mediated SOCE and Ca2+ current, while exogenous co-expression of STIM1 with TRPC1 increases SOCE [52, 53, 55]. TRPC1 co-localizes and interacts with STIM1 following store depletion and conversely, refilling of the ER-Ca2+ stores causes dissociation of STIM1 from TRPC1 and inactivation of TRPC1 function [53, 56–60]. The gating of TRPC1 by STIM1 involves electrostatic interactions between the negatively charged aspartate residues in TRPC1 (639DD640) with the positively charged lysines in the STIM1 polybasic domain (684KK685). Charge-swap (STIM1-684EE685) or deletion of polybasic domain (STIM1ΔK; deletion of aa 672-685) in STIM1 eliminates its ability to activate TRPC1, but not Orai1. Conversely, the charge-swap mutant of TRPC1 (TRPC1-639KK640) is not gated by STIM1. Swapping the charged residues that are involved in STIM1 with those in TRPC1 induces recovery of channel function [61]. In addition, the STIM1-ezrin/radixin/moesin (ERM) domain (aa 251–535) is reported to bind to TRPC1 [53], whereas the coiled-coil (CC) regions located in the N and C terminal domains of TRPC1 bind to STIM1-SOAR domain [58, 62]. It was previously shown that Caveolin-1 (Cav-1) binds to TRPC1, retaining the channel in an inactive state. Binding of STIM1 to TRPC1 displaces Cav-1 resulting in channel activation. Conversely, Cav-1-TRPC1 re-associates with TRPC1 when it is inactivated following refill of ER-Ca2+ stores [63]. The exact TRPC1 domains involved in these molecular rearrangements have not yet been determined. Additionally, it is not clear whether SOAR binding to TRPC1 limits the availability of STIM1 in the cell.
As noted above, activation of TRPC1 is absolutely dependent on the presence of functional Orai1 in cells. This was first reported by Ambudkar and colleagues [52, 55, 57] and subsequently by other groups [54, 64]. While knockdown of TRPC1 reduces SOCE by about 60%, loss of Orai1 or STIM1 induces complete elimination of TRPC1-mediated SOCE. Further, overexpression of STIM1 and TRPC1 failed to induce SOCE in the absence of endogenous Orai1 and TRPC1 function was not supported by a pore-deficient mutant of Orai1 (E106Q). These data conclusively established that Orai1 plays an essential role in the activation of TRPC1 by store depletion. Several models were proposed to explain these unexpected findings: (i) TRPC1 and Orai1 form a heteromeric channel; (ii) Ca2+ entering via Orai1 directly activates TRPC1; (iii) a secondary mechanism acting downstream from Orai1-mediated Ca2+ entry regulates TRPC1. This rather contentious puzzle was resolved by studies showing that store depletion initiates the assembly of TRPC1 into a Ca2+ signaling complex containing Orai1 and STIM1 that is localized within ER-PM junctions. This has now been demonstrated in HSG cells [57], human parathyroid cells [65], human liver cells [66], human colon cancer cells [67], mouse pulmonary arterial smooth muscle cells [68, 69], rat kidney fibroblasts [70], rat insulinoma cells [71] and mouse acinar cells from the pancreas and salivary glands [26, 72]. Further, the assembly of the TRPC1/Orai1/STIM1 complex is dependent on the clustering of STIM1 in ER-PM junctions and eliminated by knockdown of STIM1. Final resolution of the role of Orai1 in TRPC1 function came from the observation that Orai1-mediated Ca2+ entry triggers recruitment of TRPC1 into the plasma membrane where it is activated by STIM1 (Figure 1). In contrast, non-functional Orai1 mutants, Orai1E106Q or Orai1R91W, do not support the regulated surface expression of TRPC1 [52, 54, 57]. Knocking down TRPC1 did not completely abolished Tg-induced currents in HSG cells and the residual current that remained resembled that of ICRAC. However, loss of Orai1 abolished the store-dependent currents, which confirms that a preceding Orai1-mediated Ca2+ entry is required for TRPC1 channel function (Figure 1). Finally, it was shown that TRPC1/STIM1 form a distinct channel complex which, although residing in close proximity to Orai1/STIM1 channels, regulates specific cellular functions that are different from those controlled by Orai1 [55].
