Abstract
Background
Scant research has been conducted on neural mechanisms underlying stress processing in individuals with alcohol dependence (AD). We examined neural substrates of stress in AD individuals compared with controls using an fMRI task previously shown to induce stress, assessing amygdala functional connectivity to medial prefrontal cortex (mPFC).
Materials and methods
For this novel pilot study, 10 abstinent AD individuals and 11 controls completed a modified Trier stress task while undergoing fMRI acquisition. The amygdala was used as a seed region for whole-brain seed-based functional connectivity analysis.
Results
After controlling for family-wise error (p = 0.05), there was significantly decreased left and right amygdala connectivity with frontal (specifically mPFC), temporal, parietal, and cerebellar regions. Subjective stress, but not craving, increased from pre-to post-task.
Conclusions
This study demonstrated decreased connectivity between the amygdala and regions important for stress and emotional processing in long-term abstinent individuals with AD. These results suggest aberrant stress processing in individuals with AD even after lengthy periods of abstinence.
Keywords: Alcohol dependence, fMRI, Stress task, Functional connectivity, Amygdala
Highlights
-
•
Individuals with AD completed a stress task in an fMRI scanner.
-
•
AD individuals had decreased connectivity between the amygdala and medial PFC.
-
•
Results are in long-term abstinent AD, indicating lasting emotion dysregulation.
1. Introduction
Experience of early stressful life events significantly increases the odds of developing alcohol dependence (AD) (Pilowsky et al., 2009), and recent stress increases alcohol consumption in the short- and long-term (Vlahov et al, 2002, Vlahov et al, 2004). In Koob's and colleagues' (Koob and Volkow, 2010, Koob and Le Moal, 1997) three-stage model of addiction, stress is hypothesized to play several key roles. For example, in the second “withdrawal and negative affect” stage, stress increases withdrawal effects through release of corticotropin-releasing factor (CRF) and norepinephrine in the extended amygdala—a brain region comprised of the bed nucleus of the stria terminalis, the central nucleus of the amygdala, and the shell subregion of the nucleus accumbens (Koob and Volkow, 2010). Subsequent to this withdrawal phase, the third stage is further influenced by stress, which often leads to relapse. Importantly, Koob and colleagues hypothesize that addiction leads to an overall allostatic shift, a readjustment of hedonic response as a result of repeated and compulsive drug use and overcompensating by the stress response system (Koob and Le Moal, 1997, Koob, 2013). As physiological adjustments occur, it may be that there are not enough resources available to effectively inhibit the stress response. Alternatively, the stress response may also become sensitized, making it easier to be triggered in response to a stressor.
The relationship between stress and AD is complex. Stress may predispose vulnerable individuals to develop alcohol problems (Koob and Kreek, 2007), with stress system dysfunction conversely being suggested as a consequence of AD (Adinoff et al., 2005). Individuals with AD are often characterized as having a blunted cortisol response (Lovallo et al., 2000) and elevated basal cortisol levels (Lovallo et al, 2000, Thayer et al, 2006), yet as Stephens and Wand (2012) point out, specific glucocorticoid supply levels differ depending on what stage in the addiction cycle an individual is in, amongst other factors. To date, only one known study specifically examined the neural response to stress in an AD sample (Seo et al., 2013). Results showed blunted activity in the ventromedial PFC and anterior cingulate cortex (ACC) during an idiographic stress script, with hyperactivity in the amygdala and other regions during a neutral script. Since recently abstinent AD individuals have a blunted cortisol response (Lovallo et al., 2000), they may have an altered response to a stressor as a result of an inhibited negative feedback loop. For example, connectivity between the amygdala and medial PFC (mPFC) may be important since the mPFC has been suggested to play a key role in controlling motivated behavior in alcohol consumption (George et al., 2012) as well as the mPFC being key to modulate the inhibitory response to stress in healthy individuals (Kern et al., 2008).
The present study investigated the effects of AD on amygdala functional connectivity during an fMRI stress task, given the important role proposed for the amygdala in the neurobiology of addiction (Koob and Volkow, 2010). It was hypothesized that abstinent AD subjects demonstrate decreased connectivity (George et al., 2012) between the amygdala and mPFC during the stress task, compared with controls.
2. Materials and methods
The present study analyzed data previously collected as part of a pilot imaging genetics study derived from two larger parent studies (NCT00226694, NCT01200901). Twenty-one participants (10 AD, 11 controls) were recruited from the original parent studies that examined hormonal changes with stress (for details see (Anthenelli et al., 2009)). Participants completed psychological questionnaires related to stress and trauma history and performed an fMRI stress task.
2.1. Participants
A total of 21 individuals (23-55 years-old) completed the fMRI stress task. Ten abstinent individuals with AD (6 females) were recruited from the parent study that examined endocrine and behavioral responses to pharmacological stressors (Anthenelli et al., 2009) (NCT00226694). Eleven non-depressed controls (6 females) were recruited from the community as part of a larger study examining stress and brain response in depression (NCT01200901). The IRBs at the University of Cincinnati and Cincinnati Veterans Affairs Medical Center approved all aspects of the study, and all participants provided written informed consent.
