Abstract
Pulmonary alveolar microlithiasis (PAM) is an uncommon genetic disorder associated with alveolar cell injury. This injury is caused in most cases by inactivating mutations in SLC34A2 gene, which is responsible for the production of a sodium-dependent phosphate co-transporter. The dysfunction or deficiency of this transporter leads to the aggregation of local phosphate intra-alveolarly and formation of microliths. Most of the patients are asymptomatic at the time of the diagnosis but as the disease progress it leads to fatal respiratory or cardiac failure. We describe a case of a 63-year-old man referred to our department for a surgical lung biopsy. He has been symptomatic for one year with progressive shortness of breath and deteriorating exercise tolerance. The imaging was suggestive of extensive interstitial bilateral lung disease. Histological findings after the lung biopsy by video-assisted thoracic surgery (VATS) established the diagnosis of pulmonary alveolar microlithiasis. His sister suffered from the same disease and passed away at the age of 54. It is remarkably rare for PAM to have such a late onset with a previous normal X-ray and only a few cases have been reported worldwide.
Keywords: Genetic interstitial lung disease, Pulmonary alveolar microlithiasis, VATS biopsy
1. Introduction
Pulmonary alveolar microlithiasis (PAM) is a pulmonary entity included in the heterogeneous group of metabolic lung diseases. It is characterized by biochemical abnormalities of the alveolar type II cells that cause bilateral intra-alveolar calcium and phosphate deposition through the lung parenchyma [1]. Francis Harbitz first mentioned the existence of ''Extensive calcification of the lungs as a distinct disease'' in January of 1918 (Harbitz' syndrome) [2]. However, pulmonary alveolar microlithiasis was recognized as a unique pathological entity fifteen years later by Ludwig Puhr [3]. There have been reported approximately 1000 cases worldwide and most of them with predominance of younger age (mean age at diagnosis 35 years) [4]. Although PAM follows an autosomal recessive inheritance pattern, environmental factors seem to accelerate the progression of this disease (heavy smoking, infection) [5]. In most cases the only affected organs causing clinical symptoms or abnormal radiological findings are the lungs, where intra-alveolar microliths are formed. However, extrapulmonary calcifications such as medullary nephrolithiasis, calcifications in lumbar sympathetic chain, tesicles, punctuate calcifications in seminal vesicles, periurethral and epididymal calcifications and cardiac Co-morbidities are also reported in association with PAN [6]. Typical feature of PAM is the dissociation between clinical and radiological findings. The characteristic picture of PAM on the chest radiographs is most times an incidental finding in asymptomatic patients. However, as the disease progresses, the patient presents with cyanosis, clubbing, dyspnea followed by dry cough, chest pain, hemoptysis, weight loss and weakness. Gradually it leads to fatal respiratory or cardiac failure [7]. Regarding the chest radiography, ''sandstorm'' appearance (calcific micro nodules involving in middle and lower zones of both lungs) and ''black pleura'' sign (an area of linear hyperlucency caused by sub pleural cysts) are usually the first findings of chest CT [8]. Herein, we report a case of extremely late onset of pulmonary alveolar microlithiasis which was diagnosed with VATS biopsy of the lung.
2. Illustrative case presentation
A 63 year old man working as a salesman was referred to the respiratory team for clinical management of his progressive shortness of breath and deteriorating exercise tolerance that significantly worsened over the last 12 months. In terms of medical history, he mentioned an occasional left-sided pleuritic chest pain with no other exercise related pain, no peripheral swelling, no weight loss and no night sweats. He suffered for one episode of spontaneous pneumothorax on each side during the last year which was treated conservatively. He also mentioned diet controlled diabetes and hypercholesterolemia managed with Simvastatin. He is a non-smoker, he denied exposure to birds or pets, he does not have any allergies and he has no history of tuberculosis. With reference to the family history, his sister passed away from pulmonary alveolar microlithiasis at the age of 54. His 37 year old daughter is treated for hypertension and hypercholesterolemia with no evidence of lung disease. No information was available in regards of his parents. On physical examination, there were marked discrete inspiratory crackles, cyanosis and digital clubbing, while all laboratory investigations were within normal limits. His chest X-ray (Fig. 1) was suggestive of extensive interstitial bilateral lung disease and the differential diagnosis was suggested to be either a microlithiasis or an atypical infection. A high resolution computed tomography (HRCT) (Fig. 2) of the chest was performed and revealed several calcifications throughout the parenchyma, ground-glass opacities, sub-pleural cystic changes and calcified interlobular septa. Considering the findings on the CT scan, his case was discussed in our multidisciplinary meeting and patient was referred to our Department for surgical lung biopsy. After written consent was obtained from the patient, a right uniportal VATS biopsy of the lung was performed. The right lung was diffusely involved and the parenchyma was thickened with occasional superficial bulla. The postoperative course was unremarkable and the patient was discharged in stable condition on the 2nd postoperative day.
