Abstract
The administration of intravesical chemotherapy or BCG often can prolong the progression-free interval after initial transurethral resection in select bladder cancer (BCa) patients. However, 60% of these patients will recur and up to 30% of patients with recurrent BCa will progress and succumb to their disease over a 15 year period, while another 50% will cystectomy in an attempt to control their disease. Thus better therapeutic strategies are needed for patients who have failed intravesical therapy. In this article, we report the treatment of a 91-year-old man with NMIBC with high-risk features that had failed multiple intravesical therapies.
Keywords: Bladder cancer, Bacillus Calmette–Guérin, Interleukin 15, Natural killer, Non-muscle invasive bladder cancer
Introduction
The administration of intravesical chemotherapy or BCG can often prolong the progression-free interval after initial transurethral resection in select bladder cancer (BCa) patients.1 However, over a 15 year period, 60% of patients will recur and up to 30% of these patients will progress and succumb to their disease, while another 50% will undergo cystectomy in an attempt to control their disease.2 Thus, novel therapies are needed for the treatment of non-muscle invasive bladder cancer (NMIBC) to reduce recurrence rate, prevent disease progression and allow for bladder preservation to maintain quality of life for patients.
Recently, our group completed enrollment in a Phase Ib open label trial utilizing intravesical ALT-803, a recombinant IL-15 superagonist complex in combination with BCG in subjects with BCG-naïve NMIBC. Treatment was weekly for six consecutive weeks (induction). Maintenance therapy was not mandatory in this phase Ib study. The dose of BCG was set (50 mg/instillation, TICE®, Merck & Co. Inc., Whitehouse Station, NJ, USA), while ALT-803 dose was escalated (cohort 1, 100 μg/instillation; cohort 2, 200 μg/instillation; cohort 3, 400 μg/instillation) in a standard 3 + 3 study design. ALT-803 in combination with BCG was well tolerated. No dose limiting toxicity of ALT-803 was identified; therefore, the recommended dose of ALT-803 for the phase II study was 400 μg/instillation. Herein, we report a case of BCG-unresponsive NMIBC in a patient who was treated with ALT-803 (400 μg/instillation) in combination with BCG.
Case presentation
A now 92-year-old male with a history of Ta high-grade NMIBC diagnosed in October 2012 was initially treated with a standard induction course of intravesical BCG (50 mg/instillation) over 6 weeks without maintenance. Within 6 months, he was noted to have a recurrence (Tis high-grade) and was treated with another standard 6-week induction course of intravesical BCG. No maintenance BCG was administered. In November 2013, he was noted to have another recurrence of Tis. The decline of the patient's immune system with age3 may have led to sub-optimal outcomes after BCG administration. Because of co-morbidities, which included Crohn's disease, previous bowel resection for entero-vesical fistula, cobalt x-ray therapy for non-Hodgkin lymphoma, and radical prostatectomy for prostate cancer over 25 years ago, he was also not an ideal surgical candidate. Thus, he was subsequently treated with Valrubicin (200 mg/instillation) weekly for 6 consecutive weeks. In December 2014 during cystourethroscopy, the bladder mucosa appeared normal; however, the urinary cytology noted suspicious cells. The patient underwent cystoscopy and bladder biopsy, which noted Tis. Pathologic slides were reviewed by local pathologist as well as the Joint Pathology Center (Washington D.C.). The patient started intravesical gemcitabine (2 g/instillation) but developed myelosuppression (grade 3), febrile neutropenia (grade 3) and lymphocytopenia (grade 3) after the second intravesical instillation. Treatment was halted and supportive care was provided until resolution of symptoms. After 3 days, the patient was at his baseline but due to the severity of the symptoms a decision was made to abandon any further intravesical treatment with gemcitabine. In May 2015, the patient underwent cystourethroscopy and the bladder mucosa was noted to be erythematous and urinary cytology noted cancerous cells, which was confirmed on biopsy. Having exhausted all viable bladder preservation therapeutic options (i.e., two-induction courses of BCG, Valrubicin, and Gemcitabine), the patient was referred to us for consideration of our ongoing NMIBC clinical trial.
In an NMIBC rat model, the combination of ALT-803 and BCG increased NK cells and CD8+ T cells in the bladder when compared to either agent alone.4 Thus, our group believed for this specific patient, BCG and ALT-803 may provide an advantage that was absent with BCG alone. In June 2015, the patient received 6 weekly intravesical doses of ALT-803 at the 400 μg/instillation dose level in combination with the standard dose of BCG (50 mg/instillation). During treatment, the patient only reported sporadic fatigue (grade 1), which was present during his previous BCG treatments. In addition, during week 5 treatment he noted bladder spasm (grade 1) that occurred once on Day 2 after the fourth dose. Symptoms were relieved with Detrol (2 mg tablet). On Week 6 of treatment, the patient reported urgency (grade 1) post treatment that resolved after a few days.