AD participants met lifetime DSM-IV-TR criteria for AD in sustained or early full remission and were in treatment when they enrolled in the parent study. Participants were abstinent from all substances except for tobacco for at least one month prior to the MRI session. AD participants had largely maintained abstinence since their participation in the parent study, as measured by Timeline Follow-Back, though only one month of abstinence was required for this pilot imaging study. Although current PTSD was exclusionary, sub-threshold symptoms of PTSD and history of trauma were not. Controls had no history of any Axis I or Axis II disorders, including substance use disorders (SUD). Current use of psychotropic medication; lifetime history of serious neurologic injuries or disorders; severe medical illness; diagnosis of an independent Axis I anxiety, mood or psychotic disorder (or Axis II personality disorder in controls); use of oral contraceptives; current pregnancy or lactation in women; or MRI contraindications were exclusionary in both groups. Recent (past several days) abstinence from substance use was confirmed by drug toxicology testing (DrugTestStrips.com™12 Panel drug test), and breathalyzer (FC10 Breath Alcohol Tester® to verify 0.000 BAC) in AD individuals.
2.2. Procedure
Eligible participants were consented to this phase of the study, and Timeline Follow-Back (TLFB) data were collected to fill in alcohol and drug use from the parent study's conclusion to the present study. Women underwent urine pregnancy testing. All participants were given psychological questionnaires to assess mood and trauma history. Participants then completed the neuroimaging protocol.
2.3. Measures
2.3.1. Stress and craving measures
Subjective stress and craving for alcohol was measured by calculating change scores from baseline (initial moments in the scanner) to post-scan (immediately after the stress task) with participants rating their level subjective stress and craving on a 100-point scale.
2.4. Data acquisition
Imaging was conducted at the University of Cincinnati's Center for Imaging Research, using a 4.0 T Varian, Unity INOVA Whole Body MRI/MRS System (Varian, Inc., Palo Alto, CA). To provide an anatomical reference for the fMRI data, a T1-weighted, 3-D anatomical brain scan was first obtained using a modified driven equilibrium Fourier transform sequence (TMD = 1.1 s, TR = 13 ms, TE = 6 ms, FOV = 25.6 × 19.2 × 19.2 cm, matrix 256 × 192 x 96 pixels, flip angle = 20°, 15″). fMRI scans were acquired using an RF-spoiled FAST 3-D acquisition technique. Functional images were collected while performing the stress task using a T2*-weighted gradient-echo echoplanar imaging (EPI) pulse sequence (TR/TE = 2000/30 ms, FOV = 25.6 × 25.6 cm, matrix 64 × 64 pixels, slice-thickness = 4 mm, flip angle = 75°, 35 slices in coronal orientation). Sixteen minutes of the hour-long scan were dedicated to the control (5 min) and stress tasks (11 min), respectively (see fMRI Stress Task below). A neuroradiologist assessed each scan for brain abnormalities and found none.
2.5. Amygdala mask
Automated left and right amygdala masks were created for each subject, and then hand nudged to more accurately reflect neuroanatomy.
2.6. fMRI stress task
Stress was induced through a variation on the Trier Social Stress Test (TSST) (Allendorfer et al, 2014, Kirschbaum et al., 1993). The task includes two math components; a stress-inducing test and a “control” test. The control task (not used in the present study) was first and included 60 different basic subtraction problems. They then completed the stress task, which consisted of 80 subtraction problems that were considerably more difficult and contained three possible answers rather than two. As this task was about to begin, participants saw a video on their goggles worn into the scanner of two confederate “doctors” sitting in the scanner console room. Participants were told these “experimenters,” who introduced themselves as doctors, would be rating them and giving feedback on their performance (six different pre-recorded messages that informed them they were not performing up to the task, regardless of their actual performance). Participants were also told that they would have between 1 and 5 s to answer each question, but would not be told how long was left. If they went over the allotted time, their answer would not count. Finally, participants were told they had to get enough questions correct for their data to be usable; if they did not succeed, they would not receive full compensation. At the completion of the task, participants were debriefed and informed that there were no experimenters rating performance and that the feedback was not based on their performance. Each participant was fully compensated. The stress task took approximately 11 min to complete and was administered in one block. Mean amygdala activation at each TR over the course of the 11 min was then calculated and summed for AUC analysis to assess potential habituation patterns in response to the stress task.
2.7. Data processing and analysis
2.7.1. fMRI processing
fMRI data were processed using Analysis of Functional NeuroImages (AFNI) (Cox, 1996). Standard pre-processing for each participant was done, including: align slices into correct anatomical space, remove the first three repetition times (TRs, or the length of time between excitation pulses), correct for timing differences in slice acquisition, correct for motion, blur at 1.8 times the voxel size (5.4 mm), resample into 3.0 mm3 voxels, create a brain mask, warp to standard space (Talairach and Tournoux, 1988), and create time and curve regressors. Motion parameters were analyzed in 3dToutCount and trials were censored if value was above 0.3 mm. Trained research personnel visually inspected all images for excessive motion and noise; TRs with excessive movement were censored. Greater than 15% of TR removal due to censoring resulted in the participant's data not being used; no subjects surpassed this criterion. Motion for three rotational and three translational parameters were also used to create a temporal mask that was used in the analyses as nuisance regressors. Responses were visually monitored to ensure participant engagement in the task.
