Abstract
Despite the high prevalence of neuropsychiatric disorders, their aetiology and molecular mechanisms remain poorly understood. The zebrafish (Danio rerio) is increasingly utilized as a powerful animal model in neuropharmacology research and in vivo drug screening. Collectively, this makes zebrafish a useful tool for drug discovery and the identification of disordered molecular pathways. Here, we discuss zebrafish models of selected human neuropsychiatric disorders and drug‐induced phenotypes. As well as covering a broad range of brain disorders (from anxiety and psychoses to neurodegeneration), we also summarize recent developments in zebrafish genetics and small molecule screening, which markedly enhance the disease modelling and the discovery of novel drug targets.
Abbreviations
- AD
Alzheimer's disease
- ALS
amyotrophic lateral sclerosis
- APP
amyloid β A4 precursor protein
- BMAA
β‐mythylamino‐alanine
- CPP
conditioned place preference
- dpf
days post fertilization
- GR
glucocorticoid receptors
- HPA
hypothalamus‐pituitary‐adrenal
- HPI
hypothalamus‐pituitary‐interrenal
- HSR
heat‐shock stress response
- MO
morpholino‐modified antisense oligonucleotide
- NAC
N‐acetylcysteine
- NMJ
neuromuscular junction
- PPI
pre‐pulse inhibition
- PSEN1
presenilin1
- PSEN2
presenlinin2
- PTZ
pentylenetetrazole
- SNRI
selective noradrenaline reuptake inhibitors
- SSRI
selective 5‐HT (serotonin) reuptake inhibitor
- UCMS
unpredictable chronic mild stressors
- WGD
whole‐genome duplication
Tables of Links
| TARGETS | |
|---|---|
| Other protein targets a | Enzymes d |
| TNF‐α | AChE |
| Nuclear hormone receptors b | COX‐2 http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=257 |
| GR | PSEN1 |
| Transporters c | PSEN2 |
| VMAT2 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,c,dAlexander et al., 2015a,b,c,d).
Introduction: zebrafish as an emerging animal model
Widespread and debilitating, neuropsychiatric disorders have poorly understood mechanisms and often lack effective therapies (Garakani et al., 2006; Griebel and Holmes, 2013). The identification of clinically relevant biomarkers, the underlying neurobiological mechanisms and genetic and environmental factors of psychopathology, are critical steps in discovering efficacious treatments (Caspi and Moffitt, 2006; Nestler, 2013). While rodent models of human brain disorders have long been employed in this effort, they are often impeded by high‐costs and experimental inefficiency (Cryan and Holmes, 2005).
The zebrafish (Danio rerio) has recently received attention as a powerful animal model for a wide range of human brain disorders (Kalueff et al., 2014a,b; Stewart et al., 2015c). Zebrafish is a small, low‐cost and genetically tractable aquatic vertebrate species with a high degree of morphological, physiological and genetic homology to humans (Kalueff et al., 2014a,b). The zebrafish genome, fully sequenced, shows orthologues corresponding to ~82% of disease‐related genes in humans (Howe et al., 2013). Gene expression databases (e.g. http://zfin.org/) and atlases of zebrafish brain are also available to explore the genomics and neuroanatomy of brain areas associated with neuropsychiatric disorders (Ullmann et al., 2010; Wulliman et al., 2012; Mueller and Wullimann, 2015).
Modelling human conditions in zebrafish enables the discovery of potential therapeutic targets and their underlying molecular interactions (Table 1). For example, in a recent study the therapeutic potential of methylene blue (MB) was revealed in a mutant mTDP‐43 zebrafish; this was found by analysing the efficacy of various compounds to ameliorate the amyotrophic lateral sclerosis (ALS)‐like phenotype (Vaccaro et al., 2012). Likewise, the mTDP‐43 mutant zebrafish presents with short, abnormally branched motor axons, increased oxidative stress and an aberrant escape response (Vaccaro et al., 2012). The administration of MB, a neuroprotective agent, corrects the swimming and axonal phenotypes, while reducing the endoplasmic reticulum (ER) stress that occurs as a result of an accumulation of unfolded mutant proteins (Vaccaro et al., 2012, 2013). The identification of ER stress as a potential target for ALS drug treatment prompted further testing of the efficacy of several related agents in a G93A mtSOD1 transgenic mouse model, which led to the identification and repositioning of guanabenz, an approved drug for hypertension, as a potential new treatment for ALS (Vaccaro et al., 2013). Clearly, the zebrafish mutant model played a critical role in the identification of new ALS treatment options.
Table 1.
Particular examples of translational successes using the zebrafish model for drug discovery
| Human disease | Zebrafish model | Outcome | References |
|---|---|---|---|
| Pontocerebellar hyperplasia | Tsen54 antisense morpholino | Linking a loss‐of‐function mutation in the tsen54 gene to brain hypoplasia | (Kasher et al., 2011) |
| R44X‐loss‐of‐function mutant | Linking homozygous mutation of CLP1 (a member of the tRNA splicing endonuclease complex, TSEN) to abnormal spinal neurons, curved body, small head and eyes, and an early death in fish helped identify this mutation as a risk factor for human conditions | (Schaffer et al., 2014) | |
| Spinal cord injury | Heat shock transgenic lines | Zebrafish show high capacity for axonal regeneration following spinal cord injury, especially through the activation of Fgf signalling. Increasing Fgf signalling in mammalian spinal injury sites may encourage glial cell differentiation and lead to favourable conditions for axonal regeneration | (Goldshmit et al., 2012) |
| Schizophrenia | Tg(huC:eGFP) | The Rgs4 gene is associated with the onset and development of schizophrenia. Using the transgenic zebrafish line, rgs4 was found to be essential for axon formation, providing the first in vivo evidence supporting the role of rgs4 in schizophrenia | (Cheng et al., 2013) |
Another example of bringing laboratory findings to the bedside includes two modulators of haematopoietic stem cells (HSC) recently discovered in zebrafish (Zon, 2014), which have now become therapies in patients (North et al., 2007). The original screening of nearly 2500 small molecules in zebrafish identified 35 ‘leads’ that up‐regulate vital HSC genes, runx1 and c‐myb, 10 of which modulate the prostaglandin pathway, indicating that it is involved in HSC regulation. One of these potent candidates, 16,16‐dimethyl PGE2 (dmPGE2), was next tested in a mouse model where it was shown to increase the number of HSC grafted (North et al., 2007; Zon, 2014). Subsequent preclinical testing using a primate blood model yielded successful results, allowing the drug to move to an approved Phase I clinical trial (Goessling et al., 2011). These studies have recently yielded positive results in leukaemia patients and demonstrated the safety of the treatment, allowing it to move to Phase II testing (Cutler et al., 2013). Thus, the translatability of original zebrafish results was critical for the application of this drug in mice and in humans (see other examples of translational approaches in Table 1).
Both larval and adult zebrafish are useful preclinical in vivo models highly amenable to experimental, pharmacological and genetic manipulations (Barros et al., 2008; Brennan, 2011; Bruni et al., 2016). Due to their transparency and small size, larval zebrafish are particularly useful for optical manipulation and imaging of neural activity, as well as for large‐scale high‐throughput screens of molecular drug targets and candidate genes (Brennan, 2011; Wyart and Del Bene, 2011; Stewart et al., 2015a). Together with recent developments in genome editing techniques (e.g. CRISPR/Cas) and automated 3D behavioural phenotyping, this makes zebrafish an ideal model to study genotype–phenotype and genotype‐drug‐phenotype relationships (Kokel et al., 2010; Cachat et al., 2011b; Hwang et al., 2013; Stewart et al., 2015b). Furthermore, zebrafish develop externally to the maternal organism, reach sexual maturity fast (in ~90 days) and live for ~4–5 years in the laboratory, allowing for direct and easy analyses of pathogenetic trajectories (Kalueff et al., 2014b; Fonseka et al., 2016). Complementing larval models, adult zebrafish exhibit complex behaviours (Kalueff et al., 2013) relevant to cognition (Blaser and Vira, 2014; Gerlai, 2016), reward (Collier et al., 2014; von Trotha et al., 2014), social behaviour (Gerlai, 2014; Qin et al., 2014) and effects (Jesuthasan, 2012; Gerlai, 2013; Wang et al., 2016a). Numerous experimental paradigms have been converted for aquatic models to investigate major behavioural phenotypes, which are well‐conserved in zebrafish and mammals (Renier et al., 2007; Stewart et al., 2014a).
Rats and mice are currently the most commonly employed animals to study normal and abnormal brain functioning; nearly 1/3 of all published neuroscience papers in 2015 utilized rodent models, and <11% used other animal models, including zebrafish (Keifer and Summers, 2016). However, the rate of zebrafish publications is growing faster than any other model organisms, and experimental tools and resources for this organism are becoming increasingly available (Kalueff et al., 2014a; Wyatt et al., 2015). As a new animal model that still requires validation across multiple domains, the zebrafish has a growing utility in high‐throughput phenotyping, gene and drug screening, thus becoming increasingly useful in neuropsychopharmacology and drug discovery research. Here, we highlight recent successes and challenges in this rapidly expanding field.
Zebrafish CNS
The overall architecture, neuroanatomical features and cellular morphology of the zebrafish CNS are generally similar to those of mammals (Kalueff et al., 2014b). For example, the medial teleost pallium contains homologous structures to the mammalian amygdala (Martín et al., 2011; Mueller et al., 2011; Portavella et al., 2004; von Trotha et al., 2014) – the brain structure key for affective processing and emotionality in humans. The amygdala is pathologically hyperactivated in clinical anxiety (Rauch et al., 2000; Shin et al., 2006), social anxiety disorders (Stein et al., 2002; Furmark et al., 2004) and drug abuse (Mead et al., 1999; Buffalari and See, 2010). The zebrafish medial pallium shows increased Fos protein expression, a measure of neuronal activation, following both acute administration of D‐amphetamine and during drug‐seeking behaviour in a conditioned place preference (CPP) assay (von Trotha et al., 2014), collectively supporting the role of zebrafish medial pallium as a homologous structure to the mammalian amygdala, with evolutionarily conserved functions in modulating key behaviours.
The visualization of CNS activity through imaging methods is an important step to discern how the brain contributes to normal and abnormal behaviour. The small size and optical transparency of larval zebrafish allows for high resolution in vivo imaging and manipulation of neural activity in behaviourally active animals (Orger and Portugues, 2016). For example, the imaging of neuronal activity of larval zebrafish behaviour has been achieved by expressing a genetically‐encoded calcium indicator and recording whole‐brain activity using light‐sheet microscopy (Ahrens et al., 2013). Optogenetic neuromodulation of the transparent and genetically accessible larval zebrafish is particularly useful for investigating the neural circuitry underlying behaviours relevant to brain disorders (Knafo and Wyart, 2015). Neuronal excitation and inhibition of targeted neuronal populations has been successfully triggered in behaving larval zebrafish by expressing optogenetic actuators, including channelrhodopsin‐2 and halorhodopsin (Douglass et al., 2008; Arrenberg et al., 2009). To date, optogenetic studies in zebrafish have largely focused on several simpler behaviours, such as escape (Douglass et al., 2008), locomotion (Arrenberg et al., 2009; Ljunggren et al., 2014) and sensory processing (Kubo et al., 2014). However, optogenetic neuromodulation in zebrafish helps future research to create robust models of complex human neuropsychiatric disorders (Tye and Deisseroth, 2012; Stewart et al., 2015c). Furthermore, the imaging of neural activity in adult zebrafish is more challenging due to their larger and opaque brains. Contrast‐enhanced X‐ray micro‐computer tomography with iodine as a contrasting agent has been recently applied in adult zebrafish, and this provided 3D visualization of zebrafish brain anatomy in intact animals (Babaei et al., 2016). Optical coherence tomography has also recently been used in vivo in adult zebrafish to non‐invasively generate real‐time cross‐sectional images at high resolution, that are then reconstructed in 3D (Rao et al., 2009; Zhang and Yuan, 2015).
Neurochemistry is generally conserved across vertebrate species, as they share major neurotransmitters, receptors and transporters (Panula et al., 2006, 2010; Alsop and Vijayan, 2008). Thus, zebrafish are sensitive to major classes of pharmacological agents, such as psychostimulants (Ninkovic and Bally‐Cuif, 2006), opiates (Lau et al., 2006), ethanol (Tran et al., 2015), hallucinogens (Stewart et al., 2013), anxiolytics (Bencan et al., 2009), antidepressants (Stewart et al., 2014b) and antiopsychotics (Bruni et al., 2016). The spatial and temporal distribution of major neurotransmitter systems in zebrafish is also similar to that of mammals and has been well described in zebrafish for glutamate, GABA, acetylcholine, dopamine, 5‐HT, noradrenaline and histamine (Stewart et al., 2015c). For instance, the major pathways and receptor subtypes of the dopamine system are all present in zebrafish, with the exception of the D5 receptor (Panula et al., 2006, 2010; Maximino and Herculano, 2010). The amino acid sequence recently compared between zebrafish and humans for D1–D4 receptors shows 100% amino acid homology in the binding site for D1 and D3, and 85–95% for D2 and D4 receptors (Ek et al., 2016). Consequently, pharmacological agents that act on the dopamine system produce similar phenotypes, as dopamine antagonists or depletors impair locomotion (Giacomini et al., 2006; Kyzar et al., 2014) and agonists predictably increase zebrafish locomotion (Irons et al., 2013), paralleling similar effects in rodents (Mobini et al., 2000; Akhisaroglu et al., 2005). The dopamine agonist apomorphine produces a U‐shaped dose–response relationship for distance travelled in larval zebrafish, with low‐doses increasing time spent in the centre (anxiolytic effect) and high‐doses increasing thigmotaxis (anxiogenic effect) (Ek et al., 2016). Strikingly, by paralleling similar drug actions in rats (Ek et al., 2016), these findings further support the translational value of neuropharmacological studies in zebrafish.
There is mounting evidence implicating alterations in the neuroendocrine system in various brain disorders, including depression (Herbert, 2013; Holsboer, 2001), anxiety (Hek et al., 2013; Korte, 2001), addiction (Keedwell et al., 2001; Lovallo, 2006) and Alzheimer's disease (AD) (Belanoff et al., 2001; Wahbeh et al., 2008). Activation of the neuroendocrine hypothalamus‐pituitary‐interrenal (HPI) axis of zebrafish releases cortisol that acts on glucocorticoid receptors (GR), similar to the hypothalamus‐pituitary‐adrenal (HPA) axis in humans (Alsop and Vijayan, 2009; Griffiths et al., 2012b; Pavlidis et al., 2015). The zebrafish neuroendocrine system can be easily modulated by experimental, pharmacological and genetic manipulations, and fish cortisol can be sampled using various invasive and non‐invasive methods (Canavello et al., 2011; Pavlidis et al., 2011; Félix et al., 2013). For example, genetic mutation of the GR gene in adult grs357 mutant zebrafish disrupts negative feedback and cortisol signalling by abolishing the transcriptional activity of GR upon cortisol binding (Ziv et al., 2013). This elevates blood cortisol levels and evokes aberrant phenotypes, such as freezing, reduced exploration, impaired habituation and potentiated startle, most of which can be rescued in grs357 mutants by a selective 5‐HT (serotonin) reuptake inhibitor (SSRI) fluoxetine. This also emphasizes the high degree of evolutionarily conservation between the neuroendocrine system and its modulation between zebrafish and humans (Griffiths et al., 2012b; Ziv et al., 2013).
