Abstract
Background
Epithelioid haemangioendothelioma (EHE) is a rare low-grade vascular neoplasm that can arise in the lung, liver, soft tissues or, less commonly, bone. Due to its low prevalence of less than one in a million and its non-specific clinical features, EHE is often misdiagnosed and managed inappropriately. Here we discuss the case of a 58 year-old gentleman with mediastinal EHE and review existing literature on pulmonary EHE (PEH).
Case history
A 58 year-old gentleman presented to our outpatient Clinic with chest discomfort and palpitations. A whole-body FDG-CT-PET showed an FDG-avid single 6.3cm nodule in the superior anterior mediastinum which was fully excised by robotic approach. Histology showed a nodular structure with clusters of epithelioid and spindled cells with a low proliferative index and mitotic count, suspended in a sclerotic stroma. Immunohistochemistry staining was positive for CD3 and CD34, confirming endothelial lineage, and SMA, identifying smooth muscle clusters.
Discussion
PEH typically presents in young Caucasian women, either incidentally as multiple small pulmonary nodules on CT or with respiratory symptoms that include cough, dyspnoea, chest pain and occasionally pleural effusions. Aetiology and prognosis remain unclear, although indicators of poor prognosis include the presence of respiratory symptoms, male gender, older age and multi-organ disease. Diagnosis is difficult and PEH is often misidentified as chronic granulomatous disease, amyloidosis or other malignancy of the lung. Histological features suggestive of PEH include nodules of hypocellular sclerotic stroma containing spindle-shaped tumour cells with abundant eosinophilic cytoplasm, vacuoles containing erythrocytes and low mitotic counts. CD31, CD34 and Fli-1 positive immunohistochemistry is strongly indicative of epithelioid lineage. There is no standard treatment for PEH but curative resection is the preferred treatment option where possible, with chemotherapy being used as adjuvant treatment or in widespread inoperable disease.
Conclusion
This case report outlines the clinicopathological features that are characteristic of EHE with the hope of facilitating correct and early diagnosis in the future.
Keywords: Hemangioendothelioma, Thoracic oncology
1. Introduction
Epitelioid haemangioendothelioma (EHE) is a rare low-grade vascular tumour that typically involves the lung, liver, soft tissues or bone of young Caucasian women. Pulmonary EHE (PEH) commonly presents as an incidental radiological finding of bilateral multiple small nodules in the lung, occasionally combined with ground-glass alveolar opacities, hilar lymphadenopathy or pleural involvement [1]. Non-specific symptoms include cough, dyspnoea, chest pain and pleurlal effusion in locally advanced disease. Due to its non-specific presentation and low incidence, PEH is often misdiagnosed as other benign or malignant disease of the lung, leading to delayed or inappropriate management [2].
In this report we describe a case of mediastinal epithelioid haemangioendothelioma in a 58 year-old gentleman presenting with chest discomfort and palpitations. We discuss patient presentation, investigations and management. Histology and immunohistochemistry of the lesion are analysed.
The second part of the report includes a review of current literature on EHE, summarising existing knowledge of disease aetiology, presentation, diagnosis, management and prognosis. We compare our clinical and pathological findings with previously documented cases of PEH.
This report will highlight the clinicopathological features of EHE in order to set up a framework to improve early diagnosis and appropriate management in the future.
2. Case history
A 58 year-old gentleman working as an IT consultant presented to our outpatient Clinic complaining of mild chest discomfort and occasional palpitations.
An echocardiogram and coronary angiogram were performed to exclude cardiac disease. A chest x-ray showed a left side homogenous mass with no evidence of effusion or pneumothorax. Blood tests were unremarkable. Alpha-fetoprotein and beta-human-chorionic-gonadotropin levels were measured to exclude a germ cell tumour and were found to be in the normal range. A whole-body FDG-CT-PET scan showed a partly calcified mass with areas of macroscopic fat at the level of the left lung apex. The mass was FDG avid (SUV 6.5) with a maximum size of 6.3cm. It appeared to be within the superior mediastinum rather than the lung. No other mediastinal masses were noted and there was no chest lymphadenopathy. The FEV1 was 125% of predicted and the TLCO was 111% of predicted, according to lung function tests.