2.7.2. Functional connectivity (fcMRI)
Average BOLD signal across the time-series in the seed regions (both left and right amygdala) was extracted for each subject. Next, for each subject, the time-series activation in the seed regions (left and right amygdala) were used as predictors for activation in the rest of the brain (voxel-wise analysis), betas and t-statistics for each individual were saved.
2.7.3. Statistical analysis
In order to determine whether group status predicted functional connectivity between the seed regions and the rest of the brain, using 3dttest++ in AFNI, we assessed whether coefficients significantly differed by group. A Monte Carlo simulation was run to correct for family-wise error (Forman et al., 1995), finding that for an individual voxel threshold of p = 0.01 and a family-wise error corrected significance of 0.05, 13 contiguous voxels had to be activated (351 μl).
Groups differed on handedness (see Table 1). In order to rule-out influence of handedness, two MANOVAs were run with both the left and right amygdala with handedness as the independent variable. Any brain regions that were significantly predicted by handedness were then followed-up with a regression including group status and handedness. If the regression by group status and handedness predicted the brain cluster, then it was considered null and excluded from the results. Only one cluster met these criteria.
Table 1.
Demographic and clinical characteristics.
| Alcohol Dependent (N = 10) M (SD), Range; % | Healthy Control (N = 11) M (SD), Range; % | |
|---|---|---|
| Age | 43.3 (8.3) 30-55 | 37.6 (10.6) 23-55 |
| % Female | 60% | 54% |
| % Caucasian | 70% | 64% |
| % Right Handed* | 60% | 100% |
| Education (in years) | 13.7 (2.1) 10-17 | 15.4 (1.5) 14-18 |
| BDI Total Score | 8.8 (9.7) 0-27 | – |
| HAM-D Total Score | – | 0.4 (0.8) 0-2 |
Notes: * indicates p < 0.05 between groups.
3. Results
3.1. Demographic and clinical characteristics
Groups did not differ significantly on age, education, racial composition, or gender (see Table 1). Groups differed on handedness, with all left handed participants in the AD group [x2(1) = 3.85, p = 0.05]. Therefore, handedness was used as a covariate in subsequent analyses. In the AD group, at the time of the fMRI scan participants had been abstinent from alcohol a mean of 1628 days (SD = 2123, range = 68–4759).
3.2. Primary results
3.2.1. Amygdala connectivity
After controlling for family-wise error in AFNI (p = 0.05), significant differences were found between the seed regions of the left and right amygdala voxel-wise connectivity in the whole-brain analysis. Decreased connectivity was found between the left and right amygdala and the mPFC and other areas, with full results listed in Table 2, Table 3. In addition, 4 (out of 30) clusters in the left amygdala and 3 (out of 27) clusters in the right amygdala showed increased connectivity in the AD individuals relative to controls.
Table 2.
Left amygdala connectivity.
| Talairach Coordinates | ||||
|---|---|---|---|---|
| Region | CM x | CM y | CM z | Volume (μl) |
| Decreased Connectivity | ||||
| Right Cerebellar Tonsil | −23.7 | 69.5 | −29.1 | 1026 |
| Left Cerebellar Tonsil | 31.5 | 34.6 | −43.3 | 594 |
| Right Declive | −4.9 | 79.2 | −19.5 | 540 |
| Right Culmen | −5.5 | 62.7 | −5.8 | 513 |
| Left Uncus | 11.8 | 7.3 | −28.6 | 405 |
| Left Uvula | 25.3 | 74.5 | −25.5 | 351 |
| Right Caudate | −8 | 2.2 | 20 | 702 |
| Left Precuneus | 1.4 | 45.1 | 47.4 | 540 |
| Right Posterior Cingulate | −2.9 | 47.8 | 19.2 | 1080 |
| Left Cingulate Gyrus+ | 12 | 5.9 | 27.8 | 567 |
| Left Parahippocampal Gyrus | 16 | 38.4 | 6.2 | 486 |
| Left Posterior Cingulate | 1.7 | 27.7 | 17.2 | 378 |
| Right Superior Parietal Lobule | 6 | 56.3 | 58.3 | 6399 |
| Left Inferior Parietal Lobule | 40.8 | 43.2 | 54 | 378 |
| Left Inferior Temporal Gyrus | 50.6 | 49.2 | −22.9 | 756 |
| Right Superior Temporal Gyrus | −53 | −5.7 | −2.6 | 729 |
| Right Middle Temporal Gyrus | −63.4 | 43.5 | −10.2 | 378 |
| Right Inferior Temporal Gyrus | −57.7 | 59.2 | −3.7 | 378 |
| Left Inferior Frontal Gyrus | 10.8 | −25.4 | −19.2 | 540 |
| Right Superior Frontal Gyrus | −23 | −24.5 | 55.1 | 486 |
| Left Middle Frontal Gyrus | 30.6 | −35.8 | 37.2 | 432 |
| Left Postcentral Gyrus | 36.6 | 29.5 | 62.4 | 432 |
| Left Inferior Frontal Gyrus | 20 | −14.8 | −17.9 | 378 |
| Left Inferior Frontal Gyrus | 24.9 | −22.8 | −11.6 | 378 |
| Right Superior Frontal Gyrus | −23.6 | −37.3 | 33.6 | 351 |
| Right Medial Frontal Gyrus | −2 | 17.4 | 70.7 | 351 |
| Increased Connectivity | ||||
| Right Declive | −3.6 | 62.3 | −23.6 | 1161 |
| Right Posterior Cingulate | −28.5 | 55.9 | 11.2 | 702 |
| Right Precentral Gyrus | −33.4 | −0.2 | 33.3 | 1134 |
| Right Lingual Gyrus | −30.9 | 73.5 | −6.9 | 513 |
Notes: +denotes cluster that differed by handedness in MANOVA, but handedness did not predict connectivity in the follow-up multiple regression analysis.
