Abstract
Diabetes is a complex metabolic disease that exposes patients to the deleterious effects of hyperglycemia on various organs. Achievement of normoglycemia with exogenous insulin treatment requires the use of high doses of hormone, which increases the risk of life-threatening hypoglycemic episodes. We developed a gene therapy approach to control diabetic hyperglycemia based on co-expression of the insulin and glucokinase genes in skeletal muscle. Previous studies proved the feasibility of gene delivery to large diabetic animals with adeno-associated viral (AAV) vectors. Here, we report the long-term (∼8 years) follow-up after a single administration of therapeutic vectors to diabetic dogs. Successful, multi-year control of glycemia was achieved without the need of supplementation with exogenous insulin. Metabolic correction was demonstrated through normalization of serum levels of fructosamine, triglycerides, and cholesterol and remarkable improvement in the response to an oral glucose challenge. The persistence of vector genomes and therapeutic transgene expression years after vector delivery was documented in multiple samples from treated muscles, which showed normal morphology. Thus, this study demonstrates the long-term efficacy and safety of insulin and glucokinase gene transfer in large animals and especially the ability of the system to respond to the changes in metabolic needs as animals grow older.
Keywords: diabetes, adeno-associated viral vectors, dogs, gene therapy, long-term, insulin, glucokinase
Introduction
The field of gene therapy has recently seen the realization of its full potential, with many clinical studies providing unquestionable evidence of efficacy and safety for a variety of diseases.1 Indeed, in 2012, Glybera by UniQure, a muscle-directed, adeno-associated viral (AAV) vector-based gene therapy for the treatment of lipoprotein lipase deficiency, became the first gene therapy product of the western world to gain market approval in Europe.2, 3 Having overcome many of the challenges that hampered the success of gene therapy in the initial years, the technology has broadened its range of potential applications from monogenic to non-hereditary, complex diseases, such as cancer, cardiovascular disease, and diabetes.1, 4, 5, 6
AAV vectors can be used to engineer the skeletal muscle through single intramuscular administrations to lower diabetic hyperglycemia.7, 8 Using AAVs of serotype 1 (AAV1) encoding the insulin (Ins) and glucokinase (Gck) genes, we have shown long-term control of hyperglycemia and prevention of secondary complications in mice with streptozotocin-induced diabetes.7 Furthermore, treatment of dogs with experimental diabetes has demonstrated feasibility of Ins+Gck gene therapy in large animals.8 Conceptually, our approach is based on the co-expression of basal and low levels of Ins and the glucose-phosphorylating enzyme Gck.7, 9, 10, 11 When overt hyperglycemia is established in a situation of absolute Ins deficiency, translocation of glucose transporter type 4 (GLUT-4) to the plasma membrane and the mRNA levels and activity of HKII, the enzyme that phosphorylates glucose upon entry to the cell, are decreased in muscle cells,12, 13 compromising the ability of skeletal muscle to dispose of glucose after a meal. In contrast to HKII, hepatic Gck has a high KM for glucose (about 8 mM), is not inhibited by glucose 6-phosphate, and shows kinetic cooperativity with glucose.14 Therefore, glucose uptake can be enhanced in diabetic muscles in a glucose-dependent manner through co-expression of constant, low levels of Ins that ensure translocation of GLUT-4 and of Gck, which draws glucose uptake into engineered muscle cells. The combination of these two genes thus generates a “glucose sensor” in skeletal muscle that uptakes large quantities of glucose only when circulating glucose levels rise, such as in postprandial conditions, but does not cause hypoglycemia because Gck activity is shut down at physiological glucose concentrations.