Zebrafish models of major CNS disorders
A clear advantage of non‐human animals (like zebrafish) for modelling brain disorders is, as already mentioned, their amenability to experimental, genetic and pharmacological manipulations. Furthermore, the behavioural phenotypes, genetic factors and pharmacological sensitivity of zebrafish often show a high degree of similarity to those reported in rodent models of brain disorders and in human clinical populations (see further).
Depression and anxiety
Stress is a common risk factor for developing affective disorders, including major depressive disorder (Strüber et al., 2014; Lucassen et al., 2016) and anxiety (Bystritsky, 2006). In mammals, the stress response is mainly mediated by the interplay between the hypothalamus, the pituitary and adrenal glands, which, collectively, form the HPA axis (Smith and Vale, 2006). Prolonged stress and hyperactivation of the HPA axis have the potential to lower GR expression, ultimately reducing the ability to adapt and cope with stress events (Howell et al., 2011) and thereby triggering depression (Zhou et al., 2011).
Depression has been extensively modelled in rodents (Deussing, 2006; Krishnan and Nestler, 2011) utilizing early life (Fumagalli et al., 2007) and adulthood stresses (Seligman et al., 1975) as well as pharmacological interventions (Barr and Markou, 2005), selective breeding or genetic engineering (Deussing, 2006). Several hallmark depression symptoms (e.g. low self‐esteem and depressed mood) are difficult to evaluate in animals, as they do not clearly display a sense of self (Deussing, 2006). In contrast, evaluation of other phenotypes, including anhedonia, comorbid anxiety or sleep and neuroendocrine disturbances, can be easily modelled in animals (Seligman and Beagley, 1975; Porsolt, 2000). In zebrafish, depressive‐like states can be evoked by a battery of unpredictable chronic mild stressors (UCMS) applied for an extended period of time (Fulcher et al., 2017; Marcon et al., 2016; Piato et al., 2011). Adult fish exposed to 7–14 days of UCMS exhibit reduced locomotion, altered shoaling behaviour and body colour (Gerlai et al., 2000). When applied to zebrafish raised in social isolation for 5 months, UCMS increases anxiety‐like behaviours in the novel tank test and reduces body weight and whole‐brain dopamine and 5‐HT metabolite, 5‐HIAA, levels, compared with zebrafish raised in groups (Fulcher et al., 2017). The effects of UCMS on exploratory and group/shoaling behaviours are reversed by fluoxetine (an SSRI) and bromazepam, a benzodiazepine anxiolytic (Marcon et al., 2016). In addition, several key pro‐inflammatory molecules, such as TNF‐α, IL‐6 and COX‐2, are differentially regulated in the zebrafish following 7 days of UCMS (Marcon et al., 2016). COX‐2 transcription is greater in individuals with recurrent depressive disorder and is hypothesized to negatively affect cognitive functioning, emotionality and synaptic homeostasis (Galecki et al., 2014). Treatment with psychotropic drugs (fluoxetine, bromazepam and nortriptyline) reduces the expression of IL‐6 and TNF‐α (Marcon et al., 2016), highlighting the sensitivity of this model to established, clinically active antidepressants. Other pharmacological interventions, such as the administration of reserpine, produce depressive‐like responses in zebrafish, including social withdrawal, motor retardation and elevated cortisol that parallel clinical symptoms of depression (Nguyen et al., 2014). Finally, several genetic models have been used to study depression in the zebrafish. For instance, larval zebrafish with mutant GR (gr/s357) display heightened physiological responses (e.g. higher whole body cortisol levels) and dysfunctional HPI axis (Griffiths et al., 2012a), similar to the effect observed in humans.
Anxiety disorders are debilitating psychiatric diseases with a lifetime prevalence of ~30%, higher than any other mental disorder (Kessler et al., 2005; Kessler, 2007). There are several types of anxiety disorder, including panic disorder, post‐traumatic stress disorder, generalized anxiety disorder and specific phobias (American Psychiatric Association, 2013). The hallmark symptom of anxiety disorders is an overwhelming and exaggerated sense of worry in response to perceived threats (American Psychiatric Association, 2013), dramatically lowering patients' quality of life and work productivity (Anxiety Disorders Association of America, 2016). The first line of treatment for anxiety disorders is typically a regimen of SSRIs or cognitive behavioural therapy (Bystritsky, 2006). Patients who do not respond to these treatments are then given selective noradrenaline reuptake inhibitors (SNRIs) or tricyclic antidepressants (Bystritsky, 2006). However, SNRIs or tricyclics increase the risk for tolerance and dependence (Otto et al., 1993), thereby limiting their use. Furthermore, although many treatments for anxiety disorders exist, approximately 30% of patients show no improvement (Brown et al., 1996). This necessitates the identification and development of treatments that are devoid of these limitations in efficacy and tolerance (Griebel and Holmes, 2013).
One of the problems with developing new treatments has been the identification of biochemical targets, genetic variants or mechanisms of action for the onset of the disorder (Bystritsky, 2006; Insel et al., 2011; Griebel and Holmes, 2013), indicating the need for animal models. The zebrafish model is particularly amenable to high‐throughput anxiolytic drug screens (Lundegaard et al., 2015). The larval zebrafish hatch from its chorion within 3 days post fertilization (dpf) and are able to inflate their swim bladder by 5 dpf and produce a broad range of behaviours (Richendrfer et al., 2012); see Figure 1. For instance, staying near the periphery of the arena (thigmotaxis) reflects anxiety‐like behaviour and is heightened following exposure to anxiogenic stimuli or drugs (Stewart et al., 2012). In adult fish, measures of anxiety include a latency to explore the top or higher tendency to remain in the bottom (Stewart et al., 2012) in the novel tank test (Figure 2). In the light–dark test, the fish are allowed to freely explore brightly light and dark arenas, but when zebrafish spend more time in the dark (scototaxis) this is indicative of an anxiety‐like response, which can be bidirectionally influenced by anxiolytic or anxiogenic treatments (Kalueff et al., 2013). Genetic models of anxiety in zebrafish are also available, including the knockdown of vesicular monoamine transporter 2 (VMAT2), which produces an anxiety‐like profile with social withdrawal and reduced exploration (Wang et al., 2016b).
Figure 1.
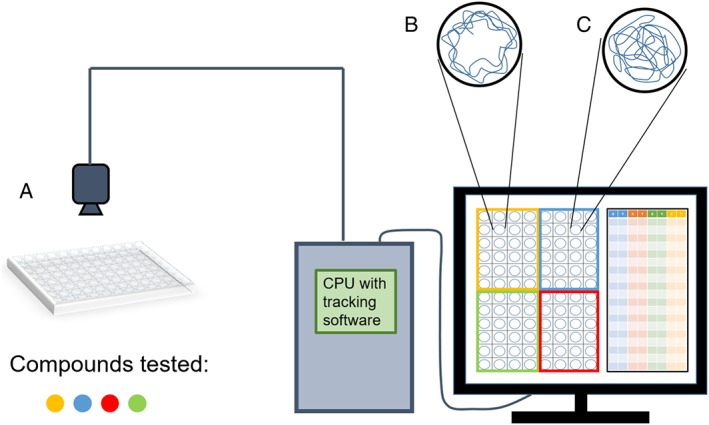
The use of automated video tracking to simultaneously assess multiple phenotypes in larval zebrafish. Panel (A) shows a 96‐well holding plate to administer several compounds to larval zebrafish. Fish behaviours are recorded by an overhead camera, and images are processed through tracking software. Swim traces garnered from the tracking software allow the researcher to assess the effects of the compounds administered. Panel (B) shows an example of a swim trace in which the larval zebrafish stay close to the walls (wall‐hugging behaviour). Panel (C) shows an example of the opposite swim pattern in which the larval zebrafish actively explore their environment, including the centre of the tank.
Figure 2.
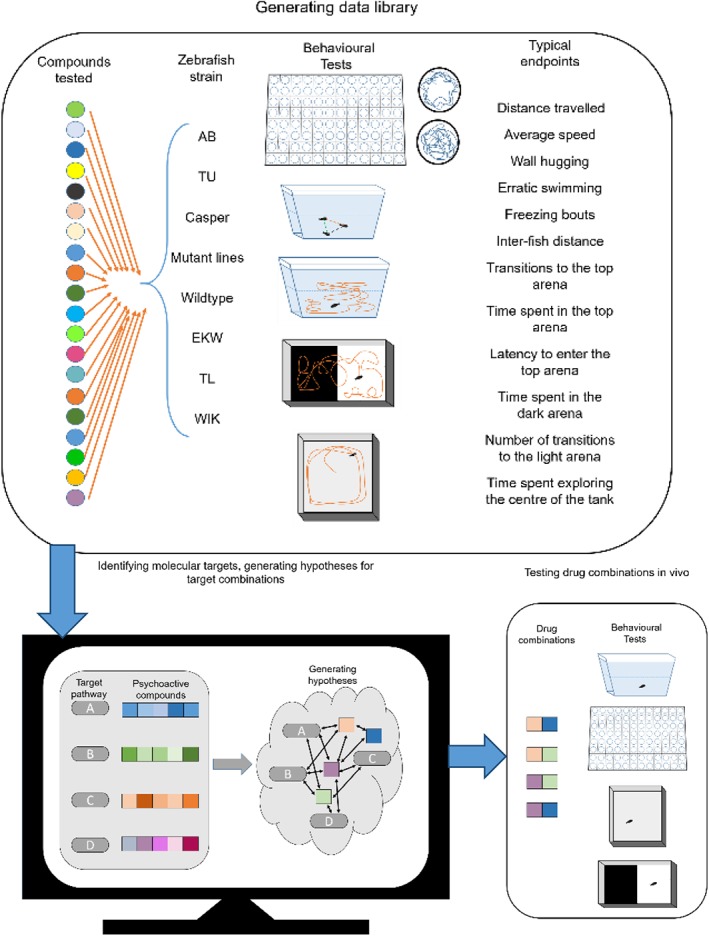
The use of computational techniques to identify novel therapeutic targets in adult zebrafish. Testing psychoactive compounds across various strains and transgenic lines of zebrafish in a wide range of behavioural and cognitive tasks can be used to generate a data library. Computational tools, such as hierarchical modelling and similarity ensemble approach (SEA), can help identify target hits, enabling the predictions about the effects of various drug combinations. The hypotheses generated may then be tested in vivo using larval or adult animals.
In the endeavour to identify new treatments for anxiety and related disorders, there has also been a call to repurpose available drugs for novel applications (Lundegaard et al., 2015). This method of drug discovery has the advantage of reducing uncertainty regarding pharmacokinetic issues or safety of the drug (Ashburn and Thor, 2004; Insel et al., 2011), thereby allowing for a more rapid drug screen and testing. For instance, N‐acetylcysteine (NAC), a common mucolytic agent and antidote for paracetamol overdose, has shown promise in the treatment of several neuropsychiatric disorders (Berk et al., 2013). NAC plays a role in maintaining oxidative balance germane to anxiety and has been shown to modulate central glutamatergic pathways (Dean et al., 2011). While a growing body of evidence supports the role of glutamate in the anxiety response, there is a clear deficit of approved glutamatergic anxiolytics (Cortese and Phan, 2005). NAC administration to adult zebrafish prevents stress‐induced anxiety (Mocelin et al., 2015), which is in line with previous reports of its clinical efficacy in depressed patients (Berk et al., 2013). In another example of drug repositioning, potential anxiolytic targets were identified using traditional cancer treatments, as cAMP mediated anxiety in the zebrafish via crosstalk of the RAS‐MAPK pathway (Lundegaard et al., 2015). This heightened anxiety‐like response is attenuated by exposure to MEK inhibitors, anti‐cancer treatment (Lundegaard et al., 2015), suggesting the MEK crosstalk as a potential alternative target for treatments of anxiety.
Epilepsy
Epilepsy, which affects approximately 50 million people worldwide (WHO, 2016), is characterized by recurrent convulsions/seizures, behavioural impairments, pathological neural activity and endocrine dysfunction (Andrea Galimberti et al., 2005; Zhang and Liu, 2008; Green et al., 2012; Engel, 2013). Epilepsy can be modelled in larval and adult zebrafish (primarily by administration of convulsant drugs and genetic modifications) and evaluated by various behavioural and physiological endpoints (Wong et al., 2010; Desmond et al., 2012; Cunliffe, 2015). Characteristic behaviours for epilepsy‐like states in adult zebrafish are hyperactivity, erratic swimming, loss of body posture, spasm‐like corkscrew swimming (Desmond et al., 2012) and electrical discharges in the CNS (Baraban et al., 2005; Zdebik et al., 2013). Experimental seizures in zebrafish can be induced by acute caffeine (250 mg·L−1), pentylenetetrazole (PTZ, 2.5 g·L−1) and picrotoxin (100 mg·L−1), causing hyperactivity, circular/corkscrew swimming, spasms and elevated whole‐body cortisol levels (Wong et al., 2010). These symptoms are suppressed by antiepileptic drugs in both larval and adult zebrafish (Green et al., 2012), enabling the discovery of more efficacious treatments for epilepsy (Alfaro et al., 2011). For instance, PTZ administration not only evokes characteristic seizures but is also accompanied by the rapid transcription of c‐fos and npas4 (Cunliffe et al., 2015), paralleling responses observed in seizure onset in mammals (Loebrich and Nedivi, 2009; Cunliffe et al., 2015). Finally, various genetic techniques enable the greater exploration of function for specific candidate genes (Teng et al., 2010; Mahmood et al., 2013; Cunliffe, 2015) or anti‐epileptic treatments using high‐throughput and rapid screening in zebrafish (Baxendale et al., 2012; Baraban et al., 2013; Cunliffe, 2015).