These studies elucidated that Orai1+STIM1 forms a basic SOCE channel in cells. However, if TRPC1 is present in the cell, it is regulated by store depletion and can modify the Ca2+ signal generated by Orai1. This modifies the global [Ca2+]i in cells and provides an additional Ca2+ microdomain that is associated with TRPC1. Together, these changes in [Ca2+]i promote regulation of cellular functions that are not modulated by Orai1-mediated Ca2+ entry. Acquisition of TRPC1 function is detected by a change in the phenotype of currents stimulated by ER-Ca2+ store depletion. ICRAC is detected when only Orai1 channels are present while ISOC is measured when both channels are activated (Figure 1). Treatment of cells with shTRPC1 or expression of STIM1-EE mutant abolished TRPC1 function, converting ISOC into ICRAC [55]. These findings have also been reproduced in cells overexpressing Orai1+STIM1, which display ICRAC, or Orai1+STIM1+TRPC1, which display ISOC [55, 73]. Together, these data provide conclusive evidence that TRPC1 and Orai1 form distinct STIM1-activated channels in cells. Notably, the change in the current phenotype is associated with a change in the pattern of [Ca2+]i. For example, when both channels are activated by maximum concentrations of agonist, there is a sustained elevation of [Ca2+]. However, when TRPC1 is knocked down, the sustained elevation of [Ca2+]i is converted into oscillations that are driven by Orai1 [74]. It is worth noting that the true TRPC1-mediated current has not yet been independently measured as most reported currents associated with TRPC1, e.g. ISOC, are combinations of currents generated by TRPC1+STIM1 and Orai1+STIM1 channels [55]. Furthermore, inhibition of Orai1 also eliminates TRPC1 function, making it particularly complicated to isolate TRPC1 function, independent from that of Orai1. Future studies should focus to develop methodologies to measure the electrophysiological properties of isolated TRPC1+STIM1-mediated currents.
Modulation of TRPC1 expression and activity at the plasma membrane
TRPC1 function in the plasma membrane is determined by proper targeting of the channel to specific regions in the cell periphery, near ER-PM junctions where it can be regulated by STIM1 and Orai1. Mechanisms regulating insertion and retention of TRPC1 in the plasma membrane, as well as those involved in internalization of the channel for degradation or recycling, all contribute to the level of functional TRPC1 in the plasma membrane.
Intracellular recycling of TRPC1
The level of TRPC1 in the plasma membrane is relatively low in unstimulated cells and increases upon activation of Orai1. Consistent with this, TRPC1 does not display constitutive activation when expressed either alone or with STIM1 in cells. However, TRPC1-containing vesicles can be detected in the sub-plasma membrane region localized in close proximity to the ER-PM junctions where Orai1 and STIM1 aggregate upon ER-Ca2+ store depletion [55]. This localization allows TRPC1-containing vesicles to sense the local Ca2+-signal generated by Orai1 which induces their recruitment to the plasma membrane. The transport of TRPC1 to this sub-plasma membrane region is critical for the regulation of its function. Internalization of TRPC1 from the plasma membrane, and its transport to this region, is determined by endocytic and exocytic vesicular trafficking pathways, respectively. A recent study has identified the essential vesicular compartments and Rab proteins involved in regulating intracellular trafficking of TRPC1 [75]. This study demonstrated that TRPC1 is trafficked via a fast endocytic recycling pathway which involves Rab5 and Rab4 (Figure 2A–C). Importantly, this recycling achieves clustering of TRPC1 within ER-PM junctions where STIM1 clusters in response to ER-Ca2+ store depletion. The authors reported that expression of Rab5 increased the retention of TRPC1 in early endosomes, leading to reduction of surface expression of TRPC1 and decrease in SOCE. As shown in the left panel of Figure 2B, expressing Rab5WT abolished the TRPC1-mediated ISOC, leaving behind a current that resembles ICRAC in HSG cells. Expression of the inactive dominant negative mutant of Rab5 (Rab5S34N) failed to suppress TRPC1 function, whereas the constitutively active mutant (Rab5Q79L) suppressed TRPC1 in a similar manner as the wild type. Importantly, co-expression of Rab4 with Rab5, but not STIM1 or Rab11, rescued routing of TRPC1 to the plasma membrane (recovery of TRPC1 function shown in Figure 1C). Notably, while STIM1 is required for recruitment of TRPC1 into the plasma membrane and activation after cell stimulation, it is not involved in intracellular trafficking of the channel [75]. This study also showed that internalization of TRPC1 occurs via an endocytic pathway mediated by ARF6 that is independent of clathrin and Cav-1. Overexpression of ARF6 suppressed TRPC1-mediated SOCE by attenuating the store-dependent increase in TRPC1 expression at the plasma membrane. It is worth mentioning that in unstimulated cells, ARF6 did not affect resting levels of TRPC1 in the plasma membrane, whereas Rab5 decreased those levels. More importantly, the effects of Rab5 and Arf6 were highly specific for TRPC1, leaving Orai1 and STIM1 unaffected. Generation of the TRPC1-mediated ISOC was suppressed in HSG cells expressing either Rab5 or ARF6, resulting instead in the generation of the Orai1-mediated ICRAC [75]. A very interesting observation reported in this paper was that under conditions when TRPC1 is inserted into the membrane and activated by STIM1, ISOC current is generated in response to ER-Ca2+ store depletion, while conditions that block TRPC1 trafficking to the plasma membrane results in generation of ICRAC. These data are consistent with the findings discussed above regarding the functional interactions between TRPC1 and Orai1; i.e. that plasma membrane insertion of TRPC1 is essential for its activation. Thus, mechanisms that regulate/affect plasma membrane expression of TRPC1 also control the modulation of Ca2+-signals generated by Orai1-STIM1. The physiological implications of this on cell function are discussed below.
Figure 2. Regulation of TRPC1 trafficking and function.