Translational efforts require the evaluation of the long-term efficacy and safety of Ins+Gck gene transfer in large animals and especially the ability of the system to respond to the changes in metabolic needs as animals grow older. Here, we report the results of the long-term (up to 8 years) follow-up of diabetic dogs treated with a single intramuscular administration of AAV1-Ins and AAV1-Gck.8
Results
Glycemic Control in Treated Dogs
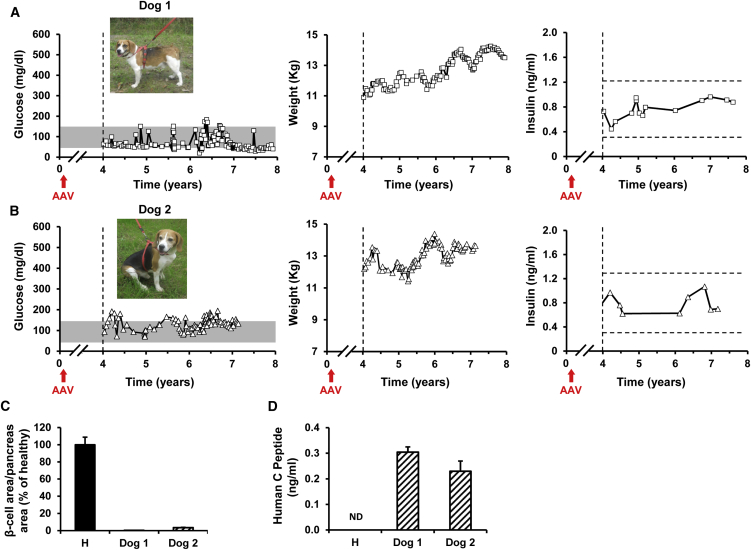
We previously demonstrated that diabetes could be treated in dogs with Ins+Gck gene therapy.8 Two of the treated dogs have now been followed for up to 8 years to assess long-term efficacy and safety. Despite aging, fasting normoglycemia was maintained in both dogs during the whole observational period (Figures 1A and 1B). Dogs gained weight as expected for healthy dogs (Figures 1A and 1B) and survived until the day of sacrifice without the need of exogenous Ins therapy. Circulating Ins levels were very similar in both dogs and remained within the range observed in healthy fasted dogs at all time points analyzed, demonstrating production of the therapeutic protein at constant low levels for ∼8 years (Figures 1A and 1B). The quantification of remaining pancreatic β cells demonstrated the absence of canine Ins-producing cells in the pancreas of dogs 1 and 2 after ∼8 years of diabetes (Figure 1C). The presence of human C-peptide in the serum of both AAV1-treated dogs (Figure 1D) further confirmed that circulating Ins derived from the production and secretion of human Ins from AAV1-injected muscles.
Figure 1.
Correction of Diabetes for ∼8 Years in Dogs that Received a Single Treatment with AAV1-Ins+Gck Gene Therapy
(A and B) Follow-up of glycemia, body weight, and insulinemia in diabetic dogs 1 and 2 treated with AAV1-Ins and AAV1-Gck vectors at 1 × 1012 vg/kg each. Figures depict the results of the monitoring from years 4 to 8 after gene transfer. “Time 0” indicates the moment of diabetes induction.8 Red arrows indicate the moment of AAV administration. Gray bars indicate the range of fasting normoglycemia in dogs.20 Insulinemia remained within the range of fasted healthy animals (dashed lines) in both AAV1-treated diabetic dogs for the whole of the follow-up period. (C) Quantification of the β cell area in pancreas samples (four sections of two to four different pancreatic regions) from a healthy dog and dogs 1 and 2 obtained at the end of the study. Diabetes induction led to >95% reduction in β cell area per pancreas area in both dogs. (D) Serum levels of human C-peptide in healthy (H) dogs and in dogs 1 and 2. Each bar represents the mean ± SEM of six measurements performed from year 4 to year 8 after treatment. The detection of human C-peptide in AAV1-Ins+Gck-treated dogs demonstrated that proinsulin was produced and processed in the engineered skeletal muscle.
Metabolic Correction of Diabetes by Ins+Gck Gene Therapy
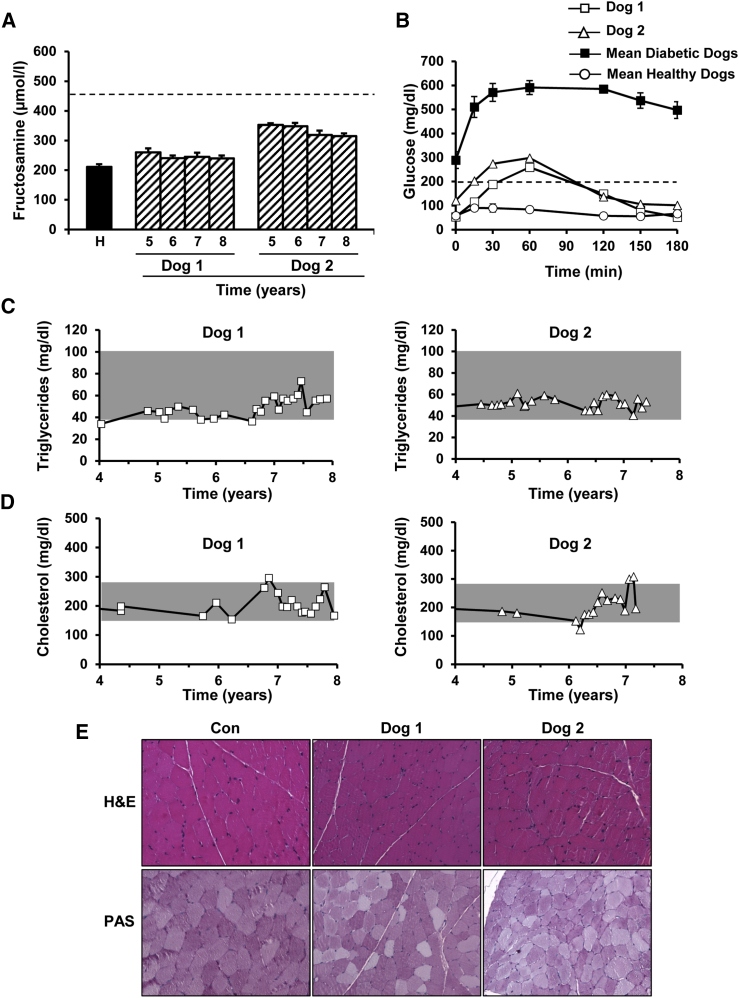
During the whole follow-up period, serum fructosamine levels ranged from 240 to 350 μmol/L in both AAV1-treated dogs (Figure 2A). These values were below the upper limit of what is considered good glycemic control in veterinary medicine (450 μmol/L),15 a control generally hard to achieve even through multiple daily administrations of exogenous Ins.8, 15, 16 In response to an oral glucose load, Ins+Gck-treated dogs showed only a slight increase in blood glucose levels compared with those documented in healthy age-matched controls, quickly returning to normoglycemic values (Figure 2B). Moreover, 2 hr after the load, the glycemia of both treated dogs was less than 200 mg/dL, a response considered non-diabetic by the American Diabetes Association guidelines.17 Serum triglyceride and total cholesterol levels also remained within the range observed in healthy dogs18 (Figures 2C and 2D). On the other hand, no signs of muscle pathology or inflammation were observed in either animal, and periodic acid-Schiff (PAS) staining demonstrated that treated muscles did not accumulate pathological amounts of glycogen as the results of the therapy (Figure 2E).