Psychosis
Psychosis manifests as disturbances in cognition, affect, motor activity and social behaviour (American Psychiatric Association, 2013) and is often accompanied by aberrant glutamatergic signalling (Merritt et al., 2013; Schobel et al., 2013). The glutamate NMDA receptor antagonists phencyclidine and ketamine produce psychotic symptoms in healthy volunteers and worsen the positive, negative and cognitive symptoms of patients with schizophrenia (Merritt et al., 2013). MK‐801 is a potent NMDA antagonist used to model schizophrenia in rodents, zebrafish and other animal models (Moghaddam and Jackson, 2003; Swain et al., 2004). Likewise, pre‐pulse inhibition (PPI) is the attenuation of startle response, when a weak non‐startling response is presented before the startling stimulus (Swerdlow et al., 2001). Schizophrenia patients show impaired PPI (Braff et al., 2001), which can be rescued by antipsychotic therapy (Kumari et al., 1999; Geyer et al., 2001). PPI is reliably reproduced in larval zebrafish, including genetic mutants with reduced PPI currently available (Burgess and Granato, 2007). Overall, the similarity in neural pathways and startle response in the zebrafish demonstrate their utility as an unbiased platform for the discovery of regulatory genes and drugs for antipsychotic treatment.
Alzheimer's disease
AD is a progressive neurodegenerative disease resulting in cognitive deficits, delusions, hallucinations and changes in mood and behaviour (Voisin and Vellas, 2009). One of the hallmark symptoms of AD is the development of neurofibrillary tangles and amyloid β plaques (Newman et al., 2011). There are two broad classes of AD: sporadic AD (developing at age > 65), and familial AD (fAD), developing much earlier (Rossor et al., 1984). Sporadic AD accounts for more than 95% of all AD cases (Newman et al., 2011) and is linked to the apolipoprotein E ε4 allele (Selkoe, 2001). The zebrafish orthologue of this gene is apoE (Babin et al., 1997). Early onset fAD is hereditary and has been linked to mutations in the presenilin1 (PSEN1), presenlinin2 (PSEN2) and amyloid β a4 precursor protein (APP) genes, orthologous to the zebrafish psen1, psen2, appa and appb genes (Newman et al., 2011). The injection of transcription‐blocking morpholinos for psen1 disrupts notch signalling and results in aberrant somite formation (Nornes et al., 2003, 2009; Campbell et al., 2006). Psen2 blocking produces notch‐signalling defects (Campbell et al., 2006) and alters the production of spinal cord interneurons in zebrafish (Nornes et al., 2009), paralleling phenotypes observed in psen1 −/− and psen2 −/− mice (Shen et al., 1997).
Zebrafish are also valuable for studying the aetiology of AD, especially the role of hypoxia as a putative risk factor (Newman et al., 2011). Under low‐oxygen conditions, mitochondria may release free radicals that increase oxidative stress (Bell et al., 2007; Moussavi Nik et al., 2014). Hypoxic conditions are easily reproduced in the zebrafish by reducing water oxygen levels or via chemical mimicry of hypoxia by sodium azide (Moussavi Nik et al., 2011). Similar to humans, hypoxic conditions in the larval and adult zebrafish up‐regulate several AD‐related genes, including sen1, psen2, appa, appb and bace1 (Moussavi Nik et al., 2011).
Pharmacological interventions may also help model the cognitive deficits associated with AD. For example, the cholinergic system (which mediates learning and memory) is affected by AD (Fibiger, 1991), as AD patients show reduced nicotinic (nAChR) and muscarinic (mAChR) binding sites, as well as reduced AChE activity (Perry et al., 1978; Lombardo and Maskos, 2015). The muscarinic antagonist scopolamine impairs zebrafish memory without causing locomotor deficits or anxiety‐like behaviour (Richetti et al., 2011; Cognato et al., 2012; Gupta, 2014). Pretreatment with quercetin and rutin, two flavonoids, protects against scopolamine‐induced memory impairment (Richetti et al., 2011). Flavonoids act as AChE inhibitors and can enhance learning/memory and synaptic plasticity (Havsteen, 2002; Spencer, 2008; Ahmed and Gilani, 2009). Scopolamine‐induced memory impairment in zebrafish is also ameliorated by pretreatment with physostigmine, an AChE inhibitor (Kim et al., 2010). The ability of scopolamine to produce amnesia while preserving normal locomotor activity provides evidence contributing to the involvement of the cholinergic system in fish learning and memory and lends credence to the use of the zebrafish as a tool for drug discovery and medicines that can treat neurodegenerative diseases, including AD.
Amyotrophic lateral sclerosis
ALS is a debilitating progressive neurodegenerative disorder affecting motor neurons in the brain and spinal cord (Rowland and Shneider, 2001). Zebrafish are a particularly attractive model for studying the function and dysfunction of spinal cord circuitry, due to visual transparency at early stages of life, and because there is a high degree of functional and anatomical similarity between the zebrafish spinal cord and humans (Fetcho and O'Malley, 1995; Friedrich et al., 2010; McGown et al., 2013). Similar to AD, there are two broad types of ALS: familial and sporadic ALS (Kiernan et al., 2011). Roughly 10% of ALS cases are inherited. The aetiology of ALS is poorly understood, with a high degree of variability in genetic mutations that contribute to ALS. Nevertheless, SOD1 is the most well‐understood gene to be associated with ALS (Rosen et al., 1993), and mutations in the SOD1 gene account for 20% of familial ALS cases (Valdmanis and Rouleau, 2008).
Larval zebrafish over‐expressing mutant Sod1 have abnormal neuromuscular junctions (NMJ) that worsen as the fish matures (Ramesh et al., 2010). Larval mutant fish present a progressive decrease in NMJ volume (Ramesh et al., 2010), poorer performance in the forced swim test (Plaut, 2000; Ramesh et al., 2010) and reduced responses to repeated stimulation (Ramesh et al., 2010). Together, this indicates a defect in the neural input to the muscle, rather than defects in the intrinsic properties of the muscle (Ramesh et al., 2010). Early identification of the pathogenic processes is also possible in the zebrafish through the heat‐shock stress response (HSR). The HSR mechanism refolds damaged proteins in stressed cells and is a useful tool for monitoring cellular perturbations (McGown et al., 2013). In sod1 mutant zebrafish harbouring the HSR reporter gene (hsp70‐DsRed), fluorescence facilitates disease mapping and spread throughout the brain (McGown et al., 2013). This method has also been used to identify neuroprotective compounds and biological targets with the potential to ameliorate early disease processes that are not yet fully understood (McGown et al., 2013).
In addition to the utility of the zebrafish in monitoring the progress of ALS symptoms, genetic mutants and pharmacological models also help identify the molecular mechanisms of this disease. For instance, the loss of function of the zebrafish orthologue C9orf72 leads to axonal degeneration of motor neurons and is accompanied by decreased swim speed and motility of larval zebrafish (Ciura et al., 2013). The motor deficits caused by knockdown of C9orf72 implicate it in ALS and related neurodegenerative disorders (Ciura et al., 2013). Gene‐editing techniques, such as TALEN‐ or CRISPR, may also be used to insert point mutations in the zebrafish genome (Armstrong et al., 2016), resulting in mutant zebrafish lines replicating ALS. This novel methodology also shows promise in the development of mutant models for other neuropsychiatric diseases (Armstrong et al., 2016). Pharmacological intervention with neurotoxins like β‐mythylamino‐alanin (BMAA) can also be relevant to modelling ALS. Pericardiac injection of BMAA during embryonic development alters protein homeostasis and glutamate signalling, whereas fish exposed to a sublethal dose of BMAA display reduced heart rate and abnormal spinal axis formation, but can be rescued pharmacologically (e.g. by inhibiting the endocannabinoid enzyme fatty acid amide hydrolase) (Purdie et al., 2009; Froyset et al., 2016).
Zebrafish sensitivity to CNS drug classes
The well‐documented similarity of zebrafish and mammalian neurotransmitter systems (Panula et al., 2006, 2010) contributes to the fact that zebrafish models display similar pharmacology and sensitivity to various CNS drugs. Using particular classes of neuroactive drugs as examples, we will further illustrate this aspect of zebrafish models and its relevance to the search for novel therapeutic approaches.
Antiepileptic drugs
PTZ is one of the most widely used convulsant agents in rodents and zebrafish and produces robust seizure phenotypes suppressed to varying degrees by a wide range of known anti‐epileptic drugs (Cunliffe, 2016). PTZ induction of seizures is also an effective way of medium‐throughput testing for the discovery of new anti‐epileptic treatments (Baxendale et al., 2012; Cunliffe, 2016). As small molecule screens may be conducted in zebrafish as early as 2 dpf, the efficacy of potential treatments is evaluated not only through behavioural testing but also through the monitoring of neural responses (e.g. c‐fos) (Baxendale et al., 2012). Exposure to PTZ increases C‐fos expression, which is attenuated by classic anti‐convulsant agents, as well as anti‐inflammatory agents, natural and synthetic steroids, antioxidants, vasodilators, pesticides and herbicides (Baxendale et al., 2012). However, while these drugs attenuate PTZ‐induced seizures, the mechanism of their action remains unclear. In addition to PTZ, other drugs evoke seizure‐like states in zebrafish (Winter et al., 2008). Kainic acid (KA) is a common convulsant agent in rodents and is able to produce similar effects in zebrafish (Alfaro et al., 2011). Glutamate receptor antagonists diminish KA‐induced seizures, underscoring the utility of the zebrafish model to study glutamatergic excitatory neurotransmission. Also pertinent to the study of anti‐epileptic treatment is the combination of genetic manipulation with pharmacological interventions (Cunliffe, 2015). For instance, clemizole (a histamine receptor antagonist) is effective at treating genetically‐evoked seizures in scn1lab zebrafish (Grone and Baraban, 2015), a model of Dravet syndrome (Baraban et al., 2013) caused by SCN1A mutations with spontaneous seizures insensitive to major anti‐epileptic drugs.
Antipsychotics
First‐generation (typical) antipsychotics are high‐affinity antagonists of dopamine D2 receptors and are the most effective treatment of psychoses (Lieberman et al., 2005). However, they produce severe side effects, including tremors, paranoia and anxiety (Miyamoto et al., 2005). The second‐generation ‘atypical’ antipsychotics demonstrate a lower affinity for D2 receptors and fewer side effects, relative to typical antipsychotics (Kane et al., 1988). However, there remains a great need for the identification of novel treatments for psychoses, and zebrafish models can be highly useful in this endeavour. For example, the administration of MK‐801 induces hyper‐locomotion [similar to psychomotor agitation, a characteristic symptom of schizophrenia (Seibt et al., 2010)] and social and cognitive deficits (Seibt et al., 2011). MK‐801‐induced locomotor effects are reversed by typical (haloperidol) and atypical (olanzapine and sulpiride) antipsychotics (Seibt et al., 2010). However, fish exposed to MK‐801 perform poorly in an inhibitory avoidance task, and their social and cognitive deficits are restored by atypical, but not typical, antipsychotics (Seibt et al., 2011). Importantly, atypical antipsychotics have affinities for dopaminergic as well as serotonergic, glutamatergic and other neurosignalling pathways. For example, resperidone acts via D2 and 5‐HT2 receptors and shows promise as an anxiolytic substance (Idalencio et al., 2015). Stressed fish exposed to resperidone spend more time in the top of the novel tank test, have fewer transitions to the dark in the light–dark test (Magno et al., 2015) and show lower cortisol levels (Idalencio et al., 2015). The purinergic system has been recently implicated in schizophrenia (Lara and Souza, 2000), especially since adenosine, the final product in the ectonucleotidase cascade, modulates dopamine and glutamate (Lara and Souza, 2000). In zebrafish, haloperidol reduces ATP hydrolysis and adenosine deamination, thereby reducing synaptic adenosine levels (Seibt et al., 2015). The sensitivity of zebrafish ATP hydrolysis to haloperidol suggests an extracellular mechanism of action, potentially relevant to pharmacological targets (Seibt et al., 2015).
Drugs of abuse
Substance abuse and addiction are easily modelled in larval and adult zebrafish (Stewart et al., 2011). For example, addiction, tolerance and withdrawal can be studied using aquatic CPP paradigms (Mathur and Guo, 2010; Collier and Echevarria, 2013; Collier et al., 2014). A typical CPP set‐up consists of two distinct environments, which differ in their colours, visual patterns or environmental cues (Darland and Dowling, 2001). The protocol consists of three steps: initial determination of environment preference, conditioning session and testing of final place preference. From the conditioning session, three outcomes are possible: preference for the non‐preferred side, aversion of the preferred side or no change. In zebrafish, CPP protocols generally take ~3 days (Collier et al., 2014), but may also run for several weeks (Kily et al., 2008). This protocol is widely used in zebrafish, rodents and other model organisms to investigate the behavioural effects of psychoactive compounds and associative learning (Lucke‐Wold, 2011) but, despite the ability to elucidate reward‐seeking behaviour, does not measure the drug's abuse potential (see further).
Alcohol and nicotine
Ethanol produces a characteristic dose‐dependent effect on zebrafish. At low doses (<0.5%), ethanol increases locomotion, swim speed and shoaling behaviours (Gerlai et al., 2000). A 20 min exposure to 1.00% ethanol is anxiolytic in zebrafish, whereas longer exposure to the same dose (or higher doses) impairs their locomotion and induces sedation (Gerlai et al., 2000; Rosemberg et al., 2012; Tran and Gerlai, 2013; Pannia et al., 2014). The rewarding effect of ethanol in zebrafish is seen after a single exposure to 0.25–1% (Collier et al., 2014) or 1.5% (Mathur et al., 2011), reliably changing fish CPP. A prolonged CPP paradigm (e.g. daily conditioning for 4 weeks) produces robust behavioural responses, which persist following abstinence, indicating the establishment of dependence‐related behaviour (Kily et al., 2008). Some reports evaluating the chronic ethanol CPP treatment note the development of tolerance, as indicated by lower drug‐induced hyperactivity and decreased anxiolytic effects (Gerlai et al., 2006). Drug abstinence following chronic (1 week) exposure produces robust withdrawal symptoms in adult zebrafish, including anxiety‐like behaviour and elevated cortisol (Cachat et al., 2011a).
Zebrafish also produce a wide range of dose‐dependent responses to nicotine (Levin et al., 2007; Kily et al., 2008). At low to moderate doses (e.g. 3–300 μM), nicotine evokes anxiolytic responses in the novel tank test (Levin et al., 2007) and robust CPP that persist following a period of abstinence (Kily et al., 2008). The behavioural effects of nicotine are also susceptible to genetic variation, allowing the researchers to identify genetic candidates for human nicotine addiction (Petzold et al., 2009). Furthermore, microarray analyses of whole brain samples from nicotine‐treated fish reveal an up‐regulation of several genes implicated in the development of drug dependence, including genes for calcineurin B and the hypocretin receptor, which have both been previously linked to synaptic plasticity and neurotransmission in drug dependence (Kily et al., 2008).