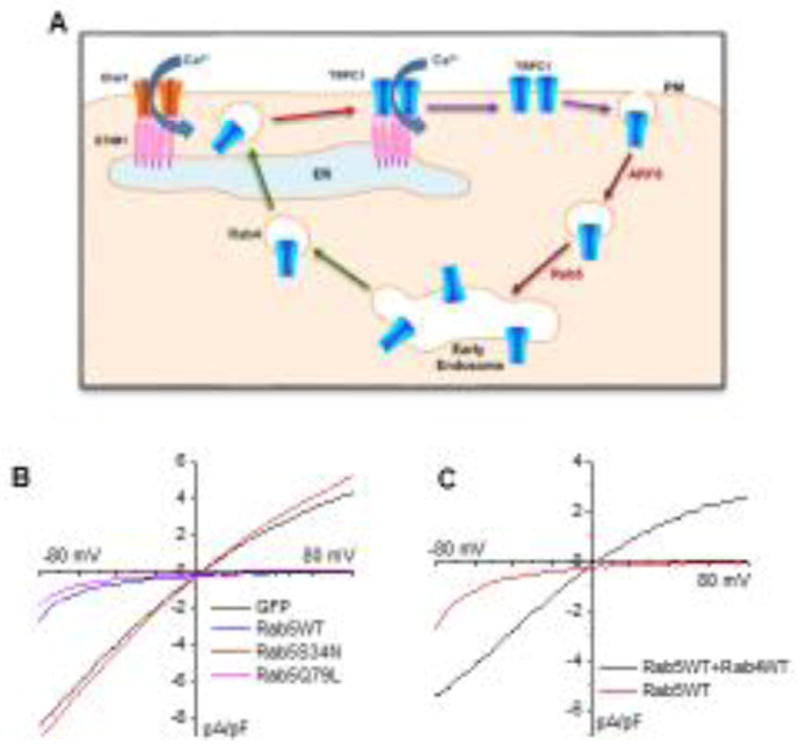
A. Model depicting the role of a rapid recycling pathway in the TRPC1 trafficking and function. TRPC1 is endocytosed from the plasma membrane by Arf6, sorted to Rab5 – early endosomes (EEs) and then is recycled back to the plasma membrane by Rab4-dependent fast recycling endosomes. This recycling carries TRPC1 to the cellular region near the plasma membrane where STIM1 clusters in response to ER – Ca2+ depletion. Within these ER–PM junctions, STIM1 interacts with and activates Orai1. Ca2+ entry via Orai1 triggers recruitment of TRPC1 from Rab4-vesicles into the plasma membrane, where the channel interacts with STIM1 and is activated. Thus, endocytic recycling via Rab4 determines clustering of TRPC1with STIM1, as well as plasma membrane insertion and function of the channel (Modified from [75]). B–C. Role of Rab4 and Rab5 on function of endogenous TRPC1 in the salivary gland cell line, HSG. Store-operated currents measured in HSG cells expressing Rab5-WT or dominant negative and spontaneously active Rab5 mutants (B) and HSG cells with Rab5 alone or Rab5+Rab4 (C).
TRPC1 insertion into the membrane
There is little information on the exact mechanisms that are involved in the final, regulated exocytosis of TRPC1-vesicles within the ER-PM junctions. Other than the important finding that it is a Ca2+ dependent process triggered by local [Ca2+]i elevations mediated by Orai1, there are no data identifying the Ca2+ sensors on TRPC1-containing vesicles or other proteins involved in driving the fusion, such as v-SNAREs and t-SNAREs. It is important to add that this final critical step is the primary determinant for the generation of functional TRPC1 channels in the plasma membrane. Based on currently available knowledge of the mechanisms of exocytosis, we can hypothesize that vesicle-associated docking and Ca2+-sensor proteins will be involved in determining where and how fast the channel-containing vesicles fuse with the plasma membrane. Ca2+-dependent exocytotic processes have been reported to involve a “kiss and run” mechanism where vesicles are fused to the plasma membrane in the presence of Ca2+ but internalized when Ca2+ is removed. The study reported by Cheng et al. argues against this type of mechanism for TRPC1 as removal of external Ca2+ after channel insertion does not alter plasma membrane levels of TRPC1 [55]. Some possible insights into the final step in TRPC1 trafficking comes from studies which show that treatment of human platelets with botulinum toxin significantly decreased SOCE by causing the cleavage and inactivation of SNAP-25. In endothelial cells, stimulation with thrombin induced assembly of TRPC1 with RhoA and IP3R and subsequent translocation of the channel to the plasma membrane. Inhibition of RhoA reduced expression of TRPC1 in the plasma membrane, adversely affected TRPC1-IP3R association, and attenuated SOCE. Further, lantrunculin inhibited TRPC1-IP3R association and SOCE suggesting a role for cytoskeletal remodeling in the process [22]. However, possible effects of this treatment on Orai1/STIM1 function need to be excluded. β-tubulin has also been reported to play a similar role as RhoA in retinal epithelial cells. Disruption of tubulin by colchicine reduced both the surface expression of TRPC1 and corresponding SOCE [76]. However, this treatment could also potentially impact STIM1 which interacts with microtubules. It will be important in future studies to delineate these final critical steps in the regulation of TRPC1.