After discussion with the patient, surgery was performed to fully excise the lesion. This was executed by robotic approach (daVinci Xi) using four ports. A thick, rounded mass was identified in the anterior mediastinum, close to the aortic arch. The mass was gently detached from surrounding adhesions and removed intact, preserving the major vascular structures. The procedure was uncomplicated and the postoperative period was unremarkable, with the patient being discharged home after two days.
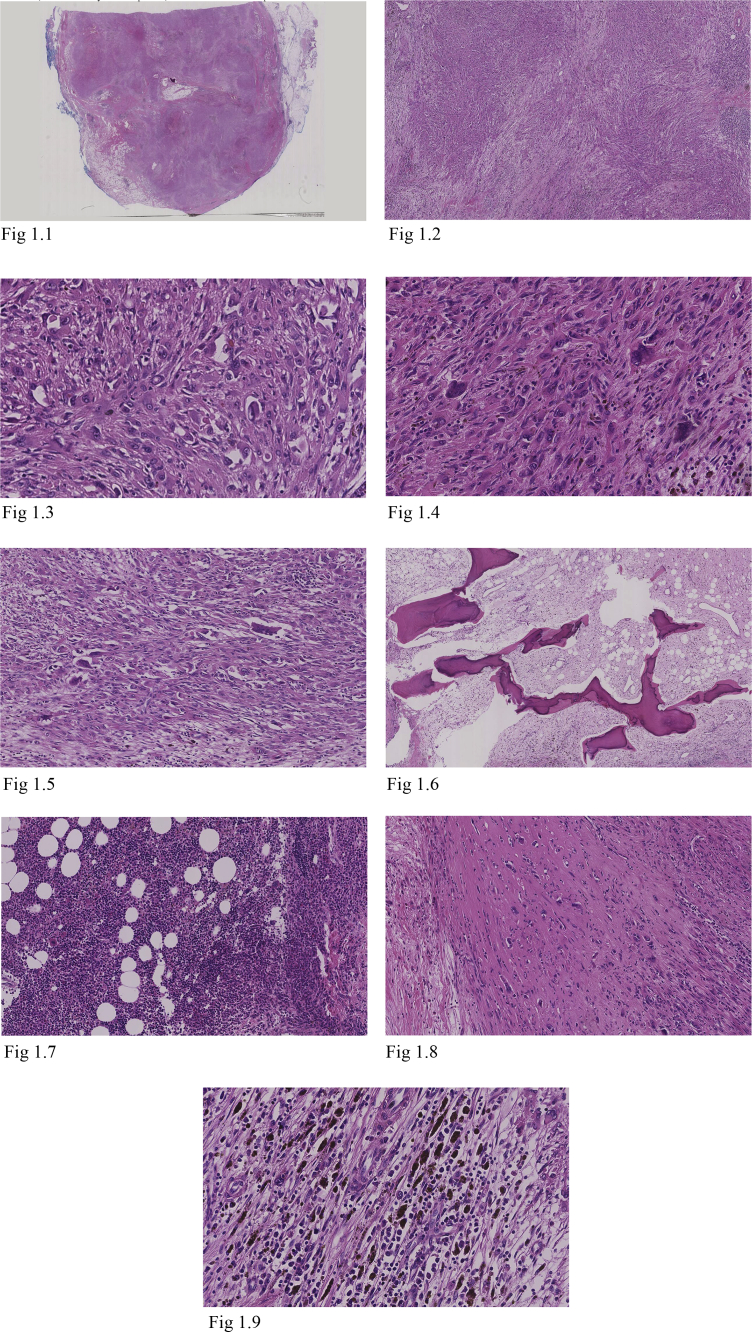
Gross pathological examination reported a 72 × 40 × 38mm mass covered in adipose tissue, with areas of calcification and infiltration. Microscopically the mass appeared well circumscribed but unencapsulated (Fig. 1.1). It was predominantly formed by vague nodular areas (Fig. 1.2) consisting of proliferating epithelioid and spindled cells (Figs. 1.3–1.4) with admixed osteoclast-like giant cells (Fig. 1.5), intervening mature adipose tissue and sclerotic stroma (Fig. 1.8). There were also areas of extramedullary haematopoiesis displaying trilineage differentiation (Fig. 1.7); metaplastic bone formation (Fig. 1.6); and smooth muscle clusters. No mitotic spindles or tumour necrosis were seen. A low mitotic count and low proliferative index were noted (Ki67 < 5%).