Table 3.
Right amygdala connectivity.
| Talairach Coordinates | ||||
|---|---|---|---|---|
| Region | CM x | CM y | CM z | Volume (μl) |
| Decreased Connectivity | ||||
| Right Pyramis | −23.4 | 69.1 | −29.3 | 1080 |
| Right Declive | −4 | 79.9 | −20.3 | 621 |
| Right Culmen | −5.8 | 62.4 | −5.4 | 594 |
| Left Cerebellar Tonsil | 33.2 | 37.8 | −4.4 | 567 |
| Right Lentiform Nucleus | −17.1 | 1.9 | −6 | 945 |
| Right Claustraum | −20.5 | −7.8 | 16 | 432 |
| Right Caudate | −8.9 | 6.9 | 17.8 | 1674 |
| Left Caudate | 12.7 | −12.5 | −1.6 | 1080 |
| Left Parahippocampal Gyrus | 14.5 | 36.5 | 6.2 | 729 |
| Left Cingulate Gyrus | 12.8 | 6.9 | 30.5 | 513 |
| Right Posterior Cingulate | −0.6 | 42.9 | 19.2 | 2187 |
| Right Precuneus | −7.6 | 55.3 | 60 | 459 |
| Right Superior Parietal Lobule | −23.5 | 58.1 | 59.2 | 864 |
| Left Superior Parietal Lobule | 9.2 | 53.6 | 59.8 | 648 |
| Left Superior Parietal Lobule | 31.9 | 58.2 | 50.7 | 540 |
| Right Middle Temporal Gyrus | −63.3 | 42.9 | −10 | 351 |
| Left Postcentral Gyrus | 1.5 | 47.6 | 65.7 | 1026 |
| Left Postcentral Gyrus | 36.3 | 29.4 | 61.8 | 567 |
| Right Postcentral Gyrus | −9.8 | 42.5 | 70.6 | 378 |
| Left Middle Frontal Gyrus | 30.4 | −39.7 | 35.1 | 621 |
| Right Medial Frontal Gyrus | −2.5 | 17.9 | 71 | 378 |
| Left Inferior Frontal Gyrus | 16.8 | −20.8 | −17.7 | 1161 |
| Right Superior Frontal Gyrus | −18.4 | −28.7 | 54.8 | 729 |
| Right Superior Frontal Gyrus | −22.8 | −37.1 | 35.1 | 621 |
| Increased Connectivity | ||||
| Right Declive | −3.7 | 63.6 | −24 | 1053 |
| Right Lingual Gyrus | −31.4 | 73 | −6.9 | 756 |
| Right Precentral Gyrus | −35 | −0.2 | 34.8 | 702 |
Results do not appear to be lateralized. For example, follow-up MANOVA analysis to control for handedness revealed that left handedness predicted connectivity in only two left cingulate gyrus regions (a) a medial anterior region of the cingulate gyrus [F(1,16) = 3.08, p < 0.10]; and (b) a lateral posterior cingulate gyrus cluster [F(1,16) = 18.18, p < 0.01]. Multiple regressions including AD group status found that left handedness predicted increased connectivity in the second (b) cluster (beta = −0.67, p = 0.002), but not the first (a) cluster (beta = 0.05, p = 0.75). This finding in the second (b) cluster was therefore excluded from the table.