TRPC1: Generation of distinct Ca2+ signals and functional specificity
Physiologically relevant Ca2+ signals display specific temporal and spatial patterns, in addition to the amplitude. For example, agonist-stimulated increase in [Ca2+]i displays an oscillatory pattern, depending on the cell type and stimulus intensity. Importantly, while global [Ca2+]i increases in cells are easily detected, changes in local [Ca2+]i , although not easily detected, maybe more relevant in regulation of cell function. Such local Ca2+ signals are detected by Ca2+ sensor proteins localized within the microdomain where the channel is located. An example of this is the regulation of NFAT by Orai1-mediated Ca2+ entry, sensed by CAM localized close to the channel which activates calcineurin the primary regulator of NFAT [77]. Thus, a Ca2+ signal detected within the vicinity of the channel pore in the cell is utilized for gene expression which occurs later and in a different cellular location, i.e. within the nucleus. When TRPC1 and Orai1 are present in cells, [Ca2+]i elevation resulting from SOCE is contributed by both channels. However, Ca2+ entry mediated by the two channels are utilized by the cell for regulation of distinct cellular functions. A major question which arises from these findings is how the cell senses and differentiates [Ca2+]i signals that originate from two different channels that are localized in close proximity to each other. Ong et al. recently demonstrated that endogenous Orai1 and TRPC1 channels contribute distinct local and global [Ca2+]i signals following agonist stimulation of HSG cells, in which both channels contribute to the net SOCE [74]. At relatively high [CCh] (>1 μM), Orai1-mediated Ca2+ entry generates baseline [Ca2+]i oscillations. When TRPC1 is also functional, this pattern is altered to oscillations over a sustained baseline [Ca2+]i. Interestingly, at very low [CCh] (300 nM), the baseline [Ca2+]i oscillations are Orai1-dependent but require Ca2+ entry via TRPC1 to maintain the oscillation frequency. Thus, Orai1 channel function in these cells results in the generation of [Ca2+]i oscillations, whereas TRPC1 channel function appears to increase signal amplitude and frequency. More importantly, cell functions that are Orai1-dependent (e.g. NFAT activation) are unaffected by the TRPC1-mediated global Ca2+ signals. In contrast, KCa activation is primarily dependent on TRPC1 while NFκB activation requires Ca2+ influx mediated by both channels [55].
SOCE is critical for driving fluid secretion in salivary gland acinar cells and protein secretion in pancreatic acinar cells. In both types of cells, TRPC1 is primarily localized in the middle to the basal end of the lateral membrane with some protein detected in the basal membrane. In contrast, Orai1 is mainly concentrated in the lateral membrane towards the apical pole, with some overlap with TRPC1. Following ER-Ca2+ depletion, STIM1 translocates to the lateral membrane where it colocalizes with both channels [26]. Since the initial intracellular Ca2+ release in acinar cells occurs at the apical pole, it can be suggested that Orai1 channels that are localized relatively close to this site, would be activated first. [Ca2+]i increase spreads from the apical to the basal pole of the cell resulting in a sustained global elevation of [Ca2+]i which is critical for the regulation of Ca2+-dependent cell function [11]. How exactly TRPC1 is regulated in the salivary gland acinar cell is not yet known, although lack of the channel causes severe loss of fluid secretion. The currently proposed model suggests that Orai1 provides an initial trigger pool of Ca2+ to induce recruitment of TRPC1 into the plasma membrane while activation of TRPC1 by STIM1 provides Ca2+ influx which sustains the global increase in [Ca2+]i. A key issue that needs to be resolved is how TRPC1 channels that are localized at sites away from Orai1 are inserted into the plasma membrane. Irrespective of the underlying mechanism that is involved in TRPC1 recruitment, it is clear that the spatio-temporal characteristics of the [Ca2+]i signal required to drive exocrine secretion are determined by the respective localization and assembly of TRPC1, Orai1 and STIM1. Very little is known about the localization of endogenous Orai1 or TRPC1 and the types of Ca2+ signals that are generated by each channel in various cell types. Indeed, it will be very important to examine these in greater detail to understand the relevance of their respective Ca2+ signal characteristics in the context of the cell function that each channel regulates.
What’s next for TRPC1?
There has been significant advance in understanding the mechanism regulating TRPC1 as well as physiological function of the channel. Basic steps involved in its activation and regulation are now known. A mechanism critical for TRPC1 function is the Ca2+-dependent exocytosis which is the last step prior to activation of the channel by STIM1. However, the molecular components of this process are currently unknown and further studies need to be focused on identifying them. Currents with varying characteristics have been attributed to TRPC1 and suggested to be mediated by a combination of TRPC1 with other channels. However, a pure TRPC1-mediated current has not yet been measured. Given the requirement for Orai1-mediated Ca2+ entry in TRPC1 channel function, simply knocking down Orai1 will affect both channels [52, 55]. Therefore, the challenge facing TRPC1 researchers is to determine the experimental conditions by which TRPC1 channel function can be verified independently of Orai1. As mentioned above, there have been few studies investigating the possible involvement of STIM2 in regulating TRPC1 channel function. Whether STIM2 also regulates the sensitivity of TRPC1 to low stimuli (as reported for Orai1 [78]) remains to be established. Along these lines, the nature of the ER-PM junctions where TRPC1 activation takes place is not known. Also not known is whether protein and structural components determining TRPC1 function are similar to those involved in Orai1 regulation. Finally, a major road block is the lack of specific inhibitors for TRPC channel, including TRPC1. Development of a TRPC1-specific inhibitor will allow researchers to clearly delineate the properties and functions of the TRPC1 channel.