Fig. 1.
H&E: Well circumscribed unencapsulated tumour with vaguely nodular areas. The tumour contains epithelioid and spindled cells showing moderate atypia, with admixed osteoclast-like giant cells. There are areas within the tumour showing metaplastic bone formation, extramedullary haematopoiesis, sclerosis and mature adipose tissue. 1.1 Epithelioid haemangioendothelioma (H&E ×3 Magnification) Well circumscribed, unencapsulated tumour. 1.2 Epithelioid haemangioendothelioma (H&E ×25 Magnification) Vaguely nodular tumour areas. 1.3 Epithelioid haemangioendothelioma (H&E ×200 Magnification) Epithelioid and spindled cells. 1.4 Epithelioid haemangioendothelioma (H&E ×200 Magnification) Epithelioid and spindled cells. 1.5 Epithelioid haemangioendothelioma (H&E ×200 Magnification) Osteoclast-like giant cells. 1.6 Epithelioid haemangioendothelioma (H&E ×200 Magnification) Metaplastic bone formation. 1.7 Epithelioid haemangioendothelioma (H&E ×200 Magnification) Extramedullary haematopoiesis. 1.8 Epithelioid haemangioendothelioma (H&E ×200 Magnification) Sclerotic areas. 1.9 Epithelioid haemangioendothelioma (H&E ×200 Magnification) Haemosiderin-laden macrophages within tumour.
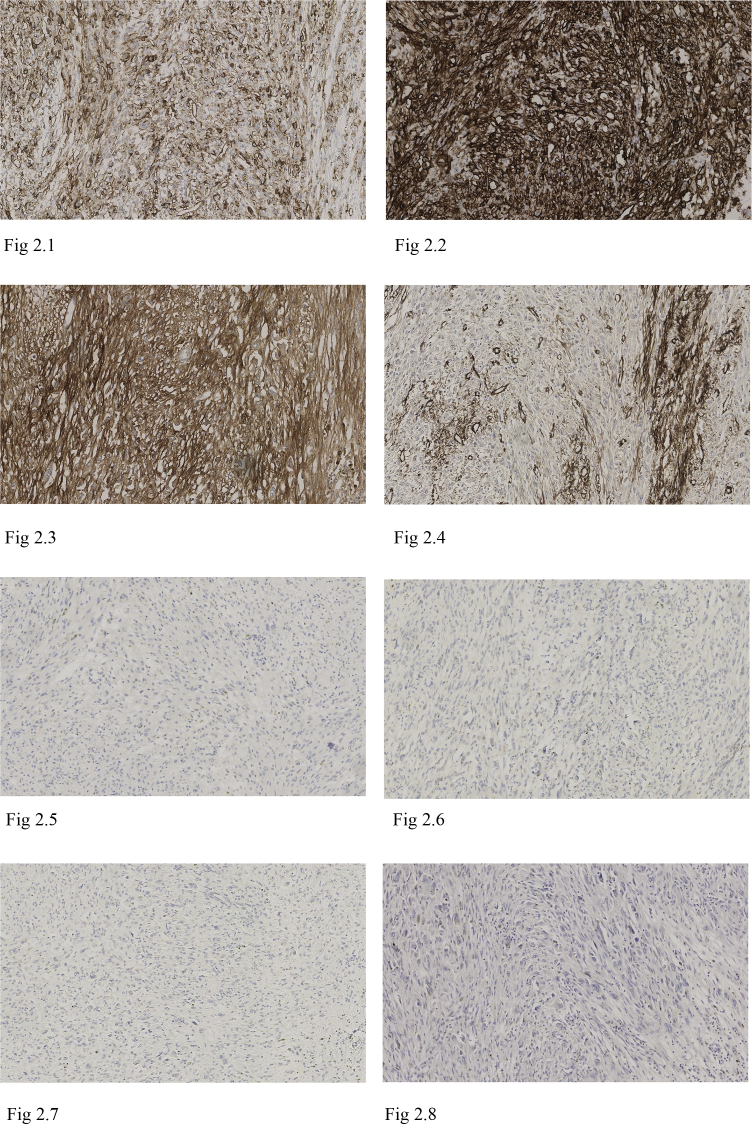
Immunohistochemistry showed the tumour cells were positive for vascular markers CD31 (Fig. 2.1) and CD34 (Fig. 2.2), with SMA evidencing intersecting smooth muscle fibres (Fig. 2.4). There was non-specific CD56 staining (Fig. 2.3). Cytokeratins (CEA, EMA, CAM2.4, MNF116), meuroendocrine markers (Synaptophysin, Chromogranin), melanoma markers (S100, Melan-A, HMB-45), skeletal muscle markers (Myogenin, MyoD1), thymic lymphocyte marker (TdT), GIST markers (CD117, DOG1) and neural markers (S100, GFAP) were all negative (Figs. 2.5–2.8). Mib-1 proliferation index was low at less than 5%.