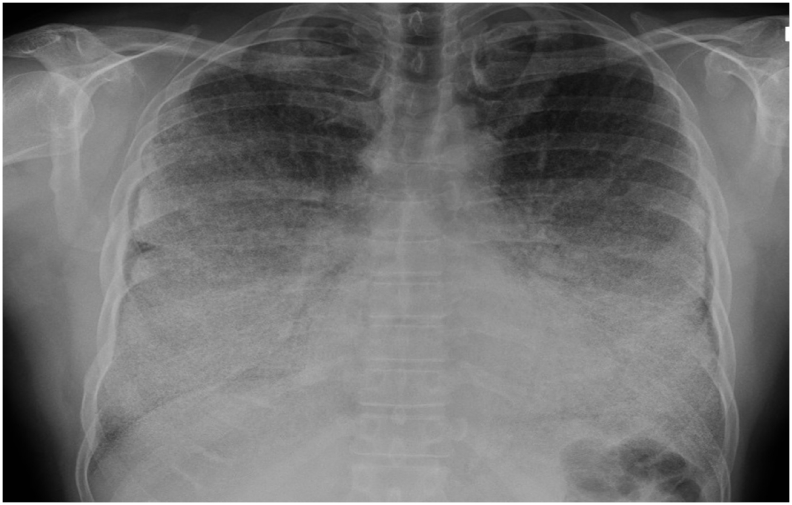
Fig. 1.
Chest radiographs in anteroposterior incidence shows a diffuse symmetric lesion with confluence of dense micronodular infiltrate, especially middle and lower zones of both lungs.
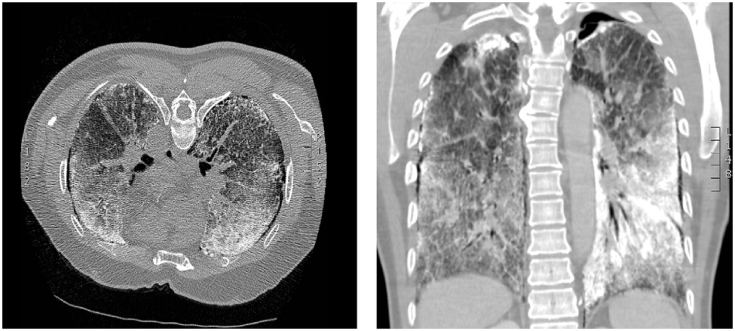
Fig. 2.
A high resolution computed tomography (HRCT) of the chest revealing innumerable calcifications throughout the parenchyma, ground-glass opacities, subpleural cystic changes and calcified interlobular septa.