Highlights.
SOCE is activated in response to depletion of ER-Ca2+ stores.
TRPC1 contributes to SOCE in many cells.
-TRPC1 is gated by STIM1 but also requires Orai1.
Ca2+ entry via Orai1 recruits TRPC1 to the plasma membrane .
-TRPC1 modifies [Ca2+]i signal generated by Orai1
TRPC1 has distinct role in cell function.
Acknowledgments
Work in ISA’s laboratory is supported by the Intramural Research Program of the NIH, NIDCR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Putney JW., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 2.Cahalan MD, Lewis RS. Functional roles of ion channels in lymphocytes. Seminars in immunology. 1990;2:107–117. [PubMed] [Google Scholar]
- 3.Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoth M, Penner R. Depletion of inracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 5.Parekh AB, Fleig A, Penner R. The store-operated calcium current ICRAC: nonlinear activation by InsP3 and dissociation from calcium release. Cell. 1997;89:973–980. doi: 10.1016/s0092-8674(00)80282-2. [DOI] [PubMed] [Google Scholar]
- 6.Penner R, Matthews G, Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988;334:499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- 7.Matthews G, Neher E, Penner R. Second messenger-activated calcium influx in rat peritoneal mast cells. J Physiol. 1989;418:105–130. doi: 10.1113/jphysiol.1989.sp017830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis RS, Cahalan MD. Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regul. 1989;1:99–112. doi: 10.1091/mbc.1.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X, Groschner K, Ambudkar IS. Distinct Ca2+-permeable cation currents are activated by internal Ca2+-store depletion in RBL-2H3 cells and human salivary gland cells, HSG and HSY. J Membr Biol. 2004;200:93–104. doi: 10.1007/s00232-004-0698-3. [DOI] [PubMed] [Google Scholar]
- 10.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 11.Cheng KT, Ong HL, Liu X, Ambudkar IS. Contribution and Regulation of TRPC Channels in Store-Operated Ca2+ Entry. In: Prakriya M, editor. Store-operated calcium channels. Elsevier; Amsterdam, The Netherlands: 2013. pp. 149–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- 13.Pollock JA, Assaf A, Peretz A, Nichols CD, Mojet MH, Hardie RC, Minke B. TRP, a protein essential for inositide-mediated Ca2+ influx is localized adjacent to the calcium stored in Drosophila photoreceptors. J Neurosci. 1995;15:3747–3760. doi: 10.1523/JNEUROSCI.15-05-03747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peretz A, Sandler C, Kirschfeld K, Hardie RC, Minke B. Genetic dissection of light-induced Ca2+ influx into Drosophila photoreceptors. J Gen Physiol. 1994;104:1057–1077. doi: 10.1085/jgp.104.6.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten S, Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. P Natl Acad Sci USA. 1995;92:9652–9656. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu X, Chu PB, Peyton M, Birnbaumer L. Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett. 1995;373:193–198. doi: 10.1016/0014-5793(95)01038-g. [DOI] [PubMed] [Google Scholar]
- 17.Brueggemann LI, Markun DR, Henderson KK, Cribbs LL, Byron KL. Pharmacological and electrophysiological characterization of store–operated currents and capacitative Ca2+ entry in vascular smooth muscle cells. J Pharmacol Exp Ther. 2006;317:488–499. doi: 10.1124/jpet.105.095067. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Bandyopadhyay BC, Singh BB, Groschner K, Ambudkar IS. Molecular analysis of a store-operated and 2-acetyl-sn-glycerol-sensitive non-selective cation channel. Heteromeric assembly of TRPC1-TRPC3. J Biol Chem. 2005;280:21600–21606. doi: 10.1074/jbc.C400492200. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Singh BB, Ambudkar IS. TRPC1 is required for functional store-operated Ca2+ channels. Role of acidic amino acid residues in the S5–S6 region. J Biol Chem. 2003;278:11337–11343. doi: 10.1074/jbc.M213271200. [DOI] [PubMed] [Google Scholar]
- 20.Dietrich A, Chubanov V, Kalwa H, Rost BR, Gudermann T. Cation channels of the transient receptor potential superfamily: their role in physiological and pathophysiological processes of smooth muscle cells. Pharmacol Ther. 2006;112:744–760. doi: 10.1016/j.pharmthera.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Wang W, Singh BB, Lockwich T, Jadlowiec J, O'Connell B, Wellner R, Zhu MX, Ambudkar IS. Trp1, a candidate protein for the store-operated Ca2+ influx mechanism in salivary gland cells. J Biol Chem. 2000;275:3403–3411. doi: 10.1074/jbc.275.5.3403. [DOI] [PubMed] [Google Scholar]