Fig. 2.
Immunohistochemistry: Immunohistochemistry highlights that the tumour cells are positive for vascular markers CD31 and CD34. Non-specific CD56 stating is present. SMA shows the presence of intersecting smooth muscle fibres, whilst Desmin is negative. Melanocytic marker Melan-A and neuroendocrine marker Synaptophysin are both negative. Mib-1 proliferation index is low; <5%. 2.1 Epithelioid haemangioendothelioma CD31 × 100 Magnification. 2.2 Epithelioid haemangioendothelioma CD34 × 100 Magnification. 2.3 Epithelioid haemangioendothelioma CD56 × 100 Magnification. 2.4 Epithelioid haemangioendothelioma SMA ×100 Magnification. 2.5 Epithelioid haemangioendothelioma Desmin ×100 Magnification. 2.6 Epithelioid haemangioendothelioma Synaptophysin ×100 Magnification. 2.7 Epithelioid haemangioendothelioma Melan-A ×100 Magnification. 2.8 Epithelioid haemangioendothelioma Mib-1×100 Magnification.
In summary, the morphological and immunohistochemical findings suggest this was a case of localised mediastinal epithelioid haemangiendothelioma with extramedullary haematopoiesis, calcification and ossification treated by curative resection.
3. Discussion
Epithelioid haemangioendothelioma (EHE) is a rare neoplasm of vascular origin, defined by the 2002 WHO classification as a low-grade locally aggressive angiosarcoma with metastatic potential [3]. It was first described by Dail and Liebow in 1975 [4]. As intravascular sclerosing bronchioalveolar tumour of the lung, being renamed EHE by Weiss and Enzinger in 1982 [5]. Here we will be focusing primarily on pulmonary EHE (PEH), but the disease is also known to affect liver, soft tissues and, less commonly, bone. PEH typically presents in 36 year-old Caucasian females (female:male 4:1). 50–75% of cases are asymptomatic and present incidentally on radiographic imaging, but common symptoms include cough, dyspnoea and chest pain. Pleural effusions are the first sign of local pleural invasion. EHE has a prevalence of less than one in a million, with less than 300 cases of PEH having been described in the literature [6].
The aetiology of EHE remains unclear, although several clonal chromosomal abnormalities have been identified. These include a translocation involving CAMTA1 (chromosome 1) and WWTR (chromosome 3) described by Errani et al. [7]: both genes have been implicated in oncogenesis, but EHE is the first instance where they have been associated together in a recurrent chromosomal translocation. Additionally, it has been suggested that Bartonella infection may play a role in the development of EHE due to its characteristic chronic intraendothelial infection, VEGF production and suppression if endothelial cell apoptosis [8].