Figure 2.
Normalization of Metabolic Parameters following AAV1-Ins+Gck Treatment
(A) Follow-up of serum fructosamine levels in dogs 1 and 2. Each bar represents the mean ± SEM of 5–12 determinations performed in a given year posttreatment. The average fructosamine value measured in four age-matched healthy (H) dogs (8–11 years of age) is provided as a reference. The dashed line indicates the limit of what is considered good glycemic control in veterinary medicine.20 Fructosamine levels between 350 and 400 mmol/L indicate excellent glycemic control, between 400 and 450 mmol/L good glycemic control, and between 450 and 500 mmol/L fair glycemic control; concentrations >500 mmol/L indicate poor glycemic control.20 (B) OGTT performed at a dose of glucose of 1.75 g/kg in 12 hr fasted dogs. After the load, glycemia declined to less than 200 mg/dL before 2 hr in both Ins+Gck-treated dogs, i.e., below the threshold for diabetes diagnosis according to American Diabetes Association guidelines (2-hr plasma glucose <200 mg/dL). Data represent the OGTT performed 1 month before sacrifice, i.e., ∼8 years after treatment. The average OGTT curve for six untreated diabetic dogs (solid squares) and four age-matched healthy dogs (empty circles) are provided as a reference. (C and D) Follow-up of serum triglycerides (C) and cholesterol (D) in AAV1-Ins+Gck-treated dogs. Throughout the years, values remained within the normal range (gray)18 in both animals. (E) Preservation of the integrity of the skeletal muscle after AAV1-Ins+Gck gene transfer. The histopathological analysis of the quadriceps (dog 1) and tibialis cranialis (dog 2) was performed on samples obtained at necropsy. No signs of muscle pathology or inflammation were observed by H&E staining. No sign of pathological glycogen storage was documented after periodic acid-Schiff (PAS) staining. Representative images obtained from a healthy control (Con) dog are provided as a reference. Original magnification ×200.
Vector Biodistribution and Persistent Expression of Therapeutic Genes
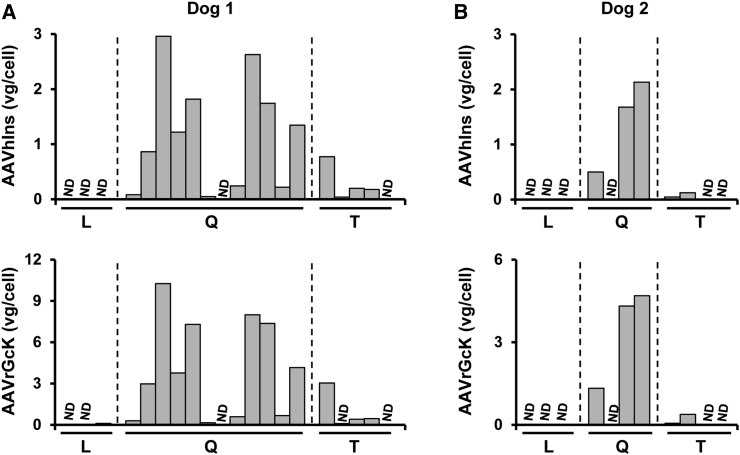
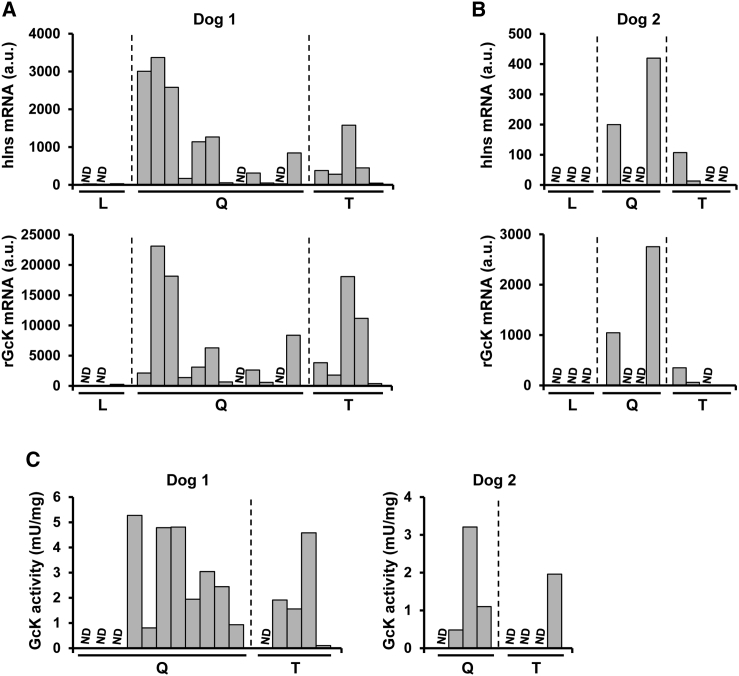
At sacrifice, multiple samples from the quadriceps femoris and tibialis cranialis muscles, the latter equivalent to human tibialis anterior, as well as liver from both AAV-injected dogs were obtained. In dog 1, 88% and 82% of the samples tested positive for AAV1-Ins and AAV1-Gck vector genomes, respectively (Figure 3A). The percentages were slightly lower for dog 2, in which 63% of the muscle samples had detectable AAV1-Ins and AAV1-Gck vector