3.2.2. Subjective stress
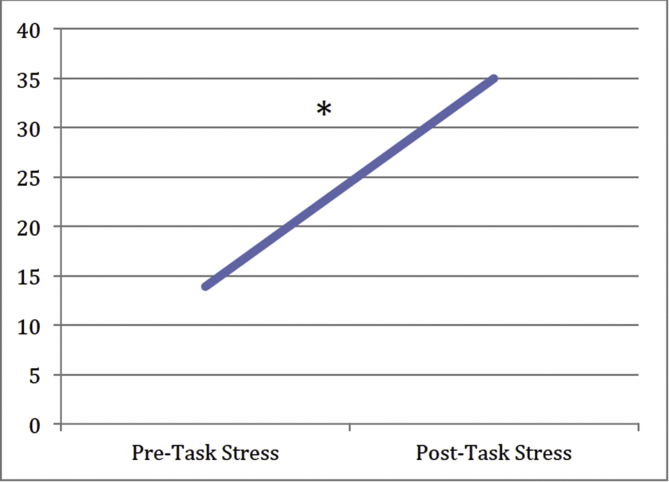
Nine of the 10 AD subjects completed 100-point visual analog scales (VAS) of their stress level before (pre-scan) and after (post-scan) the stress task (pre-scan M = 13.89; SD = 11.93; post-scan M = 35.00; SD = 24.50). Within the AD group, participants exhibited a significant increase in subjective stress from pre-scan to post-scan (t(9) = 3.967, p = 0.004; see Fig. 1); levels went from calm to moderately stressed.
Fig. 1.
Line graph of mean subjective stress level (100 point VAS) pre-scan to post-scan in the AD group. Results indicate that participants with AD experience significantly greater subjective stress after the stress task than before the stress task (* indicates p < 0.005).
3.2.3. Craving
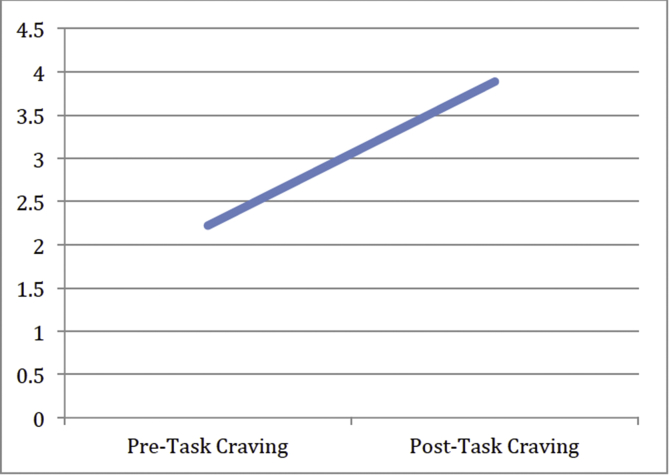
Nine of the 10 AD subjects completed 100-point VAS of their craving of alcohol before (pre-scan) and after (post-scan) the stress task (pre-scan M = 2.22; SD = 3.63; post-scan M = 3.89; SD = 8.21). There was no significant change in self-reported craving in AD subjects (t(9) = 0.535, p = 0.608; see Fig. 2), and levels remained in the no-to-minimal craving range.
Fig. 2.
Line graph of mean craving (100 point VAS) pre-scan to post-scan in the AD group. Results indicate that participants with AD did not crave alcohol significantly more after completing the stress task than before the stress task (p > 0.05).
4. Discussion
The present study investigated neural differences in processing in long-term abstinent AD during a fMRI stress task. Functional connectivity analyses using the left and right amygdala as seed regions revealed decreased connectivity between the left and right amygdala and prefrontal, temporal, parietal, and cerebellar structures in AD individuals in comparison to healthy controls. In addition, 4 (out of 30) clusters in the left amygdala and 3 (out of 27) clusters in the right amygdala showed increased connectivity in the AD individuals relative to controls.
In assessing functional connectivity of the amygdala, the AD group demonstrated decreased connectivity with many brain regions (left: 26 significant clusters; right: 24 clusters). Within the PFC, we found a range of connectivity differences in superior, inferior, middle, medial, and precentral regions. The only other known study of stress response in a scanner in individuals with AD showed blunted activity in the ventromedial PFC and anterior cingulate cortex (ACC) during an idiographic stress script, though importantly functional connectivity was not assessed. The disconnection between the prefrontal cortex and amygdala in stress, then, is particularly novel and noteworthy. Animal models of AD have most frequently been examined to assess stress processing (for review, see Koob, 2013). In humans, however, most studies have assessed emotional processing differences in fronto-limbic regions that also regulate stress. Other studies outside the stress and AD literature have shown decreased connectivity in individuals with AD compared to controls (e.g (Maurage et al, 2013, Kienast et al, 2013).,). A separate analysis of this same sample found reduced fcMRI during both fearful and happy affective processing (Padula et al.). As previous research has suggested a role for connectivity between the amygdala and medial PFC (mPFC) in controlling motivated behavior in alcohol consumption (George et al., 2012), this disrupted fcMRI may be telling of aberrant processing stress and emotional processing in general. Future research is needed to determine both overlap and unique pathways between emotional and stress processing, as well as to further understand the role of the mPFC in these functions.