Cocaine and amphetamines
Administration of cocaine (5, 10 and 15 mg·L−1) to adult zebrafish produces robust arousal states, as indicated by an extension of the fins, slow circling and remaining low in the water column (Darland and Dowling, 2001). When surrounded by conspecifics, cocaine‐treated zebrafish engage in aggressive behaviour through dominance displays and chasing (Darland and Dowling, 2001). Abstinence from the drug results in withdrawal symptoms within 72 h, wherein animals experience anxiety‐like behaviour and basal hyperlocomotion (López‐Patiño et al., 2008). Withdrawal symptoms are counteracted by the administration of a non‐sedative dose of diazepam (5 μM) or cocaine (1.5 μM) (López‐Patiño et al., 2008). Cocaine also produces dose‐dependent CPP responses, with 10 mg·L−1 causing the most robust response (Darland and Dowling, 2001). The cross‐breeding of wild‐type females with males mutagenized through repeated exposure to N‐ethyl‐nitrosourea (ENA) yielded an F1 generation, outcrossing of which results in F2 generation tested for cocaine sensitivity in the CPP task. Low‐responding F2 siblings were crossbred to yield F3 generation, which display low sensitivity to cocaine in the CPP task, demonstrating a genetic basis for the altered behaviour profile (Darland and Dowling, 2001).
Methamphetamine is a potent psychostimulant with high addiction potential, and its abuse is comorbid with psychiatric disorders, including anxiety and depression (Akindipe et al., 2014). Currently, there are no effective medications for the treatments of methamphetamine abuse. The zebrafish demonstrates sensitivity to methamphetamine and is a useful model to study effective medications and methamphetamine‐related comorbidities (Mi et al., 2016). For instance, the acute administration of methamphetamine induces avoidant behaviour and increases swim speed in the open field and mirror stimulation task (Mi et al., 2016), and this can effect can be attenuated by I‐Scoulerine, an agent acting on dopaminergic and serotonergic systems (Mi et al., 2016). The cholinergic system may also play a role in modulating the rewarding effects of various psychoactive drugs, and genetic impairment of AChE does reduce amphetamine‐induced CPP in adult zebrafish (Ninkovic et al., 2006). Finally, the pharmacological inhibition of AChE reduces the addictive potential of cocaine and morphine in mice (Hikida et al., 2003), suggesting that targeting the acetylcholine system may lead to a reduction in the addictive properties of drugs.
Hallucinogens
Hallucinogenic agents can be classified under three broad categories: (1) classic serotonergic psychedelics, (2) dissociatives, which primarily act as NMDA antagonists, and (3) deliriants, which act as anticholinergic agents (Kyzar and Kalueff, 2016). Classic serotonergic psychedelics [e.g. lysergic acid diethylamide (LSD), mescaline and psilocybin] alter zebrafish locomotion, shoaling and anxiety‐like behaviours and whole body cortisol levels (Kyzar and Kalueff, 2016). Ketamine, a dissociative psychedelic, produces a dose‐dependent anxiolytic effect in the zebrafish and decreases whole body cortisol levels (De Campos et al., 2015). The deliriant psychedelic atropine affects cholinergic neural activity in the zebrafish (Park et al., 2008). Although hallucinogens remain understudied in zebrafish, the data available demonstrate their sensitivity to various known drugs and may allow for the discovery of therapeutic targets, especially given the growing recent interest in hallucinogenic agents (Kyzar and Kalueff, 2016).
Sedatives
Sedatives are generally prescribed for the treatment of anxiety disorders and produce anxiety reduction, disinhibition and sedation, mainly modulating the histaminergic, GABA‐ergic and adrenergic systems (Koob, 1992). Zebrafish share similarities with the mammalian GABAA and GABAB receptor subunits and histamine H1 receptor (Renier et al., 2007) and are highly sensitive to a wide range of sedatives. For example, high doses of chlordiazepoxide significantly reduce swim speed (Bencan et al., 2009), whereas diazepam has a biphasic effect on anxiety, with low‐to‐moderate doses reducing bottom dwelling, and higher doses causing sedation (Bencan et al., 2009). Chronic 2 week exposure to diazepam following by abstinence produces withdrawal‐like symptoms in zebrafish, including anxiety in the light dark preference task (Cachat et al., 2011a). While this highlights the utility of zebrafish as a model for sedative‐related withdrawal, there is a clear lack of studies that evaluate the rewarding or aversive effects of sedatives in zebrafish (which can easily utilize the CPP protocol to generate invaluable information on behavioural effects of these drugs).
Perspectives on small molecule and genetic screening in zebrafish
Understanding genetic and anatomical differences from mammalian models
As a member of the teleost group, the zebrafish originated from a common ancestor ~340 million years ago (Amores et al., 2011). The ancestor had undergone an additional round of whole‐genome duplication (WGD), an event that is responsible for the diversification of gene function and phenotype in zebrafish (Meyer and Schartl, 1999). Of the homologous genes, 71.4% of human genes have at least one zebrafish orthologue and 47% of human genes have a one‐to‐one zebrafish orthologue (Amores et al., 2011). Of the genes for which zebrafish have more than one orthologue, only few have been studied and functionally characterized. Thus, the current lack of understanding of many zebrafish orthologues of human genes is a potential problem with this model. For instance, humans possess three Period (Per) genes: Per1, Per2 and Per3 (Wang, 2008) encoding regulatory elements in the circadian clock, which are also responsible for growth, rest and hormone production (Pando and Sassone‐Corsi, 2002; Danilova et al., 2004; Vatine et al., 2011). Zebrafish have two per1 genes (per1a and per1b), but only one per2 and one per3 (Wang, 2008). The per1a and per1b genes show distinct temporal and spatial expression, and their roles in the circadian clock are poorly understood (Wang, 2008). Transgenic models may help to elucidate the functions of the zebrafish per1 genes and provide insights into their role in maintaining circadian rhythms. The 5‐HT transporter (sert) genes have also been duplicated during WGD in zebrafish, which possess two sert genes: serta and sertb (Wang et al., 2006). These genes have high homology to vertebrate 5‐HT transporter genes, suggesting a conservation of function (Wang et al., 2006). Thus, despite an additional WGD, it does not render the zebrafish model unusable. Rather, the study and functional identification of genes may help better understand molecular interactions, which will further clarify the efficacy of drugs and their therapeutic targets.
Furthermore, despite significant the neuroanatomical similarity discussed above, some differences between zebrafish and mammals must be critically considered. For example, while several regions in the mammalian brain do not have clear structural homologous counterparts in zebrafish, including the substantia nigra and hippocampus (Mueller et al., 2011; Panula et al., 2010), they share functional homology with selected groups of zebrafish neurons. Thus, a small population of dopaminergic cells in the posterior tuberculum is a strong candidate for the zebrafish homologue of the substantia nigra (Kaslin and Panula, 2001), as shown by neurotoxin lesion studies (Sallinen et al., 2009). Likewise, the lateral part of the zebrafish pallium contains homologous structures to the mammalian hippocampus (von Trotha et al., 2014), thereby fostering further cognitive studies in zebrafish models. One stark difference from humans is the fact that zebrafish lack a cortex, its homologue, or even molecular markers that may be used to identify a cortex region (Northcutt, 2008; Mueller et al., 2011). This aspect may limit the translation of findings between zebrafish and humans, especially on aberrant executive functioning commonly observed in psychiatric diseases (Parker et al., 2013). However, given the potential limitations of the model, it is necessary to evaluate its face and construct validity. Face validity determines whether the model resembles the disease in question, while construct validity determines whether the model measures what it has set out to measure. Because the rodent models often fulfil these validity criteria, many zebrafish behavioural tasks have been modified from rodent paradigms (Levin et al., 2007). For instance, for modelling anxiety disorders, the zebrafish has become an adept model in the identification of stress‐inducing stimuli and psychoactive agents in various novelty‐based paradigms (Levin et al., 2007; Bencan et al., 2009; Abreu et al., 2016). Deficits in cognitive and behavioural flexibility, commonly reported in patients with psychiatric diagnoses, may also be modelled in zebrafish. Behavioural flexibility – the ability to adapt responses to changing environmental conditions – is often studied in rodents using a reversal of contingencies in choice‐discrimination tasks (Ragozzino et al., 1999; Saus et al., 2010). These tasks rely on the ability of the animal to demonstrate a reversal of learning. Zebrafish have demonstrated the ability to adapt to changing environmental contingencies, and their capacity for reversal learning shows similar patterns to that observed in rodents (Colwill et al., 2005). Thus, although zebrafish may lack a proper cortex, they retain the ability to perform executive functions, such as maintaining attention and behavioural flexibility (Parker et al., 2013). However, we still know relatively little about the neural circuits and how different neurotransmitter systems may functionally interact in zebrafish (Parker et al., 2013). Identifying the function of neural circuits in this fish and their reciprocity with other systems becomes critical to understanding the molecular basis of behaviour, and in identifying therapeutic targets for diseases.
Perspectives on automated and high‐throughput screening
Zebrafish models are highly amenable to behavioural, genomic and proteomic testing (Jones and Norton, 2015; Purushothaman et al., 2015), as they combine a relative neural simplicity with behavioural complexity sufficient for studying multiple behavioural processes from sleep (Zhdanova, 2006; Rihel et al., 2010; Purushothaman et al., 2015) to anxiety (Richendrfer et al., 2012; Stewart et al., 2012). Custom‐made and commercial video‐tracking software can record a wide range of zebrafish behavioural measures, including velocity, distance travelled, place preference (e.g. top vs. bottom, light vs. dark, centre vs. periphery) and specific patterns (e.g. erratic swimming, stereotypic circling) (Pérez‐Escudero et al., 2014; Conklin et al., 2015). The automation of zebrafish video‐tracking enables several behavioural outcomes to be recorded simultaneously, removing the need to repeat the experiment and/or watch and re‐watch videos manually each time a new outcome is measured (Pérez‐Escudero et al., 2014; Conklin et al., 2015). It is also possible to record zebrafish social groups, for example, assessing fish shoaling behaviours, presently capable of tracking multiple (e.g. 8–16) animals per arena (Noldus, 2016b) to extract rich behavioural data from average inter‐fish distance to shoal polarization and cohesion (Stewart et al., 2014a). Larval zebrafish allow for recording of even more (e.g. 96) animals, tracking their swim patterns simultaneously (Noldus, 2016a). An added advantage of technological advancements in this rapidly growing field of zebrafish phenomics is the automatization of drug administration, and the computerization of stimulus exposure – e.g. in drug addiction or fear conditioning paradigms (Saverino and Gerlai, 2008), which collectively improves the standardization of testing procedures, and provides efficient data collection, increased throughput and data reproducibility (Love et al., 2004; Stewart et al., 2015a).
Zebrafish are further amenable to high‐throughput in vivo screening as their multiple behavioural parameters can be monitored in 3D (Stewart et al., 2015b). For example, the X, Y and Z swim trajectories can be traced by two cameras, generating two 2D trajectory files integrated to produce a 3D trace of the swim pattern, which can help identify unique drug‐induced phenotypic profiles (Stewart et al., 2015b). Advances in behavioural recognitions allow for a more detailed in vivo analysis of behavioural phenotype (Stewart et al., 2015a). For instance, software that can discriminate between the tail, mid‐body and nose of the zebrafish are well capable of quantifying locomotion and interpreting complex behaviours such as chasing or nipping, chasing (Kalueff et al., 2013; Stewart et al., 2015a). These methods are especially useful in polypharmacology studies using pharmacological agents that act on multiple targets (McCarroll et al., 2016). As many psychiatric disorders are linked to deficits in several neurotransmitter systems and have multigenic aetiologies (Kendler et al., 2013; Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014), this possibility in zebrafish screens becomes particularly important. Computational techniques, such as hierarchical clustering or similarity ensemble approach, also help identify target hits and prediction of target interactions with psychoactive compounds. The combination of behavioural phenotyping and computation techniques is useful in the development and discovery of new medical targets, including in vivo behavioural phenotyping of single target compounds in 2D or 3D tracking, producing their unique swim traces (thigmotaxis, scototaxis, average swim speed, etc.), identifying the compounds that produce the desired behavioural phenotype (hit compounds) and their subsequent in‐depth analyses with algorithms that predict their biological target(s) to generate hypotheses of target combinations (McCarroll et al., 2016). Once identified, multiple hit compounds can then be tested in combination in vivo (McCarroll et al., 2016), probing their ability to work in concert to achieve a desired therapeutic outcome.
Perspectives on genetic zebrafish models
As already noted, genetic manipulations are critical on animal studies to identify candidate genes associated in the aetiology of a disease. Short‐term genetic manipulation is achieved through injection of morpholino‐modified antisense oligonucleotides (MOs) (Nasevicius and Ekker, 2000) or small interfering RNA (siRNA; de Rienzo et al., 2012) to engage in loss‐of‐function studies (Kalueff et al., 2014b). MOs target specific translational inhibitors and effectively reduce gene expression (Nasevicius and Ekker, 2000). RNA interference (RNAi) is a process in which RNA molecules inhibit the translation of targeted mRNA molecules (de Rienzo et al., 2012). These methods demonstrate efficacy in targeting specific genes and in producing altered phenotypes, although, recently, the efficacy of MOs has been questioned (Kok et al., 2015; McCammon and Sive, 2015).
The development of mutant zebrafish provides a more stable behavioural phenotype, because rather than produce a knockdown of a given gene, it completely eliminates the target gene product (Amsterdam and Hopkins, 2006; Stewart et al., 2014b). Mutants are created through retroviral insertional mutagenesis, wherein DNA basepairs are integrated into the organism's pre‐existing DNA (Amsterdam and Hopkins, 2006) or through chemical mutagenesis. Chemical mutagenesis involves exposing the male zebrafish to the methylating agent ethylnitrosourea weeks before mating in order to allow the mutation to fix in the spermatogonia just before they mature to sperm (Amsterdam and Hopkins, 2006; Wienholds et al., 2003). Mutant and morphant zebrafish are used in a wide range of studies and provide a deeper understanding of the roles and importance of specific receptors and biological targets (Griffiths et al., 2012a; Haesemeyer and Schier, 2015). For instance, in developing a mutant model of autism, a highly active set of genes was discovered with a large genetic target, providing a deeper look in to the functional changes associated with gene deletion and duplication (Blaker‐Lee et al., 2012). Furthermore, the size of the genetic target, which had previously been unknown, was elucidated allowing for targeted assays in higher vertebrates and mammals (Blaker‐Lee et al., 2012). Similarly, loss of function mutations for the synaptic machinery genes stxbp1a and stxbp1b produce robust phenotypes (Grone et al., 2016). In humans, these genes are linked to various neurodevelopmental disorders and epilepsy (Saitsu et al., 2008; Carvill et al., 2014). Homozygous stxbp1a knockdown results in immobility, reduced heart rate, reduced metabolism and early death (Grone et al., 2016). Heterozygous stxbp1a knockdown produces markedly fewer deleterious effects; aside from a slight reduction in behavioural response to a startle stimulus, larval zebrafish demonstrate normal behaviour (Grone et al., 2016). Homozygous stxbp1b mutations yield zebrafish that present with epileptic seizures, along with normal mobility, metabolism and heart rate (Grone et al., 2016). The wide range of behavioural and physiological effects of the loss of function mutations for stxbp1a and stxbp1b, coupled with the functional similarity to the mammalian genes (Saitsu et al., 2008), highlight the potential for the zebrafish model to be used in the mechanistic and epigenetic study of neurodevelopmental and neuropsychiatric diseases.