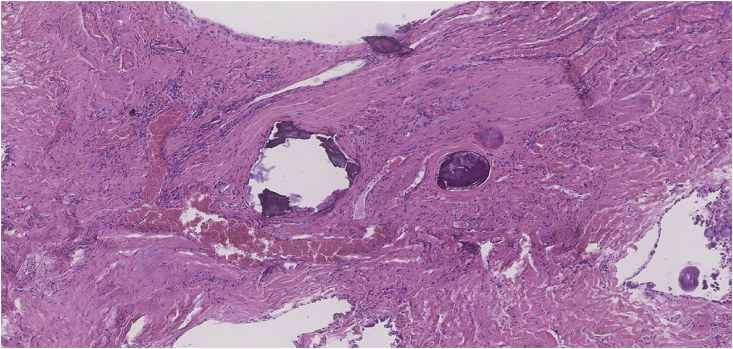
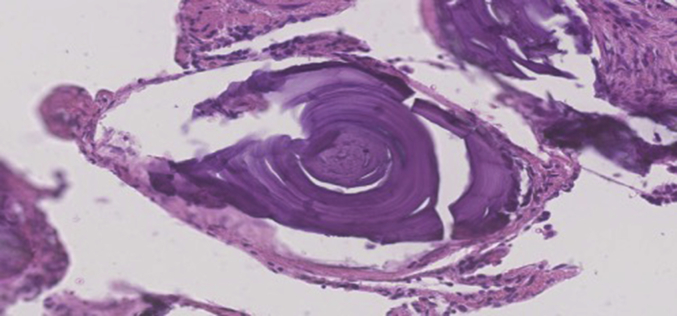
Subsequent histological examination of the specimens was performed. Section showed lung parenchyma with marked sub-pleural and interstitial fibrosis and patchy chronic inflammation. The alveolar spaces were filled with numerous calcified bodies which showed concentric lamination and radial striations (Fig. 3, Fig. 4). Microliths were also present within the areas of fibrosis (Fig. 5, Fig. 6). There was no evidence of atypia or malignancy. The diagnosis of pulmonary alveolar microlithiasis was made considering the histological features and the radiological image. Regarding the treatment plan, during a multidisciplinary meeting the conservative approach was decided with a re-evaluation period of 6 months. The option of lung transplantation was rejected due to patients' age.
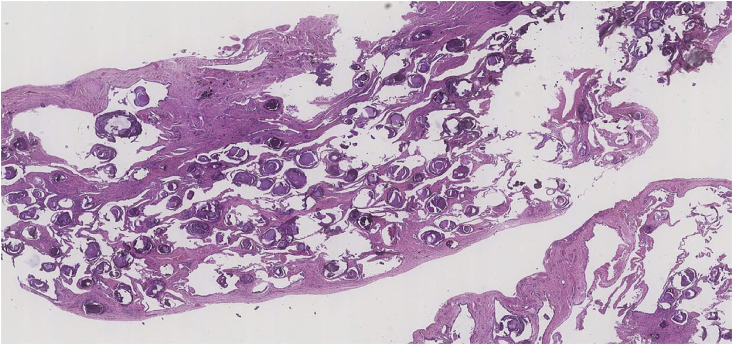
Fig. 3.
Pulmonary alveolar microlithiasis (H&E ×12.5 Magnification) Lung parenchyma showing numerous calcified bodies within alveolar air spaces. Subpleural and interstitial fibrosis is also seen.
Fig. 4.
Pulmonary alveolar microlithiasis (H&E ×50 Magnification) Alveoli filled by numerous heavily calcified lamellar bodies.
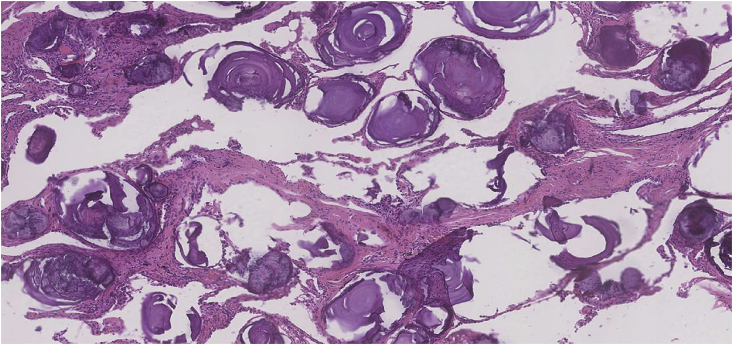
Fig. 5.
Pulmonary alveolar microlithiasis (H&E ×50 Magnification) Microlith deposition within areas of fibrosis with patchy chronic inflammation.
Fig. 6.
Epithelioid haemangioendothelioma (H&E ×200 Magnification) Alveolar microlith showing concentric laminations.