genomes (Figure 3B). In contrast, no vector genomes were detected in the liver of any of the animals with either Ins- or Gck-specific primers or probes (Figures 3A and 3B). In agreement with the vector genome data, we detected Ins and Gck mRNA in most of the quadriceps femoris and tibialis cranialis samples from dogs 1 and 2, but not in the liver (Figures 4A and 4B). Furthermore, we documented Gck activity in multiple samples from the quadriceps femoris and tibialis cranialis muscles from both dogs (Figure 4C). Overall, these results provide strong evidence of the persistence of vector genomes in injected dog muscles, which mediate long-term expression of therapeutic proteins for ∼8 years after a single vector administration and explain the tight control of glycemia achieved in these animals for such a long period of time.
Figure 3.
Viral Vector Biodistribution
(A and B) Quantification of AAV1-Ins (top panels) and AAV1-Gck (bottom panels) vector genome (vg) copy numbers, expressed as vg per cell (vg/cell), in samples of liver (L), quadriceps (Q), and tibialis cranialis (T) from dogs 1 (A) and 2 (B) obtained at necropsy. No vg were detected in any of the liver samples from either animal. ND, non-detectable.
Figure 4.
Long-Lasting Therapeutic Gene Expression following AAV-Mediated Gene Transfer to the Muscle of Dogs
(A and B) Expression of human Ins (top panels) and rat Gck (bottom panels) in the liver (L), quadriceps (Q), and tibialis cranialis (T) muscles from dogs 1 (A) and 2 (B). Human Ins and rat Gck were not expressed in any of several liver samples. a.u., arbitrary units. (C) Detection of Gck activity in quadriceps and tibialis cranialis muscles from dogs 1 and 2 detected ∼8 years after a single administration of the gene therapy vectors. ND, non-detectable.
Discussion
The goals of any therapy for Ins-deficient diabetes are to attain normoglycemia and avoid hypoglycemia. With conventional replacement therapy, the first aim requires the use of high doses of Ins, which increases the likelihood of hypoglycemic episodes (The United Kingdom Prospective Diabetes Study [UKPDS]).19 Also, to achieve optimal control, any approach should be able to rapidly respond to changes in blood sugar, such as those associated with food intake, exercise, or stress, with minimal intervention from the patient. Here, we report the successful control of diabetic hyperglycemia after a single delivery of the human Ins and rat Gck genes to the muscle of dogs, with therapeutic efficacy demonstrated for a period that essentially covered the lifetime of the animals.