A number of other regions exhibited largely decreased connectivity with the amygdala and it is interesting to speculate on the relevance of some of these. In regards to stress, the parietal lobe may potentially be linked to stress response in AD (Yang et al., 2013). The parietal lobe, in general, may also be activated by mathematical problem solving (Park et al., 2013), and in working memory and attentional tasks (Soto et al., 2014). Further research is needed to clarify both the roles of each regions in stress processing, as well as to investigate potential underlying mechanisms within the parietal lobe that may uniquely contribute to either math or stress processing. In particular, a stress task with no math component may result in no findings within the parietal lobe. However, it should be noted that our results highlight different parietal areas than those often implicated during math-processing. Again, our results suggesting decreased connectivity are broadly consistent with other findings in AD of blunted connectivity in a variety of tasks (Maurage et al, 2013, Kienast et al, 2013).
The cerebellum has been hypothesized to have a greater role in stress and emotion processing than once thought (for review, see (Stoodley and Schmahmann, 2010, Baumann and Mattingley, 2012)). In particular, the vermis has been proposed as the “limbic cerebellum” (Stoodley and Schmahmann, 2010), fitting with our findings regarding altered function of limbic regions including regions within the vermis such as the culmen and uvula. These cerebellar functional differences may also be due to structural changes, as alcohol exposure in animals (Phillips, 1990), adolescents (Lisdahl et al., 2013), and adults with AD, even after 7.5 months of abstinence (Durazzo et al., 2015), has been linked with cerebellar atrophy. As much of this cerebellum-specific research is preliminary, more research is needed to assess the influence of cerebellum structure and function on alcohol-related outcomes and the underlying mechanisms that drive cerebellar processing.
Several clusters showed increased connectivity with the amygdala. (4/30 with the left amygdala; 3/27 with the right amygdala) in the AD group. One region showing both increased and decreased connectivity is the right posterior cingulate cortex (PCC). The cingulate is important for regulating limbic activity, particularly within the amygdala (Herman et al., 2003). The PCC in particular is also key in guiding attention and internally referenced thought (Leech and Sharp, 2014). Leech and Sharp (2014) suggest the more dorsal portion of the PCC is a “transitional” pathway, coordinating complex cognition across distinct functions (e.g., balancing internal and external attention). Perhaps what the increased and decreased connectivity presented here indicates, then, is the nuanced nature of certain regions, such as the PCC, in a range of functions. This is even more likely the case given the both stressful and cognitive demanding nature of the current task.
Heavy alcohol use has known structural consequences (Rosenbloom and Pfefferbaum, 2008) that may reorganize neural systems (Crews et al., 2005), leading to aberrant stress processing. Alterations in excitatory projections from the OFC and mPFC to the amygdala in mice and monkeys have been related to inappropriate affective regulation and stress reactivity (Andolina et al, 2013, Barbas et al, 2003, Ghashghaei and Barbas, 2002). Multi-modal imaging techniques are needed to further tease apart these hypothesized connections between functional stress processing deficits and potential structural deficits in AD.
It is interesting to note that, even with aberrant neural processing in the AD individuals and increased self-reported stress levels, the AD group did not report increased alcohol craving. Though this may seem in contrast to previous studies, perhaps it is actually an indication of successful maintenance of sobriety. Craving in response to stress has previously been found to be an important mediator of relapse (Law et al, 2016, Higley et al, 2011). In heavy drinkers who are not dependent, however, craving was not predictive of alcohol use problems (Tartter and Ray, 2012). Therefore, craving may best be a predictor in those with AD who are more likely to relapse. As these participants had varying degrees of abstinence, with a minimum of two months (and up to 13 years) of abstinence, these particular individuals may not be as susceptible to stress induced craving, and, therefore, better able to sustain abstinence.
4.3 Though the present findings are robust and novel, several limitations should be noted. Participants reported stress during a “control” portion of the task (not analyzed here), which also involved solving arithmetic problems; therefore, we were unable to compare amygdala connectivity during a portion of the task that was subjectively experienced as non-stressful. Future studies should examine the relationship between stress versus non-stress response in the amygdala in abstinent individuals with AD in a task that does not include a stress-provoking cognitive component. Indeed, future studies should include a clear control task to fully determine stress processing as compared to a neutral task. Further, as the stress-inducing task presented included solving mathematical problems, there is a possibility that the findings were influenced by differences in mathematical abilities. However, it should be noted that a prior study found the task to be stress-provoking (Allendorfer et al., 2014) and subjective stress levels did increase during the task. Subjective stress analysis was conducted in the AD group only and should be replicated with a control group to provide better interpretability. This was a pilot study with a small sample size; findings need to be replicated in a larger sample to assess whole brain regional activation patterns and ensure generalization. Our sample also included left-handed individuals, which may have introduced extra variance into our between-group differences (Vingerhoets et al., 2012). However, lateralization differences predominately affect language and fine motor function (Gutwinski et al., 2011). Nonetheless, right-vs-left handedness may also have stress-related effects (Serrien and Sovijarvi-Spape, 2013). Our AD participants had histories of other substance use disorders and psychiatric comorbidities. However, AD was the primary diagnosis and the reason for enrollment in the study and these conditions are commonly comorbid in AD treatment samples. AD participants also had wide variability in the length of abstinence, and this may impact neural-related dysfunction, though this may reflect long-term stress dysregulation in individuals with AD. Future research is needed to tease apart the potential influence of length of abstinence on neural correlates of stress response. A variety of confounds may be present in this study, including tobacco smoking, mathematical and intellectual abilities, and trauma history. Therefore, additional research into the possible influence of these factors is needed to more fully assess the influence of stress relative to other confounds. Finally, future studies with larger samples of both men and women are needed to assess the functional response to stress in AD subjects, as gender is often an important moderator of emotion processing and brain connectivity (Tomasi and Volkow, 2012).