Due to its rarity and non-specific presentation, PEH is easily confused with a wide variety of benign and malignant lung disease in clinical practice. Differential diagnoses include chronic granulomatous disease, amyloidosis, hamartoma, primary and metastatic lung cancer, malignant mesothelioma and epithelioid angiosarcoma [2]. Clinical and laboratory data are non-specific and diagnosis is based primarily on radiological, histological and immunohistochemical features. Characteristically, CT scans report multiple perivascular nodules in the lower lung zones bilaterally, that are under 1cm in size [6]. Some cases of larger solitary nodules under 5cm have also been described [9]. In general, PET scans show mild FDG uptake by nodules, with a mean SUV of 3.5 [10]. Histologically, the nodules consist of a hypocellular sclerotic stroma containing spindle-shaped tumour cells with abundant eosinophilic cytoplasm and vacuoles containing erythrocytes. Nuclear atypia and the mitotic count are usually low. Occasionally, nodules with central necrosis, calcification and ossification have been described [2]. Endothelial lineage can be confirmed on immunohistochemistry, with samples staining positive for CD31, CD34, F8 and Fli-1. While CD34 is non-specific, CD31 is both specific and sensitive, being positive in 90% of cases, and the combination of CD31 and Fli-1 is considered diagnostic by some [2]. Traditionally, EHE was also diagnosed by factor VIII-related antigen expression on electron microscopy [11].
The cytological and immunohistochemical features of PEH described above allow us to differentiate it from other lung diseases presenting with multiple pulmonary nodules. Negative Congo Red staining excludes amyloidosis and the absence of non-specific inflammatory infiltration dismisses chronic granulomatous diseases such as tuberculosis, sarcoidosis and fungal infection. Hamartoma nodules are mainly comprised by lobulated mature cartilage, distinguishable from the mucous cartilage-like stroma of PEH. The atypia and increased mitosis of tumour cells in primary and metastatic lung cancer makes it easily differentiated from PEH. Malignant mesothelioma is discernible from PEH with pleural involvement through immunohistochemistry: tumour cells of the former are positive for mesothelial markers such as calretinin, cytokeratin 5/6 and WT-1. Epithelioid angiosarcoma shares similar pathology to PEH but with increased cellularity, cellular atypia and mitosis, such that some argue the latter may be a morphological continuity of the former [2].
As of yet there is no clear standard treatment for PEH. Curative surgery is an option in small size, limited and unilateral disease. Whereas adjuvant radiotherapy has been used to control localised EHE in soft tissue and bone, it is ineffective against PEH. Instead, chemotherapy with interferon-2alpha or carboplatin and etoposide has been used both as an adjuvant and in widespread inoperable disease [6]. Angiogenesis inhibition with bevacizumab and paclitaxel stopped disease progression in a case of aggressive metastatic PEH [12]. Other proposed treatment options include corticosteroids, azathioprine and multiple wedge resections [6].
Prognosis of EHE remains uncertain and reported survival ranges from 6 months to 24 years, with a mean survival of 4.6 years [13]. Indicators of poor prognosis include pulmonary disease, multi-organ involvement, disease progression, age of 55 years or above, male gender, severe pulmonary symptoms and pleural invasion. Death most commonly occurs due to respiratory failure or extrapulmonary tumour spread [6].
Having outlined the presentation and management of past cases of EHE, we can now comment on how our case compares to these. The clinical presentation can be considered atypical in that the patient was older, male and complained of non-respiratory symptoms. Radiologic findings of an FDG-avid single nodule with a diameter of 6.3cm located in the anterior superior mediastinum would make a diagnosis of EHE improbable. Nevertheless, histology and immunohistochemistry findings are highly characteristic of EHE and confirm the diagnosis. The nodule contained clusters of epithelioid and spindled cells with a low mitotic count and proliferative index, suspended in a sclerotic stroma. Areas of extramedullary haematopoiesis, calcification and ossification were also present. Endothelial lineage was corroborated by positive staining for CD31 and CD34. Interestingly, the nodule also showed evidence of smooth muscle clusters that stained positive for SMA, which have not been reported in other cases of EHE. The patient underwent curative resection that resulted in a good outcome, with no adjuvant treatment required.
In conclusion, EHE remains a rare disease that is often misdiagnosed and for which there is little understanding of the aetiology, management or prognosis. Although patterns of clinical and radiological features have been described, the definitive diagnosis remains through cytology and immunohistochemistry. This can be evidence by the case discussed above, where the clinical presentation differed greatly from that of other EHE cases but where histology and immunohistochemistry were highly characteristic. In order to improve early diagnosis and establish a clear standard of management, further studies involving larger patient cohorts are essential. We hope this case report contributes toward setting up a framework for better understanding EHE in the future.
Consent
Written informed consent was obtained from the patient for publications of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this Journal.
Conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Contributor Information
Davide Patrini, Email: davide.patrini@uclh.nhs.uk.