- 22.Mehta D, Ahmmed GU, Paria BC, Holinstat M, Voyno-Yasenetskaya T, Tiruppathi C, Minshall RD, Malik AB. RhoA interaction with inositol 1,4,5-triphosphate receptor and transient receptor potential channel-1 regulates Ca2+ entry. J Biol Chem. 2003;278:33492–33500. doi: 10.1074/jbc.M302401200. [DOI] [PubMed] [Google Scholar]
- 23.Tiruppathi C, Ahmmed GU, Vogel SM, Malik AB. Ca2+ signaling, TRP channels, and endothelial permeability. Microcirculation. 2006;13:693–708. doi: 10.1080/10739680600930347. [DOI] [PubMed] [Google Scholar]
- 24.Brownlow SL, Harper AG, Harper MT, Sage SO. A role for hTRPC1 and lipid raft domains in store-mediated calcium entry in human platelets. Cell Calcium. 2004;35:107–113. doi: 10.1016/j.ceca.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Rosado JA, Brownlow SL, Sage SO. Endogenously expressed Trp1 is involved in store-mediated Ca2+ entry by conformational coupling in human platelets. J Biol Chem. 2002;277:42157–42163. doi: 10.1074/jbc.M207320200. [DOI] [PubMed] [Google Scholar]
- 26.Hong JH, Li Q, Kim MS, Shin DM, Feske S, Birnbaumer L, Cheng KT, Ambudkar IS, Muallem S. Polarized but differential localization and recruitment of STIM1, Orai1 and TRPC channels in secretory cells. Traffic. 2011;12:232–245. doi: 10.1111/j.1600-0854.2010.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma X, Cheng KT, Wong CO, O'Neil RG, Birnbaumer L, Ambudkar IS, Yao X. Heteromeric TRPV4-C1 channels contribute to store-operated Ca2+ entry in vascular endothelial cells. Cell Calcium. 2011;50:502–509. doi: 10.1016/j.ceca.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Cheng KT, Bandyopadhyay BC, Pani B, Dietrich A, Paria BC, Swaim WD, Beech D, Yildrim E, Singh BB, Birnbaumer L, Ambudkar IS. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1−/− mice. Proc Natl Acad Sci USA. 2007;104:17542–17547. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao JN, Platoshyn O, Golovina VA, Liu L, Zou T, Marasa BS, Turner DJ, Yuan JX, Wang JY. TRPC1 functions as a store-operated Ca2+ channel in intestinal epithelial cells and regulates early mucosal restitution after wounding. Am J Physiol Gastrointest Liver Physiol. 2006;290:G782–792. doi: 10.1152/ajpgi.00441.2005. [DOI] [PubMed] [Google Scholar]
- 30.Bomben VC, Turner KL, Barclay TT, Sontheimer H. Transient receptor potential canonical channels are essential for chemotactic migration of human malignant gliomas. J Cell Physiol. 2011;226:1879–1888. doi: 10.1002/jcp.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuddapah VA, Turner KL, Seifert S, Sontheimer H. Bradykinin-induced chemotaxis of human gliomas requires the activation of KCa3.1 and ClC-3. J Neurosci. 2013;33:1427–1440. doi: 10.1523/JNEUROSCI.3980-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fiorio Pla A, Maric D, Brazer SC, Giacobini P, Liu X, Chang YH, Ambudkar IS, Barker JL. Canonical transient receptor potential 1 plays a role in basic fibroblast growth factor (bFGF)/FGF receptor-1-induced Ca2+ entry and embryonic rat neural stem cell proliferation. J Neurosci. 2005;25:2687–2701. doi: 10.1523/JNEUROSCI.0951-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li M, Chen C, Zhou Z, Xu S, Yu Z. A TRPC1-mediated increase in store-operated Ca2+ entry is required for the proliferation of adult hippocampal neural progenitor cells. Cell Calcium. 2012;51:486–496. doi: 10.1016/j.ceca.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 34.McGurk JS, Shim S, Kim JY, Wen Z, Song H, Ming GL. Postsynaptic TRPC1 function contributes to BDNF-induced synaptic potentiation at the developing neuromuscular junction. J Neurosci. 2011;31:14754–14762. doi: 10.1523/JNEUROSCI.3599-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paria BC, Vogel SM, Ahmmed GU, Alamgir S, Shroff J, Malik AB, Tiruppathi C. Tumor necrosis factor-α-induced TRPC1 expression amplifies store-operated Ca2+ influx and endothelial permeability. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1303–1313. doi: 10.1152/ajplung.00240.2004. [DOI] [PubMed] [Google Scholar]
- 36.Bollimuntha S, Singh BB, Shavali S, Sharma SK, Ebadi M. TRPC1-mediated inhibition of 1-methyl-4-phenylpyridinium ion neurotoxicity in human SH-SY5Y neuroblastoma cells. J Biol Chem. 2005;280:2132–2140. doi: 10.1074/jbc.M407384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du J, Sours-Brothers S, Coleman R, Ding M, Graham S, Kong DH, Ma R. Canonical transient receptor potential 1 channel is involved in contractile function of glomerular mesangial cells. J Am Soc Nephrol. 2007;18:1437–1445. doi: 10.1681/ASN.2006091067. [DOI] [PubMed] [Google Scholar]