The normalization of biochemical parameters in the two dogs monitored for ∼8 years provides strong evidence of the long-lasting control of glycemia achieved following gene transfer of the Ins and Gck genes. It is worth noticing that the oral glucose tolerance test (OGTT) was performed in elderly animals (∼9 years old) and ∼8 years after a single dosing of the gene therapy treatment, indicating that the capacity of treated muscles to dispose of glucose after a challenge persisted through the years. Further evidence of efficacy was provided by the fact that Ins+Gck-treated dogs showed fructosamine levels below what is considered to reflect excellent glycemic control in veterinary medicine.20 Fructosamine, which measures average glucose levels over approximately 2–3 weeks, is used in veterinary medicine to monitor treatment efficacy in a similar way to hemoglobin A1c in humans.20 Further, this was consistent with the observation that gene therapy delayed cataract development in dogs 1 and 2. In diabetic dogs that received Ins+Gck, gene therapy cataracts were evident at 8 and 9 years of age, i.e., several years after diabetes induction and similar to the age of development of cataracts in healthy Beagle dogs. Indeed, 9 out of 13 age-matched healthy Beagle dogs housed in the same facility as our Ins+Gck-treated dogs also had cataracts. In contrast, a quick cataract development is observed in diabetic dogs treated with exogenous Ins, of whom ∼80% develop cataracts within 16 months of diagnosis.21 In our hands, diabetic dogs of the same breed and housed in the same animal facility as dogs 1 and 2, but treated with recombinant human Ins, developed cataracts 3–9 months after diabetes induction.8 Also, throughout the 8-year follow-up period, treated dogs showed normal circulating levels of cholesterol and triglycerides. Hypercholesterolemia and hypertriglyceridemia, together with hyperglycemia, constitute main risk factors for cardiac disease in diabetic patients.22, 23 Diabetic dogs do not frequently develop long-term complications of diabetes.20 The only two common findings in clinical veterinary medicine are the development of cataracts and urinary tract infections.8, 20 Other long-term complications of diabetes such as nephropathy, neuropathy, retinopathy, or cardiac disease are very uncommon in diabetic dogs. This fact and the reduced number of animals included in this long-term follow-up study preclude us from drawing any strong conclusions on the efficacy of our treatment in preventing the development of secondary complications of the disease.
Some of the previous gene therapy approaches developed to counteract diabetic hyperglycemia relied on the glucose-regulated expression of Ins in surrogate non-β cells to respond to the changes in blood glucose.24, 25, 26, 27, 28, 29, 30 Both naturally occurring and engineered hybrid promoters have been used to achieve glucose-responsive expression of Ins following in vivo delivery of gene transfer vectors.24, 25, 26, 27, 28, 29, 30 None of these systems, however, has managed to mimic the quick response to glucose of the Ins promoter,31 and the slow transcriptional activation of these promoters may result in an inadequate Ins secretory response, with postprandial episodes of hyperglycemia followed by hypoglycemia several hours later.
In our system, the enzyme Gck, and not Ins, is the component that responds to glucose levels by activating glucose phosphorylation when intracellular levels of glucose are high. In engineered muscle cells, the activation of the Ins signaling is required to ensure sufficient translocation of the GLUT-4 transporter to the plasma membrane, so that glucose transport does not become a rate-limiting step to the system. Glucose uptake is then driven by the quick phosphorylation of large amounts of glucose by the enzyme Gck, active while glucose levels are high and inoperative when they are normal. Thus, despite the constitutive expression of Ins, the system does respond to glucose levels, and muscle glucose uptake is stimulated in conditions of hyperglycemia, such as in postprandial conditions, but is progressively shut down as glycemia returns to physiological levels. The good response of the system to glucose levels was evidenced by the OGTT. The peak glucose level reached in the Ins+Gck-treated dogs was substantially lower than in untreated diabetic animals, albeit slightly higher than in age-matched healthy dogs, but glycemia returned to normal values fairly quickly in the Ins+Gck-treated dogs and was less than 200 mg/dL 2 hr after the load, a profile considered non-diabetic according to American Diabetes Association guidelines.17 The fact that only very low, constant levels of Ins are required to maintain normoglycemia even after a glucose challenge increases the safety profile of our proposed therapy and constitutes an advantage over the use of glucose-regulated promoters with slow kinetics of response. We previously reported that younger Ins+Gck-treated dogs did not develop hypoglycemia even when subjected to intense exercise after 24 hr of fasting.8 In this longer follow-up we did not observe hypoglycemia as animals grew older and their metabolic needs changed, demonstrating that the risk of hypoglycemia of our approach is very low. Furthermore, the production of low, constant levels of Ins has additional beneficial effects on metabolism. We have observed in mice and dogs that the low levels of Ins produced by engineered muscles are sufficient to suppress hepatic glucose production, lipolysis, and ketoacidosis.7, 8 Indeed, diabetic animals of both species gained weight with age and had normal fat content after Ins+Gck gene therapy.7, 8
This report contains the longest efficacy data after gene transfer in a large-animal model of a non-monogenic metabolic disease. Indeed, the longest lasting efficacy for an experimental therapy for diabetes described so far was obtained following transplantation of porcine islets to dogs and non-human primates, in which 1.8 and 1.7 years of good glycemic control were reported, respectively.32, 33 For clinical translation, the approach requires further development. In this regard, the generation of single AAV vectors that encode both genes is under way. This will, in theory, improve gene transfer efficiency because the likelihood of getting both genes in a given cell will increase, allow the use of lower vector doses, minimizing potential toxicity and easing the manufacturing burden, and simplify the regulatory pathway. Toward a future application in humans, this study demonstrates in large animals the long-term efficacy and safety of Ins and Gck gene transfer, and especially the adaptability of the system to the changes in metabolic needs associated with aging. We believe gene therapy holds great potential for the treatment of diabetes in humans.