3. Discussion
The spectrum of pathologic entities which can appear as pulmonary calcifications is wide and encompasses not only neoplastic but also non-neoplastic and iatrogenic or exposure-related causes. Pulmonary alveolar microlithiasis is a rare disease included in non-neoplastic causes of pulmonary calcifications and is characterized by intra-alveolar concretion of calcium-phosphate microliths [9]. It follows an autosomal recessive inheritance manner with no gender predominance and the highest number of cases were recorded in Turkey, China, Japan, India and Italy [4]. Mariotta et al. reported that PAM usually presented in middle age patients (35.8%of patients were diagnosed before 20 years of age and 88.2% before 50 years of age) [10]. According to the genetic hypothesis, PAM is caused by mutations in SLC34A2 gene, which is mapped to locus 4p15.31-p15.2. There have been identified several mutations in SLC34A2 gene [11]. Although this gene is also expressed in small intestine, kidneys, mammary glands, pancreas, ovaries, liver, testes, placenta and prostate, extrapulmonary manifestations are not initially identified [12]. SLC34A2 gene encodes a type IIb sodium phosphate co-transporter that plays an essential role in the clearance of phospholipids of the alveolar space. Dysfunctional or deficient transporter leads in aggregation of local phosphate intra-alveolarly and formation of microliths [6]. The accumulation of these microliths in the alveolar develops the typical for the disease radiological findings. In most cases PAM is an incidental finding of chest X-ray for other purposes. Typical features of PAM in chest X-ray are the ''sandstorm'' appearance caused by the involving of calcific micro nodules in middle and lower zones of both lungs and ''black pleura'' sign which is an area of linear hyperlucency formed by sub pleural cysts. Common features found in high resolution computed tomography (HRCT) are micro nodules, interlobular septal thickening, sub pleural interstitial thickening, ground glass opacity, areas of centrilobular emphysema, pleural calcification and peripheral bronchiectasis [8]. Apical bullae are another typical feature with an associated pneumothorax. Despite the consistent imaging findings, clinical course is variable. Patients are asymptomatic in early stages of PAM, however as the disease progresses they present with cyanosis, clubbing, dyspnea followed by dry cough, chest pain, sporadic hemoptysis, weight loss and weakness, and gradually leads to fatal respiratory or cardiac failure. The progress of PAM is usually slow but environmental factors seem to accelerate the aggravation of this disease (heavy smoking, infection) [5]. Recently it has been proved that elevations in the serum concentrations of surfactant protein A and D can be ancillary to diagnosis and monitoring of the diseases progression [13].
Results from long-term follow-up reveal poor prognosis for patients with PAM as an effective treatment is yet not available. Corticosteroids and therapeutic bronchoalveolar lavage appear to have inadequate results [14]. Disodium etidronate seems to benefit lungs' function and radiological images but further research should be done to evidence their therapeutic use [15], [16], [17]. Management of PAM should include supplemental oxygen therapy and influenza and pneumococcal vaccinations [18]. Based on the reported cases, lung transplantation remains the only treatment for end-stage cases, as reccurence of the disease was not reported yet [19], [20], [21]. Gene therapy could be a promising solution and should be discussed in the future.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Chung M.J. Metabolic lung disease: imaging and histopathologic findings. Eur. J. Radiol. 2005;54(2):233–245. doi: 10.1016/j.ejrad.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Harbitz E. Extensive calcification of the lungs as a distinct diseases. Arch. Intern Med. 1918;21:139–146. [Google Scholar]
- 3.Puhr L. Mikrolithiasis alaveolaris pulmonum. Pulm. Alaveolar Microlithiasis. Virchows Arch. Pathol. Anat. 1933;290:156. [Google Scholar]