Conclusion
Neuropsychiatric conditions afflict the human population globally and have tremendous personal and societal costs (Garakani et al., 2006; Griebel and Holmes, 2013). Animal models have long been used in neuropsychiatric studies to better understand human disease states and play a key role in the identification of biological and molecular targets, with the aim of developing safer and more effective treatments (Krishnan and Nestler, 2011; Keifer and Summers, 2016). Zebrafish are a promising new animal model, which continues to provide important insights into the aetiology of CNS diseases (Kalueff et al., 2014a,b). The homology of key brain regions between zebrafish and mammals underscores the utility of zebrafish models in neurobehavioral and neuropsychiatric studies. Furthermore, the conservation of neural pathways between zebrafish and mammals allows for the bi‐directional translation of findings (Renier et al., 2007; Stewart et al., 2014a). The current genetic tools, tracking techniques and statistical algorithms foster the gaining of a deeper understanding of molecular pathways, the development of new compounds or repurposing of established drugs (Stewart et al., 2015b). Taken together with the high sensitivity of zebrafish to known anxiolytic, antipsychotic and other CNS drugs, this provides researchers with a well‐rounded model organism capable of identifying molecular targets for drug treatment and empirical testing of their hypotheses (Kokel et al., 2010; Hwang et al., 2013; Stewart et al., 2015b).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
The study was coordinated through the International Zebrafish Neuroscience Research Consortium (ZNRC), and this collaboration was funded by St. Petersburg State University, Ural Federal University and Guangdong Ocean University. A.V.K. is the Chair of ZNRC, and his research is supported by the Russian Foundation for Basic Research (RFBR) grant 16‐04‐00851.
Khan, K. M. , Collier, A. D. , Meshalkina, D. A. , Kysil, E. V. , Khatsko, S. L. , Kolesnikova, T. , Morzherin, Y. Y. , Warnick, J. E. , Kalueff, A. V. , and Echevarria, D. J. (2017) Zebrafish models in neuropsychopharmacology and CNS drug discovery. British Journal of Pharmacology, 174: 1925–1944. doi: 10.1111/bph.13754.
Contributor Information
Allan V Kalueff, Email: avkalueff@gmail.com.
David J Echevarria, Email: David.echevarria@usm.edu.
References
- Abreu MS, Giacomini AC, Kalueff AV, Barcellos LJ (2016). The smell of “anxiety”: behavioral modulation by experimental anosmia in zebrafish. Physiol Behav 157: 67–71. [DOI] [PubMed] [Google Scholar]
- Ahmed T, Gilani A‐H (2009). Inhibitory effect of curcuminoids on acetylcholinesterase activity and attenuation of scopolamine‐induced amnesia may explain medicinal use of turmeric in Alzheimer's disease. Pharmacol Biochem Behav 91: 554–559. [DOI] [PubMed] [Google Scholar]
- Ahrens MB, Orger MB, Robson DN, Li JM, Keller PJ (2013). Whole‐brain functional imaging at cellular resolution using light‐sheet microscopy. Nat Methods 10: 413–420. [DOI] [PubMed] [Google Scholar]
- Akhisaroglu M, Kurtuncu M, Manev H, Uz T (2005). Diurnal rhythms in quinpirole‐induced locomotor behaviors and striatal D2/D3 receptor levels in mice. Pharmacol Biochem Behav 80: 371–377. [DOI] [PubMed] [Google Scholar]
- Akindipe T, Wilson D, Stein DJ (2014). Psychiatric disorders in individuals with methamphetamine dependence: prevalence and risk factors. Metab Brain Dis 29: 351–357. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 172: 5729–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Nuclear hormone receptors. Br J Pharmacol 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015d). The concise guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaro JM, Ripoll‐Gómez J, Burgos JS (2011). Kainate administered to adult zebrafish causes seizures similar to those in rodent models. Eur J Neurosci 33: 1252–1255. [DOI] [PubMed] [Google Scholar]
- Alsop D, Vijayan M (2009). The zebrafish stress axis: molecular fallout from the teleost‐specific genome duplication event. Gen Comp Endocrinol 161: 62–66. [DOI] [PubMed] [Google Scholar]
- Alsop D, Vijayan MM (2008). Development of the corticosteroid stress axis and receptor expression in zebrafish. Am J Physiol Regul Integr Comp Physiol 294: R711–R719. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders: DSM, 5.ed edn. Association A.P: Washington, D.C. [Google Scholar]
- Amores A, Catchen J, Ferrara A, Fontenot Q, Postlethwait JH (2011). Genome evolution and meiotic maps by massively parallel dna sequencing: spotted gar, an outgroup for the teleost genome duplication. Genetics 188: 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A, Hopkins S (2006). Mutagenesis strategies in zebrafish for identifying genes involved in development and disease. Trends Genet 22: 473–478. [DOI] [PubMed] [Google Scholar]
- Andrea Galimberti C, Magri F, Copello F, Arbasino C, Cravello L, Casu M et al. (2005). Seizure frequency and cortisol and dehydroepiandrosterone sulfate (DHEAS) levels in women with epilepsy receiving antiepileptic drug treatment. Epilepsia 46: 517–523. [DOI] [PubMed] [Google Scholar]
- Anxiety Disorders Association of America . (2016). Website . [Online] Available from ADAA.org (accessed 12/19/2016).
- Armstrong GAB, Liao M, You Z, Lissouba A, Chen BE, Drapeau P (2016). Homology directed knockin of point mutations in the zebrafish tardbp and fus genes in ALS using the CRISPR/Cas9 system. PLoS One 11: e0150188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrenberg AB, Del Bene F, Baier H (2009). Optical control of zebrafish behavior with halorhodopsin. Proc Natl Acad Sci 106: 17968–17973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburn TT, Thor KB (2004). Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov 3: 673–683. [DOI] [PubMed] [Google Scholar]
- Babaei F, Hong TLC, Yeung K, Cheng SH, Lam YW (2016). Contrast‐enhanced X‐ray micro‐computed tomography as a versatile method for anatomical studies of adult zebrafish. Zebrafish 13: 310–316. [DOI] [PubMed] [Google Scholar]
- Babin PJ, Thisse C, Durliat M, Andre M, Akimenko M‐A, Thisse B (1997). Both apolipoprotein E and A‐I genes are present in a nonmammalian vertebrate and are highly expressed during embryonic development. Proc Natl Acad Sci 94: 8622–8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban SC, Dinday MT, Hortopan GA (2013). Drug screening in Scn1a mutant zebrafish identifies clemzole as a potential Dravet syndrome treatment. Nat Commun 4: 2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban SC, Taylor MR, Castro PA, Baier H (2005). Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c‐fos expression. Neuroscience 131: 759–768. [DOI] [PubMed] [Google Scholar]
- Barr AM, Markou A (2005). Psychostimulant withdrawal as an inducing condition in animal models of depression. Neurosci Biobehav Rev 29: 675–706. [DOI] [PubMed] [Google Scholar]
- Barros TP, Alderton WK, Reynolds HM, Roach AG, Berghmans S (2008). Zebrafish: an emerging technology for in vivo pharmacological assessment to identify potential safety liabilities in early drug discovery. Br J Pharmacol 154: 1400–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxendale S, Holdsworth CJ, Santoscoy PLM, Harrison MRM, Fox J, Parkin A et al. (2012). Identification of compounds with anti‐convulsant properties in a zebrafish model of epileptic seizures. Dis Model Mech 5: 773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanoff JK, Gross K, Yager A, Schatzberg AF (2001). Corticosteroids and cognition. J Psychiatr Res 35: 127–145. [DOI] [PubMed] [Google Scholar]
- Bell E, Klimova TA, Eisenbart J, Moraes CT, Murphy MP, Buinger GRS et al. (2007). The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol 177: 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencan Z, Sledge D, Levin ED (2009). Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol Biochem Behav 94: 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Malhi GS, Gray LJ, Dean OM (2013). The promise of N‐acetylcysteine in neuropsychiatry. Trends Pharmacol Sci 34: 167–177. [DOI] [PubMed] [Google Scholar]
- Blaker‐Lee A, Gupta S, McCammon JM, De Rienzo G, Sive HL (2012). Zebrafish homologs of genes within 16p11.2, a genomic region associated with brain disorders, are active during brain development, and include two deletion dosage sensor genes. Dis Model Mech 5: 834–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser R, Vira D (2014). Experiments on learning in zebrafish (Danio rerio): a promising model of neurocognitive function. Neurosci Biobehav Rev 42: 224–231. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR (2001). Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 156: 234–258. [DOI] [PubMed] [Google Scholar]
- Brennan CH (2011). Zebrafish behavioural assays of translational relevance for the study of psychiatric disease. Rev Neurosci 22: 37–48. [DOI] [PubMed] [Google Scholar]
- Brown C, Schulberg HC, Madonia MJ, Shear MK, Houck PR (1996). Treatment outcomes for primary care patients with major depression and lifetime anxiety disorders. Am J Psychiatry 153: 1293–1300. [DOI] [PubMed] [Google Scholar]
- Bruni G, Rennekamp AJ, Velenich A, McCarroll M, Gendelev L, Fertsch E et al. (2016). Zebrafish behavioral profiling identifies multitarget antipsychotic‐like compounds. Nat Chem Biol 12: 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, See RE (2010). Amygdala mechanisms of Pavlovian psychostimulant conditioning and relapse In: Behavioral Neuroscience of Drug Addiction. Springer: Berlin, pp. 73–99. [DOI] [PubMed] [Google Scholar]
- Burgess HA, Granato M (2007). Sensorimotor gating in larval zebrafish. J Neurosci 27: 4984–4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystritsky A (2006). Treatment‐resistant anxiety disorders. Mol Psychiatry 11: 805–814. [DOI] [PubMed] [Google Scholar]
- Cachat JM, Canavello PR, Elegante MF, Bartels BK, Hart PC, Bergner CL et al. (2011a). Modeling withdrawal syndrome in zebrafish. Behav Brain Res 208: 371–376. [DOI] [PubMed] [Google Scholar]
- Cachat JM, Stewart A, Utterback E, Hart P, Gaikwad S, Wong K et al. (2011b). Three‐dimensional neurophenotyping of adult zebrafish behavior. PLoS One 6: e17597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WA, Yang H, Zetterberg H, Baulac S, Sears JA, Liu T et al. (2006). Zebrafish lacking Alzheimer presenilin enhancer 2 (Pen‐2) demonstrate excessive p53‐dependent apoptosis and neuronal loss. J Neurochem 96: 1423–1440. [DOI] [PubMed] [Google Scholar]
- Canavello PR, Cachat JM, Beeson EC, Laffoon AL, Grimes C, Haymore WA et al. (2011). Measuring endocrine (cortisol) responses of zebrafish to stress In: Zebrafish neurobehavioral protocols. Humana Press: Totowa, NJ, pp. 135–142. [Google Scholar]
- Carvill GL, Weckhuysen S, McMahon JM, Hartmann C, Moller RS, Hjalgrim H et al. (2014). GABRA1 and STXBP1: novel genetic causes of Dravet syndrome. Neurology 82: 1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE (2006). Gene–environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci 7: 583–590. [DOI] [PubMed] [Google Scholar]
- Cheng Y‐C, Scotting PJ, Hsu L‐S, Lin S‐J, Shih H‐Y, Hsieh F‐Y et al. (2013). Zebrafish rgs4 is essential for motility and axonogenesis mediated by Akt signaling. Cell Mol Life Sci 70: 935–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciura S, Lattante S, Le Ber I, Latouche M, Tostivint H, Brice A et al. (2013). Loss of function of C9orf72 causes motor deficits in a zebrafish model of amyotrophic lateral sclerosis. Ann Neurol 74: 180–187. [DOI] [PubMed] [Google Scholar]
- Cognato GP, Bortolotto JW, Blazina AR, Christoff RR, Lara DR, Vianna MR et al. (2012). Y‐Maze memory task in zebrafish (Danio rerio): the role of glutamatergic and cholinergic systems on the acquisition and consolidation periods. Neurobiol Learn Mem 98: 321–328. [DOI] [PubMed] [Google Scholar]
- Collier AD, Echevarria DJ (2013). The utility of the zebrafish model in conditioned place preference to assess the rewarding effects of drugs. Behav Pharmacol 24: 375–383. [DOI] [PubMed] [Google Scholar]
- Collier AD, Khan KM, Caramillo EM, Mohn RS, Echevarria DJ (2014). Zebrafish and conditioned place preference: a translational model of drug reward. Prog Neuropsychopharmacol Biol Psychiatry 55: 16–25. [DOI] [PubMed] [Google Scholar]
- Colwill RM, Raymond J, Ferreira L, Escudero H (2005). Visual discrimination learning in zebrafish (Danio rerio). Behav Processes 70: 19–31. [DOI] [PubMed] [Google Scholar]
- Conklin EE, Lee KL, Schlabach SA, Woods IG (2015). VideoHacking: automated tracking and quantification of locomotor behavior with open source software and off‐the‐shelf video equipment. J Undergraduate Neurosci Educ 13: A120–A125. [PMC free article] [PubMed] [Google Scholar]
- Cortese BM, Phan KL (2005). The role of glutamate in anxiety and related disorders. CNS Spectr 10: 820–830. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Holmes A (2005). The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov 4: 775–790. [DOI] [PubMed] [Google Scholar]
- Cunliffe VT (2015). Building a zebrafish toolkit for investigating the pathobiology of epilepsy and identifying new treatments for epileptic seizures. J Neurosci Methods 260: 91–95. [DOI] [PubMed] [Google Scholar]
- Cunliffe VT (2016). Building a zebrafish toolkit for investigating the pathobiology of epilepsy and identifying new treatments for epileptic seizures. J Neurosci Methods 260: 91–95. [DOI] [PubMed] [Google Scholar]
- Cunliffe VT, Baines RA, Giachello CNG, Lin W‐H, Morgan A, Reuber M et al. (2015). Epilepsy research methods update: understanding the causes of epileptic seizures and identifying new treatments using non‐mammalian model organisms. Seizure 24: 44–51. [DOI] [PubMed] [Google Scholar]
- Cutler C, Multani P, Robbins D, Kim HT, Le T, Hoggatt J et al. (2013). Prostaglandin‐modulated umbilical cord blood hematopoietic stem cell transplantation. Blood 122: 3074–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilova NP, Krupnik VE, Sugden D, Zhdanova I (2004). Melatonin stimulates cell proliferation in zebrafish embryo and accelerates its development. FASEB J 18: 751–753. [DOI] [PubMed] [Google Scholar]