In summary, the present pilot study demonstrated decreased connectivity between the amygdala and brain areas important for stress and emotion processing in individuals with AD. Longitudinal studies would be beneficial to assess whether the stress response dysregulation causes or is a result of AD. Additional research developing early interventions that address altered amygdala response to stress in AD samples are needed, especially in youth.
Funding
This work was supported by the University of Cincinnati (UC URC Interdisciplinary Grant PIs: Medina (Lisdahl) & Anthenelli); NIDA (3R01DA030354 PI: Lisdahl); NIAAA (RO1AA13307 & RO1AA013957, PI: Anthenelli); and NIMH (NIMH K23 MH67705 PI: Nelson). Dr. Lisdahl's writing of this manuscript is supported, in part, by NIDA 3R01DA030354. Dr. Anthenelli's writing of this manuscript is supported, in part, by the Integrative Neuroscience Initiative on Alcoholism (INIA) Stress Consortium, NIAAA RO1019720, NIDA-VA CSP #s 1032 and 1033, and VA Merit Review NEUA-003-08S. Dr. Padula's writing of this manuscript is supported, in part, by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment and the Department of Veterans Affairs Sierra-Pacific Mental Illness Research, Education, and Clinical Center (MIRECC).
Financial disclosures
Dr. Anthenelli provides consultancy and/or advisory board services to Pfizer, Inc. and Arena Pharmaceuticals and Cerecor. The other authors have no financial conflicts to disclose.
Acknowledgements
Portions of these data were presented at the College on Problems of Drug Dependence Meeting (2014) and the Research Society on Alcoholism (2015). The views expressed are those of the authors and do not necessarily reflect the views of the Department of Veterans Affairs.
References
- Adinoff B. Suppression of the HPA Axis stress-response: implications for relapse. Alcohol. Clin. Exp. Res. 2005;29(7):1351–1355. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allendorfer J.B. Physiologic and cortical response to acute psychosocial stress in left temporal lobe epilepsy - a pilot cross-sectional fMRI study. Epilepsy Behav. 2014;36:115–123. doi: 10.1016/j.yebeh.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Andolina D. Prefrontal/amygdalar system determines stress coping behavior through 5-HT/GABA connection. Neuropsychopharmacology. 2013;38(10):2057–2067. doi: 10.1038/npp.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthenelli R.M. Abstracts–posters: sex differences in the stress hormone response to the combined dexamethasone/CRH stimulation test in long-term abstinent alcoholics and controls. Alcohol. Clin. Exp. Res. 2009;33(Suppl. s1):11A–265A. [Google Scholar]
- Barbas H. Serial pathways from primate PFC to autonomic areas may influence emotional expression. BMC Neurosci. 2003;4:25. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann O., Mattingley J.B. Functional topography of primary emotion processing in the human cerebellum. Neuroimage. 2012;61(4):805–811. doi: 10.1016/j.neuroimage.2012.03.044. [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crews F.T. Alcoholic neurobiology: changes in dependence and recovery. Alcohol. Clin. Exp. Res. 2005;29(8):1504–1513. doi: 10.1097/01.alc.0000175013.50644.61. [DOI] [PubMed] [Google Scholar]
- Durazzo T.C. Serial longitudinal magnetic resonance imaging data indicate non-linear regional gray matter volume recovery in abstinent alcohol-dependent individuals. Addict. Biol. 2015;20(5):956–967. doi: 10.1111/adb.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman S.D. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- George O. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. PNAS. 2012;109(44):18156–18161. doi: 10.1073/pnas.1116523109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei H.T., Barbas H. Pathways for Emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115(4):1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Gutwinski S. Understanding left-handedness. Dtsch. Arztebl Int. 2011;108(50):849–853. doi: 10.3238/arztebl.2011.0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.P., Figueiredo H., Mueller N.K., Ulrich-Lai Y., Ostrander M.M., Choi D.C., Cullinan W.E. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo–pituitary–adrenocortical responsiveness. Front. Neuroendocrinol. 2003;24(3):151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Higley A.E. Craving in response to stress induction in a human laboratory paradigm predicts treatment outcome in alcohol-dependent individuals. Psychopharmacol. Berl. 2011;218(1):121–129. doi: 10.1007/s00213-011-2355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern S. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33(4):517–529. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienast T. Dopamine-modulated aversive emotion processing fails in alcohol-dependent patients. Pharmacopsychiatry. 2013;46(04):130–136. doi: 10.1055/s-0032-1331747. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Pirke K.M., Hellhammer D.H. The 'Trier Social Stress Test'-A tool for investigating psychobiological stress responses in laboratory settings. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Koob G.F. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr. Top. Behav. Neurosci. 2013;13:3–30. doi: 10.1007/7854_2011_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G., Kreek M.J. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am. J. Psychiatry. 2007;164(8):1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F., Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278(5335):52. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law B. Craving mediates stress in predicting lapse during alcohol dependence treatment. Alcohol. Clin. Exp. Res. 2016;40(5):1058–1064. doi: 10.1111/acer.13034. [DOI] [PubMed] [Google Scholar]
- Leech R., Sharp D.J. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137(Pt1):12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl K.M. Recent binge drinking predicts smaller cerebellar volumes in adolescents. Psychiatry Res. 2013;211(1):17–23. doi: 10.1016/j.pscychresns.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo W.R. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol. Clin. Exp. Res. 2000;24(6):651–658. [PubMed] [Google Scholar]
- Maurage P. The neural network sustaining crossmodal integration is impaired in alcohol-dependence: an fMRI study. Cortex. 2013;49(6):1610–1626. doi: 10.1016/j.cortex.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Padula, C.B., et al., Functional and Structual Neural Connectivity of Affective Processing in Alcohol Dependence: a Pilot Multimodal Imaging Study. (Under Review).
- Park J., Park D.C., Polk T.A. Parietal functional connectivity in numerical cognition. Cereb. Cortex. 2013;23(9):2127–2135. doi: 10.1093/cercor/bhs193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S.C. Cerebellar white matter after long-term ethanol consumption in mice. J. Stud. Alcohol. 1990;51(1):14–18. doi: 10.15288/jsa.1990.51.14. [DOI] [PubMed] [Google Scholar]
- Pilowsky D.J., Keyes K.M., Hasin D.S. Adverse childhood events and lifetime alcohol dependence. Am. J. Public Health. 2009;99(2):258–263. doi: 10.2105/AJPH.2008.139006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom M.J., Pfefferbaum A. Magnetic resonance imaging of the living brain: evidence for brain degeneration among alcoholics and recovery with abstinence. Alcohol Res. Health. 2008;31(4):362–376. [PMC free article] [PubMed] [Google Scholar]
- Seo D. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry. 2013;70(7):727–739. doi: 10.1001/jamapsychiatry.2013.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrien D.J., Sovijarvi-Spape M.M. Cognitive control of response inhibition and switching: hemispheric lateralization and hand preference. Brain Cogn. 2013;82(3):283–290. doi: 10.1016/j.bandc.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Soto D., Rotshtein P., Kanai R. Parietal structure and function explain human variation in working memory biases of visual attention. Neuroimage. 2014;89:289–296. doi: 10.1016/j.neuroimage.2013.11.036. [DOI] [PubMed] [Google Scholar]
- Stephens M.A., Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res. 2012;34(4):468–483. [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Thieme; 1988. Co-planar Stereotaxic Atlas of the Human Brain: 3-D Proportional System: an Approach to Cerebral Imaging (Thieme Classics) [Google Scholar]
- Tartter M.A., Ray L.A. A prospective study of stress and alcohol craving in heavy drinkers. Pharmacol. Biochem. Behav. 2012;101(4):625–631. doi: 10.1016/j.pbb.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Thayer J.F. Alcohol use, urinary cortisol, and heart rate variability in apparently healthy men: evidence for impaired inhibitory control of the HPA axis in heavy drinkers. Int. J. Psychophysiol. 2006;59(3):244–250. doi: 10.1016/j.ijpsycho.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. Aging and functional brain networks. Mol. Psychiatry. 2012;17(5):471. doi: 10.1038/mp.2011.81. 549-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingerhoets G. Cerebral lateralization of praxis in right- and left-handedness: same pattern, different strength. Hum. Brain Mapp. 2012;33(4):763–777. doi: 10.1002/hbm.21247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahov D. Increased use of cigarettes, alcohol, and marijuana among Manhattan, New York residents after september 11th terrorist attacks. Am. J. Epidemiol. 2002;155(11):988–996. doi: 10.1093/aje/155.11.988. [DOI] [PubMed] [Google Scholar]
- Vlahov D. Consumption of cigarettes, alcohol, and marijuana among New York city residents six months after the september 11 terrorist attacks. Am. J. Drug Alcohol Abuse. 2004;30(2):385–407. doi: 10.1081/ada-120037384. [DOI] [PubMed] [Google Scholar]
- Yang H. Altered neural processing of threat in alcohol-dependent men. Alcohol. Clin. Exp. Res. 2013;37(12):2029–2038. doi: 10.1111/acer.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]