Laura Scolamiero, Email: laura.scolamiero.12@ucl.ac.uk.
Reena Khiroya, Email: reena.khiroya@uclh.nhs.uk.
David Lawrence, Email: david.lawrence@uclh.nhs.uk.
Elaine Borg, Email: elaine.borg@uclh.nhs.uk.
Martin Hayward, Email: martin.hayward@uclh.nhs.uk.
Nikolaos Panagiotopoulos, Email: nikolaos.panagiotopoulos@nhs.net.
References
- 1.Woo J.H., Kim T.J., Lee K.S., Kim T.S., Kim B.T. Epithelioid hemangioendothelioma in the thorax: clinicopathologic, CT, PET, and prognostic features. Medicine. 2016;95(30):e4348. doi: 10.1097/MD.0000000000004348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shao J., Zhang J. Clinicopathological characteristics of pulmonary epithelioid hemangioendothelioma: a report of four cases and review of the literature. Oncol. Lett. 2014 Dec;8(6):2517–2522. doi: 10.3892/ol.2014.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fletcher C.D.M., Unni K.K., Mertens F., editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press; Lyon: 2002. [Google Scholar]
- 4.Dail D.H., Liebow A.A., Gmelich J.T., Friedman P.J., Miyai K., Myer W., Patterson S.D., Hammar S.P. Intravascular, bronchiolar, and alveolar tumor of the lung (IVBAT). An analysis of twenty cases of a peculiar sclerosing endothelial tumor. Cancer. 1983;51(3):452–464. doi: 10.1002/1097-0142(19830201)51:3<452::aid-cncr2820510317>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 5.Weiss S.W., Enzinger F.M. Epithelioid hemangioendothelioma: a vascular tumor often mistaken for a carcinoma. Cancer. 1982;50:970–981. doi: 10.1002/1097-0142(19820901)50:5<970::aid-cncr2820500527>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 6.Sardaro A., Bardoscia L., Petruzzelli M.F., Portaluri M. Epithelioid hemangioendothelioma: an overview and update on a rare vascular tumour. Onc Rev. 2014;8(259):82–91. doi: 10.4081/oncol.2014.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Errani C., Zhang L., Sung Y.S., Hajdu M., Singer S., Maki R.G., Healey J.H., Antonescu C.R. A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes, Chromosom. Cancer. 2011;50(8):644–653. doi: 10.1002/gcc.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mascarelli P.E., Iredell J.R., Maggi R.G., Weinberg G., Breitschwerdt E.B. Bartonella species bacteremia in two patients with epithelioid hemangioendothelioma. J. Clin. Microbiol. 2011;49(11):4006–4012. doi: 10.1128/JCM.05527-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagan P., Hassan M., Le Pimpec Barthes F., Peyrard S., Souilamas R., Danel C., Riquet M. Prognostic factors and surgical indications of pulmonary epithelioid hemangioendothelioma: a review of the literature. Ann. Thorac. Surg. 2006;82(6):2010–2013. doi: 10.1016/j.athoracsur.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 10.Nizami I., Mohammed S., Abouzied Mel D. Pulmonary epitheloid hemangioendothelioma PET CT findings and review of literature. Ann. Saudi Med. 2014 Sep-Oct;34(5):447–449. doi: 10.5144/0256-4947.2014.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weldon-Linne C., Victor T., Christ M. Angiogenic nature of the “intravascular bronchioloalveolar tumor” of the lung: an electron microscopic study. Arch. Pathol. Lab. Med. 1981;105:174–179. [PubMed] [Google Scholar]
- 12.Gaur S., Torabi A., O'Neill T.J. Activity of angiogenesis inhibitors in metastatic epithelioid hemangioendothelioma: a case report. Cancer Biol. Med. 2012;9(2):133–136. doi: 10.3969/j.issn.2095-3941.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau K., Massad M., Pollak C., Rubin C., Yeh J., Wang J., Edelman G., Yeh J., Prasad S., Weinberg G. Clinical patterns and outcome in epithelioid hemangioendothelioma with or without pulmonary involvement: insights from an internet registry in the study of a rare cancer. Chest. 2011;140(5):1312–1318. doi: 10.1378/chest.11-0039. [DOI] [PubMed] [Google Scholar]