- Darland T, Dowling JE (2001). Behavioral screening for cocaine sensitivity in mutagenized zebrafish. Proc Natl Acad Sci 98: 11691–11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Campos EG, Bruni AT, De Martinis BS (2015). Ketamine induces anxiolytic effects in adult zebrafish: a multivariate statistics approach. Behav Brain Res 292: 537–546. [DOI] [PubMed] [Google Scholar]
- de Rienzo G, Gutzman JH, Sive HL (2012). Efficient shRNA‐mediated inhibition of gene expression in zebrafish. Zebrafish 9: 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean OM, Giorlando F, Berk M (2011). N‐acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action. J Psychiatry Neurosci 36: 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond D, Kyzar E, Gaikwad S, Green J, Riehl R, Roth A et al. (2012). Assessing epilepsy‐related behavioral phenotypes in adult zebrafish In: Zebrafish Protocols for Neurobehavioral Research, Humana Press: New York, pp. 313–322. [Google Scholar]
- Deussing JM (2006). Animal models of depression. Drug Discov Today Dis Model 3: 375–383. [Google Scholar]
- Douglass AD, Kraves S, Deisseroth K, Schier AF, Engert F (2008). Escape behavior elicited by single, channelrhodopsin‐2‐evoked spikes in zebrafish somatosensory neurons. Curr Biol 18: 1133–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek F, Malo M, Åberg Andersson M, Wedding C, Kronborg J, Svensson P et al. (2016). Behavioral analysis of dopaminergic activation in zebrafish and rats reveals similar phenotypes. ACS Chem Nerosci 7: 633–646. [DOI] [PubMed] [Google Scholar]
- Engel J (2013). Seizures and epilepsy, Vol. 83 Oxford University Press: New York. [Google Scholar]
- Félix AS, Faustino AI, Cabral EM, Oliveira RF (2013). Noninvasive measurement of steroid hormones in zebrafish holding‐water. Zebrafish 10: 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetcho J, O'Malley DM (1995). Visualization of active neural circuitry in the spinal cord of intact zebrafish. J Neurophysiol 73: 399–406. [DOI] [PubMed] [Google Scholar]
- Fibiger HC (1991). Cholinergic mechanisms in learning, memory and dementia: a review of recent evidence. Trends Neurosci 14: 220–223. [DOI] [PubMed] [Google Scholar]
- Fonseka TM, Wen XY, Foster JA, Kennedy SH (2016). Zebrafish models of major depressive disorders. J Neurosci Res 94: 3–14. [DOI] [PubMed] [Google Scholar]
- Friedrich RW, Jacobson GA, Zhu P (2010). Circuit neuroscience in zebrafish. Curr Biol 20: R371–R381. [DOI] [PubMed] [Google Scholar]
- Froyset AK, Khan EA, Fladmark KE (2016). Quantitative proteomics analysis of zebrafish exposed to sub‐lethal dosages of β‐methyl‐amino‐L‐alanine (BMAA). Sci Rep 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher N, Tran S, Shams S, Chatterjee D, Gerlai R (2017). Neurochemical and behavioral responses to unpredictable chronic mild stress following developmental isolation: the zebrafish as a model for major depression. Zebrafish 14: 23–34. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Molteni R, Racagni G, Riva MA (2007). Stress during development: impact on neuroplasticity and relevance to psychopathology. Prog Neurobiol 81: 197–217. [DOI] [PubMed] [Google Scholar]
- Furmark T, Tillfors M, Garpenstrand H, Marteinsdottir I, Långström B, Oreland L et al. (2004). Serotonin transporter polymorphism related to amygdala excitability and symptom severity in patients with social phobia. Neurosci Lett 362: 189–192. [DOI] [PubMed] [Google Scholar]
- Galecki P, Talarowska M, Bobińska K, Szemraj J (2014). COX‐2 gene expression is correlated with cognitive function in recurrent depressive disorder. Psychiatry Res 215: 488–490. [DOI] [PubMed] [Google Scholar]
- Garakani A, Mathew S, Charney DS (2006). Neurobiology of anxiety disorders and implications for treatment. Mt Sinai J Med 73: 941–949. [PubMed] [Google Scholar]
- Gerlai R (2013). Antipredatory behavior of zebrafish: adaptive function and a tool for translational research. Evol Psychol 11: 591–605. [PubMed] [Google Scholar]
- Gerlai R (2014). Social behavior of zebrafish: from synthetic images to biological mechanisms of shoaling. J Neurosci Methods 234: 59–65. [DOI] [PubMed] [Google Scholar]
- Gerlai R (2016). Learning and memory in zebrafish (Danio rerio). Methods Cell Biol 134: 551–586. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Lahav M, Guo S, Rosenthal A (2000). Drinks like a fish: zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behav 67: 773–782. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Lee V, Blaser RE (2006). Effects of acute and chronic ethanol exposure on the behavior of adult zebrafish (Danio rerio). Pharmacol Biochem Behav 85: 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Krebs‐Thomson K, Braff DL, Swerdlow NR (2001). Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 156: 117–154. [DOI] [PubMed] [Google Scholar]
- Giacomini NJ, Rose B, Kobayashi K, Guo S (2006). Antipsychotics produce locomotor impairment in larval zebrafish. Neurotoxicol Teratol 28: 245–250. [DOI] [PubMed] [Google Scholar]
- Goessling W, Allen RS, Guan X, Jin P, Uchida N, Dovey N et al. (2011). Prostaglandin E2 enhances human cord blood stem cell xenotransplants and shows long‐term safety in preclinical nonhuman primate transplant models. Cell Stem Cell 8: 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshmit Y, Sztal TE, Jusuf PR, Hall TE, Nguyen‐Chi M, Currie PD (2012). Fgf‐dependent glial cell bridges facilitate spinal cord regeneration in zebrafish. J Neurosci 32: 7477–7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J, Collins C, Kyzar EJ, Pham M, Roth A, Gaikwad S et al. (2012). Automated high‐throughput neurophenotyping of zebrafish social behavior. J Neurosci Methods 210: 266–271. [DOI] [PubMed] [Google Scholar]
- Griebel G, Holmes A (2013). 50 years of hurdles and hope in anxiolytic drug discovery. Nat Rev Drug Discov 12: 667–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths BB, Schoonheim PJ, Ziv L, Voelker L, Baier H, Gahtan E (2012a). A zebrafish model of glucocorticoid resistance shows serotonergic modulation of the stress response. Front Behav Neurosci 6: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths BB, Schoonheim PJ, Ziv L, Voelker L, Baier H, Gahtan E (2012b). A zebrafish model of glucocorticoid resistance shows serotonergic modulation of the stress response. Front Behav Neurosci 6: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grone BP, Baraban SC (2015). Animal models in epilepsy research: legacies and new directions. Nat Neurosci 18: 339–343. [DOI] [PubMed] [Google Scholar]
- Grone BP, Marchese M, Hamling KR, Kumar MG, Krasniak CS, Sicca F et al. (2016). Epilepsy, behavioral abnormalities, and physiological comorbidities in syntaxin‐binding protein 1 (STXBP1) mutant zebrafish. PLoS One 11: e0151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta (2014). Assessment of locomotion behavior in adult zebrafish after acute exposure to different pharmacological reference compounds. Drug Development and Therapeutics 127–133. [Google Scholar]
- Haesemeyer M, Schier AF (2015). The study of psychiatric disease genes and drugs in zebrafish. Curr Opin Neurobiol 30: 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havsteen BH (2002). The biochemistry and medical significance of the flavonoids. Pharmacol Ther 96: 67–202. [DOI] [PubMed] [Google Scholar]
- Hek K, Direk N, Newson RS, Hofman A, Hoogendijk WJ, Mulder CL et al. (2013). Anxiety disorders and salivary cortisol levels in older adults: a population‐based study. Psychoneuroendocrinology 38: 300–305. [DOI] [PubMed] [Google Scholar]
- Herbert J (2013). Cortisol and depression: three questions for psychiatry. Psychol Med 43: 449–469. [DOI] [PubMed] [Google Scholar]
- Hikida T, Kitabatake Y, Pastan I, Nakanishi S (2003). Acetylcholine enhancement in the nucleus accumbens prevents addictive behaviors of cocaine and morphine. Proc Natl Acad Sci 100: 6169–6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsboer F (2001). Stress, hypercortisolism and corticosteroid receptors in depression: implicatons for therapy. J Affect Disord 62: 77–91. [DOI] [PubMed] [Google Scholar]
- Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M et al. (2013). The zebrafish reference genome sequence and its relationship to the human genome. Nature 496: 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell KR, Kutiyanawalla A, Pillai A (2011). Long‐term continuous corticosterone treatment decreases VEGF receptor‐2 expression in frontal cortex. PLoS One 6: e20198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD et al. (2013). Efficient genome editing in zebrafish using a CRISPR‐Cas system. Nat Biotechnol 31: 227–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idalencio R, Kalichak F, Rosa JGS, de Oliveira TA, Koakoski G, Gusso D et al. (2015). Waterborne risperidone decreases stress response in zebrafish. PLoS One 10: e0140800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Voon V, Nye JS, Brown VJ, Altevogt BM, Bullmore ET et al. (2011). Innovative solutions to novel drug development in mental health. Neurosci Biobehav Rev 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons T, Kelly P, Hunter D, Macphail R, Padilla S (2013). Acute administration of dopaminergic drugs has differential effects on locomotion in larval zebrafish. Pharmacol Biochem Behav 103: 792–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesuthasan S (2012). Fear, anxiety, and control in the zebrafish. Dev Neurobiol 72: 395–403. [DOI] [PubMed] [Google Scholar]
- Jones LJ, Norton W (2015). Using zebrafish to uncover the genetic and neural basis of aggression, a frequent comorbid symptom of psychiatric disorders. Behav Brain Res 276: 171–180. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Echevarria DJ, Stewart AM (2014a). Gaining translational momentum: more zebrafish models for neuroscience research. Prog Neuropsychopharmacol Biol Psychiatry 55: 1–6. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Gebhardt M, Stewart AM, Cachat JM, Brimmer M, Chawla JS et al. (2013). Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish 10: 70–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, Stewart AM, Gerlai R (2014b). Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol Sci 35: 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J, Honigfeld G, Singer J, Meltzer H (1988). Clozapine for the treatment‐resistant schizophrenic. A double‐blind comparison with chlorpromazine. Arch Gen Psychiatry 45: 789–796. [DOI] [PubMed] [Google Scholar]
- Kasher PR, Namavar Y, van Tjin P, Fluiter K, Sizarov A, Kamermans M et al. (2011). Impairment of the tRNA‐splicing endonuclease subunit 54 (tsen54) gene causes neurological abnormalities and larval death in zebrafish models of pontocerebellar hypoplasia. Hum Mol Genet 20: 1574–1580. [DOI] [PubMed] [Google Scholar]
- Kaslin J, Panula P (2001). Comparative anatomy of the histaminergic and other aminergic systems in zebrafish (Danio rerio). J Comp Neurol 440: 342–377. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Poon L, Papadopoulos AS, Marshall E, Checkley SA (2001). Salivary cortisol measurements during a medically assisted alcohol withdrawal. Addict Biol 6: 247–257. [DOI] [PubMed] [Google Scholar]
- Keifer J, Summers CH (2016). Putting the “Biology” back into “Neurobiology”: the strength of diversity in animal model systems for neuroscience research. Front Syst Neurosci 10: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Neale MC (2013). Evidence for multiple genetic factors underlying DSM‐IV criteria for major depression. JAMA Psychiat 70: 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC (2007). The global burden of anxiety and mood disorders: putting ESEMeD findings into perspective. J Clin Psychiatry 68: 10–19. [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE (2005). Prevalence severity, and comorbidity of 12‐month DSM‐IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62: 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardimn O et al. (2011). Amyotrophic lateral sclerosis. Lancet 377: 942–955. [DOI] [PubMed] [Google Scholar]
- Kily LJ, Cowe YCM, Hussain O, Patel S, McElwaine S, Cotter FE et al. (2008). Gene expression changes in a zebrafish model of drug dependency suggest conservation of neuro‐adaptation pathways. J Exp Biol 211: 1623–1634. [DOI] [PubMed] [Google Scholar]
- Kim Y‐H, Lee Y, Kim D, Jung MW, Lee C‐J (2010). Scopolamine‐induced learning impairment reversed by physostigmine in zebrafish. Neurosci Res 67: 156–161. [DOI] [PubMed] [Google Scholar]
- Knafo S, Wyart C (2015). Optogenetic neuromodulation: new tools for monitoring and breaking neural circuits. Ann Phys Rehabil Med 58: 259–264. [DOI] [PubMed] [Google Scholar]
- Kok FO, Shin M, Ni C‐W, Gupta A, Grosse AS, van Impel A et al. (2015). Reverse genetic screening reveals poor correlation between morpholino‐induced and mutant phenotypes in zebrafish. Dev Cell 31: 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokel D, Bryan J, Laggner C, White R, Cheung CYJ, Mateus R et al. (2010). Rapid behavior‐based identification of neuroactive small molecules in the zebrafish. Nat Chem Biol 6: 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (1992). Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci 13: 177–184. [DOI] [PubMed] [Google Scholar]
- Korte S (2001). Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci Biobehav Rev 25: 117–142. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ (2011). Animal models of depression: molecular perspectives. Curr Top Behav Neurosci 7: 121–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo F, Hablitzel B, Dal Maschio M, Driever W, Baier H, Arrenberg AB (2014). Functional architecture of an optic flow‐responsive area that drives horizontal eye movements in zebrafish. Neuron 81: 1344–1359. [DOI] [PubMed] [Google Scholar]