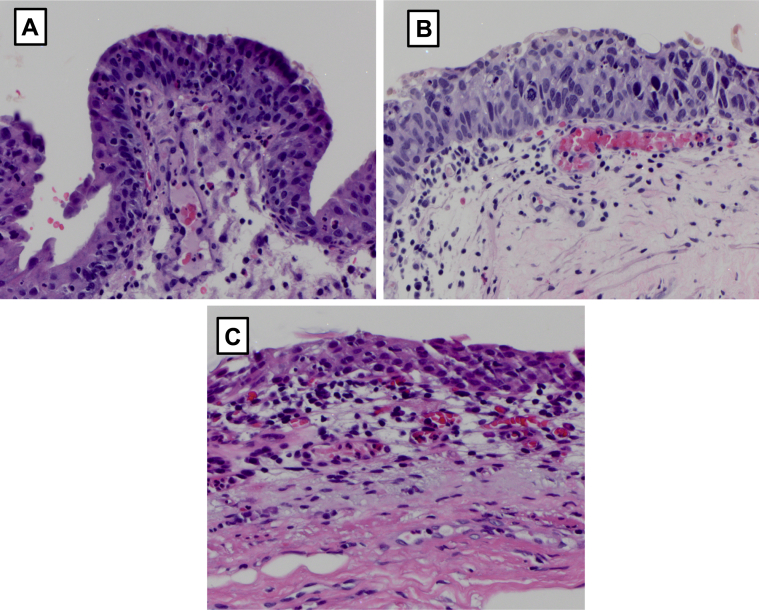
After completion of the treatment, the patient was seen by his local urologist for follow-up cystourethroscopy every 3 months. The 6-month cystourethroscopy revealed slightly erythematous bladder mucosa. Cytology revealed degenerated urothelial cells, acute inflammatory cells, rare red blood cells, and was negative for high-grade urothelial carcinoma. Bladder biopsy noted inflammatory infiltrate but no cancer (Fig. 1). The patient continues to undergo surveillance cystoscopy. Nineteen months after the first ALT-803 and BCG instillation, the patient remains without evidence of recurrent disease.
Figure 1.
Representative H&E sections illustrating BCG non-responsive tumor. Left top panel, bladder biopsy from October 2012 depicting Ta high-grade. Right top panel, bladder biopsy from December 2014 after 2 induction courses of BCG and 1 induction course of Valrubicin depicted persistent Tis. Bottom center panel, bladder biopsy after induction ALT-803 and BCG depicting inflammatory infiltrate, no evidence of cancer. Follow-up is 19 months.
Discussion
IL-15, a four-helix bundle common gamma chain cytokine, is a critical factor for the development, proliferation and activation of effector natural killer (NK) and CD8+ T cells. Although the cytokines IL-15 and IL-2 share the IL-2Rβ and IL-2Rγ chains, IL-15 is highly distinct from IL-2. IL-15 activates the immune cells without the induction of activation-induced cell death and the activation and expansion of the regulatory T cells, suggesting that IL-15 may be an effective immunotherapy for the treatment of cancer.5 Indeed, an NCI review listed IL-15 as the most promising therapeutic agent among twelve immunotherapy drugs that could potentially cure cancer. ALT-803 is a complex of an IL-15 superagonist mutant and a dimeric IL-15 receptor α Su/Fc fusion protein, which was found to exhibit enhanced biological activity in vivo with highly favorable pharmacokinetics, pharmacodynamics and toxicity profile.6 Currently, ALT-803 is undergoing clinical trials for various solid tumors and hematological malignancies.
In this report, our BCG-unresponsive NMIBC patient, unsuitable for cystectomy, was treated with 6 weekly intravesical doses of ALT-803 (400 μg/instillation) in combination with the standard dose of BCG (50 mg/instillation). Nineteen months after a well-tolerated treatment, the patient shows no evidence of disease.
Conclusion
Based on this encouraging result, we advocate formal testing of the combination therapy of ALT-803 with BCG in NMIBC patients who have failed prior BCG treatment.
Conflict of interest
The Authors have no conflict of interest.
Footnotes
Funding: This work was supported by an unrestricted grant from Altor Bioscience Corp.
References
- 1.Sylvester R.J. Bacillus Calmette-Guerin treatment of non-muscle invasive bladder cancer. Int J Urol. 2011;18(2):113–120. doi: 10.1111/j.1442-2042.2010.02678.x. [DOI] [PubMed] [Google Scholar]
- 2.Herr H.W. Tumor progression and survival of patients with high grade, noninvasive papillary (TaG3) bladder tumors: 15-year outcome. J Urol. 2000;163(1):60–61. discussion 61–2. [PubMed] [Google Scholar]
- 3.Montecino-Rodriguez E., Berent-Maoz B., Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123(3):958–965. doi: 10.1172/JCI64096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomes-Giacoia E., Miyake M., Goodison S. Intravesical ALT-803 and BCG treatment reduces tumor burden in a carcinogen induced bladder cancer rat model; a role for cytokine production and NK cell expansion. PLoS One. 2014 Jun 4;9(6):e96705. doi: 10.1371/journal.pone.0096705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waldmann T.A. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 6.Rhode P.R., Egan J.O., Xu W. Comparison of the superagonist complex, ALT-803, to IL15 as cancer immunotherapeutics in animal models. Cancer Immunol Res. 2016 Jan;4(1):49–60. doi: 10.1158/2326-6066.CIR-15-0093-T. [DOI] [PMC free article] [PubMed] [Google Scholar]