- 38.Sours S, Du J, Chu S, Ding M, Zhou XJ, Ma R. Expression of canonical transient receptor potential (TRPC) proteins in human glomerular mesangial cells. Am J Physiol Renal Physiol. 2006;290:F1507–1515. doi: 10.1152/ajprenal.00268.2005. [DOI] [PubMed] [Google Scholar]
- 39.Ong EC, Nesin V, Long CL, Bai CX, Guz JL, Ivanov IP, Abramowitz J, Birnbaumer L, Humphrey MB, Tsiokas L. A TRPC1-dependent pathway regulates osteoclast formation and function. J Biol Chem. 2013;288:22219–22231. doi: 10.1074/jbc.M113.459826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dietrich A, Fahlbusch M, Gudermann T. Classical Transient Receptor Potential 1 (TRPC1): Channel or Channel Regulator? Cells. 2014;3:939–962. doi: 10.3390/cells3040939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pani B, Bollimuntha S, Singh BB. The TR (i)P to Ca2+ signaling just got STIMy: an update on STIM1 activated TRPC channels. Front Biosci. 2012;17:805–823. doi: 10.2741/3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Y, Birnbaumer L, Singh BB. TRPC1 Regulates Calcium-Activated Chloride Channels in Salivary Gland Cells. J Cell Physiol. 2015 doi: 10.1002/jcp.25017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vig M, Beck A, Billingsley JM, Lis A, Parvez S, Peinelt C, Koomoa DL, Soboloff J, Gill DL, Fleig A, Kinet JP, Penner R. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD. Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci USA. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 46.Feske S, Prakriya M, Rao A, Lewis RS. A severe defect in CRAC Ca2+ channel activation and altered K+ channel gating in T cells from immunodeficient patients. J Exp Med. 2005;202:651–662. doi: 10.1084/jem.20050687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Velicelebi G, Stauderman KA. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 50.Gwack Y, Srikanth S, Feske S, Cruz-Guilloty F, Oh-hora M, Neems DS, Hogan PG, Rao A. Biochemical and functional characterization of Orai proteins. J Biol Chem. 2007;282:16232–16243. doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
- 51.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng KT, Liu X, Ong HL, Ambudkar IS. Functional requirement for Orai1 in store-operated TRPC1-STIM1 channels. J Biol Chem. 2008;283:12935–12940. doi: 10.1074/jbc.C800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, Icrac and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 54.Kim MS, Zeng W, Yuan JP, Shin DM, Worley PF, Muallem S. Native Store-operated Ca2+ Influx Requires the Channel Function of Orai1 and TRPC1. J Biol Chem. 2009;284:9733–9741. doi: 10.1074/jbc.M808097200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng KT, Liu X, Ong HL, Swaim W, Ambudkar IS. Local Ca2+ entry via Orai1 regulates plasma membrane recruitment of TRPC1 and controls cytosolic Ca2+ signals required for specific cell functions. PLoS Biol. 2011;9:e1001025. doi: 10.1371/journal.pbio.1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lopez JJ, Salido GM, Pariente JA, Rosado JA. Interaction of STIM1 with endogenously expressed human canonical TRP1 upon depletion of intracellular Ca2+ stores. J Biol Chem. 2006;281:28254–28264. doi: 10.1074/jbc.M604272200. [DOI] [PubMed] [Google Scholar]
- 57.Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill DL, Ambudkar IS. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem. 2007;282:9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, Muallem S. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell. 2008;32:439–448. doi: 10.1016/j.molcel.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pani B, Ong HL, Liu X, Rauser K, Ambudkar IS, Singh BB. Lipid Rafts Determine Clustering of STIM1 in Endoplasmic Reticulum-Plasma Membrane Junctions and Regulation of Store-operated Ca2+ Entry (SOCE) J Biol Chem. 2008;283:17333–17340. doi: 10.1074/jbc.M800107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11:337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee KP, Choi S, Hong JH, Ahuja M, Graham S, Ma R, So I, Shin DM, Muallem S, Yuan JP. Molecular determinants mediating gating of Transient Receptor Potential Canonical (TRPC) channels by stromal interaction molecule 1 (STIM1) J Biol Chem. 2014;289:6372–6382. doi: 10.1074/jbc.M113.546556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pani B, Ong HL, Brazer SC, Liu X, Rauser K, Singh BB, Ambudkar IS. Activation of TRPC1 by STIM1 in ER-PM microdomains involves release of the channel from its scaffold caveolin-1. Proc Natl Acad Sci USA. 2009;106:20087–20092. doi: 10.1073/pnas.0905002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jardin I, Lopez JJ, Salido GM, Rosado JA. Orai1 mediates the interaction between STIM1 and hTRPC1 and regulates the mode of activation of hTRPC1-forming Ca2+ channels. J Biol Chem. 2008;283:25296–25304. doi: 10.1074/jbc.M802904200. [DOI] [PubMed] [Google Scholar]