- Kumari V, Soni W, Sharma T (1999). Normalization of information processing deficits in schizophrenia with clozapine. Am J Psychiatry 156: 1046–1051. [DOI] [PubMed] [Google Scholar]
- Kyzar E, Stewart AM, Landsman S, Collins C, Gebhardt M, Robinson K et al. (2014). Behavioral effects of bidirectional modulation of brain monoamines by reserpine and d‐amphetamine in zebrafish. Brain Res 1527: 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyzar EJ, Kalueff AV (2016). Exploring hallucinogen pharmacology and psychedelic medicine with zebrafish models. Zebrafish 13: 379–390. [DOI] [PubMed] [Google Scholar]
- Lara DR, Souza DO (2000). Schizophrenia: a purinergic hypothesis. Med Hypotheses 54: 157–166. [DOI] [PubMed] [Google Scholar]
- Lau B, Bretaud S, Huang Y, Lin E, Guo S (2006). Dissociation of food and opiate preference by a genetic mutation in zebrafish. Genes Brain Behav 5: 497–505. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bencan Z, Cerutti DT (2007). Anxiolytic effects of nicotine in zebrafish. Physiol Behav 90: 54–58. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO et al. (2005). Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353: 1209–1223. [DOI] [PubMed] [Google Scholar]
- Ljunggren EE, Haupt S, Ausborn J, Ampatzis K, El Manira A (2014). Optogenetic activation of excitatory premotor interneurons is sufficient to generate coordinated locomotor activity in larval zebrafish. J Neurosci 34: 134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebrich S, Nedivi E (2009). The function of activity‐regulated genes in the nervous system. Physiol Rev 89: 1079–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo S, Maskos U (2015). Role of the nicotinic acetylcholine receptor in Alzheimer's disease pathology and treatment. Neuropharmacology 96: 255–262. [DOI] [PubMed] [Google Scholar]
- López‐Patiño M, Yu L, Cabral EM, Zhdanova I (2008). Anxiogenic effects of cocaine withdrawal in zebrafish. Physiol Behav 93: 160–171. [DOI] [PubMed] [Google Scholar]
- Lovallo WR (2006). Cortisol secretion patterns in addiction and addiction risk. Int J Psychophysiol 59: 195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love DR, Pichler FB, Dodd A, Copp BR, Greenwood DR (2004). Technology for high‐throughput screens: the present and future using zebrafish. Curr Opin Biotechnol 15: 564–571. [DOI] [PubMed] [Google Scholar]
- Lucassen P, Oomen C, Schouten M, Encinas J, Fitzsimons C (2016). Adult neurogenesis, chronic stress and depression In: Adult Neurogenesis in the Hippocampus: Health, Psychopathology, and Brain Disease. Academic Press, Elsevier: Amsterdam: p. 177–206. [Google Scholar]
- Lucke‐Wold B (2011). The varied uses of conditioned place preference in behavioral neuroscience research: an investigation of alcohol administration in model organisms. Impulse (Columbia, SC) 2011, 1–13. [PMC free article] [PubMed] [Google Scholar]
- Lundegaard PR, Anastasaki C, Grant NJ, Sillito RR, Zich J, Zeng Z et al. (2015). MEK inhibitors reverse cAMP‐mediated anxiety in zebrafish. Chem Biol 22: 1335–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magno LDP, Fontes A, Gonçalves BMN, Gouveia A (2015). Pharmacological study of the light/dark preference test in zebrafish (Danio rerio): waterborne administration. Pharmacol Biochem Behav 135: 169–176. [DOI] [PubMed] [Google Scholar]
- Mahmood F, Fu S, Cooke J, Wilson SW, Cooper JD, Russell C (2013). A zebrafish model of CLN2 disease is deficient in tripeptidyl peptidase 1 and displays progressive neurodegeneration accompanied by a reduction in proliferation. Brain 136: 1488–1507. [DOI] [PubMed] [Google Scholar]
- Marcon M, Herrmann AP, Mocelin R, Rambo CL, Koakoski G, Abreu MS et al. (2016). Prevention of unpredictable chronic stress‐related phenomena in zebrafish exposed to bromazepam, fluoxetine and nortriptyline. Psychopharmacology (Berl) 233: 3815–3824. [DOI] [PubMed] [Google Scholar]
- Martín I, Gómez A, Salas C, Puerto A, Rodríguez F (2011). Dorsomedial pallium lesions impair taste aversion learning in goldfish. Neurobiol Learn Mem 96: 297–305. [DOI] [PubMed] [Google Scholar]
- Mathur P, Berberoglu MA, Guo S (2011). Preferene for ethanol in zebrafish following a single exposure. Behav Brain Res 217: 128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur P, Guo S (2010). Use of zebrafish as a model to understand mechanisms of addiction and complex neurobehavioral phenotypes. Neurobiol Dis 40: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximino C, Herculano AM (2010). A review of monoaminergic neuropsychopharmacology in zebrafish. Zebrafish 7: 359–378. [DOI] [PubMed] [Google Scholar]
- McCammon JM, Sive H (2015). Challenges in understanding psychiatric disorders and developing therapeutics: a role for zebrafish. Dis Model Mech 8: 647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarroll M, Gendelev L, Keiser M, Kokel D (2016). Leveraging large‐scale behavioral profiling in zebrafish to explore neuroactive polypharmacology. ASC Chem Biol 11: 842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGown A, McDearmid JR, Panagiotaki N, Tong H, Al Mashhadi S, Redhead N et al. (2013). Early interneuron dysfunction in ALS: insights from a mutant sod1 zebrafish model. Ann Neurol 73: 246–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead AN, Vasilaki A, Spyraki C, Duka T, Stephens DN (1999). AMPA‐receptor involvement in c‐fos expression in the medial prefrontal cortex and amygdala dissociates neural substrates of conditioned activity and conditioned reward. Eur J Neurosci 11: 4089–4098. [DOI] [PubMed] [Google Scholar]
- Merritt K, McGuire P, Egerton A (2013). Relationship between glutamate dysfunction and symptoms and cognitive function in psychosis. Front Psych 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Schartl M (1999). Gene and genome duplications in vertebrates: the one‐to‐four (‐to‐eight in fish) rule and the evolution of novel gene functions. Curr Opin Cell Biol 11: 699–704. [DOI] [PubMed] [Google Scholar]
- Mi G, Gao Y, Yan H, Jin X, Ye E, Liu S et al. (2016). l‐Scoulerine attenuates behavioural changes induced by methamphetamine in zebrafish and mice. Behav Brain Res 298: 97–104. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA (2005). Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry 10: 79–104. [DOI] [PubMed] [Google Scholar]
- Mobini S, Chiang T‐J, Ho M‐Y, Bradshaw C, Szabadi E (2000). Comparison of the effects of clozapine, haloperidol, chlorpromazine and d‐amphetamine on performance on a time‐constrained progressive ratio schedule and on locomotor behaviour in the rat. Psychopharmacology (Berl) 152: 47–54. [DOI] [PubMed] [Google Scholar]
- Mocelin R, Herrmann AP, Marcon M, Rambo CL, Rohden A, Bevilaqua F et al. (2015). N‐acetylcysteine prevents stress‐induced anxiety behavior in zebrafish. Pharmacol Biochem Behav 139: 121–126. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Jackson ME (2003). Glutamatergic animal models of schizophrenia. Ann N Y Acad Sci 1003: 131–137. [DOI] [PubMed] [Google Scholar]
- Moussavi Nik SH, Croft K, Mori TA, Lardelli M (2014). The comparison of methods for measuring oxidative stress in zebrafish brains. Zebrafish 11: 248–254. [DOI] [PubMed] [Google Scholar]
- Moussavi Nik SH, Newman M, Lardelli M (2011). The response of HMGA1 to changes in oxygen availability is evolutionarily conserved. Exp Cell Res 317: 1503–1512. [DOI] [PubMed] [Google Scholar]
- Mueller T, Dong Z, Berberoglu MA, Guo S (2011). The dorsal pallium in zebrafish, Danio rerio (Cyprinidae, Teleostei). Brain Res 1381: 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller T, Wullimann M (2015). Atlas of early Zebrafish Brain Development: A Tool for Molecular Neurogenetics. Academic Press: Amsterdam, Netherlands. [Google Scholar]
- Nasevicius A, Ekker SC (2000). Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet 26: 216–220. [DOI] [PubMed] [Google Scholar]
- Nestler EJ (2013). The origins of molecular psychiatry. J Mol Psychiatry 1: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M, Verdile G, Martins RN, Lardelli M (2011). Zebrafish as a tool in Alzheimer's disease research. Biochim Biophys Acta (BBA) ‐ Mol Basis Dis 1812: 346–352. [DOI] [PubMed] [Google Scholar]
- Nguyen M, Stewart AM, Kalueff AV (2014). Aquatic blues: modeling depression and antidepressant action in zebrafish. Prog Neuropsychopharmacol Biol Psychiatry 55: 26–39. [DOI] [PubMed] [Google Scholar]
- Ninkovic J, Bally‐Cuif L (2006). The zebrafish as a model system for assessing the reinforcing properties of drugs of abuse. Methods 39: 262–274. [DOI] [PubMed] [Google Scholar]
- Ninkovic J, Folchert A, Makhankov YV, Neuhauss SCF, Sillaber I, Straehle U et al. (2006). Genetic identification of AChE as a positive modulator of addiction to the psychostimulant D‐amphetamine in zebrafish. J Neurobiol 66: 463–475. [DOI] [PubMed] [Google Scholar]
- Noldus LP (2016a). Daniovision. Retrieved from http://www.noldus.com/animal‐behavior‐research/products/daniovision (accessed 2/8/2016).
- Noldus LP (2016b). Shoaling behavior in zebrafish. Retrieved from http://www.noldus.com/animal‐behavior research/solutions/research‐fish/shoaling‐behavior (accessed 2/8/2016).
- Nornes S, Casper G, Esther C, Ey P, Lardelli M (2003). Developmental control of Presenilin1 expression, endoproteolysis, and interaction in zebrafish embryos. Exp Cell Res 289: 124–132. [DOI] [PubMed] [Google Scholar]
- Nornes S, Newman M, Wells S, Verdile G, Martins RN, Lardelli M (2009). Independent and cooperative action of Psen2 with Psen1 in zebrafish embryos. Exp Cell Res 315: 2791–2801. [DOI] [PubMed] [Google Scholar]
- North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM et al. (2007). Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 447: 1007–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcutt RG (2008). Forebrain evolution in bony fishes. Brain Res Bull 18: 191–205. [DOI] [PubMed] [Google Scholar]
- Orger MB, Portugues R (2016). Correlating whole brain neural activity with behavior in head‐fixed larval zebrafish. Methods Mol Biol (Clifton, NJ) 1451: 307. [DOI] [PubMed] [Google Scholar]
- Otto MW, Pollack MH, Sachs GS, Reiter SR, Meltzer‐Brody S, Rosenbaum JF (1993). Discontinuation of benzodiazepine treatment: efficacy of cognitive‐behavioral therapy for patients with panic disorder. Am J Psychiatry 150: 1485–1490. [DOI] [PubMed] [Google Scholar]
- Pando M, Sassone‐Corsi P (2002). Unraveling the mechanisms of the vertebrate circadian clock: zebrafish may light the way. Bioessays 24: 419–426. [DOI] [PubMed] [Google Scholar]
- Pannia E, Tran S, Rampersad M, Gerlai R (2014). Acute ethanol exposure induces behavioural differences in two zebrafish (Danio rerio) strains: a time course analysis. Behav Brain Res 259: 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula P, Chen Y‐C, Priyadarshini M, Kudo H, Semenova S, Sundvik M et al. (2010). The comparative neuroanatomy and neurochemistry of zebrafish CNS systems of relevance to human neuropsychiatric diseases. Neurobiol Dis 40: 46–57. [DOI] [PubMed] [Google Scholar]
- Panula P, Sallinen V, Sundvik M, Kolehmainen J, Torkko V, Tiittula A et al. (2006). Modulatory neurotransmitter systems and behavior: towards zebrafish models of neurodegenerative diseases. Zebrafish 3: 235–247. [DOI] [PubMed] [Google Scholar]
- Park E, Lee Y, Kim YK, Lee CJ (2008). Cholinergic modulation of neural activity in the telencephalon of the zebrafish. Neurosci Lett 439: 79–83. [DOI] [PubMed] [Google Scholar]
- Parker MO, Brock AJ, Walton RT, Brennan CH (2013). The role of zebrafish (Danio rerio) in dissecting the genetics and neural circuits of executive function. Front Neural Circuits 7: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis M, Sundvik M, Chen Y‐C, Panula P (2011). Adaptive changes in zebrafish brain in dominant–subordinate behavioral context. Behav Brain Res 225: 529–537. [DOI] [PubMed] [Google Scholar]
- Pavlidis M, Theodoridi A, Tsalafouta A (2015). Neuroendocrine regulation of the stress response in adult zebrafish, Danio rerio . Prog Neuropsychopharmacol Biol Psychiatry 60: 121–131. [DOI] [PubMed] [Google Scholar]
- Pérez‐Escudero A, Vicente‐Page J, Hinz RC, Arganda S, de Polavieja GG (2014). idTracker: tracking individuals in a group by automatic identification of unmarked animals. Nat Methods 11: 743–748. [DOI] [PubMed] [Google Scholar]
- Perry EK, Tomlinson BE, Blessed G, Bergmann K, Gibson PH, Perry RH (1978). Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br Med J 2: 1457–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold AM, Balciunas D, Sivasubbu S, Clark KJ, Bedell VM, Westcot SE et al. (2009). Nicotine response genetics in the zebrafish. Proc Natl Acad Sci 106: 18662–18667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piato AL, Capiotti KM, Tamborski AR, Oses JP, Barcellos LJG, Bogo MR et al. (2011). Unpredictable chronic stress model in zebrafish (Danio rerio): behavioral and physiological responses. Prog Neuropsychopharmacol Biol Psychiatry 35: 561–567. [DOI] [PubMed] [Google Scholar]
- Plaut I (2000). Effects of fin size on swimming performace, swimming behaviour and routine activity of zebrafish Danio rerio . J Exp Biol 203: 813–820. [DOI] [PubMed] [Google Scholar]
- Porsolt RD (2000). Animal models of depression: utility for transgenic research. Rev Neurosci 11: 53–58. [DOI] [PubMed] [Google Scholar]
- Portavella M, Torres B, Salas C (2004). Avoidance response in goldfish: emotional and temporal involvement of medial and lateral telencephalic pallium. J Neurosci 24: 2335–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdie EL, Samsudin S, Eddy FB, Codd GA (2009). Effects of the cyanobacterial neurotoxin β‐N‐methylamino‐L‐alanine on the early life stage development of zebrafish (Danio rerio). Aquat Toxicol 95: 279–284. [DOI] [PubMed] [Google Scholar]
- Purushothaman S, Saxena S, Meghah V, Lakshmi M, Singh SK, Brahmendra Swamy CV et al. (2015). Proteomic and gene expression analysis of zebrafish brain undergoing continuous light/dark stress. J Sleep Res 24: 458–465. [DOI] [PubMed] [Google Scholar]