- 4.Castellana G., Castellana G., Gentile M., Castellana R., Resta O. Pulmonary alveolar microlithiasis: review of the 1022 cases reported worldwide. Eur. Respir. Rev. 2015;24(138):607–620. doi: 10.1183/16000617.0036-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corut A, Senyigit A, Ugur SA, Altin S, Ozcelik U, Calisir H, et al. Mutations in SLC34A2 Cause Pulmonary Alveolar Microlithiasis and Are Possibly Associated with Testicular Microlithiasis. 2006;79(October):650–6. [DOI] [PMC free article] [PubMed]
- 6.Khaladkar S.M., Kondapavuluri S.K., Kamal A., Kalra R., Kuber R.S. Pulmonary alveolar microlithiasis - clinico-radiological dissociation - a case report with radiological review. J. Radiol. Case Rep. [Internet] 2016;10(1):14–21. doi: 10.3941/jrcr.v10i1.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferreira Francisco F.A., Pereira E Silva J.L., Hochhegger B., Zanetti G., Marchiori E. Pulmonary alveolar microlithiasis. State-of-the-art review. Respir. Med. 2013;107(1):1–9. doi: 10.1016/j.rmed.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Deniz O., Ors F., Tozkoparan E., Ozcan A., Gumus S., Bozlar U. High resolution computed tomographic features of pulmonary alveolar microlithiasis. Eur. J. Radiol. 2005;55(3):452–460. doi: 10.1016/j.ejrad.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Amin S.B., Slater R., Mohammed T.L.H. Pulmonary calcifications: a pictorial review and approach to formulating a differential diagnosis. Curr. Probl. Diagn Radiol. 2015;44(3):267–276. doi: 10.1067/j.cpradiol.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Mariotta S., Guidi L., Papale M., Ricci A., Bisetti A. Pulmonary alveolar microlithiasis: review of Italian reports. Eur. J. Epidemiol. 1997;13(5):587–590. doi: 10.1023/a:1007399812798. [DOI] [PubMed] [Google Scholar]
- 11.Yin X., Wang H., Wu D., Zhao G., Shao J., Dai Y. SLC34A2 Gene mutation of pulmonary alveolar microlithiasis: report of four cases and review of literatures. Respir. Med. 2013;107(2):217–222. doi: 10.1016/j.rmed.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi H., Chiba H., Shiratori M., Tachibana M., Abe S. Elevated serum surfactant protein A and D in pulmonary alveolar microlithiasis. Respirology. 2006;11(3):330–333. doi: 10.1111/j.1440-1843.2006.00844.x. [DOI] [PubMed] [Google Scholar]
- 13.Murer H., Forster I., Biber J. The sodium phosphate cotransporter family SLC34. Pflugers Arch. Eur. J. Physiol. 2004;447(5):763–767. doi: 10.1007/s00424-003-1072-5. [DOI] [PubMed] [Google Scholar]
- 14.Tachibana T., Hagiwara K., Johkoh T. Pulmonary alveolar microlithiasis: review and management. Curr. Opin. Pulm. Med. 2009;15(5):486–490. doi: 10.1097/MCP.0b013e32832d03bb. [DOI] [PubMed] [Google Scholar]
- 15.Ozcelik U., Yalcin E., Ariyurek M., Ersoz E.D., Cinel G., Gulhan B. Long-term results of disodium etidronate treatment in pulmonary alveolar microlithiasis. Pediatr. Pulmonol. 2010;45(5):514–517. doi: 10.1002/ppul.21209. [DOI] [PubMed] [Google Scholar]
- 16.Cakir E., Gedik A.H., Ozdemir A., Buyukpinarbasili N., Bilgin M., Ozgen I.T. Response to disodium etidronate treatment in three siblings with pulmonary alveolar microlithiasis. Respiration. 2015;89(6):583–586. doi: 10.1159/000375464. [DOI] [PubMed] [Google Scholar]
- 17.Gocmen A., Toppare M.F., Kiper N. Treatment of pulmonary alveolar microlithiasis with a diphosphonate. Respiration. 1992;59:250–254. doi: 10.1159/000196068. [DOI] [PubMed] [Google Scholar]
- 18.Saito A., McCormack F.X. Pulmonary alveolar microlithiasis. Clin. Chest Med. 2016;37(3):441–448. doi: 10.1016/j.ccm.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stamatis G., Zerkowski H., Doetsch N., Greschuchna D., Konietzko N., Reidemeister J.C. Sequential bilateral lung transplantation for pulmonary alveolar microlithiasis. Ann. Thorac. Surg. 1993;56(4):972–975. doi: 10.1016/0003-4975(93)90370-w. [DOI] [PubMed] [Google Scholar]
- 20.Klikovits T., Slama A., Hoetzenecker K., Waseda R., Lambers C., Murakoezy G. A rare indication for lung transplantation - pulmonary alveolar microlithiasis: institutional experience of five consecutive cases. Clin. Transpl. 2016;30(4):429–434. doi: 10.1111/ctr.12705. [DOI] [PubMed] [Google Scholar]
- 21.Shigemura N., Bermudez C., Hattler B.G., Johnson B., Crespo M., Pilewski J., Toyoda Y. 2010. Lung transplantation for pulmonary alveolar microlithiasis. J. Thorac. Cardiovasc. Surg. Mar. 2010;139(3):e50–e52. doi: 10.1016/j.jtcvs.2008.07.066. [DOI] [PubMed] [Google Scholar]