- 65.Lu M, Branstrom R, Berglund E, Hoog A, Bjorklund P, Westin G, Larsson C, Farnebo LO, Forsberg L. Expression and association of TRPC subtypes with Orai1 and STIM1 in human parathyroid. J Mol Endocrinol. 2010;44:285–294. doi: 10.1677/JME-09-0138. [DOI] [PubMed] [Google Scholar]
- 66.Zhang ZY, Pan LJ, Zhang ZM. Functional interactions among STIM1, Orai1 and TRPC1 on the activation of SOCs in HL-7702 cells. Amino Acids. 2010;39:195–204. doi: 10.1007/s00726-009-0398-5. [DOI] [PubMed] [Google Scholar]
- 67.Gueguinou M, Harnois T, Crottes D, Uguen A, Deliot N, Gambade A, Chantome A, Haelters JP, Jaffres PA, Jourdan ML, Weber G, Soriani O, Bougnoux P, Mignen O, Bourmeyster N, Constantin B, Lecomte T, Vandier C, Potier-Cartereau M. SK3/TRPC1/Orai1 complex regulates SOCE-dependent colon cancer cell migration: a novel opportunity to modulate anti-EGFR mAb action by the alkyl-lipid Ohmline. Oncotarget. 2016;7:36168–36184. doi: 10.18632/oncotarget.8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ng LC, McCormack MD, Airey JA, Singer CA, Keller PS, Shen XM, Hume JR. TRPC1 and STIM1 mediate capacitative Ca2+ entry in mouse pulmonary arterial smooth muscle cells. J Physiol. 2009;587:2429–2442. doi: 10.1113/jphysiol.2009.172254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ng LC, O'Neill KG, French D, Airey JA, Singer CA, Tian H, Shen XM, Hume JR. TRPC1 and Orai1 interact with STIM1 and mediate capacitative Ca2+ entry caused by acute hypoxia in mouse pulmonary arterial smooth muscle cells. Am J Physiol Cell Physiol. 2012;303:C1156–1172. doi: 10.1152/ajpcell.00065.2012. [DOI] [PubMed] [Google Scholar]
- 70.Almirza WH, Peters PH, van Zoelen EJ, Theuvenet AP. Role of Trpc channels, Stim1 and Orai1 in PGF2α-induced calcium signaling in NRK fibroblasts. Cell Calcium. 2012;51:12–21. doi: 10.1016/j.ceca.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 71.Sabourin J, Le Gal L, Saurwein L, Haefliger JA, Raddatz E, Allagnat F. Store-operated Ca2+ Entry Mediated by Orai1 and TRPC1 Participates to Insulin Secretion in Rat β-Cells. J Biol Chem. 2015;290:30530–30539. doi: 10.1074/jbc.M115.682583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pani B, Liu X, Bollimuntha S, Cheng KT, Niesman IR, Zheng C, Achen VR, Patel HH, Ambudkar IS, Singh BB. Impairment of TRPC1-STIM1 channel assembly and AQP5 translocation compromise agonist-stimulated fluid secretion in mice lacking caveolin1. J Cell Sci. 2013;126:667–675. doi: 10.1242/jcs.118943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Desai PN, Zhang X, Wu S, Janoshazi A, Bolimuntha S, Putney JW, Trebak M. Multiple types of calcium channels arising from alternative translation initiation of the Orai1 message. Sci Signal. 2015;8:ra74. doi: 10.1126/scisignal.aaa8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ong HL, Jang SI, Ambudkar IS. Distinct contributions of Orai1 and TRPC1 to agonist-induced [Ca2+]i signals determine specificity of Ca2+-dependent gene expression. PLoS One. 2012;7:e47146. doi: 10.1371/journal.pone.0047146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Souza LB, Ong HL, Liu X, Ambudkar IS. Fast endocytic recycling determines TRPC1-STIM1 clustering in ER-PM junctions and plasma membrane function of the channel. Biochim Biophys Acta. 2015;1853:2709–2721. doi: 10.1016/j.bbamcr.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 76.Bollimuntha S, Cornatzer E, Singh BB. Plasma membrane localization and function of TRPC1 is dependent on its interaction with β-tubulin in retinal epithelium cells. Vis Neurosci. 2005;22:163–170. doi: 10.1017/S0952523805222058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parekh AB. Decoding cytosolic Ca2+ oscillations. Trends Biochem Sci. 2011;36:78–87. doi: 10.1016/j.tibs.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 78.Ong HL, de Souza LB, Zheng C, Cheng KT, Liu X, Goldsmith CM, Feske S, Ambudkar IS. STIM2 enhances receptor-stimulated Ca2+ signaling by promoting recruitment of STIM1 to the endoplasmic reticulum-plasma membrane junctions. Sci Signal. 2015;8:ra3. doi: 10.1126/scisignal.2005748. [DOI] [PMC free article] [PubMed] [Google Scholar]