- Qin M, Wong A, Seguin D, Gerlai R (2014). Induction of social behavior in zebrafish: live versus computer animated fish as stimuli. Zebrafish 11: 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Detrick S, Kesner RP (1999). Involvement of the prelimbic–infralimbic areas of the rodent prefrontal cortex in behavioral flexibility for place and response learning. J Neurosci 19: 4585–4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh T, Lyon AN, Pineda RH, Wang C, Janssen PM, Canan BD et al. (2010). A genetic model of amyotrophic lateral sclerosis in zebrafish displays phenotypic hallmarks of motoneuron disease. Dis Model Mech 3: 652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao KD, Alex A, Verma Y, Thampi S, Gupta PK (2009). Real‐time in vivo imaging of adult Zebrafish brain using optical coherence tomography. J Biophotonics 2: 288–291. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB et al. (2000). Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry 47: 769–776. [DOI] [PubMed] [Google Scholar]
- Renier C, Faraco JH, Bourgin P, Motley T, Bonaventure P, Rosa F et al. (2007). Genomic and functional conservation of sedative‐hypnotic targets in the zebrafish. Pharmacogenet Genomics 17: 237–253. [DOI] [PubMed] [Google Scholar]
- Richendrfer H, Pelkowski SD, Colwill RM, Creton R (2012). On the edge: pharmacological evidence for anxiety‐related behavior in zebrafish larvae. Behav Brain Res 228: 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richetti S, Blank M, Capiotti K, Piato A, Bogo M, Vianna M et al. (2011). Quercetin and rutin prevent scopolamine‐induced memory impairment in zebrafish. Behav Brain Res 217: 10–15. [DOI] [PubMed] [Google Scholar]
- Rihel J, Prober D, Arvanites A, Lam K, Zimmerman S, Jang S et al. (2010). Zebrafish behavioral profiling links drugs to biological targets and rest/wake regulation. Science 327: 348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosemberg DB, Braga MM, Rico EP, Loss CM, Córdova SD, Mussulini BHM et al. (2012). Behavioral effects of taurine pretreatment in zebrafish acutely exposed to ethanol. Neuropharmacology 63: 613–623. [DOI] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A et al. (1993). Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362: 59–62. [DOI] [PubMed] [Google Scholar]
- Rossor MN, Iversen LL, Reynolds GP, Mountjoy CQ, Roth M (1984). Neurochemical characteristics of early and late onset types of Alzheimer's disease. Br Med J 288: 961–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LP, Shneider NA (2001). Amyotrophic lateral sclerosis. N Engl J Med 344: 1688–1700. [DOI] [PubMed] [Google Scholar]
- Saitsu H, Kato M, Mizuguchi T, Hamada K, Osaka H, Tohyama J et al. (2008). De novo mutations in the gene encoding STXBP1 (MUNC18‐1) cause early infantile epileptic encephalopathy. Nat Genet 40: 782–788. [DOI] [PubMed] [Google Scholar]
- Sallinen J, Torkko V, Sundvik M, Reenila I, Khrustalyov D, Kaslin J et al. (2009). MPTP and MPP+ target specific aminergic cell populations in larval zebrafish. J Neurochem 108: 719–731. [DOI] [PubMed] [Google Scholar]
- Saus E, Brunet A, Armengol I, Alonso P, Crespo JM, Fernandez‐Aranda F et al. (2010). Comprehensive copy number variant (CNV) analysis of neuronal pathways genes in psychiatric disorders identifies rare variants within patients. J Psychiatr Res 44: 971–978. [DOI] [PubMed] [Google Scholar]
- Saverino C, Gerlai R (2008). The social zebrafish: behavioral responses to conspecific, heterospecific, and computer animated fish. Behav Brain Res 191: 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer AE, Eggens VRC, Caglayan AO, Reuter MS, Scott E, Coufal NG et al. (2014). CLP1 founder mutation links tRNA splicing and maturation to cerebellar development and neurodegeneration. Cell 157: 651–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium (2014). Biological insights from 108 schizophrenia‐associated genetic loci. Nature 511: 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I et al. (2013). Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 78: 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibt KJ, da Luz Oliveira R, Bogo MR, Senger MR, Bonan CD (2015). Investigation into effects of antipsychotics on ectonucleotidase and adenosine deaminase in zebrafish brain. Fish Physiol Biochem 41: 1383–1392. [DOI] [PubMed] [Google Scholar]
- Seibt KJ, da Luz Oliveira R, Zimmermann FF, Capiotti KM, Bogo MR, Ghisleni G et al. (2010). Antipsychotic drugs prevent the motor hyperactivity induced by psychotomimetic MK‐801 in zebrafish (Danio rerio). Behav Brain Res 214: 417–422. [DOI] [PubMed] [Google Scholar]
- Seibt KJ, Piato AL, da Luz Oliveira R, Capiotti KM, Vianna MRM, Bonan CD (2011). Antipsychotic drugs reverse MK‐801‐induced cognitive and social interaction deficits in zebrafish (Danio rerio). Behav Brain Res 224: 135–139. [DOI] [PubMed] [Google Scholar]
- Seligman ME, Beagley G (1975). Learned helplessness in the rat. J Comp Physiol Psychol 88: 534–541. [DOI] [PubMed] [Google Scholar]
- Seligman ME, Rosellini RA, Kozak MJ (1975). Learned helplessness in the rat: time course, immunization, and reversibility. J Comp Physiol Psychol 88: 542–547. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ (2001). Alzheimer's disease: genes, proteins, and therapy. Physiol Rev 81: 741–766. [DOI] [PubMed] [Google Scholar]
- Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S (1997). Skeletal and CNS defects in presenilin‐1‐deficient mice. Cell 89: 629–639. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK (2006). Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci 1071: 67–79. [DOI] [PubMed] [Google Scholar]
- Smith SM, Vale WW (2006). The role of the hypothalamic‐pituitary‐adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci 8: 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JP (2008). Food for thought: the role of dietary flavonoids in enhancing human memory, learning and neuro‐cognitive performance. Proc Nutr Soc 67: 238–252. [DOI] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG (2002). Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry 59: 1027–1034. [DOI] [PubMed] [Google Scholar]
- Stewart AM, Braubach O, Spitsbergen J, Gerlai R, Kalueff AV (2014a). Zebrafish models for translational neuroscience research: from tank to bedside. Trends Neurosci 37: 264–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AM, Cachat J, Gaikwad S, Robinson KS, Gebhardt M, Kalueff AV (2013). Perspectives on experimental models of serotonin syndrome in zebrafish. Neurochem Int 62: 893–902. [DOI] [PubMed] [Google Scholar]
- Stewart AM, Gaikwad S, Kyzar E, Green J, Roth A, Kalueff AV (2012). Modeling anxiety using adult zebrafish: a conceptual review. Neuropharmacology 62: 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AM, Gerlai R, Kalueff AV (2015a). Developing higher‐throughput zebrafish screens for in‐vivo CNS drug discovery. Front Behav Neurosci 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AM, Grieco F, Tegelenbosch RA, Kyzar EJ, Nguyen M, Kaluyeva A et al. (2015b). A novel 3D method of locomotor analysis in adult zebrafish: Implications for automated detection of CNS drug‐evoked phenotypes. J Neurosci Methods 255: 66–74. [DOI] [PubMed] [Google Scholar]
- Stewart AM, Grossman L, Nguyen M, Maximino C, Rosemberg DB, Echevarria DJ et al. (2014b). Aquatic toxicology of fluoxetine: understanding the knowns and the unknowns. Aquat Toxicol (Amsterdam, Netherlands) 156: 269–273. [DOI] [PubMed] [Google Scholar]
- Stewart AM, Ullmann JFP, Norton WHJ, Parker MO, Brennan CH, Gerlai R et al. (2015c). Molecular psychiatry of zebrafish. Mol Psychiatry 20: 2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AM, Wong K, Cachat JM, Gaikwad S, Kyzar E, Wu N et al. (2011). Zebrafi sh models to study drug abuse‐related phenotypes. Rev Neurosci 22: 95–105. [DOI] [PubMed] [Google Scholar]
- Strüber N, Strüber D, Roth G (2014). Impact of early adversity on glucocorticoid regulation and later mental disorders. Neurosci Biobehav Rev 38: 17–37. [DOI] [PubMed] [Google Scholar]
- Swain H, Sigstad C, Scalzo FM (2004). Effects of dizocilpine (MK‐801) on circling behavior, swimming activity, and place preference in zebrafish (Danio rerio). Neurotoxicol Teratol 26: 725–729. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL (2001). Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 156: 194–215. [DOI] [PubMed] [Google Scholar]
- Teng Y, Xie X, Walker S, Rempala G, Kozlowski D, Mumm J et al. (2010). Knockdown of zebrafish Lgi1a results in abnormal development, brain defects and a seizure‐like behavioral phenotype. Hum Mol Genet 19: 4409–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran S, Chatterjee D, Gerlai R (2015). An integrative analysis of ethanol tolerance and withdrawal in zebrafish (Danio rerio). Behav Brain Res 276: 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran S, Gerlai R (2013). Time‐course of behavioural changes induced by ethanol in zebrafish (Danio rerio). Behav Brain Res 252: 204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Deisseroth K (2012). Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci 13: 251–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmann JF, Cowin G, Kurniawan ND, Collin SP (2010). A three‐dimensional digital atlas of the zebrafish brain. Neuroimage 51: 76–82. [DOI] [PubMed] [Google Scholar]
- Vaccaro A, Patten SA, Aggad D, Julien C, Maios C, Kabashi E et al. (2013). Pharmacological reduction of ER stress protects against TDP‐43 neuronal toxicity in vivo . Neurobiol Dis 55: 64–75. [DOI] [PubMed] [Google Scholar]
- Vaccaro A, Patten SA, Ciura S, Maios C, Therrien M, Drapeau P et al. (2012). Methylene blue protects against TDP‐43 and FUS neuronal toxicity in C. elegans and D. rerio . PLoS One 7: e42117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdmanis PN, Rouleau GA (2008). Genetics of familial amyotrophic lateral sclerosis. Neurology 70: 144–152. [DOI] [PubMed] [Google Scholar]
- Vatine G, Vallone D, Gothilf Y, Foulkes N (2011). It's time to swim! Zebrafish and the circadian clock. FEBS Lett 4: 19. [DOI] [PubMed] [Google Scholar]
- Voisin T, Vellas B (2009). Diagnosis and treatment of patients with severe Alzheimer's disease. Drugs Aging 26: 135–144. [DOI] [PubMed] [Google Scholar]
- von Trotha JW, Vernier P, Bally‐Cuif L (2014). Emotions and motivated behavior converge on an amygdala‐like structure in the zebrafish. Eur J Neurol 40: 3302–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahbeh H, Kishiyama SS, Zajdel D, Oken BS (2008). Salivary cortisol awakening response in mild Alzheimer disease, caregivers, and noncaregivers. Alzheimer Dis Assoc Disord 22: 181–183. [DOI] [PubMed] [Google Scholar]
- Wang H (2008). Comparative analysis of Period genes in teleost fish genomes. J Mol Evol 67: 29–40. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li S, Liu W, Wang F, Hu L‐F, Zhong Z‐M et al. (2016a). Vesicular monoamine transporter 2 (Vmat2) knockdown elicits anxiety‐like behavior in zebrafish. Biochem Biophys Res Commun 470: 792–797. [DOI] [PubMed] [Google Scholar]
- Wang Y, Li S, Liu W, Wang F, Hu L‐F, Zhong Z et al. (2016b). Vesicular monoamine transporter 2 (Vmat2) knockdown elicits anxiety‐like behavior in zebrafish. Biochem Biophys Res Commun 470: 792–797. [DOI] [PubMed] [Google Scholar]
- Wang Y, Takai R, Yoshioka H, Shirabe K (2006). Characterization and expression of serotonin transporter genes in zebrafish. J Exp Med 208: 267–274. [DOI] [PubMed] [Google Scholar]
- WHO (2016). Epilepsy (Fact sheet No. 999). World Health Organization.
- Wienholds E, van Eeden F, Kosters M, Mude J, Plasterk RHA, Cuppin E (2003). Efficient target‐selected mutagenesis in zebrafish. Genome Res 13: 2700–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter MJ, Redfern WS, Hayfield AJ, Owen SF, Valentin J‐P, Hutchinson TH (2008). Validation of a larval zebrafish locomotor assay for assessing the seizure liability of early stage development drugs. J Pharmacol Toxicol Methods 57: 176–187. [DOI] [PubMed] [Google Scholar]
- Wong K, Stewart AM, Gilder T, Wu N, Frank K, Gaikwad S et al. (2010). Modeling seizure‐related behavioral and endocrine phenotypes in adult zebrafish. Brain Res 1348: 209–215. [DOI] [PubMed] [Google Scholar]
- Wulliman MF, Rupp B, Reichert H (2012). Neuroanatomy of the Zebrafish Brain: A Topological Atlas. Birkhäuser: Basel, Switzerland. [Google Scholar]
- Wyart C, Del Bene F (2011). Let there be light: zebrafish neurobiology and the optogenetic revolution. Rev Neurosci 22: 121–130. [DOI] [PubMed] [Google Scholar]
- Wyatt C, Bartoszek EM, Yaksi E (2015). Methods for studying the zebrafish brain: past, present and future. Eur J Neurosci 42: 1746–1763. [DOI] [PubMed] [Google Scholar]
- Zdebik AA, Mahmood F, Stanescu HC, Kleta R, Bockenhauer D, Russell C (2013). Epilepsy in kcnj10 morphant zebrafish assessed with a novel method for long‐term EEG recordings. PLoS One 8: e79765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, & Yuan Z (2015). In vivo non‐invasive imaging of the adult zebrafish brain with a 1325 nm long range spectral‐domain optical coherence tomography system.1–3. [DOI] [PMC free article] [PubMed]
- Zhang S‐W, Liu Y‐X (2008). Changes of serum adrenocorticotropic hormone and cortisol levels during sleep seizures. Neurosci Bull 24: 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhdanova I (2006). Sleep in zebrafish. Zebrafish 3: 215–226. [DOI] [PubMed] [Google Scholar]
- Zhou QG, Zhu LJ, Wu HY, Luo CX, Chang L, Zhu DY (2011). Hippocampal neuronal nitric oxide synthase mediates the stress‐related depressive behaviors of glucocorticoids by downregulating glucocorticoid receptor. J Neurosci 31: 7579–7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv L, Muto A, Schoonheim PJ, Meijsing SH, Strasser D, Ingraham HA et al. (2013). An affective disorder in zebrafish with mutation of the glucocorticoid receptor. Mol Psychiatry 18: 681–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zon LI (2014). Translational research: the path for bringing discovery to patients. Cell Stem Cell 14: 146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]