Materials and Methods
Animals
Male Beagle dogs were purchased from Isoquimen and housed at the Servei de Granges i Camps Experimentals of the Universitat Autònoma de Barcelona (UAB). Animals were fed individually once daily at 9:00 a.m. with 30 g/kg body weight standard dry food (Nestle). Experimental diabetes was induced as previously described.8 Dogs were monitored regularly at the UAB Veterinary Clinical Hospital and sacrificed by administration of a lethal dose of pentobarbital. All experimental procedures were approved by the Ethics Committee for Animal and Human Experimentation of UAB.
AAV Production and Administration
AAV1 vectors driving the expression of human Ins containing a furin endoprotease cleavage signal and rat Gck were produced and delivered to dogs 1 and 2 at a dose of 1 × 1012 vector genomes (vg)/kg of each vector, as previously described.8
Vector Biodistribution
Tissues were digested overnight in Proteinase K (0.2 mg/mL). Total DNA was isolated with the MasterPureDNA Purification Kit (Epicenter Biotechnologies). Vg copy number was determined in 20 ng of genomic DNA by TaqMan qPCR with primers and probes specific for hINS and rGck: hINS forward primer: 5′-GTC CTG GGT GTG TAG AAG AAG-3′; hINS reverse primer: 5′-CTT TGT GAA CCA ACA CCT GTG-3′; hINS probe: 5′-TCG TTC CCC GCA CAC TAG GTA GA-3′; rGck forward primer: 5′-TGT CAA GGA AGT CAG AGA TGC-3′; rGck reverse primer: 5′-ATG CTG GTC AAA GTG GGA G-3′; rGck probe: 5′-AGG GCA GTG GAG CGT GAA GAC-3′. Vg per sample were interpolated from a standard curve built by serial dilutions of linearized plasmids bearing the target sequence spiked into 20 ng of non-transduced genomic DNA.
Transgene Expression
Total RNA was extracted from different tissues using Tripure isolation reagent (Roche Molecular Biochemicals) and the RNeasy Mini Kit (QIAGEN). Total RNA (1 μg) was retrotranscribed using the Transcriptor First Strand cDNA Synthesis Kit (Roche). Quantitative real-time (RT)-qPCR was performed in a LightCycler 480 II (Roche) using LightCycler 480 probes Master (Roche). Transgene-specific primers and probes were the same as for biodistribution studies. Results were normalized by Rplp0 expression using primers and probe specific for dogRplp0: forward primer: 5′-ACC TCT TTC TTC CAG GCT TTA G-3′; reverse primer: 5′-CCA CTT TGT CTC CCG TCT TAA T-3′; probe: 5′-ACC ATT GAA ATC TTG AGT GAT GTG CAG C-3′.
Morphological and Immunohistochemical Analysis
Samples were fixed in 10% formalin, embedded in paraffin, and sectioned. β cell area was measured on multiple sections covering the whole pancreas.8 To analyze muscle integrity, we stained muscle cross sections with H&E (Dako). PAS staining (Sigma-Aldrich) was used to evaluate muscle glycogen content.
Hormone, Metabolites, and Gck Activity
Human Ins and human C-peptide were measured in serum samples by radioimmunoassay.8 Blood glucose levels were determined using a Glucometer Elite analyzer (Bayer). Serum fructosamine, triglycerides, and cholesterol were measured by spectrophotometry.8, 34 Gck activity was measured in extracts from frozen muscle samples.8
OGTT
OGTTs were performed on 12 hr fasted dogs that received a 1.75 g/kg body weight gavage of glucose.8
Statistical Analysis
All values are expressed as means ± SEM.
Author Contributions
M.L.J., L.V., I.E., V.J., M.G., V.H., and F.B. designed experiments and interpreted the data. M.L.J., L.V., I.E., V.J., J. Rodó, L.M., R.R.-d.G., M.G., S.M., D.C., E.A., T.F., A.A., and J. Ruberte generated reagents and performed experiments. V.H. and F.B. wrote and edited the manuscript. F.B. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of Interest
F.B., D.C., E.A., V.J., M.G. and V.H. are inventors of patent applications regarding the use of insulin and glucokinase treatment of diabetes.
Acknowledgments
The authors thank Marta Moya for technical assistance. This work was supported by grants from Ministerio de Economía y Competitividad, Plan Nacional I+D+I grants SAF2011-24698 and SAF2014-54866-R from MINECO and European Regional Development Fund (ERDF), Generalitat de Catalunya grant 2014SGR-1669 (to F.B.), ICREA Academia Award (to F.B.), and Spain and the European Commission MYOCURE grant PHC-14-2015-667751. J.R. is recipient of a predoctoral fellowship (BES-2015-075799) from Ministerio de Economía y Competitividad, Spain.
References
- 1.Naldini L. Gene therapy returns to centre stage. Nature. 2015;526:351–360. doi: 10.1038/nature15818. [DOI] [PubMed] [Google Scholar]
- 2.Gaudet D., Méthot J., Déry S., Brisson D., Essiembre C., Tremblay G., Tremblay K., de Wal J., Twisk J., van den Bulk N. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther. 2013;20:361–369. doi: 10.1038/gt.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaudet D., Stroes E.S., Méthot J., Brisson D., Tremblay K., Bernelot Moens S.J., Iotti G., Rastelletti I., Ardigo D., Corzo D. Long-term retrospective analysis of gene therapy with alipogene tiparvovec and its effect on lipoprotein lipase deficiency-induced pancreatitis. Hum. Gene Ther. 2016;27:916–925. doi: 10.1089/hum.2015.158. [DOI] [PubMed] [Google Scholar]
- 4.Rincon M.Y., VandenDriessche T., Chuah M.K. Gene therapy for cardiovascular disease: advances in vector development, targeting, and delivery for clinical translation. Cardiovasc. Res. 2015;108:4–20. doi: 10.1093/cvr/cvv205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar M.D., Dravid A., Kumar A., Sen D. Gene therapy as a potential tool for treating neuroblastoma-a focused review. Cancer Gene Ther. 2016;23:115–124. doi: 10.1038/cgt.2016.16. [DOI] [PubMed] [Google Scholar]
- 6.Fesnak A.D., June C.H., Levine B.L. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat. Rev. Cancer. 2016;16:566–581. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mas A., Montané J., Anguela X.M., Muñoz S., Douar A.M., Riu E., Otaegui P., Bosch F. Reversal of type 1 diabetes by engineering a glucose sensor in skeletal muscle. Diabetes. 2006;55:1546–1553. doi: 10.2337/db05-1615. [DOI] [PubMed] [Google Scholar]
- 8.Callejas D., Mann C.J., Ayuso E., Lage R., Grifoll I., Roca C., Andaluz A., Ruiz-de Gopegui R., Montané J., Muñoz S. Treatment of diabetes and long-term survival after insulin and glucokinase gene therapy. Diabetes. 2013;62:1718–1729. doi: 10.2337/db12-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otaegui P.J., Ferre T., Riu E., Bosch F. Prevention of obesity and insulin resistance by glucokinase expression in skeletal muscle of transgenic mice. FASEB J. 2003;17:2097–2099. doi: 10.1096/fj.03-0081fje. [DOI] [PubMed] [Google Scholar]
- 10.Otaegui P.J., Ferre T., Pujol A., Riu E., Jimenez R., Bosch F. Expression of glucokinase in skeletal muscle: a new approach to counteract diabetic hyperglycemia. Hum. Gene Ther. 2000;11:1543–1552. doi: 10.1089/10430340050083270. [DOI] [PubMed] [Google Scholar]
- 11.Riu E., Mas A., Ferre T., Pujol A., Gros L., Otaegui P., Montoliu L., Bosch F. Counteraction of type 1 diabetic alterations by engineering skeletal muscle to produce insulin: insights from transgenic mice. Diabetes. 2002;51:704–711. doi: 10.2337/diabetes.51.3.704. [DOI] [PubMed] [Google Scholar]
- 12.Postic C., Leturque A., Printz R.L., Maulard P., Loizeau M., Granner D.K., Girard J. Development and regulation of glucose transporter and hexokinase expression in rat. Am. J. Physiol. 1994;266:E548–E559. doi: 10.1152/ajpendo.1994.266.4.E548. [DOI] [PubMed] [Google Scholar]
- 13.Printz R.L., Koch S., Potter L.R., O’Doherty R.M., Tiesinga J.J., Moritz S., Granner D.K. Hexokinase II mRNA and gene structure, regulation by insulin, and evolution. J. Biol. Chem. 1993;268:5209–5219. [PubMed] [Google Scholar]
- 14.Printz R.L., Magnuson M.A., Granner D.K. Mammalian glucokinase. Annu. Rev. Nutr. 1993;13:463–496. doi: 10.1146/annurev.nu.13.070193.002335. [DOI] [PubMed] [Google Scholar]
- 15.Davison L.J., Herrtage M.E., Catchpole B. Study of 253 dogs in the United Kingdom with diabetes mellitus. Vet. Rec. 2005;156:467–471. doi: 10.1136/vr.156.15.467. [DOI] [PubMed] [Google Scholar]
- 16.Fracassi F., Boretti F.S., Sieber-Ruckstuhl N.S., Reusch C.E. Use of insulin glargine in dogs with diabetes mellitus. Vet. Rec. 2012;170:52. doi: 10.1136/vr.100070. [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association Standards of medical care in diabetes--2011. Diabetes Care. 2011;34(Suppl 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaneko J., Harvey J., Bruss M. Elsevier; 2008. Clinical Biochemistry of Domestic Animals. [Google Scholar]
- 19.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 20.Nelson R.W. Canine diabetes mellitus. In: Ettinger S.J., Feldman E.C., editors. Textbook of Veterinary Internal Medicine: Diseases of the Dog and the Cat. Seventh Edition. Saunders, Elsevier; 2010. pp. 1449–1474. [Google Scholar]
- 21.Beam S., Correa M.T., Davidson M.G. A retrospective-cohort study on the development of cataracts in dogs with diabetes mellitus: 200 cases. Vet. Ophthalmol. 1999;2:169–172. doi: 10.1046/j.1463-5224.1999.00073.x. [DOI] [PubMed] [Google Scholar]
- 22.Patel T.P., Rawal K., Bagchi A.K., Akolkar G., Bernardes N., Dias Dda.S., Gupta S., Singal P.K. Insulin resistance: an additional risk factor in the pathogenesis of cardiovascular disease in type 2 diabetes. Heart Fail. Rev. 2016;21:11–23. doi: 10.1007/s10741-015-9515-6. [DOI] [PubMed] [Google Scholar]
- 23.Kannel W.B., Hjortland M., Castelli W.P. Role of diabetes in congestive heart failure: the Framingham study. Am. J. Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 24.Alam T., Wai P., Held D., Vakili S.T., Forsberg E., Sollinger H. Correction of diabetic hyperglycemia and amelioration of metabolic anomalies by minicircle DNA mediated glucose-dependent hepatic insulin production. PLoS ONE. 2013;8:e67515. doi: 10.1371/journal.pone.0067515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burkhardt B.R., Parker M.J., Zhang Y.C., Song S., Wasserfall C.H., Atkinson M.A. Glucose transporter-2 (GLUT2) promoter mediated transgenic insulin production reduces hyperglycemia in diabetic mice. FEBS Lett. 2005;579:5759–5764. doi: 10.1016/j.febslet.2005.09.060. [DOI] [PubMed] [Google Scholar]
- 26.Chen R., Meseck M.L., Woo S.L. Auto-regulated hepatic insulin gene expression in type 1 diabetic rats. Mol. Ther. 2001;3:584–590. doi: 10.1006/mthe.2001.0299. [DOI] [PubMed] [Google Scholar]
- 27.Han J., McLane B., Kim E.H., Yoon J.W., Jun H.S. Remission of diabetes by insulin gene therapy using a hepatocyte-specific and glucose-responsive synthetic promoter. Mol. Ther. 2011;19:470–478. doi: 10.1038/mt.2010.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kozlowski M., Olson D.E., Rubin J., Lyszkowicz D., Campbell A., Thulé P.M. Adeno-associated viral delivery of a metabolically regulated insulin transgene to hepatocytes. Mol. Cell. Endocrinol. 2007;273:6–15. doi: 10.1016/j.mce.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y.W., Chao C.K. Incorporation of calcium phosphate enhances recombinant adeno-associated virus-mediated gene therapy in diabetic mice. J. Gene Med. 2003;5:417–424. doi: 10.1002/jgm.353. [DOI] [PubMed] [Google Scholar]
- 30.Zhang T., Dong H.H. Glucose-regulated insulin production in the liver improves glycemic control in type 1 diabetic mice. Mol. Metab. 2014;4:70–76. doi: 10.1016/j.molmet.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong H., Woo S.L. Hepatic insulin production for type 1 diabetes. Trends Endocrinol. Metab. 2001;12:441–446. doi: 10.1016/s1043-2760(01)00491-x. [DOI] [PubMed] [Google Scholar]
- 32.Gazda L.S., Vinerean H.V., Laramore M.A., Hall R.D., Carraway J.W., Smith B.H. No evidence of viral transmission following long-term implantation of agarose encapsulated porcine islets in diabetic dogs. J. Diabetes Res. 2014;2014:727483. doi: 10.1155/2014/727483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin J.S., Kim J.M., Kim J.S., Min B.H., Kim Y.H., Kim H.J., Jang J.Y., Yoon I.H., Kang H.J., Kim J. Long-term control of diabetes in immunosuppressed nonhuman primates (NHP) by the transplantation of adult porcine islets. Am. J. Transplant. 2015;15:2837–2850. doi: 10.1111/ajt.13345. [DOI] [PubMed] [Google Scholar]
- 34.Elias I., Franckhauser S., Ferré T., Vilà L., Tafuro S., Muñoz S., Roca C., Ramos D., Pujol A., Riu E. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes. 2012;61:1801–1813. doi: 10.2337/db11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]